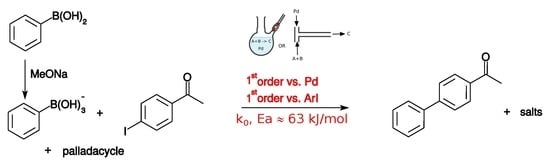
Kinetic Study of the Herrmann–Beller Palladacycle-Catalyzed Suzuki–Miyaura Coupling of 4-Iodoacetophenone and Phenylboronic Acid
Abstract
1. Introduction
2. Results
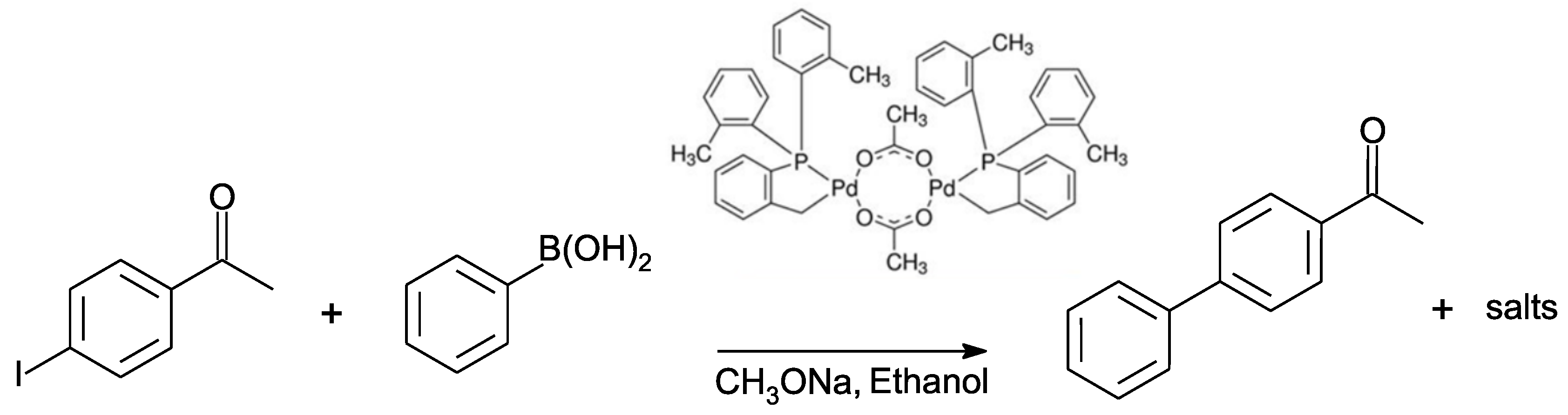
2.1. Experimental Kinetic Study
2.1.1. Preliminary Observations
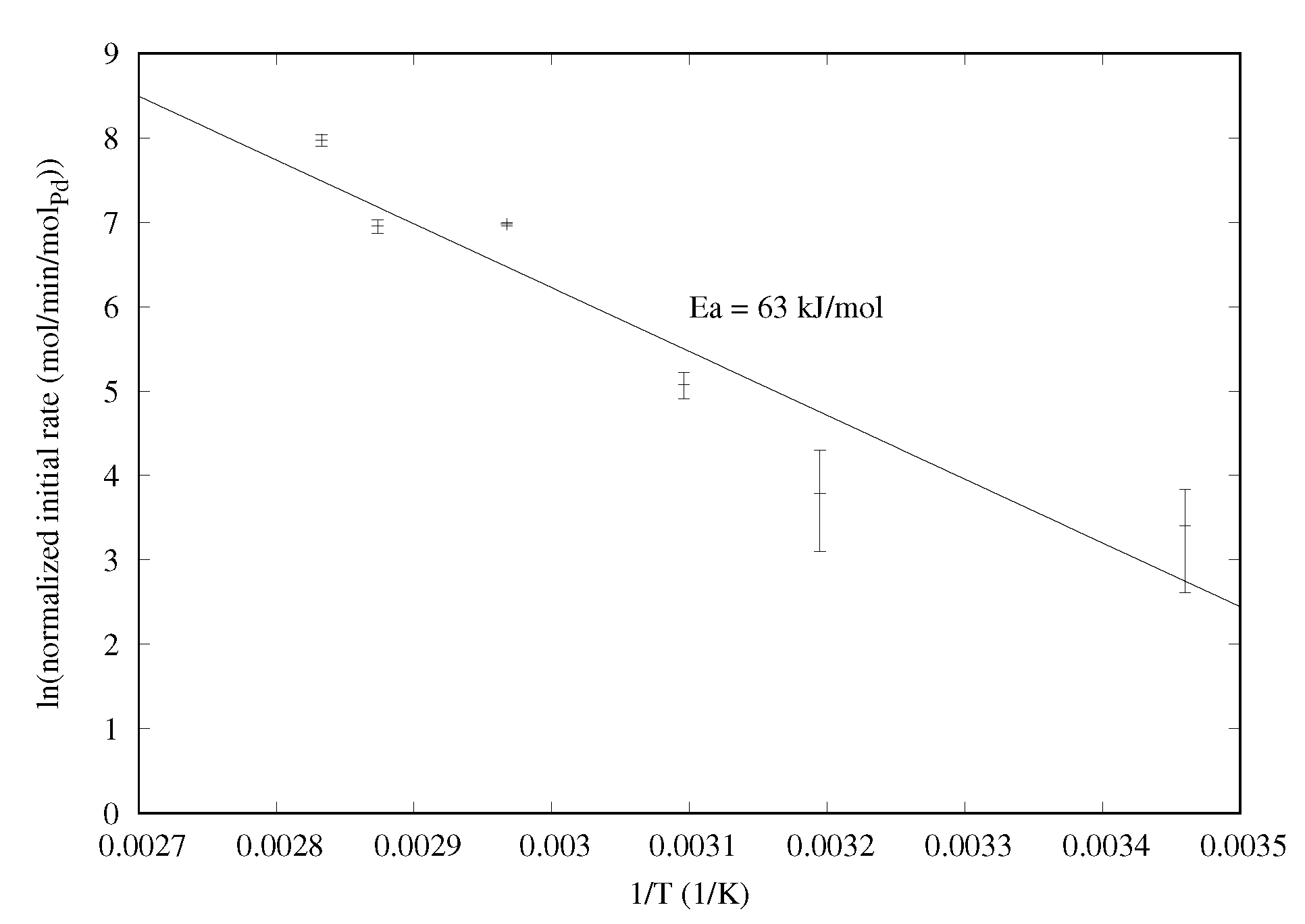
2.1.2. Temperature Effect and Activation Energy
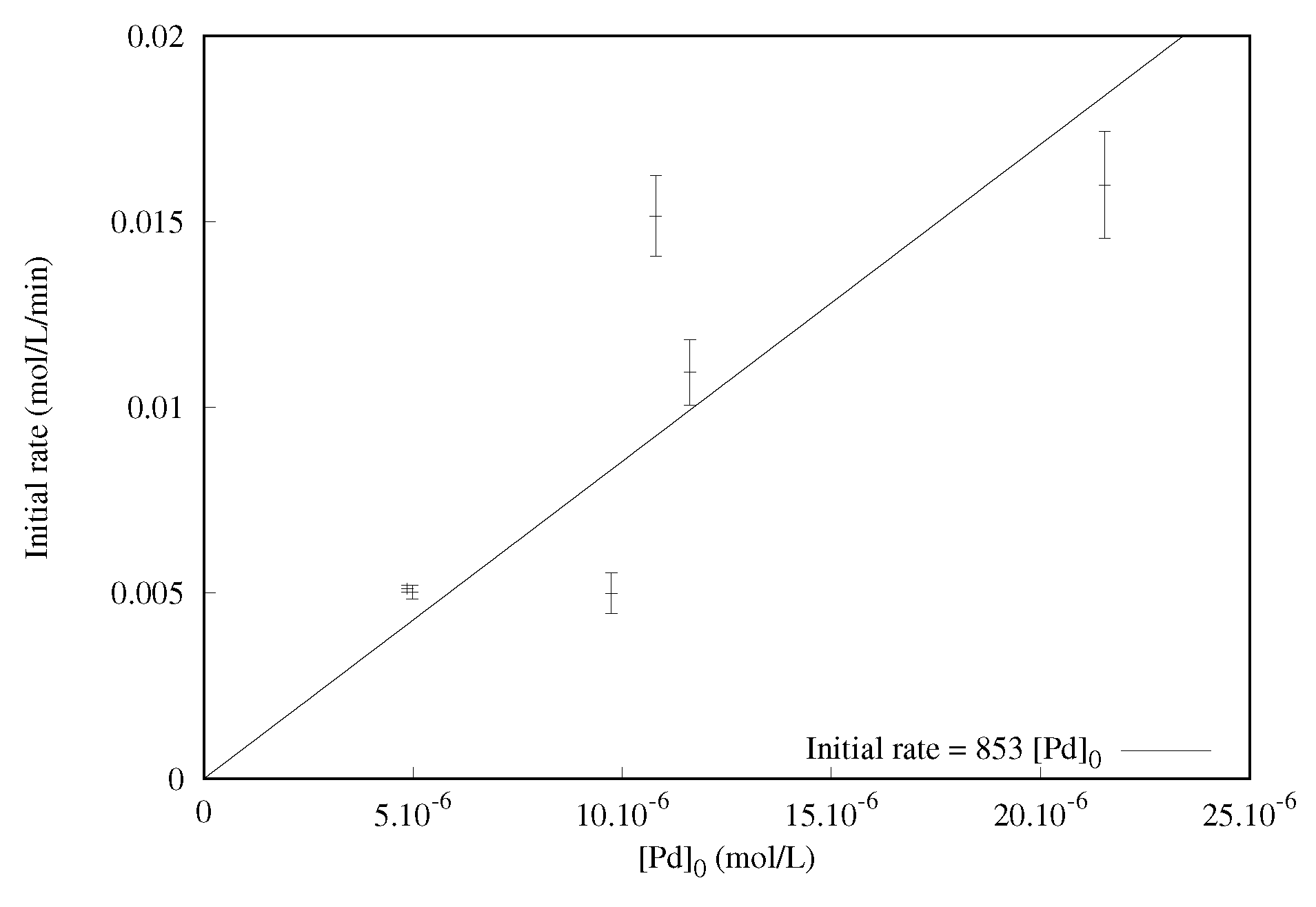
2.1.3. Effect of the Catalyst Concentration
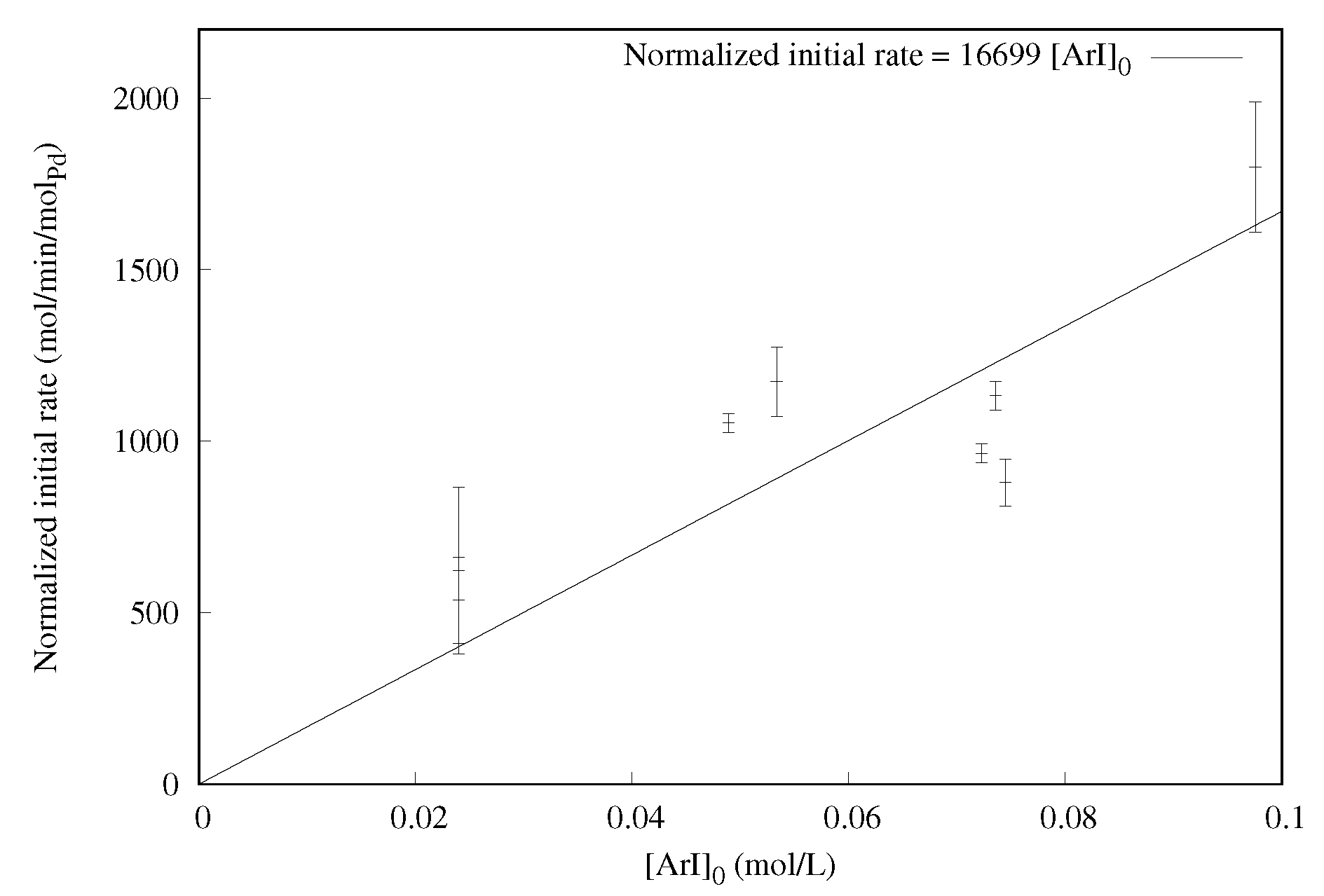
2.1.4. Effect of the Aryl Halide Concentration
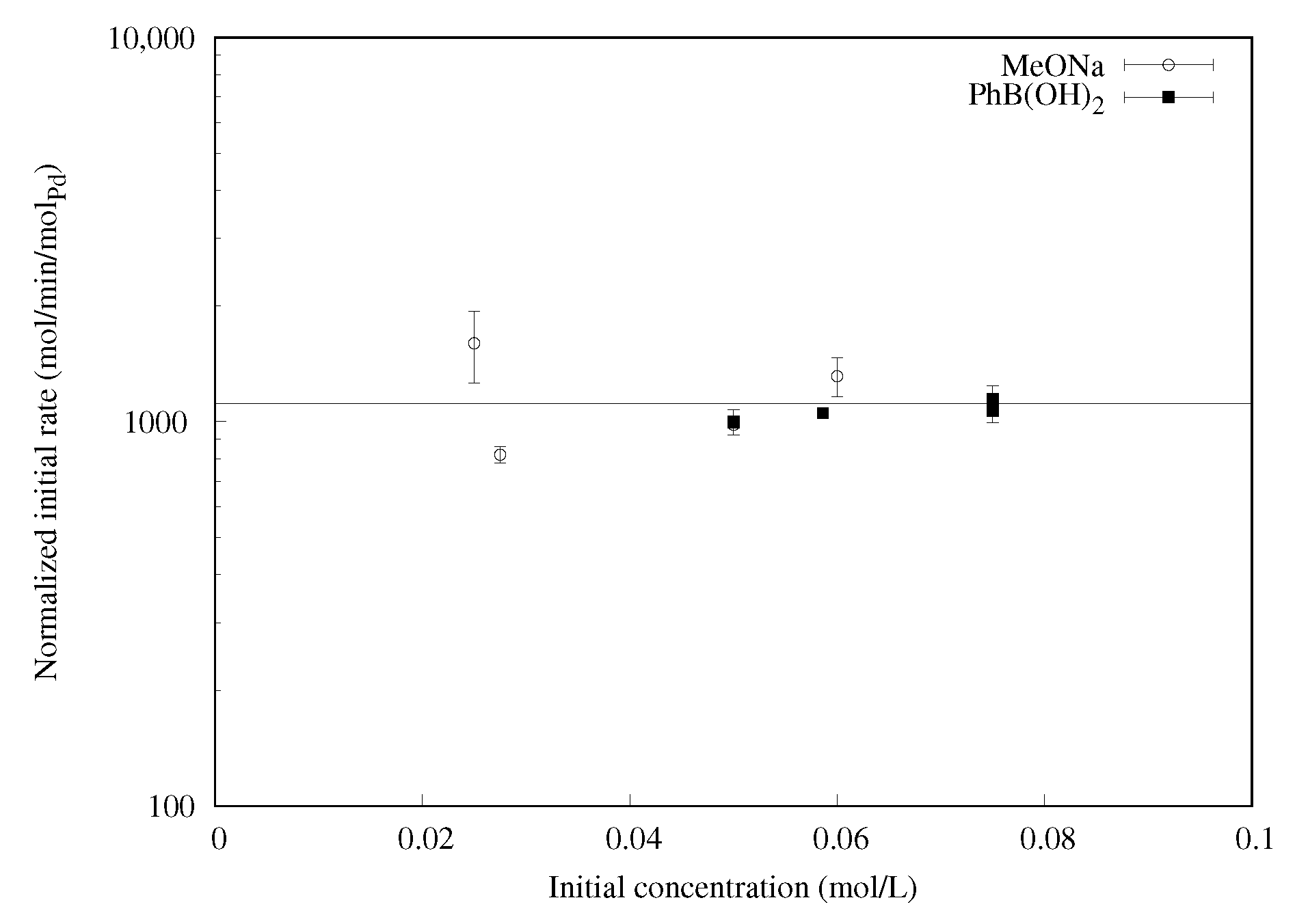
2.1.5. Effect of Base and Phenylboronic Acid Concentrations
2.2. Modeling and Discussion
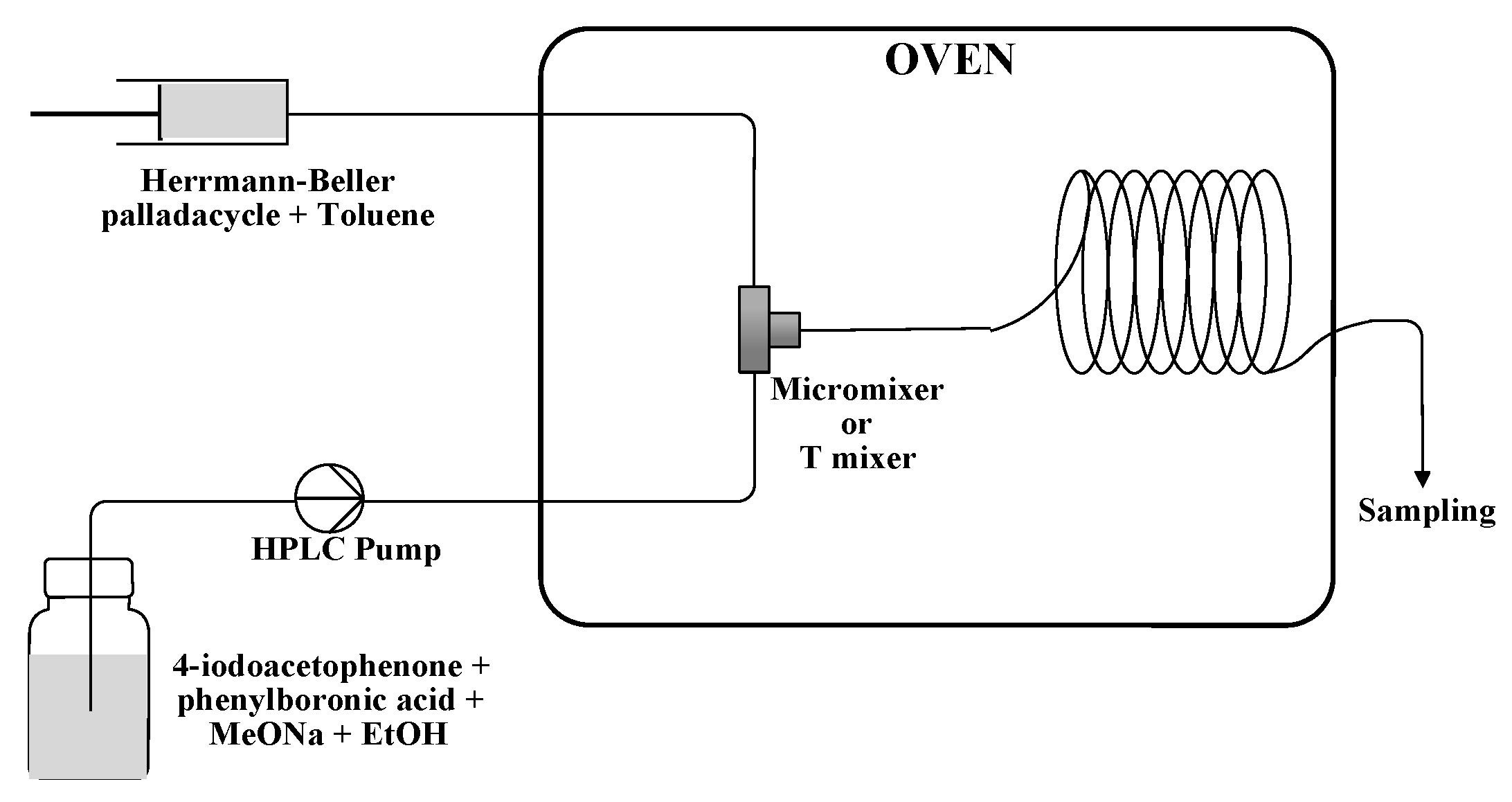
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| SM | Suzuki–Miyaura |
| ArI | 4-iodoacetophenone |
| Concentration of mononuclear palladium | |
| Ea | activation energy |
| r | rate of product formation (mol/min) |
References
- Schneider, N.; Lowe, D.M.; Sayle, R.A.; Tarselli, M.A.; Landrum, G.A. Big Data from Pharmaceutical Patents: A Computational Analysis of Medicinal Chemists’ Bread and Butter. J. Med. Chem. 2016, 59, 4385–4402. [Google Scholar] [CrossRef] [PubMed]
