Developing Low-Cost, High Performance, Robust and Sustainable Perovskite Electrocatalytic Materials in the Electrochemical Sensors and Energy Sectors: “An Overview”
Abstract
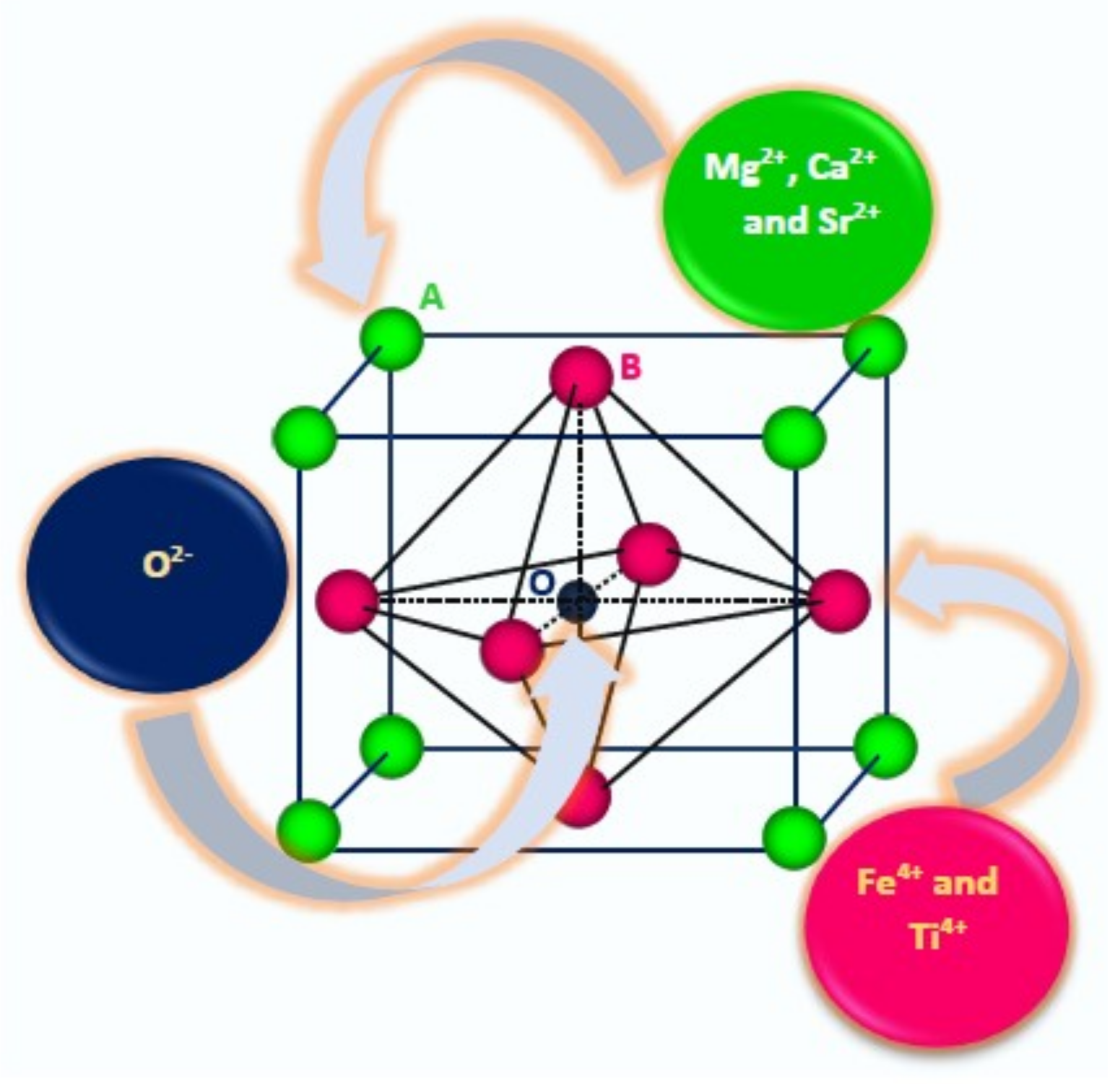
1. Introduction
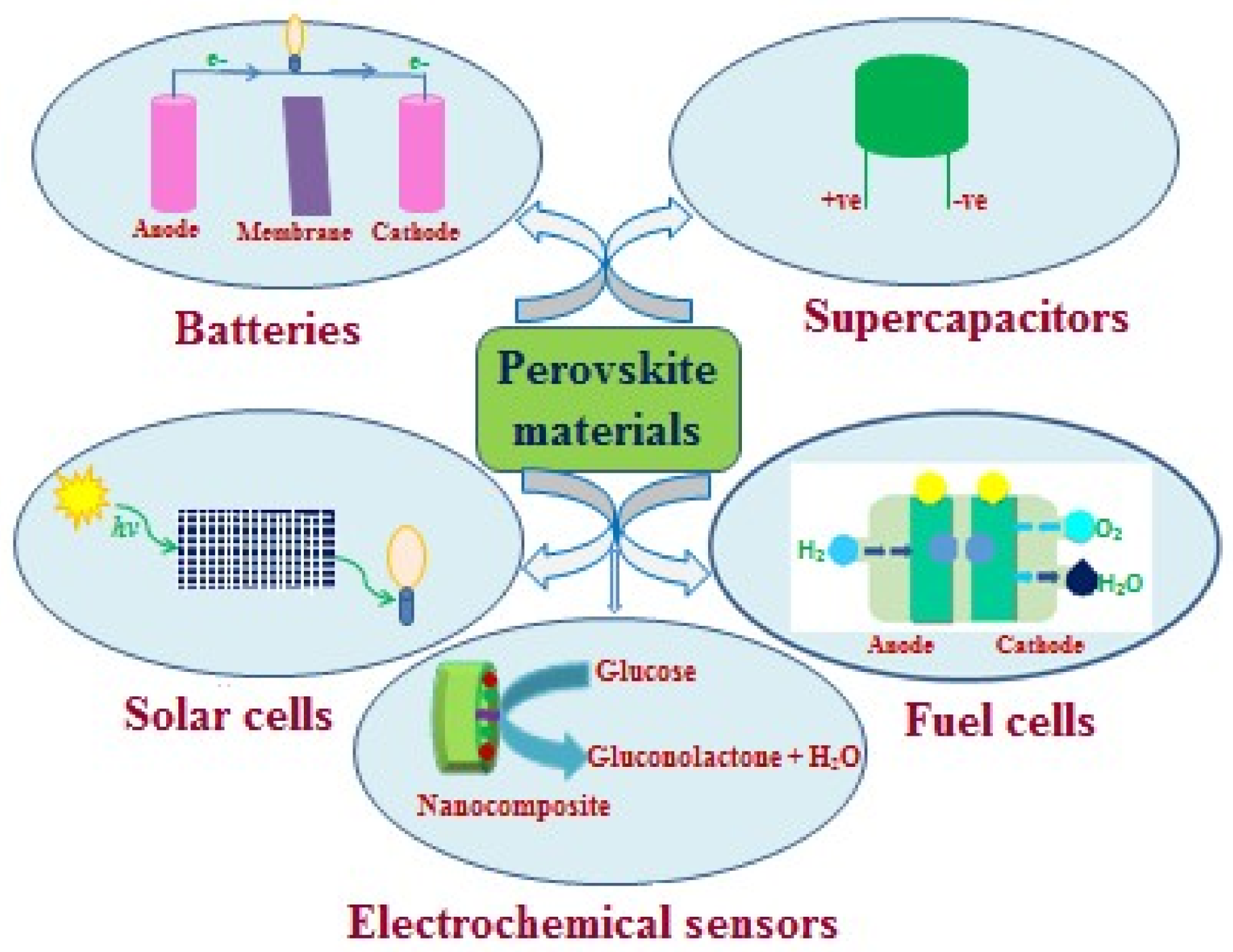
2. Energy Storage Devices
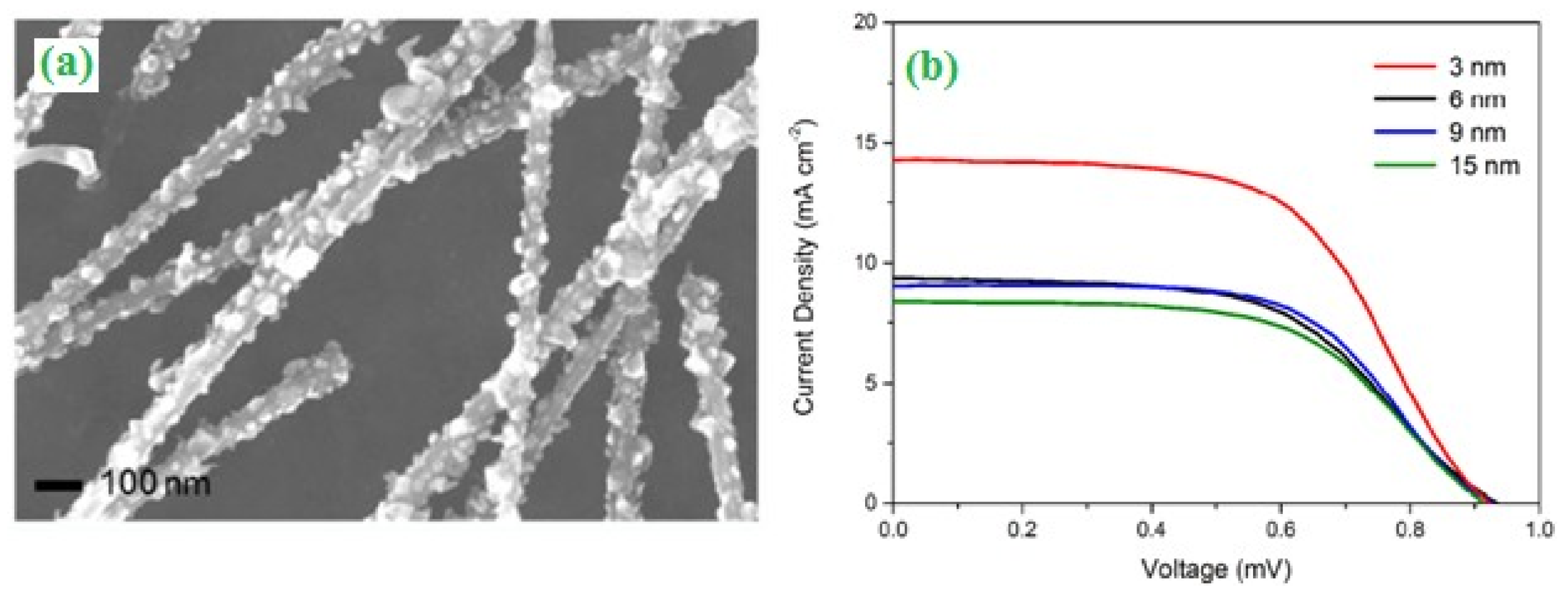
2.1. Solar Cells
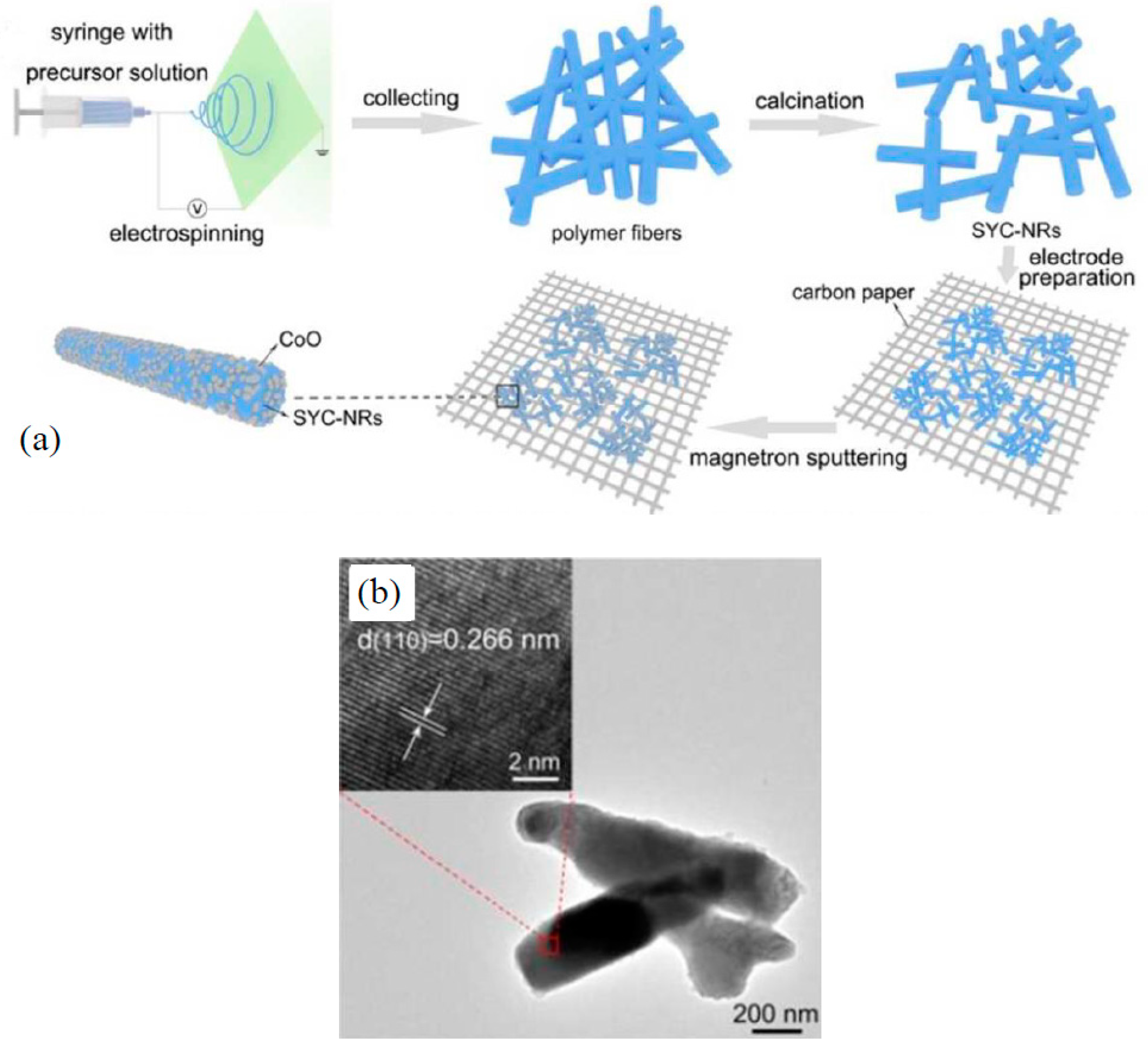
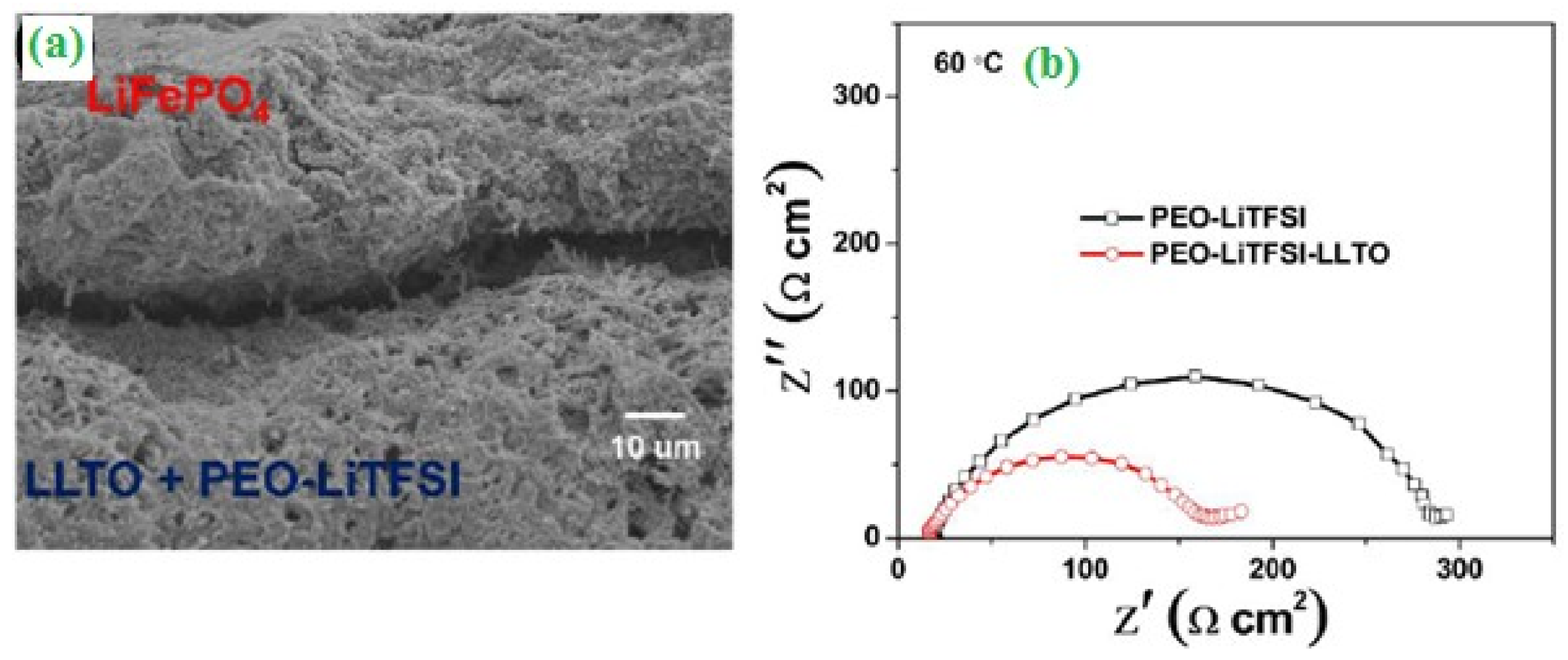
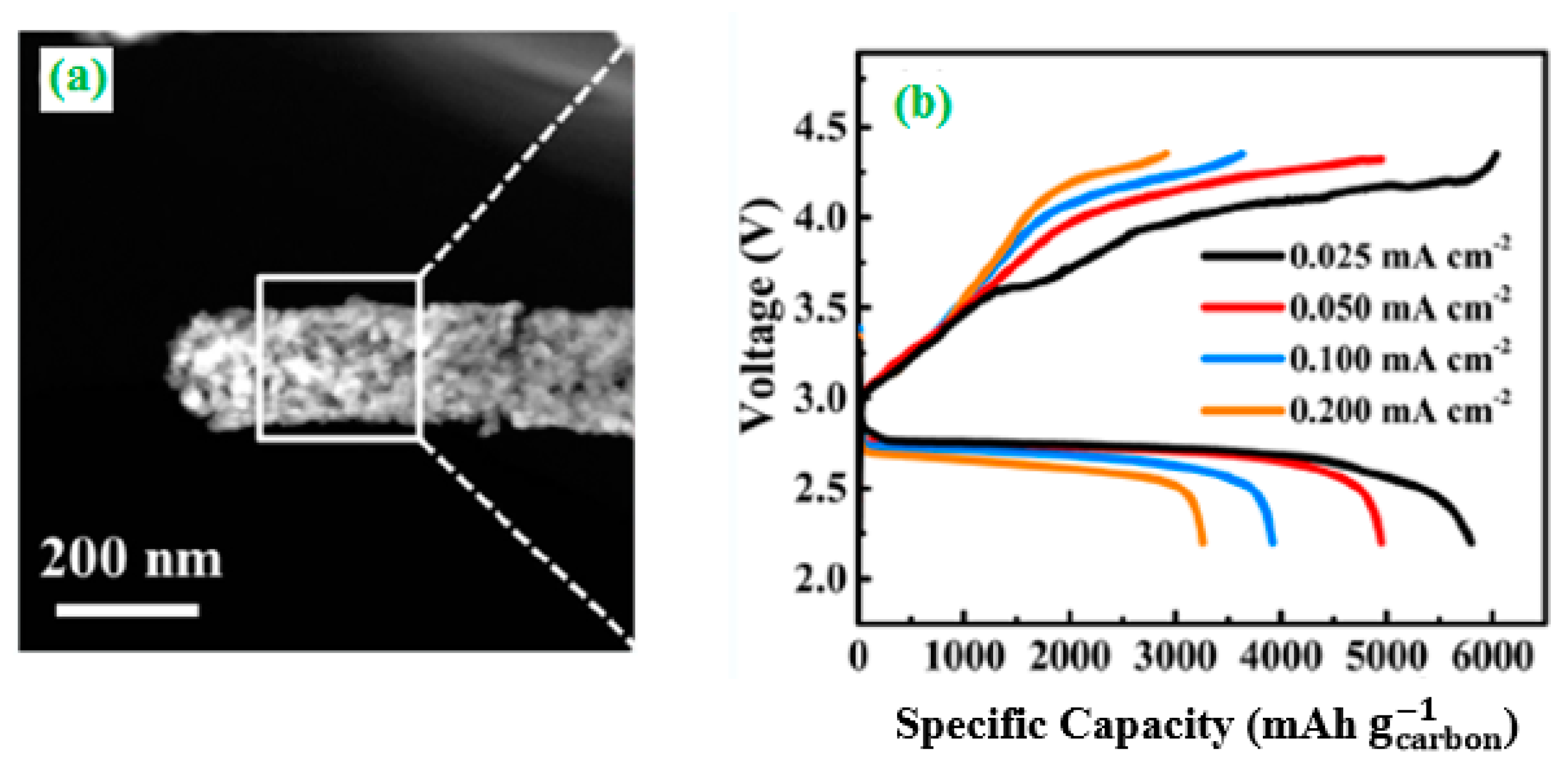
2.2. Batteries
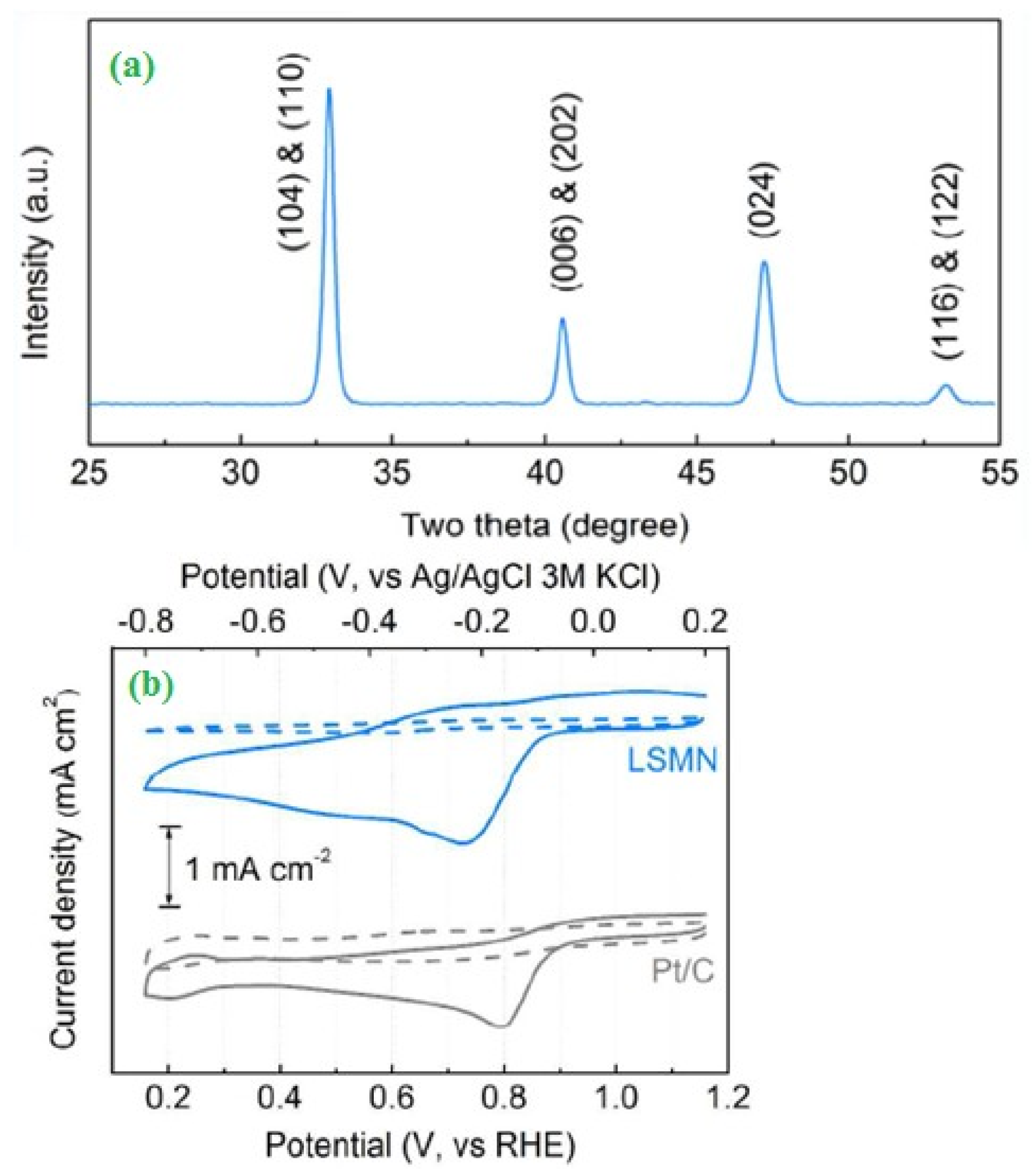
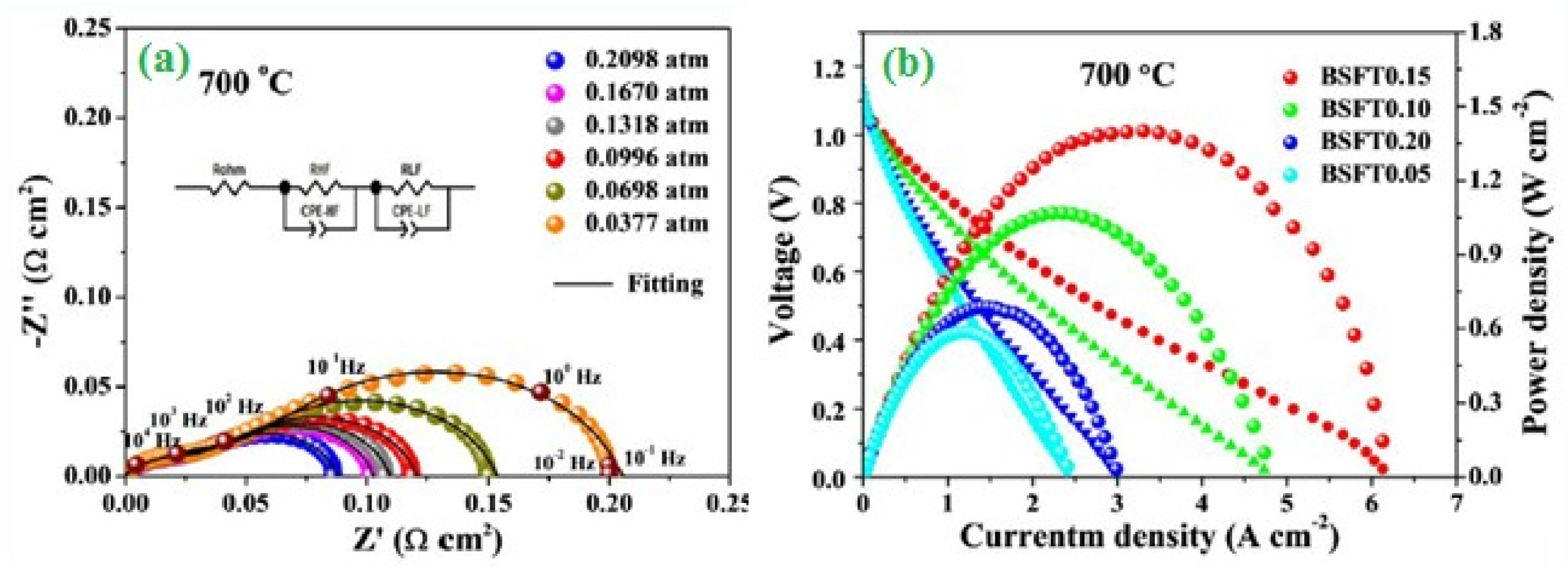
2.3. Fuel Cells (Oxygen Reduction)
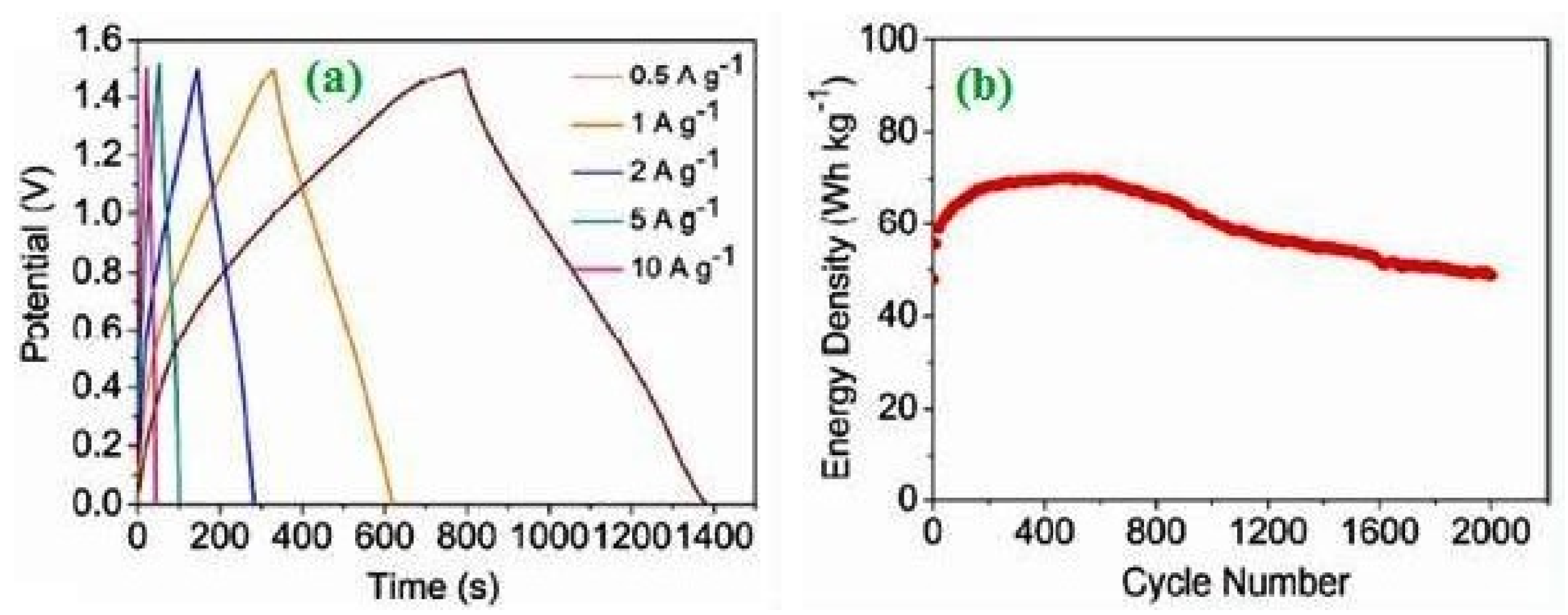
2.4. Supercapacitors
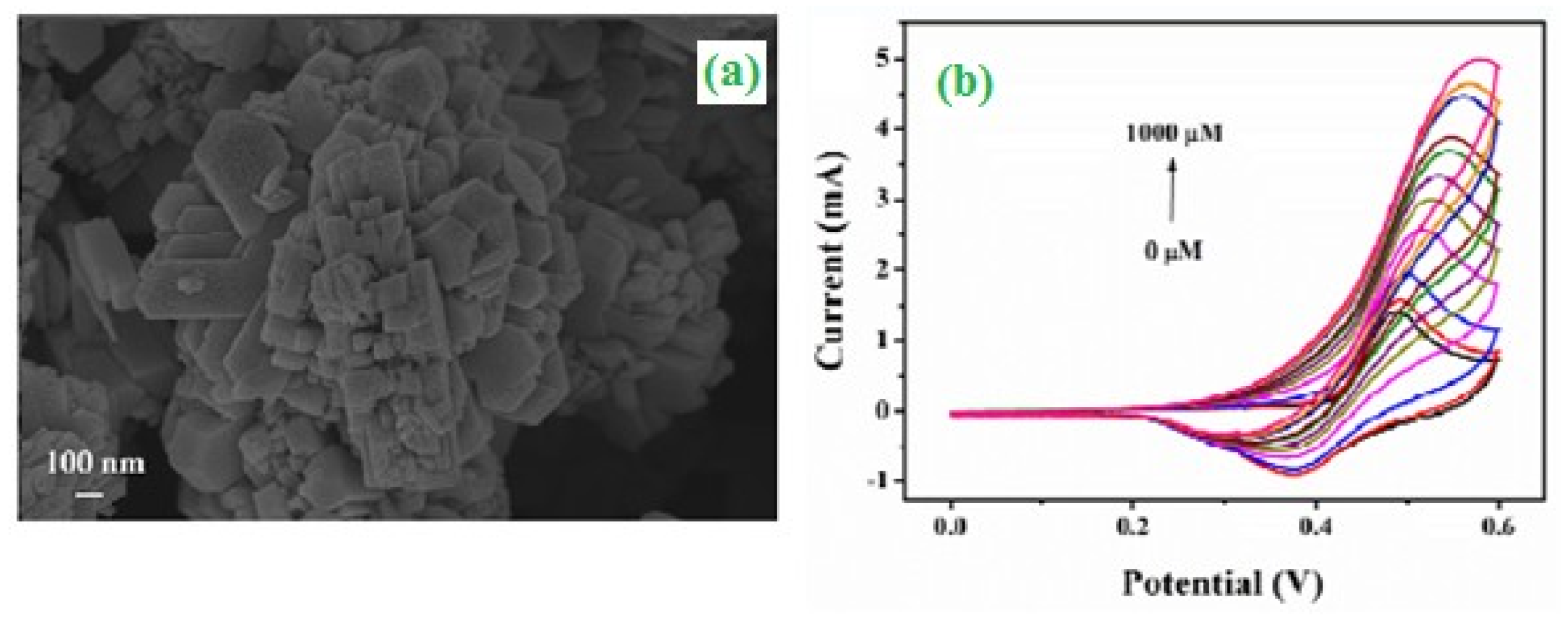
3. Electrochemical and Bio-Sensors
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PSC | Perovskite solar cell |
| LPK | Layered perovskite |
| LIBs | Lithium-ion batteries |
| SIBs | Sodium-ion batteries |
| PCE | Power conversion efficiency |
| PEDOT: PSS | Poly(3,4-ethylenedioxythiophene) and poly(styrene sulfonate) |
| FTO | Fluorinated indium tin oxide |
| CPSCs | Colorful perovskite solar cells |
| KIPIG | Potassium-ion pre-intercalated graphene |
| DSSCs | Dye-sensitized solar cells |
| IZO | Indium doped zinc oxide |
| HTM | Hole transporting materials |
| MAPbBr3 | Methyl ammonium lead halide |
| AgNWs | Silver nanowires |
| SEM | Scanning electron microscope |
| ORR | Oxygen reduction reaction |
| OER | Oxygen evolution reaction |
| LiPS | Lithium Polysulfide |
| MOF | Metal-Organic Framework |
References
- Wu, M.; Zhang, G.; Wu, M.; Prakash, J.; Sun, S. Rational design of multifunctional air electrodes for rechargeable Zn–Air batteries: Recent progress and future perspectives. Energy Storage Mater. 2019, 21, 253–286. [Google Scholar] [CrossRef]
- Wali, Q.; Iftikha, F.J.; Khan, M.E.; Ullah, A.; Iqbal, Y.; Jose, R. Advances in stabiligy of perovskite solar cells. Org. Electron. 2020, 78, 105590. [Google Scholar] [CrossRef]
- Dong, C.R.; Wang, Y.; Zhang, K.; Zeng, H. Halide perovskite materials as light harvesters for solar energy conversion. Energy Chem. 2020, 2, 100026. [Google Scholar] [CrossRef]
- Jena, A.K.; Kulkarni, A.; Miyasaka, T. Halide perovskite photovoltaics: Background, status, and future prospects. Chem. Rev. 2019, 119, 3036–3103. [Google Scholar] [CrossRef]
- Shellaiah, M.; Sun, K.W. Review on sensing applications of perovskite nanomaterials. Chemosensors 2020, 8, 55. [Google Scholar] [CrossRef]
- Zhu, Z.; Sun, Q.; Zhang, Z.; Dai, J.; Xing, G.; Li, S.; Huang, X.; Huang, W. Metal halide perovskites: Stability and sensing-ability. J. Mater. Chem. C 2018, 6, 10121–10137. [Google Scholar] [CrossRef]
- Boyd, C.C.; Cheacharoen, R.; Leijtens, T.; McGehee, M.D. Understanding degradation mechanisms and improving stability of perovskite photovoltaics. Chem. Rev. 2019, 119, 3418–3451. [Google Scholar] [CrossRef] [PubMed]
- Assirey, E.A.R. Perovskite synthesis, properties and their related biochemical and industrial application. Saudi Pharm. J. 2019, 27, 817–829. [Google Scholar] [CrossRef] [PubMed]
- Varma, P.C.R. perovskites. In Perovskite Photovoltaics; Academic Press: Thiruvananthapuram, Kerala, India, 2018; pp. 197–229. [Google Scholar]
- Galloway, K.V.; Sammes, N.M. Fuel Cells—Solid Oxide Fuel Cells/Anodes. In Encyclopedia of Electrochemical Power Sources; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Yun, S.; Qin, Y.; Uhl, A.R.; Vlachopoulos, N.; Yin, M.; Li, D.; Han, X.; Hagfeldt, A. New-generation integrated devices based on dye-sensitized and perovskite solar cell. Energy Environ. Sci. 2018, 11, 476–526. [Google Scholar] [CrossRef]
- Khan, U.; Zhinong, Y.; Khan, A.A.; Zulfiqar, A.; Khan, Q.U. Organic–inorganic hybrid perovskites based on methylamine lead halide solar cell. Sol. Energy 2019, 189, 421–425. [Google Scholar] [CrossRef]
- Salim, T.; Sun, S.; Abe, Y.; Krishna, A.; Grimsdale, A.C.; Lam, Y.M. Perovskite-based solar cells: Impact of morphology and device architecture on device performance. J. Mater. Chem. A 2015, 3, 8943. [Google Scholar] [CrossRef]
- Wang, J.; Choi, S.; Kim, J.; Cha, S.W.; Lim, J. Recent advances of first d-block metal-based perovskite oxide electrocatalyst for alkaline water splitting. Catalysts 2020, 10, 770. [Google Scholar] [CrossRef]
- Hu, Y.; Schlipf, J.; Wusslerr, M.; Petrus, M.L.; Jaegermann, W.; Bein, T.; Buschbaum, P.M.; Docampo, P. Hybrid Perovskite/Perovskite Heterojunction Solar cells. ACS Nano 2016, 10, 5999–6007. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.F.; Jeng, J.Y.; Lee, M.H.; Peng, S.R.; Chen, P.; Guo, T.F.; Wen, T.C.; Hsu, Y.J.; Hsu, C.M. High voltage and efficient bilayer heterojunction solar cells based on organic-inorganic hybrid perovskite absorber with low-cost flexible substrate. Phys. Chem. Chem. Phys. 2014, 16, 6033–6040. [Google Scholar] [CrossRef] [PubMed]
