Influence of Carrier Structure and Physicochemical Factors on Immobilisation of Fungal Laccase in Terms of Bisphenol A Removal
Abstract
1. Introduction
2. Results and Discussion
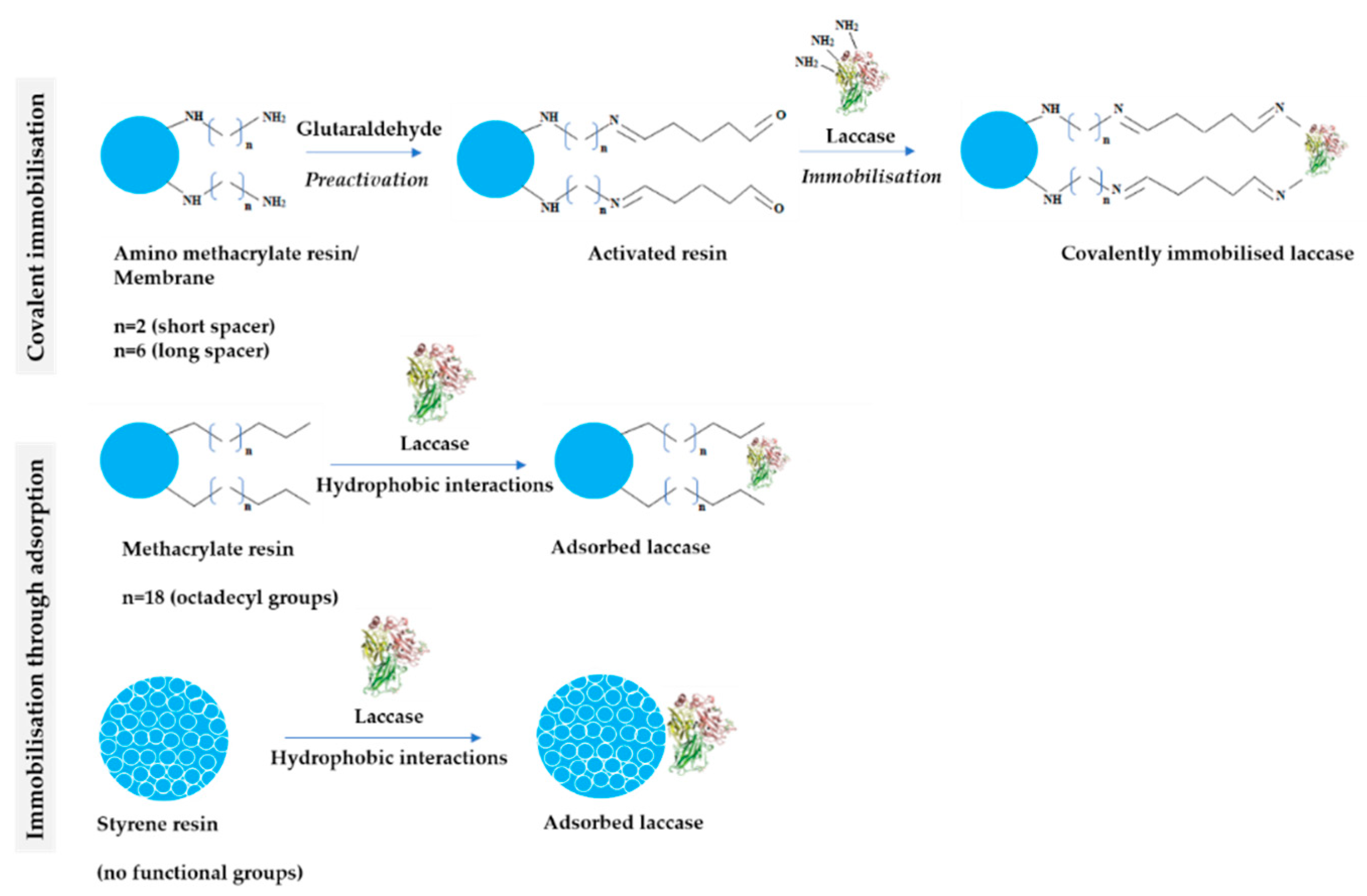
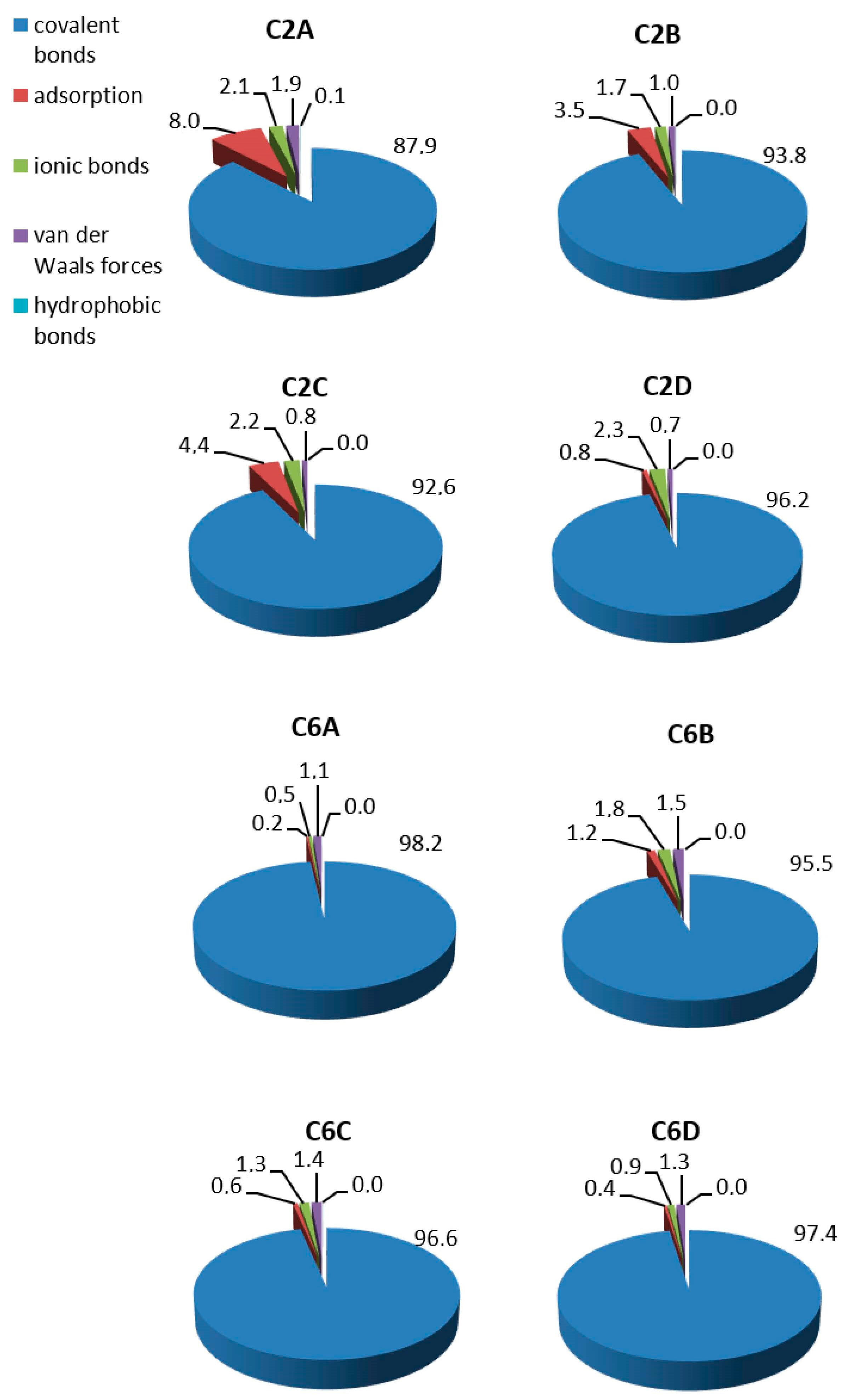
2.1. Influence of the Carrier Structure
2.2. Immobilisation Conditions
2.2.1. Influence of Phosphate Buffer pH Value
2.2.2. Temperature and Time of Immobilisation
2.2.3. Laccase Concentration
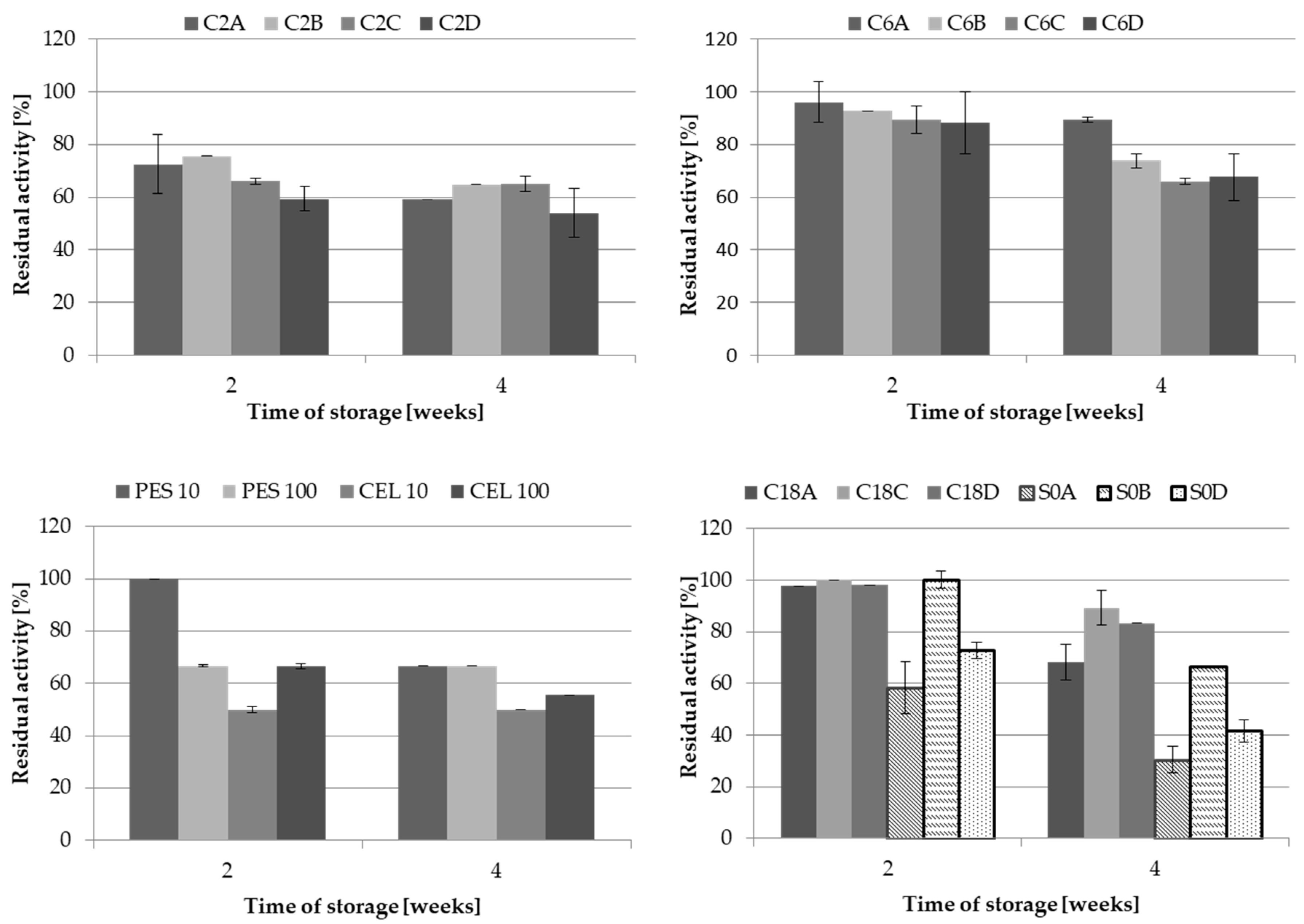
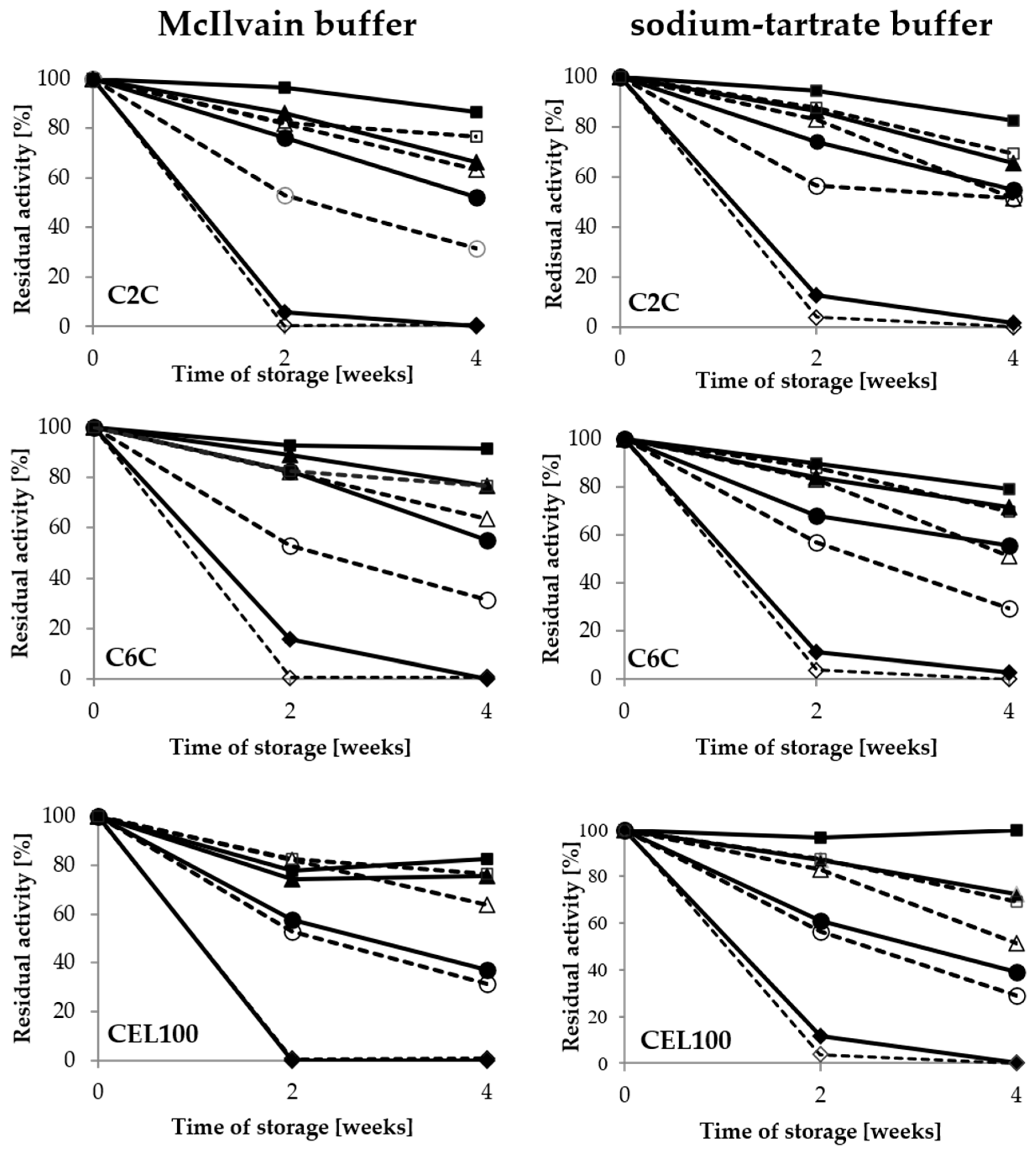
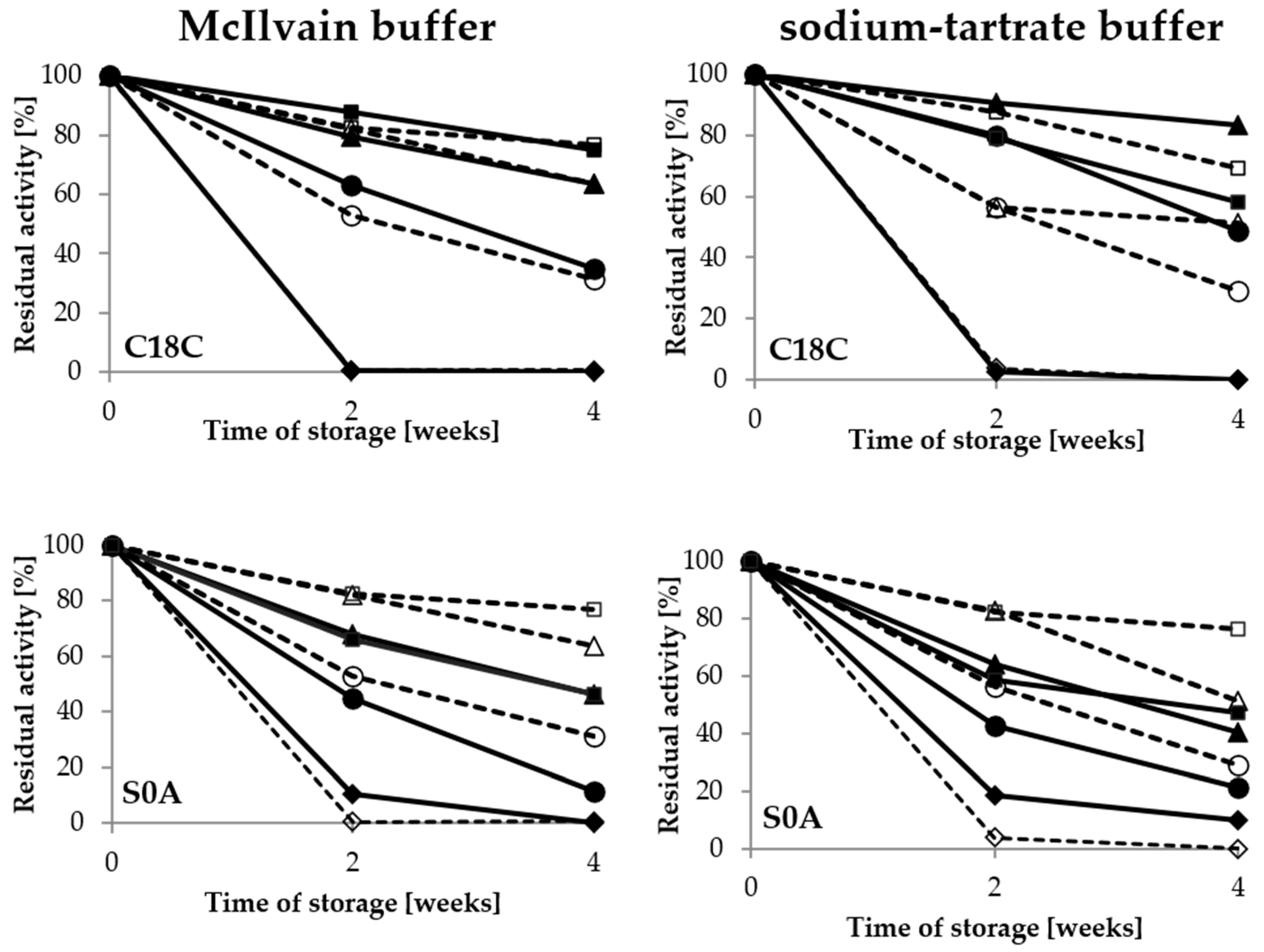
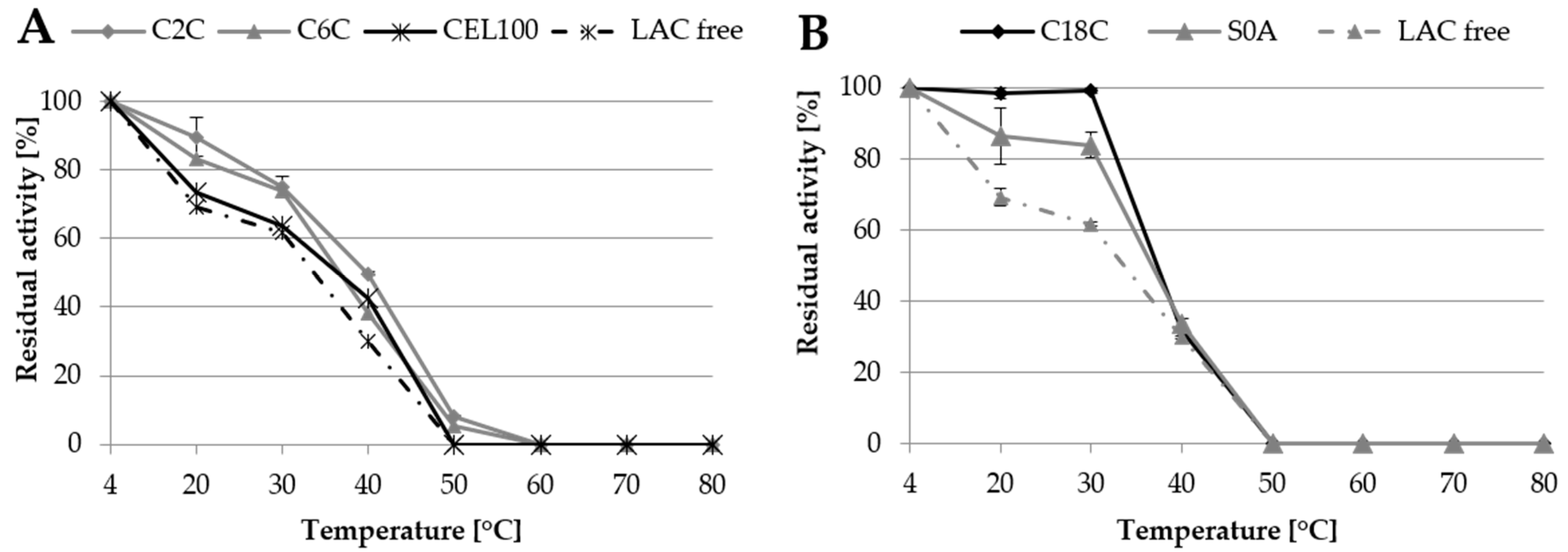
2.3. Storage Conditions and Thermostability
2.3.1. Composition and pH Value of Buffer
2.3.2. Thermostability
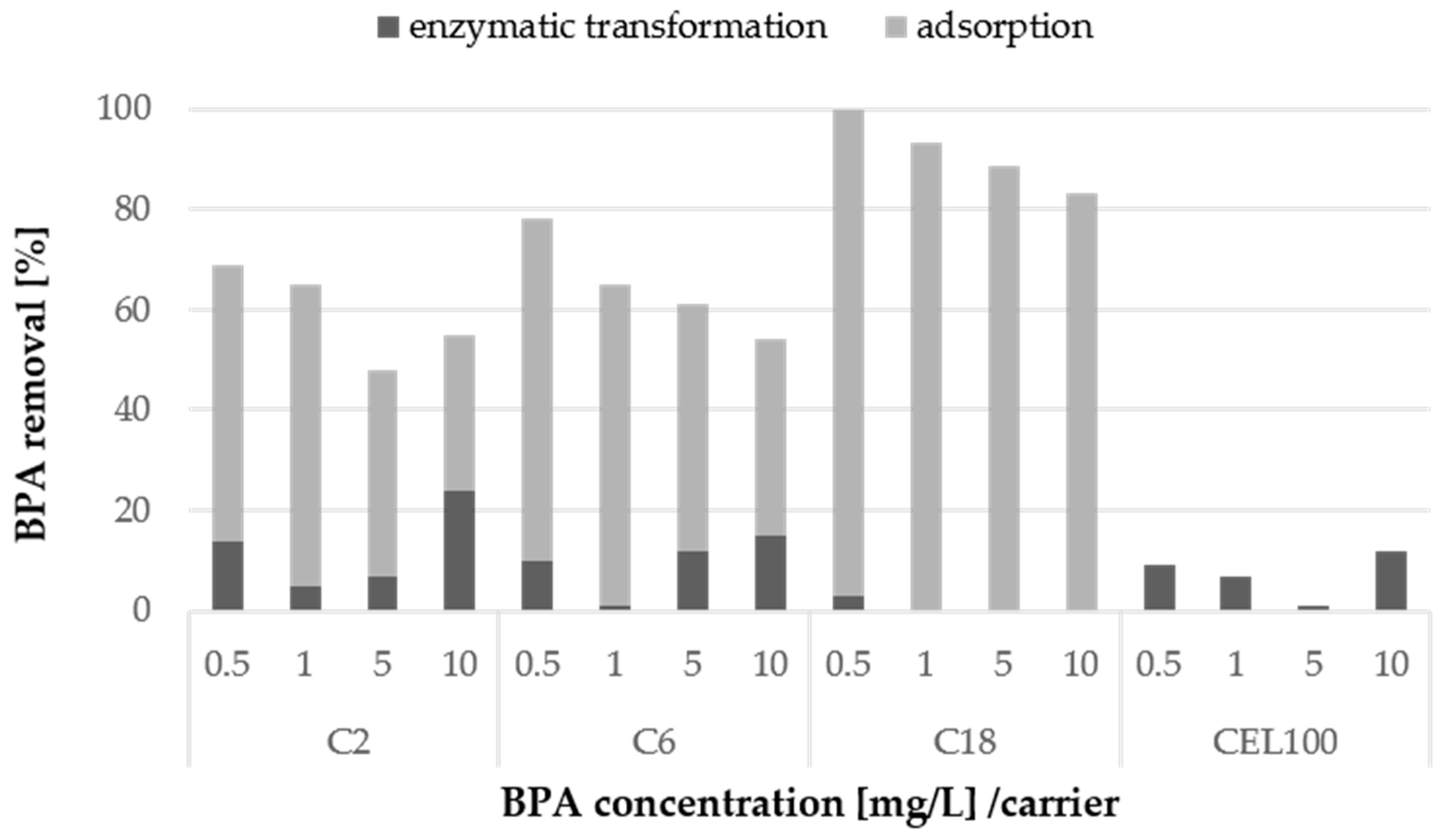
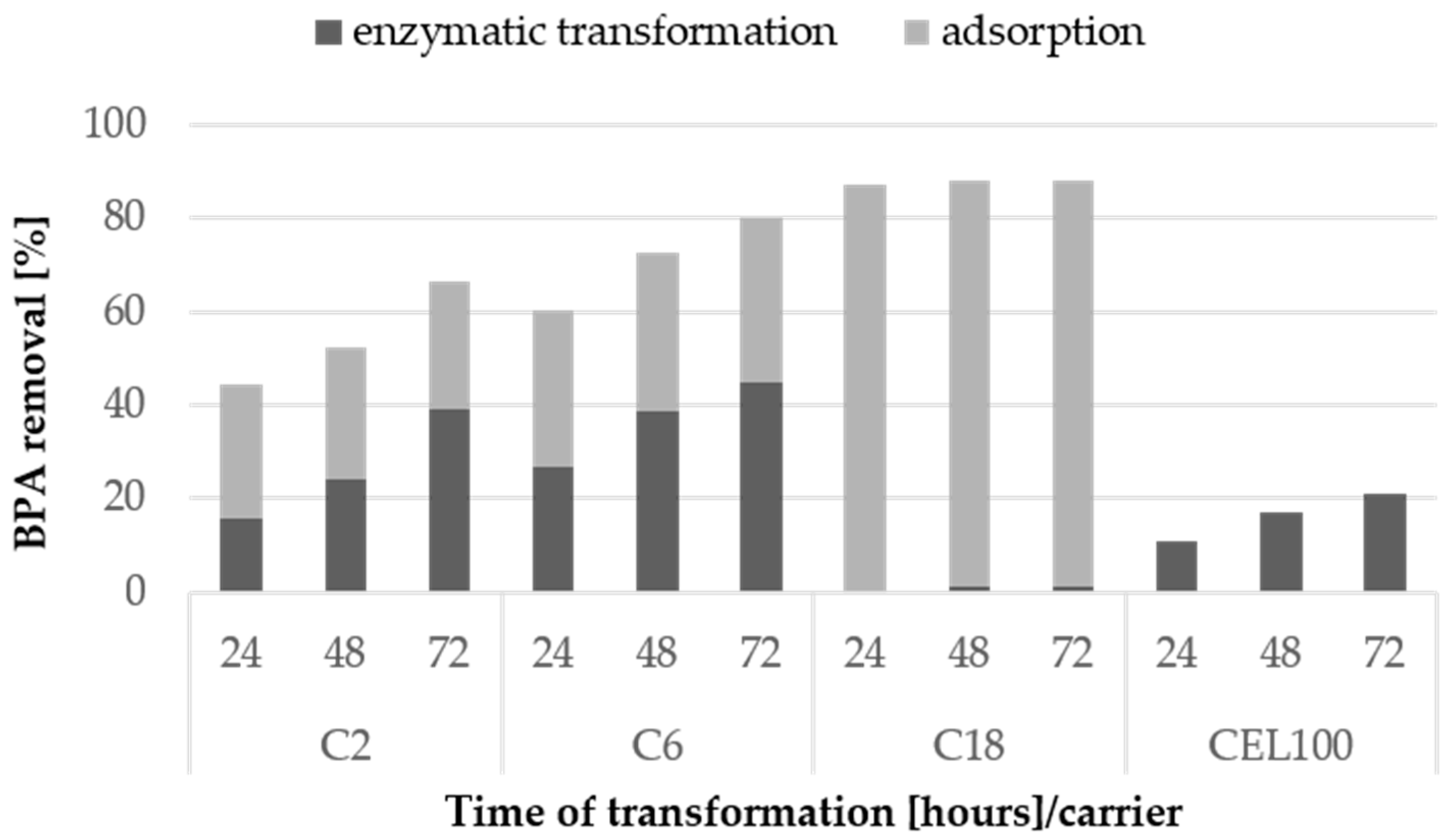
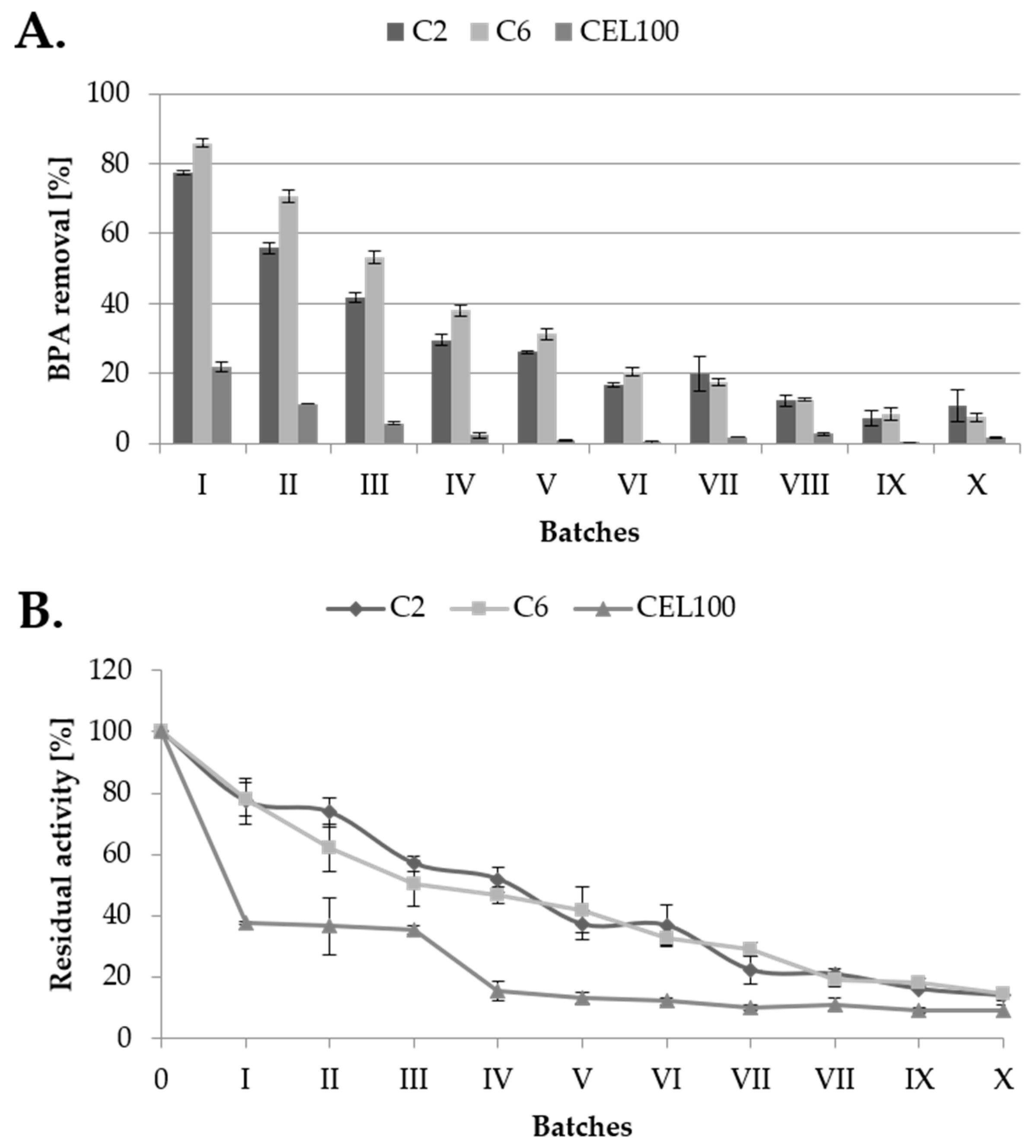
2.4. Degradation of Bisphenol A
2.5. Toxicity Evaluation
3. Materials and Methods
3.1. Microorganism and Culture Conditions
3.2. Chemicals, Reagents, and Carriers
3.3. Catalysts
3.4. Immobilisation of LAC
3.4.1. Yield of LAC Immobilisation
3.4.2. Activity of Immobilised LAC
3.4.3. Determination of the Bond Type
3.5. Stability of LAC
3.6. Transformation of Endocrine Disrupting Chemical
3.7. High Pressure Liquid Chromatogrpahy (HPLC)
3.8. Ecotoxicity Microtox® Test
3.9. MTT and LDH Cytotoxicity Tests
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| LAC | laccase |
| BPA | bisphenol A |
References
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzym. Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Peralta-Zamora, P.; Pereira, C.M.; Tiburtius, E.R.; Moraes, S.G.; Rosa, M.A.; Minussi, R.C.; Durán, N. Decolorization of reactive dyes by immobilized laccase. Appl. Catal. B Environ. 2003, 42, 131–144. [Google Scholar] [CrossRef]
- Spinelli, D.; Fatarella, E.; Di Michele, A.; Pogni, R. Immobilization of fungal (Trametes versicolor) laccase onto Amberlite IR-120 H beads: Optimization and characterization. Process Biochem. 2013, 48, 218–223. [Google Scholar] [CrossRef]
- Gonçalves, M.C.P.; Kieckbusch, T.G.; Perna, R.F.; Fujimoto, J.T.; Morales, S.A.V.; Romanelli, J.P. Trends on enzyme immobilization researches based on bibliometric analysis. Process Biochem. 2019, 76, 95–110. [Google Scholar] [CrossRef]
