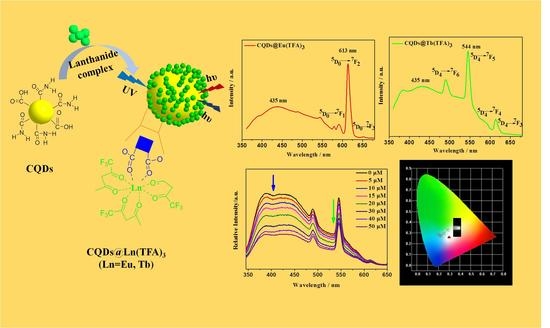
Synthesis of Lanthanide-Functionalized Carbon Quantum Dots for Chemical Sensing and Photocatalytic Application
Abstract
1. Introduction
2. Results and Discussion
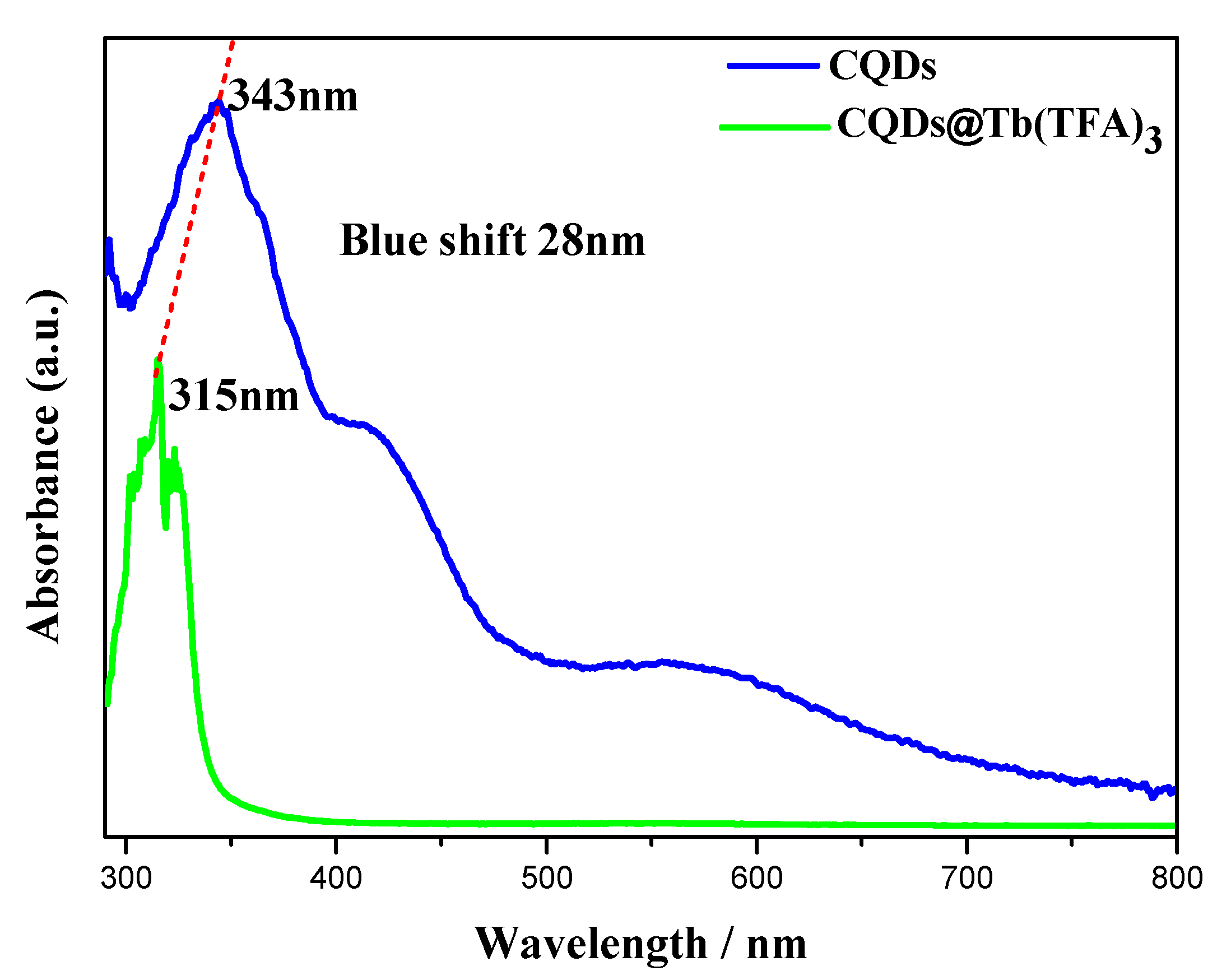
2.1. Preparation and Structural Characterization of CQDs and CQDs@Ln (TFA)3 (Ln = Eu,Tb)
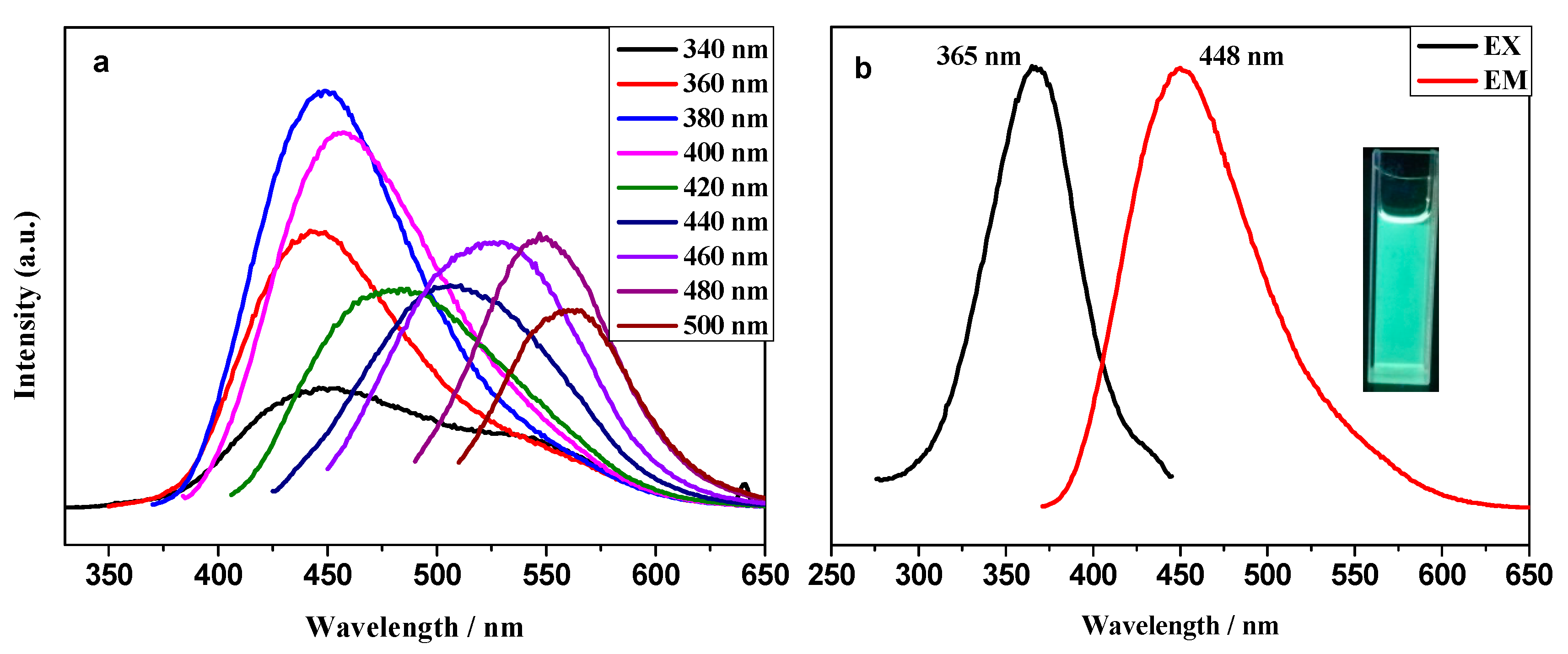
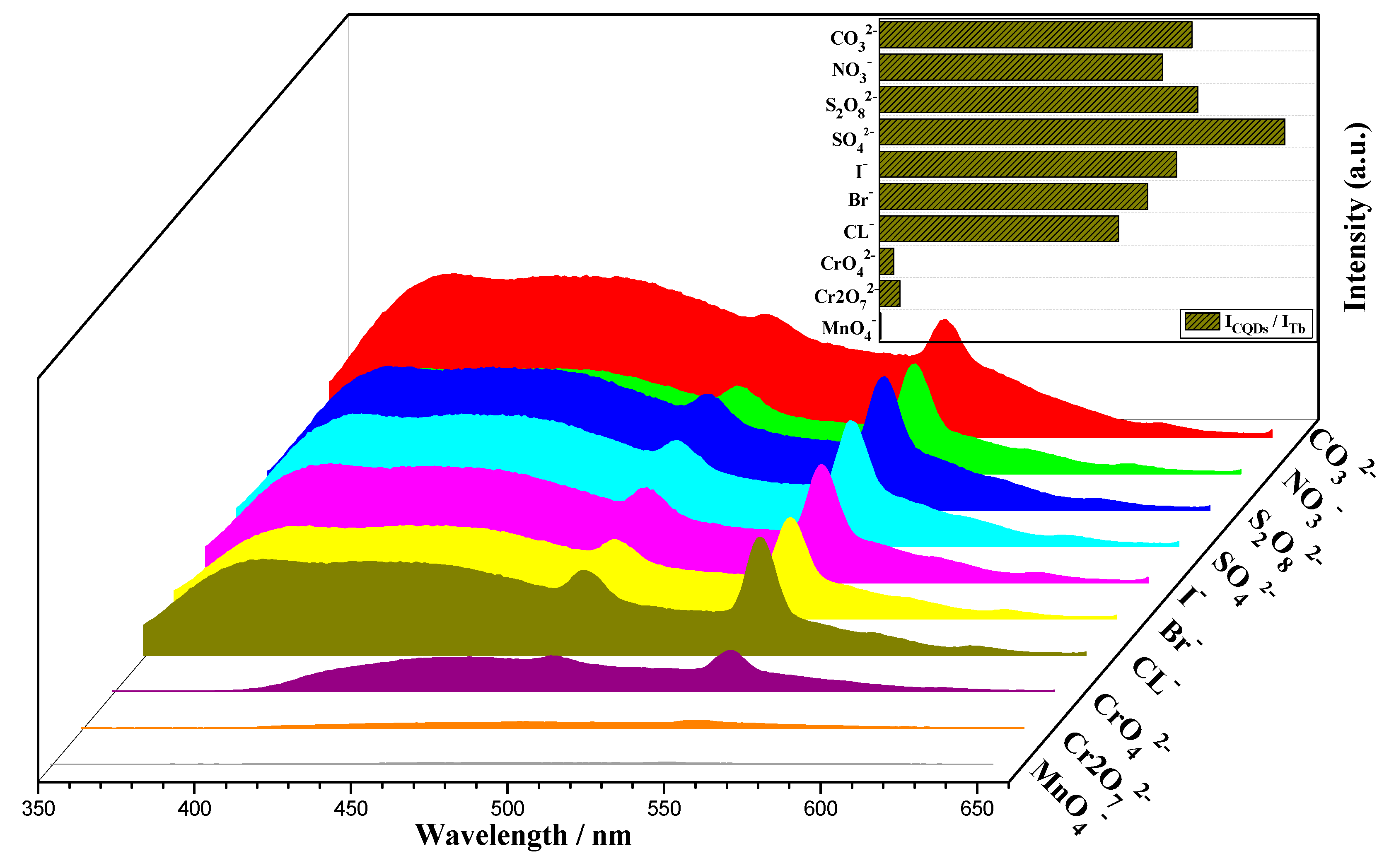
2.2. Photoluminescence Properties and Chemical Sensing Performance of CQDs@Ln (TFA)3
2.3. White-Light Tuning of CQDs@Eu/Tb (TFA)3
3. Materials and Methods
3.1. Materials
3.2. Characterization
3.3. Experimental Methods
3.3.1. Synthesis of CQDs and CQDs@Ln (TFA)3
3.3.2. Preparation of White-Light CQDs@Eu/Tb (TFA)3
3.3.3. Chemical Sensing Experiment of CQDs@Tb (TFA)3
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, Q.; Zhang, S.; Zhong, Y.; Yang, X.-F.; Li, Z.; Li, H. Preparation of yellow-green-emissive carbon dots and their application in constructing a fluorescent turn-on nanoprobe for imaging of selenol in living cells. Anal. Chem. 2017, 89, 1734–1741. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.; Zhou, L.; Tang, L.; Cai, X.; Yuan, Y. Progress in preparation of carbon quantum dots and its application in the fields of energy and environment. Chin. J. Appl. Chem. 2016, 33, 742–755. [Google Scholar]
- Wu, X.; Zhao, B.; Zhang, J.; Xu, H.; Xu, K.; Chen, G. Photoluminescence and photodetecting properties of the hydrothermally synthesized nitrogen-doped carbon quantum dots. J. Phys. Chem. C 2019, 123, 25570–25578. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Qu, S.N.; Feng, X.Y.; Xu, J.C.; Yang, Y.; Su, S.C.; Wang, S.P.; Ng, K.W. Tailoring the photoluminescence excitation dependence of the carbon dots via an alkali treatment. J. Phys. Chem. Lett. 2019, 10, 4596–4602. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Wei, J.-S.; Zhong, N.; Gao, Q.-Y.; Xiong, H.