Feasibility Study of Plasma-Catalytic Ammonia Synthesis for Energy Storage Applications
Abstract
1. Introduction
1.1. State of the Art of Plasma-Catalytic Ammonia Synthesis
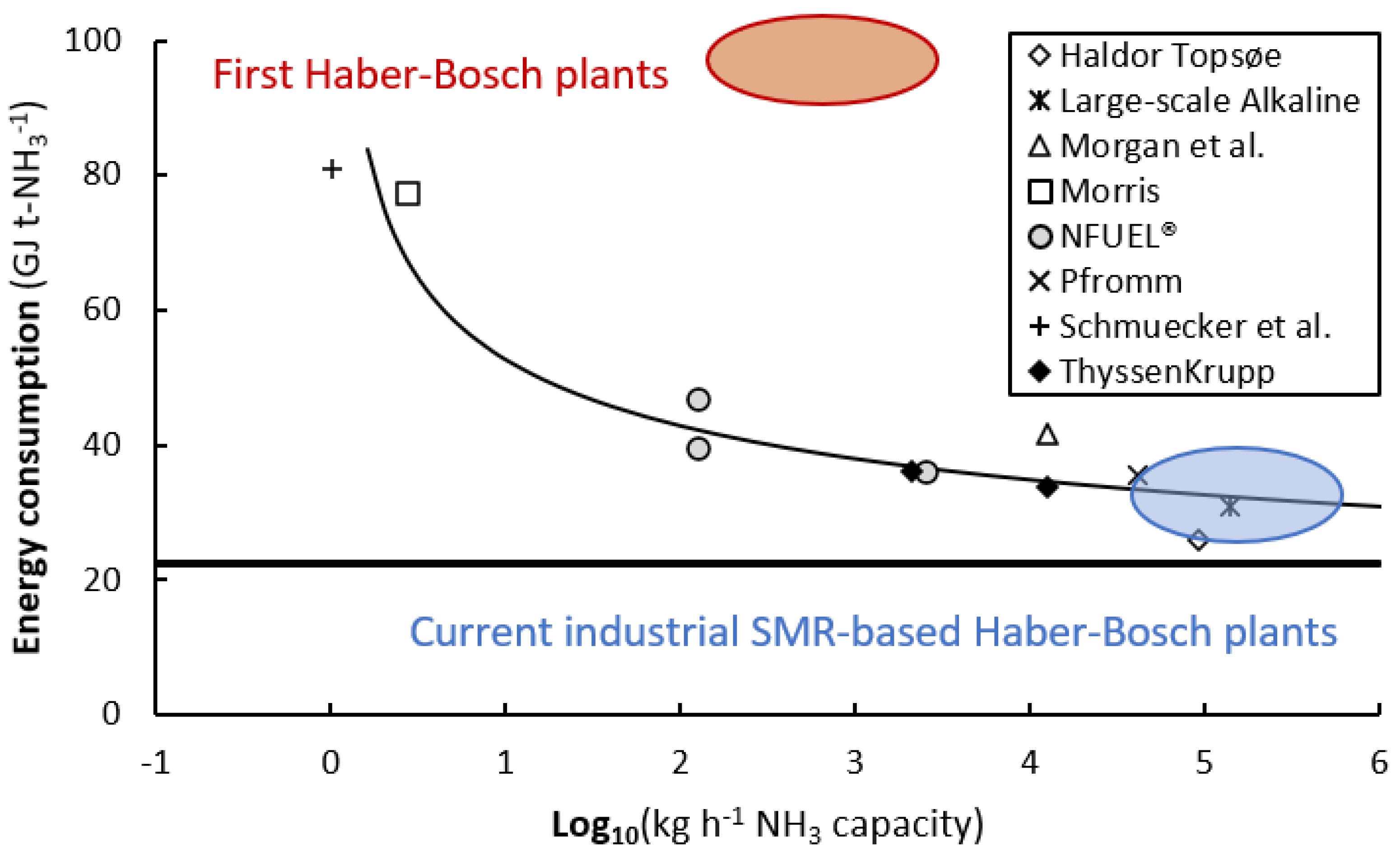
1.2. Comparison of the Small-Scale Haber-Bosch Processes and State-of-the-Art Plasma Catalysis
1.2.1. Targets and Strategies for More Energy-Efficient Plasma-Catalytic Ammonia Synthesis
2. Plasma Reactor and Catalyst Improvements to Plasma-Catalytic Ammonia Synthesis
2.1. Plasma Reactor Type
Plasma Optimization
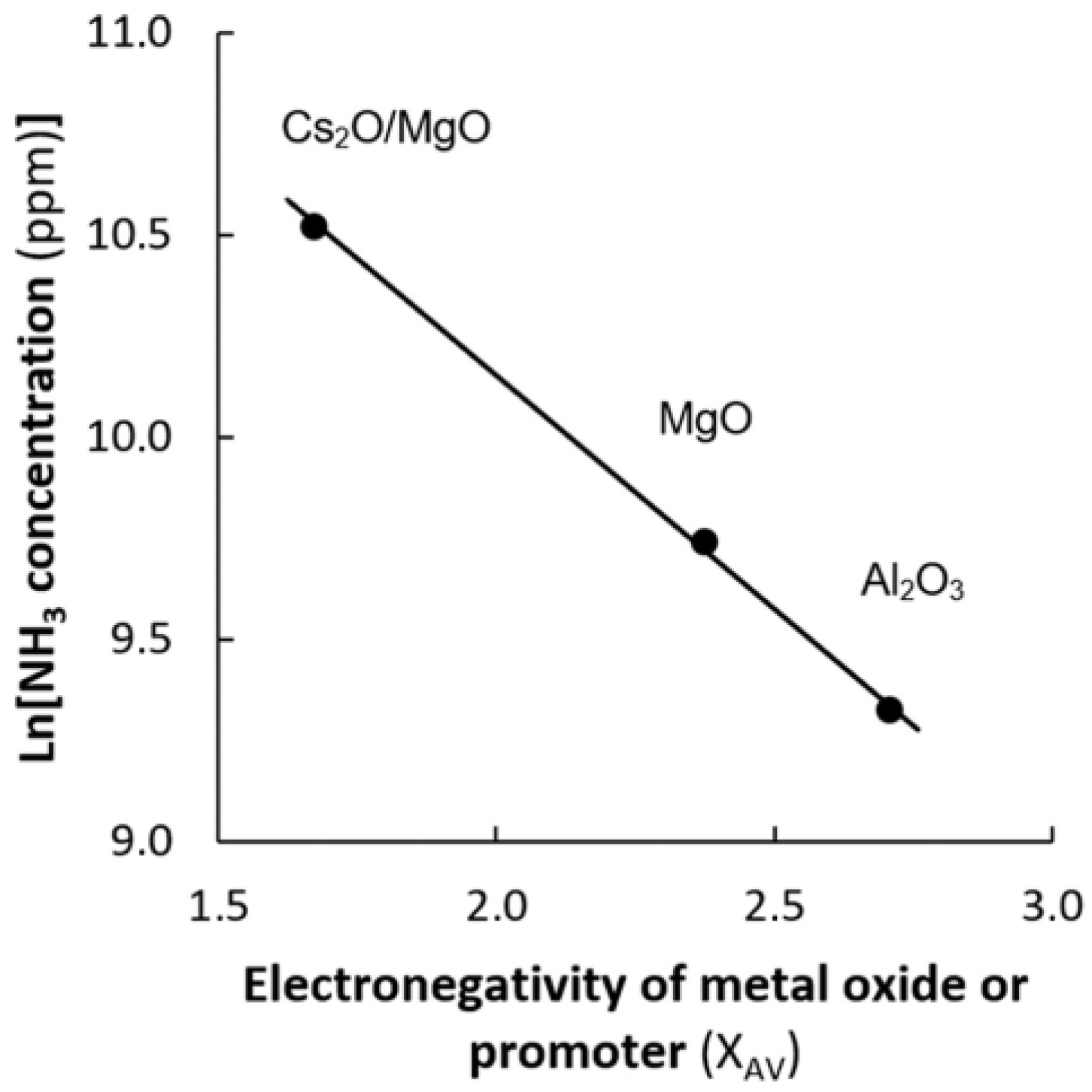
2.2. Reaction Mechanisms and Catalyst Optimization
2.2.1. Plasma-Catalytic Ammonia Synthesis with Molecular Species
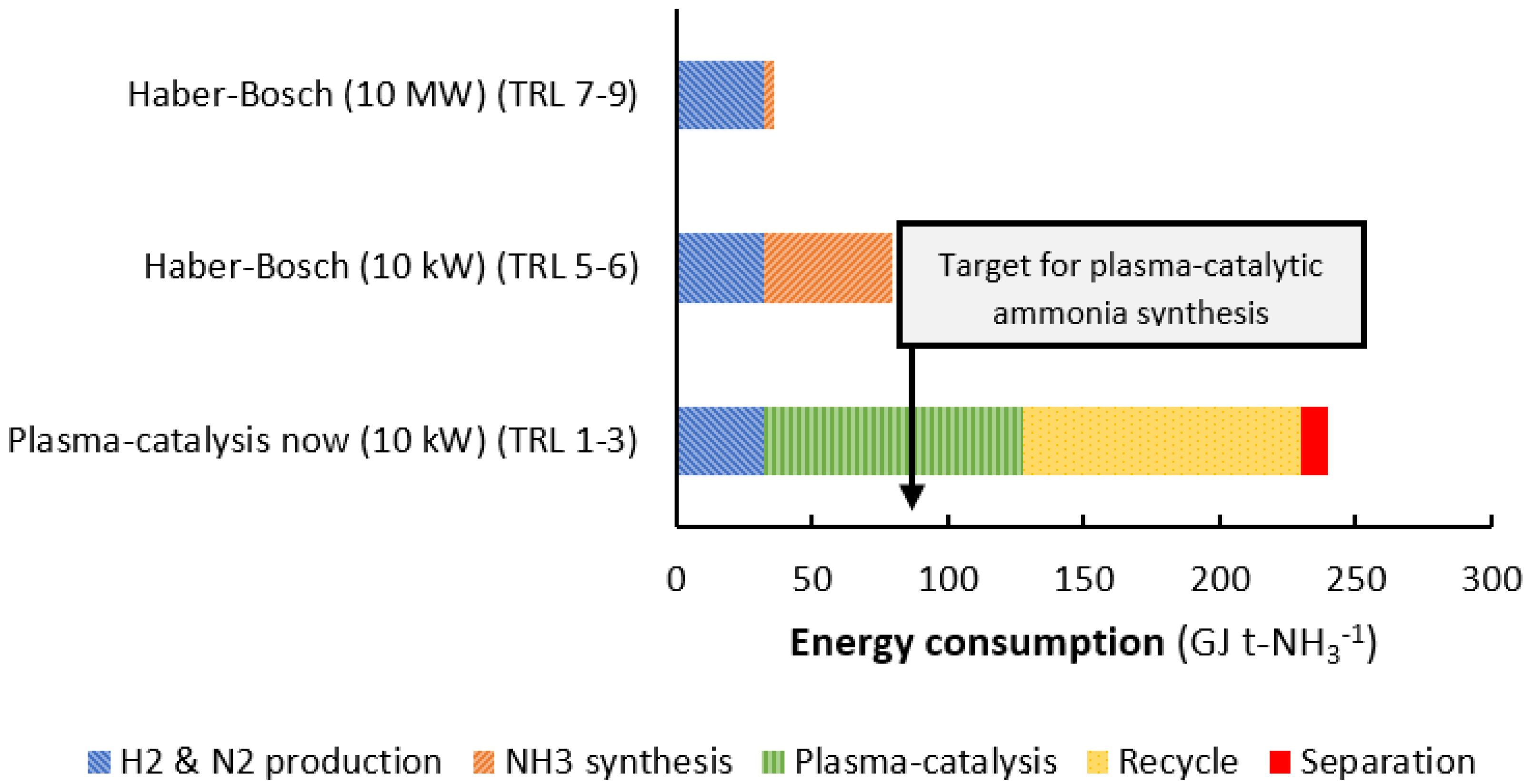
2.2.2. Best-Case Scenario for Plasma Catalysis
3. Ammonia Separation and Conceptual Process Design
3.1. Separation and Storage
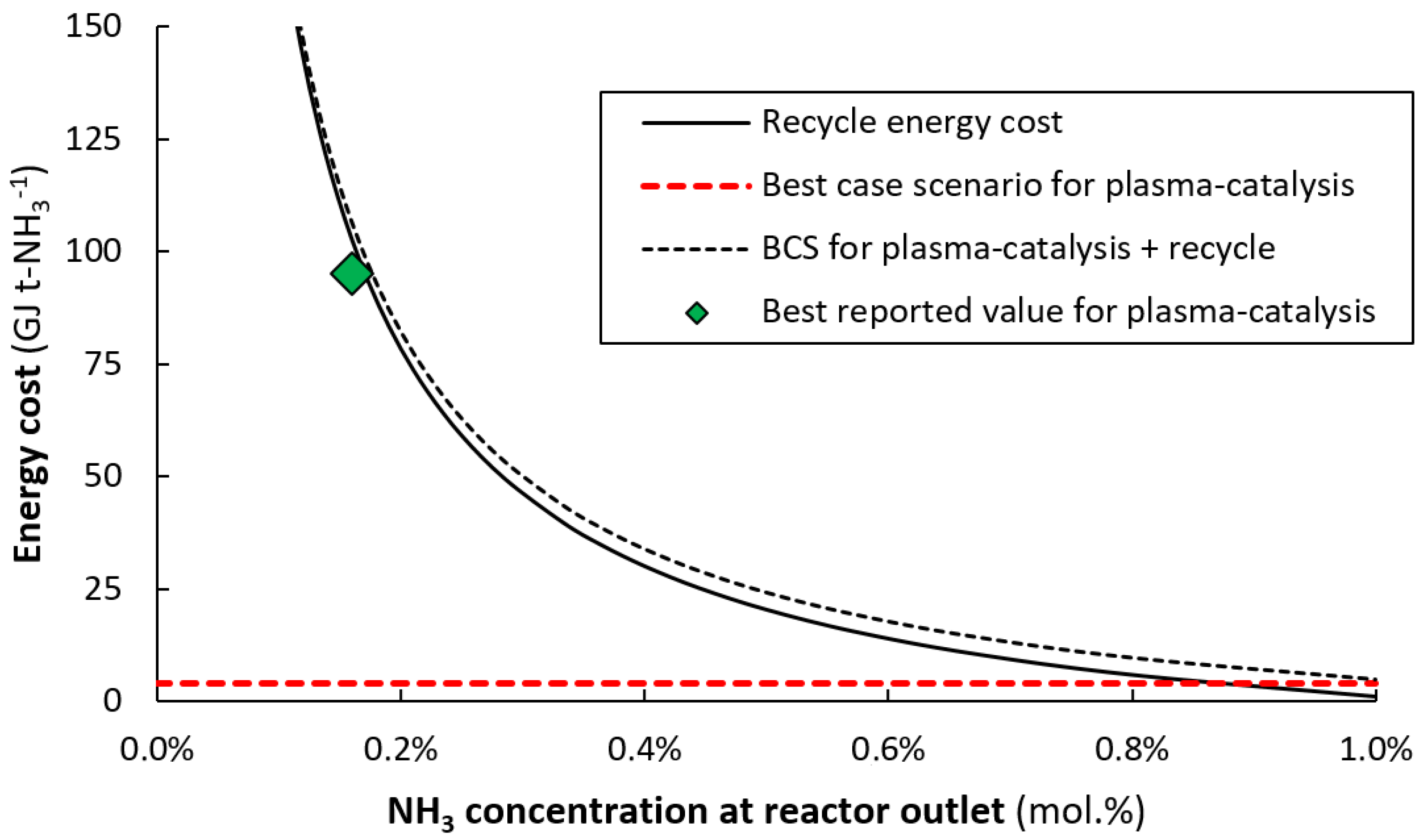
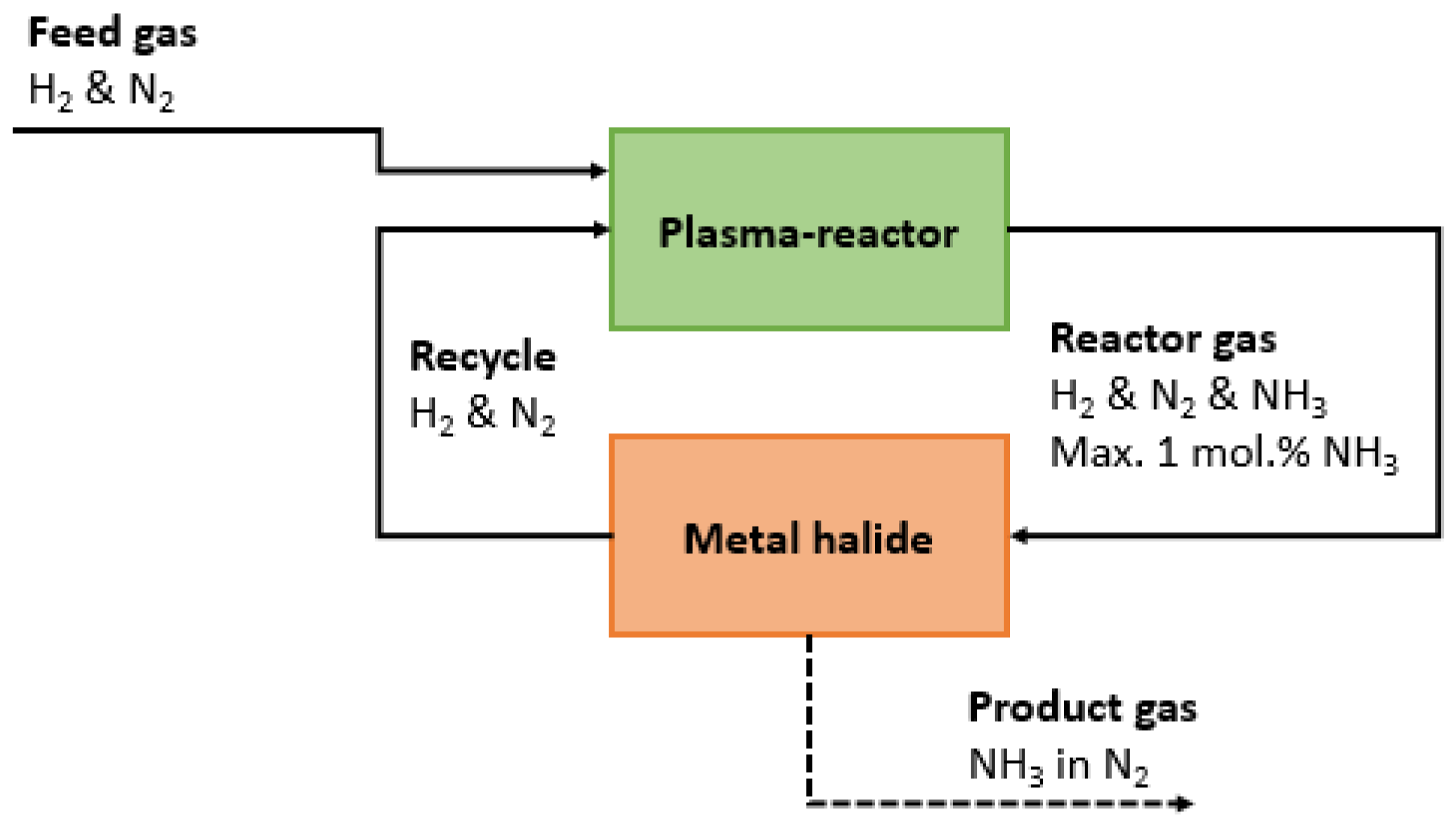
3.2. Synergy between Plasma Reactor and Ammonia Separation and Storage
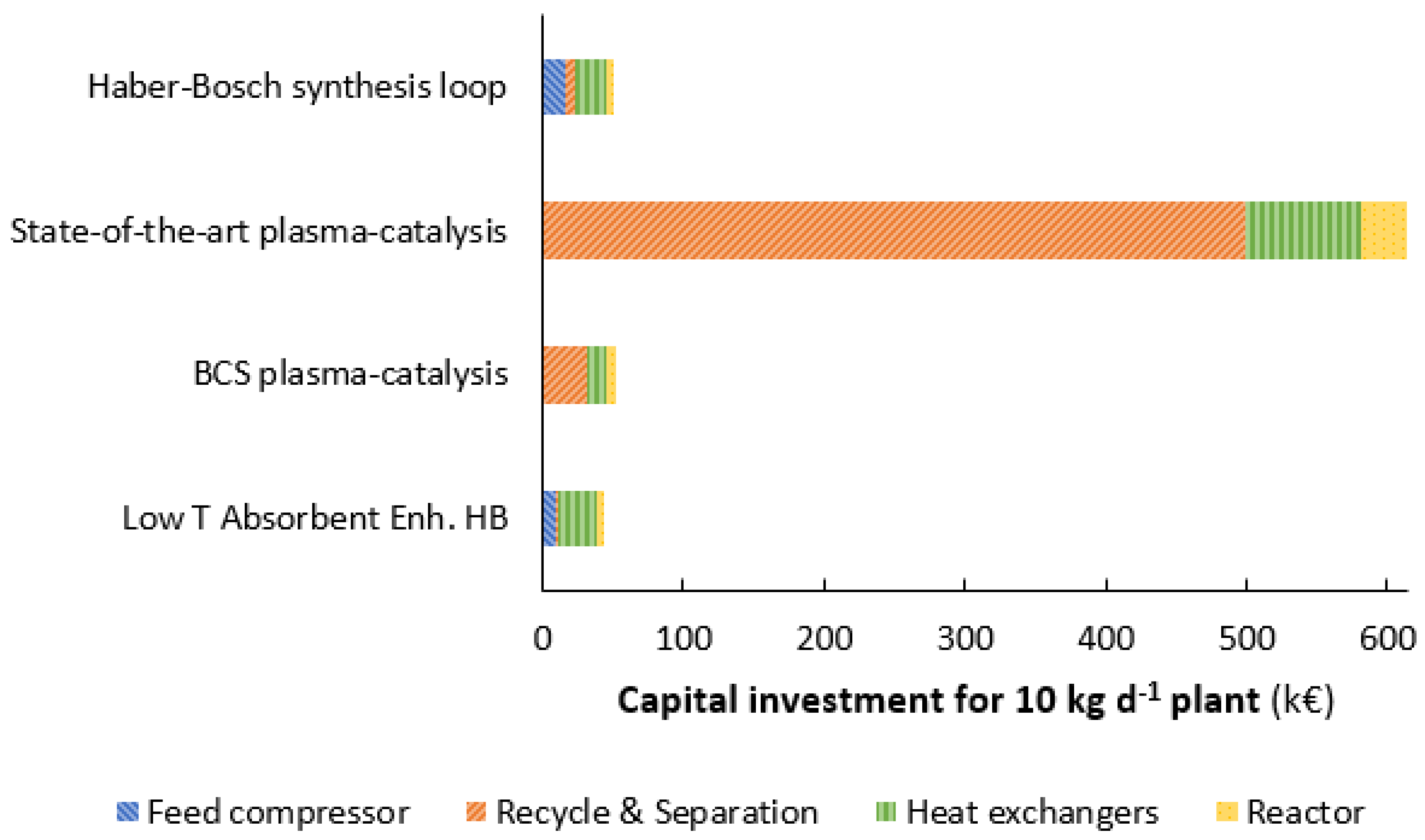
3.3. Investment Cost Comparison
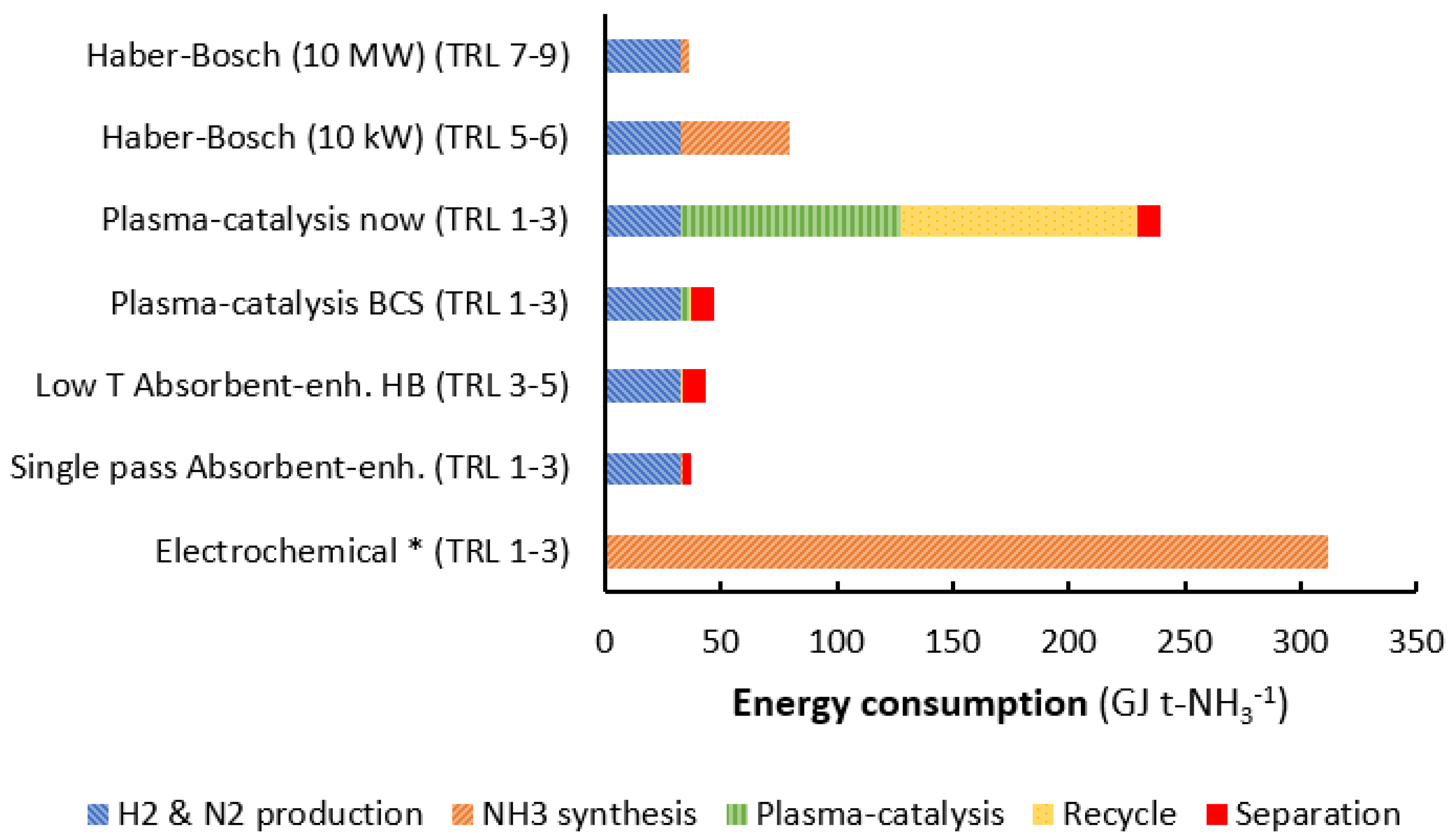
4. Plasma-Catalytic Ammonia Synthesis in Perspective
5. Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Van Geem, K.M.; Galvita, V.V.; Marin, G.B. Making chemicals with electricity. Science 2019, 364, 734–735. [Google Scholar] [CrossRef] [PubMed]
