Mechanistic Insights for Dry Reforming of Methane on Cu/Ni Bimetallic Catalysts: DFT-Assisted Microkinetic Analysis for Coke Resistance
Abstract
1. Introduction
2. Results and Discussion
2.1. Adsorption Geometries and Energies on Ni2Cu Overlayer of Ni (111) Surface
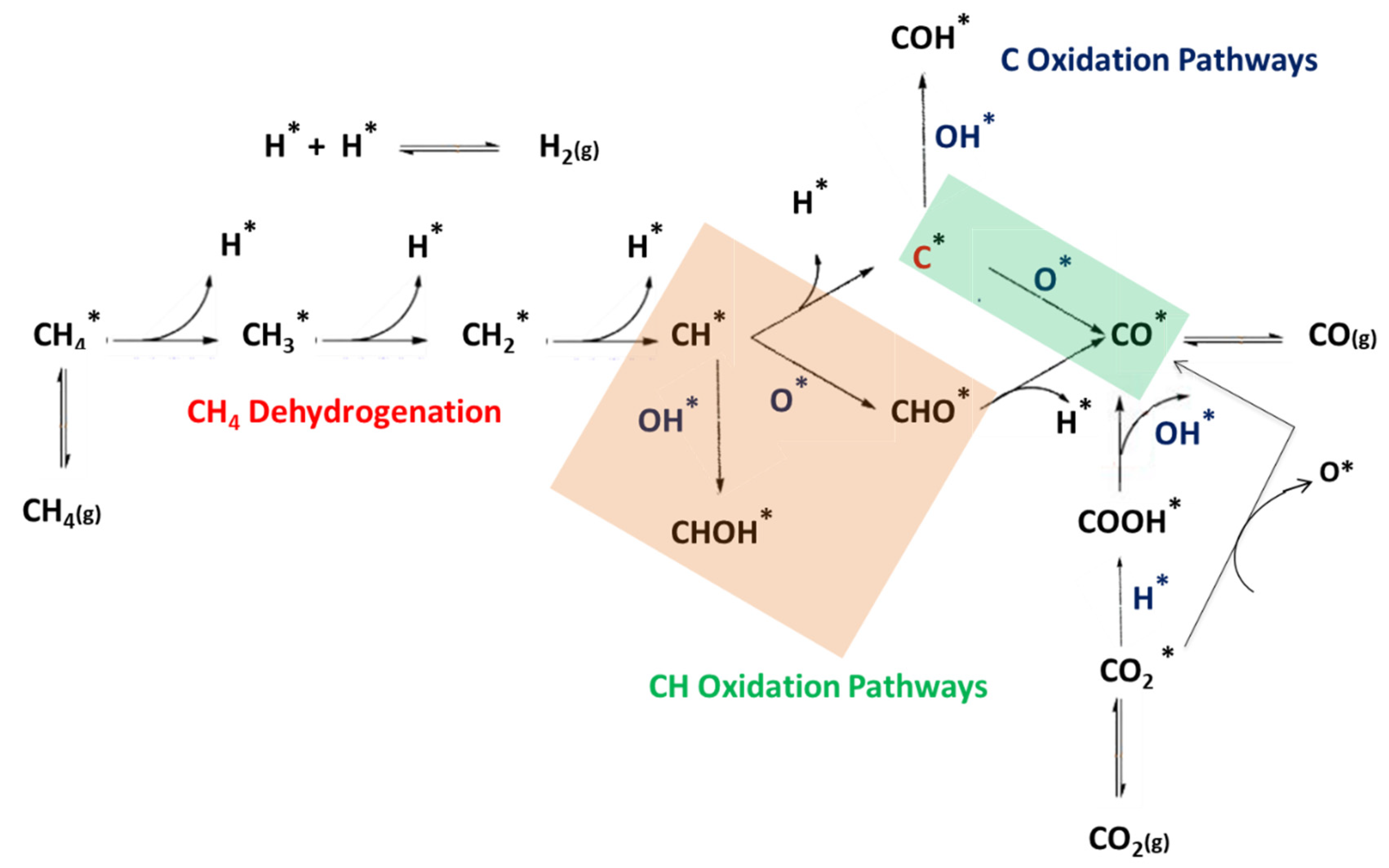
2.2. DRM Reaction Mechanism
2.2.1. CHx Dissociation (x = 1–4)
2.2.2. Two Reaction Pathways for CO2 Dissociation
2.2.3. Oxidation of C and CH
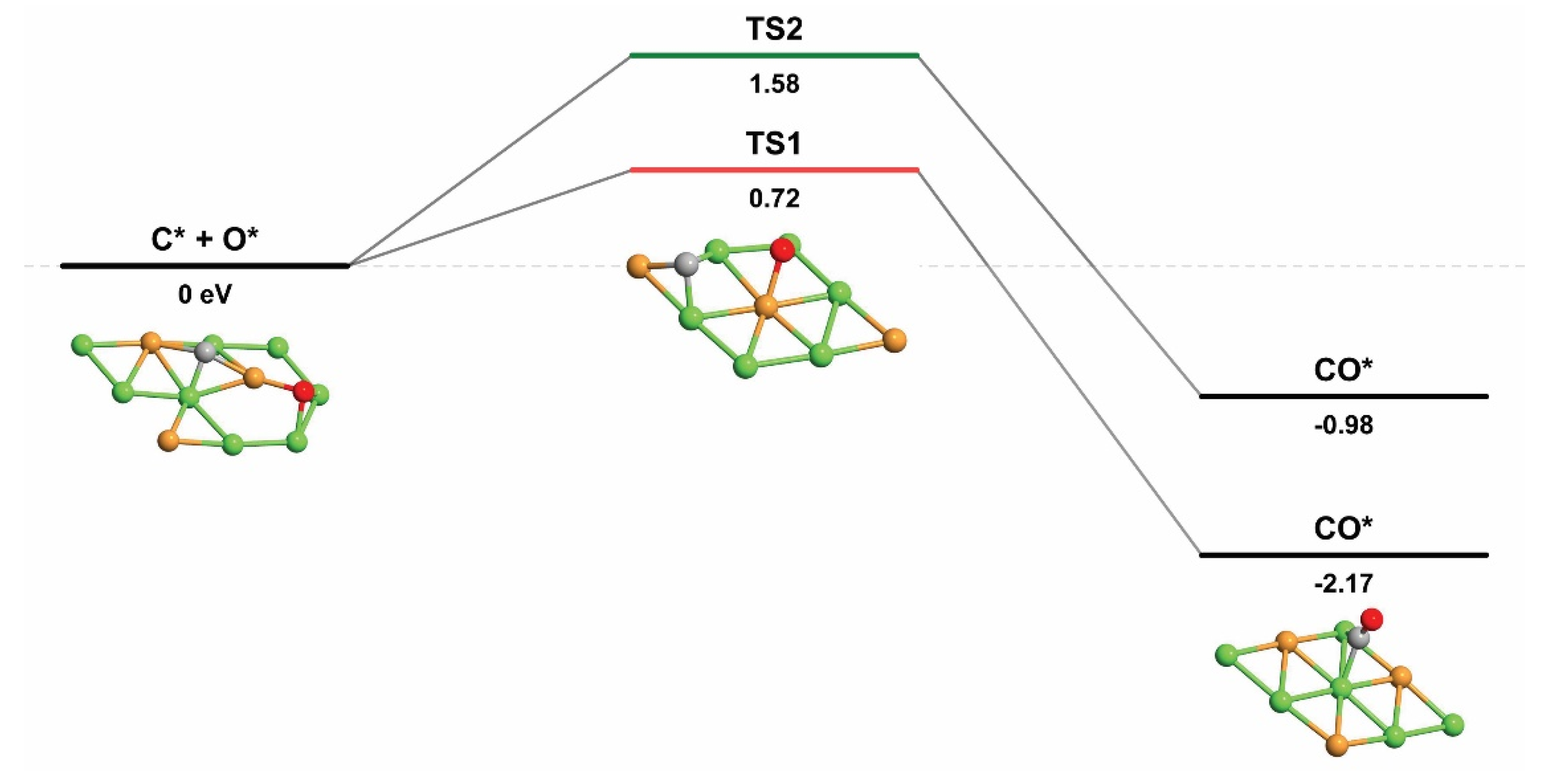
2.2.4. Carbon elimination by C + O and C + OH
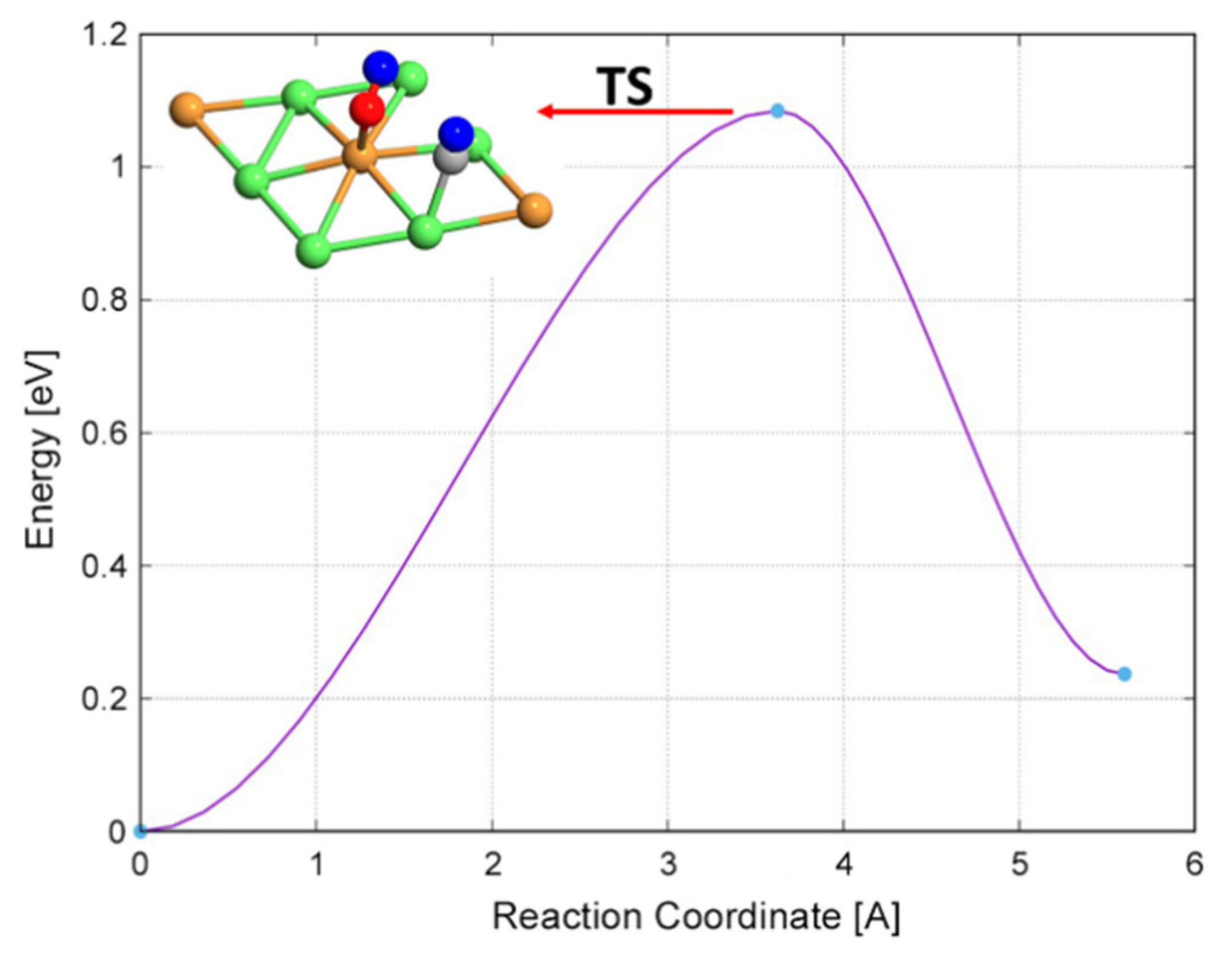
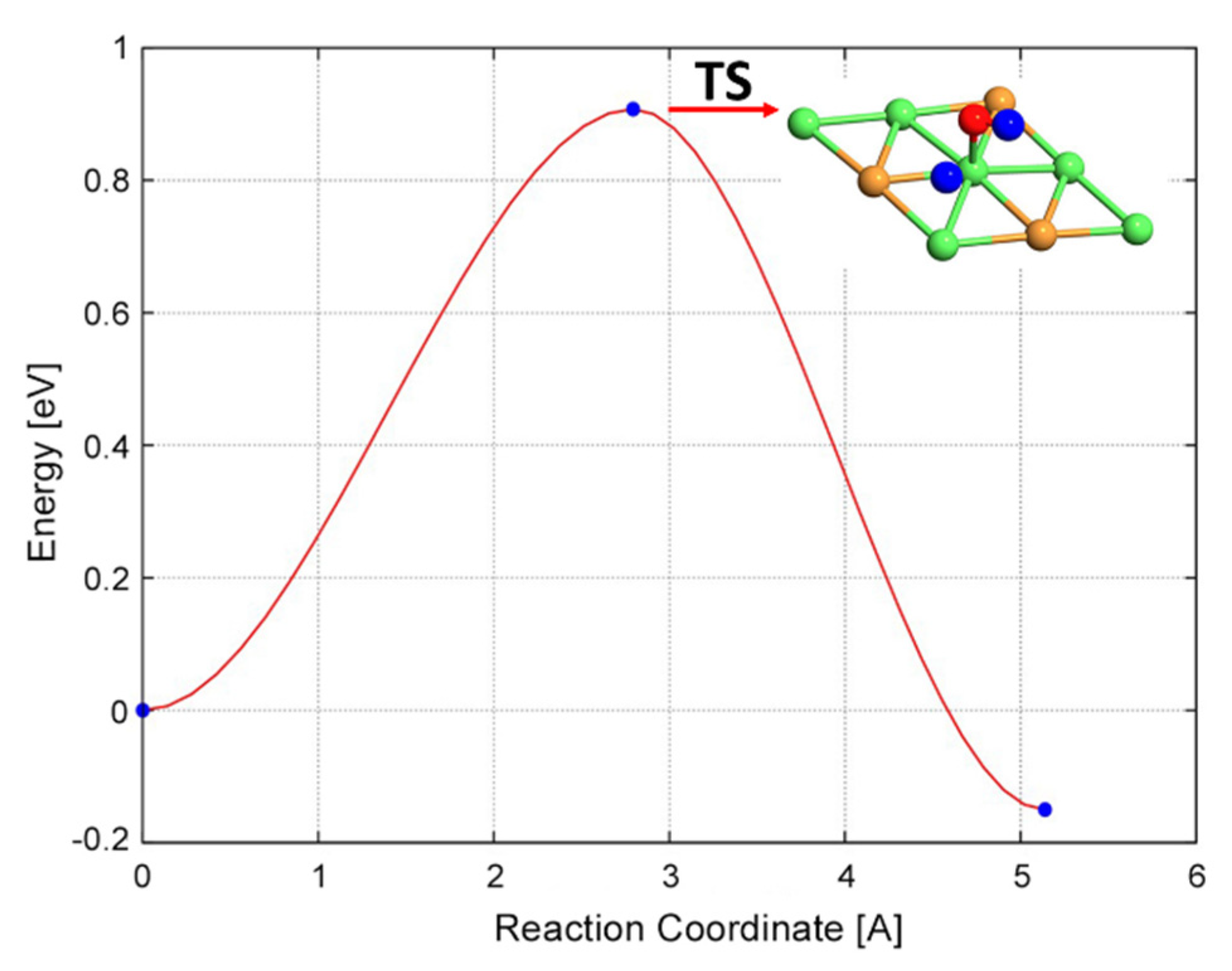
2.2.5. CH + O and CH + OH Reactions
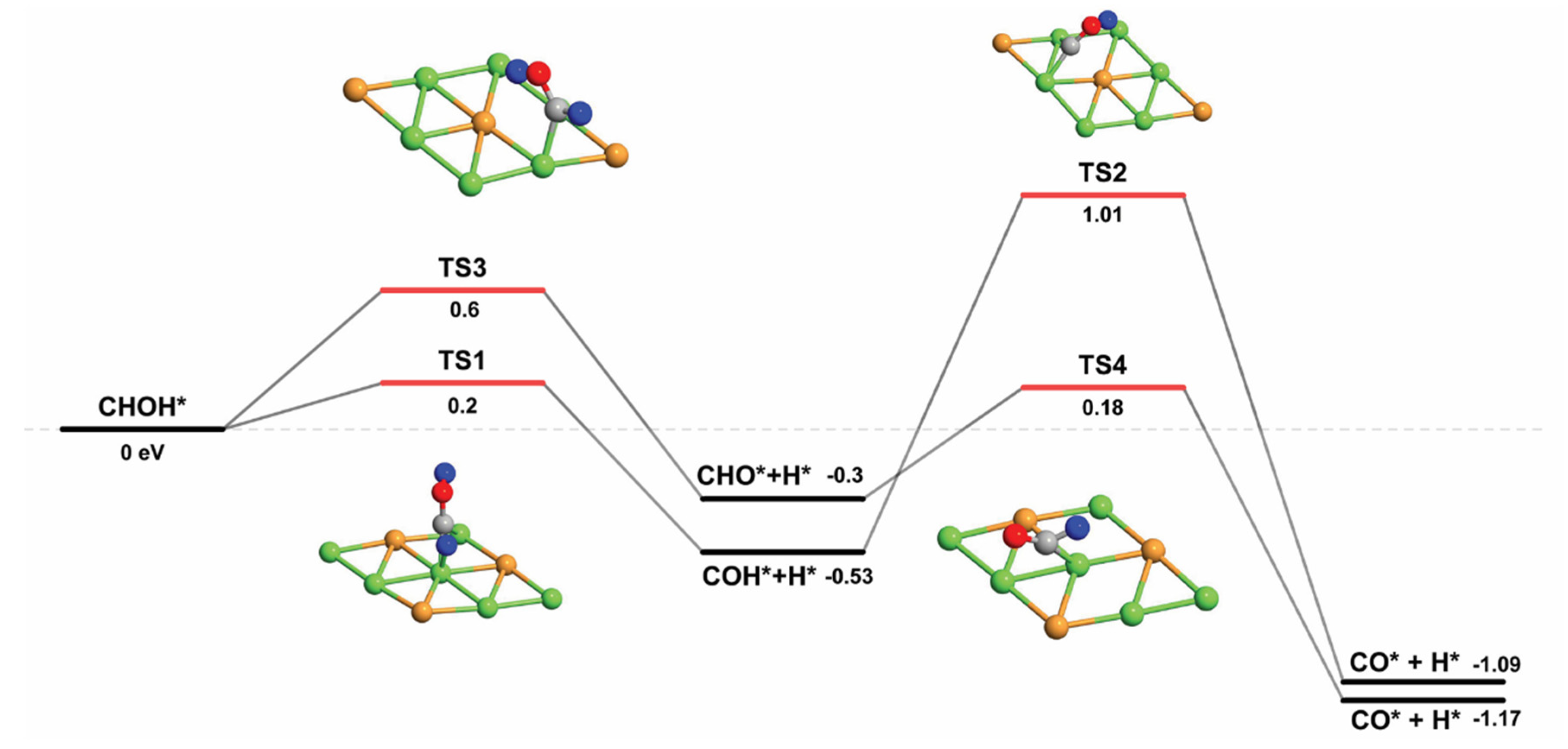
2.2.6. CHOH and COH Decomposition
2.2.7. H2 and H2O Formation
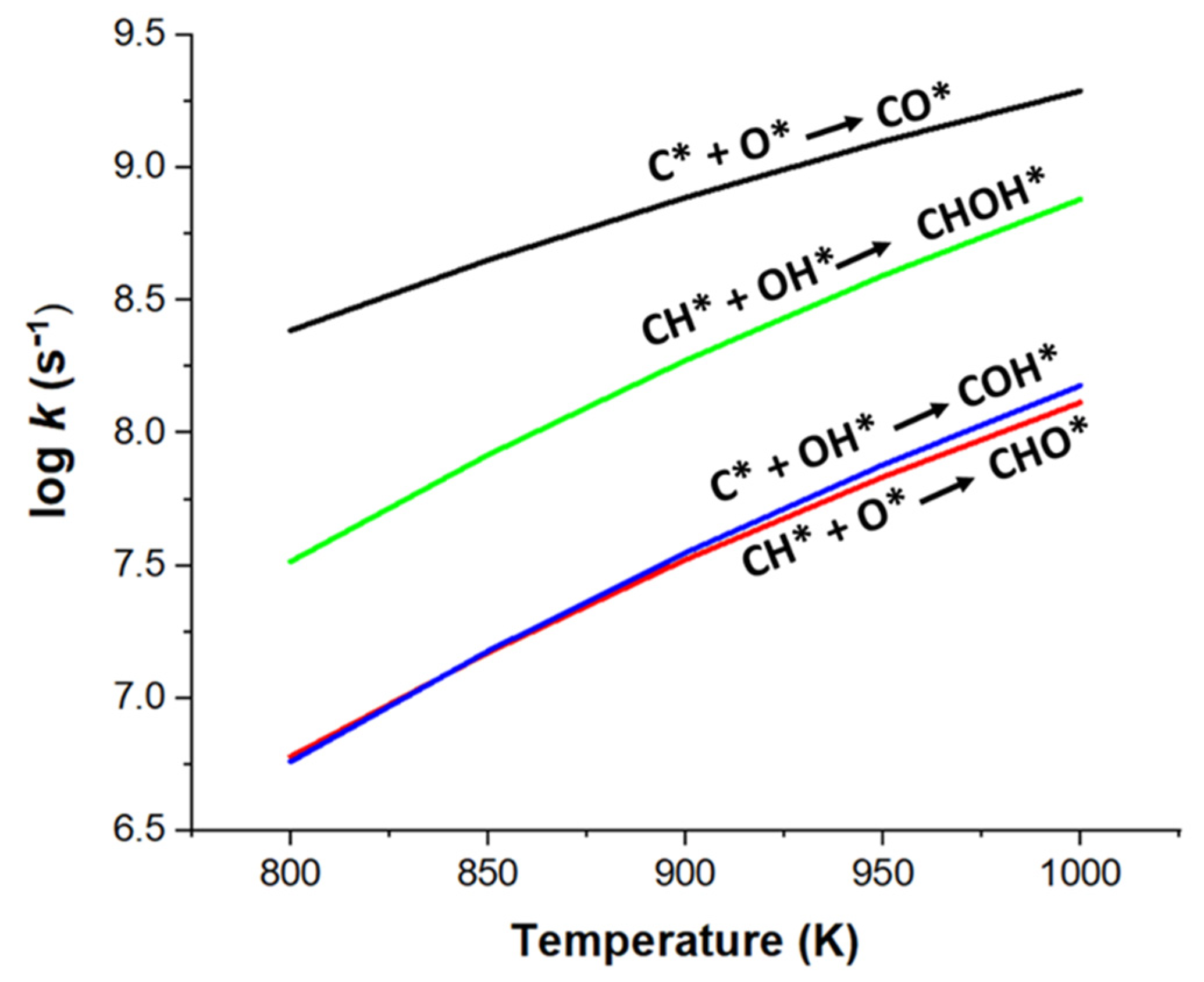
2.3. Effect of Temperature on Carbon Deposition Resistance
2.4. Dominant Reaction Pathways and Rate-Limiting Steps
3. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Uykun Mangaloğlu, D.; Baranak, M.; Ataç, Ö.; Atakül, H. Effect of the promoter presence in catalysts on the compositions of Fischer–Tropsch synthesis products. J. Ind. Eng. Chem. 2018, 66, 298–310. [Google Scholar] [CrossRef]
- Arora, S.; Prasad, R. An overview on dry reforming of methane: Strategies to reduce carbonaceous deactivation of catalysts. RSC Adv. 2016, 6, 108668–108688. [Google Scholar] [CrossRef]
- Challiwala, M.S.; Wilhite, B.A.; Ghouri, M.M.; Elbashir, N.O. Multidimensional modeling of a microfibrous entrapped cobalt catalyst Fischer-Tropsch reactor bed. AIChE J. 2018, 64, 1723–1731. [Google Scholar] [CrossRef]
- Gu, B.; Khodakov, A.Y.; Ordomsky, V.V. Selectivity shift from paraffins to α-olefins in low temperature Fischer–Tropsch synthesis in the presence of carboxylic acids. Chem. Commun. 2018, 54, 2345–2348. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Dhir, A.; Mohapatra, S.K.; Mahla, S.K. Dry reforming of methane using various catalysts in the process: Review. Biomass Convers. Biorefin. 2020, 10, 567–587. [Google Scholar] [CrossRef]
