Development of a Novel Method for the Fabrication of Nanostructured Zr(x)Ni(y) Catalyst to Enhance the Desorption Properties of MgH2
Abstract
1. Introduction
2. Results
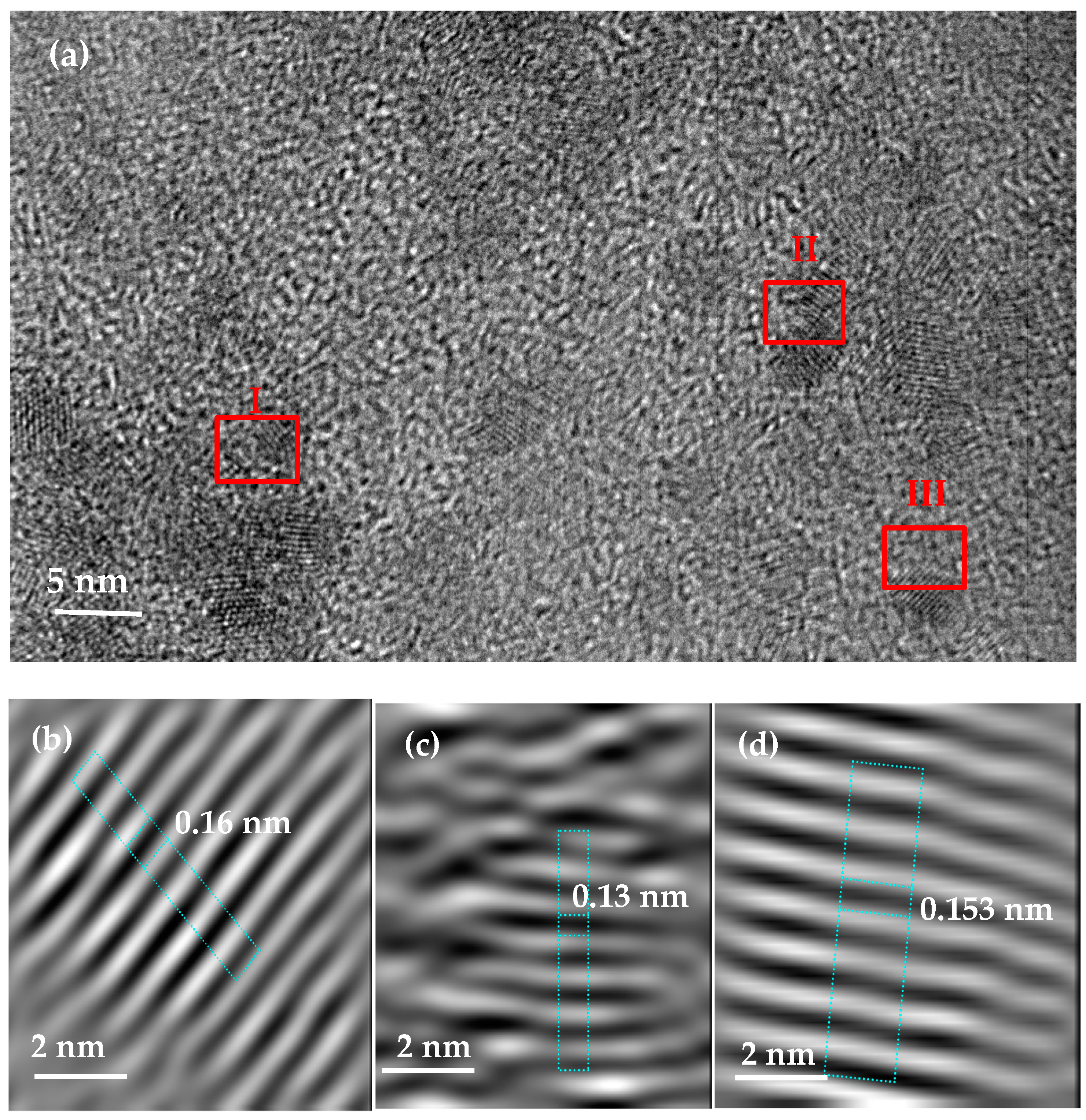
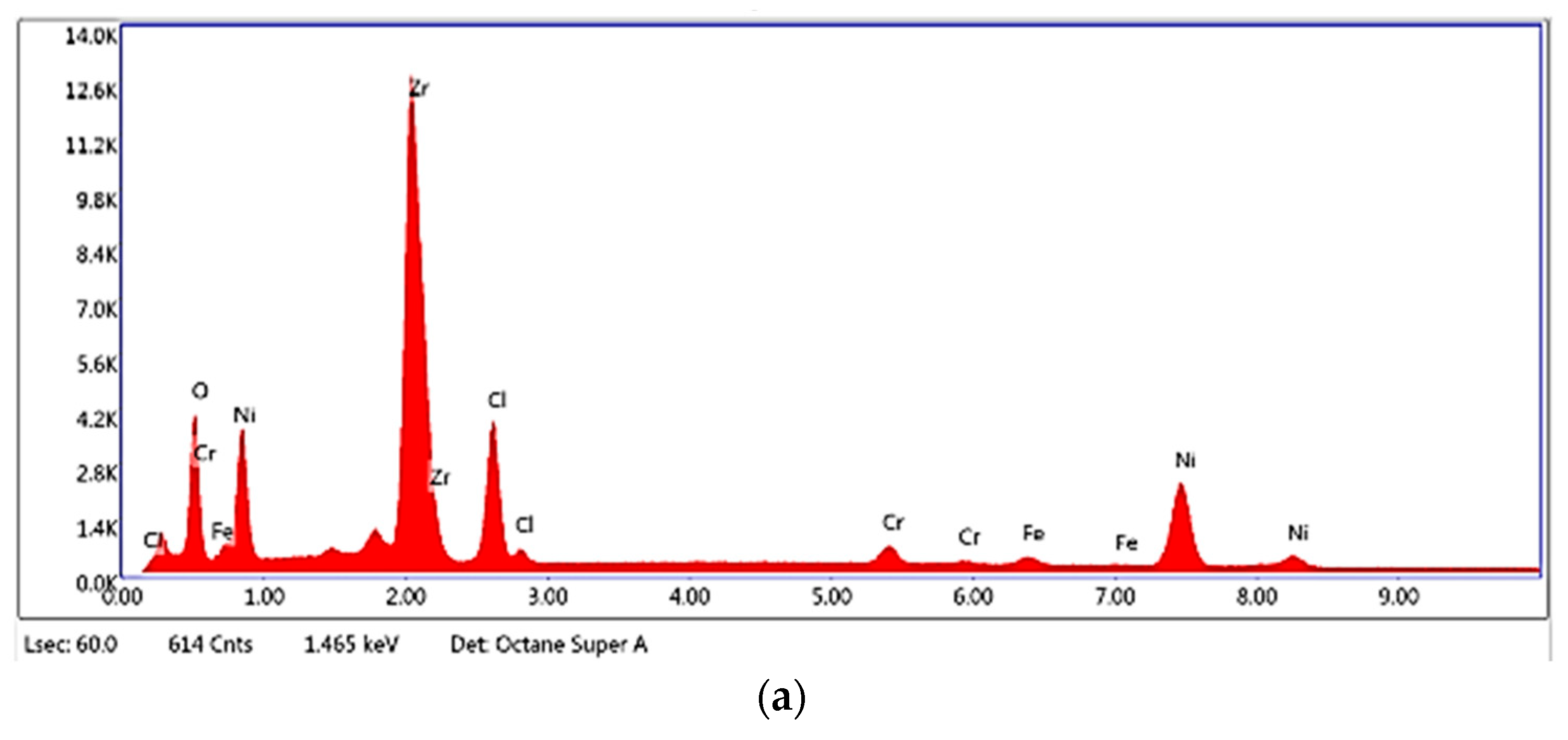
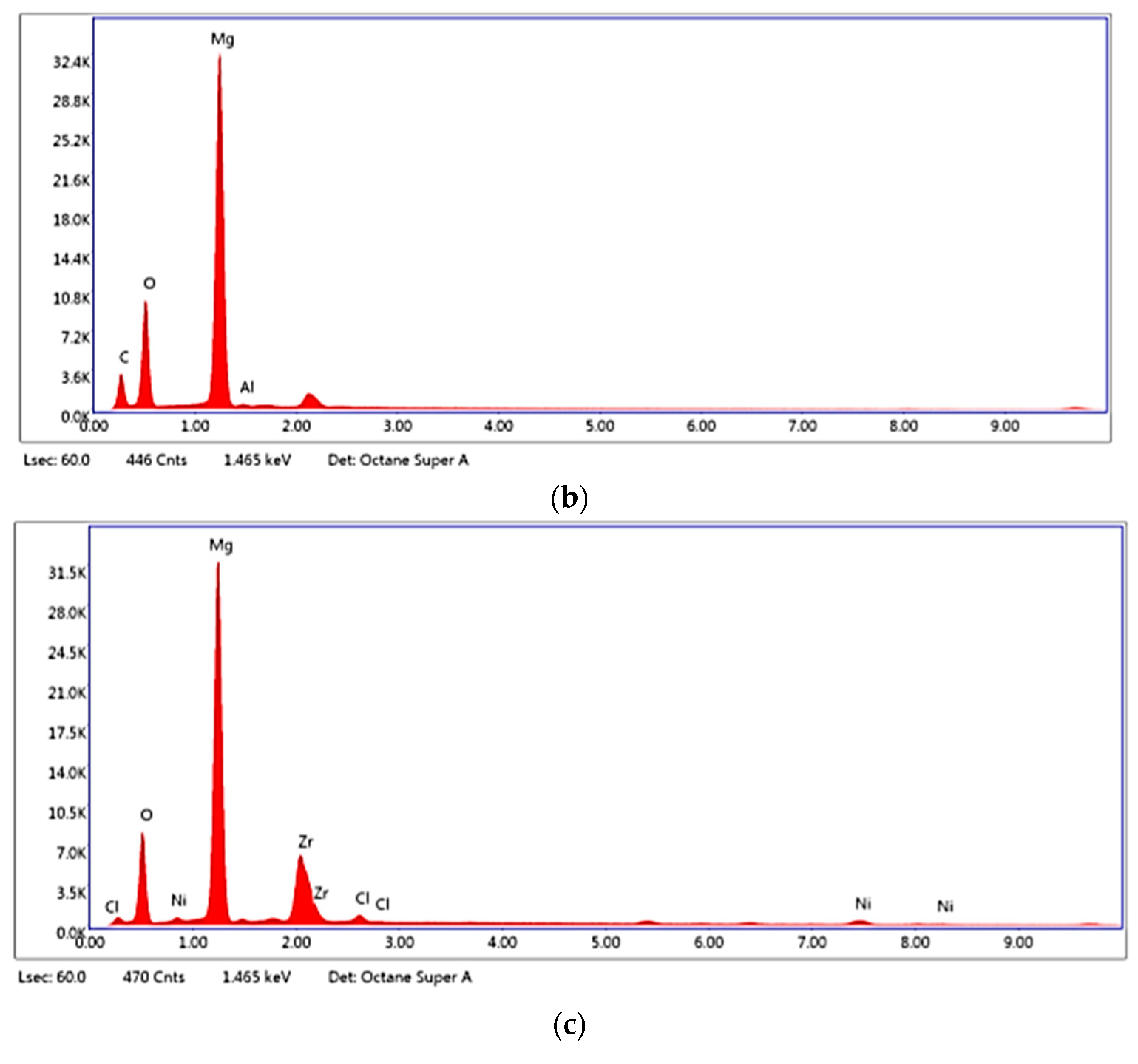
2.1. Characterisation of Zr(x)Ni(y) Catalyst
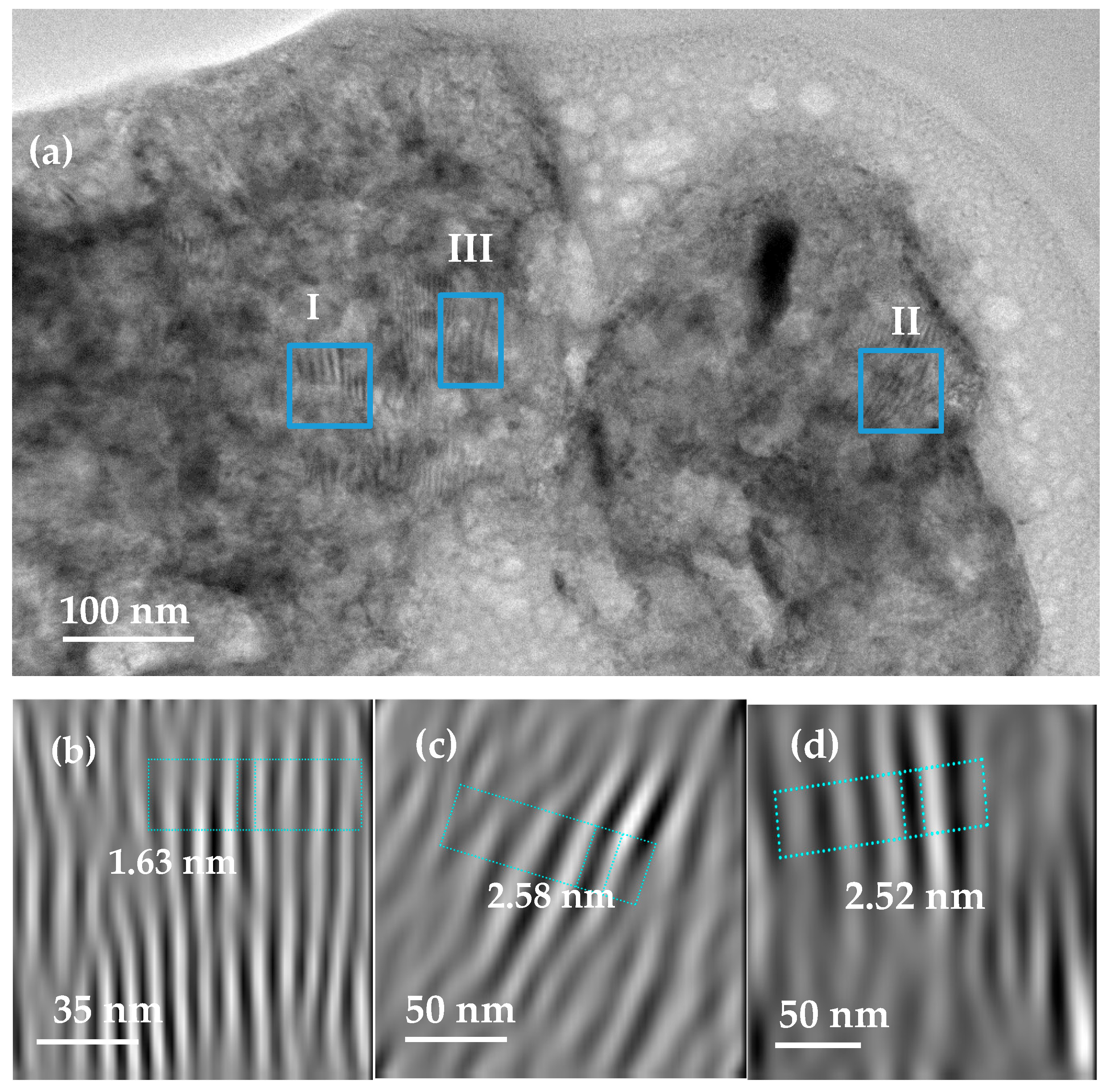
2.2. Characterisation of MgH2/10 wt.% Zr(x)Ni(y) Nanocomposite
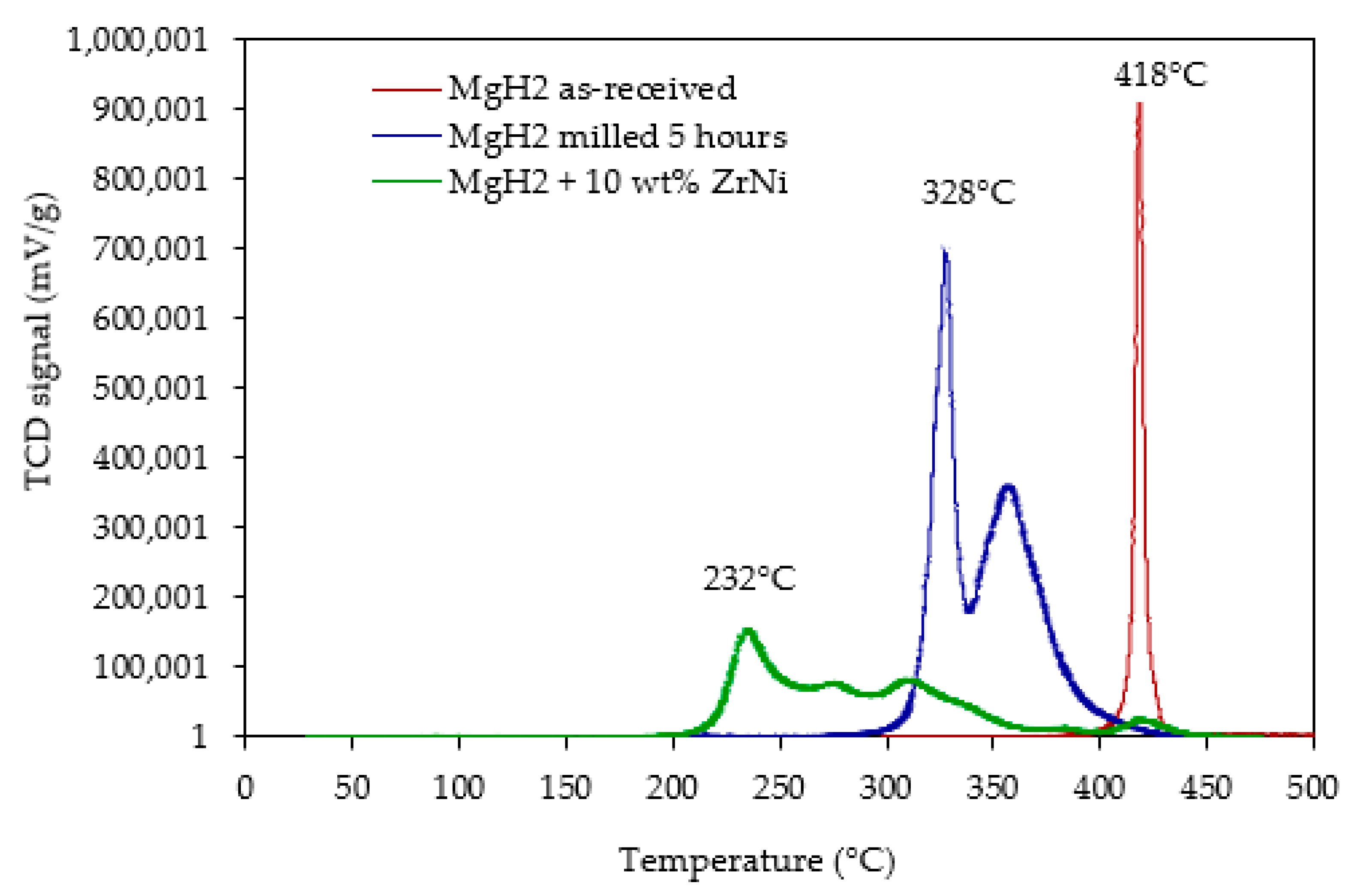
2.3. Dehydrogenation Properties of the Nanocomposite MgH2/10 wt.% Zr(x)Ni(y)
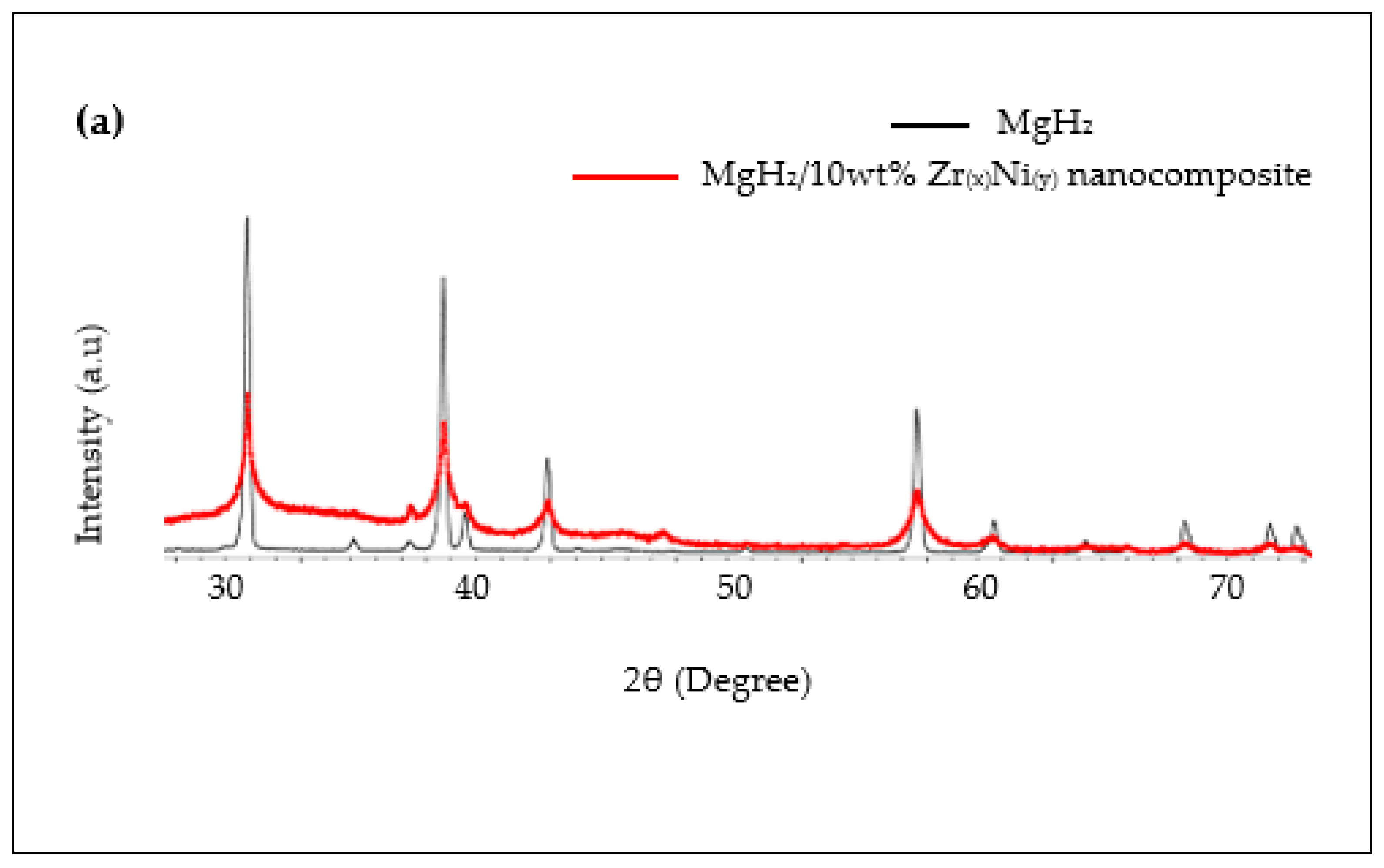
2.4. Characterisation of Crystal Structures
3. Discussion and Conclusions
4. Materials and Methods
4.1. Catalyst Synthesis
4.2. Preparation of MgH2-Based Nanocomposite Powders
4.3. Sample Characterisations
4.4. The Dehydrogenation Behaviour
Author Contributions
Funding
Conflicts of Interest
References
- Aguey-Zinsou, K.F.; Ares-Fernández, J.R. Hydrogen in magnesium: New perspectives toward functional stores. Energy Environ. Sci. 2010, 3, 526–543. [Google Scholar] [CrossRef]
