Recent Approaches to Chiral 1,4-Dihydropyridines and their Fused Analogues
Abstract
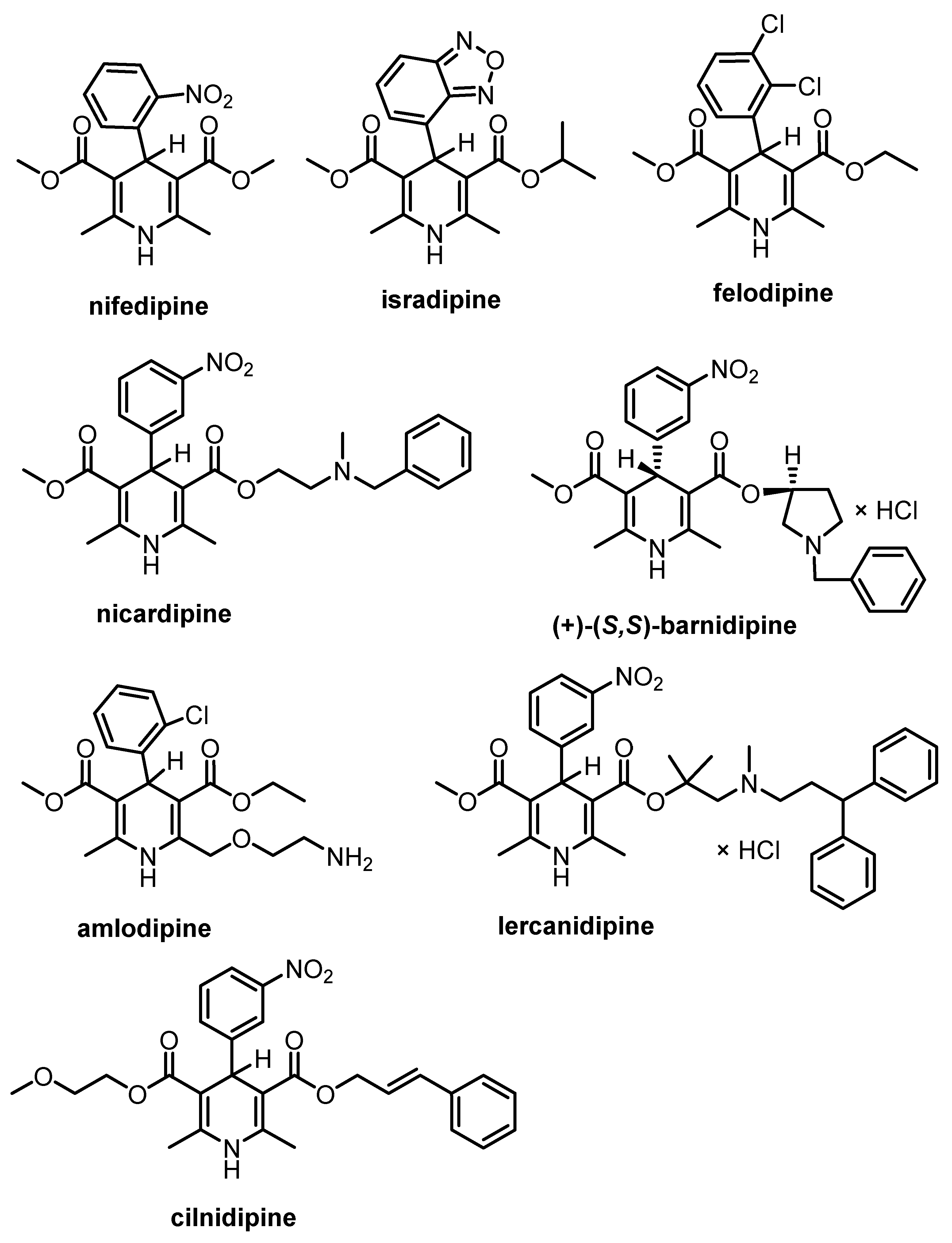
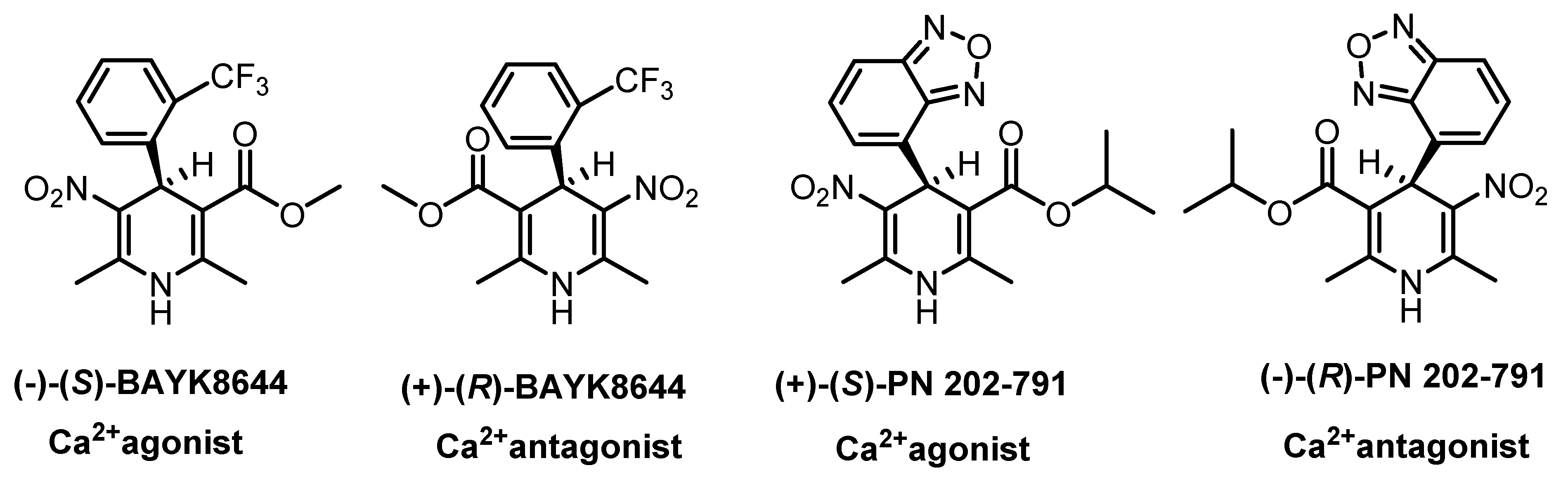
1. Introduction
2. Stereoselective Synthesis of 1,4-Dihydropyridines
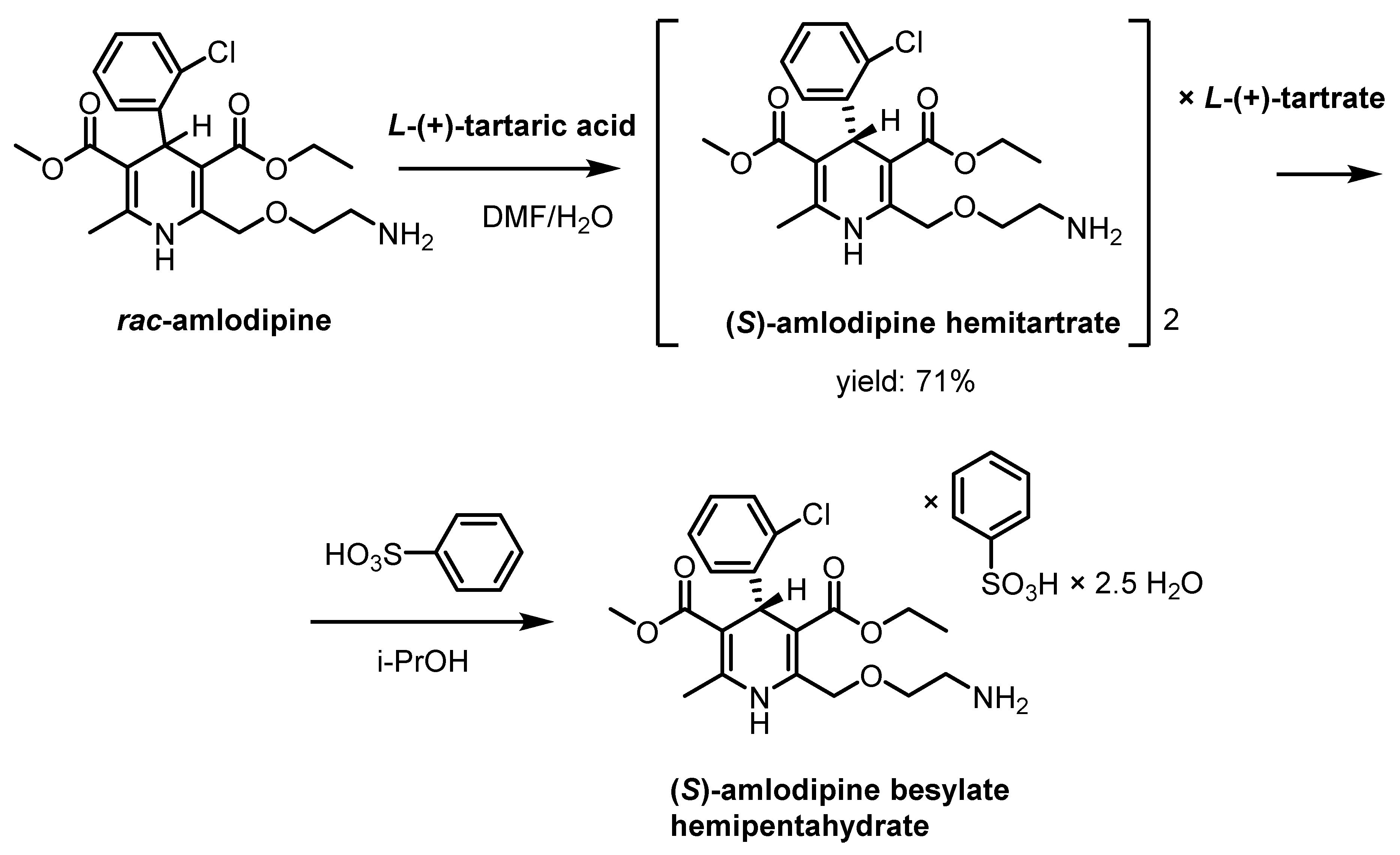
2.1. Resolution of Racemic Basic 1,4-Dihydropyridine Derivatives
2.2. Resolution of Racemic 1,4-Dihydropyridinedicarboxylic Acid Derivatives
2.3. Lipase-catalysed Kinetic Resolution of Racemic Activated Esters of 1,4-Dihydropyridinecarboxylic Acid
2.4. Organocatalytic Enantioselective Synthesis of 1,4-Dihydropyridines
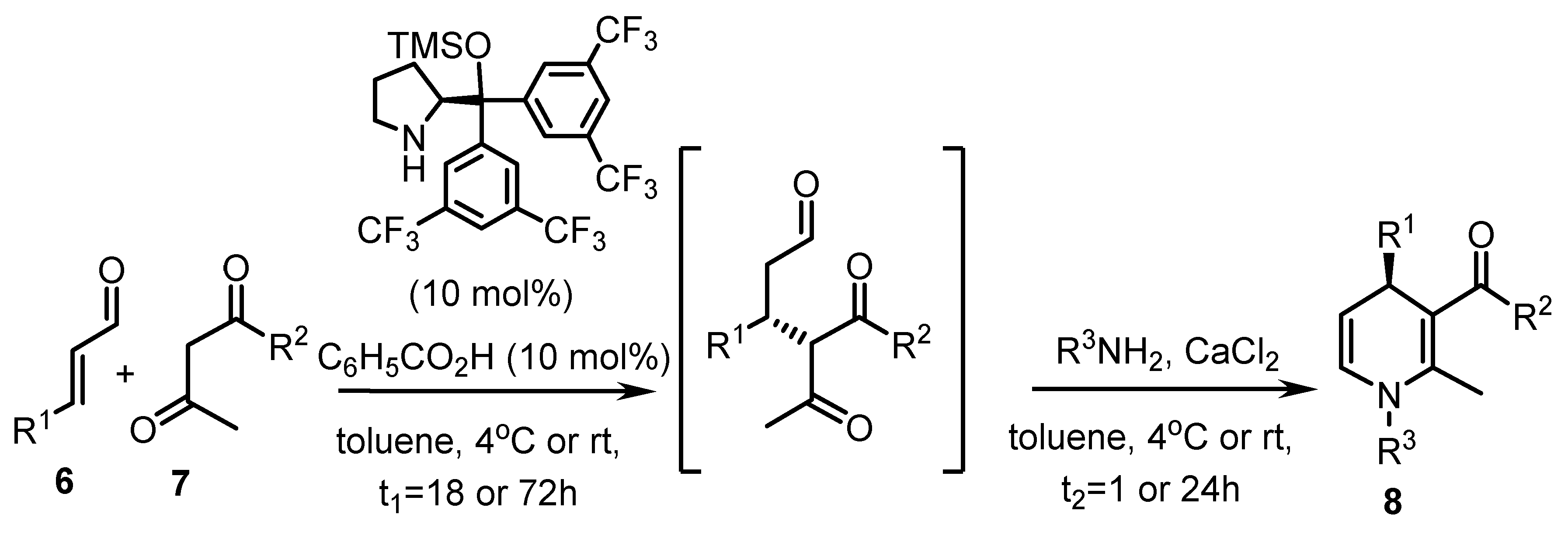
2.5. Organocatalytic Desymmetrisation of Prochiral 1,4-Dihydropyridine-3,5-dicarbaldehydes
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sharma, V.K.; Singh, S.K. Synthesis, utility and medicinal importance of 1,2- & 1,4-dihydropyridines. RSC Adv. 2017, 7, 2682–2732. [Google Scholar] [CrossRef]
- Klusa, V. Atypical 1,4-dihydropyridine derivatives, an approach to neuroprotection and memory enhancement. Pharmacol. Res. 2016, 113, 754–759. [Google Scholar] [CrossRef] [PubMed]
- Triggle, D.J. The 1,4-dihydropyridine nucleus: A pharmacophoric template part 1. Actions at ion channels. Mini Rev. Med. Chem. 2003, 3, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Godfraind, T. Discovery and development of calcium channel blockers. Front. Pharmacol. 2017, 8, 286. [Google Scholar] [CrossRef] [PubMed]
- Vater, W.; Kroneberg, G.; Hoffmeister, F.; Saller, H.; Meng, K.; Oberdorf, A.; Puls, W.; Schlossmann, K.; Stoepel, K. Pharmacology of 4-(2′-nitrophenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylic acid dimethyl ester (Nifedipine, BAY a 1040). Arzneimittel-Forschung 1972, 22, 1–14. [Google Scholar] [PubMed]
- Chandra, K.S.; Ramesh, G. The fourth-generation Calcium channel blocker: Cilnidipine. Indian Heart J. 2013, 65, 691–695. [Google Scholar] [CrossRef]
- Vardanyan, R.; Hruby, V. Chapter 22—Antihypertensive Drugs. In Synthesis of Best-Seller Drugs; Vardanyan, R., Hruby, V., Eds.; Academic Press: Boston, MA, USA, 2016; pp. 329–356. ISBN 978-0-12-411492-0. [Google Scholar]
- Wang, A.L.; Iadecola, C.; Wang, G. New generations of dihydropyridines for treatment of hypertension. J. Geriatr. Cardiol. 2017, 14, 67–72. [Google Scholar]
- Goldmann, S.; Stoltefuss, J. 1,4-Dihydropyridines: Effects of Chirality and Conformation on the Calcium Antagonist and Calcium Agonist Activities. Angew. Chem. Int. Ed. Engl. 1991, 30, 1559–1578. [Google Scholar] [CrossRef]
- Goldmann, S.; Stoltefuss, J.; Born, L. Determination of the absolute configuration of the active amlodipine enantiomer as (-)-S: A correction. J. Med. Chem. 1992, 35, 3341–3344. [Google Scholar] [CrossRef]
- Cataldi, M.; Taglialatela, M.; Palagiano, F.; Secondo, A.; de Caprariis, P.; Amoroso, S.; di Renzo, G.; Annunziato, L. Effects of manidipine and nitrendipine enantiomers on the plateau phase of K+-induced intracellular Ca2+ increase in GH3 cells. Eur. J. Pharmacol. 1999, 376, 169–178. [Google Scholar] [CrossRef]
- Mikus, G.; Mast, V.; Ratge, D.; Wisser, H.; Eichelbaum, M. Stereoselectivity in cardiovascular and biochemical action of calcium antagonists: Studies with the enantiomers of the dihydropyridine nitrendipine. Clin. Pharmacol. Ther. 1995, 57, 52–61. [Google Scholar] [CrossRef]
- Sakai, T.; Teramura, T.; Okamiya, H.; Inagaki, O. A Review on Barnidipine: A Novel Calcium Antagonist. Cardiovasc. Drug Rev. 2007, 15, 273–290. [Google Scholar] [CrossRef]