- Challiwala, M.S.; Afzal, S.; Choudhury, H.A.; Sengupa, D.; El-Halwagi, M.M.; Elbashir, N.O. Alternative via Pathways Dry Reforming for CO of 2 Utilization Methane. In Advances in Carbon Management Technologies Volume 1: Carbon Removal, Renewable and Nuclear Energy; CRC Press: Boca Raton, FL, USA, 2020; p. 253. Available online: https://www.taylorfrancis.com/books/9780429243608/chapters/10.1201/9780429243608-14 (accessed on 10 September 2020).
- Wei, J.; Iglesia, E. Isotopic and kinetic assessment of the mechanism of reactions of CH4 with CO2 or H2O to form synthesis gas and carbon on nickel catalysts. J. Catal. 2004, 224, 370–383. [Google Scholar] [CrossRef]
- Niu, J.; Ran, J.; Chen, D. Understanding the mechanism of CO2 reforming of methane to syngas on Ni@Pt surface compared with Ni(111) and Pt(111). Appl. Surf. Sci. 2020, 513, 145840. [Google Scholar] [CrossRef]
- Takami, D.; Yamamoto, A.; Yoshida, H. Dry reforming of methane over alumina-supported rhodium catalysts at low temperatures under visible and near-infrared light. Catal. Sci. Technol. 2020, 10, 5811–5814. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, K.; Barat, R.; Mitra, S. Dry reforming of methane over ruthenium/carbon nanotube catalyst. Chem. Eng. 2020, 4, 16. [Google Scholar] [CrossRef]
- Maina, S.C.P.; Ballarini, A.D.; Vilella, J.I.; de Miguel, S.R. Study of the performance and stability in the dry reforming of methane of doped alumina supported iridium catalysts. Catal. Today 2020, 344, 129–142. [Google Scholar] [CrossRef]
- Leba, A.; Yıldırım, R. Determining most effective structural form of nickel-cobalt catalysts for dry reforming of methane. Int. J. Hydro. Energy 2020, 45, 4268–4283. [Google Scholar] [CrossRef]
- Pakhare, D.; Spivey, J. A review of dry (CO2) reforming of methane over noble metal catalysts. Chem. Soc. Rev. 2014, 43, 7813–7837. [Google Scholar] [CrossRef]
