Performance of 1-(3-Sulfopropyl)-3-Methylimidazolium Hydrogen Sulfate as a Catalyst for Hardwood Upgrading into Bio-Based Platform Chemicals
Abstract
1. Introduction
- FA is a commodity chemical employed in many industries, and it may also play a role in the development of fuel cells [15].
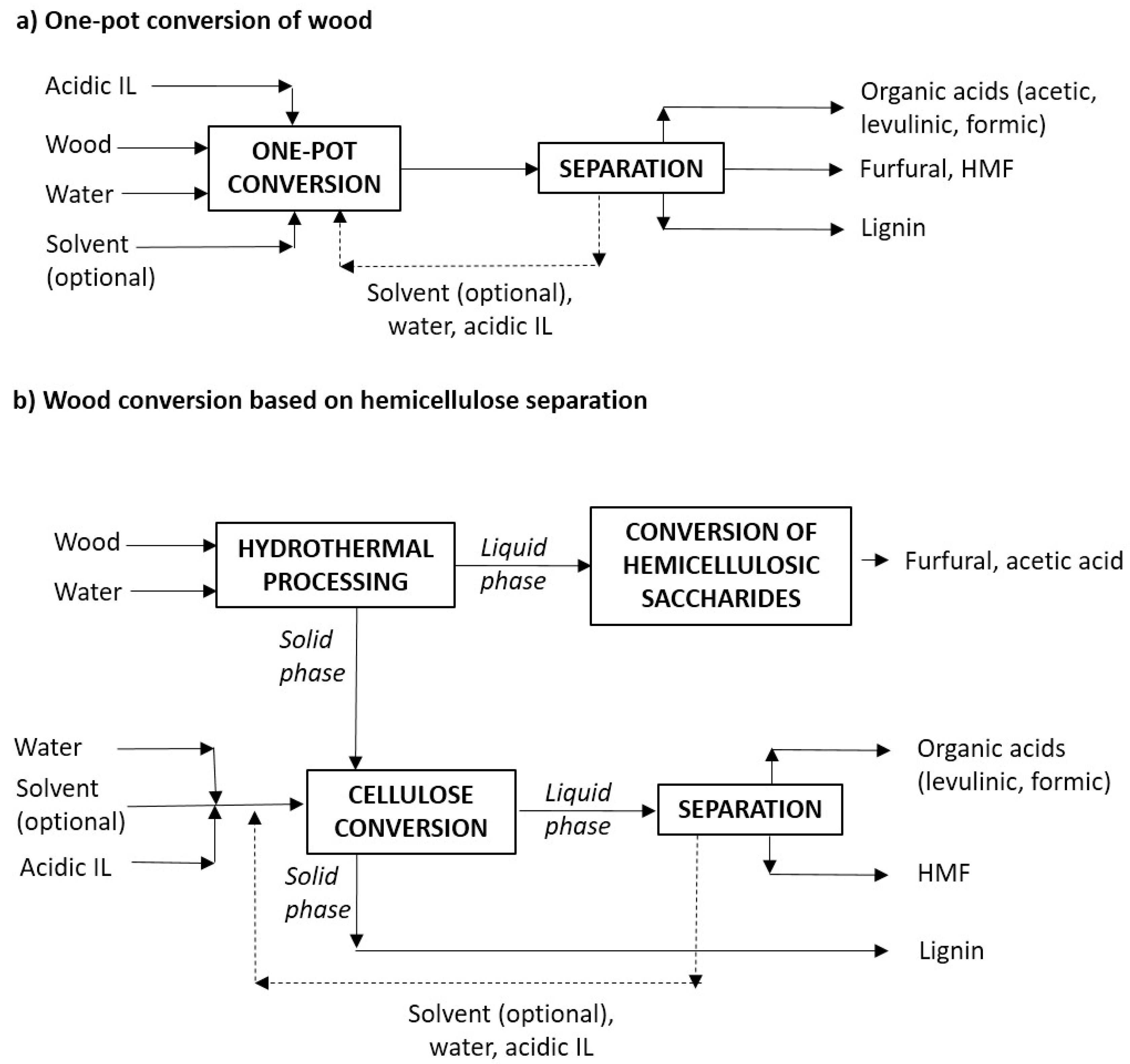
- (a)
- One-pot processing of wood in aqueous media containing an acidic catalyst, in order to obtain FF from hemicelluloses, and HMF or organic acids (LevA, FA) from cellulose. Ionic liquids (IL) with acidic characteristics (acidic ionic liquids, AIL) can be used as the acidic catalyst.
- (b)
- Solubilization of hemicelluloses by hydrothermal processing with hot, compressed water (leading to the breakdown of xylan chains into soluble saccharides), with further acid-catalyzed conversion of the reaction products into FF, and the acid-catalyzed manufacture of HMF and/or organic acids from the cellulose-rich, solid phase. As before, AIL can be employed as catalysts.
- Utilization of reaction media containing an immiscible solvent able to extract the target products, avoiding the unwanted reactions taking place in aqueous media [25,26,27]. This approach has been followed in this study, in which methyl isobutyl ketone (MIBK), a green solvent recommended by the CHEM21 guide [28], has been employed.
- Utilization of microwave (MW)-heated reactors enabling fast heating profiles, which are considered favorable to improve the experimental results [29], and to allow energy savings [30]. Following this idea, a stirred, MW-heated reactor has been employed in this work in experiments aiming at the manufacture of furans and/or organic acids.