- Simon, E. Green ammonia. In Proceedings of the REFUEL Kickoff Meeting, Denver, CO, USA, 17–18 August 2017; Available online: https://arpa-e.energy.gov/sites/default/files/04cDenver-GreenAmmonia-Siemens-final.pdf (accessed on 1 September 2019).
- Valera-Medina, A.; Xiao, H.; Owen-Jones, M.; David, W.I.F.; Bowen, P. Ammonia for power. Prog. Energy Combust. Sci. 2018, 69, 63–102. [Google Scholar] [CrossRef]
- Hunt, J.D.; Byers, E.; Wada, Y.; Parkinson, S.; Gernaat, D.E.H.J.; Langan, S.J.; Van Vuuren, D.P.; Riahi, K. Global resource potential of seasonal pumped hydropower storage for energy and water storage. Nat. Commun. 2020, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Chen, P. Catalyst: NH3 as an Energy Carrier. Chem 2017, 3, 709–712. [Google Scholar] [CrossRef]
- Nielsen, A. Ammonia: Catalysis and Manufacture, 1st ed.; Nielsen, A., Ed.; Springer: Berlin/Heidelberg, Germany, 1995. [Google Scholar]
- Liu, H. Ruthenium Based Ammonia Synthesis Catalysts; World Scientific Pub Co Pte Ltd.: Singapore, 2013; pp. 425–542. [Google Scholar]
- Jennings, J.M. Catalytic Ammonia Synthesis: Fundamentals and Practice, 1st ed.; Plenum Press: New York, NY, USA, 1991. [Google Scholar]