- Fernández-Fernández, M.; Sanromán, M.Á.; Moldes, D. Recent developments and applications of immobilized laccase. Biotechnol. Adv. 2013, 31, 1808–1825. [Google Scholar] [CrossRef]
- Galliker, P.; Hommes, G.; Schlosser, D.; Corvini, P.F.X.; Shahgaldian, P. Laccase-modified silica nanoparticles efficiently catalyze the transformation of phenolic compounds. J. Colloid Interface Sci. 2010, 349, 98–105. [Google Scholar] [CrossRef]
- Costa, J.B.; Lima, M.J.; Sampaio, M.J.; Neves, M.C.; Faria, J.L.; Morales-Torres, S.; Tavarez, A.P.M.; Silva, C.G. Enhanced biocatalytic sustainability of laccase by immobilization on functionalized carbon nanotubes/polysulfone membranes. Chem. Eng. J. 2019, 355, 974–985. [Google Scholar] [CrossRef]
- Wlizło, K.; Polak, J.; Jarosz-Wilkołazka, A.; Pogni, R.; Petricci, E. Novel textile dye obtained through transformation of 2-amino-3-methoxybenzoic acid by free and immobilised laccase from a Pleurotus ostreatus strain. Enzym. Microb. Technol. 2020, 132, 109398. [Google Scholar] [CrossRef]
- Mogharabi, M.; Nassiri-Koopaei, N.; Bozorgi-Koushalshahi, M.; Nafissi-Varcheh, N.; Bagherzadeh, G.; Faramarzi, M.A. Immobilization of laccase in alginate-gelatin mixed gel and decolorization of synthetic dyes. Bioinorg. Chem. Appl. 2012, 2012, 1–6. [Google Scholar] [CrossRef]
- Lassouane, F.; Aït-Amar, H.; Amrani, S.; Rodriguez-Couto, S. A promising laccase immobilization approach for Bisphenol A removal from aqueous solutions. Bioresour. Technol. 2019, 271, 360–367. [Google Scholar] [CrossRef]
- Mohamad, N.R.; Marzuki, N.H.C.; Buang, N.A.; Huyop, F.; Wahab, R.A. An overview of technologies for immobilization of enzymes and surface analysis techniques for immobilized enzymes. Biotechnol. Biotechnol. Equip. 2015, 29, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Nisha, S.; Karthick, S.A.; Gobi, N. A review on methods, application and properties of immobilized enzyme. Chem. Sci. Rev. Lett. 2012, 1, 148–155. [Google Scholar]
- de Bezerra, T.M.S.; Bassan, J.C.; de Santos, V.T.O.; Ferraz, A.; Monti, R. Covalent immobilization of laccase in green coconut fiber and use in clarification of apple juice. Process Biochem. 2015, 50, 417–423. [Google Scholar] [CrossRef]