-M. Highly efficient red-emitting carbon dots with gram-scale yield for bioimaging. Langmuir 2017, 33, 12635–12642. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Saha, A.; Jana, N.R.; Sarkar, R. Fluorescent carbon nanoparticles: Synthesis, characterization, and bioimaging application. J. Phys. Chem. C 2009, 113, 18546–18551. [Google Scholar] [CrossRef]
- Cao, L.; Wang, X.; Meziani, M.J.; Lu, F.; Wang, H.; Luo, P.G.; Lin, Y.; Harruff, B.A.; Veca, L.M.; Murray, D. Carbon dots for multiphoton bioimaging. J. Am. Chem. Soc. 2007, 129, 11318–11319. [Google Scholar] [CrossRef]
- Zhu, S.; Meng, Q.; Wang, L.; Zhang, J.; Song, Y.; Jin, H.; Zhang, K.; Sun, H.; Wang, H.; Yang, B. Highly photoluminescent carbon dots for multicolor patterning, sensors, and bioimaging. Angew. Chem. Int. Ed. 2013, 52, 3953–3957. [Google Scholar] [CrossRef]
- Hola, K.; Zhang, Y.; Wang, Y.; Giannelis, E.P.; Zboril, R.; Rogach, A.L. Carbon dots—Emerging light emitters for bioimaging, cancer therapy and optoelectronics. Nano Today 2014, 9, 590–603. [Google Scholar] [CrossRef]
- Zheng, X.T.; Ananthanarayanan, A.; Luo, K.Q.; Chen, P. Glowing graphene quantum dots and carbon dots: Properties, syntheses, and biological applications. Small 2015, 11, 1620–1636. [Google Scholar] [CrossRef]
- Li, Q.; Ohulchanskyy, T.Y.; Liu, R.; Koynov, K.; Wu, D.; Best, A.; Kumar, R.; Bonoiu, A.; Prasad, P.N. Photoluminescent carbon dots as biocompatible nanoprobes for targeting cancer cells in vitro. J. Phys. Chem. C 2010, 114, 12062–12068. [Google Scholar] [CrossRef]
- Li, D.; Jing, P.; Sun, L.; An, Y.; Shan, X.; Lu, X.; Zhou, D.; Han, D.; Shen, D.; Zhai, Y. Near-infrared excitation/emission and multiphoton-induced fluorescence of carbon dots. Adv. Mater. 2018, 30, 1705913. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Zhao, W.; Zhang, S.; Guo, X.; Li, Y.; Du, B. Dual-functional carbon dot-labeled heavy-chain ferritin for self-targeting bio-imaging and chemo-photodynamic therapy. J. Mater. Chem. B 2018, 6, 3107–3115. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Li, Y.; Li, W.; Zhang, H.; Liu, Y.; Ma, L.; Wang, X.; Lei, B. Recent developments in luminescent nanoparticles for plant imaging and photosynthesis. J. Rare Earths 2019, 37, 903–915. [Google Scholar] [CrossRef]
- Mirtchev, P.; Henderson, E.J.; Soheilnia, N.; Yip, C.M.; Ozin, G.A. Solution phase synthesis of carbon quantum dots as sensitizers for nanocrystalline TiO2 solar cells. J. Mater. Chem. 2012, 22, 1265–1269. [Google Scholar] [CrossRef]
- Tang, Q.; Zhu, W.; He, B.; Yang, P. Rapid conversion from carbohydrates to large-scale carbon quantum dots for all-weather solar cells. ACS Nano 2017, 11, 1540–1547. [Google Scholar] [CrossRef]
- Yu, H.; Zhao, Y.; Zhou, C.; Shang, L.; Peng, Y.; Cao, Y.; Wu, L.-Z.; Tung, C.-H.; Zhang, T. Carbon quantum dots/TiO2 composites for efficient photocatalytic hydrogen evolution. J. Mater. Chem. A 2014, 2, 3344–3351. [Google Scholar] [CrossRef]
- Fei, H.; Ye, R.; Ye, G.; Gong, Y.; Peng, Z.; Fan, X.; Samuel, E.L.; Ajayan, P.M.; Tour, J.M. Boron-and nitrogen-doped graphene quantum dots/graphene hybrid nanoplatelets as efficient electrocatalysts for oxygen reduction. ACS Nano 2014, 8, 10837–10843. [Google Scholar] [CrossRef]
- Wang, R.; Lu, K.-Q.; Tang, Z.-R.; Xu, Y.-J. Recent progress in carbon quantum dots: Synthesis, properties and applications in photocatalysis. J. Mater. Chem. A 2017, 5, 3717–3734. [Google Scholar] [CrossRef]
- Sakdaronnarong, C.; Sangjan, A.; Boonsith, S.; Kim, D.C.; Shin, H.S. Recent developments in synthesis and photocatalytic applications of carbon dots. Catalysts 2020, 10, 320. [Google Scholar] [CrossRef]
- He, J.; Zhang, H.; Zou, J.; Liu, Y.; Zhuang, J.; Xiao, Y.; Lei, B. Carbon dots-based fluorescent probe for “off-on” sensing of Hg (II) and I−. Biosens. Bioelectron. 2016, 79, 531–535. [Google Scholar] [CrossRef]