- Sadhasivam, T.; Kim, H.T.; Jung, S.; Roh, S.H.; Park, J.H.; Jung, H.Y. Dimensional effects of nanostructured Mg/MgH2 for hydrogen storage applications: A review. Renew. Sustain. Energy Rev. 2017, 72, 523–534. [Google Scholar] [CrossRef]
- Zhang, X.X.; Liu, Y.; Zhang, X.X.; Hu, J.; Gao, M.; Pan, H. Empowering hydrogen storage performance of MgH2 by nanoengineering and nanocatalysis. Mater. Today Nano 2019, 9, 100064. [Google Scholar] [CrossRef]
- Matar, S.F. Intermetallic hydrides: A review with ab initio aspects. Prog. Solid State Chem. 2010, 38, 1–37. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S. Metallic glassy Zr70Ni20Pd10 powders for improving the hydrogenation/dehydrogenation behavior of MgH2. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Shaban, E.; Al-Matrouk, H.; Behbehani, M.; Alkandary, A.; Aldakheel, F.; Ali, N.; Ahmed, S.A. Structure, morphology and hydrogen storage kinetics of nanocomposite MgH2/10 wt% ZrNi5 powders. Mater. Today Energy 2017, 3, 60–71. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y. Recent advances in additive-enhanced magnesium hydride for hydrogen storage. Prog. Nat. Sci. Mater. Int. 2017, 27, 41–49. [Google Scholar] [CrossRef]