- Durán, N.; Rosa, M.A.; D’Annibale, A.; Gianfreda, L. Applications of laccases and tyrosinases (phenoloxidases) immobilized on different supports: A review. Enzym. Microb. Technol. 2002, 31, 907–931. [Google Scholar] [CrossRef]
- Asgher, M.; Shahid, M.; Kamal, S.; Iqbal, H.M.N. Recent trends and valorization of immobilization strategies and ligninolytic enzymes by industrial biotechnology. J. Mol. Catal. B Enzym. 2014, 101, 56–66. [Google Scholar] [CrossRef]
- Bautista, L.F.; Morales, G.; Sanz, R. Immobilization strategies for laccase from Trametes versicolor on mesostructured silica materials and the application to the degradation of naphthalene. Bioresour. Technol. 2010, 101, 8541–8548. [Google Scholar] [CrossRef]
- Hou, J.; Dong, G.; Ye, Y.; Chen, V. Enzymatic degradation of bisphenol-A with immobilized laccase on TiO2 sol–gel coated PVDF membrane. J. Membr. Sci. 2014, 469, 19–30. [Google Scholar] [CrossRef]
- Fernández-Lorente, G.; Lopez-Gallego, F.; Bolivar, J.M.; Rocha-Martin, J.; Moreno-Perez, S.; Guisán, J.M. Immobilization of proteins on glyoxyl activated supports: Dramatic stabilization of enzymes by multipoint covalent attachment on pre-existing supports. Curr. Org. Chem. 2015, 19, 13. [Google Scholar] [CrossRef]
- Pezzella, C.; Russo, M.E.; Marzocchella, A.; Salatino, P.; Sannia, G. Immobilization of a Pleurotus ostreatus laccase mixture on perlite and its application to dye decolourisation. BioMed. Res. Int. 2014, 2014, 308613. [Google Scholar] [CrossRef]
- Tentori, F.; Bavaro, T.; Brenna, E.; Colombo, D.; Monti, D.; Semproli, R.; Ubiali, D. Immobilization of Old Yellow Enzymes via Covalent or Coordination Bonds. Catalysts 2020, 10, 260. [Google Scholar] [CrossRef]
- Wang, F.; Guo, C.; Yang, L.R.; Liu, C.Z. Magnetic mesoporous silica nanoparticles: Fabrication and their laccase immobilization performance. Bioresour. Technol. 2010, 101, 8931–8935. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Guo, C.; Liu, H.Z.; Liu, C.Z. Immobilization of Pycnoporus sanguineus laccase by metal affinity adsorption on magnetic chelator particles. J. Chem. Technol. Biotechnol. 2008, 83, 97–104. [Google Scholar] [CrossRef]