- Utilization of efficient and selective catalysts. Mineral acids such as sulfuric acid have been widely used for biomass processing. Alternatively, acidic ionic liquids have been reported to have potential as catalysts with improved activity, selectivity, and stability, and to allow an easier separation and reutilization [31]. Specifically, AILs have been used as catalysts for processing LB (or fractions derived from it) in aqueous or biphasic media [9,32]. In this work, a Brønsted acidic, imidazolium-type IL has been used for manufacturing FF, LevA, and FA from Eucalyptus wood (or from fractions derived from it), following the general ideas shown in Figure 1. Additional literature information of the utilization of ILs in the framework of biorefineries is provided in the next paragraphs.
2. Results
2.1. Composition of Eucalyptus Globulus Wood
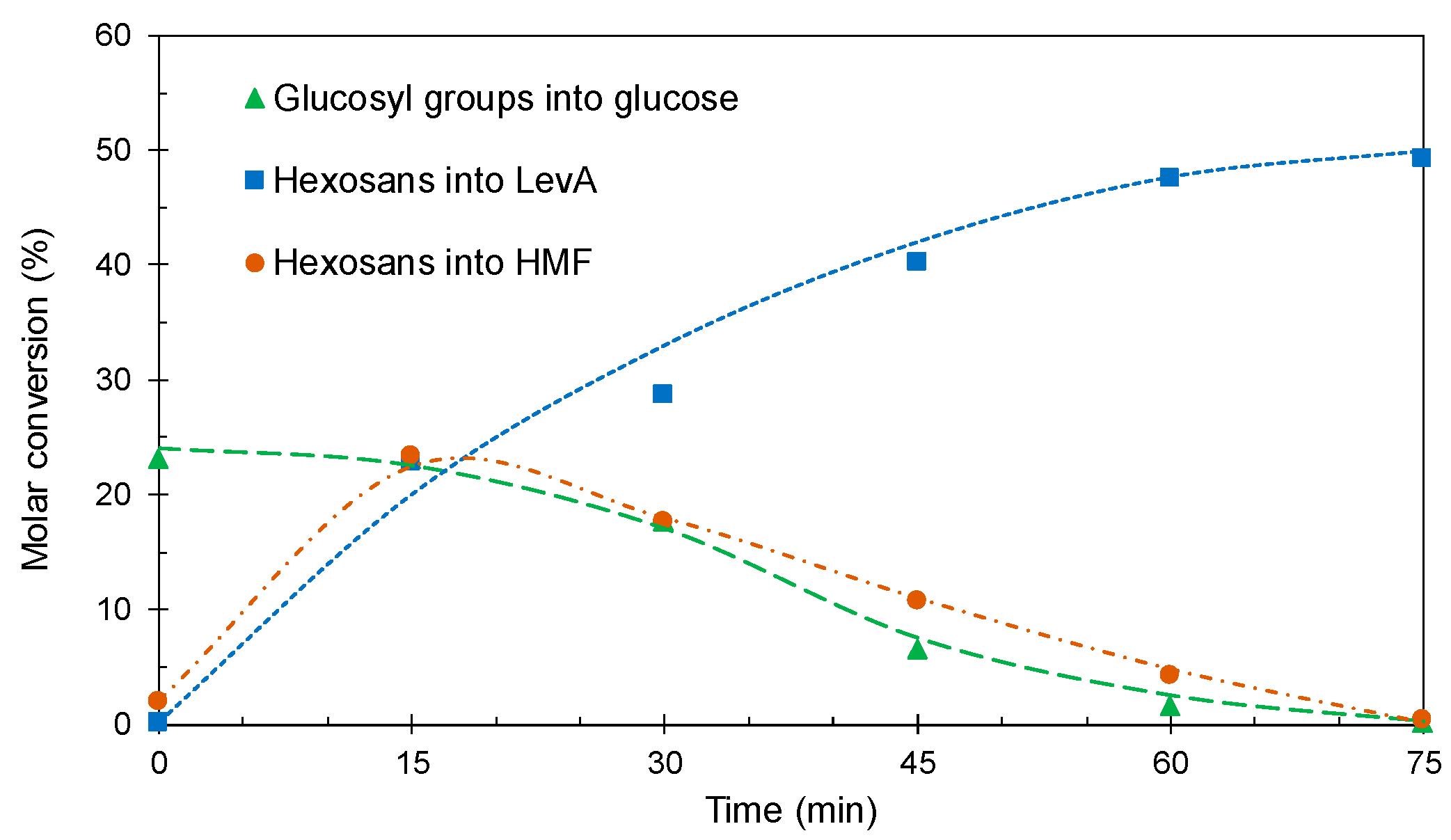
2.2. One-Pot Conversion of Eucalyptus Globulus Wood
2.3. Eucalyptus Globulus Wood Conversion Based on Hemicellulose Removal
2.3.1. Hydrothermal Processing
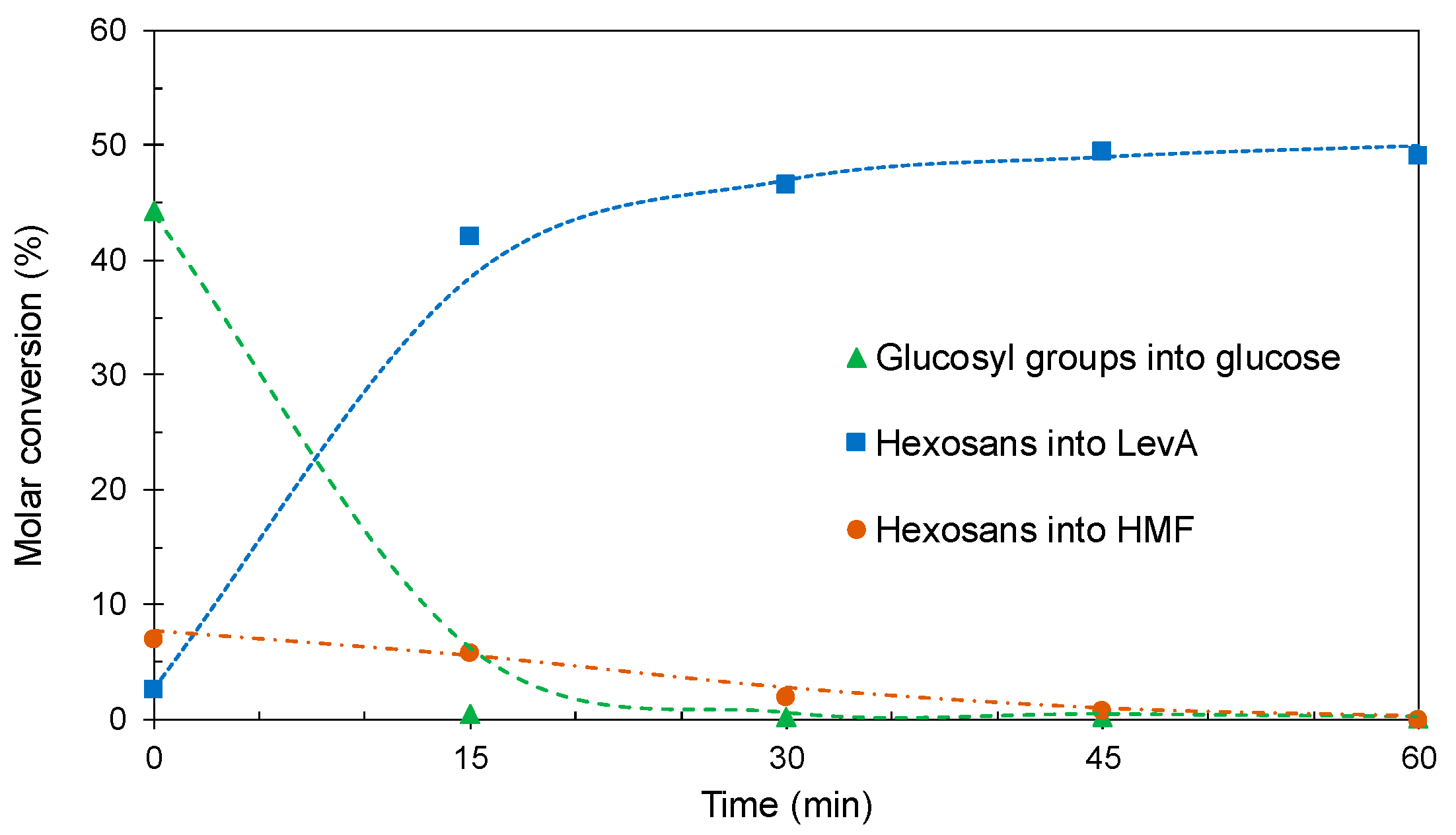
2.3.2. Conversion of the Solids from Hydrothermal Processing
2.3.3. Conversion of the Liquid Phase from Hydrothermal Processing
2·(VCCj − 2.84 × 10−2)/(4.26 × 10−2 − 1.42 × 10−2)
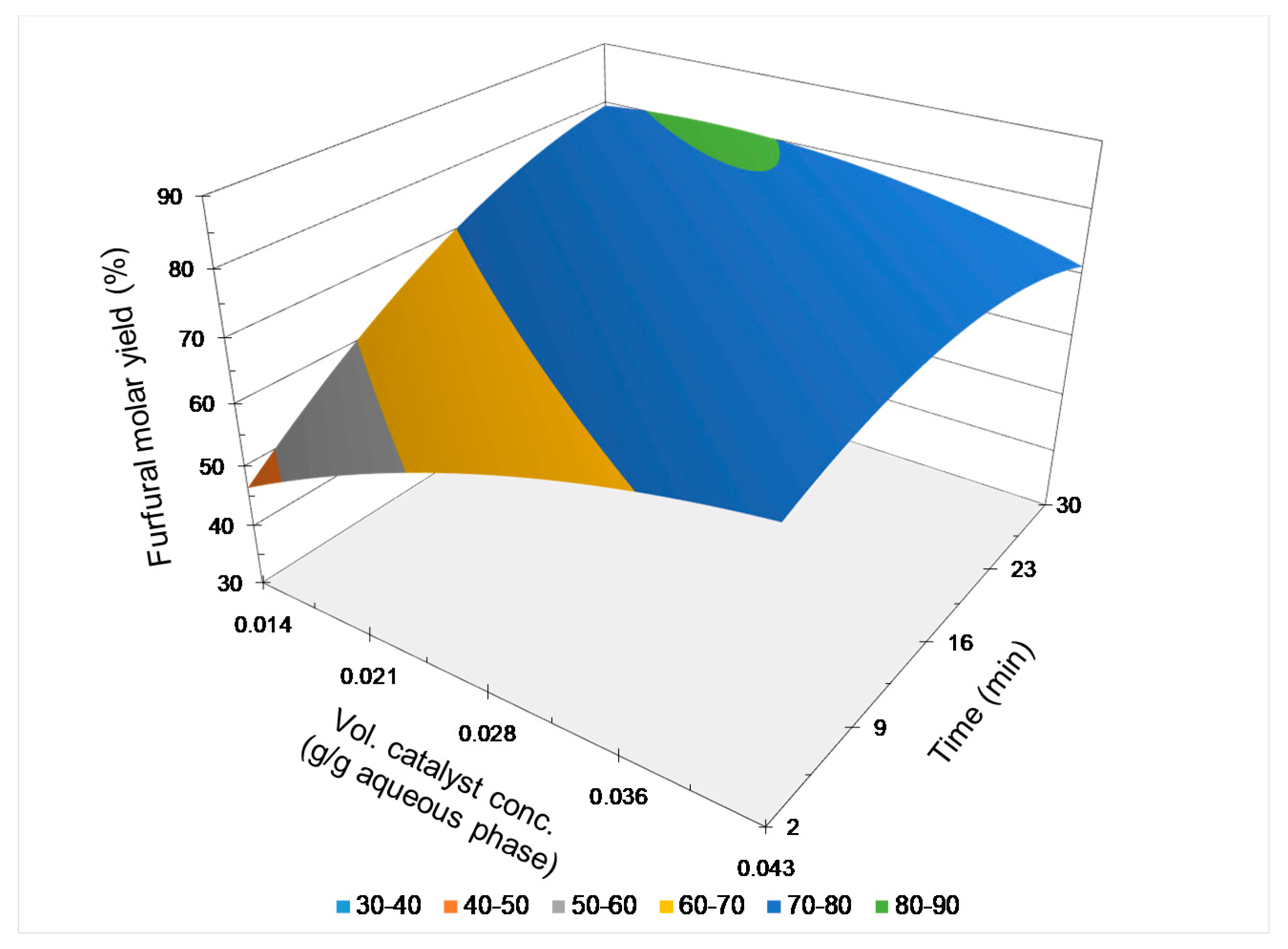