- Mondal, T.K.; Ghorai, U.K.; Saha, S.K. Dual-emissive carbon quantum dot-Tb nanocomposite as a fluorescent indicator for a highly selective visual detection of Hg (II) in water. ACS Omega 2018, 3, 11439–11446. [Google Scholar] [CrossRef] [PubMed]
- Mondal, T.K.; Gupta, A.; Shaw, B.K.; Mondal, S.; Ghorai, U.K.; Saha, S.K. Highly luminescent N-doped carbon quantum dots from lemon juice with porphyrin-like structures surrounded by graphitic network for sensing applications. RSC Adv. 2016, 6, 59927–59934. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Konkena, B.; Jayaramulu, K.; Schuhmann, W.; Maji, T.K. Synthesis of nano-porous carbon and nitrogen doped carbon dots from an anionic MOF: A trace cobalt metal residue in carbon dots promotes electrocatalytic ORR activity. J. Mater. Chem. A 2017, 5, 13573–13580. [Google Scholar] [CrossRef]
- Jiang, K.; Wang, Y.; Cai, C.; Lin, H. Conversion of carbon dots from fluorescence to ultralong room-temperature phosphorescence by heating for security applications. Adv. Mater. 2018, 30, 1800783. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yuan, F.; Li, X.; Li, Y.; Zhong, H.; Fan, L.; Yang, S. 53% efficient red emissive carbon quantum dots for high color rendering and stable warm white-light-emitting diodes. Adv. Mater. 2017, 29, 1702910. [Google Scholar] [CrossRef]
- Zhang, F.; Feng, X.; Zhang, Y.; Yan, L.; Yang, Y.; Liu, X. Photoluminescent carbon quantum dots as a directly film-forming phosphor towards white LEDs. Nanoscale 2016, 8, 8618–8632. [Google Scholar] [CrossRef]
- Yuan, F.; Wang, Y.-K.; Sharma, G.; Dong, Y.; Zheng, X.; Li, P.; Johnston, A.; Bappi, G.; Fan, J.Z.; Kung, H. Bright high-colour-purity deep-blue carbon dot light-emitting diodes via efficient edge amination. Nat. Photonics 2020, 14, 171–176. [Google Scholar] [CrossRef]
