Kinetic Analysis of the Lipase-Catalyzed Hydrolysis of Erythritol and Pentaerythritol Fatty Acid Esters: A Biotechnological Application for Making Low-Calorie Healthy Food Alternatives
Abstract
1. Introduction
2. Results and Discussion
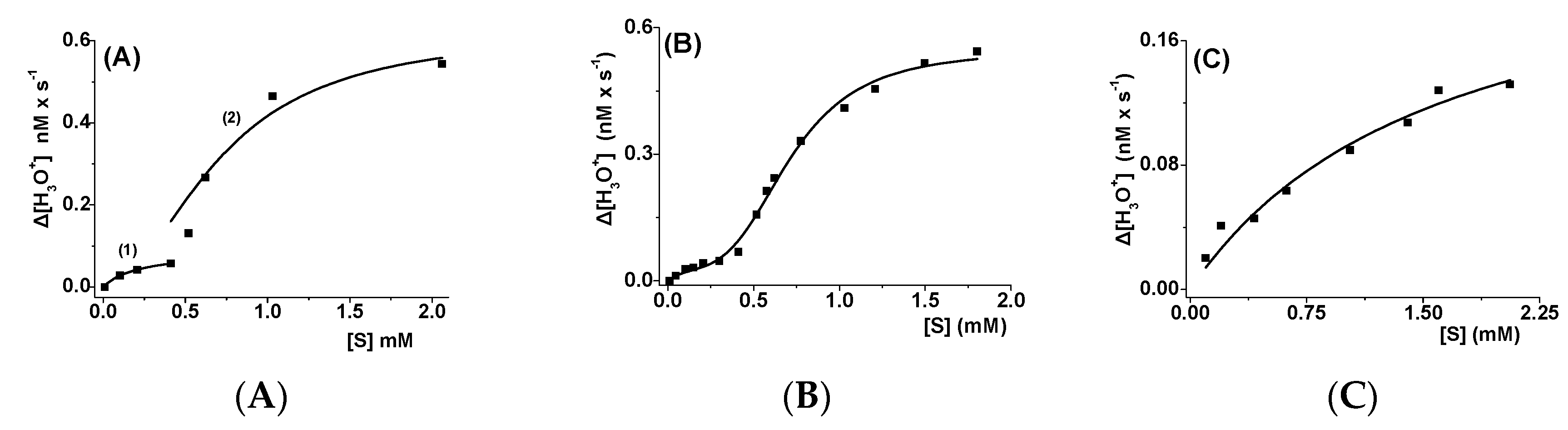
2.1. Hydrolysis of the Tetraesters of Erythritol and Pentaerythritol by PPL and CALB
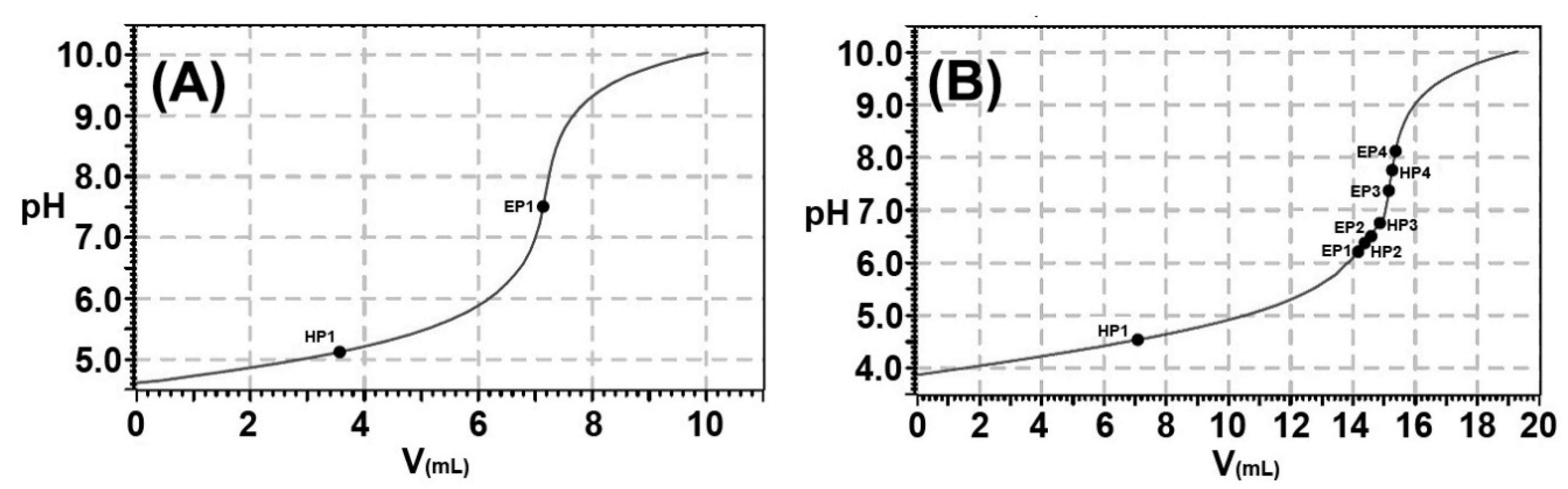
2.2. The Estimated pKa Values of the Released Fatty Acids
3. Materials and Methods
3.1. Materials
3.2. Solutions and Devices
3.3. Kinetic Measurements
3.4. Estimation of the pKa Values
3.5. Analysis and Curve Fitting of the Experimental Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Ethical Approval
References
- Röper, H.; Goossens, J. Erythritol, a New Raw Material for Food and Non-food Applications. Starch/Stärke 1993, 45, 400–405. [Google Scholar] [CrossRef]
- Moon, H.J.; Jeya, M.; Kim, I.W.; Lee, J.K. Biotechnological production of erythritol and its applications. Appl. Microbiol. Biotechnol. 2010, 86, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Hartog, G.J.M.D.; Boots, A.W.; Adam-Perrot, A.; Brouns, F.; Verkooijen, I.W.C.M.; Weseler, A.R.; Haenen, G.R.M.M.; Bast, A. Erythritol is a sweet antioxidant. Nutrition 2010, 26, 449–458. [Google Scholar] [CrossRef]
- Regnat, K.; Mach, R.L.; Mach-Aigner, A.R. Erythritol as sweetener-wherefrom and whereto? Appl. Microbiol. Biotechnol. 2018, 102, 587–595. [Google Scholar] [CrossRef]
- Bringmann, G.; Kuhn, R. Results of toxic action of water pollutants on Daphnia magna Straus tested by an improved standardized procedure. In Z. Wasser Abwasser-Forsch; Aqua Publishing: Richmond, BC, Canada, 1982; Volume 15, pp. 1–6. [Google Scholar]
- Ioannou, P.V.; Lala, M.A.; Tsivgoulis, G.M. Preparation and properties of fully esterified erythritol. Eur. J. Lipid Sci. Technol. 2011, 113, 1357–1362. [Google Scholar] [CrossRef]
- Cygler, M.; Schrag, J.D. Structure as basis for understanding interfacial properties of lipases. Meth. Enzymol. 1997, 284, 3–27. [Google Scholar] [CrossRef]