- Heck, R.F. Palladium-catalyzed reactions of organic halides with olefins. Acc. Chem. Res. 1979, 12, 146–151. [Google Scholar] [CrossRef]
- Negishi, E.i. Magical Power of Transition Metals: Past, Present, and Future (Nobel Lecture). Angew. Chem. Int. Ed. 2011, 50, 6738–6764. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A. Cross-Coupling Reactions Of Organoboranes: An Easy Way To Construct C-C Bonds (Nobel Lecture). Angew. Chem. Int. Ed. 2011, 50, 6722–6737. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.F.; Anbarasan, P.; Neumann, H.; Beller, M. From Noble Metal to Nobel Prize: Palladium-Catalyzed Coupling Reactions as Key Methods in Organic Synthesis. Angew. Chem. Int. Ed. 2010, 49, 9047–9050. [Google Scholar] [CrossRef] [PubMed]
- Widegren, J.A.; Finke, R.G. A review of the problem of distinguishing true homogeneous catalysis from soluble or other metal-particle heterogeneous catalysis under reducing conditions. J. Mol. Catal. A Chem. 2003, 198, 317–341. [Google Scholar] [CrossRef]
- Phan, N.T.S.; Van Der Sluys, M.; Jones, C.W. On the Nature of the Active Species in Palladium Catalyzed Mizoroki-Heck and Suzuki-Miyaura Couplings - Homogeneous or Heterogeneous Catalysis, A Critical Review. Adv. Synth. Catal. 2006, 348, 609–679. [Google Scholar] [CrossRef]
- Pérez-Lorenzo, M. Palladium Nanoparticles as Efficient Catalysts for Suzuki Cross-Coupling Reactions. J. Phys. Chem. Lett. 2012, 3, 167–174. [Google Scholar] [CrossRef]
- Zotto, A.D.; Zuccaccia, D. Metallic palladium, PdO, and palladium supported on metal oxides for the Suzuki-Miyaura cross-coupling reaction: A unified view of the process of formation of the catalytically active species in solution. Catal. Sci. Technol. 2017, 7, 3934–3951. [Google Scholar] [CrossRef]
- Len, C.; Bruniaux, S.; Delbecq, F.; Parmar, V.S. Palladium-Catalyzed Suzuki-Miyaura Cross-Coupling in Continuous Flow. Catalysts 2017, 7, 146. [Google Scholar] [CrossRef]
- Hussain, I.; Capricho, J.; Yawer, M.A. Synthesis of Biaryls via Ligand-Free Suzuki-Miyaura Cross-Coupling Reactions: A Review of Homogeneous and Heterogeneous Catalytic Developments. Adv. Synth. Catal. 2016, 358, 3320–3349. [Google Scholar] [CrossRef]
