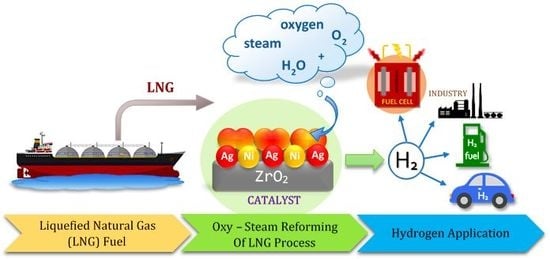
Effect of Ag-Addition on the Catalytic and Physicochemical Properties of Ni/ZrO2 Catalyst in Oxy-Steam Reforming of CH4 and LNG Processes
Abstract
1. Introduction
2. Results and Discussion
2.1. The Syngas Production via OSR of Methane and LNG Reactions
2.2. The Characterisation of the Physicochemical Properties of the Investigated Catalysts
2.2.1. The Specific Surface Area of the Support and Catalysts
2.2.2. The Surface Acidity of the Catalysts Surface
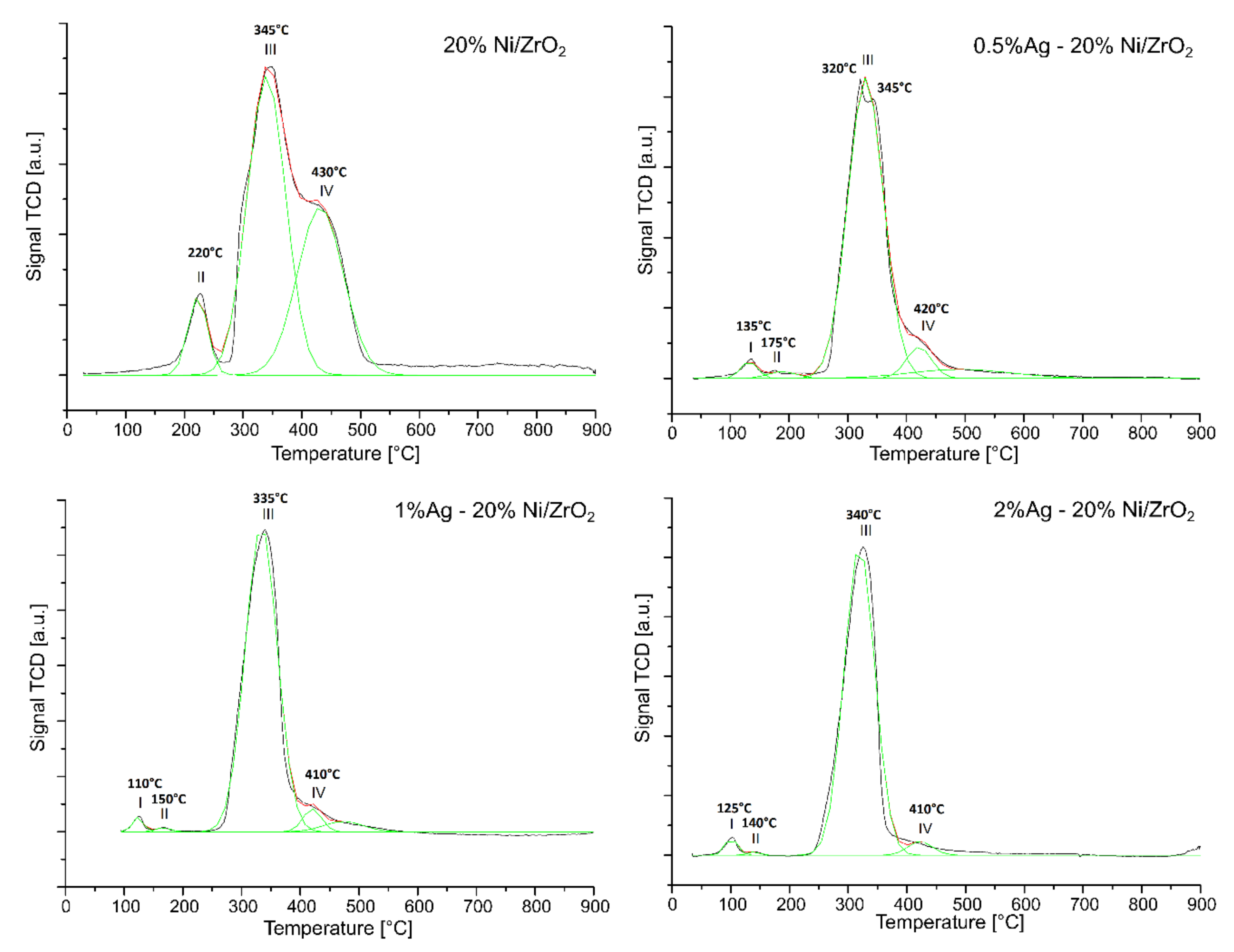
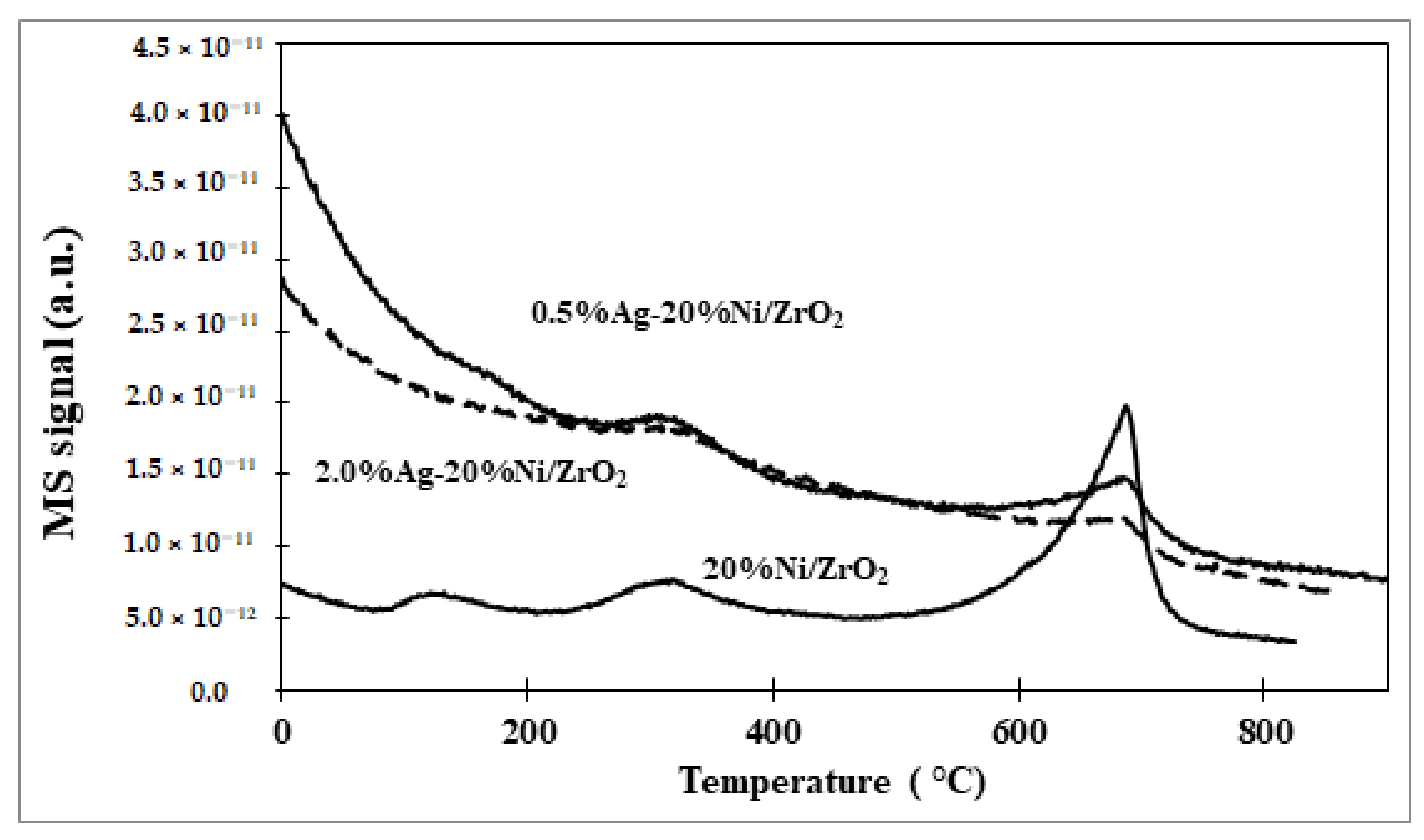
2.2.3. Reduction Behaviour of the Catalytic Materials
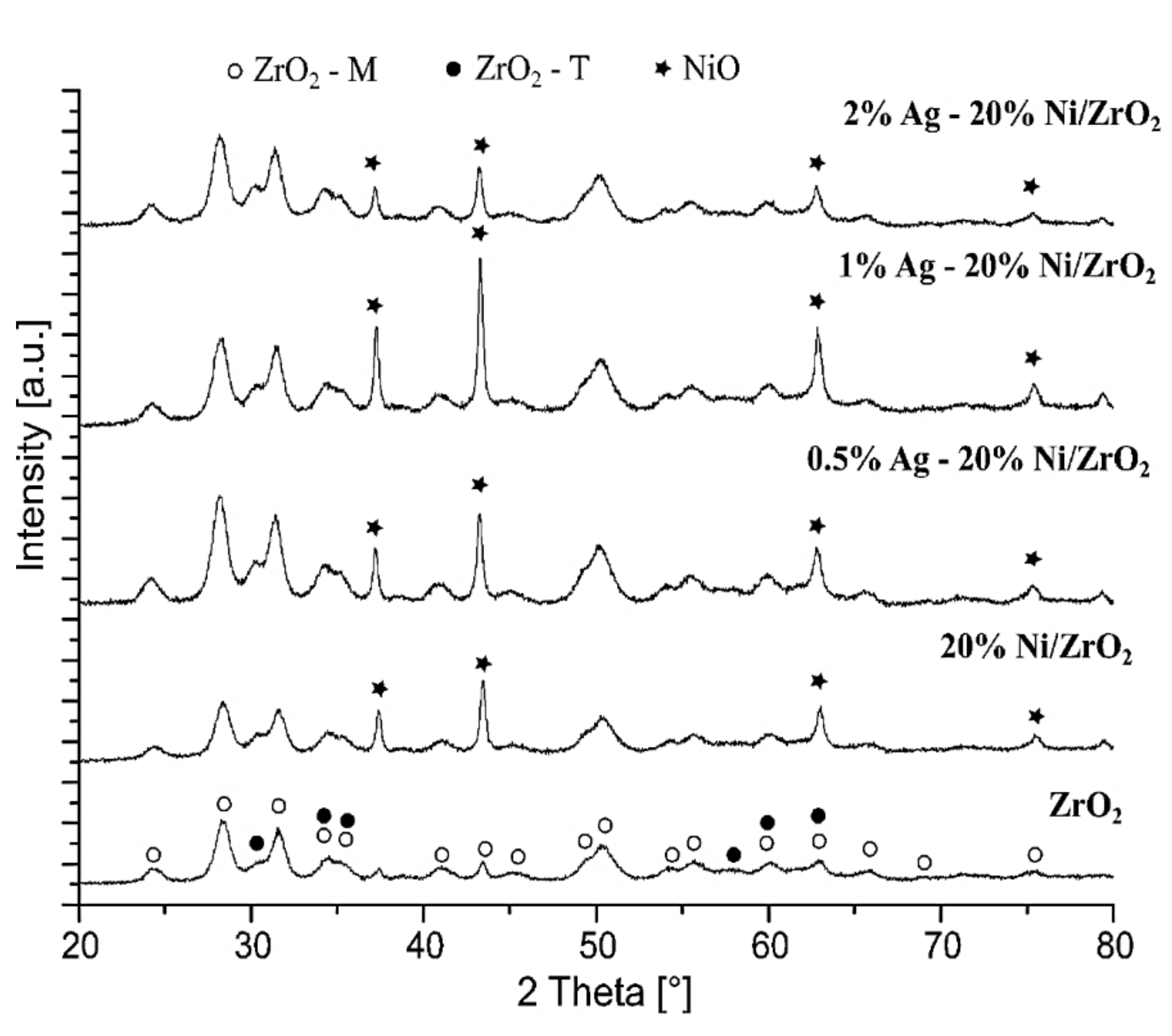
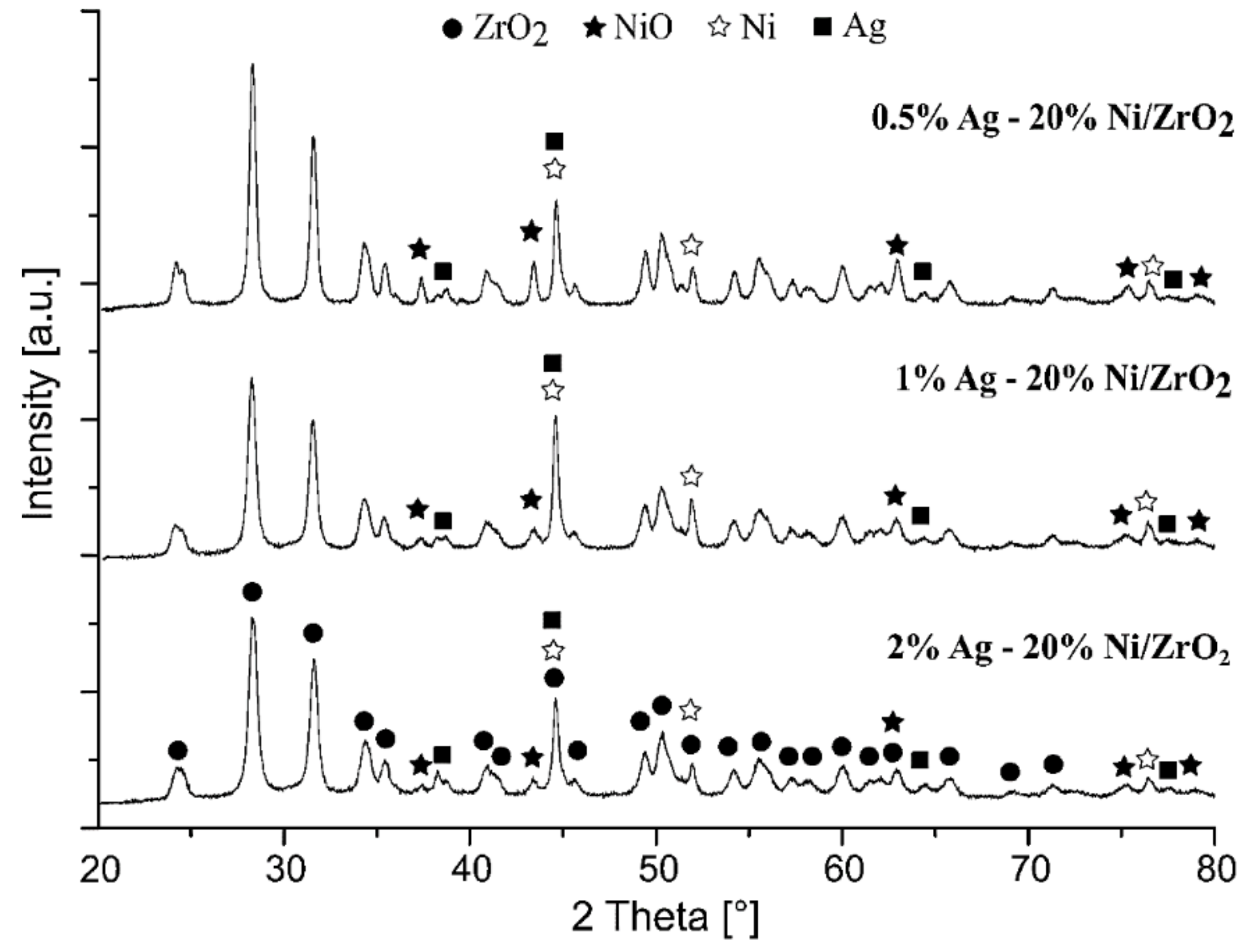
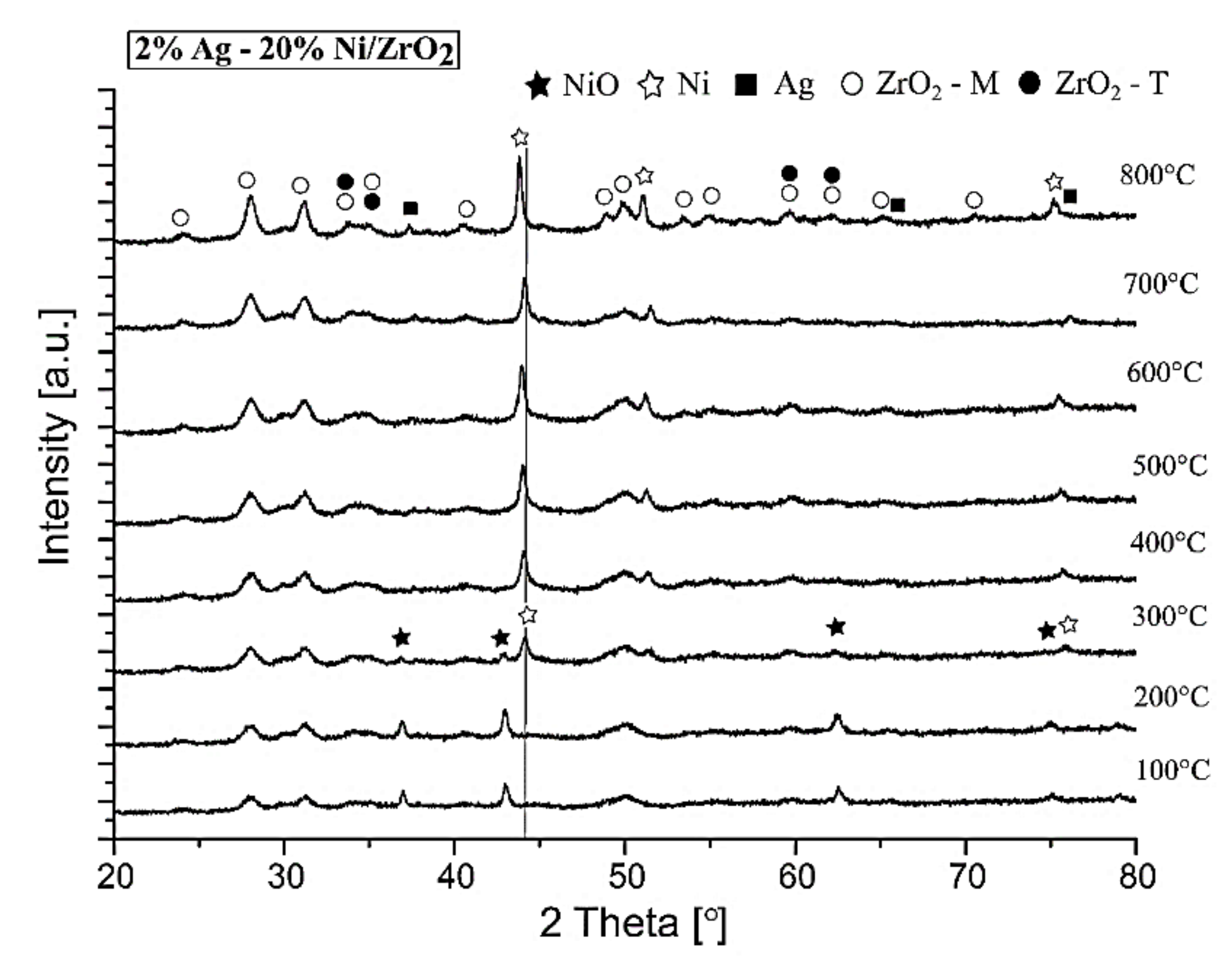
2.2.4. The Crystalline Structure of the Investigated Catalysts
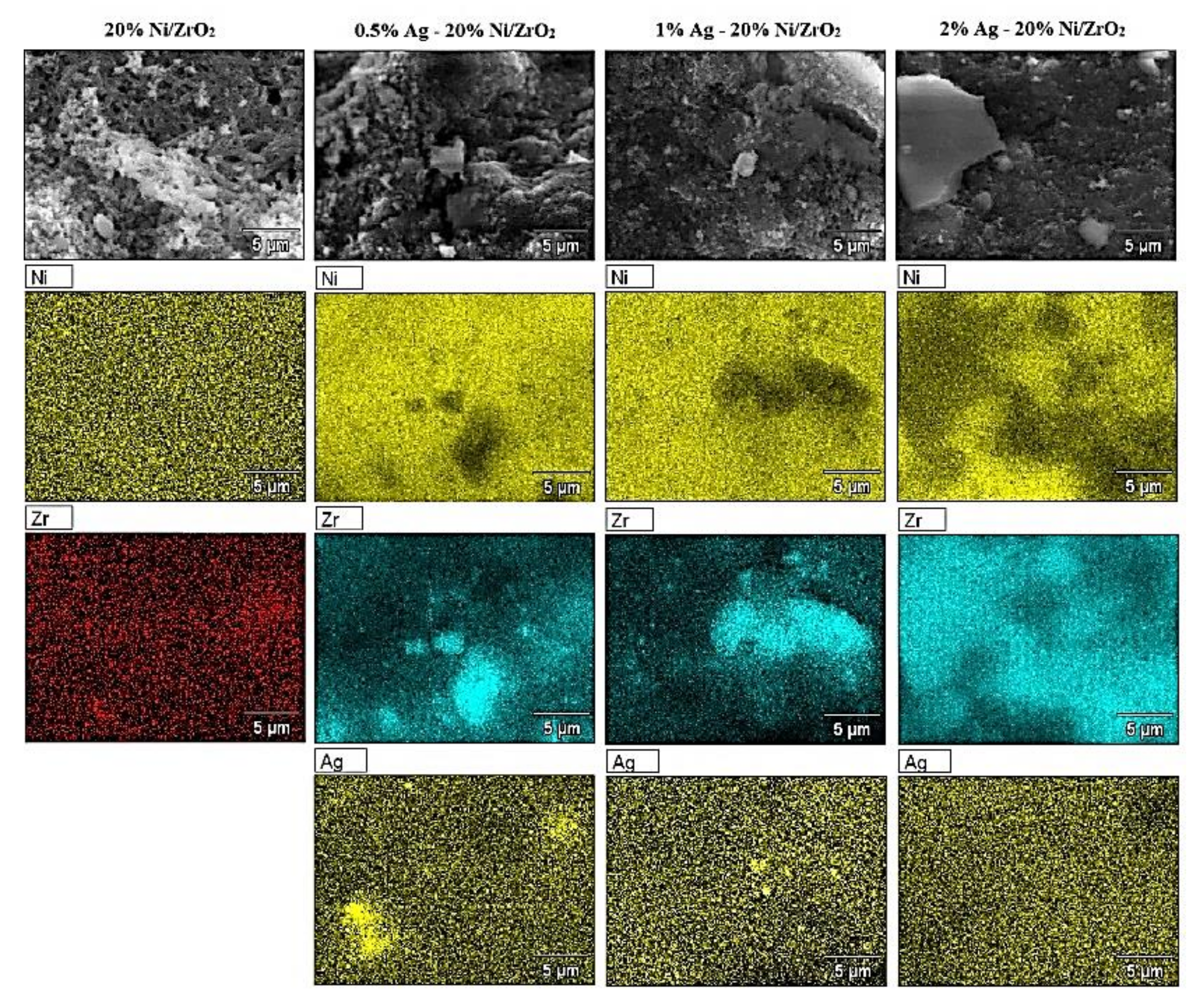
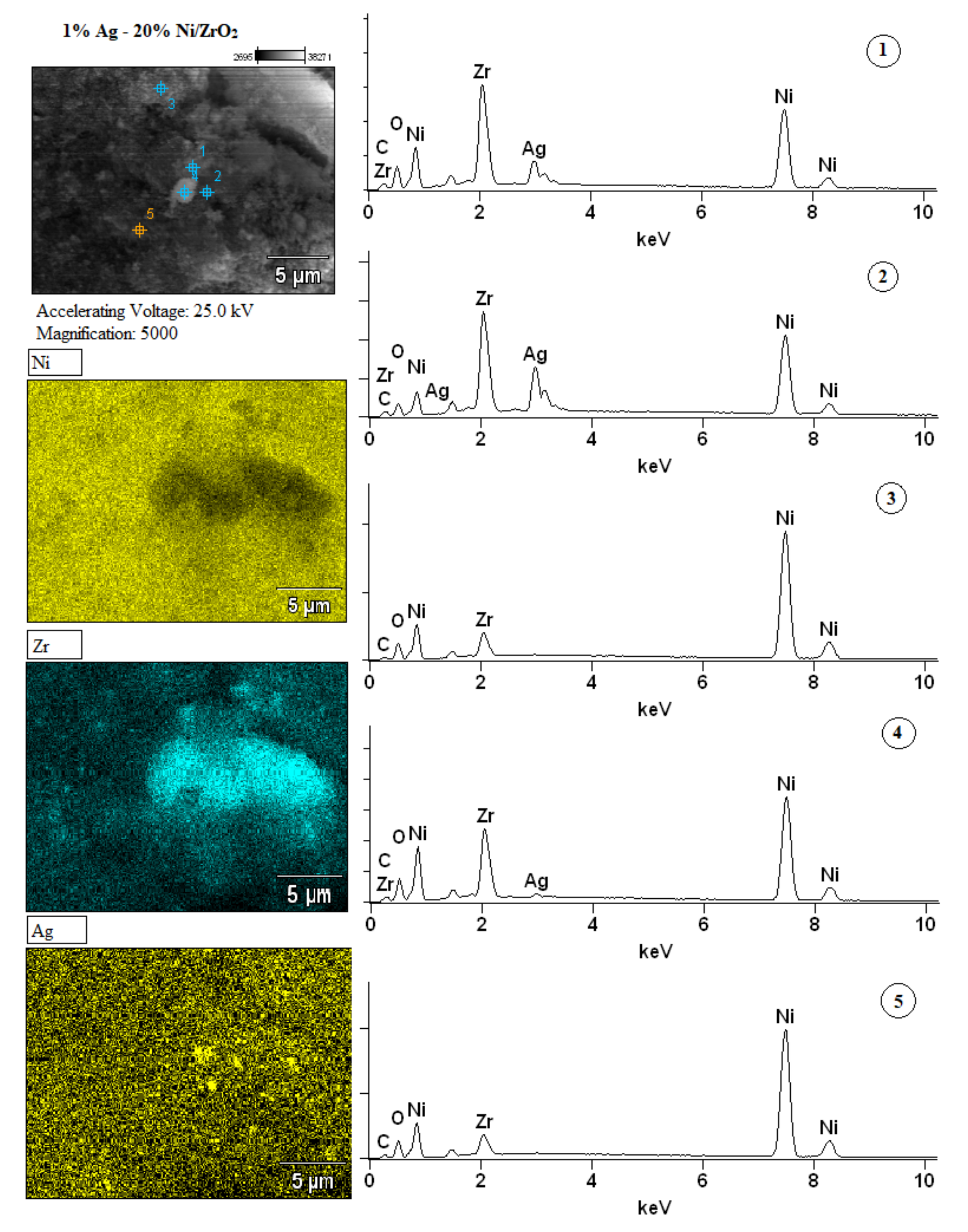
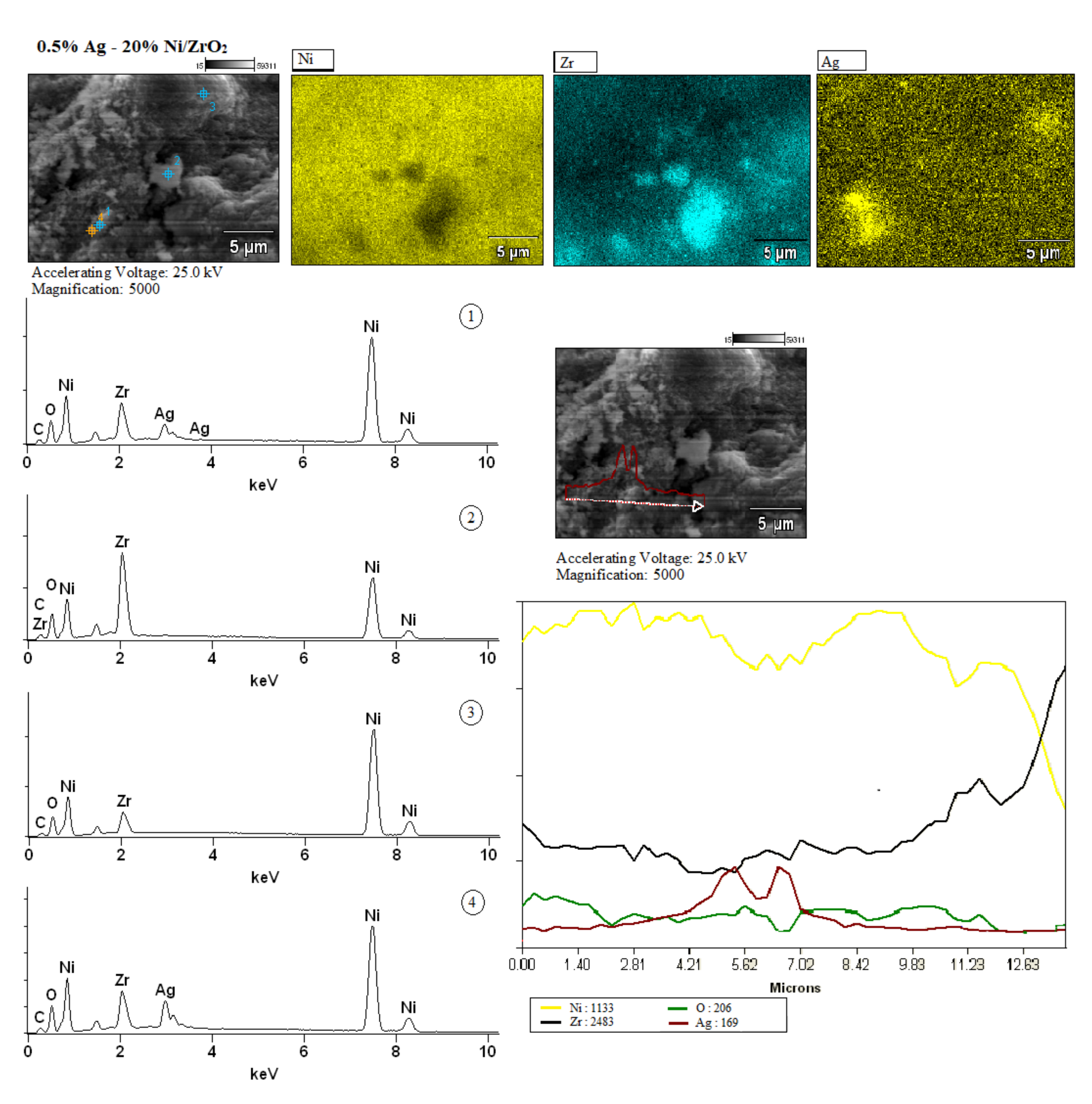
2.2.5. The Morphology and the Elemental Composition of the Surface of the Investigated Catalysts
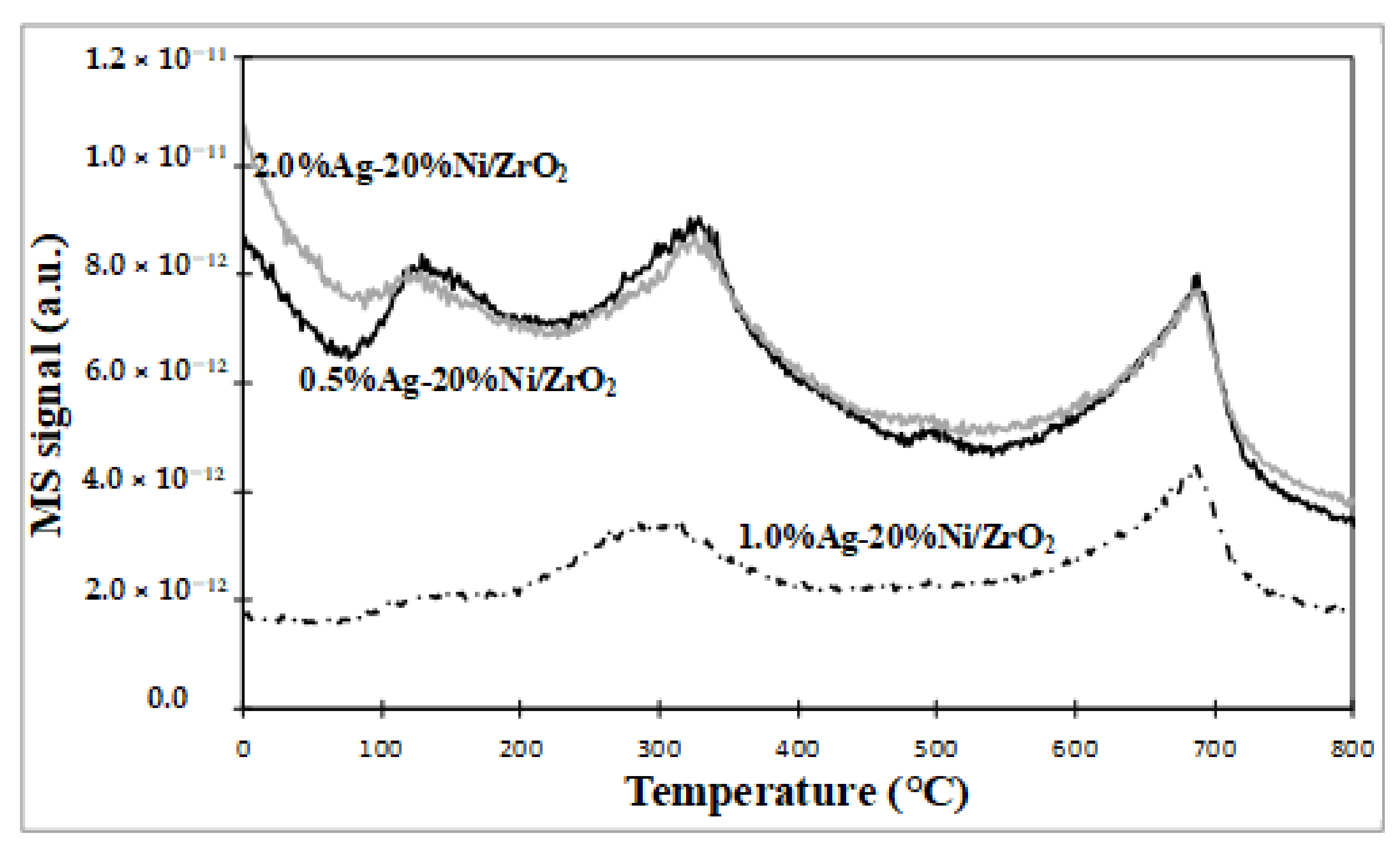
2.2.6. Thermal Analysis of the Selected Catalytic Materials
3. Experimental
3.1. Supports and Catalysts Preparation
3.2. Characterization of the Catalytic Material
3.3. Catalytic Activity Measurements in Oxy-Steam Reforming of Methane or LNG
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mazloomi, K.; Gomes, C. Hydrogen as an energy carrier: Prospects and challenges. Renew. Sustain. Energy Rev. 2012, 16, 3024–3033. [Google Scholar] [CrossRef]
- Moon, D.J. Hydrogen Production by Catalytic Reforming of Gaseous Hydrocarbons (Methane & LPG). Catal. Surv. Asia 2008, 12, 188–202. [Google Scholar] [CrossRef]
- Vita, A.; Cristiano, G.; Italiano, C.; Specchia, S.; Cipitì, F.; Specchia, V. Methane oxy-steam reforming reaction: Performances of Ru/γ-Al2O3 catalysts loaded on structured cordierite monoliths. Int. J. Hydrog. Energy 2014, 39, 18592–18603. [Google Scholar] [CrossRef]
