Abstract
Nowadays, biocatalysts have received much more attention in chemistry regarding their potential to enable high efficiency, high yield, and eco-friendly processes for a myriad of applications. Nature’s vast repository of catalysts has inspired synthetic chemists. Furthermore, the revolutionary technologies in bioengineering have provided the fast discovery and evolution of enzymes that empower chemical synthesis. This article attempts to deliver a comprehensive overview of the last two decades of investigation into enzymatic reactions and highlights the effective performance progress of bio-enzymes exploited in organic synthesis. Based on the types of enzymatic reactions and enzyme commission (E.C.) numbers, the enzymes discussed in the article are classified into oxidoreductases, transferases, hydrolases, and lyases. These applications should provide us with some insight into enzyme design strategies and molecular mechanisms.
1. Introduction
1.1. A Brief History of Enzymes and Their Application
Currently, enzymes are applied extensively in many fields, including the food, medical, chemical, and cosmetic industries [1]. Following their discovery in the late 18th century, enzymes were predominantly employed to produce digested food for humans and animals. The concept of catalysis was well defined in the middle of the 19th century when scientists started to study the mechanism of catalysis and the relationship between chemistry and biology. The study of enzymes bloomed in the 20th century. Models resulting from the further study of enzyme kinetics, including the Michaelis-Menten equation, provided more insight into how enzymes work in reactions in vitro and in vivo. Mechanistic studies led to the discovery of the enzyme-substrate intermediate and the first crystal structure of an enzyme (lysozyme), which marked huge milestones in the history of science and technology [1]. At the same time, more and more enzymes were applied in clinical trials, which led to the discovery of many therapeutic enzymes, like collagenases to treat skin ulcers [2] and antibody-drug conjugates to treat cancer [3]. In addition, enzymes started to play an important role in producing organics like polymer materials and inorganics used in treating wastewater in the chemical industry [4]. During this time, enzymes were also widely applied in the cosmetic industry, helping to produce body care products such as skin protective and soothing agents [5].
Among enzyme applications, utilizing enzymes as a catalyst in an organic reaction to obtain the specific target product is ubiquitous in the chemical industry. The advantages of using enzymes instead of the traditional chemical catalyst include higher stereoselectivity, higher yield, higher efficiency, and less waste [4]. In addition to the advantages of enzyme application, there are several drawbacks of applying enzymes in this industry. Enzymes are sensitive to the temperature and pH of the environment, and their activity is changed drastically with the changes in the parameters. The cost of isolation and preparation of enzymes is high. Furthermore, a lack of long-term operational stability and the difficulty of their recovery or reuse discourage their wide application [6]. To circumvent these issues, three methods of enzyme immobilization have been developed, including binding to a support (carrier), entrapment (encapsulation), and crosslinking [7]. With the increasing number of techniques to solve these problems, the application of enzymes has become an essential part of industrial development and will continue to make significant contributions.
1.2. A Brief Introduction to Regioselective and Stereoselective Reactions
A critical advantage related to the three-dimensional structure of enzymes is that they catalyze the reaction in which a target functional group is inserted into a specific position of the reactant. Compared to traditional organic synthesis, the product obtained from an enzymatic reaction usually leans towards a selective structure with a specific configuration [8]. Two main types of selectivity are often observed in enzymatic reactions: regioselectivity and stereoselectivity. Regioselectivity refers to the preference of a functional group to bond to one atom over another atom in the reactant, while stereoselectivity indicates a favoring of one stereoisomer over another stereoisomer. Related to stereoselectivity, “enantioselectivity” is defined as the preference for producing one enantiomer over another. Regioselectivity and stereoselectivity exist ubiquitously in the plant, animal, bacterial, and fungal kingdoms. A known example is melanin, which functions as a pigment and as a radiation protection agent [9]. Tyrosinase catalyzes the hydroxylation of L-tyrosine to regioselectively produce L-DOPA in the melanin biosynthesis pathway [10]. Another important compound existing in humans is norepinephrine (NE), whose main functions are as a hormone and as a neurotransmitter. In its biosynthesis, a dopamine-β-hydroxylase inserts a hydroxyl group to β-carbon on the side chain of dopamine, stereoselectively producing an R-enantiomer [11].
To date, more than half of the small molecule drugs in clinical use are chiral, but the chirality of drug structure was not valued initially [12]. People started to spotlight chiral drugs following the huge thalidomide tragedy that occurred in the 1960s. Thalidomide entered the market in 1957 to treat insomnia and morning sickness as an over-the-counter drug and soon expanded to markets in 46 countries. Four years later, the side effect of severe birth defects was associated with thalidomide. Use of thalidomide by women during pregnancy was linked to the delivery of babies with shortened or flipper-like limbs [13]. More than 10,000 cases were reported, with 40 percent of families losing their babies and those who survived having additional problems such as dysmelia, facial anomalies, and systemic anomalies [14]. Stereochemistry figures into this tragedy because thalidomide sold in the market was a mixture of two stereoisomers, R- and S- forms. R-enantiomer was therapeutically active, while S-enantiomer caused severe side effects. The thalidomide disaster prompted changes to US-FDA regulations that tightened restrictions surrounding the surveillance and approval process, including requiring manufacturers to provide all data on the safety and effectiveness of a drug, including the evaluation of the absolute stereochemistry as part of the approval process [15].
The broad application of enzymes in stereochemistry drives the rapid development of chiral compounds, expanding the drug candidate pool and opportunities to obtain a therapeutically active drug. Herein, we provide a brief overview of the application of enzymes in regioselective and stereoselective reactions, which also involve enantioselective reactions.
2. Examples of Enzyme Application in Regioselective and Stereoselective Reactions
Depending on the types of enzymatic reactions and enzyme commission (E.C.) numbers, the enzymes are classified into different groups. In this review, four main groups of enzymes, including oxidoreductases, transferases, hydrolases, and lyases, will be discussed.
2.1. Oxidoreductases E.C.1
2.1.1. Enzymes Applied for Reduction
Ketoreductases. The enantioselective reduction of ketones to the corresponding alcohols that have significant bioactivity has attracted more and more attention in organic synthesis. However, the reduction of tert-butyl and isopropyl ketones still represents a significant challenge in both enzymatic and chemical reductions. Enzymatic protocols, such as utilizing carbonyl reductase extracted from Sporobolomyces salmonicolor (SSCR), proved to be more effective in catalyzing the enantioselective reduction of aryl alkyl ketones than traditional chemical formation (Scheme 1). Notably, the enzyme demonstrated excellent enantioselectivity (>96% ee, enantiomeric excess), reducing ketones with bulky alkyl groups (tert-butyl, cyclopropyl, etc.) [16].
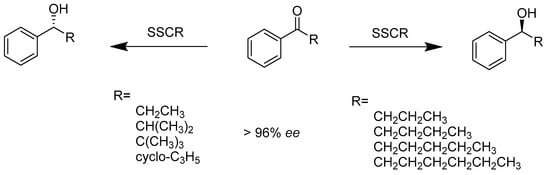
Scheme 1.
The enantioselective reductions of aryl alkyl ketones.
Alpha-hydroxy ketones were experimentally synthesized using three different mechanisms. The first method involved reacting aldehydes with thiamine diphosphate-dependent lyases to catalyze the umpolung carboligation of aldehydes; this allows one aldehyde to become an active enamine carbanion. Another aldehyde attacks the carbanion to form an alpha-hydroxy ketone. The second method occurred when a substrate containing a ketone with an adjacent alcohol group (either R or S) was reacted using hydrolases via dynamic kinetic resolutions. The substrate being either (R) or (S) allows for high conversions and enantiomeric excess of the desired alpha-hydroxy ketone. The third method elaborated on reacting diketones and vicinal diols with whole-cell redox reactions. By employing free enzymes or whole-cells, Hoyos et al. were able to reduce the diketones and oxidize the diols to produce the alpha-hydroxy ketones, which is racemic [17].