- Bourouina, A.; Meille, V.; de Bellefon, C. A flow split test to discriminating between heterogeneous and homogeneous contributions in Suzuki coupling. J. Flow Chem. 2018, 8, 117–121. [Google Scholar] [CrossRef]
- Bourouina, A.; Meille, V.; de Bellefon, C. About Solid Phase vs. Liquid Phase in Suzuki-Miyaura Reaction. Catalysts 2019, 9, 60. [Google Scholar] [CrossRef]
- Sherwood, J.; Clark, J.H.; Fairlamb, I.J.S.; Slattery, J.M. Solvent effects in palladium catalysed cross-coupling reactions. Green Chem. 2019, 21, 2164–2213. [Google Scholar] [CrossRef]
- Van Vaerenbergh, B.; Lauwaert, J.; Thybaut, J.W.; Vermeir, P.; De Clercq, J. Pd nanoparticle and molecular Pd2+ leaching pathways for a strongly acid versus strongly basic resin supported Pd nanoparticle catalyst in Suzuki coupling. Chem. Eng. J. 2019, 374, 576–588. [Google Scholar] [CrossRef]
- Amatore, C.; Jutand, A.; Le Duc, G. Kinetic Data for the Transmetalation/Reductive Elimination in Palladium-Catalyzed Suzuki-Miyaura Reactions: Unexpected Triple Role of Hydroxide Ions Used as Base. Chem. Eur. J. 2011, 17, 2492–2503. [Google Scholar] [CrossRef]