- El Khatabi, M.; Naji, S.; Bhihi, M.; Benyoussef, A.; El Kenz, A.; Loulidi, M. Effects of double substitution on MgH2 hydrogen storage properties: An Ab initio study. J. Alloys Compd. 2018, 743, 666–671. [Google Scholar] [CrossRef]
- Galey, B.; Auroux, A.; Sabo-Etienne, S.; Dhaher, S.; Grellier, M.; Postole, G. Improved hydrogen storage properties of Mg/MgH2 thanks to the addition of nickel hydride complex precursors. Int. J. Hydrogen Energy 2019, 44, 28848–28862. [Google Scholar] [CrossRef]
- Zaluska, A.; Zaluski, L.; Ström–Olsen, J.O. Nanocrystalline magnesium for hydrogen storge. J. Alloys Compd. 1999, 288, 217–225. [Google Scholar] [CrossRef]
- Zaluski, L.; Zaluska, A.; Ström-Olsen, J.O. Hydrogen absorption in nanocrystalline Mg2Ni formed by mechanical alloying. J. Alloys Compd. 1995, 217, 245–249. [Google Scholar] [CrossRef]
- Jain, I.P.; Lal, C.; Jain, A. Hydrogen storage in Mg: A most promising material. Int. J. Hydrogen Energy 2010, 35, 5133–5144. [Google Scholar] [CrossRef]