A large number of reductases were encoded in Saccharomyces cerevisiae yeast genome, many of which have the ability to reduce α-chloro-β-keto esters. Kaluzna’s study revealed that in nearly all the targeted reductases, more than one α-chloro-β-hydroxy ester diastereomer was obtained, with up to 98% ee. The only deficiency in this approach is a lack of D-specific reductases [18]. Zhu et al. determined the activity and enantioselectivity of a carbonyl reductase from Candida magnoliae. Using NADPH as a cofactor, the reductase catalyzed the enantioselective reduction of a series of ketones, including aliphatic, aromatic ketones, α, and β ketoesters, to give anti-Prelog configurated alcohols with high optical purity (Scheme 2). This reductase with a new application complemented the known ketoreductase pool and could be employed to synthesize chiral alcohols. This strategy also solves the problems in the chemical synthesis, such as the complicated process operation, high metal residue, low enantiomeric purity, and productivity [19]. The backbones of bacterial aromatic polyketides could be synthesized from acyl-CoA-derived building blocks by polyketide synthases (PKSs). A recent study revealed that some polyketides were attained from nonactate precursors. Exemplarily, the engineered PKSs catalyzed the synthesis of regioselectively modified anthraquinones with predictable functional moiety that have improved pharmacological properties over the estrogen receptor antagonist R1128 [20].
Scheme 2.
Enantioselectivity of carbonyl reductase from Candida magnolia toward the reduction of aryl ketones.
Dehydrogenases. Classen et al. investigated the utilization of the enoate reductase YqjM as well as alcohol dehydrogenases and a glucose dehydrogenase to synthesize chiral γ-butyrolactones. Their results revealed that the process is scalable, with up to 90% yields and excellent enantioselectivity (over 98% ee) [21]. Furthermore, the substrate ethyl 4-oxo-pent-2-enoates are readily available through simple Wittig-type chemical reactions. Notably, the enoate reductase YqjM is strongly substrate-dependent and hardly predictable, unlike ADHs that follow a simple rule of prediction [15,22]. Studies show that the log P of the organic solvent has a dramatic impact on enzyme activity. Exemplarily, alcohol dehydrogenase ADH-A from Rhodococcus ruber demonstrated robust enzymatic efficiency (ee >99%), catalyzing the reduction of a large variety of ketones in micro-aqueous media, a nonconventional aqueous organic solvent system (99% v/v). This organic media showed great biocompatibility, with 10-fold improvement, in contrast to previous observations using an aqueous solvent [23,24]. Two methods were used to synthesize optically pure cis- and trans-isomers of whisky lactone. The first method employed enzyme-mediated reactions that involved eight alcohol dehydrogenases as biocatalysts to enantioselectively oxidize the racemic products of erythron- and threo-3-methyloctane-1,4-diols. The second approach involved biotransformation, using microorganisms to produce naturally occurring opposite isomers of the whiskey lactone. For this method, the trans-isomer of 3-methyl-4-oxooctanoic acid was produced. Boratynski and colleagues were able to synthesize enantiomerically enriched isomers of oak lactones and opposite enantiomerically enriched isomers of whisky lactones based on the method of reaction used, and they found that the lactonization of whole-cell microorganisms is a significant alternative to the enzyme-mediated oxidation strategy [25]. The chemical synthesis of stereoselective 1,2-diols is a significant challenge due to the lack of a precursor and toxic catalyst. To discover new alcohol dehydrogenases (ADHs) used to reduce 2-hydroxy ketones, Kulig et al. investigated eight ADHs and found that all of them had good activities. Among the enzymes tested, alcohol dehydrogenases from Thermoanaerobacter sp. (ADHT), Lactobacillus brevis (LBADH), and Ralstonia sp. (RADH) showed high activities and stereoselectivities in the enzymatic reactions of reducing all reactants to anti-diols. In addition, all three ADHs showed promising activity towards 2-hydroxy ketones, and RADH was the most active ADH towards bulky-bulky 2-hydroxy ketones [26].
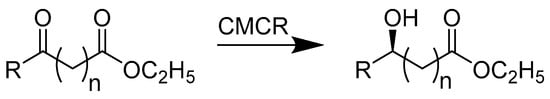
O’Reilly et al. experimentally determined a mechanism to regio- and stereoselectively synthesize 2,5-disubstirutred pyrrolidines from 1,4-diketones (Scheme 3). By using a one-pot ω-transaminase and monoamine oxidase from Aspergillus niger, the reaction was accomplished in one step, without intermediation due to the compatibility of the enzymes. The transaminase mediates the reductive amination of prochiral ketones and favors the production of chiral amines, while the monoamine oxidase favors the (S)-enantiomer during the conversion of amines to imines. The enzymes do not hinder each other and therefore can be used in a two-step, one-pot synthesis reaction of 2,5-disubstituted pyrrolidines, exhibiting exceptional enantioselectivity of greater than 94% ee and diastereoselectivity greater than 98% de [27].
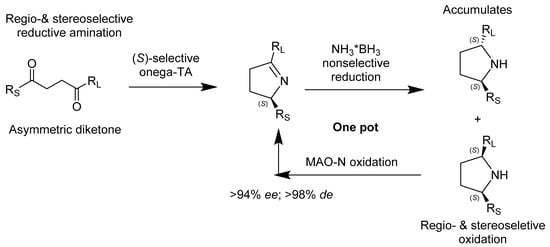
Scheme 3.
A chemoenzymatic approach for the synthesis of 2,5-disubstituted pyrrolidines by employing an ω-transaminase (TA) and a monoamine oxidase (MAO-N).
Single-electron-transfer steps involve alkali metals, radical anion transition states, etc., that lie in the center of Birch reductions of aromatic compounds. Conversely, 2-naphthoyl-coenzyme A (2-NCoA) and 5,6-dihydro-2-NCoA (5,6-DHNCoA) reductases such as NCR and DHNCR reduce two different naphthoyl-ring systems to tetrahydronaphthoyl-CoA by different mechanisms that are categorized as two-electron reductions. Both enzymatic reactions have demonstrated high stereoselectivity (30-fold improvement) in aqueous solution at ambient temperature. Moreover, these bio-catalysts provide an enantioselective pathway to products that are not readily accessible via Birch reductions [28]. The biosynthesis of polyunsaturated fatty acids (PUFAs) requires Δ12 and ω3 fatty acid desaturases to form the second and third double bonds in the molecule, respectively, as PUFAs are crucial building blocks of membrane glycerolipids and predecessors of signaling molecules in cells. Sk-FAD2 and Sk-FAD3 that are Δ12 and ω3 fatty acid desaturase genes from Saccharomyces kluyveri demonstrated excellent substrate specificity and regioselectivity. Sk-FAD2 introduced a double bond to C16–20 monounsaturated fatty acids at the ν + 3 position, whereas Sk-FAD3 selected the ω3 position on C18 and C20. Moreover, studies suggested that both enzymes showed little to no specificity toward the polarity of the head group or the sn positions of acyl groups in phospholipids [29].
2.1.2. Enzymes Applied for Oxidation
Cytochrome P450s. Linear alkanes are difficult to hydroxylate by using chemical synthesis due to the high carbon–hydrogen bond strength (~97 kcal/mol) and high activation energy. Therefore, numerous hydroxylations rely on enzymatic catalysis, of which some require the use of cytochrome P450s to initiate the electron transfer between molecules. A common cytochrome P450 enzyme exploited for this purpose is cytochrome P450 BM-3, an enzyme that uses heme as a cofactor and imitates monooxygenases. Studies revealed that the cofactor increases the chemical reaction rates and allows the enzyme to function efficiently. Thus, monooxygenases are essential to synthetic chemistry to not only increase efficiency but also enable a reaction within the constraints of a living system, such as temperature or excessive amounts of substrate. The modification of biocatalysts for natural synthetic use is a relevant field in biochemistry today. There has been much work done on the modification of enzymes that perform hydroxylation reactions. The engineering of these enzymes has been investigated to modify aspects such as regioselectivity, stereoselectivity, and enantioselectivity, among others. Much focus is placed on different variations of the P450 BM-3 enzyme. Other enzymes being studied for their hydroxylation reactions include heme-containing biocatalysts such as the PikC enzyme. Variations of the P450 BM-3 can improve its industrial applications since it is capable of hydroxylating alkanes [30]. A light-activated hybrid P450 BM-3, WT/L407C-Ru1, hydroxylates 10-undecenoic acid exclusively at the allylic position. The reaction obtains the highly enantiomerically enriched (R)-9-hydroxy-10-undecnoic acid in 85% ee [31]. Moreover, cytochrome P450 ΒΜ-3 extracted from Bacillus megaterium was engineered by directed evolution to hydroxylate linear alkanes. Variant 9-10A-A328V has the ability to produce S-2-octanol from octane. This product is obtained in 40% ee, with excellent total turnovers of 2000. Another variant, 1-12G, was made from the same cytochrome as P450 BM-3 via mutagenesis. This variant also hydroxylates in the 2-position on alkanes larger than hexane, including octane, nonane, and decane, to produce R-2-alcohols in 40%–55% ee [30].