- Amatore, C.; Le Duc, G.; Jutand, A. Mechanism of Palladium-Catalyzed Suzuki-Miyaura Reactions: Multiple and Antagonistic Roles of Anionic “Bases” and Their Countercations. Chem. A Eur. J. 2013, 19, 10082–10093. [Google Scholar] [CrossRef]
- Thomas, A.A.; Zahrt, A.F.; Delaney, C.P.; Denmark, S.E. Elucidating the Role of the Boronic Esters in the Suzuki-Miyaura Reaction: Structural, Kinetic, and Computational Investigations. J. Am. Chem. Soc. 2018, 140, 4401–4416. [Google Scholar] [CrossRef]
- Thomas, A.A.; Denmark, S.E. Pre-transmetalation intermediates in the Suzuki-Miyaura reaction revealed: The missing link. Science 2016, 352, 329–332. [Google Scholar] [CrossRef]
- Matos, K.; Soderquist, J.A. Alkylboranes in the Suzuki-Miyaura Coupling: Stereochemical and Mechanistic Studies. J. Org. Chem. 1998, 63, 461–470. [Google Scholar] [CrossRef]
- Larina, E.V.; Kurokhtina, A.A.; Schmidt, A.F. Approach to the Determination of Kinetic Order of Catalyst Deactivation: Observation of Unusual Kinetics in the Suzuki- Miyaura Reaction. Mendeleev Commun. 2014, 2, 96–97. [Google Scholar] [CrossRef]