- Kawi, S.; Kathiraser, Y.; Ni, J.; Oemar, U.; Li, Z.W.; Saw, E.T. Progress in synthesis of highly active and stable nickel-based catalysts for carbon dioxide reforming of methane. Chemsuschem. 2015, 8, 3556–3575. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yao, L.; Wang, S.H.; Mao, D.H.; Hu, C.W. Low-temperature catalytic CO2 dry reforming of methane on Ni-based catalysts: A review. Fuel Process. Technol. 2018, 169, 199–206. [Google Scholar] [CrossRef]
- Ren, J.; Qin, X.; Yang, J.Z.; Qin, Z.F.; Guo, H.L.; Lin, J.Y.; Li, Z. Methanation of carbon dioxide over Ni-M/ZrO2 (M = Fe, Co, Cu) catalysts: Effect of addition of a second metal. Fuel Process. Technol. 2015, 137, 204–211. [Google Scholar] [CrossRef]
- Bian, Z.F.; Das, S.; Wai, M.H.; Hongmanorom, P.; Kawi, S. A review on bimetallic nickel-based catalysts for CO2 reforming of methane. Chemphyschem 2017, 18, 3117–3134. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.Y.; Fu, Z.M.; Yang, Z.X. The carbon-tolerance mechanism of Ni-based alloy with coinage metals. Phys. Lett. A 2013, 377, 2189–2194. [Google Scholar] [CrossRef]
- Xu, L.-L.; Wen, H.; Jin, X.; Bing, Q.-M.; Liu, J.-Y. DFT study on dry reforming of methane over Ni2Fe overlayer of Ni (1 1 1) surface. Appl. Surf. Sci. 2018, 443, 515–524. [Google Scholar] [CrossRef]
- Theofanidis, S.A.; Batchu, R.; Galvita, V.V.; Poelman, H.; Marin, G.B. Carbon gasification from Fe–Ni catalysts after methane dry reforming. Appl. Catal. B Environ. 2016, 185, 42–55. [Google Scholar] [CrossRef]
- Theofanidis, S.A.; Galvita, V.V.; Poelman, H.; Marin, G.B. Enhanced carbon-resistant dry reforming Fe–Ni catalyst: Role of Fe. ACS Catal. 2015, 5, 3028–3039. [Google Scholar] [CrossRef]
- Lo Faro, M.; Frontera, P.; Antonucci, P.; Arico, A.S. Ni-Cu based catalysts prepared by two different methods and their catalytic activity toward the ATR of methane. Chem. Eng. Res. Des. 2015, 93, 269–277. [Google Scholar] [CrossRef]
- Rahemi, N.; Haghighi, M.; Babaluo, A.A.; Allahyari, S.; Jafari, M.F. Syngas production from reforming of greenhouse gases CH4/CO2 over Ni–Cu/Al2O3 nanocatalyst: Impregnated vs. plasma-treated catalyst. Energ. Convers. Manag. 2014, 84, 50–59. [Google Scholar] [CrossRef]