- Singh, V.K.; Singh, V.; Yadav, P.K.; Chandra, S.; Bano, D.; Kumar, V.; Koch, B.; Talat, M.; Hasan, S.H. Bright-blue-emission nitrogen and phosphorus-doped carbon quantum dots as a promising nanoprobe for detection of Cr (vi) and ascorbic acid in pure aqueous solution and in living cells. New J. Chem. 2018, 42, 12990–12997. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, L.; Li, Y.; Deng, R.; Zhang, H. Highly fluorescent nitrogen-doped carbon dots with excellent thermal and photo stability applied as invisible ink for loading important information and anti-counterfeiting. Nanoscale 2017, 9, 491–496. [Google Scholar] [CrossRef]
- Hoan, B.T.; Thanh, T.T.; Tam, P.D.; Trung, N.N.; Cho, S.; Pham, V.-H. A green luminescence of lemon derived carbon quantum dots and their applications for sensing of V5+ ions. Mater. Sci. Eng. B 2019, 251, 114455. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, S.; Wu, P.; Ma, C.; Wu, X.; Xu, M.; Li, W.; Liu, S. Hydrothermal synthesis of green fluorescent nitrogen doped carbon dots for the detection of nitrite and multicolor cellular imaging. Anal. Chimica Acta 2019, 1090, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.; Zhou, D.; Li, D.; Ji, W.; Jing, P.; Han, D.; Liu, L.; Zeng, H.; Shen, D. Toward efficient orange emissive carbon nanodots through conjugated sp2-domain controlling and surface charges engineering. Adv. Mater. 2016, 28, 3516–3521. [Google Scholar] [CrossRef]
- Ding, H.; Yu, S.-B.; Wei, J.-S.; Xiong, H.-M. Full-color light-emitting carbon dots with a surface-state-controlled luminescence mechanism. ACS Nano 2015, 10, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Li, X.; Chen, M.; Gong, Y.; Tang, A.; Wang, Z.; Wei, Z.; Guan, L.; Teng, F. Controllable synthesis highly efficient red, yellow and blue carbon nanodots for photo-luminescent light-emitting devices. Chem. Eng. J. 2020, 380, 122503. [Google Scholar] [CrossRef]
- Liu, Y.; Chao, D.; Zhou, L.; Li, Y.; Deng, R.; Zhang, H. Yellow emissive carbon dots with quantum yield up to 68.6% from manganese ions. Carbon 2018, 135, 253–259. [Google Scholar] [CrossRef]