- Wei, X.Y.; Luchowski, E.M.; Rutledge, A.; Su, C.M.; Triggle, D.J. Pharmacologic and radioligand binding analysis of the actions of 1,4-dihydropyridine activator-antagonist pairs in smooth muscle. J. Pharmacol. Exp. Ther. 1986, 239, 144–153. [Google Scholar] [PubMed]
- Franckowiak, G.; Bechem, M.; Schramm, M.; Thomas, G. The optical isomers of the 1,4-dihydropyridine BAY K 8644 show opposite effects on Ca channels. Eur. J. Pharmacol. 1985, 114, 223–226. [Google Scholar] [CrossRef]
- Hof, R.P.; Rüegg, U.T.; Hof, A.; Vogel, A. Stereoselectivity at the calcium channel: Opposite action of the enantiomers of a 1,4-dihydropyridine. J. Cardiovasc. Pharmacol. 1985, 7, 689–693. [Google Scholar] [CrossRef]
- Kawano, S.; Shoji, S.; Ichinose, S.; Yamagata, K.; Tagami, M.; Hiraoka, M. Characterization of Ca2+ signaling pathways in human mesenchymal stem cells. Cell Calcium 2002, 32, 165–174. [Google Scholar] [CrossRef]
- Uzieliene, I.; Bernotiene, E.; Rakauskiene, G.; Denkovskij, J.; Bagdonas, E.; Mackiewicz, Z.; Porvaneckas, N.; Kvederas, G.; Mobasheri, A. The Antihypertensive Drug Nifedipine Modulates the Metabolism of Chondrocytes and Human Bone Marrow-Derived Mesenchymal Stem Cells. Front. Endocrinol. 2019, 10, 756. [Google Scholar] [CrossRef]
- Guilak, F.; Zell, R.A.; Erickson, G.R.; Grande, D.A.; Rubin, C.T.; McLeod, K.J.; Donahue, H.J. Mechanically induced calcium waves in articular chondrocytes are inhibited by gadolinium and amiloride. J. Orthop. Res. 1999, 17, 421–429. [Google Scholar] [CrossRef]
- Uzieliene, I.; Bernotas, P.; Mobasheri, A.; Bernotiene, E. The role of physical stimuli on calcium channels in chondrogenic differentiation of mesenchymal stem cells. Int. J. Mol. Sci. 2018, 19, 2998. [Google Scholar] [CrossRef]
- Vaca-González, J.J.; Guevara, J.M.; Moncayo, M.A.; Castro-Abril, H.; Hata, Y.; Garzón-Alvarado, D.A. Biophysical Stimuli: A Review of Electrical and Mechanical Stimulation in Hyaline Cartilage. Cartilage 2019, 10, 157–172. [Google Scholar] [CrossRef]
- Steward, A.J.; Kelly, D.J.; Wagner, D.R. The role of calcium signalling in the chondrogenic response of mesenchymal stem cells to hydrostatic pressure. Eur. Cells Mater. 2014, 28, 358–371. [Google Scholar] [CrossRef]
- Siddiqi, F.H.; Menzies, F.M.; Lopez, A.; Stamatakou, E.; Karabiyik, C.; Ureshino, R.; Ricketts, T.; Jimenez-Sanchez, M.; Esteban, M.A.; Lai, L.; et al. Felodipine induces autophagy in mouse brains with pharmacokinetics amenable to repurposing. Nat. Commun. 2019, 10, 1718. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, J.; Li, Y.; Wang, F.; Yang, F.; Xu, W. Synthesis and Radioprotective Activity of Mitochondria Targeted Dihydropyridines in Vitro. Int. J. Mol. Sci. 2017, 18, 2233. [Google Scholar] [CrossRef] [PubMed]
- Leonova, E.; Ošiņa, K.; Duburs, G.; Bisenieks, E.; Germini, D.; Vassetzky, Y.; Sjakste, N. Metal ions modify DNA-protecting and mutagen-scavenging capacities of the AV-153 1,4-dihydropyridine. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2019, 845, 403077. [Google Scholar] [CrossRef]