- Wu, T.; Cai, W.Y.; Zhang, P.; Song, X.F.; Gao, L. Cu-Ni@ SiO2 alloy nanocomposites for methane dry reforming catalysis. RSC Adv. 2013, 3, 23976–23979. [Google Scholar] [CrossRef]
- Liu, H.Y.; Zhang, R.G.; Yan, R.X.; Li, J.R.; Wang, B.J.; Xie, K.C. Insight into CH4 dissociation on NiCu catalyst: A first-principles study. Appl. Surf. Sci. 2012, 258, 8177–8184. [Google Scholar] [CrossRef]
- An, W.; Zeng, X.C.; Turner, C.H. First-principles study of methane dehydrogenation on a bimetallic Cu/Ni (111) surface. J. Chem. Phys. 2009, 131, 174702. [Google Scholar] [CrossRef] [PubMed]
- Chatla, A.; Ghouri, M.M.; El Hassan, O.W.; Mohamed, N.; Prakash, A.V.; Elbashir, N.O. An experimental and first principles DFT investigation on the effect of Cu addition to Ni/Al2O3 catalyst for the dry reforming of methane. Appl. Catal. A-Gen. 2020, 602, 117699. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Cao, X.M.; Zhu, J.H.; Hu, P. Activity and coke formation of nickel and nickel carbide in dry reforming: A deactivation scheme from density functional theory. J. Catal. 2014, 311, 469–480. [Google Scholar] [CrossRef]
- Arevalo, R.L.; Aspera, S.M.; Escano, M.C.S.; Nakanishi, H.; Kasai, H. Tuning methane decomposition on stepped Ni surface: The role of subsurface atoms in catalyst design. Sci. Rep. 2017, 7, 13963. [Google Scholar] [CrossRef]
- Heiland, W. Charge-exchange processes and surface-chemistry. Surf. Sci. 1991, 251, 942–946. [Google Scholar] [CrossRef]
- Jang, W.J.; Shim, J.O.; Kim, H.M.; Yoo, S.Y.; Roh, H.S. A review on dry reforming of methane in aspect of catalytic properties. Catal. Today 2019, 324, 15–26. [Google Scholar] [CrossRef]
- Kim, S.M.; Abdala, P.M.; Margossian, T.; Hosseini, D.; Foppa, L.; Armutlulu, A.; van Beek, W.; Comas-Vives, A.; Copéret, C.; Müller, C. Cooperativity and dynamics increase the performance of NiFe dry reforming catalysts. J. Am. Chem. Soc. 2017, 139, 1937–1949. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Liu, Z.; Zhu, Y.-A.; Tong, G.; Zhang, J.; Engelbrekt, C.; Ulstrup, J.; Zhu, K.; Zhou, X. Tuning the composition of metastable CoxNiyMg100−x−y(OH)(OCH3) nanoplates for optimizing robust methane dry reforming catalyst. J. Catal. 2015, 330, 106–119. [Google Scholar] [CrossRef]
