Insights into Interface Charge Extraction in a Noble-Metal-Free Doped Z-Scheme NiO@BiOCl Heterojunction
Abstract
1. Introduction
2. Results and Discussions
2.1. Identification of Phases and Components
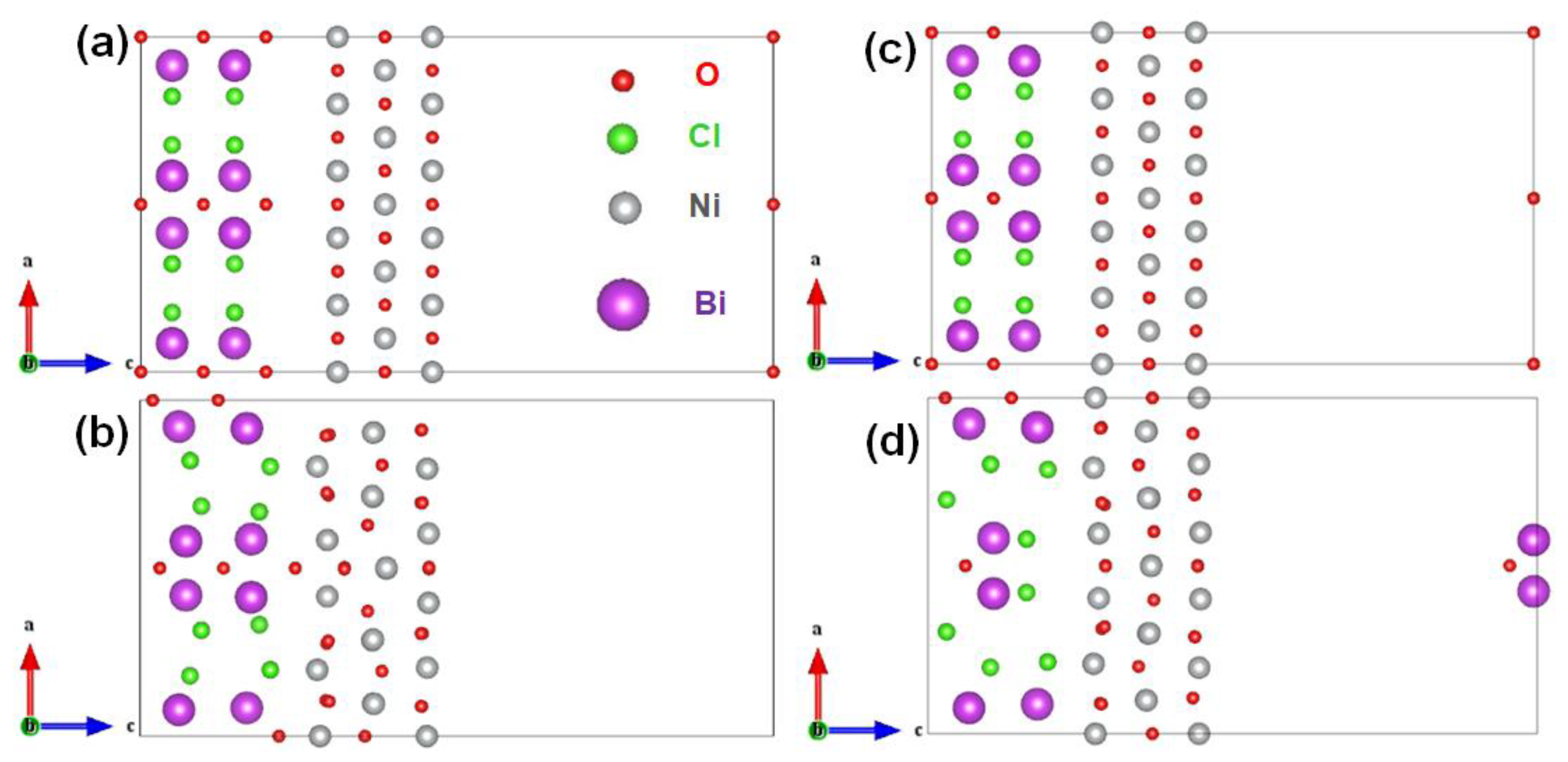
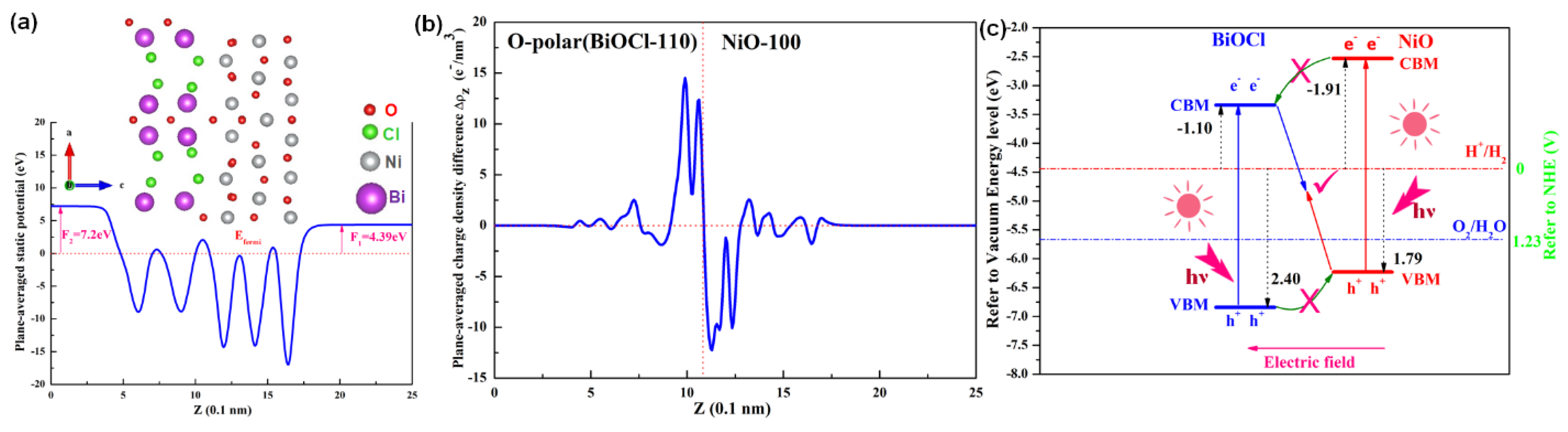
2.2. Heterostructure Fabrication and Interface Electron Extraction
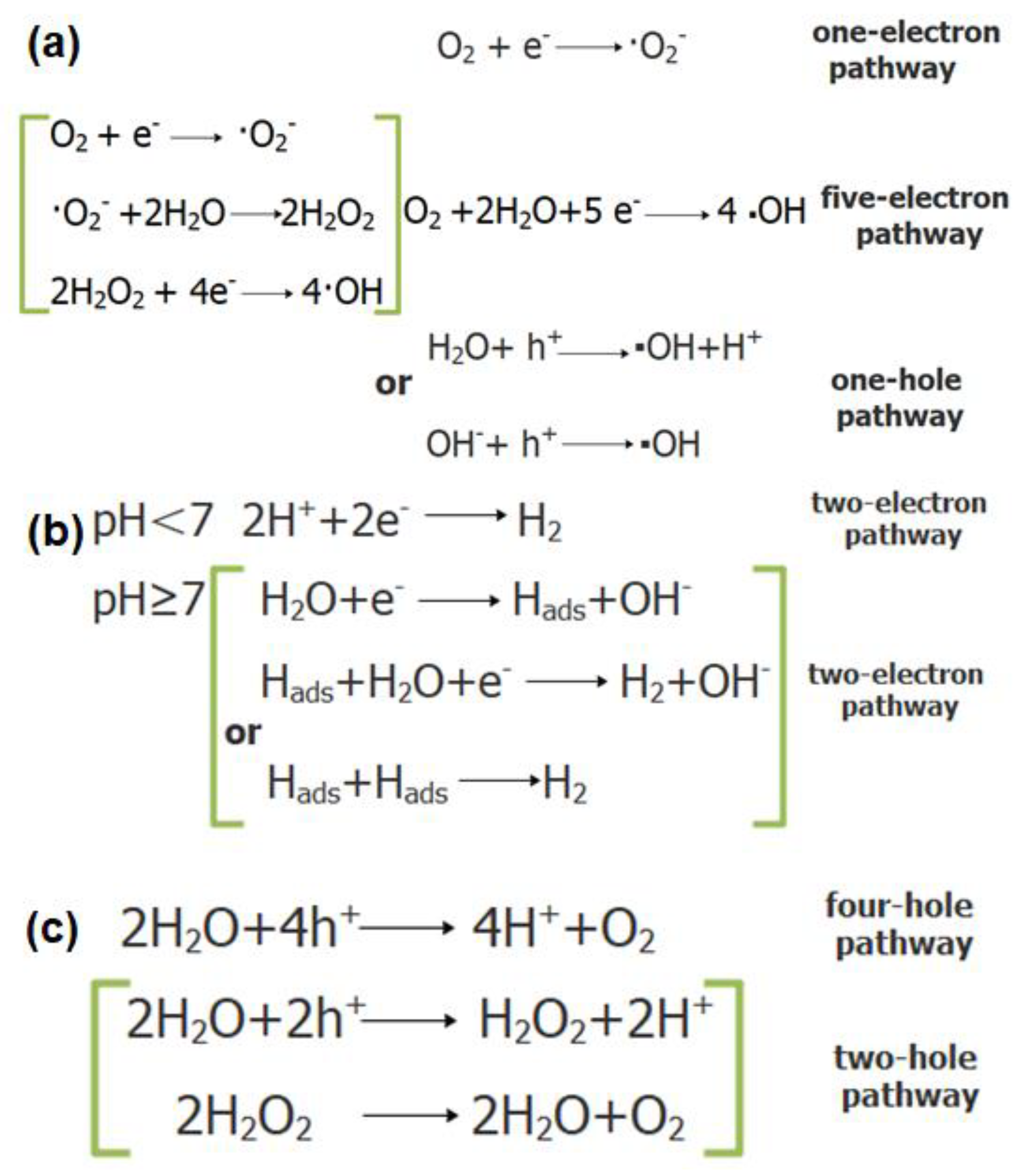
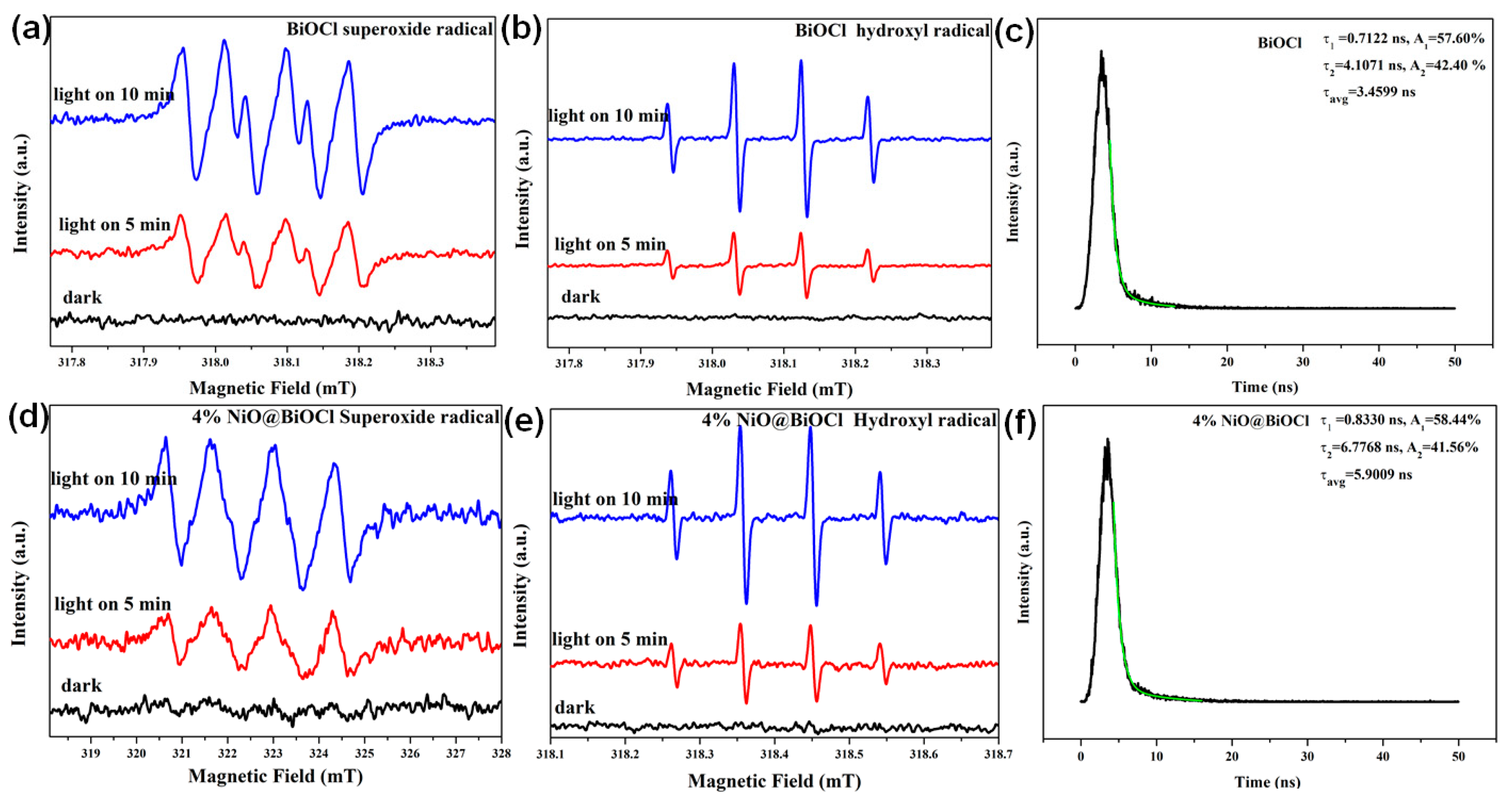
2.3. Optical Properties and Photocatalytic Activity
3. Experimental Procedures
3.1. Materials Synthesis
3.2. Material Characterization
3.3. First-Principles Calculation Method
3.4. Evaluation of Photocatalytic Activity
4. Conclusions and Outlooks
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, X.; Hong, M.Z.; Zhang, F.W.; Zhuang, Z.Y.; Yu, Y. Recyclable Nanoscale Zero Valent Iron Doped g-C3N4/MoS2 for Efficient Photocatalytic of RhB and Cr (VI) Driven by Visible Light. ACS Sustain. Chem. Eng. 2016, 4, 4055–4063. [Google Scholar] [CrossRef]
- Fu, H.B.; Zhang, S.C.; Xu, T.G.; Zhu, Y.F.; Chen, J.M. Photocatalytic Degradation of RhB by Fluorinated Bi2WO6 and Distributions of the Intermediate Products. Environ. Sci. Technol. 2008, 42, 2085–2091. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.D.; Dai, W.X.; Tian, Q.F.; Li, Z.H.; Xie, L.Y.; Wang, J.X.; Liu, P. Photocatalytic Degradation of RhB over TiO2 Bilayer Films: Effect of Defects and Their Location. Langmuir 2010, 26, 9686–9694. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yu, J.H.; Seo, H.J.; Jeong, R.H.; Boo, J.H. Improved electrochromic properties of nanoporous NiO film by NiO flake with thickness controlled by aluminum. Appl. Surf. Sci. 2018, 461, 88–92. [Google Scholar] [CrossRef]