- Avery, W. A role for ammonia in the hydrogen economy. Int. J. Hydrogen Energy 1988, 13, 761–773. [Google Scholar] [CrossRef]
- Christensen, C.H.; Johannessen, T.; Sørensen, R.Z.; Nørskov, J.K. Towards an ammonia-mediated hydrogen economy? Catal. Today 2006, 111, 140–144. [Google Scholar] [CrossRef]
- Appl, M. Ammonia, 2. Production Processes. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Hoboken, NJ, USA, 2012. [Google Scholar] [CrossRef]
- European Fertilizer Manufacturers’ Association. Production of Ammonia. Best Available Techniques for Pollution Prevention and Control in the European Fertilizer Industry; European Fertilizer Manufacturers’ Association: Brussels, Belgium, 2000; Available online: https://www.ocinitrogen.com/MediaLibrary/Ammoniaprocess—BATProductionofammonia(2000)—Brochure.pdf (accessed on 1 September 2019).
- Brightling, J. Ammonia and the Fertiliser Industry: The Development of Ammonia at Billingham. Johns. Matthey Technol. Rev. 2018, 62, 32–47. [Google Scholar] [CrossRef]
- Rouwenhorst, K.H.R.; Krzywda, P.M.; Benes, N.E.; Mul, G.; Lefferts, L. Ammonia, 4. Green Ammonia Production. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Hoboken, NJ, USA, 2012. [Google Scholar] [CrossRef]