- Michaelides, A.; Hu, P. Methyl chemisorption on Ni (111) and C-H-M multicentre bonding: A density functional theory study. Surf. Sci. 1999, 437, 362–376. [Google Scholar] [CrossRef]
- Zhu, Y.-A.; Dai, Y.-C.; Chen, D.; Yuan, W.-K. First-principles calculations of CH4 dissociation on Ni (100) surface along different reaction pathways. J. Mol. Catal. A Chem. 2007, 264, 299–308. [Google Scholar] [CrossRef]
- Bradford, M.C.J.; Vannice, M.A. Catalytic reforming of methane with carbon dioxide over nickel catalysts II. Reaction kinetics. Appl. Catal. A-Gen. 1996, 142, 97–122. [Google Scholar] [CrossRef]
- Maslov, M.M.; Openov, L.A.; Podlivaev, A.I. On the vineyard formula for the pre-exponential factor in the Arrhenius law. Phys. Solid State 2014, 56, 1239–1244. [Google Scholar] [CrossRef][Green Version]
- Monkhorst, H.J.; Pack, J.D. Special points for brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Jørgensen, M. Microkinetic Modeling of Nanoparticl Catalysis Using Density Functional Theory. Ph.D. Thesis, Chalmers University of Technology, Gothenburg, Sweden, 2017. [Google Scholar]
- Jørgensen, M.; Grönbeck, H. First-principles microkinetic modeling of methane oxidation over Pd (100) and Pd (111). ACS Catal. 2016, 6, 6730–6738. [Google Scholar] [CrossRef]
- Ray, D.; Ghosh, S.; Tiwari, A.K. Controlling heterogeneous catalysis of water dissociation using Cu-Ni bimetallic alloy surfaces: A quantum dynamics study. J. Phys. Chem. A 2018, 122, 5698–5709. [Google Scholar] [CrossRef]
- Zhang, R.G.; Guo, X.Q.; Wang, B.J.; Ling, L.M. Insight Into the effect of CuNi (111) and FeNi (111) surface structure and second metal composition on surface carbon elimination by O or OH: A comparison study with Ni (111) surface. J. Phys. Chem. C 2015, 119, 14135–14144. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Blochl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Hammer, B.; Hansen, L.B.; Norskov, J.K. Improved adsorption energetics within density-functional theory using revised Perdew-Burke-Ernzerhof functionals. Phys. Rev. B 1999, 59, 7413–7421. [Google Scholar] [CrossRef]
