Degradation of Acid Orange 7 Azo Dye in Aqueous Solution by a Catalytic-Assisted, Non-Thermal Plasma Process
Abstract
1. Introduction
2. Results
2.1. Characterization Results
2.1.1. Specific Surface Area (SSA)
2.1.2. SEM Images Results
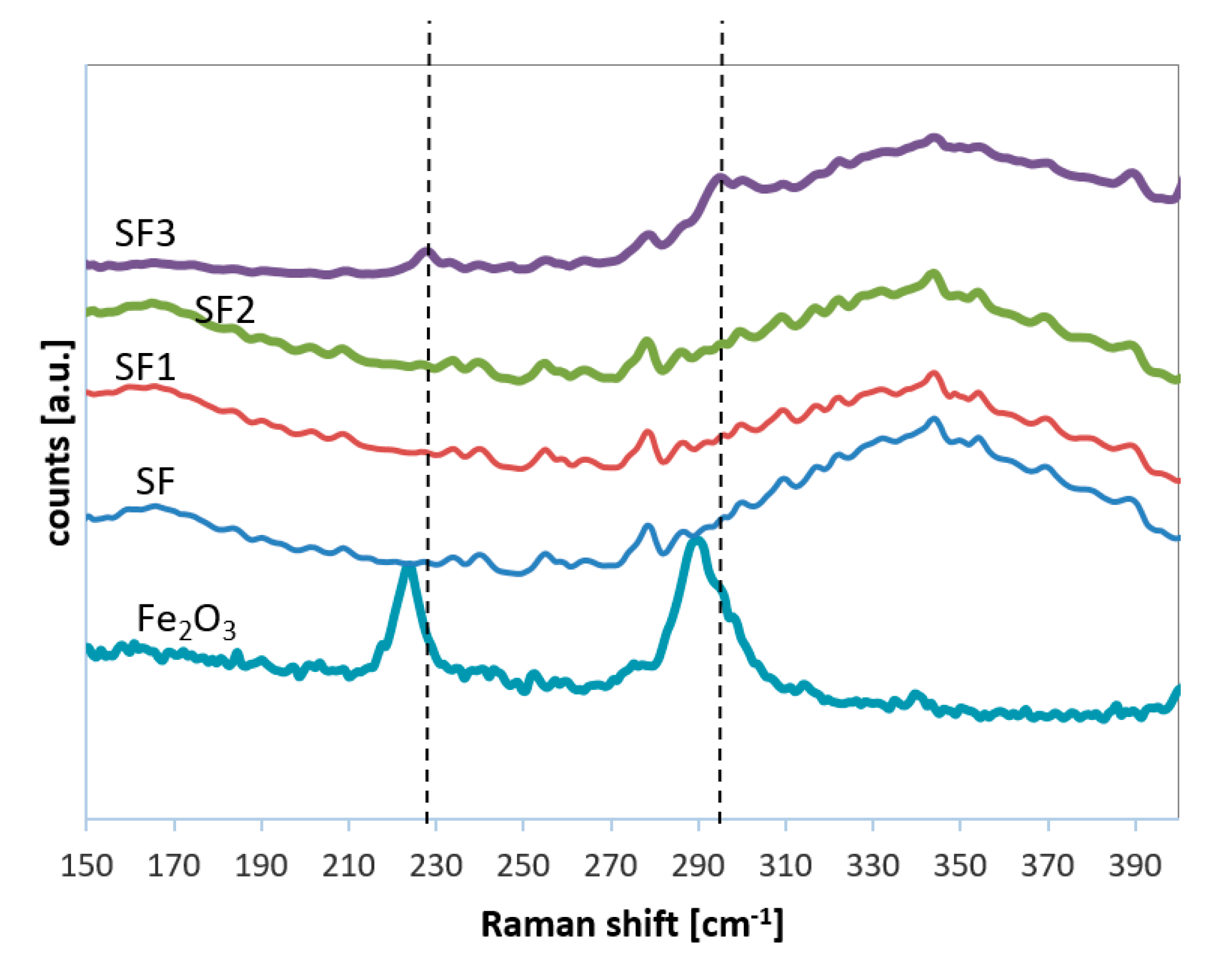
2.1.3. Raman Analysis
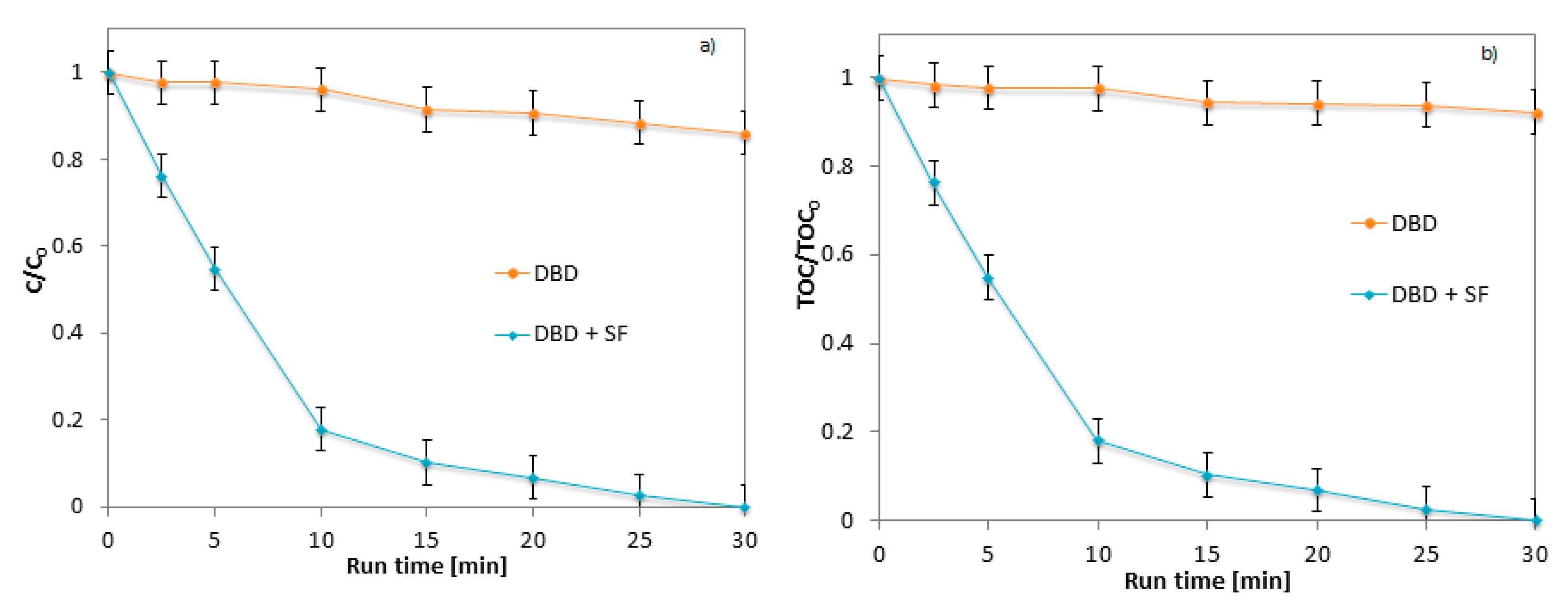
2.2. Influence of Packed Material on DBD Performances
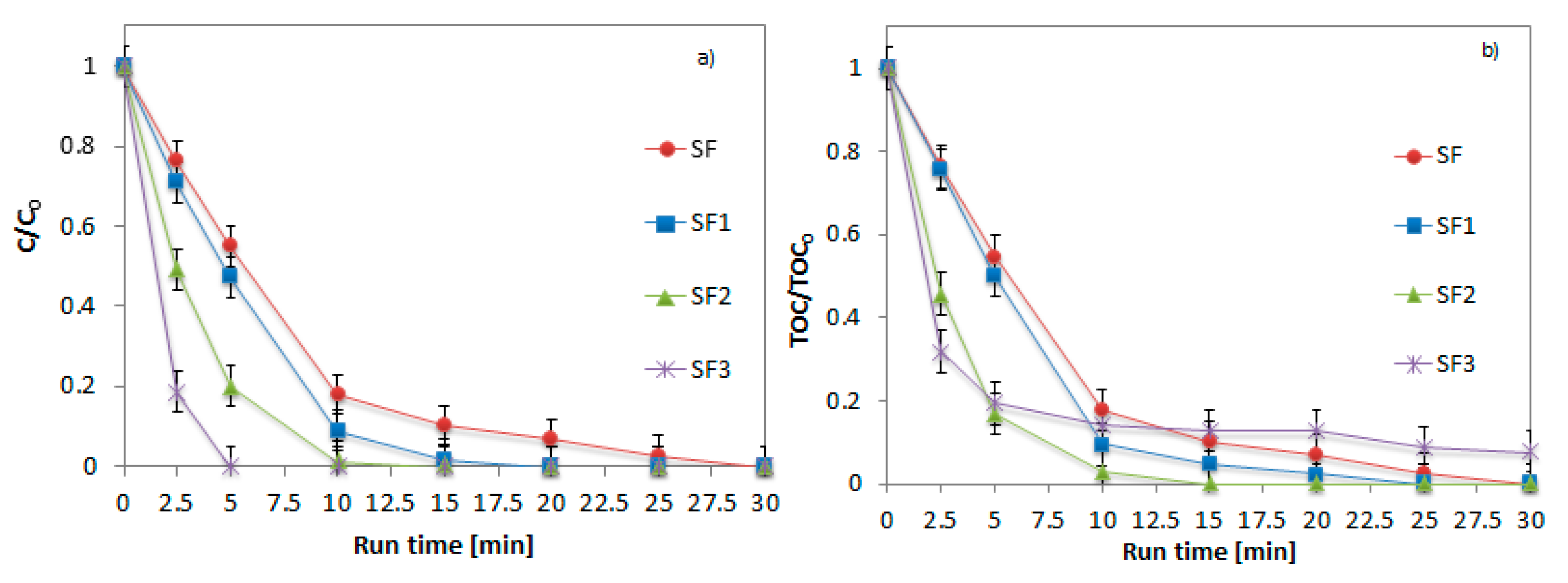
2.3. Effect of Fe2O3 Amount on Glass Spheres Surface
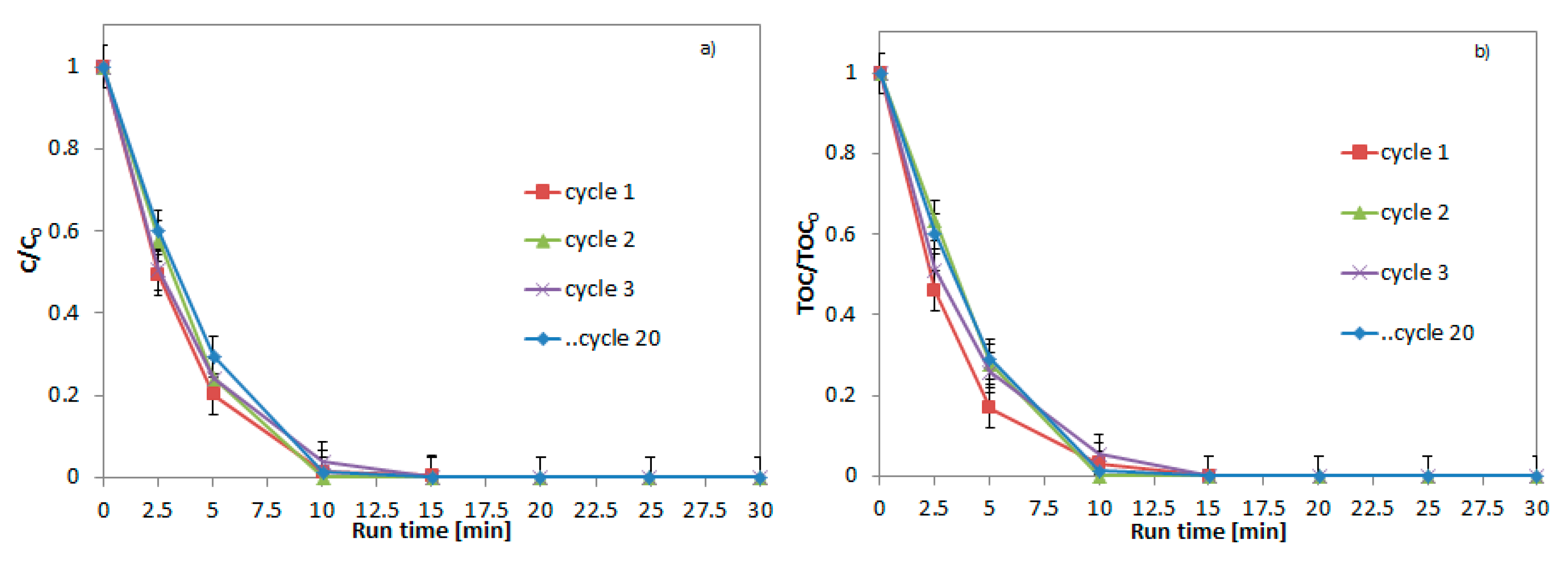
2.4. Recyclability Tests of SF2 Catalyst in DBD Reactor
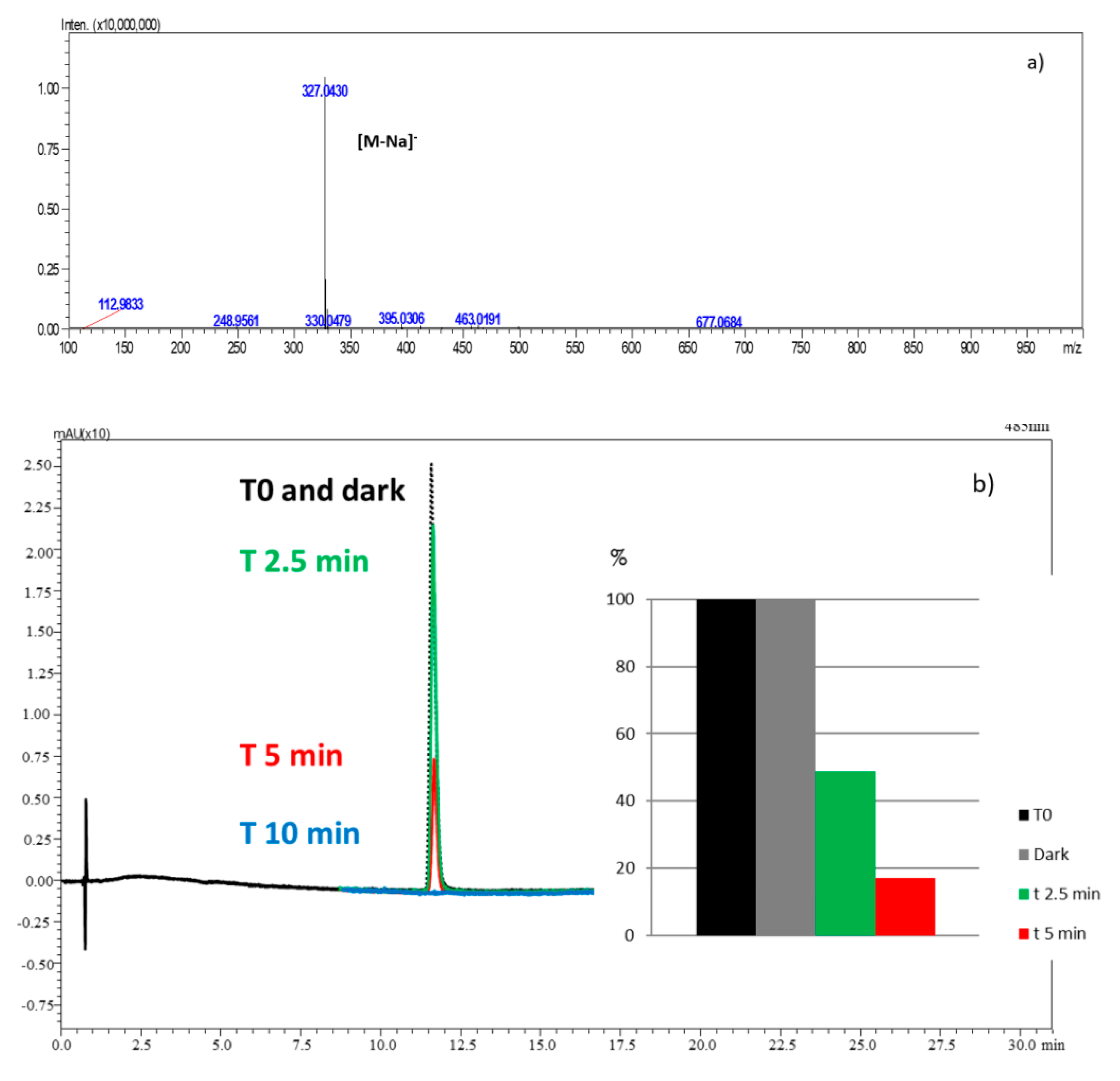
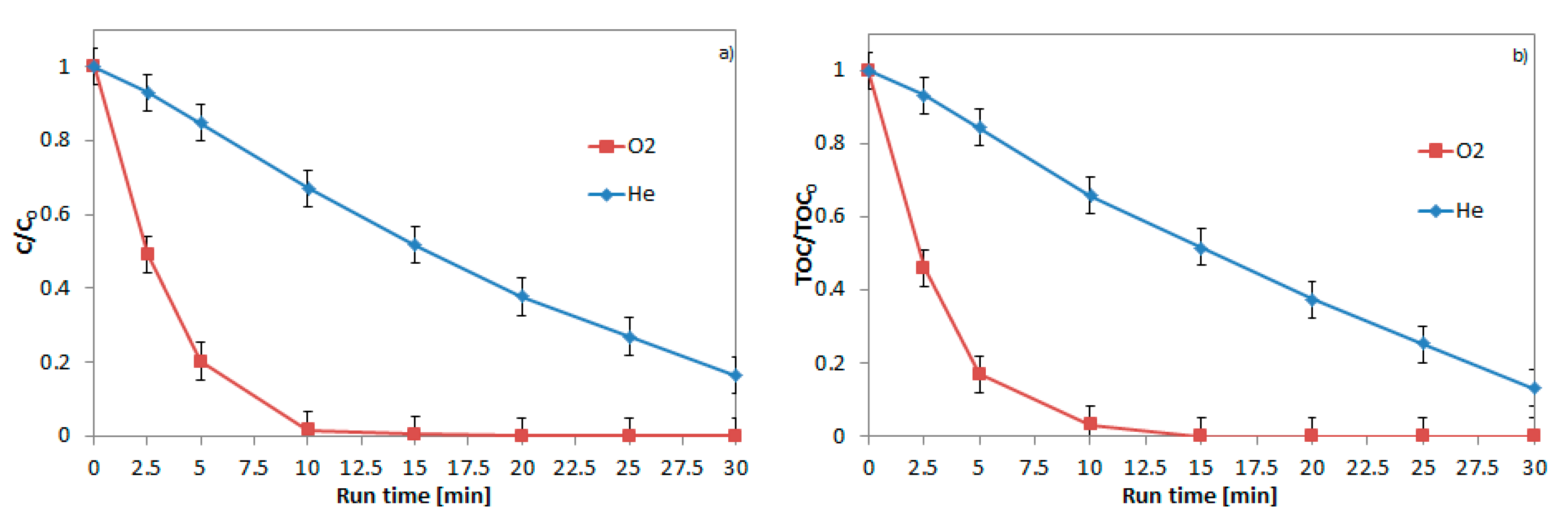
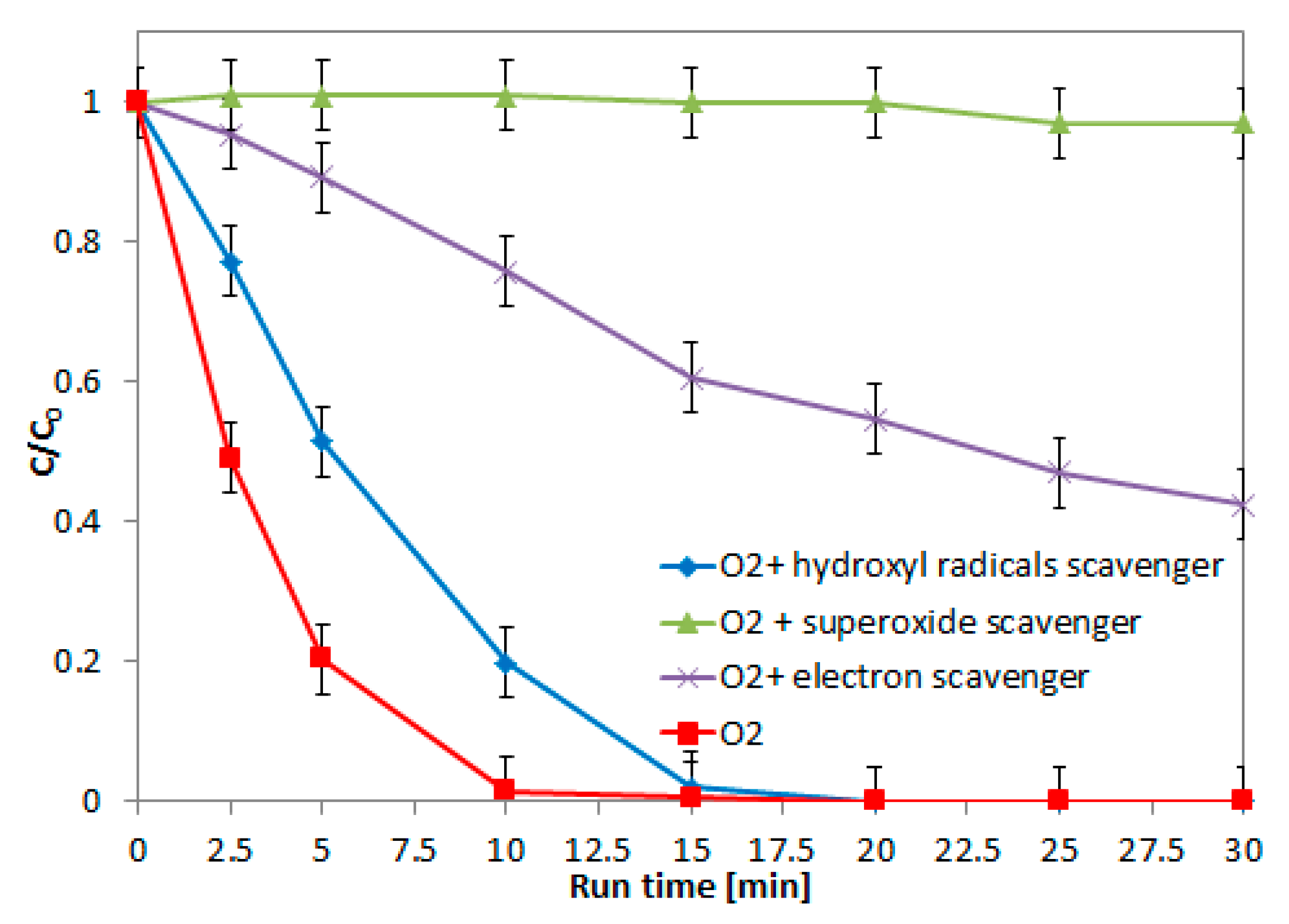
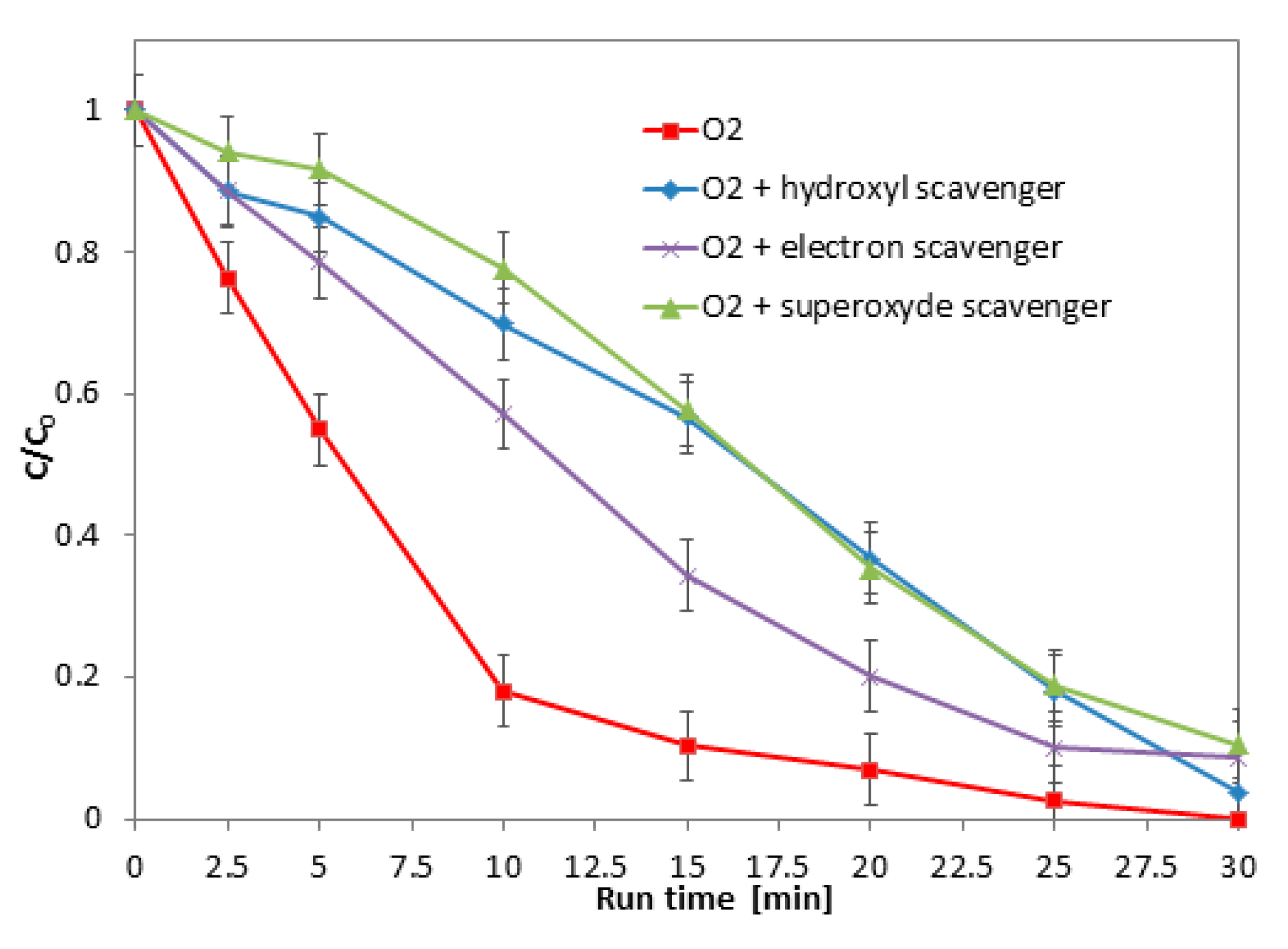
2.5. Role of the Main Oxidizing Species
3. Materials and Methods
3.1. Structured Photocatalysts Preparation
3.2. Characterization Techniques
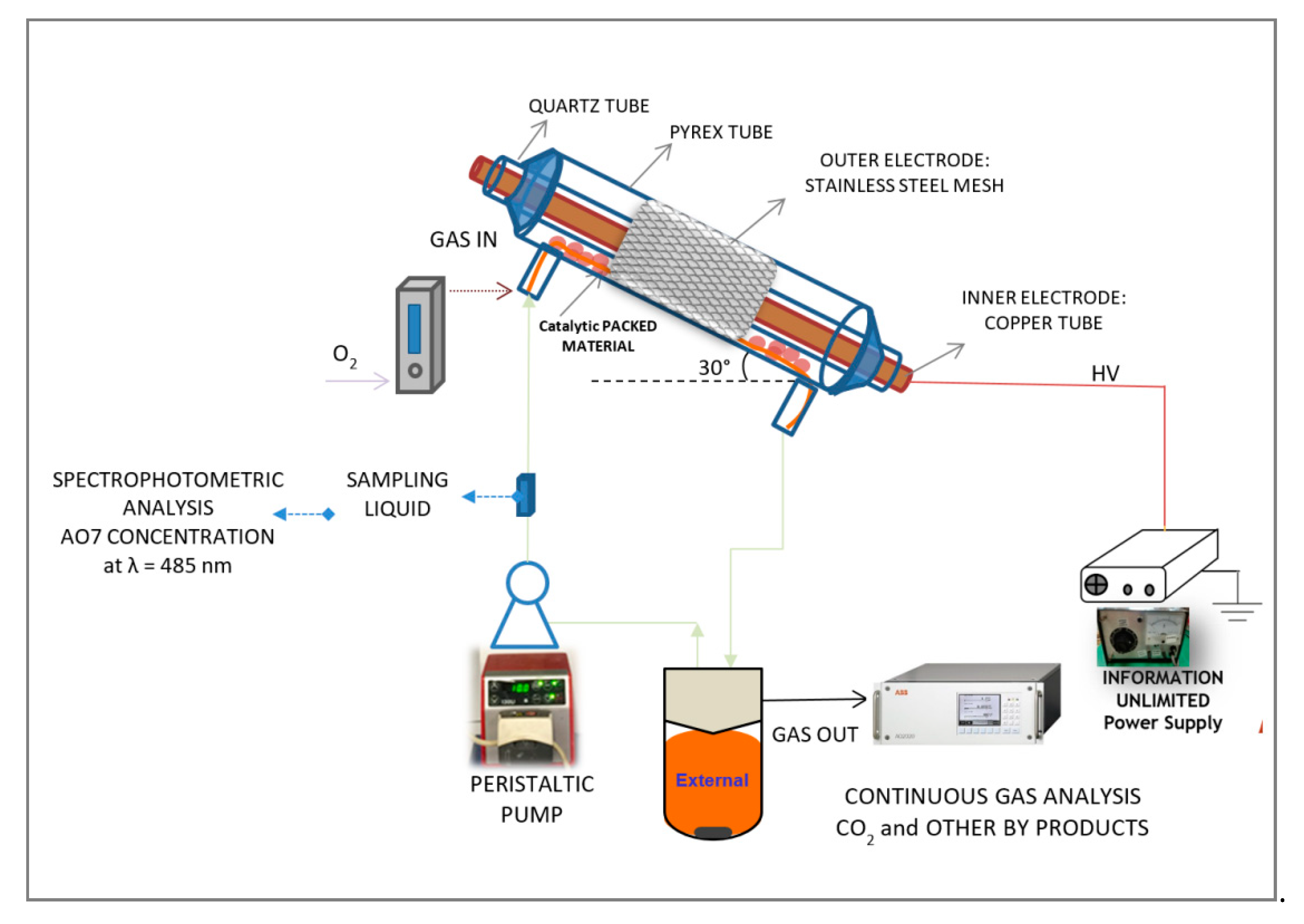
3.3. Experimental Setup and Cold Plasma Tests
3.4. Analytical Measurements
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yaseen, D.; Scholz, M. Textile dye wastewater characteristics and constituents of synthetic effluents: A critical review. Int. J. Environ. Sci. Technol. 2018, 16, 1193–1226. [Google Scholar] [CrossRef]
- Clarke, E.A.; Anliker, R.; Butler, G.C.; Förstner, U.; Funke, W.; Hyslop, C.; Kaiser, G.; Rappe, C.; Russow, J.; Tölg, G.; et al. Organic Dyes and Pigments; Springer Science and Business Media LLC: Berlin, Germany, 1980; pp. 181–215. [Google Scholar]
- Fu, Y.; Viraraghavan, T. Fungal decolorization of dye wastewaters: A review. Bioresour. Technol. 2001, 79, 251–262. [Google Scholar] [CrossRef]