- Tong, G.; Chen, T.; Li, H.; Song, W.; Chang, Y.; Liu, J.; Yu, L.; Xu, J.; Qi, Y.; Jiang, Y. High Efficient Hole Extraction and Stable All-Bromide Inorganic Perovskite Solar Cells via Derivative-Phase Gradient Bandgap architecture. Sol. RRL 2019, 1900030. [Google Scholar] [CrossRef]
- Wang, W.; Fleischer, C.; Sauer, D.U. Critical review of the methods for monitoring of lithium-ion batteries in electric and hybrid vehicles. J. Power Sources 2014, 258, 321–339. [Google Scholar]
- Sun, N.; Liu, H.; Yu, Z.; Zhenning, Z.; Shao, C. Mn-doped La0.6Sr0.4CoO3 perovskite catalysts with enhanced performances for non-aqueous electrolyte Li-O2 batteries. RSC Adv. 2016, 6, 13522. [Google Scholar] [CrossRef]
- Liu, J.; Sheha, E.; Dek, S.I.E.; Goonetilleke, D.; Harguindeguey, M.; Sharma, N. SmFeO3 and Bi-doped SmFeO3 perovskites as an alternative class of electrodes in lithium-ion batteries. Cryst. Eng. Commun. 2018, 20, 6165–6172. [Google Scholar] [CrossRef]
- Kong, L.; Chen, X.; Li, B.Q.; Peng, H.J.; Huang, J.Q.; Xie, J.; Zhang, Q. A Bifunctional Perovskite Promoter for Polysulfide Regulation toward Stable Lithium–Sulfur batteries. Adv. Mater. 2018, 30, 1705219. [Google Scholar] [CrossRef]
- Cao, D.; Yin, C.; Shi, D.; Fu, Z.; Zhang, J.; Li, C. Cubic Perovskite Fluoride as Open Framework Cathode for Na-Ion batteries. Adv. Funct. Mater. 2017, 1701130. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, W.; Ding, D.; Liu, M.; Ciucci, F.; Tade, M.; Shao, Z. Advances in Cathode Materials for Solid Oxide Fuel Cells: Complex Oxides without Alkaline Earth metal elements. Adv. Energy Mater. 2015, 5, 1500537. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, M.; Choudhury, P.; Luo, H. Perovskite oxides as bifunctional oxygen electrocatalysts for oxygen evolution/reduction reactions-A mini review. Appl. Mater. Today 2019, 16, 5671. [Google Scholar] [CrossRef]
- Ge, X.; Sumboja, A.; Wu, D.; An, T.; Li, B.; Goh, F.W.T.; Hor, T.S.A.; Zong, Y.; Liu, Z. Oxygen Reduction in Alkaline Media: From Mechanisms to Recent Advances catalysts. ACS Catal. 2015, 5, 4643–4667. [Google Scholar] [CrossRef]
- Zhou, W.; Ge, L.; Chen, Z.G.; Liang, F.; Xu, H.Y.; Motuzas, J.; Julbe, A.; Zhu, Z. Amorphous Iron Oxide Decorated 3D Hetero structured Electrode for Highly Efficient Oxygen reduction. Chem. Mater. 2011, 23, 4193–4198. [Google Scholar] [CrossRef]
- Fabbri, E.; Mohamed, R.; Levecque, P.; Conrad, O.; Kotz, R.; Schmidt, T.J. Composite Electrode Boosts the Activity of Ba0.5Sr0.5Co0.8Fe0.2O3−δPerovskite and Carbon toward Oxygen Reduction in alkaline media. ACS Catal. 2014, 4, 1061–1070. [Google Scholar] [CrossRef]
- Zhou, W.; Sunarso, J.; Zhao, M.; Liang, F.; Klande, T.; Feldhoff, A. A Highly Active Perovskite Electrode for the Oxygen Reduction Reaction below 600 °C. Angew. Chem. Int. Ed. 2013, 52, 14036–14040. [Google Scholar] [CrossRef]
- Jeyalakshmi, M.; Balasubramanian, K. Simple Capacitors to Supercapacitors—An Overview. Int. J. Electrochem. Sci. 2018, 3, 1196–1217. [Google Scholar]
- Zhang, Y.; Ding, J.; Xu, W.; Wang, M.; Shao, R.; Sun, Y.; Lin, B. Mesoporous LaFeO3 perovskite derived from MOF gel for all-solid-state symmetric supercapacitors. Chem. Eng. J. 2020, 386, 124030. [Google Scholar] [CrossRef]
- Cao, Y.; Lin, B.; Sun, Y.; Yang, H.; Zhang, X. Symmetric/Asymmetric Supercapacitor Based on the Perovskite-type Lanthanum Cobaltate Nanofibers with Sr-substitution. Electrochim. Acta 2015, 178, 398–406. [Google Scholar] [CrossRef]
- Hussain, S.; Javed, M.S.; Ullah, N.; Shaheen, A.; Aslam, N.; Ashraf, I.; Abbas, Y.; Wang, M.; Liu, G.; Qiao, G. Unique hierarchical mesoporous LaCrO3 perovskite oxides for highly efficient electrochemical energy storage applications. Ceram. Int. 2019, 45, 15164–15170. [Google Scholar] [CrossRef]
- Park, S.; Boo, H.; Chung, T.D. Electrochemical non-enzymatic glucose sensors. Anal. Chim. Acta 2006, 556, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Supraja, P.; Sudarshan, V.; Tripathy, S.; Agarwal, A.; Singh, S.G. Label free electrochemical detection of cardiac biomarker troponin T using ZnSnO3 perovskite nanomaterials. Anal. Methods 2019, 11, 744–751. [Google Scholar] [CrossRef]
- Hu, X.; Meng, X.; Zhang, L.; Zhang, Y.; Cai, Z.; Huang, Z.; Su, M.; Wang, Y.; Li, Y.; Yao, X.; et al. A Mechanically Robust Conducting Polymer Network Electrode for Efficient Flexible Perovskite solar cells. Joule 2019, 3, 2205–2218. [Google Scholar] [CrossRef]