- Kokkinou, M.; Theodorou, L.G.; Papamichael, E.M. Aspects on the Catalysis of Lipase from Porcine Pancreas (type VI-s) in Aqueous Media: Development of Ion-pairs. Braz. Arch. Biol. Technol. 2012, 55, 231–236. [Google Scholar] [CrossRef]
- Stauch, B.; Fisher, S.J.; Cianci, M. Open and closed states of Candida antarctica lipase B: Protonation and the mechanism of interfacial activation. J. Lipid Res. 2015, 56, 2348–2358. [Google Scholar] [CrossRef] [PubMed]
- Abdul Rahman, R.N.Z.R.; Salleh, A.B.; Basri, M. Lipases: Introduction. In New Lipases and Proteases; Salleh, A.B., Abdul Rahman, R.N.Z.R., Basri, M., Eds.; Nova Science Publishers Inc.: New York, NY, USA, 2006; pp. 1–22. ISBN 1-60021-068-6. [Google Scholar]
- Dulęba, J.; Czirson, K.; Siódmiak, T.; Marszałł, M.P. Lipase B from Candida antarctica—The wide applicable biocatalyst in obtaining pharmaceutical compounds. Med. Res. J. 2019, 4, 174–177. [Google Scholar] [CrossRef]
- Stergiou, P.-Y.; Foukis, A.; Filippou, M.; Koukouritaki, M.; Parapouli, M.; Theodorou, L.G.; Hatziloukas, E.; Afendra, A.; Pandey, A.; Papamichael, E.M. Advances in lipase-catalyzed esterification reactions. Biotechnol. Adv. 2013, 31, 1846–1859. [Google Scholar] [CrossRef] [PubMed]
- Foukis, A.; Gkini, O.A.; Stergiou, P.-Y.; Papamichael, E.M. New insights and tools for the elucidation of lipase catalyzed esterification reaction mechanism in n-hexane: The synthesis of ethyl butyrate. Mol. Catal. 2018, 455, 159–163. [Google Scholar] [CrossRef]
- Strohmeyer, G.; Martin, G.A.; Khingsmuller, V. Changes of alpha-ketoglutaric acid, pyruvic acid and diphosphopyridine nucleotide in chronic liver diseases. Klin. Wochenschr. 1957, 35, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Lymperopoulos, K.; Kosmas, M.; Papamichael, E.M. A Formulation of Different Equations Applied in Enzyme Kinetics. J. Sci. Ind. Res. India 1998, 57, 604–606. [Google Scholar]
- Jenke, D. Establishing the pH of Extraction Solvents Used to Simulate Aqueous Parenteral Drug Products during Organic Extractables Studies. Pharm. Outsourcing 2014, 15. [Google Scholar] [CrossRef]
- Chemical Book. Available online: https://www.chemicalbook.com/ProductMSDSDetailCB4853859_EN.htm (accessed on 27 July 2020).