- Yang, J.; Xie, T.P.; Zhu, Q.X.; Wang, J.K.; Xu, L.J.; Liu, C.L. Boosting the photocatalytic activity of BiOX under solar light via selective crystal facet growth. J. Mater. Chem. C 2020, 8, 2579–2588. [Google Scholar] [CrossRef]
- Yang, J.; Xie, T.P.; Liu, C.L.; Xu, L.J. Dy(III) Doped BiOCl Powder with Superior Highly Visible-Light-Driven Photocatalytic Activity for Rhodamine B Photodegradation. Nanomaterials 2018, 8, 697. [Google Scholar] [CrossRef]
- He, R.G.; Xu, D.F.; Cheng, B.; Yu, J.G.; Ho, W.K. Review on Nanoscale Bi-based Photocatalysts. Nanoscale Horiz. 2018, 3, 464–504. [Google Scholar] [CrossRef]
- Jiang, J.; Zhao, K.; Xiao, X.Y.; Zhang, L.Z. Synthesis and Facet-Dependent Photoreactivity of BiOCl Single Crystalline Nanosheets. J. Am. Chem. Soc. 2012, 134, 4473–4476. [Google Scholar] [CrossRef]
- Xie, T.P.; Xu, L.J.; Liu, C.L.; Yang, J.; Wang, M. Magnetic composite BiOCl–SrFe12O19: A novel p-n type heterojunction with enhanced photocatalytic activity. Dalton Trans. 2014, 43, 2211–2220. [Google Scholar] [CrossRef]
- Li, Y.W.; Zhao, Y.; Wu, G.J.; Ma, H.M.; Zhao, J.Z. Bi superlattice nanopolygons at BiOCl (001) nanosheet assembled architectures for visible-light photocatalysis. Mater. Res. Bull. 2018, 101, 39–47. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Ai, Z.H.; Jia, F.L.; Zhang, L.Z. Oxygen Vacancy-Mediated Photocatalysis of BiOCl: Reactivity, Selectivity, and Perspectives. Angew. Chem. Int. Ed. 2018, 57, 122–138. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, V.G.; Paloly, A.R.; Divya, N.G.; Bushiri, M.J. Photocatalytic and ferromagnetic properties of electrically conducting multifunctional Ni/NiO nanocomposites in amorphous carbon matrix. Mater. Sci. Eng. B 2018, 228, 132–141. [Google Scholar] [CrossRef]
- Ghosh, C.; Majumder, S.; Bhattachatjee, S. NiO/Ag heterostructure: Enhanced UV emission intensity, exchange interaction and photocatalytic activity. RSC Adv. 2016, 6, 56503–56510. [Google Scholar]
- Fechete, I.; Ivan, S.B.; Popescu, I.; Garin, F.; Parvulescu, V.I.; Marcu, I.C. The effect of phosphorus on the catalytic performance of nickel oxide in ethane oxidative dehydrogenation. Catal. Sci. Technol. 2016, 6, 6953–6964. [Google Scholar]
- Faisal, M.; Harraz Farid, A.; Ismail Adel, A.; El-Tonib, A.M.; Al-Sayari, S.A.; Al-Hajry, A.; Al-Assiri, M.S. Novel mesoporous NiO/TiO2 nanocomposites with enhanced photocatalytic activity under visible light illumination. Ceram. Int. 2018, 44, 7047–7056. [Google Scholar] [CrossRef]
- Varshney, D.; Dwivedi, S. Synthesis, structural, Raman spectroscopic and paramagnetic properties of Sn doped NiO nanoparticles. Superlattices Microstruct. 2015, 86, 430–437. [Google Scholar] [CrossRef]