- Pighin, S.A.; Capurso, G.; Lo Russo, S.; Peretti, H.A. Hydrogen sorption kinetics of magnesium hydride enhanced by the addition of Zr8Ni21 alloy. J. Alloys Compd. 2012, 530, 111–115. [Google Scholar] [CrossRef]
- Dehouche, Z.; Peretti, H.A.; Hamoudi, S.; Yoo, Y.; Belkacemi, K. Effect of activated alloys on hydrogen discharge kinetics of MgH2 nanocrystals. J. Alloys Compd. 2008, 455, 432–439. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Al-ajmi, F.; Banyan, M. Mechanically-Induced Catalyzation of MgH2 Powders with Zr2 Ni-Ball Milling Media. Catalysts 2019, 9, 382. [Google Scholar] [CrossRef]
- Nei, J.; Young, K.; Regmi, R.; Lawes, G.; Salley, S.O.; Ng, K.Y.S. Gaseous phase hydrogen storage and electrochemical properties of Zr8Ni21, Zr7Ni10, Zr9Ni11, and ZrNi metal hydride alloys. Int. J. Hydrogen Energy 2012, 37, 16042–16055. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Al-Matrouk, H.; Behbehani, M.; Shaban, E.; Alkandary, A.; Aldakheel, F.; Al-Saidi, M. Amorphous-versus big cube-Zr2Ni for improving the kinetics of hydrogenation/dehydrogenation behaviors for MgH2powders. Mater. Chem. Phys. 2018, 203, 17–26. [Google Scholar] [CrossRef]