- Vella, L.D.; Villoria, J.A.; Specchia, S.; Mota, N.; Fierro, J.L.G.; Specchia, V. Catalytic partial oxidation of CH4 with nickel–lanthanum-based catalysts. Catal. Today 2011, 171, 84–96. [Google Scholar] [CrossRef]
- Vella, L.D.; Specchia, S. Alumina-supported nickel catalysts for catalytic partial oxidation of methane in short-contact time reactors. Catal. Today 2011, 176, 340–346. [Google Scholar] [CrossRef]
- Pino, L.; Vita, A.; Cordaro, M.; Recupero, V.; Hegde, M.S. A comparative study of Pt/CeO2 catalysts for catalytic partial oxidation of methane to syngas for application in fuel cell electric vehicles. Appl. Catal. A Gen. 2003, 243, 135–146. [Google Scholar] [CrossRef]
- Christian Enger, B.; Lødeng, R.; Holmen, A. A review of catalytic partial oxidation of methane to synthesis gas with emphasis on reaction mechanisms over transition metal catalysts. Appl. Catal. A Gen. 2008, 346, 1–27. [Google Scholar] [CrossRef]
- Peela, N.R.; Kunzru, D. Oxidative steam reforming of ethanol over Rh based catalysts in a micro-channel reactor. Int. J. Hydrog. Energy 2011, 36, 3384–3396. [Google Scholar] [CrossRef]
- Mierczynski, P.; Mosinska, M.; Maniukiewicz, W.; Nowosielska, M.; Czylkowska, A.; Szynkowska, M.I. Oxy-steam reforming of methanol on copper catalysts. React. Kinet. Mech. Catal. 2019, 127, 857–874. [Google Scholar] [CrossRef]
- Mierczynski, P.; Mosinska, M.; Zakrzewski, M.; Dawid, B.; Ciesielski, R.; Maniukiewicz, W.; Maniecki, T. Influence of the Zn–Al binary oxide composition on the physicochemical and catalytic properties of Ni catalysts in the oxy-steam reforming of methanol. React. Kinet. Mech. Catal. 2017, 121, 453–472. [Google Scholar] [CrossRef][Green Version]
- Mierczynski, P.; Mierczynska, A.; Ciesielski, R.; Mosinska, M.; Nowosielska, M.; Czylkowska, A.; Maniukiewicz, W.; Szynkowska, I.M.; Vasilev, K. High Active and Selective Ni/CeO2–Al2O3 and Pd–Ni/CeO2–Al2O3 Catalysts for Oxy-Steam Reforming of Methanol. Catalysts 2018, 8, 380. [Google Scholar] [CrossRef]