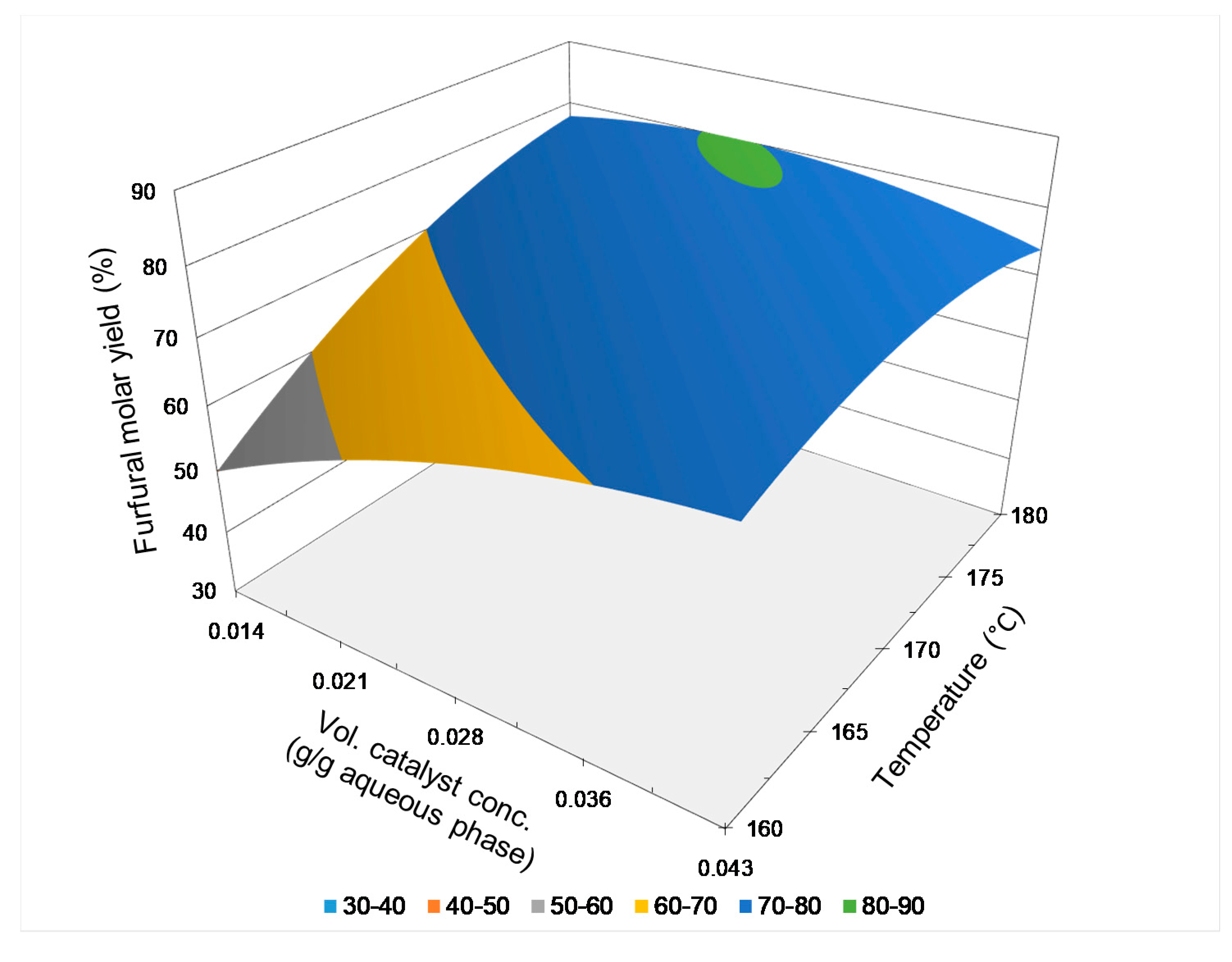
2.3.4. Comparative Analysis of Results
3. Materials and Methods
3.1. Raw Material
3.2. Reaction
3.3. Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mika, L.T.; Cséfalvay, E.; Németh, Á. Catalytic Conversion of Carbohydrates to Initial Platform Chemicals: Chemistry and Sustainability. Chem. Rev. 2018, 118, 505–613. [Google Scholar] [CrossRef] [PubMed]
- Oak Ridge National Laboratory. U.S.; Department of Energy. U.S. Billion-Ton Update: Biomass Supply for a Bioenergy and Bioproducts Industry. 2011. Available online: https://www1.eere.energy.gov/bioenergy/pdfs/billion_ton_update.pdf (accessed on 20 July 2020).