- Da Costa Cabrera, D.; Santa-Helena, E.; Leal, H.P.; de Moura, R.R.; Nery, L.E.M.; Gonçalves, C.A.N.; Russowsky, D.; Montes D’Oca, M.G. Synthesis and antioxidant activity of new lipophilic dihydropyridines. Bioorg. Chem. 2019, 84, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Nkosi, S.M.; Anand, K.; Anandakumar, S.; Singh, S.; Chuturgoon, A.A.; Gengan, R.M. Design, synthesis, anticancer, antimicrobial activities and molecular docking studies of novel quinoline bearing dihydropyridines. J. Photochem. Photobiol. B Biol. 2016, 165, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Lentz, F.; Reiling, N.; Spengler, G.; Kincses, A.; Csonka, A.; Molnár, J.; Hilgeroth, A. Dually acting nonclassical 1,4-dihydropyridines promote the anti-tuberculosis (Tb) activities of clofazimine. Molecules 2019, 24, 2873. [Google Scholar] [CrossRef] [PubMed]
- González, A.; Casado, J.; Chueca, E.; Salillas, S.; Velázquez-Campoy, A.; Angarica, V.E.; Bénejat, L.; Guignard, J.; Giese, A.; Sancho, J.; et al. Repurposing dihydropyridines for treatment of helicobacter pylori infection. Pharmaceutics 2019, 11, 681. [Google Scholar] [CrossRef]
- Rucins, M.; Dimitrijevs, P.; Pajuste, K.; Petrichenko, O.; Jackevica, L.; Gulbe, A.; Kibilda, S.; Smits, K.; Plotniece, M.; Tirzite, D.; et al. Contribution of Molecular Structure to Self-Assembling and Biological Properties of Bifunctional Lipid-Like 4-(N-Alkylpyridinium)-1,4-Dihydropyridines. Pharmaceutics 2019, 11, 115. [Google Scholar] [CrossRef]
- Hyvönen, Z.; Plotniece, A.; Reine, I.; Chekavichus, B.; Duburs, G.; Urtti, A. Novel cationic amphiphilic 1,4-dihydropyridine derivatives for DNA delivery. Biochim. Biophys. Acta Biomembr. 2000, 1509, 451–466. [Google Scholar] [CrossRef]
- Apsite, G.; Timofejeva, I.; Vezane, A.; Vigante, B.; Rucins, M.; Sobolev, A.; Plotniece, M.; Pajuste, K.; Kozlovska, T.; Plotniece, A. Synthesis and comparative evaluation of novel cationic amphiphile C12-Man-Q as an efficient DNA delivery agent in vitro. Molecules 2018, 23, 1540. [Google Scholar] [CrossRef] [PubMed]
- Pajuste, K.; Hyvonen, Z.; Petrichenko, O.; Kaldre, D.; Rucins, M.; Cekavicus, B.; Ose, V.; Skrivele, B.; Gosteva, M.; Morin-Picardat, E.; et al. Gene delivery agents possessing antiradical activity: Self-assembling cationic amphiphilic 1,4-dihydropyridine derivatives. New J. Chem. 2013, 37, 3062–3075. [Google Scholar] [CrossRef]
- Petrichenko, O.; Rucins, M.; Vezane, A.; Timofejeva, I.; Sobolev, A.; Cekavicus, B.; Pajuste, K.; Plotniece, M.; Gosteva, M.; Kozlovska, T.; et al. Studies of the physicochemical and structural properties of self-assembling cationic pyridine derivatives as gene delivery agents. Chem. Phys. Lipids 2015, 191, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Rucins, M.; Gosteva, M.; Domracheva, I.; Kanepe-Lapsa, I.; Belyakov, S.; Plotniece, M.; Pajuste, K.; Cekavicus, B.; Jekabsone, M.; Sobolev, A.; et al. Synthesis and Evaluation of Reducing Capacity and Calcium Channel Blocking Activity of Novel 3,5-Dipropargylcarbonyl-Substituted 1,4-Dihydropyridines. Chem. Heterocycl. Compd. 2015, 50, 1432–1443. [Google Scholar] [CrossRef]
- Rucins, M.; Kaldre, D.; Pajuste, K.; Fernandes, M.A.S.; Vicente, J.A.F.; Klimaviciusa, L.; Jaschenko, E.; Kanepe-Lapsa, I.; Shestakova, I.; Plotniece, M.; et al. Synthesis and studies of calcium channel blocking and antioxidant activities of novel 4-pyridinium and/or N-propargyl substituted 1,4-dihydropyridine derivatives. Comptes Rendus Chim. 2014, 17, 69–80. [Google Scholar] [CrossRef]
- Eisner, U.; Kuthan, J. Chemistry of dihydropyridines. Chem. Rev. 1972, 72, 1–42. [Google Scholar] [CrossRef]
- Kuthan, J.; Kurfurst, A. Development in dihydropyridine chemistry. Ind. Eng. Chem. Prod. Res. Dev. 1982, 21, 191–261. [Google Scholar] [CrossRef]