- Proton Ventures, B.V. Sustainable ammonia for food and power. In Proceedings of the Nitrogen + Syngas, Gotheburg, Sweden, 6 February–1 March 2018; pp. 1–10. Available online: http://www.protonventures.com/wp-content/uploads/2018/09/NS-354-Small-scale-plant-design-PROTON-VENTURES-3-1.pdf (accessed on 1 September 2019).
- Rouwenhorst, K.H.R.; Van Der Ham, A.G.J.; Mul, G.; Kersten, S.R. Islanded ammonia power systems: Technology review & conceptual process design. Renew. Sustain. Energy Rev. 2019, 114. [Google Scholar] [CrossRef]
- Vrijenhoef, H. Dutch initiatives to store sustainable energy in the form of ammonia. In Proceedings of the NH3 Fuel Conference, Minneapolis, MN, USA, 1–2 November 2017; Available online: https://nh3fuelassociation.org/2017/09/25/dutch-initiatives-to-store-sustainable-energy-in-the-form-of-ammonia/ (accessed on 1 September 2019).
- Reese, M.; Marquart, C.; Malmali, M.; Wagner, K.; Buchanan, E.; McCormick, A.; Cussler, E.L. Performance of a Small-Scale Haber Process. Ind. Eng. Chem. Res. 2016, 55, 3742–3750. [Google Scholar] [CrossRef]
- Brown, T. Ammonia Technology Portfolio: Optimize for Energy Efficiency and Carbon Efficiency. Available online: https://ammoniaindustry.com/ammonia-technology-portfolio-optimize-for-energy-efficiency-and-carbon-efficiency/ (accessed on 23 March 2018).