- Rampe, T.; Heinzel, A.; Vogel, B. Hydrogen generation from biogenic and fossil fuels by autothermal reforming. J. Power Sources 2000, 86, 536–541. [Google Scholar] [CrossRef]
- Cutillo, A.; Specchia, S.; Antonini, M.; Saracco, G.; Specchia, V. Diesel fuel processor for PEM fuel cells: Two possible alternatives (ATR versus SR). J. Power Sources 2006, 154, 379–385. [Google Scholar] [CrossRef]
- Villoria, J.A.; Alvarez-Galvan, M.C.; Al-Zahrani, S.M.; Palmisano, P.; Specchia, S.; Specchia, V.; Fierro, J.L.G.; Navarro, R.M. Oxidative reforming of diesel fuel over LaCoO3 perovskite derived catalysts: Influence of perovskite synthesis method on catalyst properties and performance. Appl. Catal. B Environ. 2011, 105, 276–288. [Google Scholar] [CrossRef]
- Krumpelt, M.; Krause, T.R.; Carter, J.D.; Kopasz, J.P.; Ahmed, S. Fuel processing for fuel cell systems in transportation and portable power applications. Catal. Today 2002, 77, 3–16. [Google Scholar] [CrossRef]
- Mierczynski, P.; Mosinska, M.; Maniukiewicz, W.; Vasilev, K.; Szynkowska, M.I. Novel Rh(Pd)-Cu(Ni) supported catalysts for oxy-steam reforming of methanol. Arab. J. Chem. 2018. [Google Scholar] [CrossRef]
- Gonçalves, G.; Lenzi, M.K.; Santos, O.A.A.; Jorge, L.M.M. Preparation and characterization of nickel based catalysts on silica, alumina and titania obtained by sol–gel method. J. Non Cryst. Solids 2006, 352, 3697–3704. [Google Scholar] [CrossRef]
- Nieva, M.A.; Villaverde, M.M.; Monzón, A.; Garetto, T.F.; Marchi, A.J. Steam-methane reforming at low temperature on nickel-based catalysts. Chem. Eng. J. 2014, 235, 158–166. [Google Scholar] [CrossRef]
- Kim, J.-H.; Suh, D.J.; Park, T.-J.; Kim, K.-L. Effect of metal particle size on coking during CO2 reforming of CH4 over Ni–alumina aerogel catalysts. Appl. Catal. A Gen. 2000, 197, 191–200. [Google Scholar] [CrossRef]
- Pino, L.; Vita, A.; Cipitì, F.; Laganà, M.; Recupero, V. Performance of Pt/CeO2 catalyst for propane oxidative steam reforming. Appl. Catal. A Gen. 2006, 306, 68–77. [Google Scholar] [CrossRef]