- Sausins, A.; Duburs, G. Synthesis of 1,4-dihydropyridines by cyclocondensation reactions. Heterocycles 1988, 27, 291–314. [Google Scholar] [CrossRef]
- Stout, D.M.; Meyers, A.I. Recent advances in the chemistry of dihydropyridines. Chem. Rev. 1982, 82, 223–243. [Google Scholar] [CrossRef]
- Yamamoto, T.; Ohno, S.; Niwa, S.; Tokumasu, M.; Hagihara, M.; Koganei, H.; Fujita, S.; Takeda, T.; Saitou, Y.; Iwayama, S.; et al. Asymmetric synthesis and biological evaluations of (+)- and (−)-6-dimethoxymethyl-1,4-dihydropyridine-3-carboxylic acid derivatives blocking N-type calcium channels. Bioorg. Med. Chem. Lett. 2011, 21, 3317–3319. [Google Scholar] [CrossRef]
- Auria-Luna, F.; Marqués-López, E.; Herrera, R.P. Organocatalytic Enantioselective Synthesis of 1,4-Dihydropyridines. Adv. Synth. Catal. 2017, 359, 2161–2175. [Google Scholar] [CrossRef]
- Cheng, Z.G.; Dai, X.Y.; Li, L.W.; Wan, Q.; Ma, X.; Xiang, G.Y. Synthesis and characterization of impurities of barnidipine hydrochloride, an antihypertensive drug substance. Molecules 2014, 19, 1344–1352. [Google Scholar] [CrossRef]
- Achiwa, K.; Kato, T. Asymmetric Synthesis of Optically Active 1,4-Dihydropyridines as Calcium Antagonist. Curr. Org. Chem. 1999, 3, 77–106. [Google Scholar]
- Decroix, B.; Marchalín, S.; Chudík, M.; Mastihuba, V. Use of Enzymes in Preparation of Enantiopure 1,4-Dihydropyridines. Heterocycles 1998, 48, 1943. [Google Scholar] [CrossRef]
- Sobolev, A.; Franssen, M.C.R.; Duburs, G.; de Groot, A. Chemoenzymatic synthesis of enantiopure 1,4-dihydropyridine derivatives. Biocatal. Biotransform. 2004, 22, 231–252. [Google Scholar] [CrossRef]
- Arrowsmith, J.E.; Campbell, S.F.; Cross, P.E.; Stubbs, J.K.; Burges, R.A.; Gardiner, D.G.; Blackburn, K.J. Long-acting dihydropyridine calcium antagonists. 1. 2-Alkoxymethyl derivatives incorporating basic substituents. J. Med. Chem. 1986, 29, 1696–1702. [Google Scholar] [CrossRef]
- Shibanuma, T.; Iwanani, M.; Okuda, K.; Takenaka, T.; Murakami, M. Synthesis of Optically Active 2-(N-Benzyl-N-methylamino)ethyl Methyl 2,6-Dimethyl-4-(m-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate (Nicardipine). Chem. Pharm. Bull. 1980, 28, 2809–2812. [Google Scholar] [CrossRef]
- Gotrane, D.M.; Deshmukh, R.D.; Ranade, P.V.; Sonawane, S.P.; Bhawal, B.M.; Gharpure, M.M.; Gurjar, M.K. A Novel Method for Resolution of Amlodipine. Org. Process Res. Dev. 2010, 14, 640–643. [Google Scholar] [CrossRef]
- Zhang, B.; He, W.; Shi, X.; Huan, M.; Huang, Q.; Zhou, S. Synthesis and biological activity of the calcium modulator (R) and (S)-3-methyl 5-pentyl 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate. Bioorg. Med. Chem. Lett. 2010, 20, 805–808. [Google Scholar] [CrossRef]
- Sobolev, A.; Franssen, M.C.R.; Vigante, B.; Cekavicus, B.; Makarova, N.; Duburs, G.; de Groot, A. An efficient chemoenzymatic approach to enantiomerically pure 4-(2-(difluoromethoxy)phenyl) substituted 1,4-dihydropyridine-3,5-dicarboxylates. Tetrahedron Asymmetry 2001, 12, 3251–3256. [Google Scholar] [CrossRef]
- Sobolev, A.; Franssen, M.C.R.; Vigante, B.; Cekavicus, B.; Zhalubovskis, R.; Kooijman, H.; Spek, A.L.; Duburs, G.; de Groot, A. Effect of acyl chain length and branching on the enantioselectivity of Candida rugosa lipase in the kinetic resolution of 4-(2-difluoromethoxyphenyl)-substituted 1,4-dihydropyridine 3,5-diesters. J. Org. Chem. 2002, 67, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Sobolev, A.; Franssen, M.C.R.; Poikans, J.; Duburs, G.; de Groot, A. Enantioselective lipase-catalysed kinetic resolution of acyloxymethyl and ethoxycarbonylmethyl esters of 1,4-dihydroisonicotinic acid derivatives. Tetrahedron Asymmetry 2002, 13, 2389–2397. [Google Scholar] [CrossRef]