- Pereira, J.S.; Linder, S.; Araujo, M.C.; Pereira, H.; Ericsson, T.; Borralho, N.; Leal, L.C. Optimization of Biomass Production in Eucalyptus Globulus Plantations—A Case Study. In Biomass Production by Fast-Growing Trees, 1st ed.; Pereira, J.S., Landsberg, J.J., Eds.; Springer: Dordrecht, The Netherlands, 1989; Volume 166, pp. 101–121. [Google Scholar] [CrossRef]
- Penín, L.; López, M.; Santos, V.; Alonso, J.L.; Parajó, J.C. Technologies for Eucalyptus wood processing in the scope of biorefineries: A comprehensive review. Bioresour. Technol. 2020, 311, 123528. [Google Scholar] [CrossRef] [PubMed]
- Rockwood, D.L.; Rudie, A.W.; Ralph, S.A.; Zhu, J.Y.; Winandy, J.E. Energy product options for Eucalyptus species grown as short rotation woody crops. Int. J. Mol. Sci. 2008, 9, 1361–1378. [Google Scholar] [CrossRef] [PubMed]
- FAO. Planted Forests and Trees Working Paper FP38E. Global Planted Forests Thematic Study: Results and Analysis 2006. Available online: http://www.fao.org/forestry/12139-03441d093f070ea7d7c4e3ec3f306507.pdf (accessed on 20 July 2020).
- Chandel, A.K.; Garlapati, V.K.; Singh, A.K.; Antunes, F.A.F.; da Silva, S.S. The path forward for lignocellulose biorefineries: Bottlenecks, solutions, and perspective on commercialization. Bioresour. Technol. 2018, 264, 370–381. [Google Scholar] [CrossRef]
- Naidu, D.S.; Hlangothi, S.P.; John, M.J. Bio-based products from xylan: A review. Carbohydr. Polym. 2018, 179, 28–41. [Google Scholar] [CrossRef]
- Peleteiro, S.; Rivas, S.; Alonso, J.L.; Santos, V.; Parajó, J.C. Furfural production using ionic liquids: A review. Bioresour. Technol. 2016, 202, 181–191. [Google Scholar] [CrossRef]
- Cai, C.M.; Zhang, T.; Kumar, R.; Wyman, C.E. Integrated furfural production as a renewable fuel and chemical platform from lignocellulosic biomass. J. Chem. Technol. Biotechnol. 2014, 89, 2–10. [Google Scholar] [CrossRef]
- Bohre, A.; Dutta, S.; Saha, B.; Abu-Omar, M.M. Upgrading furfurals to drop-in biofuels: An overview. ACS Sustainable Chem. Eng. 2015, 3, 1263–1277. [Google Scholar] [CrossRef]
- Frankiewicz, A. Overview of 4-oxopentanoic (levulinic) acid production methods—An intermediate in the biorefinery process. CHEMIK 2016, 70, 203–208. [Google Scholar]
- Kang, S.; Fu, J.; Zhang, G. From lignocellulosic biomass to levulinic acid: A review on acid-catalyzed hydrolysis. Renew. Sustain. Energy Rev. 2018, 94, 340–362. [Google Scholar] [CrossRef]
- Pileidis, F.D.; Titirici, M.M. Levulinic acid biorefineries: New challenges for efficient utilization of biomass. ChemSusChem 2016, 9, 562–582. [Google Scholar] [CrossRef] [PubMed]
- Aslam, N.M.; Masdar, M.S.; Kamarudin, S.K.; Daud, W.R.W. Overview on Direct Formic Acid Fuel Cells (DFAFCs) as an energy sources. APCBEE Proc. 2012, 3, 33–39. [Google Scholar] [CrossRef]
- Badgujar, K.C.; Wilson, L.D.; Bhanage, B.M. Recent advances for sustainable production of levulinic acid in ionic liquids from biomass: Current scenario, opportunities and challenges. Renew. Sustain. Energy Rev. 2019, 102, 266–284. [Google Scholar] [CrossRef]
- Penín, L.; Peleteiro, S.; Rivas, S.; Santos, V.; Parajó, J.C. Production of 5-hydroxymethylfurfural from pine wood via biorefinery technologies based on fractionation and reaction in ionic liquids. BioResources 2019, 14, 4733–4747. [Google Scholar]
- Sweygers, N.; Harrer, J.; Dewil, R.; Appels, L. A microwave-assisted process for the in-situ production of 5-hydroxymethylfurfural and furfural from lignocellulosic polysaccharides in a biphasic reaction system. J. Cleaner Prod. 2018, 187, 1014–1024. [Google Scholar] [CrossRef]
- Cai, C.M.; Zhang, T.; Kumar, R.; Wyman, C.E. THF co-solvent enhances hydrocarbon fuel precursor yields from lignocellulosic biomass. Green Chem. 2013, 15, 3140–3145. [Google Scholar] [CrossRef]