- Jakobsen, J.G.; Jørgensen, T.L.; Chorkendorff, I.; Sehested, J. Steam and CO2 reforming of methane over a Ru/ZrO2 catalyst. Appl. Catal. A Gen. 2010, 377, 158–166. [Google Scholar] [CrossRef]
- Carvalho, L.S.; Martins, A.R.; Reyes, P.; Oportus, M.; Albonoz, A.; Vicentini, V.; Rangel, M.d.C. Preparation and characterization of Ru/MgO-Al2O3 catalysts for methane steam reforming. Catal. Today 2009, 142, 52–60. [Google Scholar] [CrossRef]
- Hosseini, S.M.S.; Hashemipour Rafsanjani, H.; Talebizadeh, A.R. Methane oxy-steam reforming over a highly efficient Ni/Al2O3 nanocatalyst prepared by microwave-assisted impregnation method. Iran. J. Chem. Eng. 2017, 14, 3–16. [Google Scholar]
- Yoshida, K.; Begum, N.; Ito, S.-i.; Tomishige, K. Oxidative steam reforming of methane over Ni/α-Al2O3 modified with trace noble metals. Appl. Catal. A Gen. 2009, 358, 186–192. [Google Scholar] [CrossRef]
- Parizotto, N.V.; Rocha, K.O.; Damyanova, S.; Passos, F.B.; Zanchet, D.; Marques, C.M.P.; Bueno, J.M.C. Alumina-supported Ni catalysts modified with silver for the steam reforming of methane: Effect of Ag on the control of coke formation. Appl. Catal. A Gen. 2007, 330, 12–22. [Google Scholar] [CrossRef]
- Roh, H.-S.; Jun, K.-W.; Dong, W.-S.; Park, S.-E.; Baek, Y.-S. Highly stable Ni catalyst supported on Ce–ZrO2 for oxy-steam reforming of methane. Catal. Lett. 2001, 74, 31–36. [Google Scholar] [CrossRef]
- Dong, W.-S.; Roh, H.-S.; Jun, K.-W.; Park, S.-E.; Oh, Y.-S. Methane reforming over Ni/Ce-ZrO2 catalysts: Effect of nickel content. Appl. Catal. A Gen. 2002, 226, 63–72. [Google Scholar] [CrossRef]
- Wang, H.; Blaylock, D.W.; Dam, A.H.; Liland, S.E.; Rout, K.R.; Zhu, Y.-A.; Green, W.H.; Holmen, A.; Chen, D. Steam methane reforming on a Ni-based bimetallic catalyst: Density functional theory and experimental studies of the catalytic consequence of surface alloying of Ni with Ag. Catal. Sci. Technol. 2017, 7, 1713–1725. [Google Scholar] [CrossRef]