- Hammer, B.; Norskov, J.K. Theoretical surface science and catalysis—Calculations and concepts. Adv. Catal. 2000, 45, 71–129. [Google Scholar] [CrossRef]
- Methfessel, M.; Paxton, A.T. High-precision sampling for brillouin-zone integration in metals. Phys. Rev. B 1989, 40, 3616–3621. [Google Scholar] [CrossRef]
- Zhu, Y.A.; Chen, D.; Zhou, X.G.; Yuan, W.K. DFT studies of dry reforming of methane on Ni catalyst. Catal. Today 2009, 148, 260–267. [Google Scholar] [CrossRef]
- Henkelman, G.; Jonsson, H. Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points. J. Chem. Phys. 2000, 113, 9978–9985. [Google Scholar] [CrossRef]
- Yoon, S.H.; Kang, U.; Park, H.; Abdel-Wahab, A.; Han, D.S. Computational density functional theory study on the selective conversion of CO2 to formate on homogeneously and heterogeneously mixed CuFeO2 and CuO surfaces. Catal. Today 2019, 335, 345–353. [Google Scholar] [CrossRef]
- Henkelman, G.; Jonsson, H. A dimer method for finding saddle points on high dimensional potential surfaces using only first derivatives. J. Chem. Phys. 1999, 111, 7010–7022. [Google Scholar] [CrossRef]
- Yoon, S.H.; Yu, C.; Han, A.; Park, H.; Elbashir, N.; Han, D.S. Computational characterization of nitrogen-doped carbon nanotube functionalized by Fe adatom and Fe substituent for oxygen reduction reaction. Appl. Surf. Sci. 2019, 485, 342–352. [Google Scholar] [CrossRef]
- Henkelman, G.; Arnaldsson, A.; Jónsson, H. Theoretical calculations of CH4 and H2 associative desorption from Ni (111): Could subsurface hydrogen play an important role? J. Chem. Phys. 2006, 124, 044706. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Zhu, Y.A.; Yang, M.L.; Sui, Z.J.; Zhou, X.G.; Chen, D. Density functional theory-assisted microkinetic analysis of methane dry reforming on Ni catalyst. Ind. Eng. Chem. Res. 2015, 54, 5901–5913. [Google Scholar] [CrossRef]