- Hu, J.L.; Fan, W.J.; Ye, W.Q.; Huang, C.J.; Qiu, X.Q. Insights into the photosensitivity activity of BiOCl under visible light irradiation. Appl. Catal. B 2014, 158–159, 182–189. [Google Scholar] [CrossRef]
- Wang, M.G.; Han, J.; Hu, Y.M.; Guo, R.; Yin, Y.D. Carbon-Incorporated NiO/TiO2 Mesoporous Shells with p-n Heterojunctions for Efficient Visible Light Photocatalysis. ACS Appl. Mater. Interfaces 2016, 8, 29511–29521. [Google Scholar] [CrossRef]
- Xing, P.X.; Zhang, W.Q.; Chen, L.; Dai, X.Q.; Zhang, J.L.; Zhao, L.H.; He, Y.M. Preparation of a NiO/KNbO3 nanocomposite via a photodeposition method and its superior performance in photocatalytic N2 fixation. Sustain. Energy Fuels 2020, 4, 1112–1117. [Google Scholar] [CrossRef]
- Mallows, J.; Planells, M.; Thakare, V.; Bhosale, R.; Ogale, S.; Robertson, N. p-Type NiO Hybrid Visible Photodetector. ACS Appl. Mater. Interfaces 2015, 7, 27597–27601. [Google Scholar] [CrossRef]
- Fu, G. Tuning the Electronic Structure of NiO via Li Doping for the Fast Oxygen Evolution Reaction. Chem. Mater. 2019, 31, 419–428. [Google Scholar] [CrossRef]
- He, Z.; Shi, Y.; Gao, C.; Wen, L.; Chen, J.; Song, S. BiOCl/BiVO4 P–N Heterojunction with Enhanced Photocatalytic Activity under Visible-Light Irradiation. J. Phys. Chem. C 2014, 118, 389–398. [Google Scholar] [CrossRef]
- Zhang, X.; Fan, C.; Wang, Y.; Wang, Y.; Liang, Z.; Han, P. Dft+U Predictions: The Effect of Oxygen Vacancy on the Structural, Electronic and Photocatalytic Properties of Mn-Doped BiOCl. Comp. Mater. Sci. 2013, 71, 135–145. [Google Scholar] [CrossRef]