- Rahmani, K.; Faramarzi, M.A.; Mahvi, A.H.; Gholami, M.; Esrafili, A.; Forootanfar, H.; Farzadkia, M. Elimination and detoxification of sulfathiazole and sulfamethoxazole assisted by laccase immobilized on porous silica beads. Int. Biodeterior. Biodegrad. 2015, 97, 107–114. [Google Scholar] [CrossRef]
- Lin, J.; Liu, Y.; Chen, S.; Le, X.; Zhou, X.; Zhao, Z.; Ou, Y.; Yang, J. Reversible immobilization of laccase onto metal-ion-chelated magnetic microspheres for bisphenol A removal. Int. J. Biol. Macromol. 2016, 84, 189–199. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, Z.; Zeng, G.; Tang, L.; Pang, Y.; Li, Z.; Liu, C.; Lei, X.; Wu, M.; Ren, P.; et al. Immobilization of laccase on magnetic bimodal mesoporous carbon and the application in the removal of phenolic compounds. Bioresour. Technol. 2012, 115, 21–26. [Google Scholar] [CrossRef]
- Xu, R.; Si, Y.; Wu, X.; Li, F.; Zhang, B. Triclosan removal by laccase immobilized on mesoporous nanofibers: Strong adsorption and efficient degradation. Chem. Eng. J. 2014, 255, 63–70. [Google Scholar] [CrossRef]
- Sathishkumar, P.; Kamala-Kannan, S.; Cho, M.; Kim, J.S.; Hadibarata, T.; Salim, M.R.; Oh, B.T. Laccase immobilization on cellulose nanofiber: The catalytic efficiency and recyclic application for simulated dye effluent treatment. J. Mol. Catal. B Enzym. 2014, 100, 111–120. [Google Scholar] [CrossRef]
- Michałowicz, J. Bisphenol A—Sources, toxicity and biotransformation. Environ. Toxicol. Pharmacol. 2014, 37, 738–758. [Google Scholar] [CrossRef]
- Rogala, D.; Kulik-Kupka, K.; Śnieżek, E.; Janicka, A.; Moskalenko, O. Bisfenol A—Niebezpieczny związek ukryty w tworzywach sztucznych. Probl. Hig. Epidemiol. 2016, 97, 213–219. [Google Scholar]
- Sifakis, S.; Androutsopoulos, V.P.; Tsatsakis, A.M.; Spandidos, D.A. Human exposure to endocrine disrupting chemicals: Effects on the male and female reproductive systems. Environ. Toxicol. Pharmacol. 2017, 51, 56–70. [Google Scholar] [CrossRef]
- Castro, B.; Sanchez, P.; Torres, J.M.; Preda, O.; del Moral, R.G.; Ortega, E. Bisphenol A exposure during adulthood alters expression of aromatase and 5α-reductase isozymes in rat prostate. PLoS ONE 2013, 8, e55905. [Google Scholar] [CrossRef] [PubMed]