- Wei, W.; Li, M.; Hu, Y. Applications of 3D Potassium-Ion Pre-Intercalated Graphene for Perovskite and Dye-Sensitized solar cells. Ind. Eng. Chem. Res. 2019, 58, 8743–8749. [Google Scholar] [CrossRef]
- Lee, W.J.; Ramasamy, E.; Lee, D.Y.; Song, J.S. Efficient Dye-Sensitized Solar Cells with Catalytic Multiwall Carbon Nanotube Counter electrodes. ACS Appl. Mater. Interfaces 2009, 6, 1145–1149. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.S.; Lin, J.C. Dual doping of mesoporous carbon pillars with oxygen and sulfur as counter electrodes for iodide/triiodide redox mediated dye-sensitized solar cells. Appl. Surf. Sci. 2019, 471, 455–461. [Google Scholar] [CrossRef]
- Dong, G.; Xia, D.; Yang, Y.; Zhang, W.; Fan, R.; Sui, L.; Su, L.; Zhao, Y.; Yang, P.; Li, Y. In-situ passivation of TiO2 mesoporous scaffold with nano-sized heteropolyacid for boosting the efficiency of the perovskite solar cells. Electrochim. Acta 2020, 332, 135427. [Google Scholar] [CrossRef]
- Joshi, P.; Zhang, L.; Chen, Q.; Glipeau, D.; Fong, H.; Qiao, Q. Electrospun Carbon Nanofibers as Low-Cost Counter Electrode for Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2010, 12, 3572–3577. [Google Scholar] [CrossRef]
- Yue, H.; Wu, J.; Xiao, Y.; Lin, J.; Huang, M.; Lan, Z.; Fan, L. Functionalized graphene/poly(3,4-ethylenedioxythiophene):polystyrenesulfonate as counter electrode catalyst for dye-sensitized solar cells. Energy 2013, 54, 315–321. [Google Scholar] [CrossRef]
- Kavan, L.; Yum, J.H.; Gratzel, M. Optically Transparent Cathode for Dye-Sensitized Solar Cells Based on Graphene nanoplates. ACS Nano 2011, 5, 165–172. [Google Scholar] [CrossRef]
- Wang, G.; Kuang, S.; Zhang, J.; Hou, S.; Nian, S. Graphitic carbon nitride/multiwalled carbon nanotubes composite as Pt-free counter electrode for high-efficiency dye-sensitized solar cells. Electrochim. Acta 2016, 187, 243–248. [Google Scholar] [CrossRef]
- Hino, T.; Ogawa, Y.; Kuramoto, N. Preparation of functionalized and non-functionalized fullerene thin films on ITO glasses and the application to a counter electrode in a dye-sensitized solar cell. Carbon 2006, 44, 880–887. [Google Scholar] [CrossRef]
- Torres, D.; Sebastian, D.; Lazzaro, M.J.; Pinilla, J.L.; Suelves, I.; Arico, A.S.; Baglio, V. Performance and stability of counter electrodes based on reduced few-layer graphene oxide sheets and reduced graphene oxide quantum dots for dye-sensitized solar cells. Electrochim. Acta 2019, 306, 396–406. [Google Scholar] [CrossRef]
- Jost, M.; Alrecht, S.; Kegelmann, L.; Wolf, C.M.; Lang, F.; Lipovsek, B.; Krc, J.; Korte, L.; Neher, D.; Rech, B.; et al. Efficient Light Management by Textured Nanoimprinted Layers for Perovskite Solar Cells. ACS Photonics 2017, 4, 1232–1239. [Google Scholar] [CrossRef]
- Ye, S.; Sun, W.; Li, Y.; Yan, W.; Peng, H.; Bian, Z.; Liu, Z.; Huang, C. CuSCN-Based Inverted Planar Perovskite Solar Cell with an Average PCE of 15.6%. Nano Lett. 2015, 15, 3723–3728. [Google Scholar] [CrossRef] [PubMed]
- Berhe, T.A.; Su, W.N.; Chen, C.H.; Pan, C.J.; Cheng, J.H.; Chen, H.M. Organometal halide perovskite solar cells: Degradation and stability. Energy Environ. Sci. 2016, 9, 323–356. [Google Scholar] [CrossRef]
- Jiang, X.; Xiong, Y.; Mei, A.; Rong, Y.; Hu, Y.; Jin, Y.; Liu, Q.; Han, H. Efficient Compact-Layer-Free, Hole-Conductor Free, Fully Printable Mesoscopic Perovskite Solar cell. J. Phys. Chem. Lett. 2016, 7, 4142–4146. [Google Scholar] [CrossRef]
- Jiang, Y.; Luo, B.; Jiang, F.; Hernandez, C.F.; Liu, T.; Mao, L.; Xiong, S.; Li, Z.; Wang, T.; Kippelen, B.; et al. Efficient colorful perovskite solar cells using a top polymer electrode simultaneously as spectrally selective antireflection coating. Nano Lett. 2016, 16, 7829–7835. [Google Scholar] [CrossRef]
- Denegri, G.M.; Colodrero, S.; Kamarenko, M.; Martorrell, J. All-Nanoparticle SnO2/TiO2 Electron-transporting layers processed at low temperature for efficient thin-film perovskite solar cells. ACS Appl. Energy Mater. 2018, 1, 5548–5556. [Google Scholar]
- Chiang, Y.H.; Shih, C.K.; Sie, A.S.; Li, M.H.; Peng, C.C.; Shen, P.S.; Wang, Y.P.; Guo, T.F.; Chen, P. Highly stable perovskite solar cells with all-inorganic selective contacts from microwave-synthesized oxide nanoparticles. J. Mater. Chem. A 2017, 5, 25485. [Google Scholar] [CrossRef]
- Li, Z.; Boix, P.P.; Xing, G.; Fu, K.; Kulkarnai, S.A.; Batabyal, S.K.; Xu, W.; Cao, A.; Sum, T.C.; Mathews, N.; et al. Carbon nanotubes as an efficient hole collector for high voltage methylammonium lead bromide perovskite solar cells. Nano Scale 2016, 8, 6352–6360. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Zhang, Y.; Shen, H.; Luo, Q.; Zhao, X.; Li, J.; Lin, H. Working from Both Sides: Composite Metallic Semitransparent Top Electrode for High Performance Perovskite solar cells. ACS Appl. Mater. Interfaces 2016, 8, 4523–4531. [Google Scholar] [CrossRef] [PubMed]