- Molinas, B.; Ghilarducci, A.A.; Melnichuk, M.; Corso, H.L.; Peretti, H.A.; Agresti, F.; Bianchin, A.; Lo Russo, S.; Maddalena, A.; Principi, G. Scaled-up production of a promising Mg-based hydride for hydrogen storage. Int. J. Hydrogen Energy 2009, 34, 4597–4601. [Google Scholar] [CrossRef]
- Ruiz, F.C.; Castro, E.B.; Peretti, H.A.; Visintin, A. Study of the different ZrxNiy phases of Zr- based AB2 materials. Int. J. Hydrogen Energy 2010, 35, 9879–9887. [Google Scholar] [CrossRef]
- Nei, J.; Young, K.; Salley, S.O.; Ng, K.Y.S. Effects of annealing on Zr8Ni19X2(X = Ni, Mg, Al, Sc, V, Mn, Co, Sn, La, and Hf): Hydrogen storage and electrochemical properties. Int. J. Hydrogen Energy 2012, 37, 8418–8427. [Google Scholar] [CrossRef]
- Parayil, S.K.; Psota, R.J.; Koodali, R.T. Modulating the textural properties and photocatalytic hydrogen production activity of TiO2 by high temperature supercritical drying. Int. J. Hydrogen Energy 2013, 38, 10215–10225. [Google Scholar] [CrossRef]
- Galey, B.; Auroux, A.; Sabo-Etienne, S.; Grellier, M.; Postole, G. Enhancing hydrogen storage properties of the Mg/MgH2 system by the addition of bis(tricyclohexylphosphine)nickel(II) dichloride. Int. J. Hydrogen Energy 2019, 44, 11939–11952. [Google Scholar] [CrossRef]