- Du, C.; Shi, T.; Sun, Y.; Zhuang, X. Decolorization of Acid Orange 7 solution by gas–liquid gliding arc discharge plasma. J. Hazard. Mater. 2008, 154, 1192–1197. [Google Scholar] [CrossRef] [PubMed]
- Ramachandra, T.; Ahalya, N.; Kanamadi, R. Biosorption: Techniques and Mechanisms, A Report. Available online: http:/ces.iisc.ernet.in/energy; http/ces.iisc.ernet.in/biodiversity; cestvr@ us. iisc. ernet (accessed on 3 August 2020).
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of various recent wastewater dye removal methods: A review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Vaiano, V.; Iervolino, G.; Rizzo, L.; Sannino, D. Advanced Oxidation Processes for the Removal of Food Dyes in Wastewater. Curr. Org. Chem. 2017, 21, 1068–1073. [Google Scholar] [CrossRef]
- Vaiano, V.; Iervolino, G.; Sannino, D. Photocatalytic removal of tartrazine dye from aqueous samples on LaFeO3/ZnO photocatalysts. Chem. Eng. Trans. 2016, 52, 847–852. [Google Scholar]
- Vaiano, V.; Iervolino, G.; Sannino, D.; Rizzo, L.; Sarno, G.; Ciambelli, P.; Isupova, L.A. Food Azo-Dyes Removal from Water by Heterogeneous Photo-Fenton with LaFeO3 Supported on Honeycomb Corundum Monoliths. J. Environ. Eng. 2015, 141, 04015038. [Google Scholar] [CrossRef]
- Zammit, I.; Vaiano, V.; Iervolino, G.; Rizzo, L. Inactivation of an urban wastewater indigenous Escherichia coli strain by cerium doped zinc oxide photocatalysis. RSC Adv. 2018, 8, 26124–26132. [Google Scholar] [CrossRef]
- Arzate, S.; Pfister, S.; Oberschelp, C.; Sánchez-Pérez, J. Environmental impacts of an advanced oxidation process as tertiary treatment in a wastewater treatment plant. Sci. Total. Environ. 2019, 694, 133572. [Google Scholar] [CrossRef]