- Ito, N.; Kamarudin, M.A.; Hirotani, D.; Zhang, Y.; Shen, Q.; Ogomi, Y.; Iikubo, S.; Minemoto, T.; Yoshino, K.; Hayase, S. Mixed Sn-Ge Perovskite for Enhanced Perovskite Solar Cell Performance in Air. J. Phys. Chem. Lett. 2018, 9, 1682–1688. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, M.M.; Tavakoli, R.; Hasanzadeh, S.; Mirfasin, M.H. Interface Engineering of Perovskite Solar Cell Using a Reduced-Graphene Scaffold. J. Phys. Chem. C 2016, 120, 19531–19536. [Google Scholar] [CrossRef]
- Bhaskar, A.; Mikhailova, D.; Yuvuz, N.K.; Nikolowski, K.; Oswald, S.; Braminik, N.N.; Ehrenberg, H. 3d-Transition metal doped spinels as high-voltage cathode materials for rechargeable lithium-ion batteries. Prog. Solid State Chem. 2014, 42, 128–148. [Google Scholar] [CrossRef]
- Goriparti, S.; Miele, E.; Angelis, F.D.; Fabrizio, E.D.; Zaccaria, R.P.; Capiglia, C. Review on recent progress of nanostructured anode materials for Li-ion batteries. J. Power Sources 2014, 257, 421–443. [Google Scholar] [CrossRef]
- Krass, S.; Vijn, A.; Falk, M.; Ufer, B.; Luerben, B.; Janek, J.; Froba, M. Nanostructured and nanoporous LiFePO4and LiNi0.5Mn1.5O4-δ as cathode materials for lithium-ion batteries. Prog. Solid State Chem. 2014, 42, 218–241. [Google Scholar] [CrossRef]
- Zhang, X.; Gong, Y.; Li, S.; Sun, C. Porous Perovskite La0.6Sr0.4Co0.8Mn0.2O3 Nanofibers Loaded with RuO2 Nano sheets as an Efficient and Durable Bifunctional Catalyst for Rechargeable Li-O2 Batteries. ACS Catal. 2017, 7, 7737–7747. [Google Scholar] [CrossRef]
- Miao, H.; Wang, Z.; Wang, Q.; Sun, S.; Xue, Y.; Wang, F.; Zhao, J.; Liu, Z.; Yuan, J. A new family of Mn-based perovskite (La1−xYxMnO3) with improved oxygen electrocatalytic activity for metal-air batteries. Energy 2018, 154, 561–570. [Google Scholar] [CrossRef]
- Wang, J.; Cheng, X.; Li, Z.; Xu, M.; Lu, Y.; Liu, S.; Zhang, Y.; Sun, C. Perovskite Sr0.9Y0.1CoO3−δ Nano rods Modified with CoO Nanoparticles as a Bifunctional Catalyst for Rechargeable Li–O2 Batteries. ACS Appl. Energy Mater. 2018, 1, 5557–5566. [Google Scholar]
- Tong, W.; Yoon, W.S.; Hagh, N.M.; Amatucci, G.G. A Novel Silver Molybdenum Oxy fluoride Perovskite as a Cathode Material for Lithium batteries. Chem. Mater. 2009, 21, 2148. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, R.; Sun, J.; Wu, M.; Zhao, T. Polyoxyethylene (PEO)|PEO–Perovskite|PEO Composite Electrolyte for All-Solid-State Lithium Metal batteries. ACS Appl. Mater. Interfaces 2019, 11, 46930–46937. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Ramirez, D.; Navarro, N.C.R.; Jullian, D.; Miura, A.; Jaramillo, F.; Tadanaga, K. Two-Dimensional Hybrid Halide Perovskite as Electrode Materials for All-Solid-State Lithium Secondary Batteries Based on Sulfide Solid Electrolytes. ACS Appl. Energy Mater. 2019, 2, 6569–6576. [Google Scholar] [CrossRef]
- Ramirez, D.; Suto, Y.; Cavarro, C.R.; Miura, A.; Tadanaga, K. Structural and Electrochemical Evaluation of Three and Two Dimensional Organohalide Perovskites and Their Influence on the Reversibility of Lithium intercalation. Inorg. Chem. 2018, 57, 4181–4188. [Google Scholar] [CrossRef]
- Ogunniran, K.O.; Murugadoss, G.; Thangamuthu, R.; Periyasamy, P. All inorganic based Nd0.9Mn0.1FeO3 perovskite for Li-ion battery application: Synthesis, structural and morphological investigation. J. Alloys Compd. 2018, 766, 1014–1023. [Google Scholar] [CrossRef]
- Veerappan, G.; Yoo, S.; Zhang, K.; Ma, M.; Kang, B.; Park, J.H. High-reversible capacity of Perovskite BaSnO3/rGO composite for Lithium-Ion Battery Anodes. Electrochim. Acta 2016, 214, 31–37. [Google Scholar] [CrossRef]
- Liu, G.; Chen, H.; Xia, L.; Wang, S.; Ding, L.X.; Li, D.; Xiao, K.; Dai, S.; Wang, H. Hierarchical Mesoporous/Macroporous Perovskite La0.5Sr0.5CoO3–xNanotubes: A Bifunctional Catalyst with Enhanced Activity and Cycle Stability for Rechargeable Lithium Oxygen batteries. ACS Appl. Mater. Interfaces 2015, 7, 22478–22486. [Google Scholar] [CrossRef]
- Ma, Z.; Yuan, X.; Li, L.; Ma, Z.F. Double Perovskite Oxide Sr2CrMoO6-δ as an Efficient Electrocatalyst for Rechargeable Lithium Air batteries. Chem. Comm. 2014, 50, 14855–14858. [Google Scholar] [CrossRef]
- Oh, M.Y.; Lee, J.J.; Zuhoor, A.; Gnanakumar, G.; Nahm, K.S. Enhanced electrocatalytic activity of three-dimensionally-ordered macroporous La0.6Sr0.4CoO3−δ perovskite oxide for Li–O2 battery applications. RSC Adv. 2016, 6, 32212–32219. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Tao, H.B.; Chen, Z.; Li, M.; Sun, Y.F.; Hua, B.; Luo, J.L. In situ grown cobalt phosphide (CoP) on perovskite nanofibers as an optimized trifunctional electrocatalyst for Zn–air batteries and overall water splitting. J. Mater. Chem. A 2019, 7, 26607–26617. [Google Scholar] [CrossRef]