- Seo, J.G.; Youn, M.H.; Jung, J.C.; Song, I.K. Hydrogen production by steam reforming of liquefied natural gas (LNG) over mesoporous nickel–alumina aerogel catalyst. Int. J. Hydrog. Energy 2010, 35, 6738–6746. [Google Scholar] [CrossRef]
- Seo, J.G.; Youn, M.H.; Park, S.; Chung, J.S.; Song, I.K. Hydrogen production by steam reforming of liquefied natural gas (LNG) over Ni/Al2O3–ZrO2 xerogel catalysts: Effect of calcination temperature of Al2O3–ZrO2 xerogel supports. Int. J. Hydrog. Energy 2009, 34, 3755–3763. [Google Scholar] [CrossRef]
- Harun, N.; Abidin, S.Z.; Osazuwa, O.U.; Taufiq-Yap, Y.H.; Azizan, M.T. Hydrogen production from glycerol dry reforming over Ag-promoted Ni/Al2O3. Int. J. Hydrog. Energy 2019, 44, 213–225. [Google Scholar] [CrossRef]
- Mierczynski, P.; Stępińska, N.; Mosinska, M.; Chalupka, K.; Albinska, J.; Maniukiewicz, W.; Rogowski, J.; Nowosielska, M.; Szynkowska, M.I. Hydrogen Production via the Oxy-Steam Reforming of LNG or Methane on Ni Catalysts. Catalysts 2020, 10, 346. [Google Scholar] [CrossRef]
- Hoang, D.L.; Lieske, H. Effect of hydrogen treatments on ZrO2 and Pt/ZrO2 catalysts. Catal. Lett. 1994, 27, 33–42. [Google Scholar] [CrossRef]
- Calafat, Á.; Sánchez, N. Production of carbon nanotubes through combination of catalyst reduction and methane decomposition over Fe–Ni/ZrO2 catalysts prepared by the citrate method. Appl. Catal. A Gen. 2016, 528, 14–23. [Google Scholar] [CrossRef]
- Ren, J.; Qin, X.; Yang, J.-Z.; Qin, Z.-F.; Guo, H.-L.; Lin, J.-Y.; Li, Z. Methanation of carbon dioxide over Ni–M/ZrO2 (M=Fe, Co, Cu) catalysts: Effect of addition of a second metal. Fuel Process. Technol. 2015, 137, 204–211. [Google Scholar] [CrossRef]
- Hengne, A.M.; Malawadkar, A.V.; Biradar, N.S.; Rode, C.V. Surface synergism of an Ag–Ni/ZrO2 nanocomposite for the catalytic transfer hydrogenation of bio-derived platform molecules. RSC Adv. 2014, 4, 9730–9736. [Google Scholar] [CrossRef]
- Mierczynski, P.; Vasilev, K.; Mierczynska, A.; Maniukiewicz, W.; Ciesielski, R.; Rogowski, J.; Szynkowska, I.M.; Trifonov, A.Y.; Dubkov, S.V.; Gromov, D.G.; et al. The effect of gold on modern bimetallic Au-Cu/MWCNT catalysts for the oxy-steam reforming of methanol. Catal. Sci. Technol. 2016. [Google Scholar] [CrossRef]