- Mandal, T.; Maity, S.; Dasgupta, D.; Datta, S. Advanced oxidation process and biotreatment: Their roles in combined industrial wastewater treatment. Desalination 2010, 250, 87–94. [Google Scholar] [CrossRef]
- Suhaimy, S.H.M.; Hamid, S.B.A.; Lai, C.W.; Hasan, R.; Johan, M.R. TiO2 Nanotubes Supported Cu Nanoparticles for Improving Photocatalytic Degradation of Simazine under UV Illumination. Catalysts 2016, 6, 167. [Google Scholar] [CrossRef]
- Zuorro, A.; Lavecchia, R.; Monaco, M.M.; Iervolino, G.; Vaiano, V. Photocatalytic Degradation of Azo Dye Reactive Violet 5 on Fe-Doped Titania Catalysts under Visible Light Irradiation. Catalysts 2019, 9, 645. [Google Scholar] [CrossRef]
- Khurana, P.; Thatai, S.; Kumar, D. Chapter 6—Destruction of recalcitrant nanomaterials contaminants in industrial wastewater. In Emerging and Nanomaterial Contaminants in Wastewater; Mishra, A.K., Anawar, H.M.D., Drouiche, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 137–158. [Google Scholar]
- Liao, X.; Liu, N.; Chen, S.; Ye, X.; Ding, T. Degradation of antibiotic resistance contaminants in wastewater by atmospheric cold plasma: Kinetics and mechanisms. Environ. Technol. 2019, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-N.; Xu, H.; Zhu, S.-F.; Zhou, M.; Miao, J. Enhanced Degradation of Acid Orange 7 Solution by Non-thermal Plasma Discharge with TiO2. Plasma Chem. Plasma Process. 2014, 34, 1403–1413. [Google Scholar] [CrossRef]
- Schiavon, M.; Schiorlin, M.; Torretta, V.; Brandenburg, R.; Ragazzi, M. Non-thermal plasma assisting the biofiltration of volatile organic compounds. J. Clean. Prod. 2017, 148, 498–508. [Google Scholar] [CrossRef]
- Iervolino, G.; Sacco, O.; Vaiano, V.; Palma, V. Non-thermal plasma technology for the effective regeneration of macroscopic adsorbent materials used in the removal of patent blue V dye from aqueous solutions. Chem. Eng. Trans. 2019, 73, 151–156. [Google Scholar]