- Larramendi, I.R.; Vitoriano, N.O.; Dzul Bautista, I.B.; Rojo, T. Chapter 20, Designing perovskite oxides for solid oxide fuel cells. In Perovskite Materials—Synthesis, Characterisation, Properties, and Applications; Intech Open Limited: London, UK, 2016; pp. 590–617. [Google Scholar] [CrossRef]
- Chen, Y.; Nie, X.; Wang, B.; Xia, C.; Dong, W.; Wang, X.; Wang, H.; Zhu, B. Tuning La0.6Sr0.4Co0.2Fe0.8O3−δ perovskite cathode as functional electrolytes for advanced low-temperature SOFCs. Catal. Today 2019, 1–9. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, X.; Zhang, W.; Wei, Z.; Guan, K.; Meng, J.; Meng, F.; Meng, J.; Liu, X. Enhancing catalysis activity of La0.6Sr0.4Co0.8Fe0.2O3−δ cathode for solid oxide fuel cell by a facile and efficient impregnation process. Int. J. Hydrogen Energy 2019, 44, 13757. [Google Scholar] [CrossRef]
- Wang, L.; Wang, P.; Geng, C.; Cao, H.; Xu, C.; Cheng, J.; Hong, T. A novel core-shell LSCF perovskite structured electro catalyst with local hetero-interface for solid oxide fuel cells. Int. J. Hydrogen Energy 2020, 45, 11824–11833. [Google Scholar] [CrossRef]
- Budiman, R.A.; Bangarinao, K.D.; Ishiyama, T.; Krishimoto, H.; Yamaji, K.; Horita, T.; Yokokawa, T. Influence of Sr and Co deficiency on the transport properties and oxygen reduction reaction of La0.6Sr0.4Co0.2Fe0.8O3−δ. Solid State Ion. 2020, 348, 115285. [Google Scholar] [CrossRef]
- Gomez, A.E.M.; Sacanell, J.; Iriart, C.H.; Ramos, C.P.; Soldati, A.L.; Figueroa, S.J.A.; Tabacniks, M.H.; Fantini, M.C.A.; Craievich, A.F.; Lamas, D.G. Crystal structure, cobalt and iron speciation and oxygen nonstoichiometry of La0.6Sr0.4Co1-yFeyO3-d nano rods for IT-SOFC cathodes. J. Alloys Compd. 2020, 817, 153250. [Google Scholar] [CrossRef]
- Li, P.; Yang, Q.; Zhang, H.; Yao, M.; Yan, F.; Fu, D. Effect of Fe, Ni and Zn dopants in La0·9Sr0·1CoO3 on the electrochemical performance of single-component solid oxide fuel cell. Int. J. Hydrogen Energy 2020, 45, 11802. [Google Scholar] [CrossRef]
- Xie, D.; Li, K.; Yang, J.; Yan, D.; Jia, L.; Chi, B.; Pu, J.; Li, J. High-performance La0.5(Ba0.75Ca0.25)0.5Co0.8Fe0.2O3−δ cathode for proton-conducting solid oxide fuel cells. Int. J. Hydrogen Energy 2020, 1–8. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, H.; Guan, K.; Wei, Z.; Zhang, X.; Meng, J.; Liu, X.; Meng, J. La0.6Sr0.4Co0.2Fe0.8O3−δ/CeO2 Hetero structured Composite Nanofibers as a Highly Active and Robust Cathode Catalyst for Solid Oxide Fuel Cells. ACS Appl. Mater. Interfaces 2019, 11, 26830–26841. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, W.; Guan, K.; Wei, Z.; Meng, J.; Meng, J.; Liu, X. Enhancing Activity and Durability of A-Site-Deficient (La0.6Sr0.4)0.95Co0.2Fe0.8O3−δ Cathode by Surface Modification with PrO2−δ Nanoparticles. ACS Sustain. Chem. Eng. 2020, 8, 3367–3380. [Google Scholar] [CrossRef]
- Nadeem, M.; Wan, Y.; Xia, C. The effect of group IIIA oxides on the oxygen reduction reaction at cathodes for intermediate-temperature solid oxide fuel cells. Compos. Part B Eng. 2020, 189, 107924. [Google Scholar] [CrossRef]
- Lai, Y.W.; Lee, K.R.; Yang, S.Y.; Tseng, C.J.; Jang, S.C.; Tsao, I.Y.; Chen, S.Y.; Lee, S.W. Production of La0.6Sr0.4Co0.2Fe0.8O3−δ cathode with graded porosity for improving proton-conducting solid oxide fuel cells. Ceram. Int. 2019, 45, 22479–22485. [Google Scholar] [CrossRef]
- Ryu, S.; Lee, S.; Jeong, W.; Pandiyan, A.; Moorthy, S.B.K.; Chang, I.; Park, T.; Cha, S.W. Pulsed laser deposition of BaCo0.4Fe0.4Zr0.1Y0.1O3−δ cathode for solid oxide fuel cell. Surf. Coat. Technol. 2019, 369, 265–268. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Zhang, Z.; Xu, X.; Chen, Y.; Song, Y.; Dai, J.; Yang, G.; Ran, R.; Zhou, W.; et al. A new highly active and CO2-stable perovskite-type cathode material for solid oxide fuel cells developed from A- and B-site cation synergy. J. Power Sources 2020, 457, 227995. [Google Scholar] [CrossRef]
- Namgung, Y.; Hong, J.; Kumar, A.; Lim, D.K.; Song, S.J. One step infiltration induced multi-cation oxide nanocatalyst for load proof SOFC application. Appl. Catal. B Environ. 2020, 267, 118374. [Google Scholar] [CrossRef]
- Ge, X.; Du, Y.; Li, B.; Hor, T.S.A.; Sindoro, M.; Zong, Y.; Zhang, H.; Liu, Z. Intrinsically Conductive Perovskite Oxides with Enhanced Stability and Electrocatalytic Activity for Oxygen Reduction Reactions. ACS Catal. 2016, 6, 7865–7871. [Google Scholar] [CrossRef]