- Climent, M.J.; Corma, A.; Iborra, S. Converting carbohydrates to bulk chemicals and fine chemicals over heterogeneous catalysts. Green Chem. 2011, 13, 520–540. [Google Scholar] [CrossRef]
- Mok, W.S.L.; Antal, M.J.; Varhegyi, G. Productive and parasitic pathways in dilute acid-catalyzed hydrolysis of cellulose. Ind. Eng. Chem. Res. 1992, 31, 94–100. [Google Scholar] [CrossRef]
- Patil, S.K.R.; Lund, C.R.F. Formation and growth of humins via aldol addition and condensation during acid-catalyzed conversion of 5-hydroxymethylfurfural. Energy Fuels 2011, 25, 4745–4755. [Google Scholar] [CrossRef]
- Girisuta, B.; Janssen, L.P.B.M.; Heeres, H.J. A kinetic study on the decomposition of 5-hydroxymethylfurfural into levulinic acid. Green Chem. 2006, 8, 701–709. [Google Scholar] [CrossRef]
- Girisuta, B.; Dussan, K.; Haverty, D.; Leahy, J.J.; Hayes, M.H.B. A kinetic study of acid catalysed hydrolysis of sugar cane bagasse to levulinic acid. Chem. Eng. J. 2013, 217, 61–70. [Google Scholar] [CrossRef]
- Rivas, S.; Vila, C.; Alonso, J.L.; Santos, V.; Parajó, J.C.; Leahy, J.J. Biorefinery processes for the valorization of Miscanthus polysaccharides: From constituent sugars to platform chemicals. Ind. Crops Prod. 2019, 134, 309–317. [Google Scholar] [CrossRef]
- Ren, H.; Zhou, Y.; Liu, L. Selective conversion of cellulose to levulinic acid via microwave-assisted synthesis in ionic liquids. Bioresour. Technol. 2013, 129, 616–619. [Google Scholar] [CrossRef] [PubMed]
- Sorokina, K.N.; Taran, O.P.; Medvedeva, T.B.; Samoylova, Y.V.; Piligaev, A.V.; Parmon, V.N. Cellulose Biorefinery Based on a Combined Catalytic and Biotechnological Approach for Production of 5-HMF and Ethanol. ChemSusChem 2017, 10, 562–574. [Google Scholar] [CrossRef]
- Prat, D.; Wells, A.; Hayler, J.; Sneddon, H.; McElroy, C.R.; Abou-Shehada, S.; Dunn, P.J. CHEM21 selection guide of classical- and less classical-solvents. Green Chem. 2015, 18, 288–296. [Google Scholar] [CrossRef]
- Ren, H.; Girisuta, B.; Zhou, Y.; Liu, L. Selective and recyclable depolymerization of cellulose to levulinic acid catalyzed by acidic ionic liquid. Carbohydr. Polym. 2015, 117, 569–576. [Google Scholar] [CrossRef]
- Hayes, B.L. Recent advances in microwave-assisted synthesis. Aldrichimica Acta 2004, 37, 66–77. [Google Scholar] [CrossRef]
- Song, J.; Han, B. Green chemistry: A tool for the sustainable development of the chemical industry. Natl. Sci. Rev. 2015, 2, 255–256. [Google Scholar] [CrossRef]
- Peleteiro, S.; Santos, V.; Parajó, J.C. Furfural production in biphasic media using an acidic ionic liquid as a catalyst. Carbohydr. Polym. 2016, 153, 421–428. [Google Scholar] [CrossRef]
- Peleteiro, S.; Rivas, S.; Alonso, J.L.; Santos, V.; Parajó, J.C. Utilization of ionic liquids in lignocellulose biorefineries as agents for separation, derivatization, fractionation, or pretreatment. J. Agric. Food Chem. 2015, 63, 8093–8102. [Google Scholar] [CrossRef]
- Serrano-Ruiz, J.C.; Campelo, J.M.; Francavilla, M.; Romero, A.A.; Luque, R.; Menéndez-Vázquez, C.; García, A.B.; García-Suárez, E.J. Efficient microwave-assisted production of furfural from C5 sugars in aqueous media catalyzed by Brønsted acidic ionic liquids. Catal. Sci. Technol. 2012, 2, 1828–1832. [Google Scholar] [CrossRef]
- Carvalho, A.V.; Da Costa Lopes, A.M.; Bogel-Łukasik, R. Relevance of the acidic 1-butyl-3-methylimidazolium hydrogen sulphate ionic liquid in the selective catalysis of the biomass hemicellulose fraction. RSC Advances 2015, 5, 47153–47164. [Google Scholar] [CrossRef]
- Peleteiro, S.; Santos, V.; Garrote, G.; Parajó, J.C. Furfural production from Eucalyptus wood using an acidic ionic liquid. Carbohydr. Polym. 2016, 146, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Sun, J.K.; Yi, Y.X.; Wang, B.; Xu, F.; Sun, R.C. One-pot synthesis of levulinic acid from cellulose in ionic liquids. Bioresour. Technol. 2015, 192, 812–816. [Google Scholar] [CrossRef] [PubMed]