- Morgan, E.R.; Manwell, J.F.; McGowan, J.G. Sustainable Ammonia Production from U.S. Offshore Wind Farms: A Techno-Economic Review. ACS Sustain. Chem. Eng. 2017, 5, 9554–9567. [Google Scholar] [CrossRef]
- Pfromm, P.H. Towards sustainable agriculture: Fossil-free ammonia. J. Renew. Sustain. Energy 2017, 9, 034702. [Google Scholar] [CrossRef]
- Will, M. Realisation of Large-Scale Green Ammonia Plants. In Proceedings of the NH3 Fuel Conference, Pittsburgh, PA, USA, 31 October–1 November 2018. [Google Scholar]
- Will, M.; Lüke, L. Realisation of large-scale Green Ammonia plants. In Proceedings of the NH3 Event, Rotterdam, The Netherlands, 2–5 June 2018; Available online: https://nh3event.com/day-2/ (accessed on 15 July 2018).
- Vrijenhoef, J.P. Opportunities for Small scale Ammonia Production; International Fertiliser Society: London, UK, 2017; pp. 1–16. Available online: http://www.protonventures.com/wp-content/uploads/2017/07/Paper-Opportunities-for-small-scale-ammonia-production_ProtonVentures_HansVrijenhoef.pdf (accessed on 1 September 2019).
- Schmuecker, J.; Toyne, D. Making demonstration amounts of renewable ammonia and using it to fuel a farm tractor. In Proceedings of the NH3 Event, Rotterdam, The Netherlands, 6–7 June 2019. [Google Scholar]
- Hansen, J.B.; Han, P. The SOC4NH3 Project in Denmark. In Proceedings of the NH3 Event, Rotterdam, The Netherlands, 6–7 June 2019. [Google Scholar]
- Bogaerts, A.; Neyts, E.C. Plasma Technology: An Emerging Technology for Energy Storage. ACS Energy Lett. 2018, 3, 1013–1027. [Google Scholar] [CrossRef]
- Mehta, P.; Barboun, P.; Go, D.B.; Hicks, J.C.; Schneider, W.F. Catalysis Enabled by Plasma Activation of Strong Chemical Bonds: A Review. ACS Energy Lett. 2019, 4, 1115–1133. [Google Scholar] [CrossRef]
- Brandenburg, R.; Bogaerts, A.; Bongers, W.; Fridman, A.; Fridman, G.; Locke, B.R.; Miller, V.; Reuter, S.; Schiorlin, M.; Verreycken, T.; et al. White paper on the future of plasma science in environment, for gas conversion and agriculture. Plasma Process. Polym. 2018, 16, 1–18. [Google Scholar] [CrossRef]
- Bogaerts, A.; Tu, X.; Whitehead, J.C.; Centi, G.; Lefferts, L.; Guaitella, O.; Azzolina-Jury, F.; Kim, H.-H.; Murphy, A.B.; Schneider, W.F.; et al. The 2020 Plasma Catalysis Roadmap. J. Phys. D Appl. Phys. 2020, 53, 1–51. [Google Scholar] [CrossRef]
- Rouwenhorst, K.H.R.; Engelmann, Y.; van’t Veer, K.; Postma, R.S.; Bogaerts, A.; Lefferts, L. Plasma-Driven catalysis: Green ammonia synthesis from intermittent electricity. Green Chem. 2020, in press. [Google Scholar]
- Hong, J.; Prawer, S.; Murphy, A.B. Plasma Catalysis as an Alternative Route for Ammonia Production: Status, Mechanisms, and Prospects for Progress. ACS Sustain. Chem. Eng. 2017, 6, 15–31. [Google Scholar] [CrossRef]
- Carreon, M.L. Plasma catalytic ammonia synthesis: State of the art and future directions. J. Phys. D Appl. Phys. 2019, 52, 483001. [Google Scholar] [CrossRef]
- Kim, H.H.; Teramoto, Y.; Ogata, A.; Takagi, H.; Nanba, T. Plasma Catalysis for Environmental Treatment and Energy Applications. Plasma Chem. Plasma Process. 2015, 36, 45–72. [Google Scholar] [CrossRef]
- Peng, P.; Chen, P.; Schiappacasse, C.; Zhou, N.; Anderson, E.; Chen, D.; Liu, J.; Cheng, Y.; Hatzenbeller, R.; Addy, M.; et al. A review on the non-thermal plasma-assisted ammonia synthesis technologies. J. Clean. Prod. 2018, 177, 597–609. [Google Scholar] [CrossRef]
- Peng, P.; Schiappacasse, C.; Zhou, N.; Addy, M.; Cheng, Y.; Zhang, Y.; Ding, K.; Wang, Y.; Chen, P.; Ruan, R. Sustainable Non-Thermal Plasma-Assisted Nitrogen Fixation—Synergistic Catalysis. ChemSusChem 2019, 12, 3702–3712. [Google Scholar] [CrossRef]
- Neyts, E.C. Plasma-Surface Interactions in Plasma Catalysis. Plasma Chem. Plasma Process. 2015, 36, 185–212. [Google Scholar] [CrossRef]
- Rouwenhorst, K.H.R.; Kim, H.-H.; Lefferts, L. Vibrationally Excited Activation of N2 in Plasma-Enhanced Catalytic Ammonia Synthesis: A Kinetic Analysis. ACS Sustain. Chem. Eng. 2019, 7, 17515–17522. [Google Scholar] [CrossRef]
- Barboun, P.M.; Mehta, P.; Herrera, F.A.; Go, D.B.; Schneider, W.F.; Hicks, J.C. Distinguishing Plasma Contributions to Catalyst Performance in Plasma-Assisted Ammonia Synthesis. ACS Sustain. Chem. Eng. 2019, 7, 8621–8630. [Google Scholar] [CrossRef]
- Akay, G.; Zhang, K. Process Intensification in Ammonia Synthesis Using Novel Coassembled Supported Microporous Catalysts Promoted by Nonthermal Plasma. Ind. Eng. Chem. Res. 2017, 56, 457–468. [Google Scholar] [CrossRef]
- Peng, P.; Cheng, Y.; Hatzenbeller, R.; Addy, M.; Zhou, N.; Schiappacasse, C.; Chen, D.; Zhang, Y.; Anderson, E.; Liu, Y.; et al. Ru-Based multifunctional mesoporous catalyst for low-pressure and non-thermal plasma synthesis of ammonia. Int. J. Hydrog. Energy 2017, 42, 19056–19066. [Google Scholar] [CrossRef]
- Li, S.; Van Raak, T.; Gallucci, F. Investigating the operation parameters for ammonia synthesis in dielectric barrier discharge reactors. J. Phys. D Appl. Phys. 2019, 53, 014008. [Google Scholar] [CrossRef]
- Kim, H.H.; Teramoto, Y.; Ogata, A.; Takagi, H.; Nanba, T. Atmospheric-Pressure nonthermal plasma synthesis of ammonia over ruthenium catalysts. Plasma Process. Polym. 2016, 14, 1600157. [Google Scholar] [CrossRef]
- Shah, J.R.; Gorky, F.; Lucero, J.; Carreon, M.A.; Carreon, M.L. Ammonia Synthesis via Atmospheric Plasma Catalysis: Zeolite 5A, a Case of Study. Ind. Eng. Chem. Res. 2020, 59, 5167–5176. [Google Scholar] [CrossRef]
- Iwamoto, M.; Akiyama, M.; Aihara, K.; Deguchi, T. Ammonia Synthesis on Wool-Like Au, Pt, Pd, Ag, or Cu Electrode Catalysts in Nonthermal Atmospheric-Pressure Plasma of N2 and H2. ACS Catal. 2017, 7, 6924–6929. [Google Scholar] [CrossRef]
- Hong, J.; Prawer, S.; Murphy, A.B. Production of Ammonia by Heterogeneous Catalysis in a Packed-Bed Dielectric-Barrier Discharge: Influence of Argon Addition and Voltage. IEEE Trans. Plasma Sci. 2014, 42, 2338–2339. [Google Scholar] [CrossRef]
- Gómez-Ramírez, A.M.; Cotrino, J.; Lambert, R.M.; González-Elipe, A.R. Efficient synthesis of ammonia from N2 and H2 alone in a ferroelectric packed-bed DBD reactor. Plasma Sources Sci. Technol. 2015, 24, 65011. [Google Scholar] [CrossRef]
- Gómez-Ramírez, A.M.; Montoro-Damas, A.M.; Cotrino, J.; Lambert, R.M.; González-Elipe, A.R. About the enhancement of chemical yield during the atmospheric plasma synthesis of ammonia in a ferroelectric packed bed reactor. Plasma Process. Polym. 2016, 14, 1600081. [Google Scholar] [CrossRef]
- Mizushima, T.; Matsumoto, K.; Sugoh, J.-I.; Ohkita, H.; Kakuta, N. Tubular membrane-like catalyst for reactor with dielectric-barrier-discharge plasma and its performance in ammonia synthesis. Appl. Catal. A Gen. 2004, 265, 53–59. [Google Scholar] [CrossRef]
- Srinath, N.V. Plasma Catalytic Ammonia Synthesis at Atmospheric Pressure in a Dielectric Barrier Discharge Reactor; Technische Universiteit Eindhoven: Eindhoven, The Netherlands, 2017; Available online: https://pure.tue.nl/ws/files/91518802/Afstudeerverslag_Nadadur_Veeraraghavan_Srinath_0980194_.pdf (accessed on 1 September 2019).