- Gao, J.; Li, Q.; Xia, W.; Sun, L.; Huo, L.H.; Zhao, H. Advanced Electrochemical Performance and CO2 Tolerance of Bi0.5 Sr0.5 Fe1−x Tix O3−δ Perovskite Materials as Oxygen Reduction Cathodes for Intermediate—Temperature Solid Oxide Fuel Cells. ACS Sustain. Chem. Eng. 2019, 7, 18647–18656. [Google Scholar] [CrossRef]
- Wang, J.; Saccoccio, M.; Chen, D.; Gao, Y.; Chen, C. The effect of A-site and B-site substitution on BaFeO3−δ; An investigation as a cathode material for intermediate-temperature solid oxide fuel cell. J. Power Sources 2015, 297, 511–518. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef]
- Zhou, F.; Ren, Z.; Zhao, Y.; Shen, X.; Wang, A.; Li, Y.Y.; Surya, C.; Chai, Y. Perovskite Photovoltachromic Supercapacitor with All-Transparent Electrodes. ACS Nano 2016, 10, 5900–5908. [Google Scholar] [CrossRef]
- Li, X.; Zhao, H.; Xu, N.; Zhou, X.; Zhang, C.; Chen, N. Electrical conduction behavior of La, Co co-doped SrTiO3 perovskite as anode material for solid oxide fuel cells. Int. J. Hydrogen Energy 2009, 34, 6407–6414. [Google Scholar] [CrossRef]
- Li, X.; Zhao, X.; Zhou, W.; Xu, N.; Xie, Z.; Chen, N. Electrical conductivity and structural stability of La-doped SrTiO3 with A-site deficiency as anode materials for solid oxide fuel cells. Int. J. Hydrogen Energy 2010, 35, 7913–7918. [Google Scholar] [CrossRef]
- Yoo, K.B.; Choi, G.M. Performance of La-doped strontium titanate (LST) anode on LaGaO3-based SOFC. Solid State Ion. 2009, 180, 867–871. [Google Scholar] [CrossRef]
- Lang, X.; Mo, H.; Hu, X.; Tian, H. Supercapacitor performance of perovskite La1−xSrxMnO3. Dalton Trans. 2017, 46, 13720–13730. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Dinh, J.; Tade, M.O.; Shao, Z. Design of Perovskite Oxides as Anion-Intercalation-Type Electrodes for Supercapacitors: Cation Leaching Effect. ACS Appl. Mater. Interfaces 2016, 8, 23774–23783. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, Y.; Su, C.; Zhou, W.; Liu, M.; Shao, Z. Perovskite SrCo0.9Nb0.1O3-δ as an Anion-Intercalated Electrode Material for Supercapacitors with Ultrahigh Volumetric Energy Density. Angew. Chem. 2016, 128, 9728–9731. [Google Scholar] [CrossRef]
- Alexander, C.T.; Mefford, J.T.; Saunders, J.; Forslund, R.P.; Johnston, K.P.; Stevenson, K.J. Anion-Based Pseudocapacitance of the Perovskite Library La1−xSrx BO3−δ (B = Fe, Mn, Co). ACS Appl. Mater. Interfaces 2019, 11, 5084–5094. [Google Scholar] [CrossRef]
- Tomar, A.K.; Singh, G.; Sharma, R.K. Charge storage characteristics of mesoporous strontium titanate perovskite aqueous as well as flexible solid-state supercapacitor cell. J. Power Sources 2019, 426, 223–232. [Google Scholar] [CrossRef]
- Shi, W.; Ding, R.; Li, X.; Xu, Q.; Ying, D.; Huang, Y.; Liu, E. Bimetallic Co-Mn perovskite fluorides as high-stable electrode materials for supercapacitors. Chem. Eur. J. 2017, 23, 15305–15311. [Google Scholar] [CrossRef]
- Ng, C.H.; Lim, H.N.; Hayase, S.; Zainal, Z.; Shafie, S.; Lee, H.W.; Huang, N.M. Cesium Lead Halide Inorganic-Based Perovskite-Sensitized Solar Cell for PhotoSupercapacitor Application Under High Humidity Condition. ACS Appl. Energy Mater. 2018, 1, 692–699. [Google Scholar] [CrossRef]
- Luqman, E.; Oloore, E.; Gondal, M.A.; Idris, K.; Popoola, A. CdS Quantum Dots-Organometallic Halide Perovskites Bilayer Electrodes Structures for Supercapacitor Applications. Chem. Electron. Chem. 2019, 7, 486–492. [Google Scholar]
- Popoola, I.; Gondal, M.; Oloore, L.; Popoola, A.; AlGhamdi, J. Fabrication of organometallic halide perovskite electrochemical supercapacitors utilizing quasi-solid-state electrolytes for energy storage devices. Electrochim. Acta 2020, 332, 135536. [Google Scholar] [CrossRef]
- Kutty, T.R.N.; Padmini, P. Wet chemical formation of nanoparticles of binary perovskites through isothermal gel to crystallite conversion. Mater. Res. Bull. 1992, 27, 945–952. [Google Scholar] [CrossRef]
- Ishikawa, K.; Uemori, T. Surface relaxation in ferroelectric perovskites. Phys. Rev. B 1991, 60, 11841. [Google Scholar] [CrossRef]
- Jose, R.; James Asha, J.; John, M.; Divakar, R.; Koshy, J. Synthesis and characterization of nanoparticles of Ba2EuZrO5.5: A new complex perovskite ceramic oxide. J. Mater. Res. 2000, 15, 2125–2130. [Google Scholar] [CrossRef]
- Giannakas, A.E.; Vaimakis, T.C.; Ladavos, A.K.; Trikalitis, P.N.; Pomonis, P.J. Variation of surface properties and textural features of spinel ZnAl2O4 and perovskite LaMnO3 nanoparticles prepared via CTAB–butanol–octane–nitrate salt microemulsions in the reverse and bicontinuous states. J. Colloid Interface Sci. 2003, 259, 244–253. [Google Scholar] [CrossRef]