- López, M.; Vila, C.; Santos, V.; Parajó, J.C. Manufacture of Platform Chemicals from Pine Wood Polysaccharides in Media Containing Acidic Ionic Liquids. Polymers 2020, 12, 1215. [Google Scholar] [CrossRef]
- Garrote, G.; Parajó, J.C. Non-isothermal autohydrolysis of Eucalyptus wood. Wood Sci. Technol. 2002, 36, 111–123. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, Z.K. Microwave-assisted conversion of lignocellulosic biomass into furans in ionic liquid. Bioresour. Technol. 2010, 101, 1111–1114. [Google Scholar] [CrossRef]
- Sievers, C.; Valenzuela-Olarte, M.B.; Marzialetti, T.; Musin, I.; Agrawal, P.K.; Jones, C.W. Ionic-liquid-phase hydrolysis of pine wood. Ind. Eng. Chem. Res. 2009, 48, 1277–1286. [Google Scholar] [CrossRef]
- Teng, J.; Ma, H.; Wang, F.; Wang, L.; Li, X. Catalytic fractionation of raw biomass to biochemicals and organosolv lignin in a methyl isobutyl ketone/H2O biphasic system. ACS Sustain. Chem. Eng. 2016, 4, 2020–2026. [Google Scholar] [CrossRef]
- Rivas, S.; González-Muñoz, M.J.; Santos, V.; Parajó, C.J. Production of furans from hemicellulosic saccharides in biphasic reaction systems. Holzforschung 2013, 67, 923–929. [Google Scholar] [CrossRef]
| EXPER | OPERATIONAL CONDITIONS | EXPERIMENTAL RESULTS | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SY | Concentrations in Aqueous Phase, g/L | Concentrations in Organic Phase, g/L | ||||||||||||||||
| CC | WSR | OSR | T | t | Glc | Xyc | FAc | AcHc | LevAc | HMFc | FFc | FAc | AcHc | LevAc | HMFc | FFc | ||
| 1 | 0.15 | 6 | 12 | 170 | 0 | 53.7 | 12.4 | 18.6 | 2.14 | 3.02 | 0.10 | 0.20 | 0.31 | 0.10 | 1.54 | 0.05 | 0.19 | 2.14 |
| 2 | 0.15 | 6 | 12 | 180 | 0 | 38.5 | 28.0 | 8.18 | 1.06 | 3.11 | 0.98 | 0.68 | 0.61 | 0.28 | 1.54 | 0.34 | 0.54 | 3.52 |
| 3 | 0.10 | 6 | 12 | 180 | 16 | 19.5 | 17.9 | 1.00 | 3.96 | 3.11 | 6.54 | 1.71 | 0.66 | 1.64 | 1.57 | 3.26 | 1.84 | 5.73 |
| 4 | 0.15 | 6 | 12 | 190 | 0 | 26.1 | 32.2 | 4.38 | 1.44 | 3.07 | 2.99 | 1.17 | 0.74 | 0.59 | 1.55 | 0.88 | 0.85 | 4.12 |
| 5 | 0.15 | 6 | 12 | 190 | 30 | 18.1 | 0.16 | 0.17 | 5.74 | 3.50 | 10.4 | 0.04 | 0.52 | 2.44 | 1.77 | 5.27 | 0.04 | 4.25 |
| 6 | 0.15 | 6 | 12 | 190 | 50 | 17.9 | 0.17 | 0.20 | 5.40 | 3.41 | 10.1 | <0.01 | 0.42 | 2.27 | 1.73 | 5.15 | <0.01 | 3.58 |
| 7 | 0.10 | 10 | 20 | 180 | 15 | 8.10 | 16.9 | 1.16 | 1.27 | 1.95 | 1.95 | 2.11 | 0.43 | 0.58 | 0.99 | 0.95 | 2.30 | 3.89 |
| 8 | 0.10 | 10 | 20 | 180 | 30 | 8.00 | 9.14 | 0.60 | 2.25 | 2.10 | 3.58 | 1.81 | 0.39 | 0.86 | 1.06 | 1.83 | 2.07 | 3.82 |
| 9 | 0.10 | 10 | 20 | 180 | 45 | 6.90 | 1.00 | 0.29 | 3.55 | 2.17 | 6.08 | 0.62 | 0.40 | 1.46 | 1.11 | 3.13 | 0.69 | 3.49 |
| 10 | 0.10 | 10 | 20 | 190 | 15 | 5.60 | 1.26 | 0.32 4.70 | 2.99 | 2.15 | 4.97 | 1.24 | 0.44 | 1.26 | 1.08 | 2.59 | 1.41 | 4.03 |
| 11 | 0.10 | 10 | 20 | 190 | 30 | 5.43 | 0.16 | 0.26 | 3.61 | 2.35 | 6.27 | 0.17 | 0.35 | 1.47 | 1.20 | 3.28 | 0.22 | 3.56 |
| 12 | 0.10 | 10 | 20 | 190 | 45 | 4.69 | 0.14 | 0.29 | 3.58 | 2.31 | 6.44 | 0.03 | 0.35 | 1.53 | 1.17 | 3.34 | 0.03 | 3.30 |
| (a) Solid Phase | |
| COMPONENT | CONTENT (g/100 g Treated Solid, Oven-Dry Basis) |
| Cellulose | 58.8 |
| Xylan | 3.20 |
| Arabinan | 0.07 |
| Acetyl groups | 0.69 |
| Klason lignin | 30.6 |
| (b) Liquid Phase | |
| COMPONENT | CONCENTRATION (g/L) |
| Glucose | 0.51 |
| Xylose | 6.40 |
| Arabinose | 0.51 |
| FA | 0.66 |
| AcH | 1.41 |
| LevA | 0.02 |
| HMF | 0.09 |
| FF | 0.57 |
| Glucosyl groups in oligomers | 1.00 |
| Xylosyl groups in oligomers | 12.9 |
| Arabinosyl groups in oligomers | 0.00 |
| Acetyl groups in oligomers | 3.32 |