- Alfonso Albiñana, P.; El Haskouri, J.; Dolores Marcos, M.; Estevan, F.; Amorós, P.; Úbeda, M.A.; Pérez-Pla, F. A new efficient, highly dispersed, Pd nanoparticulate silica supported catalyst synthesized from an organometallic precursor. Study of the homogeneous vs. heterogeneous activity in the Suzuki-Miyaura reaction. J. Catal. 2018, 367, 283–295. [Google Scholar] [CrossRef]
- Boztepe, C.; Künkül, A.; Gürbüz, N.; Özdemir, I. The kinetics and mechanism of polymer-based NHC-Pd-pyridine catalyzed heterogeneous Suzuki reaction in aqueous media. Int. J. Chem. Kinet. 2019, 51, 931–942. [Google Scholar] [CrossRef]
- Liu, Y.; Hartman, R.L. Reaction kinetics of a water-soluble palladium-β-cyclodextrin catalyst for a Suzuki-Miyaura cross-coupling in continuous flow. React. Chem. Eng. 2019, 4, 1341–1346. [Google Scholar] [CrossRef]
- Beller, M.; Fischer, H.; Herrmann, W.A.; Öfele, K.; Brossmer, C. Palladacycles as Efficient Catalysts for Aryl Coupling Reactions. Angew. Chem. Int. Ed. Engl. 1995, 34, 1848–1849. [Google Scholar] [CrossRef]
- Fauvarque, J.F.; Pflüger, F.; Troupel, M. Kinetics of oxidative addition of zerovalent palladium to aromatic iodides. J. Organomet. Chem. 1981, 208, 419–427. [Google Scholar] [CrossRef]