- Gao, X.; Gong, X.; Nguyen, T.T.; Du, W.; Chen, X.; Song, Z.; Chai, R.; Guo, M. Luminescent materials comprised of wood-based carbon quantum dots adsorbed on a Ce0.7Zr0.3O2 solid solution: Synthesis, photoluminescence properties, and applications in light-emitting diode devices. J. Mater. Sci. 2019, 54, 14469–14482. [Google Scholar] [CrossRef]
- Zhu, X.; Pang, X.; Zhang, Y.; Yao, S. Titanium carbide MXenes combined with red-emitting carbon dots as a unique turn-on fluorescent nanosensor for label-free determination of glucose. J. Mater. Chem. B 2019, 7, 7729–7735. [Google Scholar] [CrossRef]
- Jia, H.; Wang, Z.; Yuan, T.; Yuan, F.; Li, X.; Li, Y.; Tan, Z.A.; Fan, L.; Yang, S. Electroluminescent warm white light-emitting diodes based on passivation enabled bright red bandgap emission carbon quantum dots. Adv. Sci. 2019, 6, 1900397–1900405. [Google Scholar] [CrossRef]
- Yuan, F.; Xi, Z.; Shi, X.; Li, Y.; Li, X.; Wang, Z.; Fan, L.; Yang, S. Ultrastable and low-threshold random lasing from narrow-bandwidth-emission triangular carbon quantum dots. Adv. Opt. Mater. 2019, 7, 1801202. [Google Scholar] [CrossRef]
- Yuan, F.; Yuan, T.; Sui, L.; Wang, Z.; Xi, Z.; Li, Y.; Li, X.; Fan, L.; Tan, Z.A.; Chen, A. Engineering triangular carbon quantum dots with unprecedented narrow bandwidth emission for multicolored LEDs. Nat. Commun. 2018, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; He, P.; Xi, Z.; Li, X.; Li, Y.; Zhong, H.; Fan, L.; Yang, S. Highly efficient and stable white LEDs based on pure red narrow bandwidth emission triangular carbon quantum dots for wide-color gamut backlight displays. Nano Res. 2019, 12, 1669–1674. [Google Scholar] [CrossRef]
- Yan, B. Lanthanide-functionalized metal–organic framework hybrid systems to create multiple luminescent centers for chemical sensing. Acc. Chem. Res. 2017, 50, 2789–2798. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhu, P.; Xiong, J.; Gao, L.; Tan, K. A new dual-recognition strategy for hybrid ratiometric and ratiometric sensing perfluorooctane sulfonic acid based on high fluorescent carbon dots with ethidium bromide. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 224, 117362. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-X.; Yan, B. A dual-emission probe to detect moisture and water in organic solvents based on green-Tb3+ post-coordinated metal-organic frameworks with red carbon dots. Dalton Trans. 2017, 46, 7098–7105. [Google Scholar] [CrossRef]
- Chen, L.; Gao, Z.; Li, Y. Immobilization of Pd (II) on MOFs as a highly active heterogeneous catalyst for Suzuki-Miyaura and Ullmann-type coupling reactions. Catal. Today 2015, 245, 122–128. [Google Scholar] [CrossRef]
- Dang, H.X.; Li, Y.; Zou, H.; Liu, S.H. Tunable White-light Emission Hybrids Based on Lanthanide Complex Functionalized Poly (Ionic Liquid): Assembly and Chemical Sensing. Dyes Pigment. 2020, 172, 107804. [Google Scholar] [CrossRef]
- Zeng, Y.C.; Qiu, B.W.; Wang, F.F.; Zhou, L.; Li, Y. Transparent films based on functionalized Poly(ionic liquids) coordinating to photoactive Lanthanide(Eu3+,Tb3+) and Poly(methyl methacrylate): Luminescence and chemical sensing. Opt. Mater. 2020, 107, 110149. [Google Scholar] [CrossRef]
- Wu, J.-X.; Yan, B. Eu (III)-functionalized In-MOF (In (OH) bpydc) as fluorescent probe for highly selectively sensing organic small molecules and anions especially for CHCl3 and MnO4−. J. Colloid Interface Sci. 2017, 504, 197–205. [Google Scholar] [CrossRef]
- Wang, Y.N.; Wang, S.D.; Gao, Y.M.; Yang, L.L.; Xie, L.X. 3D cadmium (II)-based coordination polymer constructed from v-shaped semirigid ligand: Selective detection of oxoanion pollutants CrO42−, Cr2O72−, MnO4− in Water. Zeitschrift für Anorganische und Allgemeine Chemie 2019, 645, 1358–1364. [Google Scholar] [CrossRef]