- Iervolino, G.; Vaiano, V.; Palma, V. Enhanced azo dye removal in aqueous solution by H2O2 assisted non-thermal plasma technology. Environ. Technol. Innov. 2020, 19, 100969. [Google Scholar] [CrossRef]
- Zhou, L.; Ma, C.; Horlyck, J.; Liu, R.; Yun, J. Development of Pharmaceutical VOCs Elimination by Catalytic Processes in China. Catalysts 2020, 10, 668. [Google Scholar] [CrossRef]
- Guo, X.; Ha, K.H.; Du, D. New Experiment of Diesel Exhaust Treatment by Atmospheric Pressure Plasma–Wood Fiber Combination. Catalysts 2020, 10, 577. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Nguyen, D.B.; Heo, I.; Mok, Y.S. Plasma-Assisted Selective Catalytic Reduction for Low-Temperature Removal of NOx and Soot Simulant. Catalysts 2019, 9, 853. [Google Scholar] [CrossRef]
- Ajo, P.; Kornev, I.; Preis, S. Pulsed Corona Discharge in Water Treatment: The Effect of Hydrodynamic Conditions on Oxidation Energy Efficiency. Ind. Eng. Chem. Res. 2015, 54, 7452–7458. [Google Scholar] [CrossRef]
- Rezaei, F.; Vanraes, P.; Nikiforov, A.; Morent, R.; De Geyter, N. Applications of Plasma-Liquid Systems: A Review. Materials 2019, 12, 2751. [Google Scholar] [CrossRef]
- Chen, J.; Du, Y.; Shen, Z.; Lu, S.; Su, K.; Yuan, S.; Hu, Z.; Zhang, A.; Feng, J. Non-thermal plasma and BiPO4 induced degradation of aqueous crystal violet. Sep. Purif. Technol. 2017, 179, 135–144. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, G.; Liu, X.; Phan, A.N.; Luo, K. A Study on CO2 Decomposition to CO and O2 by the Combination of Catalysis and Dielectric-Barrier Discharges at Low Temperatures and Ambient Pressure. Ind. Eng. Chem. Res. 2017, 56, 3204–3216. [Google Scholar] [CrossRef]