- Konieczna, A.; Rutkowska, A.; Rachoń, D. Health risk of exposure to bisfenol A (BPA). Rocz. Państ. Zakł. Hig. 2015, 66, 5–11. [Google Scholar]
- Nair, R.R.; Demarche, P.; Agathos, S.N. Formulation and characterization of an immobilized laccase biocatalyst and its application to eliminate organic micropollutants in wastewater. New Biotechnol. 2013, 30, 814–823. [Google Scholar] [CrossRef] [PubMed]
- Zdarta, J.; Antecka, K.; Frankowski, R.; Zgoła-Grześkowiak, A.; Ehrlich, H.; Jesionowski, T. The effect of operational parameters on the biodegradation of bisphenols by Trametes versicolor laccase immobilized on Hippospongia communis spongin scaffolds. Sci. Total Environ. 2018, 615, 784–795. [Google Scholar] [CrossRef] [PubMed]
- Sadeghzadeh, S.; Nejad, Z.G.; Ghasemi, S.; Khafaji, M.; Borghei, S.M. Removal of bisphenol A in aqueous solution using magnetic cross-linked laccase aggregates from Trametes hirsuta. Bioresour. Technol. 2020, 306, 123169. [Google Scholar] [CrossRef]
- Dudziak, M. Wpływ warunków środowiska wodnego na rozkład bisfenolu A. Proc. ECOpolen 2017, 11, 131–139. [Google Scholar]
- Varga, B.; Somogyi, V.; Meiczinger, M.; Kováts, N.; Domokos, E. Enzymatic treatment and subsequent toxicity of organic micropollutants using oxidoreductases—A review. J. Clean. Prod. 2019, 221, 306–322. [Google Scholar] [CrossRef]
- Spina, F.; Cordero, C.; Schilirò, T.; Sgorbini, B.; Pignata, C.; Gilli, G.; Bichi, C.; Varese, G.C. Removal of micropollutants by fungal laccases in model solution and municipal wastewater: Evaluation of estrogenic activity and ecotoxicity. J. Clean. Prod. 2015, 100, 185–194. [Google Scholar] [CrossRef]
- de Freitas, E.N.; Bubna, G.A.; Brugnari, T.; Kato, C.G.; Nolli, M.; Rauen, T.G.; Moreira, R.F.P.M.; Peralta, R.A.; Bracht, A.; de Souza, C.G.M.; et al. Removal of bisphenol A by laccases from Pleurotus ostreatus and Pleurotus pulmonarius and evaluation of ecotoxicity of degradation products. Chem. Eng. J. 2017, 330, 1361–1369. [Google Scholar] [CrossRef]
- Wlizło, K.; Polak, J.; Jarosz-Wilkołazka, A. Związki biologicznie aktywne i metody ich usuwania na drodze biokatalizy. Postęp. Biochem. 2017, 63, 304–314. [Google Scholar]