- Torres, S.Y.; Verdecia, Y.; Rebolledo, F. Chemoenzymatic approach to optically active 1,4-dihydropyridine derivatives. Tetrahedron 2015, 71, 3976–3984. [Google Scholar] [CrossRef]
- Torres, S.Y.; Rebolledo, F. A Highly Efficient Synthesis of Optically Active Hybrid 1H-1,5-Benzodiazepine-1,4-Dihydropyridines. Synthesis 2016, 48, 1414–1420. [Google Scholar] [CrossRef]
- Andzans, Z.; Adlere, I.; Versilovskis, A.; Krasnova, L.; Grinberga, S.; Duburs, G.; Krauze, A. Effective method of lipase-catalyzed enantioresolution of 6-alkylsulfanyl-1,4-dihydropyridines. Heterocycles 2014, 89, 43–58. [Google Scholar] [CrossRef]
- Chen, C.S.; Fujimoto, Y.; Girdaukas, G.; Sih, C.J. Quantitative Analyses of Biochemical Kinetic Resolutions of Enantiomers. J. Am. Chem. Soc. 1982, 104, 7294–7299. [Google Scholar] [CrossRef]
- Franke, P.T.; Johansen, R.L.; Bertelsen, S.; Jørgensen, K.A. Organocatalytic Enantioselective One-Pot Synthesis and Application of Substituted 1,4-Dihydropyridines-Hantzsch Ester Analogues. Chem. Asian J. 2008, 3, 216–224. [Google Scholar] [CrossRef]
- Noole, A.; Borissova, M.; Lopp, M.; Onis Kanger, T. Enantioselective Organocatalytic Aza-Ene-Type Domino Reaction Leading to 1,4-Dihydropyridines. J. Org. Chem. 2011, 76, 1538–1545. [Google Scholar] [CrossRef]
- Auria-Luna, F.; Marqués-López, E.; Gimeno, M.C.; Heiran, R.; Mohammadi, S.; Herrera, R.P. Asymmetric Organocatalytic Synthesis of Substituted Chiral 1,4-Dihydropyridine Derivatives. J. Org. Chem. 2017, 82, 5516–5523. [Google Scholar] [CrossRef]
- Auria-Luna, F.; Marqués-López, E.; Herrera, R.P. First Organocatalytic Asymmetric Synthesis of 1-Benzamido-1,4-Dihydropyridine Derivatives. Molecules 2018, 23, 2692. [Google Scholar] [CrossRef]
- Auria-Luna, F.; Marqués-López, E.; Mohammadi, S.; Heiran, R.; Herrera, R. New Organocatalytic Asymmetric Synthesis of Highly Substituted Chiral 2-Oxospiro-(indole-3,4′- (1′,4′-dihydropyridine)) Derivatives. Molecules 2015, 20, 15807–15826. [Google Scholar] [CrossRef] [PubMed]
- Inokuma, T.; Hoashi, Y.; Takemoto, Y. Thiourea-catalyzed asymmetric michael addition of activated méthylene compounds to α,β-unsaturated imides: Dual activation of imide by intra- and intermolecular hydrogen bonding. J. Am. Chem. Soc. 2006, 128, 9413–9419. [Google Scholar] [CrossRef] [PubMed]
- Moreau, J.; Duboc, A.; Hubert, C.; Hurvois, J.P.; Renaud, J.L. Metal-free Brønsted acids catalyzed synthesis of functional 1,4-dihydropyridines. Tetrahedron Lett. 2007, 48, 8647–8650. [Google Scholar] [CrossRef]
- Jiang, J.; Yu, J.; Sun, X.-X.; Rao, Q.-Q.; Gong, L.-Z. Organocatalytic Asymmetric Three-Component Cyclization of Cinnamaldehydes and Primary Amines with 1,3-Dicarbonyl Compounds: Straightforward Access to Enantiomerically Enriched Dihydropyridines. Angew. Chem. 2008, 47, 2458–2462. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.G.; Gestwicki, J.E. Enantioselective organocatalytic hantzsch synthesis of polyhydroquinolines. Org. Lett. 2009, 11, 2957–2959. [Google Scholar] [CrossRef]