- Neyts, E.C.; Ostrikov, K.; Sunkara, M.K.; Bogaerts, A. Plasma Catalysis: Synergistic Effects at the Nanoscale. Chem. Rev. 2015, 115, 13408–13446. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ma, C.; Zhu, S.; Yu, F.; Dai, B.; Yang, D. A Review of Recent Advances of Dielectric Barrier Discharge Plasma in Catalysis. Nanomaterials 2019, 9, 1428. [Google Scholar] [CrossRef] [PubMed]
- Veerapandian, S.K.P.; De Geyter, N.; Giraudon, J.-M.; Lamonier, J.-F.; Morent, R. The Use of Zeolites for VOCs Abatement by Combining Non-Thermal Plasma, Adsorption, and/or Catalysis: A Review. Catalysts 2019, 9, 98. [Google Scholar] [CrossRef]
- Trinh, Q.H.; Mok, Y.S. Non-Thermal Plasma Combined with Cordierite-Supported Mn and Fe Based Catalysts for the Decomposition of Diethylether. Catalysts 2015, 5, 800–814. [Google Scholar] [CrossRef]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A review of ZnO nanoparticles as solar photocatalysts: Synthesis, mechanisms and applications. Renew. Sust. Energ. Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Pekarek, S.; Mikeš, J.; Krýsa, J. Comparative study of TiO2 and ZnO photocatalysts for the enhancement of ozone generation by surface dielectric barrier discharge in air. Appl. Catal. A: Gen. 2015, 502, 122–128. [Google Scholar] [CrossRef]
- Nian, P.; Peng, L.; Feng, J.; Han, X.; Cui, B.; Lu, S.; Zhang, J.; Liu, Q.; Zhang, A. Aqueous methylparaben degradation by dielectric barrier discharge induced non-thermal plasma combined with ZnO-rGO nanosheets. Sep. Purif. Technol. 2019, 211, 832–842. [Google Scholar] [CrossRef]