In another study, a cytochrome P450 BM-3 variant was produced to aid in the production of indirubin, an anticancer drug. The enzyme was engineered using site-directed saturation mutagenesis. The mutant, D168W, altered the enzyme so that the enzyme produced indirubin (~90%) instead of indigo (~85%), which was synthesized by its parent enzyme. This change was caused by the shift of hydroxylation C-3 to C-2 [32]. Furthermore, BM-3 variant 9-10A was engineered to hydroxylate aromatic carboxylic acid. This enzyme has the highest efficiency with propyl and butyl esters. When variant 9-10A was constructed with the F87A mutation, the total turnover numbers (TTN) increased to its highest amount of 1640. This enzyme obtained a propyl mandelate in 93% ee. When 9-10A-F87A is combined with an NADPH regeneration system, utilizing NADPH as the cofactor, the TTN improves to over 5800 [33]. Regioselectivity is highly affected by the anchoring group of an enzyme. This can be demonstrated by the biosynthetic enzyme, P450 monooxygenase PikCD50N-RhFRED. The natural anchoring group desoamine gives a 1:1 mixture of methymycin and neomethymycin, but by using a synthetic anchor, the ratio can be altered [34].
Although stereoselective epoxidation of terminal alkenes has been reported in a few cases, using a chemical catalyst in the epoxidation of simple linear terminal alkenes has not yet been reported. Cytochrome P450 BM-3 enzymes (Scheme 4) paired with saturated mutagenesis and recombination have been applied to analyze the effective epoxidation catalysts for linear terminal alkenes. In the presence of cell lysate and alcohol dehydrogenase, various P450 enzymes could reproduce NADPH cofactor. Mutagenesis and screening helped us to achieve optimal results. Exemplarily, P450 BM-3 from Bacillus megaterium was utilized for the enantioselective epoxidation of terminal alkenes and forms (R)- and (S)-epoxides with desirable catalytic turnovers; this catalyst and the NADPH oxidize the terminal alkene into a terminal epoxide product [35]. Denard et al. experimentally determined a one-pot tandem reaction to produce aryl epoxides from a mixture of stilbene-derived alkenes in the presence of ruthenium. This novel development combines alkene metathesis with enzymatic epoxidation. It favors the utilization of (Z)-stilbene substrates and produced an epoxide product with moderate yields and good enantioselectivity. Substantial enzyme and reaction engineering with cytochrome P450 BM-3 is currently under investigation to improve catalytic activity and increase enzymatic reaction rates [36]. The enantioselective total synthesis of norditerpenoid alkaloid nigelladine A was accomplished within an expedient 12 steps and with an overall yield of 5%. The engineered enzyme cytochrome P450 BM-3 was used to catalyze the chemo- and regioselective allylic C–H bond oxidation in the intermediate step of the reaction. The reaction relied on the asymmetric allylic alkylation that constructed the quaternary center of the product, with prominent yield and enantioselectivity; the cytochrome P450 BM-3 enzyme allowed Steven A. Loskot and coworkers to site-select the secondary allylic oxidation without major intermediate interference. Their results have revealed that bio-transformations have the potential to circumvent the limitations of traditional chemical syntheses [37].
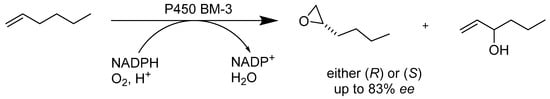
Scheme 4.
Oxidation of 1-hexene by P450 BM-3.
Experimentation with intermolecular nitrene transfer accomplished by utilizing the cytochrome P450 BM-3 enzyme found in Bacillus megaterium was performed by Farwell et al. The catalyst allows for the intermolecular insertion of nitrogen-containing functional groups into thioethers and subsequently sulfimides. Two factors that heavily impact the reaction are the electronic properties of the substrates used, which can influence reactivity, and the amino acid groups attached, which can vary the rate and stereoselectivity of the sulfimidation reaction. Sulfide substituents may also impact sulfimide formation, as sulfimide side products were introduced, indicating that the nitrenoid may only permit insertion into reactive sulfides. The orientation of the redox state heme group, in either the presence or absence of the nitrene source and the sulfide acceptor, including the redox state of the heme group, helped to determine the nitrene transfer, but the usage of cytochrome P450 BM-3 allows for intermolecular nitrene transfer in the form of sulfimidation [38].
Enzymatic mutation also proved to alter cyclopropanation reactions dealing with a heteroatom. Cytochrome P450 BM3 variants were engineered to catalyze the cyclopropanation of heteroatom-bearing alkenes. Cytochrome P411 had the capability of synthesizing heteroatom-bearing cyclopropanes. Furthermore, evolved P411 provided nitrogen, oxygen, and sulfur-cyclopropanes with high diasteroselectivities and enantioselectivities. With certain mutations, a single parent enzyme became a selective catalyst for cis and trans heteroatom substituted cyclopropanes synthesis with preferred diasteroselectivities and enantioselectivities [39].
Hemeprotein biocatalyst is another type of hydroxylase. As a heme-containing enzyme, SfmD is used to hydroxylate the aromatic ring of tyrosine in the presence of hydrogen peroxide and an oxidant (Scheme 5). It is possibly responsible for producing 3-OH-5-Me-Tyr from 3-Me-Tyr. This has significance for the biosynthesis of tetrahydroisoquinoline family members [40]. Another hemeprotein is wild-type Rhodothermus marinus (Rma) cytochrome c. The variant, Rma cytochrome c TQL, transforms alkenes to chiral 1,2-amino alcohols via direct amino-hydroxylation. This enzyme is thermally stable and demonstrated high enantioselectivity, with total turnovers of up to 2500 and 90% ee under optimized conditions [41]. G. Sello et al. (Scheme 6) analyzed various reactions that would turn a styrene substrate into enantiopure 1,2-amino alcohol. The styrene oxidized into enantiopure phenyl epoxide via an E. coli containing monooxygenase from P. fluorescens. The epoxide then reacted with a nitrogen nucleophile to open up the ring, leaving an alcohol group and an amine group. Their work indicated that 2-amino, 1-, and 2-phenyl ethanols were produced with high enantiopurities and in good yields [42].
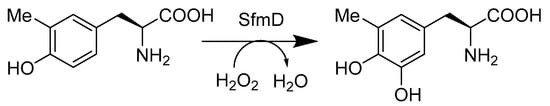
Scheme 5.
Biosynthetic pathway of 3-OH-5-Me-Tyr.
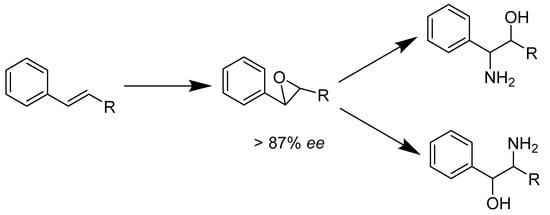
Scheme 6.
Regioisomeric addition of nitrogen to epoxides.
A three-enzyme tandem reaction was used to stereoselectively synthesize γ-oxyfunctionalyzed amino acids (Scheme 7). Combining N-acylamino acid racemase (NAAAR), L-selective aminoacylase from Geobacillus thermoglucosidasius, and isoleucine dioxygenase from Bacillus thuringiensis paired with dynamic kinetic resolution allows racemic N-acetylmethionine to be converted to L-methionine-(S)-sulfoxide, with a very high experimental yield of 97% and 95% de under optimized conditions. The three-enzyme cascade reaction results in the separation of the oxyfunctionalization step from the dynamic kinetic resolution step, which makes it possible for each enzyme to work at the ideal temperature [43].
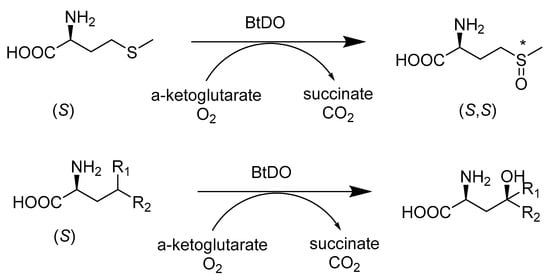
Scheme 7.
BtDO catalyzes the stereoselective γ-specific oxidation of aliphatic L-amino acids such as L-methionine (above) and several branched-chain amino acids (below).