- Amatore, C.; Pfluger, F. Mechanism of oxidative addition of palladium(0) with aromatic iodides in toluene, monitored at ultramicroelectrodes. Organometallics 1990, 9, 2276–2282. [Google Scholar] [CrossRef]
- Perego, L.A.; Payard, P.A.; Haddou, B.; Ciofini, I.; Grimaud, L. Evidence for a Cooperative Mechanism Involving Two Palladium(0) Centers in the Oxidative Addition of Iodoarenes. Chem. Eur. J. 2018, 24, 2192–2199. [Google Scholar] [CrossRef]
- Hoops, S.; Sahle, S.; Gauges, R.; Lee, C.; Pahle, J.; Simus, N.; Singhal, M.; Xu, L.; Mendes, P.; Kummer, U. COPASI—A COmplex PAthway SImulator. Bioinformatics 2006, 22, 3067–3074. [Google Scholar] [CrossRef]
- Schmidt, A.F.; Kurokhtina, A.A.; Smirnov, V.V.; Larina, E.V.; Chechil, E.V. Competing reaction method for identification of fast and slow steps of catalytic cycles: Application to heck and Suzuki reactions. Kinet. Catal. 2012, 53, 214–221. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bourouina, A.; Oswald, A.; Lido, V.; Dong, L.; Rataboul, F.; Djakovitch, L.; de Bellefon, C.; Meille, V. Kinetic Study