- Lan, H.C.; Wu, K.Y.; Lin, I.W.; Yang, Z.J.; Chang, A.A.; Hu, M.C. Bisphenol A disrupts steroidogenesis and induces a sex hormone imbalance through c-Jun phosphorylation in Leydig cells. Chemosphere 2017, 185, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Zhang, T.; Yang, P.; Li, M.; Yang, Y.; Wang, Y.; Du, J.; Pan, K.; Zhang, K. Low doses of bisphenol A stimulate the proliferation of breast cancer cells via ERK1/2/ERRγ signals. Toxicol. In Vitro 2015, 302, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Lindeberg, G.; Holm, G. Occurrence of tyrosinase and laccase in fruit bodies and mycelia of some Hymenomycetes. Physiol. Plant. 1952, 5, 100–114. [Google Scholar] [CrossRef]
- Bryjak, J.; Kruczkiewicz, P.; Rekuć, A.; Pęczyńska-Czoch, W. Laccase immobilization on copolymer of butyl acrylate and ethylene glycol dimethacrylate. Biochem. Eng. J. 2007, 35, 325–332. [Google Scholar] [CrossRef]
- Rekuć, A.; Kruczkiewicz, P.; Jastrzembska, B.; Liesiene, J.; Pęczyńska-Czoch, W.; Bryjak, J. Laccase immobilization on the tailored cellulose-based Granocel carriers. Int. J. Biol. Macromol. 2008, 42, 208–215. [Google Scholar] [CrossRef]
- Ginalska, G.; Kowalczuk, D.; Osińska, M. A chemical method of gentamicin bonding to gelatin-sealed prosthetic vascular grafts. Int. J. Pharm. 2005, 288, 131–140. [Google Scholar] [CrossRef]
- Polak, J.; Jarosz-Wilkolazka, A.; Szuster-Ciesielska, A.; Wlizlo, K.; Kopycinska, M.; Sojka-Ledakowicz, J.; Lichawska-Olczyk, J. Toxicity and dyeing properties of dyes obtained through laccase-mediated synthesis. J. Clean. Prod. 2016, 112, 4265–4272. [Google Scholar] [CrossRef]

| Immobilisation Technique | Carrier | Aimm [U/g] | Yield [%] |
|---|---|---|---|
| Covalent | C2A | 2.47 ± 0.1 | 40.5 ± 5.4 |
| C2B | 1.58 ± 0.3 | 51 ± 1 | |
| C2C | 5.34 ± 0.4 | 80.5 ± 2.5 | |
| C2D | 1.86 ± 0.1 | 55.5 ± 5.5 | |
| C6A | 2.75 ± 0.2 | 76 ± 3.6 | |
| C6B | 2.12 ± 0 | 62 ± 2.1 | |
| C6C | 2.12 ± 0.3 | 68 ± 0.1 | |
| C6D | 2.65 ± 0.2 | 92 ± 0.5 | |
| CEL10 | 0.30 ± 0 | 1.23 ± 0.1 | |
| CEL100 | 0.33 ± 0 | 1.54 ± 0.1 | |
| PES10 | 0.55 ± 0 | 1.0 ± 0.5 | |
| PES100 | 0.88 ± 0 | 1.15 ± 0 | |
| Adsorption | C18A | 1.04 ± 0.1 | 100 ± 0 |
| C18C | 7.43 ± 0.3 | 100 ± 0.1 | |
| C18D | 2.55 ± 0.2 | 100 ± 0.1 | |