- Pei, G.X.; Liu, X.Y.; Wang, A.; Su, Y.; Li, L.; Zhang, T. Selective hydrogenation of acetylene in an ethylene-rich stream over silica supported Ag-Ni bimetallic catalysts. Appl. Catal. A Gen. 2017, 545, 90–96. [Google Scholar] [CrossRef]
- Roh, H.-S.; Jun, K.-W. Carbon Dioxide Reforming of Methane over Ni Catalysts Supported on Al2O3 Modified with La2O3, MgO, and CaO. Catal. Surv. Asia 2008, 12, 239–252. [Google Scholar] [CrossRef]
- Yagi, F.; Kanai, R.; Wakamatsu, S.; Kajiyama, R.; Suehiro, Y.; Shimura, M. Development of synthesis gas production catalyst and process. Catal. Today 2005, 104, 2–6. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, H.; Li, W. Morphology observation of carbon deposition by CH4 decomposition over Ni-based catalysts. Nanotechnology 2004, 16, 129–132. [Google Scholar] [CrossRef]
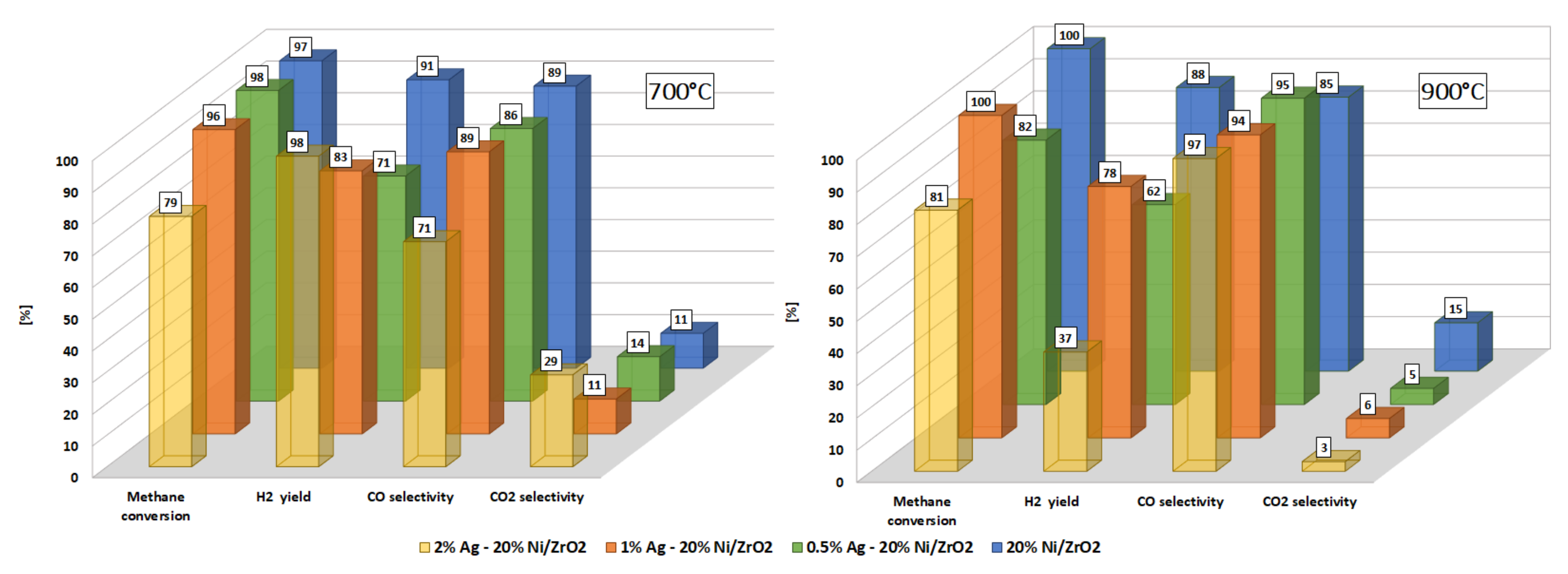
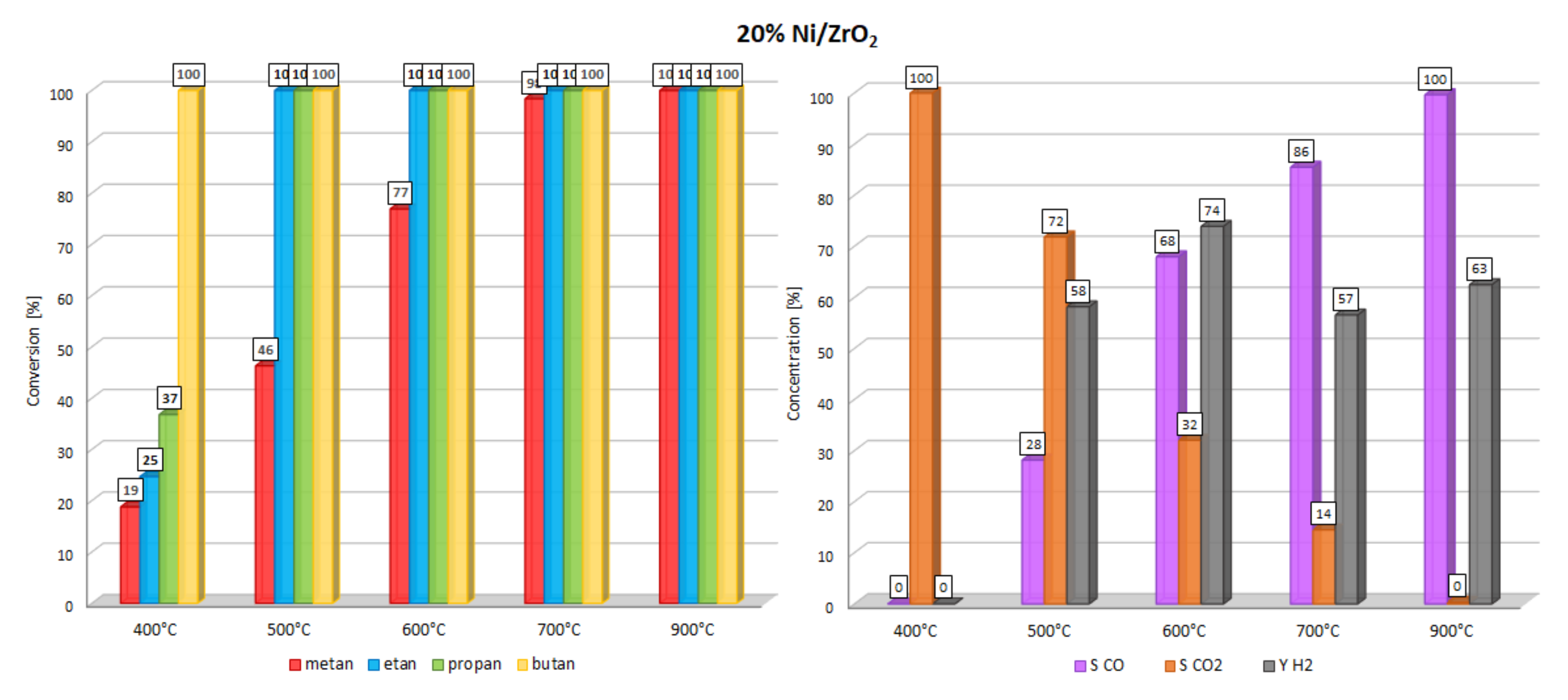



| Catalysts | Temp. [°C] | Methane Conversion [%] | CO2 Selectivity [%] | CO Selectivity [%] | H2 Yield [%] |
|---|---|---|---|---|---|
| 20% Ni/ZrO2 | 700 | 97 | 11 | 89 | 91 |
| 900 | 100 | 15 | 85 | 88 | |
| 0.5% Ag-20% Ni/ZrO2 | 700 | 98 | 14 | 86 | 71 |
| 900 | 82 | 5 | 95 | 62 | |
| 1% Ag-20% Ni/ZrO2 | 700 | 96 | 11 | 89 | 83 |
| 900 | 100 | 6 | 94 | 78 | |
| 2% Ag-20% Ni/ZrO2 | 700 | 79 | 29 | 71 | 98 |
| 900 | 81 | 3 | 97 | 37 |
| Catalysts | Temp [°C] | Methane Conversion [%] | Ethane Conversion [%] | Propane Conversion [%] | Butane Conversion [%] |
|---|---|---|---|---|---|
| 20% Ni/ZrO2 | 400 | 19 | 25 | 37 | 100 |
| 500 | 46 | 100 | 100 | 100 | |
| 600 | 77 | 100 | 100 | 100 | |
| 700 | 98 | 100 | 100 | 100 | |
| 900 | 100 | 100 | 100 | 100 | |
| 0.5% Ag-20% Ni/ZrO2 | 400 | 2 | 14 | 26 | 100 |
| 500 | 6 | 34 | 83 | 100 | |
| 600 | 57 | 100 | 100 | 100 | |
| 700 | 73 | 100 | 100 | 100 | |
| 900 | 81 | 100 | 100 | 100 | |
| 1% Ag-20% Ni/ZrO2 | 400 | 0 | 10 | 25 | 100 |