- Patil, B.S. Plasma (Catalyst)—Assisted Nitrogen Fixation: Reactor Development for Nitric Oxide and Ammonia Production; Technische Universiteit Eindhoven: Eindhoven, The Netherlands, 2017; Available online: https://pure.tue.nl/ws/files/64000562/20170510_Patil.pdf (accessed on 1 September 2019).
- Peng, P.; Li, Y.; Cheng, Y.; Deng, S.; Chen, P.; Ruan, R. Atmospheric Pressure Ammonia Synthesis Using Non-Thermal Plasma Assisted Catalysis. Plasma Chem. Plasma Process. 2016, 36, 1201–1210. [Google Scholar] [CrossRef]
- Peng, P.; Chen, P.; Addy, M.; Cheng, Y.; Anderson, E.; Zhou, N.; Schiappacasse, C.; Zhang, Y.; Chen, D.; Hatzenbeller, R.; et al. Atmospheric Plasma-Assisted Ammonia Synthesis Enhanced via Synergistic Catalytic Absorption. ACS Sustain. Chem. Eng. 2018, 7, 100–104. [Google Scholar] [CrossRef]
- Yin, K.S.; Venugopalan, M. Plasma chemical synthesis. I. Effect of electrode material on the synthesis of ammonia. Plasma Chem. Plasma Process. 1983, 3, 343–350. [Google Scholar] [CrossRef]
- Wildfire, C.; Abdelsayed, V.; Shekhawat, D.; Spencer, M.J. Ambient pressure synthesis of ammonia using a microwave reactor. Catal. Commun. 2018, 115, 64–67. [Google Scholar] [CrossRef]
- Uyama, H.; Nakamura, T.; Tanaka, S.; Matsumoto, O. Catalytic effect of iron wires on the syntheses of ammonia and hydrazine in a radio-frequency discharge. Plasma Chem. Plasma Process. 1993, 13, 117–131. [Google Scholar] [CrossRef]
- Nakajima, J.; Sekiguchi, H. Synthesis of ammonia using microwave discharge at atmospheric pressure. Thin Solid Films 2008, 516, 4446–4451. [Google Scholar] [CrossRef]
- Bai, X.; Tiwari, S.; Robinson, B.; Killmer, C.P.; Li, L.; Hu, J. Microwave catalytic synthesis of ammonia from methane and nitrogen. Catal. Sci. Technol. 2018, 8, 6302–6305. [Google Scholar] [CrossRef]
- Siemsen, L.G. The Synthesis of Ammonia from Hydrogen and Atomic Nitrogen on the Rh(110) Surface; Iowa State University: Ames, IA, USA, 1990; Available online: https://lib.dr.iastate.edu/cgi/viewcontent.cgi?article=12220&context=rtd (accessed on 1 September 2019).
- Shah, J.; Gorky, F.; Psarras, P.; Seong, B.; Gómez-Gualdrón, D.A.; Carreon, M.L. Ammonia yield enhancement by hydrogen sink effect during plasma catalysis. ChemCatChem 2019, 12, 1200–1211. [Google Scholar] [CrossRef]
- Shah, J.; Wu, T.; Lucero, J.; Carreon, M.A.; Carreon, M.L. Nonthermal Plasma Synthesis of Ammonia over Ni-MOF-74. ACS Sustain. Chem. Eng. 2018, 7, 377–383. [Google Scholar] [CrossRef]
- Shah, J.; Wang, W.; Bogaerts, A.; Carreon, M.L. Ammonia Synthesis by Radio Frequency Plasma Catalysis: Revealing the Underlying Mechanisms. ACS Appl. Energy Mater. 2018, 1, 4824–4839. [Google Scholar] [CrossRef]
- Uyama, H.; Uchikura, T.; Niijima, H.; Matsumoto, O. Synthesis of ammonia with RF discharge. Adsorption of products on zeolite. Chem. Lett. 1987, 16, 555–558. [Google Scholar] [CrossRef]
- Uyama, H.; Matsumoto, O. Synthesis of ammonia in high-frequency discharges. Plasma Chem. Plasma Process. 1989, 9, 13–24. [Google Scholar] [CrossRef]
- Shah, J.; Harrison, J.M.; Carreon, M.L. Ammonia Plasma-Catalytic Synthesis Using Low Melting Point Alloys. Catalysts 2018, 8, 437. [Google Scholar] [CrossRef]
- Rouwenhorst, K.H.R.; Krzywda, P.M.; Benes, N.E.; Mul, G.; Lefferts, L. Green Ammonia Production. In Techno-Economic Challenges of Green Ammonia as Energy Vector; Bañares-Alcántara, R., Valera-Medina, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Smith, C.; Hill, A.K.; Torrente-Murciano, L. Current and future role of Haber-Bosch ammonia in a carbon-free energy landscape. Energy Environ. Sci. 2020, 13, 331–344. [Google Scholar] [CrossRef]
- Buchner, G.A.; Stepputat, K.J.; Zimmermann, A.W.; Schomäcker, R. Specifying Technology Readiness Levels for the Chemical Industry. Ind. Eng. Chem. Res. 2019, 58, 6957–6969. [Google Scholar] [CrossRef]
- Eliasson, B.; Kogelschatz, U.; Xue, B.; Zhou, L.-M. Hydrogenation of Carbon Dioxide to Methanol with a Discharge-Activated Catalyst. Ind. Eng. Chem. Res. 1998, 37, 3350–3357. [Google Scholar] [CrossRef]
- Teramoto, Y.; Kim, H.H. Effect of vibrationally excited N2(v) on atomic nitrogen generation using two consecutive pulse corona discharges under atmospheric pressure N2. J. Phys. D Appl. Phys. 2019, 52, 494003. [Google Scholar] [CrossRef]
- Mehta, P.; Barboun, P.; Herrera, F.; Kim, J.; Rumbach, P.; Go, D.B.; Hicks, J.C.; Schneider, W.F. Overcoming ammonia synthesis scaling relations with plasma-enabled catalysis. Nat. Catal. 2018, 1, 269–275. [Google Scholar] [CrossRef]
- Rouwenhorst, K.H.R.; Burbach, H.G.B.; Núñez Paulí, J.; Vogel, D.W.; Geerdink, B.; Lefferts, L. Plasma-Catalytic Ammonia Synthesis beyond Thermal Equilibrium over Ru-based Catalysts. In preparation.