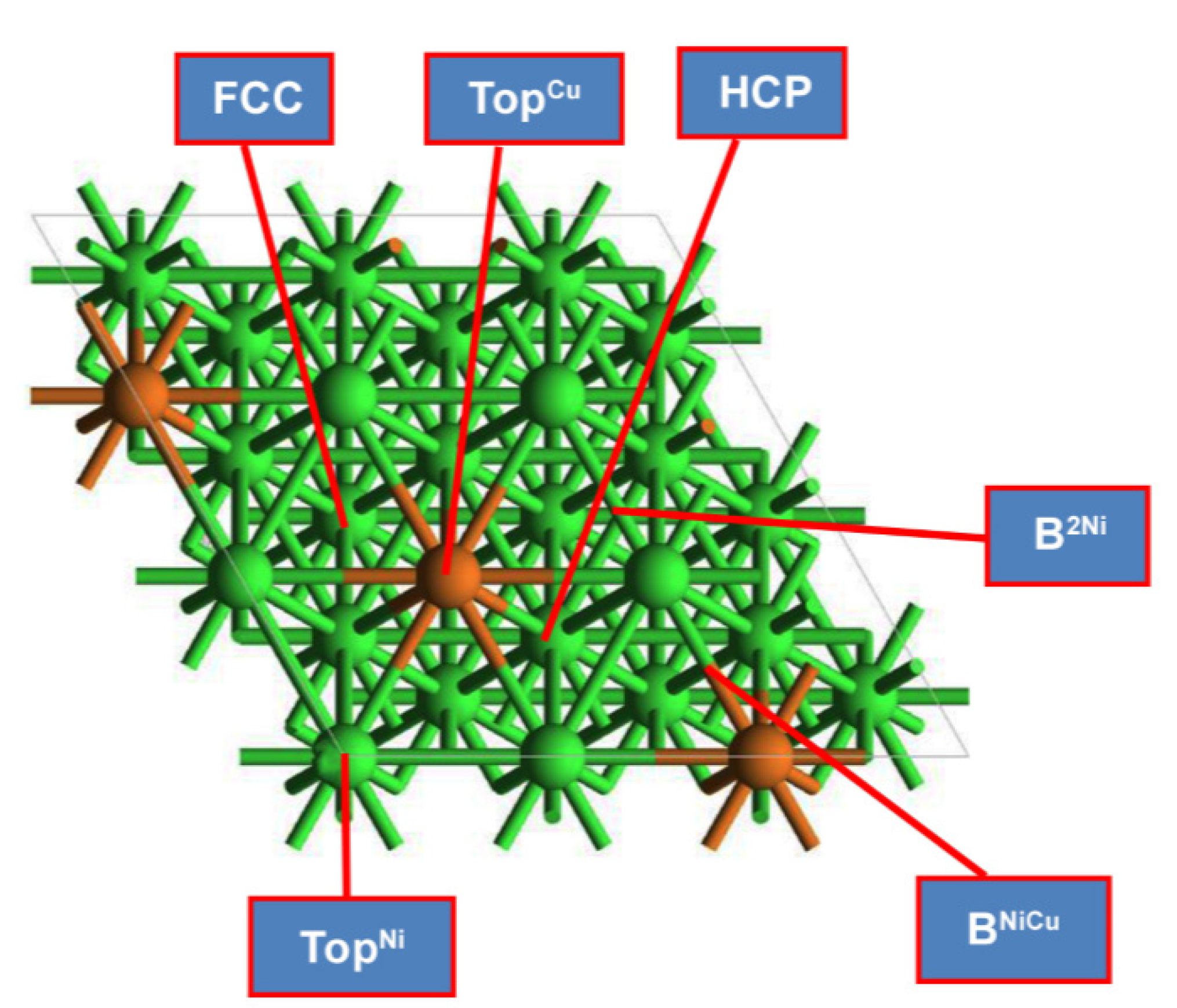
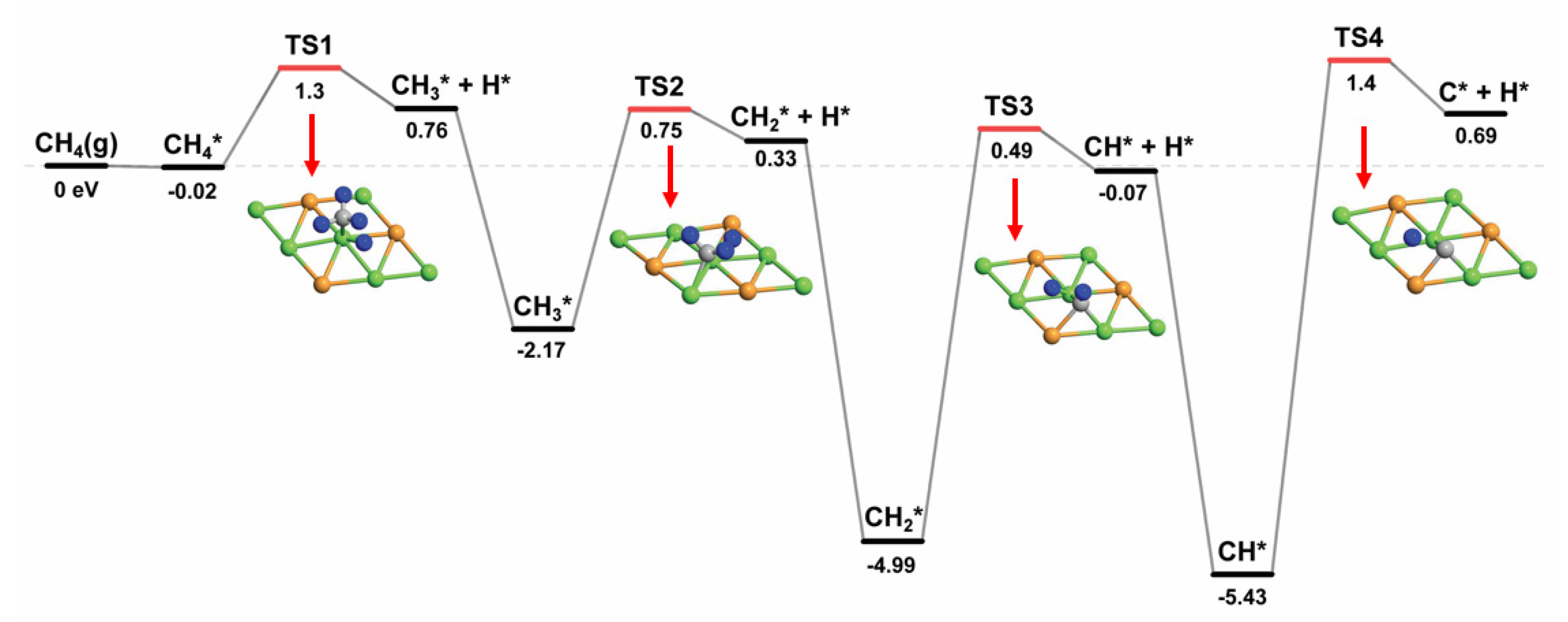
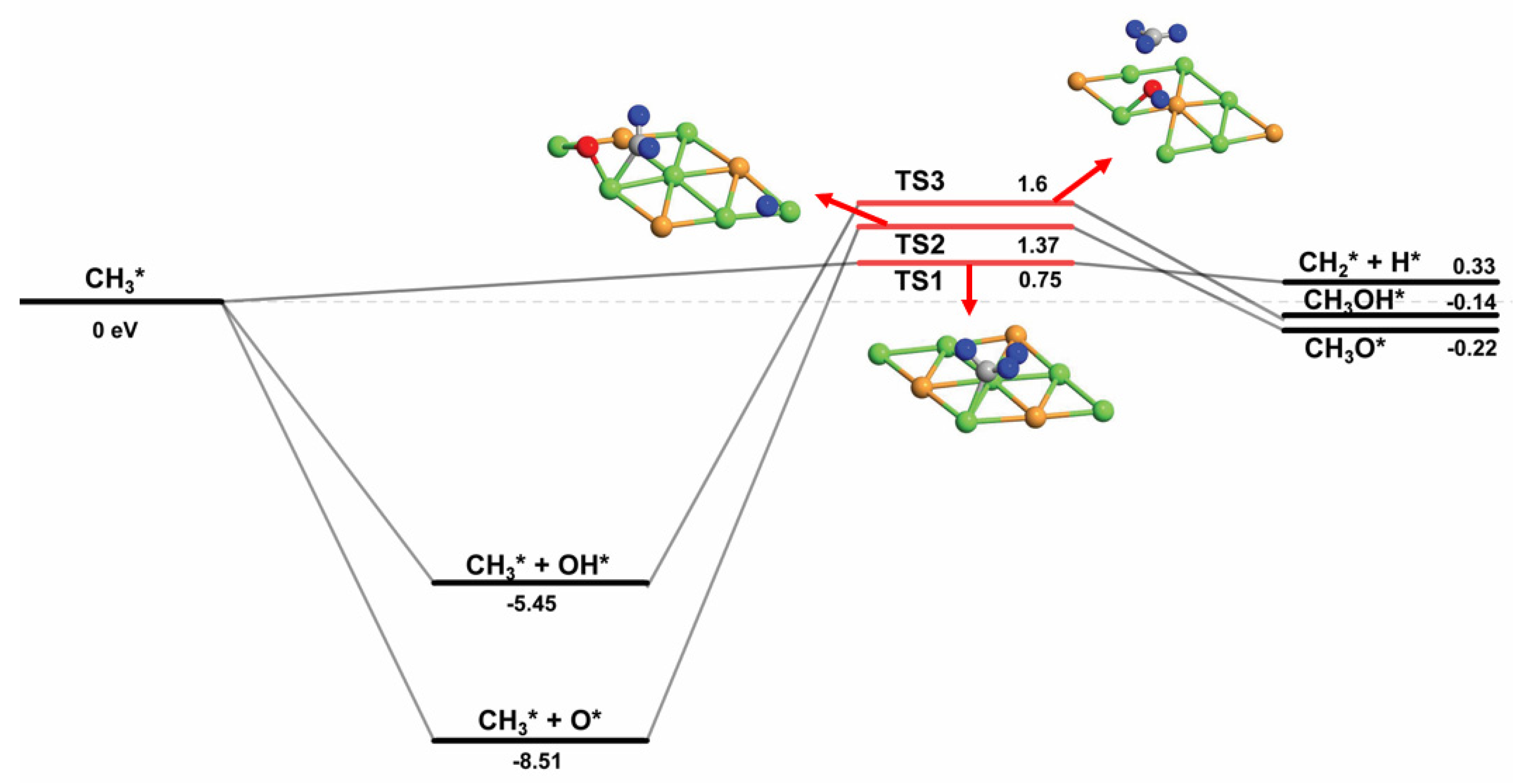
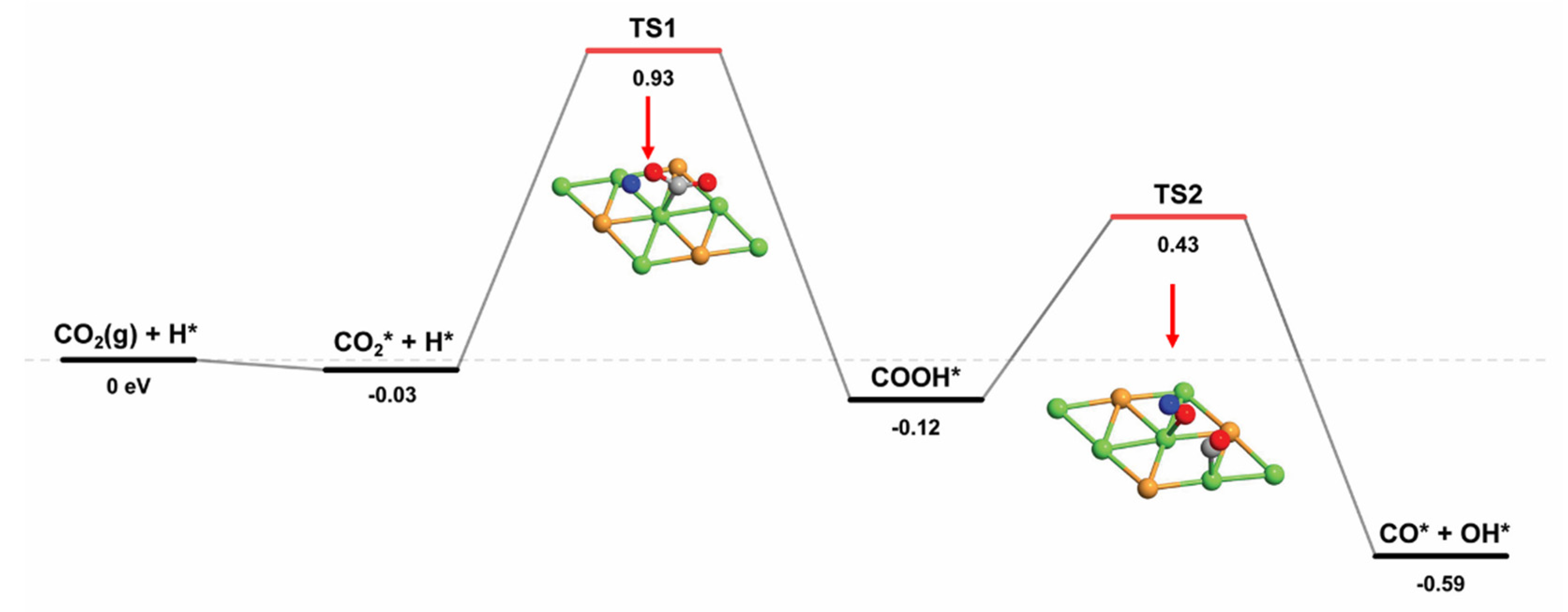
| Species | Eads (eV) | Favored Adsorption Site | |
|---|---|---|---|
| 1 | CH4* | −0.02 | N/A |
| 2 | CH3* | −2.17 | FCC |
| 3 | CH2* | −4.99 | FCC |
| 4 | CH* | −5.43 | FCC |
| 5 | C* | −7.04 | HCP |
| 6 | O* | −6.34 | FCC |
| 7 | OH* | −3.28 | B2Ni |
| 8 | CO* | −1.52 | B2Ni |
| 9 | COH* | −4.07 | B2Ni |
| 10 | CHOH* | −2.40 | B2Ni |
| 11 | CO2* | −0.03 | N/A |
| 12 | H* | −3.60 | FCC |
| 13 | H2* | −0.06 | N/A |
| 14 | CH3O* | −2.43 | B2Ni |
| 15 | CH2O | −0.04 | N/A |
| 16 | CH2OH* | −1.87 | TopNi |
| 17 | CH3OH* | −0.05 | N/A |
| 18 | CHO*a | −2.05 | TopNi |
| 19 | COH*a | −4.07 | B2Ni |
| 20 | H2O* | −0.06 | N/A |
| 21 | COOH* | −2.28 | TopNi |
| Reaction | Ea,f+ (eV) | Hf+ (eV) | Ea,r+ (eV) |
|---|---|---|---|
| CH4* → CH3* + H* | 1.30 | 0.78 | 0.52 |
| CH3* → CH2* + H* | 0.75 | 0.33 | 0.42 |
| CH2* → CH* + H* | 0.49 | −0.06 | 0.55 |
| CH* → C* + H* | 1.40 | 0.69 | 0.71 |
| C* + O* → CO* | 0.72 | −2.17 | 2.89 |
| CH* + O* → CHO* | 1.06 | −0.30 | 1.36 |
| CHO* → CO* + H* | 0.18 | −1.17 | 1.35 |
| C* + OH* → COH* | 1.13 | −0.95 | 2.07 |
| H* + H* → H2 | 0.73 | 0.57 | 0.16 |
| CH* + OH* → CHOH* | 1.08 | 0.24 | 0.84 |
| CHOH* → CHO* + H* | 0.60 | −0.31 | 0.91 |
| CO2* + H* → COOH* | 0.93 | −0.12 | 1.05 |
| COOH* → CO* + OH* | 0.43 | −0.59 | 1.02 |
| O* + H* → OH* | 0.94 | −0.24 | 1.18 |
| H* + OH* → H2O* | 0.91 | −0.15 | 1.06 |
| CO2* → CO* + O* | 1.69 | 0.48 | 1.21 |
| CH3* + O* → CH3O* | 1.37 | −0.22 | 1.59 |
| CH2* + O* → CH2O* | 0.76 | −0.43 | 1.19 |
| CHOH* → COH* + H* | 0.20 | −0.53 | 0.73 |
| COH* → CO* + H* | 1.01 | −1.09 | 2.10 |
| CH3* + OH* → CH3OH* | 1.60 | −0.14 | 1.74 |
| CH2* + OH* → CH2OH* | 0.78 | −0.10 | 0.88 |
| CH3O* → CH2O* + H* | 0.96 | 0.57 | 0.39 |
| CH2OH* → CH2O* + H* | 0.75 | −0.07 | 0.82 |