- Reddy, P.M.K.; Dayamani, A.; Mahammadunnisa, S.; Subrahmanyam, C. Mineralization of Phenol in Water by Catalytic Non-T hermal Plasma Reactor–An Eco-F riendly Approach for Wastewater Treatment. Plasma Process. Polym. 2013, 10, 1010–1017. [Google Scholar] [CrossRef]
- Wang, J.; Sun, Y.; Jiang, H.; Feng, J. Removal of caffeine from water by combining dielectric barrier discharge (DBD) plasma with goethite. J. Saudi Chem. Soc. 2017, 21, 545–557. [Google Scholar] [CrossRef]
- Jović, M.; Dojčinović, B.; Kovačević, V.V.; Obradović, B.M.; Kuraica, M.M.; Gašić, U.M.; Roglic, G. Effect of different catalysts on mesotrione degradation in water falling film DBD reactor. Chem. Eng. J. 2014, 248, 63–70. [Google Scholar] [CrossRef]
- Fahmy, A.; El-Zomrawy, A.; Saeed, A.M.; Sayed, A.Z.; El-Arab, M.A.E.; Shehata, H.A. Modeling and optimizing Acid Orange 142 degradation in aqueous solution by non-thermal plasma. Chemosphere 2018, 210, 102–109. [Google Scholar] [CrossRef]
- Liu, H.; Du, C.M.; Wang, J.; Li, H.X.; Zhang, L.L. Comparison of Acid Orange 7 Degradation in Solution by Gliding Arc Discharge with Different Forms of TiO2. Plasma Process. Polym. 2012, 9, 285–297. [Google Scholar] [CrossRef]
- Reddy, P.M.K.; Raju, B.R.; Karuppiah, J.; Reddy, E.L.; Subrahmanyam, C. Degradation and mineralization of methylene blue by dielectric barrier discharge non-thermal plasma reactor. Chem. Eng. J. 2013, 217, 41–47. [Google Scholar] [CrossRef]
- Van Durme, J.; Dewulf, J.; Leys, C.; Van Langenhove, H. Combining non-thermal plasma with heterogeneous catalysis in waste gas treatment: A review. Appl. Catal. B: Environ. 2008, 78, 324–333. [Google Scholar] [CrossRef]
- Mahadik, M.; Shinde, S.; Mohite, V.; Kumbhar, S.; Moholkar, A.; Rajpure, K.; Ganesan, V.; Nayak, J.; Barman, S.; Bhosale, C.H. Visible light catalysis of rhodamine B using nanostructured Fe2O3, TiO2 and TiO2/Fe2O3 thin films. J. Photochem. Photobiol. B Boil. 2014, 133, 90–98. [Google Scholar] [CrossRef]