A stereoselective, one-pot biocatalytic total synthesis reaction was employed to synthesize bisorbicillinoid natural products and unnatural side-chain analogues by utilizing recombinant oxidoreductase SorbC from the sorbicillin biosynthetic gene cluster. Applying an enzymatic oxidative dearomatization or dimerization cascade allows for the direct preparation of sorbiquinol, trichodimeral, and disorbicillinol and the synthesis of bisorbibutenolide. The bio-enzyme SorbC has high stereoselectivity that allows synthetic access for natural products. Sib et al. also found that the spontaneous formation of bisorbicillinoids can be accomplished without biocatalysts, indicating that the natural compounds produced can be assembled in non-enzymatic natural producers. More investigation is in progress to determine the full synthetic potential of the SorbC enzyme [44].
In 2016, Priyanka Bajaj and her team (Scheme 8) developed a new strategy to engineer myoglobin variants that increase the synthesis of 1-carboxyl-2-aryl-cyclopropanes with trans- (1R, 2R), a product used in conjunction with its stereo complement of trans- (1S, 2S). This method improved the essential asymmetric cyclopropanation utilized in drugs (such as Tranylcypromine, Tasimelteon, Ticagrelor, and a TRPV1 inhibitor), thereby showing that the additive mutations of myoglobin gave way to the higher control of stereoselectivity in cyclopropanation reactions [45].
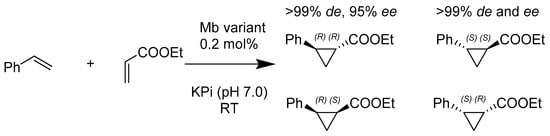
Scheme 8.
Activity and selectivity of representative myoglobin variants for styrene cyclopropanation with ethyl-α-diazoacetate (EDA).
2.2. Transferases E.C.2
2.2.1. Enzymes Applied for Alkylation
Polymethoxy flavonoids (PMFs) have shown myriad health-supporting applications. However, one of the greatest challenges that limits their use is small production from plant cells. Plant cells exploit the methylation of hydroxyl groups on flavonoids catalyzed by O-methyltransferases (FOMT) to produce PMFs. This category of enzymes has very high substrate specificity and regioselectivity during the synthetic process. Recombinant CdFOMT5 (one of the genes encoding FOMT from Citrus depressa) expressed in E. coli cells exhibited a preference for flavonol to other types of flavonoids. Moreover, it converted 3-, 5-, 6-, and 7-hydroxyl groups on flavone to methyl groups under mild conditions. Furthermore, taking advantage of this engineering strategy of mutation of CdFOMT5 could provide alternative routes to synthesizing various PMFs [46].
The installation of fluoroalkyl groups is an abiological process that could significantly alter the pharmacological properties of a molecule. Despite the ubiquity of C–H bonds in complex bioactive molecules, the fluoroalkylation of enantioselective C(sp3)−H remains a challenge due to inherent problems coupled with cross-coupling pathways of the C- fluoroalkyl bond in the transition metal-catalyzed reaction. To circumvent this issue, the engineered cytochrome P450s could catalyze the addition of fluoroalkyl carbene intermediates to C(sp3)−H bonds (Scheme 9). The catalyst demonstrated excellent selectivity toward α-amino C−H bonds, with up to 99% ee. Additionally, the enzyme is capable of introducing a variety of fluoroalkyl groups including pentafluoropropyl entity through the same process, with great activity and enantioselectivity. This versatile enzyme could potentially synthesize fluorinated bioactive compounds with directed evolution [47].
Scheme 9.
Substrate scope of P411-PFA-catalyzed C−H trifluoroethylation reaction.
Prenyltransferases such as FtmPT1 from Aspergillus fumigatus transfer a prenyl moiety regiospecifically to the C-2 position of brevianamide F (cyclo-L-Trp-L-Pro). Studies have suggested that the C-2 prenylation of various tryptophan-containing cyclic dipeptides can be catalyzed by the same enzyme in the presence of dimethylallyl diphosphate. However, HPLC and NMR analyses detected C-3 prenylated derivatives as by-products, with yields of up to 19%, while regularly C-2-prenylated indolines were still perceived as main products. This indicates that prenylation catalyzed by FtmPT1 can be intermittent, which increases the challenge for purification; however, it also increases the chemoenzymatic synthesis structure diversity [48].
2.2.2. Enzymes Applied for Amination
Optically active amines are very important constituents for various bioactive compounds, with important pharmaceutical, agrochemical, or industrial applications. In principle, various synthetic routes catalyzed by different enzymes, including hydrolases, oxidoreductases, or transferases, can be applied for the production of optically active primary amines. However, amine transaminases offer a unique way to obtain pure amine enantiomers directly from prochiral ketones. Moreover, these catalysts elucidate pronounced enantio- and regioselectivity. Until now, compared to many (S)-selective amines transaminases (S-ATAs), only a few (R)-amine transaminases (R-ATAs) were commercially available and extensively investigated. R-ATAs such as AspTer from Aspergillus terreus, PenChry from Penicillium, etc., showed excellent conversion rates (>99%) and enantiomeric purities of >99% ee when they were exploited to catalyze the asymmetric synthesis of targeted amines (Scheme 10) [49].
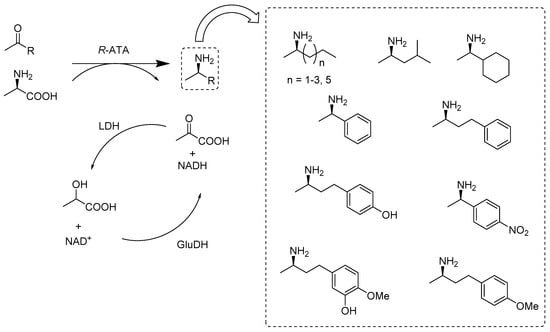
Scheme 10.
Reaction scheme for the asymmetric synthesis of chiral (R)-amines with the (R)-amine transaminases (R-ATAs) in combination with the lactate dehydrogenase (LDH)/lactate dehydrogenase (GDH) system to shift the equilibrium towards product formation.
Regioselectivity is a major challenge in amine transformations due to two or more reactive centers in the molecule. Various (S)- and (R)-stereoselective ω-transaminases have provided protecting group-free strategies for the bio-amination of diketone compounds. A number of 1,5-diketones were selected to synthesize the corresponding optically pure amino ketones catalyzed by ω-transaminases (>92% ee). Simon’s study also suggested that the intermediate underwent a spontaneous ring-closure reaction (Scheme 11), in which Δ1-piperideines were produced. Diastereoselectively reducing these products yields a second chiral center, which will provide the possibility of making chiral 2,6-disubstituted piperidines. Their method is one of the shortest synthetical protocols to date that expands the toolbox for more selective amine transformations [50].
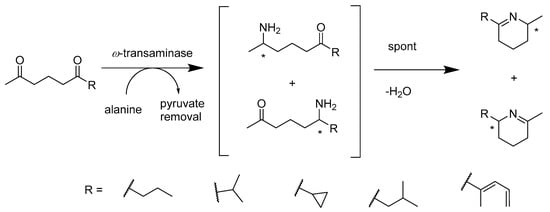
Scheme 11.
Regioselective amination of various 1,5-diketones.
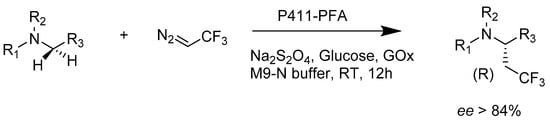
Compared to inserting oxygen atoms into the inactivated carbon–hydrogen bond, enzyme-catalyzed oxidative amination is more constrained. Where suitable enzymes were absent in nature, synthetic chemists started exploiting evolutionary optimization to engineer natural enzymes. Mutants of P450 enzymes such as P411Bm3-CIS are capable of the amination of 2,4,6-triethylben-zene-1-sulfonylazide and its analogues, with excellent enantioselectivity of 430 total turnover numbers and 86% ee for benzosultam (Scheme 12) [51].
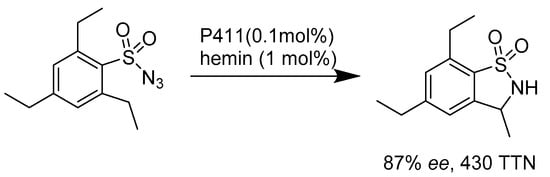
Scheme 12.
Enantioselective intramolecular C–H amination catalyzed by P411.