| EXPER | OPERATIONAL CONDITIONS | EXPERIMENTAL RESULTS | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SY | Concentrations in Aqueous Phase, g/L | Concentrations in Organic Phase, g/L | |||||||||||
| CC | WSR | OSR | T | t | Glc | FAc | LevAc | HMFc | FAc | LevAc | HMFc | ||
| 1 | 0.1 | 10 | 20 | 180 | 0 | 61.7 | 14.9 | 0.17 | 0.03 | 0.25 | 0.03 | 0.02 | 0.27 |
| 2 | 0.1 | 10 | 20 | 180 | 15 | 15.0 | 14.8 | 2.45 | 4.18 | 2.64 | 0.95 | 2.09 | 3.13 |
| 3 | 0.1 | 10 | 20 | 180 | 30 | 15.3 | 11.2 | 2.93 | 5.23 | 2.18 | 1.20 | 2.62 | 2.29 |
| 4 | 0.1 | 10 | 20 | 180 | 45 | 14.8 | 4.16 | 4.04 | 7.38 | 1.32 | 1.60 | 3.65 | 1.41 |
| 5 | 0.1 | 10 | 20 | 180 | 60 | 15.2 | 1.10 | 4.51 | 8.69 | 0.52 | 1.86 | 4.34 | 0.58 |
| 6 | 0.1 | 10 | 20 | 180 | 75 | 13.4 | 0.15 | 4.62 | 8.98 | 0.06 | 1.89 | 4.50 | 0.06 |
| 7 | 0.1 | 10 | 20 | 190 | 0 | 42.0 | 28.4 | 0.45 | 0.60 | 0.91 | 0.11 | 0.21 | 0.90 |
| 8 | 0.1 | 10 | 20 | 190 | 15 | 22.0 | 0.35 | 4.32 | 7.66 | 0.68 | 1.72 | 3.85 | 0.76 |
| 9 | 0.1 | 10 | 20 | 190 | 30 | 21.9 | 0.19 | 4.54 | 8.41 | 0.23 | 1.82 | 4.31 | 0.27 |
| 10 | 0.1 | 10 | 20 | 190 | 45 | 13.2 | 0.16 | 4.72 | 8.94 | 0.09 | 1.95 | 4.54 | 0.10 |
| 11 | 0.1 | 10 | 20 | 190 | 60 | 12.0 | 0.12 | 4.55 | 8.82 | <0.01 | 1.83 | 4.45 | <0.01 |
| Exper. | T, °C | T, min | VCC, g/g Aqueous Phase | FFMY, % |
|---|---|---|---|---|
| 1a | 170 | 16 | 2.84 × 10−2 | 77.6 |
| 1b | 170 | 16 | 2.84 × 10−2 | 77.0 |
| 2a | 160 | 30 | 2.84 × 10−2 | 75.6 |
| 2b | 160 | 30 | 2.84 × 10−2 | 75.4 |
| 3a | 180 | 16 | 1.42 × 10−2 | 78.7 |
| 3b | 180 | 16 | 1.42 × 10−2 | 76.3 |
| 4a | 170 | 2 | 4.26 × 10−2 | 73.8 |
| 4b | 170 | 2 | 4.26 × 10−2 | 73.4 |
| 5a | 160 | 16 | 1.42 × 10−2 | 51.0 |
| 5b | 160 | 16 | 1.42 × 10−2 | 53.3 |
| 6a | 170 | 30 | 1.42 × 10−2 | 77.3 |
| 6b | 170 | 30 | 1.42 × 10−2 | 80.3 |
| 7a | 160 | 16 | 4.26 × 10−2 | 70.7 |
| 7b | 160 | 16 | 4.26 × 10−2 | 74.3 |
| 8a | 180 | 2 | 2.84 × 10−2 | 77.0 |
| 8b | 180 | 2 | 2.84 × 10−2 | 79.4 |
| 9a | 180 | 16 | 4.26 × 10−2 | 72.0 |
| 9b | 180 | 16 | 4.26 × 10−2 | 70.9 |
| 10a | 170 | 2 | 1.42 × 10−2 | 45.5 |
| 10b | 170 | 2 | 1.42 × 10−2 | 44.3 |
| 11a | 160 | 2 | 2.84 × 10−2 | 43.4 |
| 11b | 160 | 2 | 2.84 × 10−2 | 37.8 |
| 12a | 180 | 30 | 2.84 × 10−2 | 72.3 |
| 12b | 180 | 30 | 2.84 × 10−2 | 72.7 |
| 13a | 170 | 30 | 4.26 × 10−2 | 72.0 |
| 13b | 170 | 30 | 4.26 × 10−2 | 73.1 |
| Coefficient | Value | Statistical Significance |
|---|---|---|
| a0 | 77.30 | >99% |
| a1 | 7.36 | >99% |
| a2 | 7.77 | >99% |
| a3 | 4.59 | >99% |
| a12 | −10.15 | >99% |
| a13 | −6.59 | >99% |
| a23 | −8.74 | >99% |
| a11 | −4.84 | >99% |
| a22 | −5.76 | >99% |
| a33 | −4.08 | >99% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López, M.; Rivas, S.; Vila, C.; Santos, V.; Parajó, J.C. Performance of 1-(3-Sulfopropyl)-3-Methylimidazolium Hydrogen Sulfate as a Catalyst for Hardwood Upgrading into Bio-Based Platform Chemicals. Catalysts 2020, 10, 937. https://doi.org/10.3390/catal10080937
López M, Rivas S, Vila C, Santos V, Parajó JC. Performance of 1-(3-Sulfopropyl)-3-Methylimidazolium Hydrogen Sulfate as a Catalyst for Hardwood Upgrading into Bio-Based Platform Chemicals. Catalysts. 2020; 10(8):937. https://doi.org/10.3390/catal10080937
Chicago/Turabian StyleLópez, Mar, Sandra Rivas, Carlos Vila, Valentín Santos, and Juan Carlos Parajó. 2020. "Performance of 1-(3-Sulfopropyl)-3-Methylimidazolium Hydrogen Sulfate as a Catalyst for Hardwood Upgrading into Bio-Based Platform Chemicals" Catalysts 10, no. 8: 937. https://doi.org/10.3390/catal10080937
APA StyleLópez, M., Rivas, S., Vila, C., Santos, V., & Parajó, J. C. (2020). Performance of 1-(3-Sulfopropyl)-3-Methylimidazolium Hydrogen Sulfate as a Catalyst for Hardwood Upgrading into Bio-Based Platform Chemicals. Catalysts, 10(8), 937. https://doi.org/10.3390/catal10080937