| CH2OH* → CHOH* + H* | 0.92 | 0.42 | 0.50 |
| CH3OH* → CH3O* + H* | 4.29 | 0.01 | 4.28 |
| CH3OH* → CH2OH* + H* | 2.46 | 1.39 | 1.10 |
| Reactions | 800 K | 850 K | 900 K | 950 K | 1000 K |
|---|---|---|---|---|---|
| CO* → C* + O* | 4.36 × 10−7 | 5.11 × 10−6 | 4.56 × 10−5 | 3.23 × 10−4 | 1.88 × 10−3 |
| CH* → C* + H* | 1.18 × 105 | 3.90 × 105 | 1.13 × 106 | 2.92 × 106 | 6.88 × 106 |
| C* + O* → CO* | 2.43 × 108 | 4.50 × 108 | 7.74 × 108 | 1.25 × 109 | 1.95 × 109 |
| C* + OH* → COH* | 5.75 × 106 | 1.50 × 107 | 3.53 × 107 | 7.59 × 107 | 1.51 × 108 |
| CH* + O* → CHO* | 6.01 × 106 | 1.49 × 107 | 3.32 × 107 | 6.83 × 108 | 1.31 × 108 |
| CH* + OH* → CHOH* | 3.28 × 107 | 8.27 × 107 | 1.88 × 108 | 3.93 × 108 | 7.62 × 108 |
| kCH/kC(O) | 0.02 | 0.03 | 0.04 | 0.05 | 0.07 |
| kCH/kC(OH) | 5.70 | 5.50 | 5.33 | 5.18 | 5.05 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omran, A.; Yoon, S.H.; Khan, M.; Ghouri, M.; Chatla, A.; Elbashir, N. Mechanistic Insights for Dry Reforming of Methane on Cu/Ni Bimetallic Catalysts: DFT-Assisted Microkinetic Analysis for Coke Resistance. Catalysts 2020, 10, 1043. https://doi.org/10.3390/catal10091043
Omran A, Yoon SH, Khan M, Ghouri M, Chatla A, Elbashir N. Mechanistic Insights for Dry Reforming of Methane on Cu/Ni Bimetallic Catalysts: DFT-Assisted Microkinetic Analysis for Coke Resistance. Catalysts. 2020; 10(9):1043. https://doi.org/10.3390/catal10091043
Chicago/Turabian StyleOmran, Ahmed, Sun Hee Yoon, Murtaza Khan, Minhaj Ghouri, Anjaneyulu Chatla, and Nimir Elbashir. 2020. "Mechanistic Insights for Dry Reforming of Methane on Cu/Ni Bimetallic Catalysts: DFT-Assisted Microkinetic Analysis for Coke Resistance" Catalysts 10, no. 9: 1043. https://doi.org/10.3390/catal10091043
APA StyleOmran, A., Yoon, S. H., Khan, M., Ghouri, M., Chatla, A., & Elbashir, N. (2020). Mechanistic Insights for Dry Reforming of Methane on Cu/Ni Bimetallic Catalysts: DFT-Assisted Microkinetic Analysis for Coke Resistance. Catalysts, 10(9), 1043. https://doi.org/10.3390/catal10091043