- An, D.; Zhu, Z.; Zhang, G.; Gao, Y.; Gao, J.; Han, X.; Zheng, L.; Zhang, S. H8-BINOL chiral imidodiphosphoric acids catalyzed cyclization reactions of β,γ-unsaturated α-ketoesters, arylamines and 1,3-dicarbonyl compounds: Enantioselective synthesis of 1,4-dihydropyridines. Tetrahedron Asymmetry 2015, 26, 897–906. [Google Scholar] [CrossRef]
- Deng, Y.; Kumar, S.; Wheeler, K.; Wang, H. Trio catalysis merging enamine, brønsted acid, and metal lewis acid catalysis: Asymmetric three-component aza-diels-alder reaction of substituted cinnamaldehydes, cyclic ketones, and arylamines. Chem. Eur. J. 2015, 21, 7874–7880. [Google Scholar] [CrossRef]
- Steiger, S.A.; Li, C.; Campana, C.F.; Natale, N.R. Lanthanide and asymmetric catalyzed syntheses of sterically hindered 4-isoxazolyl-1,4-dihydropyridines and 4-isoxazolyl-quinolones. Tetrahedron Lett. 2016, 57, 423–425. [Google Scholar] [CrossRef]
- Di Carmine, G.; Ragno, D.; Brandolese, A.; Bortolini, O.; Pecorari, D.; Sabuzi, F.; Mazzanti, A.; Massi, A. Enantioselective Desymmetrization of 1,4-Dihydropyridines by Oxidative NHC Catalysis. Chem. Eur. J. 2019, 25, 7469–7474. [Google Scholar] [CrossRef]

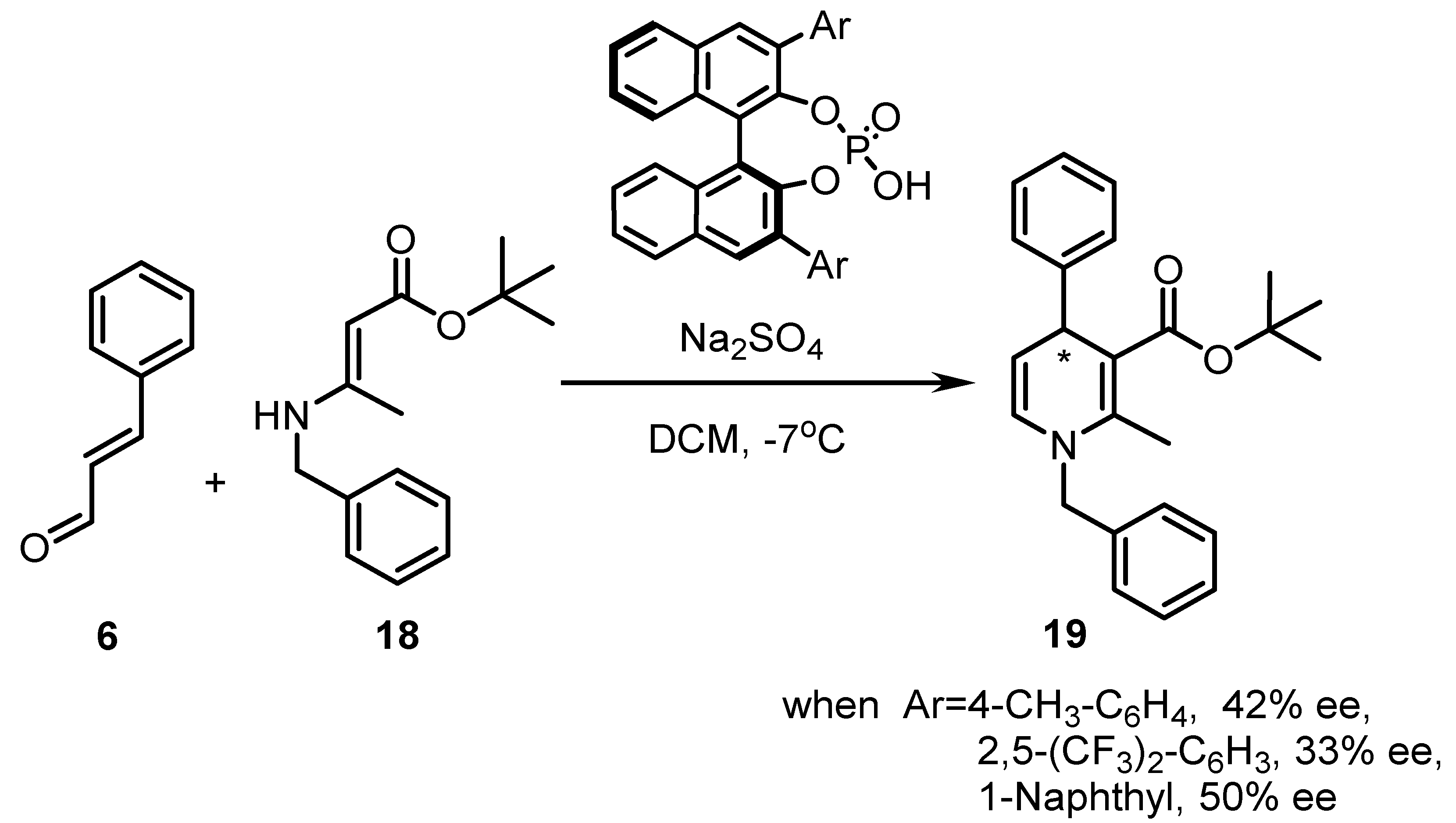
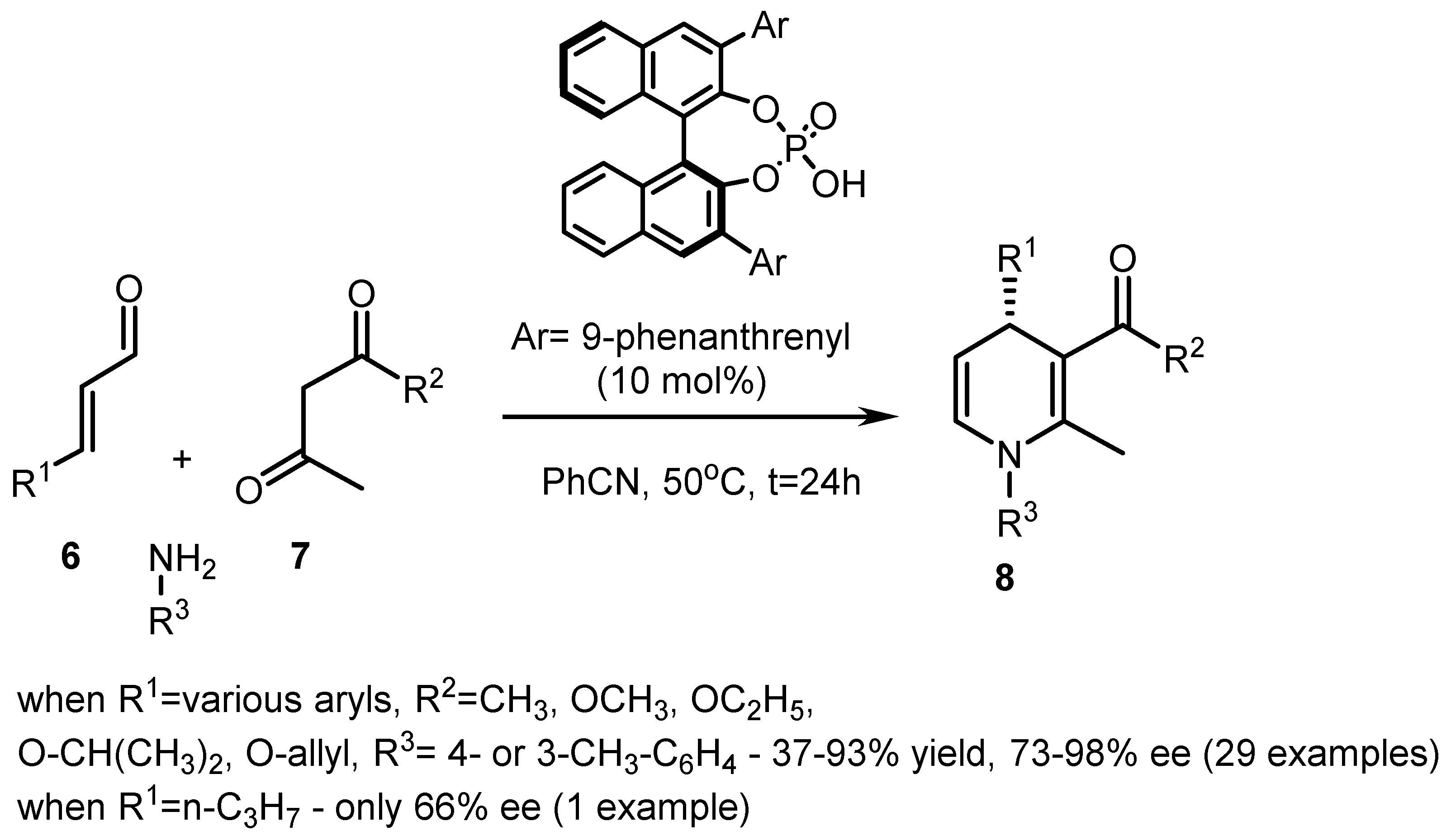
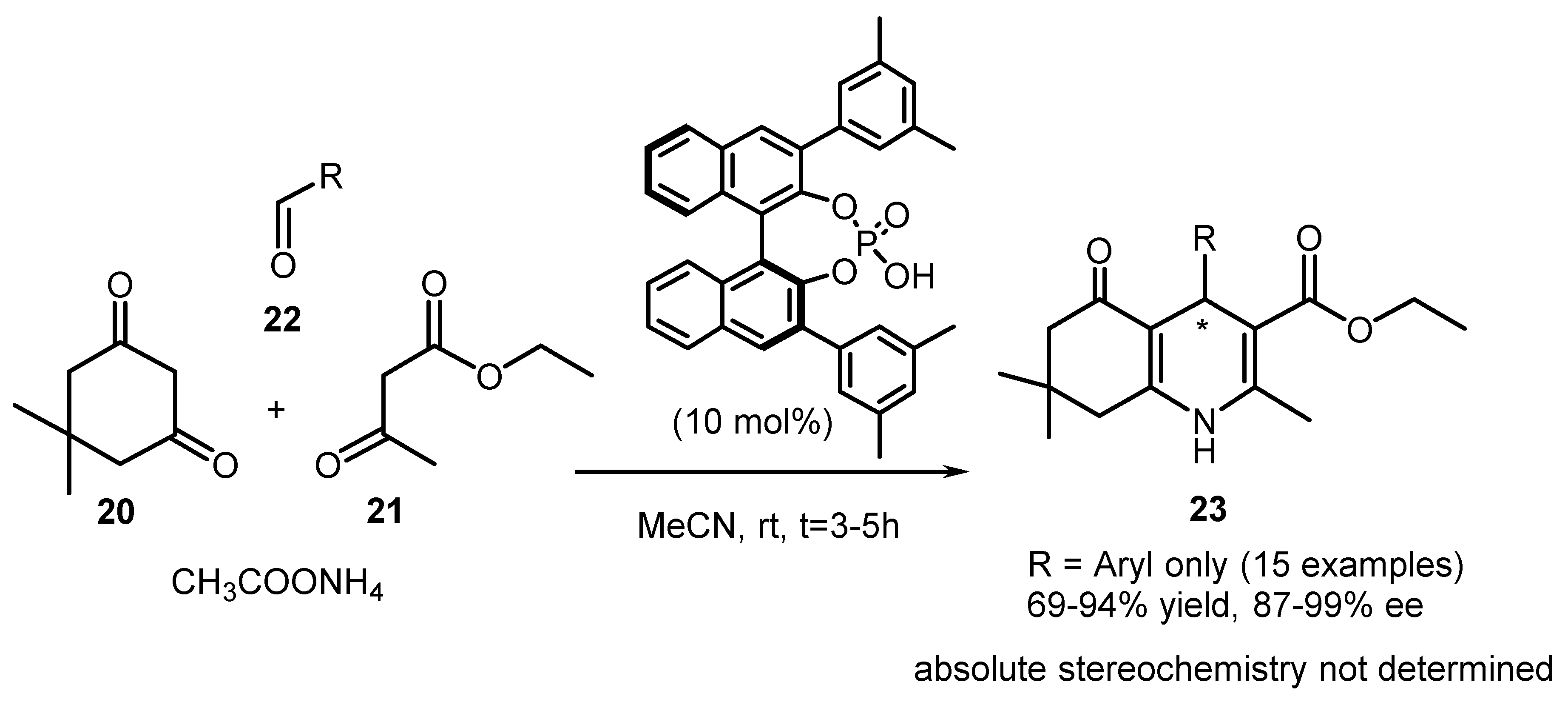
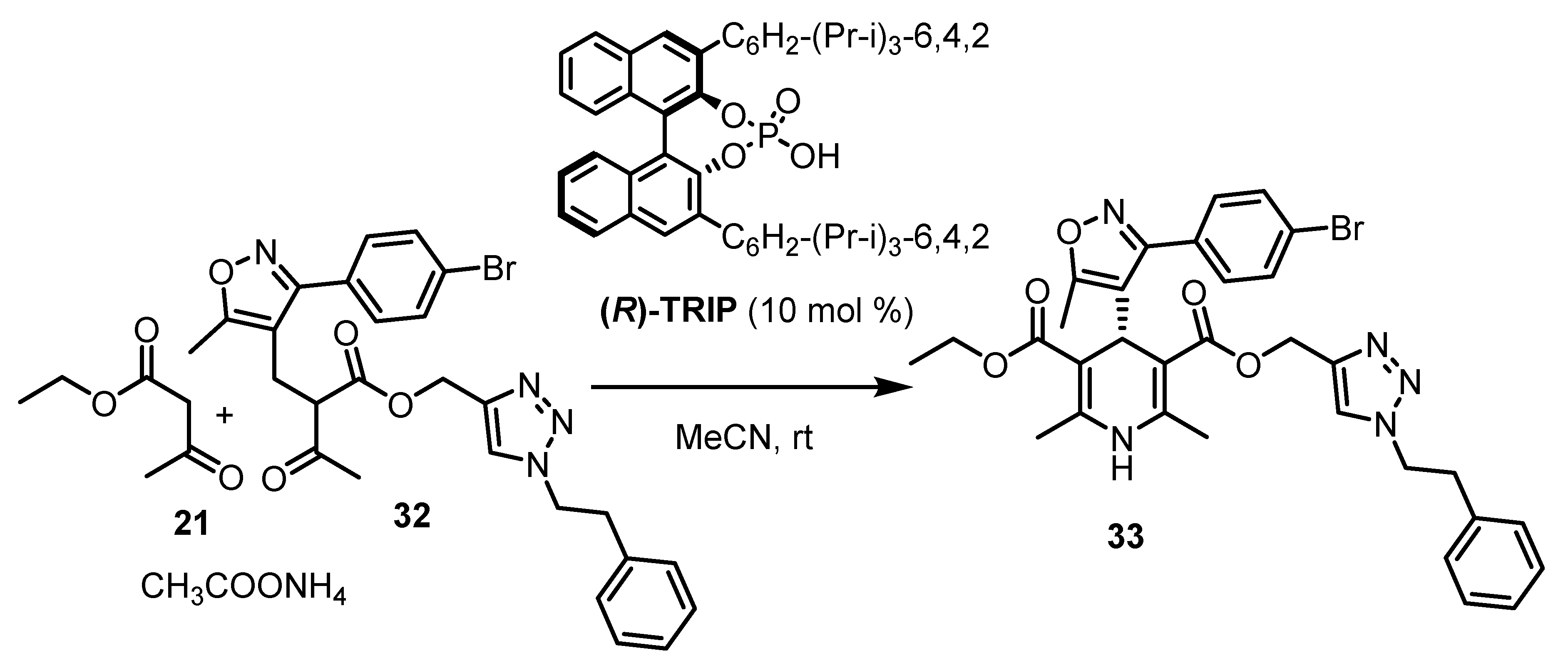
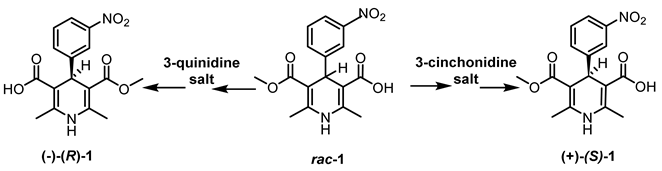
| Entry | Resolving Agent | Solvent Ratio | Yield, (%) | ee, (%) | Abs. conf. * |
|---|---|---|---|---|---|
| 1 | cinchonidine | DMF-H2O (4:1) | 32 | 99.5 | S |
| 2 | cinchonidine | DMF-H2O (8:5) | 41 | >99.5 | S |
| 3 | cinchonidine | DMF-H2O (1:1) | 44 | 97.8 | S |
| 4 | quinidine | DMF-H2O (8:5) | 43 | >99.5 | R |
| 5 | quinidine | DMF-H2O (1:1) | 46 | 96.6 | R |
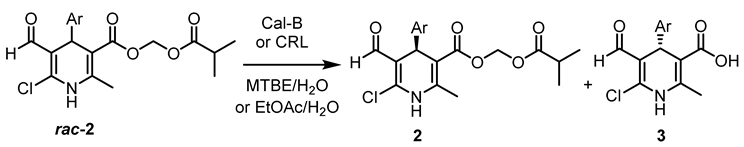
| Entry | Ar | Lipase | Solvent | t *, h | Conv. †, (%) | 2 Abs. conf. /ees, (%) | 3 Abs. conf. /eep, (%) | E-value ‡ |
|---|---|---|---|---|---|---|---|---|
| 1 | 2-NO2-C6H4 | CRL | EtOAc | 0.7 | 47 | S/84 | R/95 | 103 |
| 2 | 3-NO2-C6H4 | CRL | EtOAc | 1.5 | 50 | S/89 | R/88 | 46 |
| 3 | 2-Cl-NO2-C6H3 | CRL | EtOAc | 0.8 | 49 | R/95 | S/98 | >200 |
| 4 | Naphthyl | CRL | EtOAc | 2.5 | 46 | S/84 | R/97 | 175 |
| 5 | 4-Br-C6H4 | CRL | EtOAc | 23 | 51 | S/60 | R/58 | 7 |
| 6 | 3-CH3O-C6H4 | CRL | EtOAc | 10 | 39 | S/40 | R/62 | 6 |
| 7 | 4-Br-C6H4 | Cal-B | MTBE | 6 | 47 | S/75 | R/85 | 27 |
| 8 | 4-Br-C6H4 | Cal-B | EtOAc | 25 | 43 | S/67 | R/89 | 34 |
| 9 | 3-CH3O-C6H4 | Cal-B | MTBE | 9 | 41 | S/62 | R/88 | 29 |
| 10 | 3-CH3O-C6H4 | Cal-B | EtOAc | 21 | 34 | S/49 | R/95 | 63 |

| Entry | Compound | Enzyme | Temp *, °C | t, h | (-)-4 Yield, (%) | (-)-4 ees, (%) | (-)-3 Yield, (%) | Es-value † | Abs. conf. |
|---|---|---|---|---|---|---|---|---|---|
| 1 | a | Cal-B | 25 | 48 | 49 | 82 | 47 | 22 | Not known |
| 2 | b | Cal-B | 25 | 165 | 46 | 80 | 48 | 20 | |
| 3 | c | Cal-B | 25 | 51 | 46 | 77 | 47 | 16 | |
| 4 | d | Cal-B | 25 | 264 | 46 | 65 | 46 | 8 | |
| 5 | a | Cal-B | 45 | 18 | 49 | 95 | 47 | 104 | |
| 6 | b | Cal-B | 45 | 48 | 46 | 92 | 48 | 65 | |
| 7 | c | Cal-B | 45 | 26 | 46 | 99 | 46 | >200 | |
| 8 | d | Cal-B | 45 | 168 | 46 | 86 | 46 | 29 | |
| 9 | a | Acylase | 25 | 96 | 47 | 52 | 48 | 5 | |
| 10 | b | Acylase | 25 | 310 | 45 | 89 | 48 | 44 | |
| 11 | c | Acylase | 25 | 100 | 48 | 38 | 47 | 3 | |
| 12 | d | Acylase | 25 | 336 | 45 | 48 | 48 | 4 |
| Entry | R1 | R2 | R3 | Temp, °C | t1, h | t2, h | 8 | |
|---|---|---|---|---|---|---|---|---|
| Yield, (%) | ee, (%) | |||||||
| 1 | C2H5 | CH3 | C6H5 | rt | 18 | 1 | 55 | 90 |
| 2 | Hex-3-en-yl | CH3 | C6H5 | rt | 18 | 1 | 45 | 92 |