- Zhang, Q.; Zang, L.; Huang, Y.; Gao, P.; Jiao, L.; Yuan, H.; Wang, Y. Improved hydrogen storage properties of MgH2 with Ni-based compounds. Int. J. Hydrogen Energy 2017, 42, 24247–24255. [Google Scholar] [CrossRef]
- Galey, B.; Auroux, A.; Sabo-Etienne, S.; Grellier, M.; Dhaher, S.; Postole, G. Impact of the addition of poly-dihydrogen ruthenium precursor complexes on the hydrogen storage properties of the Mg/MgH2 system. Sustain. Energy Fuels 2018, 2, 2335–2344. [Google Scholar] [CrossRef]
- Chen, B.H.; Chuang, Y.S.; Chen, C.K. Improving the hydrogenation properties of MgH2 at room temperature by doping with nano-size ZrO2 catalyst. J. Alloys Compd. 2016, 655, 21–27. [Google Scholar] [CrossRef]
- Dong, J.; Panda, S.; Zhu, W.; Zou, J.; Ding, W. Enhanced hydrogen sorption properties of MgH2 when doped with mechanically alloyed amorphous Zr0·67Ni0.33 particles. Int. J. Hydrogen Energy 2020. [Google Scholar] [CrossRef]
- Zavaliy, I.Y. Effect of oxygen content on hydrogen storage capacity of Zr-based h-phases. J. Alloys Compd. 1999, 291, 102–109. [Google Scholar] [CrossRef]