- Serrano, A.; Rubio-Zuazo, J.; Sánchez, J.L.; Arnay, I.; Colera, E.S.; Castro, G.R. Stabilization of Epitaxial α-Fe2O3 Thin Films Grown by Pulsed Laser Deposition on Oxide Substrates. J. Phys. Chem. C 2018, 122, 16042–16047. [Google Scholar] [CrossRef]
- Ray, D.; Subrahmanyam, C. CO2 decomposition in a packed DBD plasma reactor: Influence of packing materials. RSC Adv. 2016, 6, 39492–39499. [Google Scholar] [CrossRef]
- Mei, D.; Zhu, X.; He, Y.-L.; Yan, J.D.; Tu, X. Plasma-assisted conversion of CO2 in a dielectric barrier discharge reactor: Understanding the effect of packing materials. Plasma Sources Sci. Technol. 2014, 24, 15011. [Google Scholar] [CrossRef]
- Yap, D.; Tatibouët, J.-M.; Batiot-Dupeyrat, C. Carbon dioxide dissociation to carbon monoxide by non-thermal plasma. J. CO2 Util. 2015, 12, 54–61. [Google Scholar] [CrossRef]
- Wu, L.; Xie, Q.; Lv, Y.; Zhang, Z.; Wu, Z.; Liang, X.; Lu, M.; Nie, Y. Degradation of methylene blue by dielectric barrier discharge plasma coupled with activated carbon supported on polyurethane foam. RSC Adv. 2019, 9, 25967–25975. [Google Scholar] [CrossRef]
- Gao, B.; Liu, L.; Liu, J.; Yang, F. Photocatalytic degradation of 2,4,6-tribromophenol on Fe2O3 or FeOOH doped ZnIn2S4 heterostructure: Insight into degradation mechanism. Appl. Catal. B: Environ. 2014, 147, 929–939. [Google Scholar] [CrossRef]
- Vaiano, V.; Sacco, O.; Sannino, D.; Ciambelli, P. Nanostructured N-doped TiO2 coated on glass spheres for the photocatalytic removal of organic dyes under UV or visible light irradiation. Appl. Catal. B Environ. 2015, 170, 153–161. [Google Scholar] [CrossRef]