- Li, B.; Zhou, J.; Bai, F.; Xing, Y. Lanthanide-organic framework based on a 4, 4-(9, 9-dimethyl-9H-fluorene-2, 7-diyl) dibenzoic acid: Synthesis, structure and fluorescent sensing for a variety of cations and anions simultaneously. Dyes Pigment. 2020, 172, 107862. [Google Scholar] [CrossRef]
- Shi, J.L.; Xu, P.; Wang, X.G.; Ding, B.; Zhao, X.J.; Yang, E.C. A dual-responsive luminescent terbium (III) chain for selective sensing of Fe3+ and MnO4− Ions. Zeitschrift für Anorganische und Allgemeine Chemie 2018, 644, 1598–1606. [Google Scholar] [CrossRef]




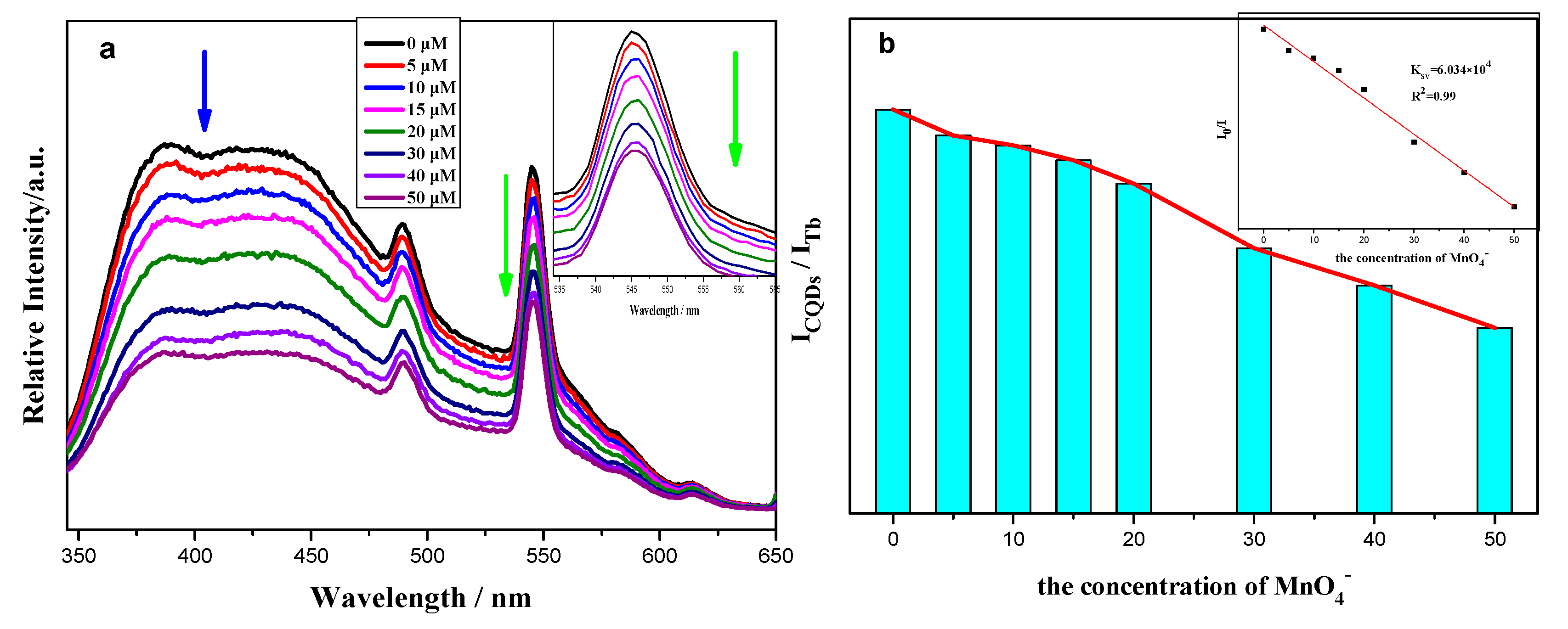

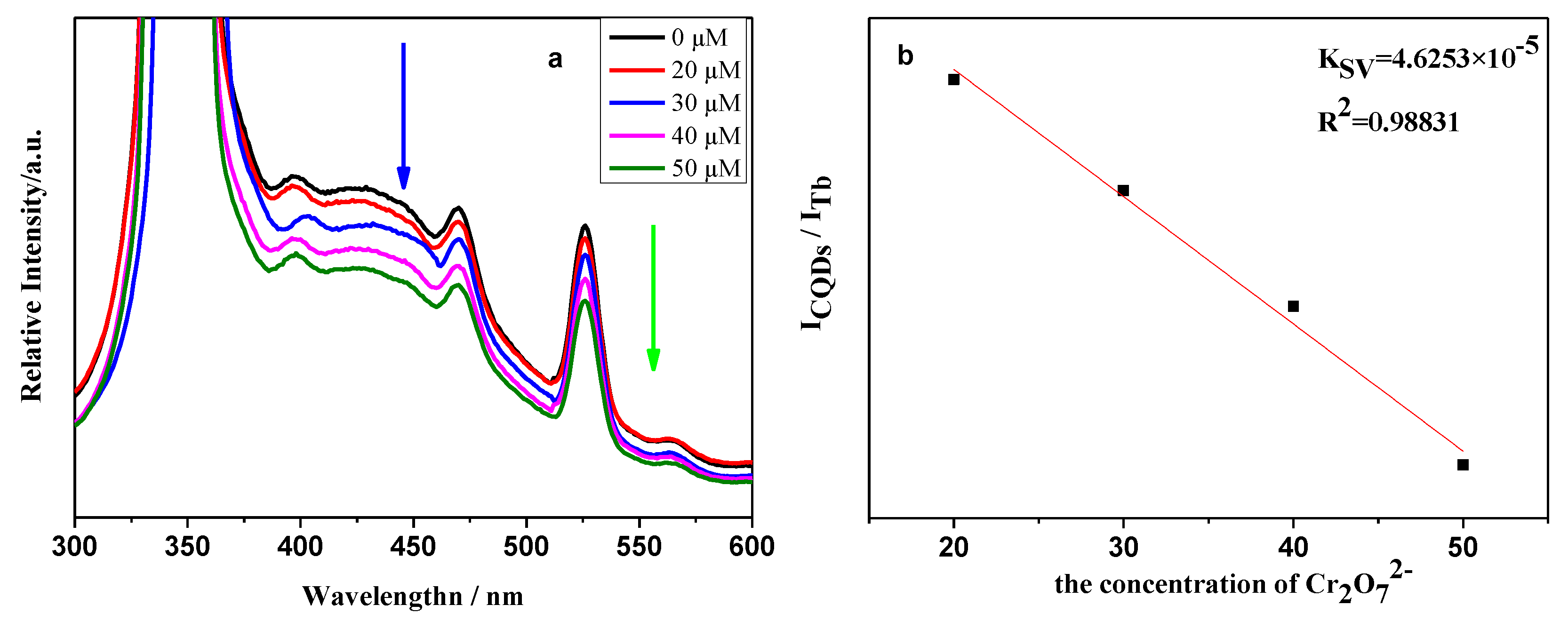
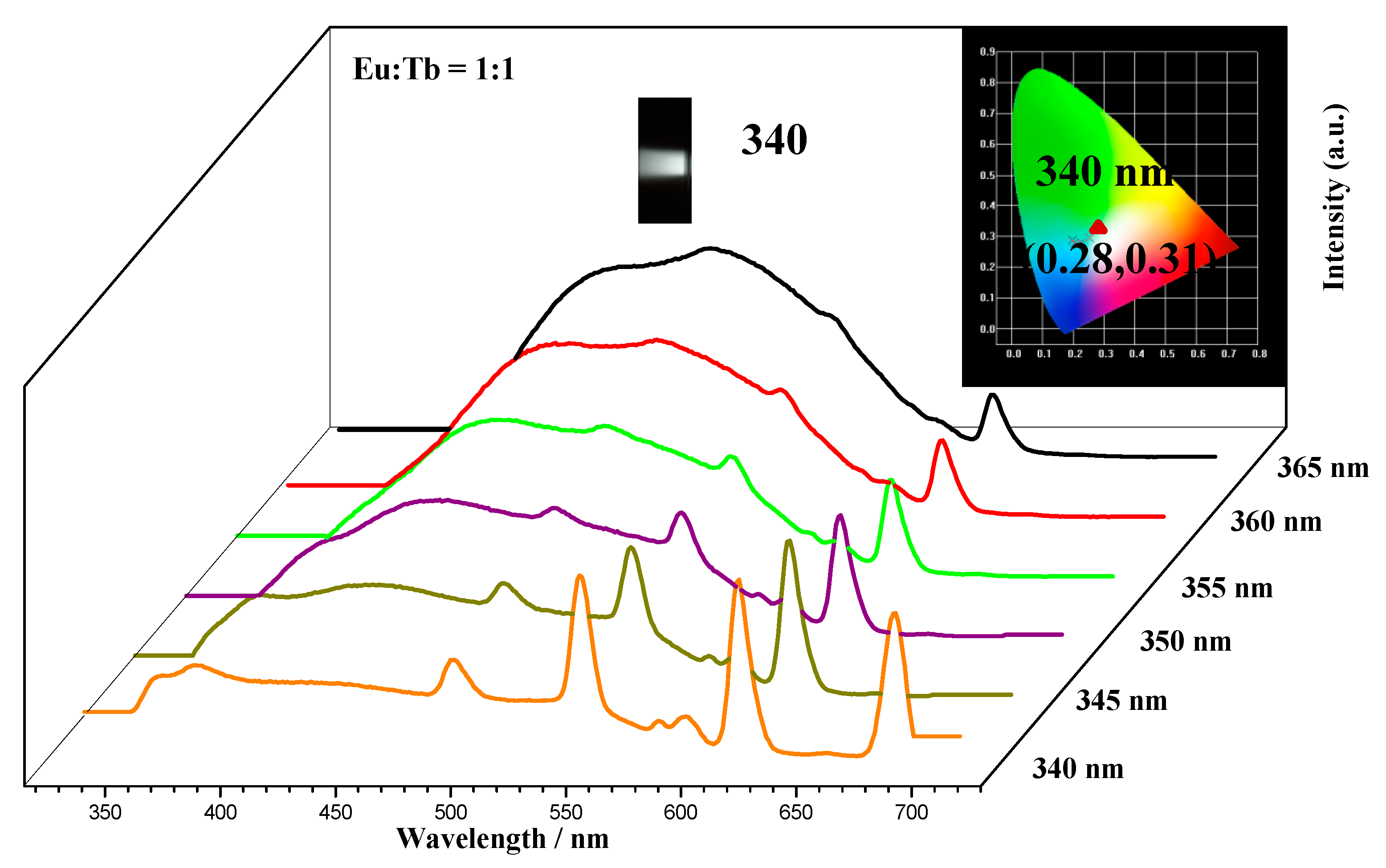
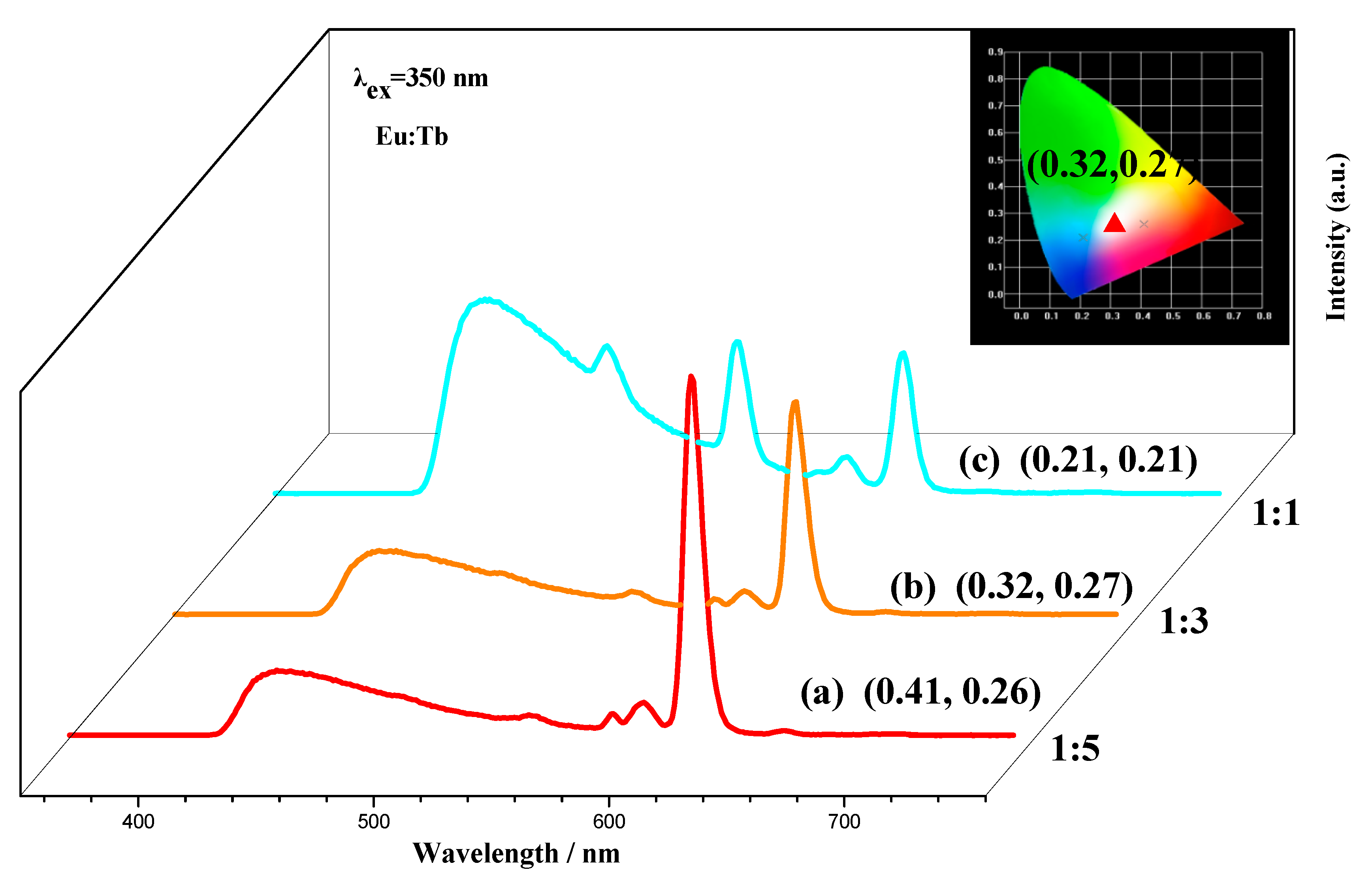
| Detection Method | Linear Range (μM) | Detection Limit | Ref. |
|---|---|---|---|
| n-MOF-Eu | 0–500 | 1.47μM | [49] |
| Cd(HL)(4,4′-bipy) | 40–100 | 0.647 μM | [50] |
| I [Eu(DLDA)(DMF)(H2O)(COO)] n | 0–200 | 10.8μM | [51] |
| A dual-responsive Luminescent Terbium(III) Chain | 0–476 | 1.43 μM | [52] |
| CQDs@Tb(TFA)3 | 0–50 | 0.55 μM | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, F.-R.; Wang, S.-N.; Wang, F.-F.; Liu, D.; Li, Y. Synthesis of Lanthanide-Functionalized Carbon Quantum Dots for Chemical Sensing and Photocatalytic Application. Catalysts 2020, 10, 833. https://doi.org/10.3390/catal10080833
Zou F-R, Wang S-N, Wang F-F, Liu D, Li Y. Synthesis of Lanthanide-Functionalized Carbon Quantum Dots for Chemical Sensing and Photocatalytic Application. Catalysts. 2020; 10(8):833. https://doi.org/10.3390/catal10080833
Chicago/Turabian StyleZou, Fu-Ran, Sai-Nan Wang, Fang-Fang Wang, Dan Liu, and Ying Li. 2020. "Synthesis of Lanthanide-Functionalized Carbon Quantum Dots for Chemical Sensing and Photocatalytic Application" Catalysts 10, no. 8: 833. https://doi.org/10.3390/catal10080833
APA StyleZou, F.-R., Wang, S.-N., Wang, F.-F., Liu, D., & Li, Y. (2020). Synthesis of Lanthanide-Functionalized Carbon Quantum Dots for Chemical Sensing and Photocatalytic Application. Catalysts, 10(8), 833. https://doi.org/10.3390/catal10080833