- Available online: https://pubchem.ncbi.nlm.nih.gov/compound/985#section=Dissociation-Constants&fullscreen=true (accessed on 27 July 2020).
- Available online: https://pubchem.ncbi.nlm.nih.gov/compound/3893#section=pKa&fullscreen=true (accessed on 27 July 2020).
- Po, H.N.; Senozan, N.M. The Henderson-Hasselbalch Equation: Its History and Limitations. J. Chem. Educ. 2001, 78, 1499–1503. [Google Scholar] [CrossRef]
- Namazian, M.; Halvani, S. Calculations of pKa values of carboxylic acids in aqueous solution using density functional theory. J. Chem. Thermodyn. 2006, 38, 1495–1502. [Google Scholar] [CrossRef]
- Salentinig, S.; Sagalowicz, L.; Glatter, O. Self-Assembled Structures and pKa Value of Oleic Acid in Systems of Biological Relevance. Langmuir 2010, 26, 11670–11679. [Google Scholar] [CrossRef] [PubMed]
- Foukis, A.; Gkini, O.A.; Stergiou, P.-Y.; Sakkas, V.A.; Dima, A.; Boura, K.; Koutinas, A.A.; Papamichael, E.M. Sustainable production of a new generation biofuel by lipasecatalyzed esterification of fatty acids from liquid industrial waste biomass. Bioresour. Technol. 2017, 238, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Papamichael, E.M.; Lymperopoulos, K. Elastase and Cathepsin-G from Human PMN activated by PAF: Relation between their Kinetic Behavior and Pathophysiological Role. In Advances in Biotechnology, 1st ed.; Pandey, A., Ed.; Educational Publishers & Distributers: New Delhi, India, 1998; pp. 221–234. ISBN 81-87198-03-6. [Google Scholar]


| SUBSTRATE | kcat (s−1) | Km (mM) | kcat/Km (M−1 × s−1) | |||
|---|---|---|---|---|---|---|
| CALB | PPL | CALB | PPL | CALB | PPL | |
| Erythritol tetraolate | 9.9 × 10−4 | 1.2 | 1.7 | 3.5 | 0.6 | 342.9 |
| Erythritol tetrapalmitate | 7.0 × 10−4 | 1.5 | 1.8 | 2.1 | 0.4 | 714.3 |
| Erythritol tetralaurate | 2.8 × 10−4 | 2.9 | 0.8 | 5.8 | 0.4 | 500.0 |
| Pentaerythritol tetrapalmitate | 6.2 × 10−3 | 7.3 | 5.1 | 4.8 | 1.2 | 1520.8 |
| Pentaerythritol tetrastearate | (A1) 4.7 × 10−5 a (A1) 5.5 × 10−4 b (A2) 3.7 × 10−4 a (A2) 4.3 × 10−4 b (B) 3.2 × 10−4 | (C) 0.3 | (A1) 0.3 c (A1) 11.2 d (A2) 0.7 c (A2) 0.3 d (B) 1.9 | (C) 1.6 | (A1) 0.2 e (A1) 0.1 f (A2) 0.5 e (A2) 1.4 f (B) 0.2 | (C) 187.5 |
| (B) Virial coefficients ⇒ V1 = −3.9; V2 = 9.4 | ||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gkini, O.A.; Stergiou, P.-Y.; Foukis, A.; Ioannou, P.V.; Papamichael, E.M. Kinetic Analysis of the Lipase-Catalyzed Hydrolysis of Erythritol and Pentaerythritol Fatty Acid Esters: A Biotechnological Application for Making Low-Calorie Healthy Food Alternatives. Catalysts 2020, 10, 965. https://doi.org/10.3390/catal10090965
Gkini OA, Stergiou P-Y, Foukis A, Ioannou PV, Papamichael EM. Kinetic Analysis of the Lipase-Catalyzed Hydrolysis of Erythritol and Pentaerythritol Fatty Acid Esters: A Biotechnological Application for Making Low-Calorie Healthy Food Alternatives. Catalysts. 2020; 10(9):965. https://doi.org/10.3390/catal10090965
Chicago/Turabian StyleGkini, Olga A., Panagiota-Yiolanda Stergiou, Athanasios Foukis, Panayiotis V. Ioannou, and Emmanuel M. Papamichael. 2020. "Kinetic Analysis of the Lipase-Catalyzed Hydrolysis of Erythritol and Pentaerythritol Fatty Acid Esters: A Biotechnological Application for Making Low-Calorie Healthy Food Alternatives" Catalysts 10, no. 9: 965. https://doi.org/10.3390/catal10090965
APA StyleGkini, O. A., Stergiou, P.-Y., Foukis, A., Ioannou, P. V., & Papamichael, E. M. (2020). Kinetic Analysis of the Lipase-Catalyzed Hydrolysis of Erythritol and Pentaerythritol Fatty Acid Esters: A Biotechnological Application for Making Low-Calorie Healthy Food Alternatives. Catalysts, 10(9), 965. https://doi.org/10.3390/catal10090965