- Hwang, D.K.; Kim, S.; Lee, J.H.; Hwang, I.S.; Kim, I.D. Phase evolution of perovskite LaNiO3 nanofibers for supercapacitor application and p-type gas sensing properties of LaOCl–NiO composite nanofibers. J. Mater. Chem. 2011, 21, 1959–1965. [Google Scholar] [CrossRef]
- Ding, R.; Li, X.; Shi, W.; Xu, Q.; Han, X.; Zhou, Y.; Hong, W.; Liu, E. Flexible high efficiency perovskite solar cells. Energy Environ. Sci. 2015, 8, 3208–3214. [Google Scholar]
- Cheng, Y.; Yang, Q.D.; Xiao, J.; Xue, Q.; Li, H.W.; Guan, Z.; Yip, H.L.; Tsang, S.W. On the Decomposition of Organometal Halide Perovskite Films on Zinc Oxide Nanoparticles. ACS Appl. Mater. Interfaces 2015, 7, 19986–19993. [Google Scholar] [CrossRef]
- Lee, D.U.; Park, H.W.; Park, M.G.; Ismayilov, V.; Chen, Z. Synergistic Bifunctional Catalyst Design based on Perovskite Oxide Nanoparticles and Intertwined Carbon Nanotubes for Rechargeable Zinc−Air Battery Applications. ACS Appl. Mater. Interfaces 2015, 7, 902–910. [Google Scholar] [CrossRef]
- Che, W.; Wei, M.; Sang, Z.; Ou, Y.; Liu, Y.; Liu, J. Perovskite LaNiO3−δ oxide as an anion-intercalated pseudocapacitor electrode. J. Alloys Compd. 2018, 731, 381–388. [Google Scholar] [CrossRef]
- Liu, P.; Liu, J.; Cheng, S.; Cai, W.; Yu, F.; Zhang, Y.; Wu, P.; Liu, M. A high-performance electrode for supercapacitors: Silver nanoparticles grown on a porous perovskite-type material La0.7S0.3CoO3−δ substrate. Chem. Eng. J. 2017, 328, 1–10. [Google Scholar] [CrossRef]
- Shankar, J.; Prasad, B.V.; Suresh, M.B.; Kumar, R.V.; Babu, D.S. Electrical Properties of NdCr1−xFexO3 Perovskite Ceramic Nanoparticles-An Impedance Spectroscopy studies. Mater. Res. Bull. 2017, 94, 385–398. [Google Scholar] [CrossRef]
- George, G.; Jackson, S.L.; Luo, C.Q.; Fang, D.; Luo, D.; Hu, D.; Wen, J.; Luo, Z. Effect of doping on the performance of high-crystalline SrMnO3 perovskite nanofibers as a supercapacitor electrode. Ceram. Int. 2018, 44, 21982–21992. [Google Scholar] [CrossRef]
- Galal, A.; Hassan, H.K.; Atta, N.F.; Jacob, T. Energy and cost-efficient nano-Ru-based perovskites/RGO composites for application in high performance supercapacitors. J. Colloid Interface Sci. 2019, 538, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Hussain, N.; Wu, F.; Younasd, W.; Xu, L. Hollow sphere formation by the self-aggregation of perovskite fluoride NaNiF3 nanocrystals and the application of these spheres as an electrode in an ultrahigh performance asymmetric supercapacitor. New J. Chem. 2019, 43, 11959–11967. [Google Scholar] [CrossRef]
- Lang, X.; Sun, X.; Liu, Z.; Nan, H.; Li, C.; Hu, X.; Tian, H. Ag nanoparticles decorated perovskite La0.85Sr0.15MnO3 as electrode materials for supercapacitors. Mater. Lett. 2019, 243, 34–37. [Google Scholar] [CrossRef]
- Vinuthraj, T.N.; Hoskeri, P.A.; Muralidhara, H.B. Facile synthesis of perovskite Lanthanum aluminate and its green reduced graphene oxide composite for high performance supercapacitors. J. Electroanal. Chem. 2020, 858, 113830. [Google Scholar] [CrossRef]
- Rezanezhad, A.; Rezaie, E.; Ghadimi, L.S.; Hajalilou, A.; Lotf, E.A.; Arsalani, N. Outstanding supercapacitor performance of Nd–Mn co-doped perovskite LaFeO3@nitrogen-doped graphene oxide nanocomposites. Electrochim. Acta 2020, 335, 135699. [Google Scholar] [CrossRef]
- Ishida, N.; Wakamiya, A.; Saeki, A. Quantifying Hole Transfer Yield from Perovskite to Polymer Layer: Statistical Correlation of Solar Cell Outputs with Kinetic and Energetic Properties. ACS Photonics 2016, 3, 1678–1688. [Google Scholar] [CrossRef]
- Lacerda, G.R.D.B.S.; Santos, G.A.; Rocco, M.L.M.; Lavall, R.L.; Matencio, T.; Calado, T.H.D.R. Development of nanohybrids based on carbon nanotubes/P(EDOTco-MPy) and P(EDOT-co-PyMP) copolymers as electrode material for aqueous supercapacitors. Electrochim. Acta 2020, 335, 135637. [Google Scholar] [CrossRef]
- Xu, X.; Li, S.; Zhang, H.; Shen, Y.; Zakeeruddin, S.M.; Grätzel, M.; Cheng, Y.B.; Wang, M. A Power Pack based on Organometallic Perovskite Solar Cell and Supercapacitor. ACS Nano 2015, 9, 1782–1787. [Google Scholar] [CrossRef] [PubMed]
- Takeoka, Y.; Asai, K. Perovskite-Type Organic/Inorganic Lamellar Polymer. U.S. Patent No: US6741,6927B2, 6 April 2004. [Google Scholar]
- Jain, M.; Bauer, E.; Ronning, F.; Hundley, M.F.; Civale, L.; Wang, H.; Maiorov, B.; Burrell, A.K.; McClesky, T.M.; Foltyn, S.R.; et al. Mixed-Valence Perovskite Thin Films by Polymer-Assisted Deposition. J. Am. Ceram. Soc. 2008, 91, 1858–1863. [Google Scholar] [CrossRef]
- Hou, S.; Guo, Y.; Tang, Y.; Quan, Q. Synthesis and Stabilization of Colloidal Perovskite Nanocrystals by Multidentate Polymer Micelles. ACS Appl. Mater. Interfaces 2017, 9, 18417–18422. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Ha, S.R.; Park, S.; Oh, J.T.; Kim, D.H.; Cho, S.; Bae, S.Y.; Kang, D.; Kim, J. Water-resistant PEDOT:PSS hole transport layer by photo crosslinking agent for high-performance perovskite and polymer solar cells. Nanoscale 2018, 10, 13187–13193. [Google Scholar]