Chemically, the synthesis of optically pure L-tert-leucine has low yields and enantio-purity. Nevertheless, many enzyme-catalyzed reactions were obstructed, with merely 50% yields. Recent studies show that leucine dehydrogenase identified and cloned from Exiguobacterium sibiricum (EsLeuDH) has high enantioselectivity via the enzymatic reductive amination reaction to produce L-tert-leucine (Scheme 13). Compared to other reported LeuDH, the enzyme EsLeuDH exhibited higher activity at low temperatures and better thermostability at high temperatures. In addition, large scale experimental results indicated that the enzyme has the highest space-time yield (over 80%) among all LeuDHs, with >99% ee [52].

Scheme 13.
Substrate specificity of EsLeuDH in reductive amination and oxidative deamination.
2.2.3. Enzymes Applied for Glycosylation
Chemically, to control the regio- and stereoselectivity of glycosidic bonds, the synthesizing of these oligosaccharides involves various protective group manipulations. Moreover, the challenge of large-scale production limits their application in the food and medical industries. Enzymatic glycosylations provide highly expedient transformation approaches in synthetic chemistry and can be easily exploited broadly to the directed alteration of small molecules and polymers via the covalent attachment of glycosyl residues. Sucrose phosphorylase was successfully employed in the synthesis of (R)-2-O-α-D-glucopyranosyl glyceric acid amide from single-step diastereoselective glucosylation, utilizing sucrose as a glucosyl donor and racemic glyceric acid amide as an acceptor, with d.e. > 83% and high yield (Scheme 14). This protocol could potentially simplify the access to biomimetic glycoside via esterification processes [53]. In another study, Milosavic reported that, when using sucrose and arbutin, α-glucosidase from Saccharomyces cerevisiae was highly stereospecific for the formation of 4-hydroxyphenyl-β-isomaltoside in a molar yield of 50% with reference to arbutin (Scheme 15). Their study also suggested that α-glucosidase can be exploited to glycosylation reaction with other bioactive phenolic compounds comprising a hydroquinone moiety [54].
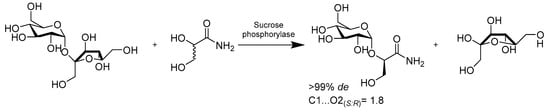
Scheme 14.
Diastereoselectivity of sucrose phosphorylase in the glucosylation of 1,2-diols.
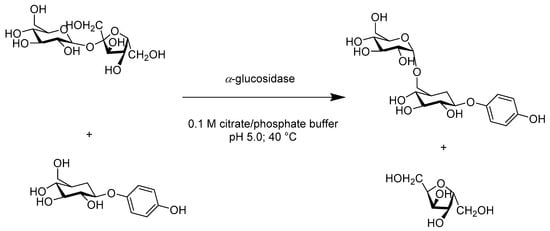
Scheme 15.
The transglucosylation reaction catalyzed by α-glucosidase was optimized with respect to pH, temperature, and time.
N-acetyl-β-D-galactosamine (GalNAc) and N-acetyl-β-D-glucosamine (GlcNAc) residue containing oligosaccharides are essential for many biological activities. They exist in the sugar unit of blood group P-related glycan, many tumor-related carbohydrate antigens gangliosides, milk oligosaccharides, and chitin. Chen’s work showed that a β-N-acetylhexosaminidase, named BbhI, isolated from B. bifidum JCM 1254 exhibited excellent efficiency and regioselectivity in transglycosylation at 3-OH of galactose (Gal) moieties in lactose (Scheme 16). BbhI has a strict regioselectivity of β 1-3 linkage when transferring GalNAc and GlcNAc residues to Gal residue of lactose, with maximal yields of 55.4% and 44.9%, respectively. The enzyme provided a powerful synthetic approach to obtain physiologically active GalNAcβ1-3Lac and GlcNAcβ1-3Lac [55].
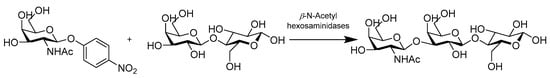
Scheme 16.
Synthesis of β-GalNAc/GlcNAc-Lactose.
Despite great progress in the enzymatic synthesis of sialic acid-containing molecules using sialyltransferase from mammalian sources, a major challenge exists in the low expression levels in the processes. A bacterial based enzyme (α2−6-sialyltransferase) extracted from Photobacterium damselae (Pd2, 6ST) [56] could easily circumvent this issue. Pd2, 6ST could identify the terminal galactose (Gal) and N-Acetylgalactosamine (GalNAc) to produce Neu5Acα2−6Gal and Neu5Acα2−6GalNAc, respectively, in the presence of N-acetylneuraminic acid (Neu5Ac). This novel protocol opens a toolbox for the synthesis of disialyl tetrasaccharide epitopes and their derivatives [57].
The Pictet–Spengler reaction is a stepwise reaction in which a β-arylethylamine proceeds in a condensation reaction with a ketone or aldehyde to produce the addition product followed by ring closure. To synthesize benzyl-isoquinoline alkaloid, plants utilize the stereoselective Pictet–Spengler reaction between dopamine and 4-hydroxyphenylacetaldehyde catalyzed by norcoclaurine synthase (NCS). Recent studies (Scheme 17) revealed that the enzyme could catalyze the manufacturing of artificial, optically active tetrahydroisoquinolines due to its versatility toward aldehydes. Especially when exploiting CjNCS (N-terminally truncated) from Coptis japonica expressed in Escherichia coli, over 85.0% molar yields and excellent e.e. could be achieved by synthesizing 6,7-dihy-droxy-1-propyl-1,2,3,4-tetrahydroisoquinoline and 6,7-dihydroxy-1-phen-ethyl-1,2,3,4-tetrahydroisoquinoline [58]. Alessandra Bonamore’s work (Scheme 18) also suggests that recombinant (S)-NCS produced in E. coli is an efficient and stereoselective environmentally friendly enzyme that catalyzes tyrosine and dopamine substrates in a one-pot, simple, two-step process to produce (S)-norcoclaurine (higenamine), with ee of 93% and a yield higher than 80% in the optimized condition. The process allowed the recycling of NCS with good recovery. Their strategy provided a novel, efficient, and green route for producing plant-derived metabolites such as benzyl-isoquinoline alkaloids [59].
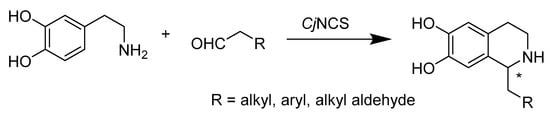
Scheme 17.
Enzymatic synthesis of unnatural 1-substituted 1,2,3,4-tetrahydroisoquinolines.
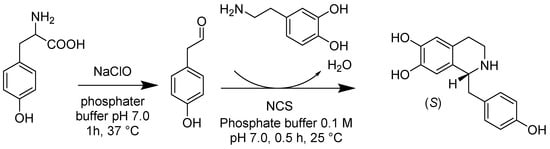
Scheme 18.
The stereospecific chemoenzymatic synthesis of (S)-norcoclaurine from tyrosine and dopamine.
2.2.4. Enzymes Applied for Halogenation
In organic synthesis, the introduction of halogen substituents, especially in mechanistically less-favored positions, remains a challenge. The antibiotic compound pyrroindomycin B was initially isolated by Ding’s research group in 1994 [60]. The molecule is composed of a pyrroloindole entity and a polyketide macro-ring system with a tetramic acid functional group, covalently appended to each end of an unbranched deoxytrisaccharide. The indole ring moiety of pyrroindomycin B is halogenated at the C5 position. As the indole ring is a derivative of tryptophan, typtophan 5-halogenase is responsible for the biosynthesis of pyrroindomycin B in Streptomyces rugosporus LL-42D005. The disruption mutation of the tryptophan 5-halogenase gene resulting in the nonhalogenated pyrroindomycin confirmed this theory [61].
C-1027 or lidamycin is an antitumor antibiotic comprising a reactive enediyne chromophore and an apoprotein unit (CagA). The chromophore readily generates benzenoid diradical intermediate, which is capable of separating hydrogen atoms from the DNA backbone in the presence of free oxygen. The destruction of DNA is associated well with cytotoxicity. The bioactive chromophore has three chemical subunits that are covalently bonded to the nine-membered enediynes, including (S)-3-chloro-4,5-dihydroxy-β-phenylalanine component. This moiety is synthesized from L-α-tyrosine catalyzed by six proteins, one of which is SgcC3. The intermediate (S)-β-tyrosyl-S-SgcC2 can be regioselectively chlorinated by SgcC3 (Scheme 19). The study indicated that the activity of that enzyme is oxygen and FADH2 dependent [62].