| S0A | 5.80 ± 0.9 | 96 ± 0.4 | |
| S0B | 1.05 ± 0.1 | 85 ± 3.5 | |
| S0D | 3.87 ± 0.3 | 97 ± 2.1 |
| Immobilisation Technique | Carrier | Buffer pH | |||
|---|---|---|---|---|---|
| 5 | 6 | 7 | 8 | ||
| Covalent | C2C | 5.41 ± 0.2 | 4.88 ± 0 | 4.5 ± 0.3 | 3.13 ± 0 |
| C6C | 5.26 ± 0.2 | 4.65 ± 0.2 | 3.99 ± 0 | 3.76 ± 0 | |
| CEL100 | 0.45 ± 0 | 0.45 ± 0 | 0.35 ± 0 | 0.23 ± 0 | |
| Adsorption | C18C | 4.78 ± 0 | 4.95 ± 0 | 5.14 ± 0 | 4.72 ± 0 |
| S0A | 1.89 ± 0 | 2.06 ± 0 | 2.09 ± 0 | 1.5 ± 0 | |
| S0D | 1.56 ± 0 | 1.53 ± 0 | 1.32 ± 0 | 1.07 ± 0 | |
| Carrier | Temperature | Time of LAC Incubation [h] | |||
|---|---|---|---|---|---|
| 1 | 3 | 5 | 24 | ||
| C2C | 4 °C | 3.43 ± 0.4 | 4.73 ± 0.1 | 4.26 ± 0.3 | 4.44 ± 0.3 |
| 22 °C | 4.41 ± 0.2 | 5.18 ± 0.1 | 4.55 ± 0.2 | 4.30 ± 0.4 | |
| C6C | 4 °C | 3.91 ± 0.2 | 4.16 ± 0.2 | 4.37 ± 0.2 | 4.38 ± 0.7 |
| 22 °C | 4.55 ± 0.2 | 4.61 ± 0.4 | 3.72 ± 0.2 | 4.01 ± 0.6 | |
| CEL100 | 4 °C | 0.55 ± 0.1 | 0.63 ± 0.1 | 0.61 ± 0 | 0.83 ± 0.1 |
| 22 °C | 0.73 ± 0 | 0.68 ± 0 | 0.78 ± 0.1 | 0.83 ± 0.1 | |
| C18C | 4 °C | 4.02 ± 0.2 | 3.97 ± 0.2 | 3.63 ± 0.5 | 4.44 ± 0.3 |
| 22 °C | 4.22 ± 0 | 4.37 ± 0.2 | 4.01 ± 0.5 | 4.59 ± 0.1 | |
| S0A | 4 °C | 2.20 ± 0.1 | 2.15 ± 0.1 | 2.26 ± 0.1 | 2.33 ± 0.3 |
| 22 °C | 2.06 ± 0 | 2.63 ± 0.2 | 2.13 ± 0.1 | 2.51 ± 0.2 | |
| S0D | 4 °C | 2.40 ± 0.1 | 2.44 ± 0.1 | 2.57 ± 0.1 | 2.40 ± 0.1 |
| 22 °C | 2.33 ± 0 | 2.99 ± 0.2 | 2.41 ± 0.2 | 2.83 ± 0.2 | |
| Carrier | LAC Concentration [mg/g] | |||
|---|---|---|---|---|
| 0.5/0.25 * | 1/0.5 * | 2/0.75 * | 4/1 * | |
| C2C | 5.38 ± 0.3 | 12.18 ± 0 | 8.14 ± 0.3 | 19.14 ± 0 |
| C6C | 3.82 ± 0.5 | 5.79 ± 0.2 | 10.73 ± 1.3 | 14.09 ± 1.4 |
| CEL100 | 0.74 ± 0 | 0.77 ± 0.2 | 0.92 ± 0.1 | 0.97 ± 0.1 |
| C18C | 5.56 ± 0 | 7.51 ± 0.6 | 5.31 ± 0.2 | 5 ± 0.5 |
| S0A | 1.51 ± 0.1 | 3.13 ± 0.1 | 3.27 ± 0.2 | 3.56 ± 0.4 |
| Time | EC50 [mg/L] | |
|---|---|---|
| BPA | Products | |
| 5 min | 0.116 ± 0 | 0.316 ± 0.01 |
| 15 min | 0.156 ± 0.01 | 0.375 ± 0.02 |
| Time | MTT | LDH | ||
|---|---|---|---|---|
| BPA | Products | BPA | Products | |
| 24 h | 0 | 0 | 15 ± 1.82 | 5 ± 0.23 |
| 48 h | 10 ± 0.77 | 7 ± 1.23 | 17 ± 2.5 | 3 ± 0.71 |