- Xu, Z.; Liu, Y.; Zhou, W.; Tade, M.O.; Shao, Z. B-Site Cation-Ordered Double-Perovskite Oxide as an Outstanding Electrode Material for Supercapacitive Energy Storage Based on the Anion Intercalation mechanism. ACS Appl. Mater. Interfaces 2018, 10, 9415–9423. [Google Scholar] [CrossRef]
- Dai, H.; Zhang, Y.; Wu, X.; Hu, R.; Wang, L.; Zhang, Y.; Fan, G.; Hu, X.; Li, T.; Yang, Z. Synthesis of perovskite-type SrTiO3 nanoparticles for sensitive electrochemical bio-sensing applications. J. Electroanal. Chem. 2018, 810, 95–99. [Google Scholar] [CrossRef]
- Govindasamy, M.; Wang, S.F.; Pan, W.C.; Subramanian, B.; Ramalingam, R.J.; Lohedan, H.A. Facile sonochemical synthesis of perovskite-type SrTiO3 nanocubes with reduced graphene oxide nanocatalyst for an enhanced electrochemical detection of α-amino acid (tryptophan). Ultrason. Sonochem. 2019, 56, 193–199. [Google Scholar] [CrossRef]
- Atta, N.F.; Ali, S.M.; Ads, E.H.E.; Galal, A. Nano-perovskite carbon paste composite electrode for the simultaneous determination of dopamine, ascorbic acid and uric acid. Electrochim. Acta 2014, 128, 16–64. [Google Scholar] [CrossRef]
- Deganello, F.; Liotta, L.F.; Loonardi, S.G.; Neri, G. Electrochemical properties of Ce-doped SrFeO3 perovskites-modified electrodes towards hydrogen peroxide oxidation. Electrochim. Acta 2016, 190, 939–947. [Google Scholar] [CrossRef]
- Ponnusamy, R.; Chakraborty, B.; Rout, C.S. Pd Doped WO3 Nanostructures as Potential Glucose Sensor with Insight from Electronic Structure simulations. J. Phys. Chem. B 2018, 122, 2737–2746. [Google Scholar] [CrossRef]
- Jia, F.F.; Zhong, H.; Zhang, W.G.; Li, X.R.; Wang, G.Y.; Song, J.; Chen, Z.P.; Yin, J.Z.; Guo, L.P. A novel nonenzymatic ECL glucose sensor based on perovskite LaTiO3-Ag0.1 nanomaterials. Sens. Actuators B 2015, 212, 174–182. [Google Scholar] [CrossRef]
- Ye, D.; Xu, Y.; Luo, L.; Ding, Y.; Wang, Y.; Liu, X.; Xing, L.; Peng, J. A novel hydrogen peroxide sensor based on LaNi0.5Ti0.5O3/CoFe2O4 modified electrode. Colloids Surf. B 2012, 89, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Luque, G.L.; Ferreyra, N.F.; Leyva, A.G.; Rivas, G.A. Characterization of carbon paste electrodes modified with manganese based perovskites-type oxides from the amperometric determination of hydrogen peroxide. Sens. Actuators B 2009, 142, 331–336. [Google Scholar] [CrossRef]
- Ahmad, K.; Mohammad, A.; Mathur, P.; Mobin, S.M. Preparation of SrTiO3 perovskite decorated rGO and electrochemical detection of nitroaromatics. Electrochim. Acta 2016, 215, 435–446. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Luo, L.; Ding, Y.; Liu, X.; Huang, A. A novel sensitive nonenzymatic glucose sensor based on perovskite LaNi0.5Ti0.5O3-modified carbon paste electrode. Sens. Actuators B 2010, 151, 65–70. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.-W.; Ramachandran, R.; Chen, S.-M.; Kavitha, N.; Dinakaran, K.; Kannan, R.; Anushya, G.; Bhuvana, N.; Jeyapragasam, T.; Mariyappan, V.; et al. Developing Low-Cost, High Performance, Robust and Sustainable Perovskite Electrocatalytic Materials in the Electrochemical Sensors and Energy Sectors: “An Overview”. Catalysts 2020, 10, 938. https://doi.org/10.3390/catal10080938
Chen T-W, Ramachandran R, Chen S-M, Kavitha N, Dinakaran K, Kannan R, Anushya G, Bhuvana N, Jeyapragasam T, Mariyappan V, et al. Developing Low-Cost, High Performance, Robust and Sustainable Perovskite Electrocatalytic Materials in the Electrochemical Sensors and Energy Sectors: “An Overview”. Catalysts. 2020; 10(8):938. https://doi.org/10.3390/catal10080938
Chicago/Turabian StyleChen, Tse-Wei, Rasu Ramachandran, Shen-Ming Chen, Narayanasamy Kavitha, Kannaiyan Dinakaran, Ramanjam Kannan, Ganesan Anushya, Nagulan Bhuvana, Tharini Jeyapragasam, Vinitha Mariyappan, and et al. 2020. "Developing Low-Cost, High Performance, Robust and Sustainable Perovskite Electrocatalytic Materials in the Electrochemical Sensors and Energy Sectors: “An Overview”" Catalysts 10, no. 8: 938. https://doi.org/10.3390/catal10080938
APA StyleChen, T.-W., Ramachandran, R., Chen, S.-M., Kavitha, N., Dinakaran, K., Kannan, R., Anushya, G., Bhuvana, N., Jeyapragasam, T., Mariyappan, V., Divya Rani, S., & Chitra, S. (2020). Developing Low-Cost, High Performance, Robust and Sustainable Perovskite Electrocatalytic Materials in the Electrochemical Sensors and Energy Sectors: “An Overview”. Catalysts, 10(8), 938. https://doi.org/10.3390/catal10080938