- Vojvodic, A.; Medford, A.J.; Studt, F.; Abild-Pedersen, F.; Khan, T.S.; Bligaard, T.; Nørskov, J. Exploring the limits: A low-pressure, low-temperature Haber-Bosch process. Chem. Phys. Lett. 2014, 598, 108–112. [Google Scholar] [CrossRef]
- Aika, K.-I. Role of alkali promoter in ammonia synthesis over ruthenium catalysts—Effect on reaction mechanism. Catal. Today 2017, 286, 14–20. [Google Scholar] [CrossRef]
- Ozaki, A. Development of alkali-promoted ruthenium as a novel catalyst for ammonia synthesis. Acc. Chem. Res. 1981, 14, 16–21. [Google Scholar] [CrossRef]
- Mortensen, J.J.; Hammer, B.; Nørskov, J.K. Alkali Promotion of N2 Dissociation over Ru(0001). Phys. Rev. Lett. 1998, 80, 4333–4336. [Google Scholar] [CrossRef]
- Inoue, Y.; Kitano, M.; Tokunari, M.; Taniguchi, T.; Ooya, K.; Abe, H.; Niwa, Y.; Sasase, M.; Hara, M.; Hosono, H. Direct Activation of Cobalt Catalyst by 12CaO·7Al2O3 Electride for Ammonia Synthesis. ACS Catal. 2019, 9, 1670–1679. [Google Scholar] [CrossRef]
- Muhler, M.; Rosowski, F.; Hinrichsen, O.; Hornung, A.; Ertl, G. Ruthenium as catalyst for ammonia synthesis. Stud. Surf. Sci. Catal. 1996, 101, 317–326. [Google Scholar] [CrossRef]
- Ruan, R.; Deng, S.; Le, Z.; Chen, P. Non-Thermal Plasma Synthesis of Ammonia; United States, 2009. Available online: https://patentimages.storage.googleapis.com/b8/04/25/03baed26ca11d4/WO2009025835A1.pdf (accessed on 1 September 2019).
- Liu, C.Y.; Aika, K.-I. Effect of the Cl/Br Molar Ratio of a CaCl2−CaBr2 Mixture Used as an Ammonia Storage Material. Ind. Eng. Chem. Res. 2004, 43, 6994–7000. [Google Scholar] [CrossRef]
- Beach, J.D.; Kintner, J.D.; Welch, A.W. Removal of Gaseous NH3 from an NH3 Reactor Product Stream. United States, 2018. Available online: https://patentimages.storage.googleapis.com/62/ef/a4/83ada6d54f30fe/US20180339911A1.pdf (accessed on 1 September 2019).
- Malmali, M.; Le, G.; Hendrickson, J.; Prince, J.; McCormick, A.V.; Cussler, E.L. Better Absorbents for Ammonia Separation. ACS Sustain. Chem. Eng. 2018, 6, 6536–6546. [Google Scholar] [CrossRef]
- Liu, C.Y.; Aika, K.-I. Modification of active carbon and zeolite as ammonia separation materials for a new de-NOx process with ammonia on-site synthesis. Res. Chem. Intermed. 2002, 28, 409–417. [Google Scholar] [CrossRef]
- Zhang, T.; Miyaoka, H.; Miyaoka, H.; Ichikawa, T.; Kojima, Y. Review on Ammonia Absorption Materials: Metal Hydrides, Halides, and Borohydrides. ACS Appl. Energy Mater. 2018, 1, 232–242. [Google Scholar] [CrossRef]
- Kitano, M.; Inoue, Y.; Sasase, M.; Kishida, K.; Kobayashi, Y.; Nishiyama, K.; Tada, T.; Kawamura, S.; Yokoyama, T.; Hara, M.; et al. Self-Organized Ruthenium-Barium Core-Shell Nanoparticles on a Mesoporous Calcium Amide Matrix for Efficient Low-Temperature Ammonia Synthesis. Angew. Chem. 2018, 130, 2678–2682. [Google Scholar] [CrossRef]
- Kitano, M.; Kanbara, S.; Inoue, Y.; Kuganathan, N.; Sushko, P.V.; Yokoyama, T.; Hara, M.; Hosono, H. Electride support boosts nitrogen dissociation over ruthenium catalyst and shifts the bottleneck in ammonia synthesis. Nat. Commun. 2015, 6, 6731. [Google Scholar] [CrossRef] [PubMed]
- Kitano, M.; Inoue, Y.; Yamazaki, Y.; Hayashi, F.; Kanbara, S.; Matsuishi, S.; Yokoyama, T.; Kim, S.-W.; Hara, M.; Hosono, H. Ammonia synthesis using a stable electride as an electron donor and reversible hydrogen store. Nat. Chem. 2012, 4, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Zhang, X.; Waterhouse, G.I.N.; Zhao, Y.; Zhang, T. The Journey toward Low Temperature, Low Pressure Catalytic Nitrogen Fixation. Adv. Energy Mater. 2020, 10. [Google Scholar] [CrossRef]
- Smith, C.; Malmali, M.; Liu, C.-Y.; McCormick, A.V.; Cussler, E.L. Rates of Ammonia Absorption and Release in Calcium Chloride. ACS Sustain. Chem. Eng. 2018, 6, 11827–11835. [Google Scholar] [CrossRef]
- Armijo, J.; Philibert, C. Flexible production of green hydrogen and ammonia from variable solar and wind energy: Case study of Chile and Argentina. Int. J. Hydrog. Energy 2020, 45, 1541–1558. [Google Scholar] [CrossRef]
- Van Rooij, G.J.; Akse, H.N.; A Bongers, W.A.; Van De Sanden, M.C.M. Plasma for electrification of chemical industry: A case study on CO2 reduction. Plasma Phys. Control. Fusion 2017, 60, 014019. [Google Scholar] [CrossRef]
- Singh, A.R.; Rohr, B.A.; Statt, M.J.; Schwalbe, J.A.; Cargnello, M.; Nørskov, J.K. Strategies toward Selective Electrochemical Ammonia Synthesis. ACS Catal. 2019, 9, 8316–8324. [Google Scholar] [CrossRef]
- Andersen, S.Z.; Čolić, V.; Yang, S.; Schwalbe, J.A.; Nielander, A.C.; McEnaney, J.M.; Enemark-Rasmussen, K.; Baker, J.; Singh, A.R.; Rohr, B.A.; et al. A rigorous electrochemical ammonia synthesis protocol with quantitative isotope measurements. Nature 2019, 570, 504–508. [Google Scholar] [CrossRef]
- Kibsgaard, J.; Nørskov, J.K.; Chorkendorff, I. The Difficulty of Proving Electrochemical Ammonia Synthesis. ACS Energy Lett. 2019, 4, 2986–2988. [Google Scholar] [CrossRef]
- Hollevoet, L.; De Ras, M.; Roeffaers, M.; Hofkens, J.; Martens, J.A. Energy-Efficient Ammonia Production from Air and Water Using Electrocatalysts with Limited Faradaic Efficiency. ACS Energy Lett. 2020, 5, 1124–1127. [Google Scholar] [CrossRef]
- Liu, H.; Han, W.; Huo, C.; Cen, Y. Development and application of wüstite-based ammonia synthesis catalysts. Catal. Today 2019, 1–18. [Google Scholar] [CrossRef]
- Mittasch, A.; Frankenburg, W. Early Studies of Multicomponent Catalysts. Adv. Catal. 1950, 2, 81–104. [Google Scholar] [CrossRef]
- Hattori, M.; Iijima, S.; Nakao, T.; Hosono, H.; Hara, M. Solid solution for catalytic ammonia synthesis from nitrogen and hydrogen gases at 50 °C. Nat. Commun. 2020, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kammert, J.; Moon, J.; Cheng, Y.; Daemen, L.L.; Irle, S.; Fung, V.; Liu, J.; Page, K.; Ma, X.; Phaneuf, V.; et al. Nature of Reactive Hydrogen for Ammonia Synthesis over a Ru/C12A7 Electride Catalyst. J. Am. Chem. Soc. 2020, 142, 7655–7667. [Google Scholar] [CrossRef]
- Hara, M.; Kitano, M.; Hosono, H. Ru-Loaded C12A7:e− Electride as a Catalyst for Ammonia Synthesis. ACS Catal. 2017, 7, 2313–2324. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Kawamura, S.; Kitano, M.; Yokoyama, T.; Hosono, H. Kinetic evidence: The rate-determining step for ammonia synthesis over electride-supported Ru catalysts is no longer the nitrogen dissociation step. Catal. Sci. Technol. 2017, 7, 47–50. [Google Scholar] [CrossRef]