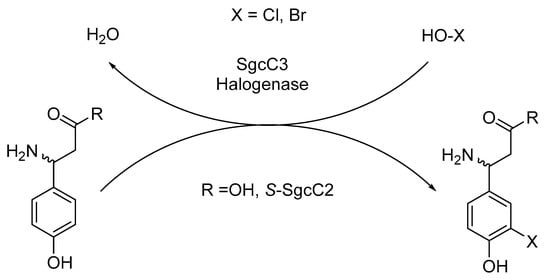
Scheme 19.
SgcC3-catalyzed halogenation of (S)-β-tyrosyl-S-SgcC2.
Another FAD-dependent halogenase, RebH, was translated from Lechevalieria aerocolonigenes that could halogenate rebeccamycin. This enzyme is competent at overriding the distinct rules of halogenation, which is regioselective. For instance, in comparison to the halogenation of indole or tryptophan at the C-3 or C-2 position correspondingly, RebH would chlorinate or brominate L-tryptophan at the C-7 position, which is electronically unfavored (Scheme 20), even in the presence of ortho/para-directing groups [63].
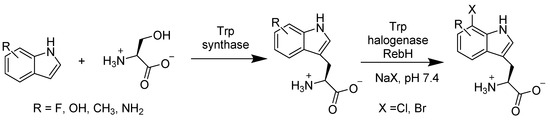
Scheme 20.
Combination of tryptophan synthase from Salmonella enterica and tryptophan-7-halogenase RebH from Lechevalieria aerocolonigenes for the regioselective halogenation of substituted tryptophan derivatives.
2.3. Hydrolases E.C.3
Many polyhydroxylated steroids with vicinal diols on the A-ring have remarkable biological activities. Enzyme catalysis has shown a significant impact on the regio- and stereoselective transformation of functional groups in steroids. Silva and her colleagues conducted a systematic study on a series of stereoisomeric 2, 3- and 3, 4-vicinal diols with selective transesterification catalyzed by commercially available lipases (Scheme 21). Their work delineated the high regioselectivity of enzymatic acylation. The targeted lipases, such as Novozym 435 (immobilized lipase B from Candida antarctica) and C. viscosum lipase, were capable of discriminating between A-ring vicinal hydroxyl groups located on the steroids. According to their results, these lipases are sensitive to the configuration of diols, affording highly regioselective monoesters. Their findings can provide useful synthetic tools for the preparation of mono-acylated, glycosylated, or sulphated steroidal vicinal diols and their derivatives [64].
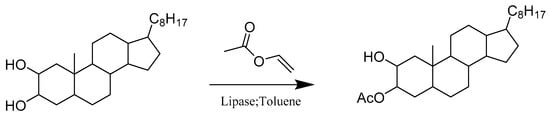
Scheme 21.
Regioselective enzymatic acylation of vicinal diols of steroids.
The chemical acylation of nucleosides has been reported in a few cases, but the reaction is usually carried out by using protecting groups. Furthermore, the tedious separation processes always result in low yields of the mono-O-acyl derivative. However, 2-methyltetrahydrofuran (MeTHF) is a versatile aprotic solvent. Compared to tetrahydrofuran (THF), MeTHF is an excellent substitute in biotransformation processes and has gained popularity in industrial synthetic applications due to its low environmental impact. In this sense, Yolanda Simeo et al. investigated the benefits of using MeTHF as a solvent in enzyme-catalyzed biotransformation. They applied CAL-B lipase to acylate 1-β-arabinofuranosyl uracil (ara-U), 9-β-arabinofuranosyl adenosine (ara-A, 15), 2′-O-(2-methoxyethyl)-5-methyluridine, adenosine, and uridine with vinyl esters and hexanoic anhydride, with yields of over 90% and exceptional regioselectivity. Both THF and MeTHF behave in a similar way, while MeTHF exhibited better conversions for the acylation of uridine and ara-U [65].
The classical chemical acylation approaches which need a base to catalyze with the acid chloride have their downside due to side isomerization reactions. Díaz-Rodríguez and coworkers utilized biocatalytic methodology to synthesize several O-crotonyl 20-deoxynucleoside derivatives (Scheme 22). All these compounds have crotonyl functional groups present, with biological activities such as anti-tumor or inhabitation of the replication of HIV-1 and HIV-2. Their research group employed lipase B from Candida Antarctica (CAL-B) and the immobilized lipase from Pseudomonas cepacia(PSL-C) to attain 5′-O-acylated nucleoside and 3′-O-crotonylated analogs, respectively, without isomerization [66].
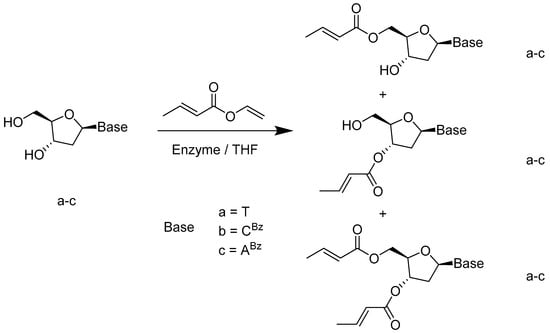
Scheme 22.
Regioselective enzymatic acylation on 2’-deoxynucleosides.
Kołodziejska’s research group also investigated the influence of the solvent on enzymatic reactions. Their study revealed that both the choice of solvent and the acyl group donor impact the activity, stability, and selectivity in enzyme-catalyzed acylation. The hydrophobic organic solvent indicated enzymatic enantioselectivity improvement [67,68]. Tert-butyl methyl ether (TBME) provided an excellent reaction environment for the lipase Amano PS from Burkholderia cepacia (BCL). Acylation reaction gave (R)-monoesters with 81%–99% ee enantiomeric purity (Scheme 23), due to the selectivity of enantio-topic hydroxyl groups [69].
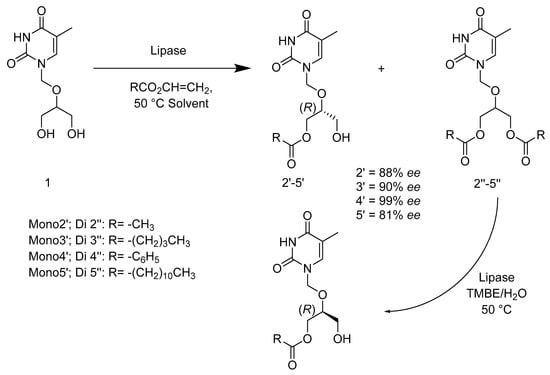
Scheme 23.
Acylation of the prochiral 1-(([1,3-dihydroxypropan-2-yl]oxy)methyl)-5-methylpyrimidine-2,4(1H,3H)-dione and the hydrolysis of the corresponding diesters in the presence of a lipase under various conditions.
Allenes are flexible synthetic precursors of numerous axially chiral compounds, with important industrial applications. Many stereoselective methods have been developed to synthesize these chiral allenes, most of which start from a racemic mixture. Thus, the yield of the allene resolution is always limited. However, exploiting prochiral substrates in enzyme-catalyzed transesterification reactions is a pronounced approach to overcome the limitation of yields. Sapu’s work suggests that porcine pancreatic lipase (PPL) is an excellent biocatalyst for the kinetic resolution of allenols (Scheme 24). The enzyme is also suitable for the transesterification of diols with similar structures. Exemplarily, treating the prochiral phenyl-substituted diol with vinyl butyrate catalyzed by PPL led to monoesters with 95% yield and 98% ee under modified conditions. One can conclude that this powerful chemoenzymatic protocol makes either enantiomer of optical allenyl monoesters accessible [70].
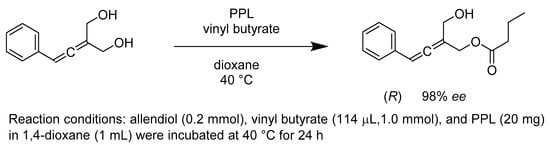
Scheme 24.
Desymmetrization of allendiols.