| Group of Carriers | Immobilisation Technique | Type of Carriers | Functional Groups | Pore Diameter (Å) | Particle Size (µm) | Acronym |
| Porous | Covalent (C2 and C6 carriers) | Amino C2 methacrylate | NH2 (short spacer) | 300–600 | 150–300 | C2A |
| 300–710 | C2B | |||||
| 1200–1800 | 150–300 | C2C | ||||
| 300–710 | C2D | |||||
| Amino C6 methacrylate | NH2 (long spacer) | 300–600 | 150–300 | C6A | ||
| 300–710 | C6B | |||||
| 1200–1800 | 150–300 | C6C | ||||
| 300–710 | C6D | |||||
| Adsorption (C18 and S0 carriers) | Octadecyl methacrylate | Octadecyl | 350–450 | 150–300 | C18A | |
| 500–700 | 150–300 | C18C | ||||
| 300–710 | C18D | |||||
| Macroporous styrene | None | 900–110 | 150–300 | S0A | ||
| 300–710 | S0B | |||||
| 950–1200 | 300–710 | S0D | ||||
| - | - | - | - | Pore Diameter (kDa) | Membrane Diameter (mm) | Acronym |
| Membrane | Covalent (CEL and PES carriers) | Cellulose (disc) | NH2 | 10 | 47 | CEL10 |
| 100 | 47 | CEL100 | ||||
| Polyethersulfone (disc) | NH2 | 10 | 47 | PES10 | ||
| 100 | 47 | PES100 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wlizło, K.; Polak, J.; Kapral-Piotrowska, J.; Grąz, M.; Paduch, R.; Jarosz-Wilkołazka, A. Influence of Carrier Structure and Physicochemical Factors on Immobilisation of Fungal Laccase in Terms of Bisphenol A Removal. Catalysts 2020, 10, 951. https://doi.org/10.3390/catal10090951
Wlizło K, Polak J, Kapral-Piotrowska J, Grąz M, Paduch R, Jarosz-Wilkołazka A. Influence of Carrier Structure and Physicochemical Factors on Immobilisation of Fungal Laccase in Terms of Bisphenol A Removal. Catalysts. 2020; 10(9):951. https://doi.org/10.3390/catal10090951
Chicago/Turabian StyleWlizło, Kamila, Jolanta Polak, Justyna Kapral-Piotrowska, Marcin Grąz, Roman Paduch, and Anna Jarosz-Wilkołazka. 2020. "Influence of Carrier Structure and Physicochemical Factors on Immobilisation of Fungal Laccase in Terms of Bisphenol A Removal" Catalysts 10, no. 9: 951. https://doi.org/10.3390/catal10090951
APA StyleWlizło, K., Polak, J., Kapral-Piotrowska, J., Grąz, M., Paduch, R., & Jarosz-Wilkołazka, A. (2020). Influence of Carrier Structure and Physicochemical Factors on Immobilisation of Fungal Laccase in Terms of Bisphenol A Removal. Catalysts, 10(9), 951. https://doi.org/10.3390/catal10090951