| MgH2 | 2020 | 2025 | Ultimate | |
|---|---|---|---|---|
| Gravimetric capacity (H2-wt.%) | 7.6 | 4.5 | 5.5 | 6.5 |
| Volumetric capacity kg H2 L−1 | 1.4 | 0.03 | 0.04 | 0.05 |
| Operating temperature (max/min) °C | >250 | −40/85 | −40/85 | −40/85 |
| Storage system cycle life (cycles) | 1500 | 1500 | 1500 | 1500 |
| Charging rate-filling time for 5 kg of H2 (min) | - | 3–5 | 3–5 | 3–5 |
| Storage system cost ($/kg H2) | Meet target | 333 | 300 | 266 |
| Samples | Peak Desorption Temperature (°C) | Discharge Rate (H-wt.% min−1) | Discharge Capacity (H-wt.%) |
|---|---|---|---|
| MgH2 as-received (un-milled) | 418 | 1.51 | 7 |
| MgH2 (milled 5 h) | 328 | 0.17 | 6.7 |
| MgH2/10 wt.% ZrNi | 232 | 0.07 | 5.9 |
| Elements | Peak Desorption Temperature °C | H2-wt.% | References |
|---|---|---|---|
| 10 wt.% Zr(x)Ni(y) | 232 | 5.9 | Present study |
| 10 wt.% Zr0.67Ni0.33 | 325 | 5.0 | Dong et al. [26] |
| 10 wt.% Zr8Ni21 | 300 | 5.9 | Pighin et al. [13] |
| 10 wt.% ZrNi5 | 275 | 5.3 | El-Eskandarany et al. [6] |