of the Herrmann–Beller Palladacycle-Catalyzed Suzuki–Miyaura Coupling of 4-Iodoacetophenone and Phenylboronic Acid. Catalysts 2020, 10, 989. https://doi.org/10.3390/catal10090989
Bourouina A, Oswald A, Lido V, Dong L, Rataboul F, Djakovitch L, de Bellefon C, Meille V. Kinetic Study of the Herrmann–Beller Palladacycle-Catalyzed Suzuki–Miyaura Coupling of 4-Iodoacetophenone and Phenylboronic Acid. Catalysts. 2020; 10(9):989. https://doi.org/10.3390/catal10090989
Chicago/Turabian StyleBourouina, Amine, Alexis Oswald, Valentin Lido, Lu Dong, Franck Rataboul, Laurent Djakovitch, Claude de Bellefon, and Valérie Meille. 2020. "Kinetic Study of the Herrmann–Beller Palladacycle-Catalyzed Suzuki–Miyaura Coupling of 4-Iodoacetophenone and Phenylboronic Acid" Catalysts 10, no. 9: 989. https://doi.org/10.3390/catal10090989
APA StyleBourouina, A., Oswald, A., Lido, V., Dong, L., Rataboul, F., Djakovitch, L., de Bellefon, C., & Meille, V. (2020). Kinetic Study of the Herrmann–Beller Palladacycle-Catalyzed Suzuki–Miyaura Coupling of 4-Iodoacetophenone and Phenylboronic Acid. Catalysts, 10(9), 989. https://doi.org/10.3390/catal10090989