| 500 | 2 | 46 | 86 | 100 | |
| 600 | 62 | 100 | 100 | 100 | |
| 700 | 72 | 100 | 100 | 100 | |
| 900 | 75 | 100 | 100 | 100 | |
| 2% Ag-20% Ni/ZrO2 | 400 | 0 | 10 | 20 | 100 |
| 500 | 4 | 36 | 71 | 100 | |
| 600 | 7 | 57 | 80 | 100 | |
| 700 | 12 | 59 | 82 | 100 | |
| 900 | 53 | 95 | 100 | 100 |
| Catalysts | Temp [°C] | CO2 Selectivity [%] | CO Selectivity [%] | H2 Yield [%] |
|---|---|---|---|---|
| 20% Ni/ZrO2 | 400 | 100 | 0 | 0 |
| 500 | 72 | 28 | 58 | |
| 600 | 32 | 68 | 74 | |
| 700 | 14 | 86 | 57 | |
| 900 | 0 | 100 | 63 | |
| 0.5% Ag-20% Ni/ZrO2 | 400 | 100 | 0 | 0 |
| 500 | 100 | 0 | 0 | |
| 600 | 56 | 44 | 63 | |
| 700 | 35 | 65 | 66 | |
| 900 | 24 | 76 | 54 | |
| 1% Ag-20% Ni/ZrO2 | 400 | 100 | 0 | 0 |
| 500 | 100 | 0 | 0 | |
| 600 | 48 | 52 | 70 | |
| 700 | 39 | 61 | 59 | |
| 900 | 18 | 82 | 65 | |
| 2% Ag-20% Ni/ZrO2 | 400 | 100 | 0 | 0 |
| 500 | 100 | 0 | 0 | |
| 600 | 100 | 0 | 0 | |
| 700 | 100 | 0 | 75 | |
| 900 | 28 | 72 | 63 |
| Catalytic Materials | BET Surface Area [m2/g] | Monolayer Capacity [cm3/g] | Average Pore Radius [nm] |
|---|---|---|---|
| ZrO2 | 110 | 0.26 | 3.4 |
| 20% Ni/ZrO2 | 77 | 0.18 | 3.4 |
| 0.5% Ag-20% Ni/ZrO2 | 66 | 0.17 | 3.8 |
| 1% Ag-20% Ni/ZrO2 | 69 | 0.18 | 3.8 |
| 2% Ag-20% Ni/ZrO2 | 69 | 0.19 | 3.8 |
| Catalytic Materials | Total Acidity [mmol/g] | Weak Centres [mmol/g] | Medium Centres [mmol/g] | Strong Centres [mmol/g] |
|---|---|---|---|---|
| 100–450 °C | 100–300 °C | 300–450 °C | >450°C | |
| ZrO2 | 0.10 | 0.05 | 0.04 | 0.01 |
| 20% Ni/ZrO2 | 0.22 | 0.12 | 0.09 | 0.01 |
| 0.5% Ag-20% Ni/ZrO2 | 0.49 | 0.23 | 0.23 | 0.03 |
| 1% Ag-20% Ni/ZrO2 | 0.44 | 0.23 | 0.18 | 0.03 |
| 2% Ag-20% Ni/ZrO2 | 0.43 | 0.23 | 0.18 | 0.02 |
| Catalysts | Total H2 Consumption [mmol/gcat] | Reduction Degree of NiO |
|---|---|---|
| 20% Ni/ZrO2 | 2.70 | 80 |
| 0.5% Ag-20% Ni/ZrO2 | 2.65 | 78 |
| 1% Ag-20% Ni/ZrO2 | 3.03 | 88 |
| 2% Ag-20% Ni/ZrO2 | 2.81 | 82 |
| Catalysts | Peak Contribution to the Overall TPR Peak Area (%) | |||
|---|---|---|---|---|