| 3 | COOC2H5 | CH3 | C6H5 | rt | 18 | 24 | 31 | 88 |
| 4 | (CH2)2OTBDMS | CH3 | C6H5 | rt | 18 | 1 | 33 | 95 |
| 5 | Furyl | CH3 | C6H5 | 4 | 18 | 24 | 35 | 64 |
| 6 | CH(CH3)2 | CH3 | C6H5 | rt | 18 | 1 | 33 | 92 |
| 7 | C2H5 | OCH3 | C6H5 | rt | 18 | 1 | 41 | 91 |
| 8 | CH3 | OCH3 | C6H5 | 4 | 18 | 24 | 39 | 82 |
| 9 | C6H5 | CH3 | C6H5 | 4 | 72 | 1 | 60 | 38 |
| 10 | C2H5 | CH3 | CH(CH3)2 | rt | 18 | 1 | 39 | 90 |
| 11 | C2H5 | CH3 | 4-Br-C6H4 | rt | 18 | 1 | 48 | 92 |
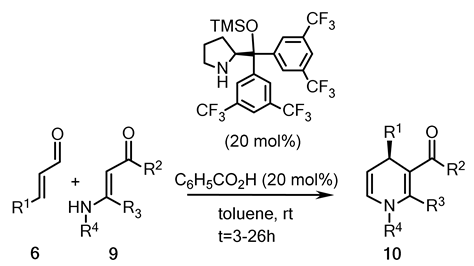
| Entry | R1 | R2 | R3 | R4 | t, h | 10 | |
|---|---|---|---|---|---|---|---|
| Yield, (%) | Abs. conf. /ee, (%) | ||||||
| 1 | C6H5 | n-C4H9 | H | C6H5 | 19 | 79 | S/76 |
| 2 | C6H5 | n-C4H9 | H | CH2C6H5 | 24 | 54 | S/87 |
| 3 | C6H5 | n-C4H9 | H | C(CH3)3 | 26 | 72 | S/89 |
| 4 | C6H5 | n-C4H9 | H | CH(CH3)2 | 20 | 75 | S/93 |
| 5 | 4-NO2-C6H4 | n-C4H9 | H | CH(CH3)2 | 3 | 83 | S/89 |
| 6 | 4-NO2-C6H4 | OC2H5 | CH3 | CH(CH3)2 | 4 | 45 | S/84 |
| 7 | 4-NO2-C6H4 | OC2H5 | CH3 | CH2C6H5 | 4 | 70 | S/89 |
| 8 | C2H5 | n-C4H9 | H | CH(CH3)2 | 18 | 69 | R/93 |
| 9 | n-C4H9 | n-C4H9 | H | CH(CH3)2 | 17 | 96 | R/96 |
| 10 | n-C4H9 | OC2H5 | CH3 | CH2C6H5 | 4 | 62 | R/71 |
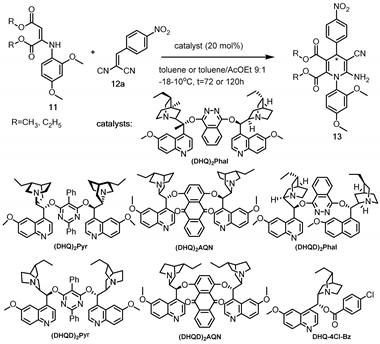
| Entry | Catalyst | R | Solvent | Temp, °C | t, h | 13 | ||
|---|---|---|---|---|---|---|---|---|
| Yield, (%) | eep, (%) | Abs. conf. | ||||||
| 1 | (DHQ)2Phal | CH3 | toluene/AcOEt (9:1) | 10 | 72 | 91 | 66 | Not known |
| 2 | (DHQ)2AQN | CH3 | toluene/AcOEt (9:1) | 10 | 72 | 22 | 54 | |
| 3 | (DHQD)2Phal | CH3 | toluene/AcOEt (9:1) | 10 | 72 | 47 | 64 | |
| 4 | (DHQD)2Pyr | CH3 | toluene/AcOEt (9:1) | 10 | 72 | 13 | 54 | |
| 5 | (DHQD)2AQN | CH3 | toluene/AcOEt (9:1) | 10 | 72 | 23 | 68 | |
| 6 | (DHQ)2Pyr | CH3 | toluene/AcOEt (9:1) | 10 | 72 | 81 | 80 | |
| 7 | (DHQ)2Pyr | CH3 | toluene | 10 | 72 | 88 | 82 | |
| 8 | (DHQ)2Pyr | CH3 | toluene/AcOEt (9:1) | 0 | 72 | 32 | 80 | |
| 9 | (DHQ)2Pyr | CH3 | toluene/AcOEt (9:1) | -18 | 120 | <5 | 85 | |
| 10 | (DHQ)2Pyr | C2H5 | toluene/AcOEt (9:1) | 10 | 72 | 97 | 76 | |
| 11 | (DHQ)2Pyr | C2H5 | toluene/AcOEt (9:1) | -18 | 72 | <5 | 90 | |
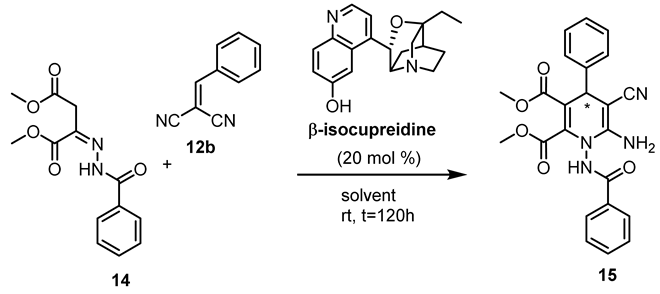
| Entry | Solvent | 15 | |
|---|---|---|---|
| Yield, (%) | eep, (%) | ||
| 1 | MeCN | 56 | 10 |
| 2 | AcOEt | 54 | 40 |
| 3 | DCM | 35 | Rac. |
| 4 | CHCl3 | 49 | 33 |
| 5 | Et2O | 39 | 40 |
| 6 | MeOH | 77 | Rac |
| 7 | THF | 48 | 52 |

| Entry | Catalyst Loading (mol %) | R | Ar | R1 | 17 Yield, (%) eep, (%) | |
|---|---|---|---|---|---|---|
| 1 | 30 | CH2C6H5 | 2,4-(H3CO)2-C6H3 | H | 30 | 42 |
| 2 | 20 | CH2C6H5 | 2,4-(H3CO)2-C6H3 | H | 25 | 23 |
| 3 | 10 | CH2C6H5 | 2,4-(H3CO)2-C6H3 | H | 29 | 11 |
| 4 | 30 | CH2C6H5 | 2,4-(H3CO)2-C6H3 | 5-Br | 82 | 48 |
| 5 | 30 | CH2C6H5 | 2,4-(H3CO)2-C6H3 | 5,7-diCH3 | 40 | 30 |
| 6 | 30 | CH2C6H5 | 2,4-(H3CO)2-C6H3 | 5-Cl | 71 | 30 |
| 7 | 30 | CH2C6H5 | 2,4-(H3CO)2-C6H3 | 5-NO2 | 65 | 58 |
| 8 | 30 | CH2C6H5 | 3-H3CO-C6H4 | H | 65 | 30 |
| 9 | 30 | Allyl | 3-H3CO-C6H4 | H | 61 | 30 |
| 10 | 30 | C2H5 | 3-H3CO-C6H4 | H | 49 | 32 |
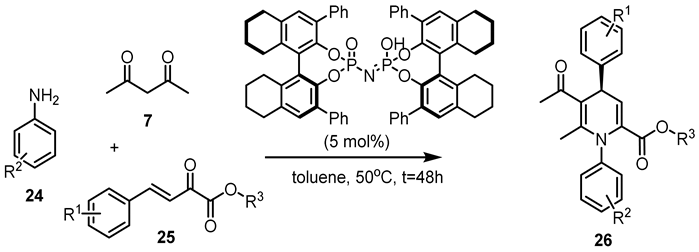
| Entry | R1 | R2 | R3 | 26 | |
|---|---|---|---|---|---|
| Yield, (%) | ee, (%) | ||||
| 1 | 4-NO2 | 3-OCH3 | CH3 | 54 | 88 |
| 2 | 4-NO2 | 3-OCH3 | C2H5 | 61 | 85 |
| 3 | 4-NO2 | 3-OCH3 | CH(CH3)2 | 54 | 90 |
| 4 | 4-NO2 | 3-OCH3 | CH2C6H5 | 51 | 89 |
| 5 | 4-NO2 | 3-Cl | CH(CH3)2 | 53 | 91 |
| 6 | 4-NO2 | 3-Br | CH(CH3)2 | 54 | 88 |