- Vaiano, V.; Iervolino, G. Facile method to immobilize ZnO particles on glass spheres for the photocatalytic treatment of tannery wastewater. J. Colloid Interface Sci. 2018, 518, 192–199. [Google Scholar] [CrossRef]
- Iervolino, G.; Vaiano, V.; Palma, V. Enhanced removal of water pollutants by dielectric barrier discharge non-thermal plasma reactor. Sep. Purif. Technol. 2019, 215, 155–162. [Google Scholar] [CrossRef]

| Sample | Number of Dip-Coating Steps | Fe2O3 Amount on Glass Spheres (wt.%) | SSA * (m2/g) |
|---|---|---|---|
| SF | 0 | - | 0.078 |
| SF1 | 1 | 0.13 | 0.10 |
| SF2 | 2 | 0.25 | 0.12 |
| SF2 | 3 | 0.37 | 0.14 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iervolino, G.; Vaiano, V.; Pepe, G.; Campiglia, P.; Palma, V. Degradation of Acid Orange 7 Azo Dye in Aqueous Solution by a Catalytic-Assisted, Non-Thermal Plasma Process. Catalysts 2020, 10, 888. https://doi.org/10.3390/catal10080888
Iervolino G, Vaiano V, Pepe G, Campiglia P, Palma V. Degradation of Acid Orange 7 Azo Dye in Aqueous Solution by a Catalytic-Assisted, Non-Thermal Plasma Process. Catalysts. 2020; 10(8):888. https://doi.org/10.3390/catal10080888
Chicago/Turabian StyleIervolino, Giuseppina, Vincenzo Vaiano, Giacomo Pepe, Pietro Campiglia, and Vincenzo Palma. 2020. "Degradation of Acid Orange 7 Azo Dye in Aqueous Solution by a Catalytic-Assisted, Non-Thermal Plasma Process" Catalysts 10, no. 8: 888. https://doi.org/10.3390/catal10080888
APA StyleIervolino, G., Vaiano, V., Pepe, G., Campiglia, P., & Palma, V. (2020). Degradation of Acid Orange 7 Azo Dye in Aqueous Solution by a Catalytic-Assisted, Non-Thermal Plasma Process. Catalysts, 10(8), 888. https://doi.org/10.3390/catal10080888