- Huang, H.; Tu, S.; Zeng, C.; Zhang, T.; Reshak, A.H.; Zhang, Y. Macroscopic Polarization Enhancement Promoting Photo- and Piezoelectric-Induced Charge Separation and Molecular Oxygen Activation. Angew. Chem. Int. Ed. 2017, 56, 11860–11864. [Google Scholar] [CrossRef] [PubMed]
- Chai, S.Y.; Kim, Y.J.; Jung, M.H.; Chakraborty, A.K.; Jung, D.; Lee, W.I. Heterojunctioned BiOCl/Bi2O3, a New Visible Light Photocatalyst. J. Catal. 2009, 262, 144–149. [Google Scholar] [CrossRef]
- Muñoz-Batista, M.; Gómez-Cerezo, M.; Kubacka, A.; Tudela, D.; Fernández-García, M. Role of Interface Contact in CeO2–TiO2 Photocatalytic Composite Materials. ACS Catal. 2014, 4, 63–72. [Google Scholar]
- Muñoz-Batista, M.; Ballari, M.; Kubacka, A.; Alfano, O.; Fernández-García, M. Braiding kinetics and spectroscopy in photo-catalysis: The spectro-kinetic approach. Chem. Soc. Rev. 2019, 48, 637–682. [Google Scholar]
- Huang, S.Y.; Wu, H.; Matsubara, K.; Cheng, J.; Pan, W. Facile assembly of n-SnO2 nanobelts p-NiO heterojunctions with enhanced ultraviolet photoresponse. Chem. Commun. 2014, 50, 2847–2850. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Y.; Zhao, Z.L.; Zhang, Q.H.; Huang, L.B.; Gu, L.; Lu, G.; Hu, J.S.; Wan, L.J. Steering Elementary Steps towards Efficient Alkaline Hydrogen Evolution via Size-Dependent Ni/NiO Nanoscale Heterosurfaces. Nat. Sci. Rev. 2020, 7, 27–36. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmuller, J. Efficient Iterative Schemes for Ab Initio Total-Energy Calculations Using a Plane-Wave Basis Set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From Ultrasoft Pseudopotentials to the Projector Augmented-Wave Method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Neugebauer, J.; Scheffler, M. Adsorbate-Substrate and Adsorbate-Adsorbe Interactions of Na and K Adlayers on Al(111). Phys. Rev. B 1992, 46, 16067–16080. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Zhu, Q.; Xie, T.; Wang, J.; Peng, Y.; Wang, Y.; Liu, C.; Xu, L. Insights into Interface Charge Extraction in a Noble-Metal-Free Doped Z-Scheme NiO@BiOCl Heterojunction. Catalysts 2020, 10, 958. https://doi.org/10.3390/catal10090958
Yang J, Zhu Q, Xie T, Wang J, Peng Y, Wang Y, Liu C, Xu L. Insights into Interface Charge Extraction in a Noble-Metal-Free Doped Z-Scheme NiO@BiOCl Heterojunction. Catalysts. 2020; 10(9):958. https://doi.org/10.3390/catal10090958
Chicago/Turabian StyleYang, Jun, Quanxi Zhu, Taiping Xie, Jiankang Wang, Yuan Peng, Yajing Wang, Chenglun Liu, and Longjun Xu. 2020. "Insights into Interface Charge Extraction in a Noble-Metal-Free Doped Z-Scheme NiO@BiOCl Heterojunction" Catalysts 10, no. 9: 958. https://doi.org/10.3390/catal10090958
APA StyleYang, J., Zhu, Q., Xie, T., Wang, J., Peng, Y., Wang, Y., Liu, C., & Xu, L. (2020). Insights into Interface Charge Extraction in a Noble-Metal-Free Doped Z-Scheme NiO@BiOCl Heterojunction. Catalysts, 10(9), 958. https://doi.org/10.3390/catal10090958