| I-Peak (Tmax) | II-Peak (Tmax) | III-Peak (Tmax) | IV-Peak (Tmax) | |
| 20% Ni/ZrO2 | - | 8 (220 °C) | 55 (345 °C) | 37 (430 °C) |
| 0.5% Ag-20% Ni/ZrO2 | 1.5 (135 °C) | 0.5 (175 °C) | 90 (320 °C) | 8 (420 °C) |
| 1% Ag-20% Ni/ZrO2 | 2 (110 °C) | 1 (150 °C) | 86 (335 °C) | 11 (410 °C) |
| 2% Ag-20% Ni/ZrO2 | 2 (125 °C) | 1 (140 °C) | 83 (340 °C) | 14 (410 °C) |
| Catalyst | The Size of NiO Crystallites [nm] |
|---|---|
| 20% Ni/ZrO2 | 32 |
| 0.5% Ag-20% Ni/ZrO2 | 33 |
| 1% Ag-20% Ni/ZrO2 | 17 |
| 2% Ag-20% Ni/ZrO2 | 18 |
| Catalyst | The Size of Metallic Ni Crystallites [nm] |
|---|---|
| 20% Ni/ZrO2 | 35 |
| 0.5% Ag-20% Ni/ZrO2 | 39 |
| 1% Ag-20% Ni/ZrO2 | 39 |
| 2% Ag-20% Ni/ZrO2 | 36 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mosinska, M.; Stepinska, N.; Chalupka, K.; Maniukiewicz, W.; Szynkowska, M.I.; Mierczynski, P. Effect of Ag-Addition on the Catalytic and Physicochemical Properties of Ni/ZrO2 Catalyst in Oxy-Steam Reforming of CH4 and LNG Processes. Catalysts 2020, 10, 855. https://doi.org/10.3390/catal10080855
Mosinska M, Stepinska N, Chalupka K, Maniukiewicz W, Szynkowska MI, Mierczynski P. Effect of Ag-Addition on the Catalytic and Physicochemical Properties of Ni/ZrO2 Catalyst in Oxy-Steam Reforming of CH4 and LNG Processes. Catalysts. 2020; 10(8):855. https://doi.org/10.3390/catal10080855
Chicago/Turabian StyleMosinska, Magdalena, Natalia Stepinska, Karolina Chalupka, Waldemar Maniukiewicz, Malgorzata I. Szynkowska, and Pawel Mierczynski. 2020. "Effect of Ag-Addition on the Catalytic and Physicochemical Properties of Ni/ZrO2 Catalyst in Oxy-Steam Reforming of CH4 and LNG Processes" Catalysts 10, no. 8: 855. https://doi.org/10.3390/catal10080855
APA StyleMosinska, M., Stepinska, N., Chalupka, K., Maniukiewicz, W., Szynkowska, M. I., & Mierczynski, P. (2020). Effect of Ag-Addition on the Catalytic and Physicochemical Properties of Ni/ZrO2 Catalyst in Oxy-Steam Reforming of CH4 and LNG Processes. Catalysts, 10(8), 855. https://doi.org/10.3390/catal10080855