| 10 wt.% Zr9Ni11 | 250 | 5.9 | Dehouche et al. [14] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shokano, G.; Dehouche, Z.; Galey, B.; Postole, G. Development of a Novel Method for the Fabrication of Nanostructured Zr(x)Ni(y) Catalyst to Enhance the Desorption Properties of MgH2. Catalysts 2020, 10, 849. https://doi.org/10.3390/catal10080849
Shokano G, Dehouche Z, Galey B, Postole G. Development of a Novel Method for the Fabrication of Nanostructured Zr(x)Ni(y) Catalyst to Enhance the Desorption Properties of MgH2. Catalysts. 2020; 10(8):849. https://doi.org/10.3390/catal10080849
Chicago/Turabian StyleShokano, Gracia, Zahir Dehouche, Basile Galey, and Georgeta Postole. 2020. "Development of a Novel Method for the Fabrication of Nanostructured Zr(x)Ni(y) Catalyst to Enhance the Desorption Properties of MgH2" Catalysts 10, no. 8: 849. https://doi.org/10.3390/catal10080849
APA StyleShokano, G., Dehouche, Z., Galey, B., & Postole, G. (2020). Development of a Novel Method for the Fabrication of Nanostructured Zr(x)Ni(y) Catalyst to Enhance the Desorption Properties of MgH2. Catalysts, 10(8), 849. https://doi.org/10.3390/catal10080849