Traditionally, the selective benzoylation of the 5′-hydroxyl group of thymidine requires highly toxic hexamethylphosphoric triamide and produces a by-product, triphenylphosphine oxide, that is difficult to remove [71]. Other alternative routes are either time consuming and/or have moderate yields [72,73]. Enzyme-catalyzed acylation is a substitute for standard synthetic methods that can avoid these complications. Enzyme Candida Antarctica lipase B (CAL-B) proved to have high selectivity over 5′-hydroxyl in the transesterification and to be reclaimed (Scheme 25). The benzoylation protocol catalyzed by CAL-B is a mild and efficient synthetic method for 5′-O-benzoyl-2′-deoxynucleosides, with excellent yields (over 89%). Easy scalability, minimal environmental impact, and recycling of both enzyme and acylating agent make this pathway very attractive for industrial applications [74].
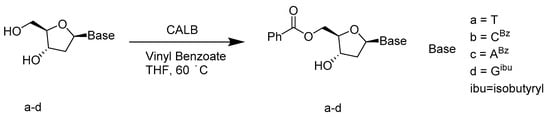
Scheme 25.
Regioselective enzymatic procedure for 5’-O-benzoylation of 2’-deoxynucleosides.
Xiao et al. (Scheme 26) developed a new method to prepare enantiopure caffeic acid amides with an asymmetric aminolysis reaction between the cinnamic acid ester and (R, S)-α-phenylethylamine, which is catalyzed by an immobilized lipase (Novozym 435) from Candida antarctica. They optimized the reaction conditions, and under those conditions, high enantioselectivity for the enzymatic reaction was gained, with an enantiomeric excess of 98.5% [75]. Intermolecular aziridination is a highly enantioselective and valuable synthetical organic reaction that has no known natural equivalent. An engineered, genetically encoded enzyme, cytochrome P450, demonstrated high enantioselectivity, with up to 99% ee for intermolecular aziridination, and it can be easily optimized (Scheme 27). Exemplarily, enzyme P-I263F-A328V-L437V, a variant of P411 (A mutation from wild-type P450), showed significant improvements in enantioselectivity (25% ee to 99% ee) and yield (1.1% to 55%) compared to its precursor, P411, when catalyzing aziridination of 4-methyl styrene [76].
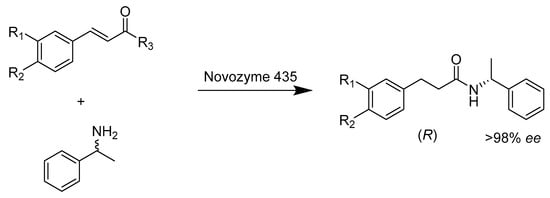
Scheme 26.
Asymmetric aminolysis of cinnamic acid ester derivatives.
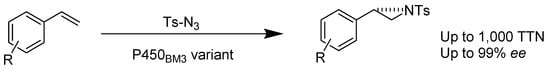
Scheme 27.
Enzyme-catalyzed olefin aziridination.
Wombacher et al. (Scheme 28) discovered an RNA enzyme (ribozyme) that catalyzes a Diels–Alder reaction between an oligo anthracene diene and a maleimide dienophile, stereoselectively synthesizing an R, R enantiomer with a high yield of ~90% (ee%) when n equals 6 or 12. The ribozyme was selected from a library of RNA conjugates generated by 120 randomized nucleotides. They proved that the size of the diene determined the stereoselectivity of the reaction and explained the mechanism in the true catalytic reaction through a crystal structure study [8]. The synthesis strategy of 5-hydroxyimino-4,5-dihydrofurans through a lipase-catalyzed reaction between β-nitrostyrenes and 1,3-dicarbonyl compounds was developed by Wu (Scheme 29) and coworkers. The highlight of their study was that a high stereoselectivity (Z/E up to 99:1) was achieved by screening a series of different β-nitrostyrenes and lipases from different organisms. Compared with the reaction using traditional catalyst NEt3 in methanol, the enzymatic reaction produced much less by-product of dihydrofuran, which led to the high stereoselectivity of the Z product [77].
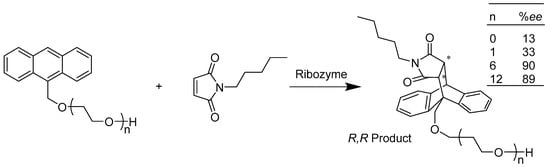
Scheme 28.
Formation of carbon–carbon bonds by a Diels–Alder reaction between an anthracene diene and a maleimide dienophile.
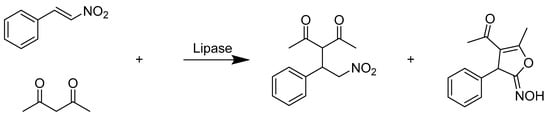
Scheme 29.
Lipase-catalyzed reaction of nitrostyrene and acetylacetone.
As already delineated, the lipase extracted from Pseudomonas aeruginosa (PAL) has been proven to be effective in catalyzing the stereoselective hydrolytic kinetic resolution of a chiral ester. Manfred T. Reetz and coworkers applied iterative saturation mutagenesis (ISM) to maximize the quality of mutant libraries. They were able to subject ISM to systematic and rigorous comparison with directed evolution methods such as error-prone polymerase chain reaction (epPCR), saturation mutagenesis, and DNA shuffling (Scheme 30). Their results revealed that the best mutant dramatically improved enantioselectivity without requiring high screening effort [78].
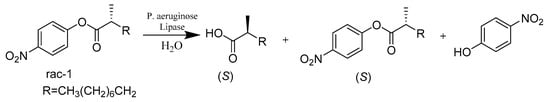
Scheme 30.
Hydrolytic kinetic resolution of the chiral ester rac-1.
Enantio-pure ethyl- and methyl-3-hydroxyglutaric monocarboxylic acids have numerous industrial applications. The hydrolysis of prochiral diethyl- and dimethyl-3-hydroxy glutarates is one way to obtain these chiral molecules. Jacobsen’s work has demonstrated that lipase B from Candida Antarctica (CALB) is suitable for catalyzing in both hydrolysis and ammonolysis reactions. Moreover, the CALB enzyme provided high efficiency in the hydrolysis of diethyl-3-hydroxyglutarate, with up to 91% ee (Scheme 31). The hydrolase is reusable, with the preservation of excellent selectivity and activity [79].
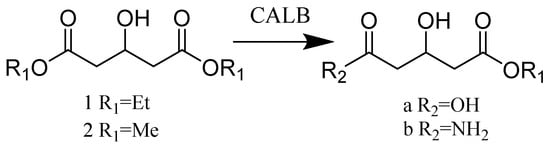
Scheme 31.
Hydrolysis of prochiral diethyl-3-hydroxyglutarate (1) and dimethyl-3-hydroxyglutarate (2) with CALB.
Chiral bicyclic diols are potentially important synthetic intermediates. In the past, dynamic kinetic resolution (DKR) has been one of the most extensively used methods of asymmetric synthesizing enantioselective pure primary amines or secondary alcohols. Thus, a modified method called enzyme- and ruthenium-catalyzed dynamic kinetic asymmetric transformation (DYKAT), developed by Patrik Krumlinde and coworkers, was employed to synthesize the diacetates of bicyclic diols, with high enantioselectivity (99.9% ee). The DYKAT protocol gives high enantio- and diastereoselectivities (Scheme 32). Compared to synthetic chemistry, their work has illustrated that the synthesis of enantioselective sertraline from racemic cis/trans diol (n = 2) can be achieved with a higher yield [80].
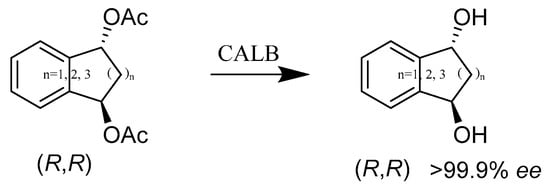
Scheme 32.
Ester cleavage of diacetates.
The carboxylation of phenol and styrene derivatives by carboxylases (Scheme 33) in carbonate buffer is highly regioselective. While benzoic acid carboxylases form the ortho-hydroxybenzoic acid derivative, the phenolic acid carboxylases act on the beta-carbon and form (E)- cinnamic acids, with the aromatic segment remaining completely intact [81].
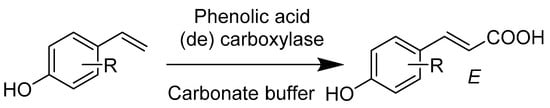
Scheme 33.
Regiocomplementary enzymatic carboxylation of phenols and hydroxystyrene derivatives.