| 7 | 4-NO2 | 3-F | CH(CH3)2 | 53 | 87 |
| 8 | 4-NO2 | 3-CH3 | CH(CH3)2 | 55 | 83 |
| 9 | 4-Br | 3-Cl | CH(CH3)2 | 54 | 92 |
| 10 | 4-CN | 3-Cl | CH(CH3)2 | 41 | 93 |
| 11 | 4-F | 3-Br | C2H5 | 51 | 94 |
| 12 | 2,3,4-triCl | 3-Cl | CH3 | 55 | 95 |
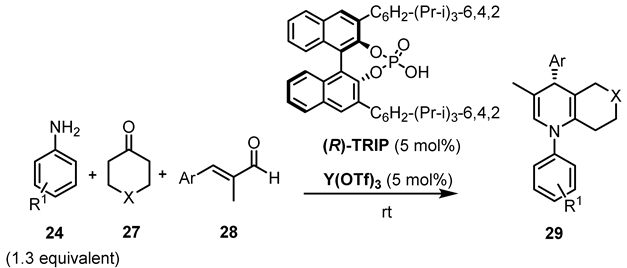
| Entry | Solvent | R1 | Ar | X | t, h | 29 | |
|---|---|---|---|---|---|---|---|
| Yield, (%) | eep, (%) | ||||||
| 1 | toluene | 4-Cl | C6H5 | CH2 | 48 | 38 | 92 |
| 2 | MeCN | 4-Cl | C6H5 | CH2 | 48 | 70 | 35 |
| 3 | Neat | 4-Cl | C6H5 | CH2 | 48 | 15 | 80 |
| 4 | MeOH | 4-Cl | C6H5 | CH2 | 48 | 67 | 93 |
| 5 | 1,4-dioxane | 4-Cl | C6H5 | CH2 | 48 | 44 | 43 |
| 6 | H2O | 4-Cl | C6H5 | CH2 | 48 | 50 | 99 |
| 7 | CHCl3 | 4-Cl | C6H5 | CH2 | 24 | 87 | 69 |
| 8 | acetone | 4-Cl | C6H5 | CH2 | 24 | 78 | 88 |
| 9 | DCM | 4-Cl | C6H5 | CH2 | 24 | 81 | 98 |
| 10 | DCE * | 4-Cl | C6H5 | CH2 | 24 | 78 | 98 |
| 11 | DCE | 4-CH3O | C6H5 | CH2 | 18 | 79 | 99 |
| 12 | DCE | H | C6H5 | CH2 | 24 | 72 | 94 |
| 13 | DCE | 4-Br | C6H5 | CH2 | 24 | 71 | 92 |
| 14 | DCE | 3-Cl | C6H5 | CH2 | 30 | 66 | 99 |
| 15 | DCE | 4-Cl | 4-CH3O-C6H4 | CH2 | 24 | 73 | 98 |
| 16 | DCE | 4-Cl | 4-Cl-C6H4 | CH2 | 24 | 71 | 99 |
| 17 | DCE | 4-Cl | 4-Br-C6H4 | CH2 | 24 | 67 | 94 |
| 18 | DCE | 4-Cl | Naphthyl | CH2 | 40 | 59 | 94 |
| 19 | DCE | 4-Cl | 4-NO2-C6H4 | CH2 | 16 | 84 | 98 |
| 20 | DCE | 4-CH3O | C6H5 | - | 20 | 68 | 91 |
| 21 | DCE | 4-Cl | C6H5 | - | 24 | 60 | 94 |
| 22 | DCE | 4-Cl | 4-NO2-C6H4 | - | 24 | 63 | 98 |
| 23 | DCE | 4-Cl | 4-CH3O-C6H4 | - | 24 | 68 | 98 |
| 24 | DCE | 4-CH3O | 4-NO2-C6H4 | S | 30 | 73 | 98 |
| 25 | DCE | 4-CH3O | 4-NO2-C6H4 | O | 30 | 70 | 95 |
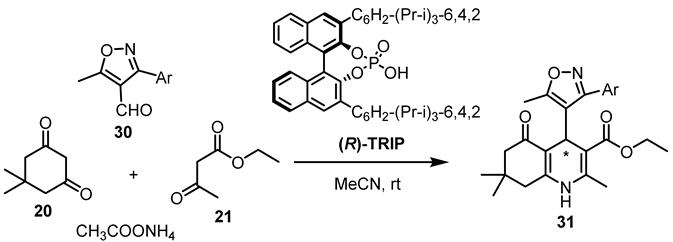
| Entry | Ar | 31 Yield, (%) |
|---|---|---|
| 1 | C6H5 | 26 |
| 2 | 2-Br-C6H4 | 64 |
| 3 | 3-Br-C6H4 | 60 |
| 4 | 4-Br-C6H4 | 65 |

| Entry | Solvent | Base (mol %) | T, °C | t, h | 37 Yield (%) | 38 Yield (%) | 37 ee (%) |
|---|---|---|---|---|---|---|---|
| 1 | DCM | DIPEA (25) | r.t. | 72 | 29 | - | 82 |
| 2 | DCM | DIPEA (100) | r.t. | 72 | 36 | - | 82 |
| 3 | THF | DIPEA (100) | r.t. | 72 | 31 | 12 | 3 |
| 4 | MeCN | DIPEA (100) | r.t. | 72 | 49 | 18 | 84 |
| 5 | DCE | DIPEA (100) | r.t. | 72 | 65 | 10 | 84 |
| 6 | CHCl3 | DIPEA (100) | r.t. | 72 | 67 | 13 | 90 |
| 7 | CHCl3 | KHDMS (25) | r.t. | 72 | 53 | 13 | 71 |
| 8 | CHCl3 | K3PO4 (25) | r.t. | 72 | 74 | 11 | 82 |
| 9 | CHCl3 | TEA (25) | r.t. | 72 | 52 | 10 | 88 |
| 10 | CHCl3 | DIPEA (100) | 40 | 16 | 70 | 7 | 91 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rucins, M.; Plotniece, A.; Bernotiene, E.; Tsai, W.-B.; Sobolev, A. Recent Approaches to Chiral 1,4-Dihydropyridines and their Fused Analogues. Catalysts 2020, 10, 1019. https://doi.org/10.3390/catal10091019
Rucins M, Plotniece A, Bernotiene E, Tsai W-B, Sobolev A. Recent Approaches to Chiral 1,4-Dihydropyridines and their Fused Analogues. Catalysts. 2020; 10(9):1019. https://doi.org/10.3390/catal10091019
Chicago/Turabian StyleRucins, Martins, Aiva Plotniece, Eiva Bernotiene, Wei-Bor Tsai, and Arkadij Sobolev. 2020. "Recent Approaches to Chiral 1,4-Dihydropyridines and their Fused Analogues" Catalysts 10, no. 9: 1019. https://doi.org/10.3390/catal10091019
APA StyleRucins, M., Plotniece, A., Bernotiene, E., Tsai, W.-B., & Sobolev, A. (2020). Recent Approaches to Chiral 1,4-Dihydropyridines and their Fused Analogues. Catalysts, 10(9), 1019. https://doi.org/10.3390/catal10091019