- Gong, Y.; Wu, J.; Kitano, M.; Wang, J.; Ye, T.-N.; Li, J.; Kobayashi, Y.; Kishida, K.; Abe, H.; Niwa, Y.; et al. Ternary intermetallic LaCoSi as a catalyst for N2 activation. Nat. Catal. 2018, 1, 178–185. [Google Scholar] [CrossRef]
- Wu, J.; Li, J.; Gong, Y.; Kitano, M.; Inoshita, T.; Hosono, H. Intermetallic Electride Catalyst as a Platform for Ammonia Synthesis. Angew. Chem. Int. Ed. 2019, 58, 825–829. [Google Scholar] [CrossRef]
- Inoue, Y.; Kitano, M.; Kishida, K.; Abe, H.; Niwa, Y.; Sasase, M.; Fujita, Y.; Ishikawa, H.; Yokoyama, T.; Hara, M.; et al. Efficient and Stable Ammonia Synthesis by Self-Organized Flat Ru Nanoparticles on Calcium Amide. ACS Catal. 2016, 6, 7577–7584. [Google Scholar] [CrossRef]
- Gao, W.; Wang, P.; Guo, J.; Chang, F.; He, T.; Wang, Q.; Wu, G.; Chen, P. Barium Hydride-Mediated Nitrogen Transfer and Hydrogenation for Ammonia Synthesis: A Case Study of Cobalt. ACS Catal. 2017, 7, 3654–3661. [Google Scholar] [CrossRef]
- Matsuishi, S.; Toda, Y.; Miyakawa, M.; Hayashi, K.; Kamiya, T.; Hirano, M.; Tanaka, I.; Hosono, H. High-Density Electron Anions in a Nanoporous Single Crystal: [Ca24Al28O64]4+(4e-). Science 2003, 301, 626–629. [Google Scholar] [CrossRef]
- McEnaney, J.M.; Singh, A.R.; Schwalbe, J.A.; Kibsgaard, J.; Lin, J.C.; Cargnello, M.; Jaramillo, T.F.; Nørskov, J.K. Ammonia synthesis from N2 and H2O using a lithium cycling electrification strategy at atmospheric pressure. Energy Environ. Sci. 2017, 10, 1621–1630. [Google Scholar] [CrossRef]
- Jiao, F.; Xu, B. Electrochemical Ammonia Synthesis and Ammonia Fuel Cells. Adv. Mater. 2018, 31, 1–5. [Google Scholar] [CrossRef]
- Smith, R.R.; Killelea, D.R.; DelSesto, D.F.; Utz, A.L. Preference for Vibrational over Translational Energy in a Gas-Surface Reaction. Science 2004, 304, 992–995. [Google Scholar] [CrossRef] [PubMed]
- Holmblad, P.M.; Wambach, J.; Chorkendorff, I. Molecular beam study of dissociative sticking of methane on Ni(100). J. Chem. Phys. 1995, 102, 8255–8263. [Google Scholar] [CrossRef][Green Version]
- Patil, B.S.; Hessel, V.; Seefeldt, L.C.; Dean, D.R.; Hoffman, B.M.; Cook, B.J.; Murray, L.J. Nitrogen Fixation. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Hoboken, NJ, USA, 2017; pp. 1–21. [Google Scholar] [CrossRef]
- Patil, B.S.; Wang, Q.; Hessel, V.; Lang, J. Plasma N2-fixation: 1900–2014. Catal. Today 2015, 256, 49–66. [Google Scholar] [CrossRef]
 [39,40,41,42,43,44,45,46,47,48,49,50,51,52], DBD (pulse)
[39,40,41,42,43,44,45,46,47,48,49,50,51,52], DBD (pulse)  [43,53], glow discharge
[43,53], glow discharge  [54], MW
[54], MW  [55,56,57,58,59] and radiofrequency (RF)
[55,56,57,58,59] and radiofrequency (RF)  [60,61,62,63,64,65]. Note that the feed composition varies among the references, and therefore the ammonia yield. In case of a stoichiometric feed ratio, the ammonia outlet concentration is equal to the yield (at 0.0 mol. % NH3 inlet). The feasible region is discussed in Section 1.2.1.
[60,61,62,63,64,65]. Note that the feed composition varies among the references, and therefore the ammonia yield. In case of a stoichiometric feed ratio, the ammonia outlet concentration is equal to the yield (at 0.0 mol. % NH3 inlet). The feasible region is discussed in Section 1.2.1.
 [39,40,41,42,43,44,45,46,47,48,49,50,51,52], DBD (pulse)
[39,40,41,42,43,44,45,46,47,48,49,50,51,52], DBD (pulse)  [43,53], glow discharge
[43,53], glow discharge  [54], MW
[54], MW  [55,56,57,58,59] and radiofrequency (RF)
[55,56,57,58,59] and radiofrequency (RF)  [60,61,62,63,64,65]. Note that the feed composition varies among the references, and therefore the ammonia yield. In case of a stoichiometric feed ratio, the ammonia outlet concentration is equal to the yield (at 0.0 mol. % NH3 inlet). The feasible region is discussed in Section 1.2.1.
[60,61,62,63,64,65]. Note that the feed composition varies among the references, and therefore the ammonia yield. In case of a stoichiometric feed ratio, the ammonia outlet concentration is equal to the yield (at 0.0 mol. % NH3 inlet). The feasible region is discussed in Section 1.2.1.

| Catalyst | Thermal Catalysis | Plasma Catalysis | |||
|---|---|---|---|---|---|
| Muhler et al. [78] | Ruan et al. [79] | Kim et al. [43] | |||
| Relative Act. | Relative Act. | Relative Act. | Energy Cost (GJ t-NH3−1) | ||
| AC Plasma | Pulsed Plasma | ||||
| Ru/Al2O3 | 1.0 | 1.0 | 1.0 | 1029–1800 | - |
| Ru/Al2O3 promoted | 2.5 | - | 2.8–3.3 | 313–563 | 101–141 |
| Ru/MgO | 9.2 | 1.5 | - | - | - |
| Ru/MgO promoted | 62 | 3.3 | - | - | - |
| Condensation | Metal Halides | Zeolites | |
|---|---|---|---|
| Separation temperature (°C) | −20 to 30 | 150–250 | 20–100 |
| Desorption temperature (°C) | - | 350–400 | 200–250 |
| Pressure (bar) | 100–450 | 10–30 | 10–30 |
| Energy consumption (GJ t-NH3−1) | 3–5 * | 6–11 | 8 |
| Ammonia at outlet (mol. %) | 2–5 | 0.1–0.3 | 0.1–0.3 |
| Ammonia capacity (wt. %) | 100 | 5–30 | 5–15 |
| Ammonia density (kg m−3) | 680 | 100–600 | 30–90 |
| Chemical stability | - | Low/Medium | High |
| Technology readiness level (TRL) | 9 | 4–5 | 4–5 |
| State-of-the-Art Plasma Reactor | BCS Plasma Reactor | Separation | |
|---|---|---|---|
| Type | DBD reactor (pulse) | DBD reactor (pulse) | Solid absorbent |
| Material | Promoted Ru/Al2O3 catalyst | More active catalyst | MgCl2/SiO2 |
| Reaction temperature (°C) | 300 | 200 | 200 |
| Desorption temperature (°C) | - | - | 300 |
| Operating pressure (bar) | 1.5 | 1.5 | 1.0 |
| Outlet NH3 concentration (mol. %) | 0.16 | 1.0 | 0.1 |
| Outlet ammonia pressure (kPa) | 1.6 | 10 | 0.3 |
| Energy consumption (GJ t-NH3−1) * | 197 (PC:95, Rec:102) | 5 (PC:4, Rec:1) | 10 |
| Syngas ratio (H2:N2) | 1:4 | 1:4 | 1:4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rouwenhorst, K.H.R.; Lefferts, L. Feasibility Study of Plasma-Catalytic Ammonia Synthesis for Energy Storage Applications. Catalysts 2020, 10, 999. https://doi.org/10.3390/catal10090999
Rouwenhorst KHR, Lefferts L. Feasibility Study of Plasma-Catalytic Ammonia Synthesis for Energy Storage Applications. Catalysts. 2020; 10(9):999. https://doi.org/10.3390/catal10090999
Chicago/Turabian StyleRouwenhorst, Kevin H. R., and Leon Lefferts. 2020. "Feasibility Study of Plasma-Catalytic Ammonia Synthesis for Energy Storage Applications" Catalysts 10, no. 9: 999. https://doi.org/10.3390/catal10090999
APA StyleRouwenhorst, K. H. R., & Lefferts, L. (2020). Feasibility Study of Plasma-Catalytic Ammonia Synthesis for Energy Storage Applications. Catalysts, 10(9), 999. https://doi.org/10.3390/catal10090999