2.4. Lyases E.C.4
Williams et al. (Scheme 34) applied the evolved tagatose-1,6-bisphosphate (TBP) aldolase to catalyze the production of tagatose 1,6-bisphosphate by using dihydroxyacetone phosphate (DHAP) and glyceraldehyde 3-phosphate (G3P) as reactants. In their study, they performed three rounds of gene mutation, including random mutation and direct mutation on the framework of TBP aldolase (agaY), followed by screening ~3000 first-generation variants created from the mutation. The evolved third-generation TBP aldolase not only increased the reaction rate 80-fold but also increased the stereoselectivity 100-fold [82].
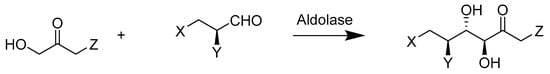
Scheme 34.
Stereochemistry of the reaction catalyzed by aldolases.
A new approach to stereoselectively synthesizing syn- or anti-2-hydroxy diols was studied by Husain and coworkers. In this study, the stereoselective synthesis of (R)-2-hydroxy carbonyl compounds catalyzed by benzaldehyde lyase from Pichia. Glucozyma (BAL) was carried out as the critical step, in which an aliphatic and an aromatic ketone were used as reactants. Corresponding compounds with high optical purity (ee%, >97%) were obtained from the enzymatic reaction. This new approach provides a new solution to selectively synthesize pure syn- or anti-2-hydroxy diols with unprotected substrate [83].
Wu et al. (Scheme 35) established a practical biocatalytic method to prepare L-norephedrine, in which an R-selective pyruvate decarboxylase from Saccharomyces cerevisiae and an S-selective ω-transaminase from Vibrio fluvialis JS17 were tandemly applied. With a molar yield of over 60%, de (diastereomeric excess) and ee (enantiomeric excess) values of greater than 99.5% were achieved through the enzyme-catalyzed reaction [84]. In the presence of carbon dioxide as a C-1 unit, the enzymatic carboxylation of para-hydroxystyrenes by phenolic acid decarboxylases synthesizes into (E)-cinnamic acids at a conversion rate of up to 40%. Wuensch’s study revealed that this novel enzyme-catalyzed β-carboxylation has no traditional chemical synthesis counterpart [85].
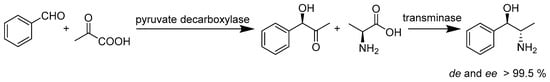
Scheme 35.
Biocatalytic preparation of L-norephedrine by ScPDC and VfTA.
3. Conclusions
Nature’s vast repertoire has inspired the chemist to exploit biocatalysts for chemical synthesis. They also utilize the evolution engineering strategy of mutation and selection to improve the efficiency, stability, and regio- and enantioselectivity of enzyme function for various applications. However, challenges to enzymatic reactions remain. Research should focus on investigating nature’s mechanisms for enzyme innovation, especially catalysts for non-natural reactions, as well as delineating the structure and molecular interactions of enzymes. All these challenges will have a huge impact on modern organic synthetical processes.
Undoubtedly, the extensive literature on enzymatic reactions highlights the extraordinary ability of the biocatalyst to adapt and acquire new functions. Protein engineering and new analytical methods have opened a toolbox for more sustainable, selective, and cost-efficient chemical reactions.
Funding
This work was carried by the Louisiana Board of Regents, grant number LEQSF-EPS (2020)-SURE-226, and the Centenary College of Louisiana Charlton H. Lyons Summer Research Award. The publication of this article was funded by the Centenary College of Louisiana.
Acknowledgments
We thank James M. Clawson for his thoughtful comments on the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Punekar, N.S. Enzymes: Catalysis, Kinetics and Mechanisms; Springer: Singapore, 2018. [Google Scholar] [CrossRef]
- Rao, D.B.; Sane, P.G.; Georgiev, E.L. Collagenase in the treatment of dermal and decubitus ulcers. J. Am. Geriatr. Soc. 1975, 23, 22–30. [Google Scholar] [CrossRef]
- Wang, Z.; Li, C.; Wei, Y. Application of Fluorescence in Studying Therapeutic Enzymes. Adv. Exp. Med. Biol. 2019, 1148, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Schmid, A.; Hollmann, F.; Park, J.B.; Bühler, B. The use of enzymes in the chemical industry in Europe. Curr. Opin. Biotechnol. 2002, 13, 359–366. [Google Scholar] [CrossRef]
- Sunar, K.; Kumar, U.; Deshmukh, S.K. Recent Applications of Enzymes in Personal Care Products. In Agro-Industrial Wastes as Feedstock for Enzyme Production; Elsevier: Philadelphia, PA, USA, 2016; pp. 279–298. [Google Scholar] [CrossRef]
- Singh, R.; Kumar, M.; Mittal, A.; Mehta, P.K. Microbial enzymes: Industrial progress in 21st century. 3 Biotech 2016, 6, 174. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A. Enzyme Immobilization: The Quest for Optimum Performance. Adv. Synth. Catal. 2007, 349, 1289–1307. [Google Scholar] [CrossRef]
- Wombacher, R.; Keiper, S.; Suhm, S.; Serganov, A.; Patel, D.J.; Jaschke, A. Control of stereoselectivity in an enzymatic reaction by backdoor access. Angew. Chem. Int. Ed. Engl. 2006, 45, 2469–2472. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; McCallum, N.C.; Ni, Q.Z.; Li, W.; Boyce, H.; Mao, H.; Zhou, X.; Sun, H.; Thompson, M.P.; Battistella, C.; et al. Selenomelanin: An Abiotic Selenium Analogue of Pheomelanin. J. Am. Chem. Soc. 2020. [Google Scholar] [CrossRef]
- Taofiq, O.; González-Paramás, A.M.; Martins, A.; Barreiro, M.F.; Ferreira, I.C.F.R. Mushrooms extracts and compounds in cosmetics, cosmeceuticals and nutricosmetics—A review. Ind. Crop. Prod. 2016, 90, 38–48. [Google Scholar] [CrossRef]
- Weinshilboum, R.; Axelrod, J. Serum dopamine-beta-hydroxylase activity. Circ. Res. 1971, 28, 307–315. [Google Scholar] [CrossRef]
- Shen, Z.; Lv, C.; Zeng, S. Significance and challenges of stereoselectivity assessing methods in drug metabolism. J. Pharm. Anal. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Vargesson, N. Thalidomide-induced teratogenesis: History and mechanisms. Birth Defects Res. C Embryo Today 2015, 105, 140–156. [Google Scholar] [CrossRef]
- Miller, M.T. Thalidomide embryopathy: A model for the study of congenital incomitant horizontal strabismus. Trans. Am. Ophthalmol. Soc. 1991, 89, 623–674. [Google Scholar] [PubMed]
- Brooks, W.H.; Guida, W.C.; Daniel, K.G. The significance of chirality in drug design and development. Curr. Top. Med. Chem. 2011, 11, 760–770. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Hua, L. Enantioselective enzymatic reductions of sterically bulky aryl alkyl ketones catalyzed by a NADPH-dependent carbonyl reductase. J. Org. Chem. 2006, 71, 9484–9486. [Google Scholar] [CrossRef] [PubMed]
- Hoyos, P.; Sinisterra, J.V.; Molinari, F.; Alcantara, A.R.; de Maria, P.D. Biocatalytic strategies for the asymmetric synthesis of alpha-hydroxy ketones. Acc. Chem. Res. 2010, 43, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Kaluzna, I.A.; Feske, B.D.; Wittayanan, W.; Ghiviriga, I.; Stewart, J.D. Stereoselective, biocatalytic reductions of alpha-chloro-beta-keto esters. J. Org. Chem. 2005, 70, 342–345. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Yang, Y.; Hua, L. Stereoselective enzymatic synthesis of chiral alcohols with the use of a carbonyl reductase from Candida magnoliae with anti-Prelog enantioselectivity. J. Org. Chem. 2006, 71, 4202–4205. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Lee, T.S.; Khosla, C. Engineered biosynthesis of regioselectively modified aromatic polyketides using bimodular polyketide synthases. PLoS Biol. 2004, 2, e31. [Google Scholar] [CrossRef]
- Classen, T.; Korpak, M.; Schölzel, M.; Pietruszka, J. Stereoselective Enzyme Cascades: An Efficient Synthesis of Chiral γ-Butyrolactones. ACS Catal. 2014, 4, 1321–1331. [Google Scholar] [CrossRef]
- Oroz-Guinea, I.; Garcia-Junceda, E. Enzyme catalysed tandem reactions. Curr. Opin. Chem. Biol. 2013, 17, 236–249. [Google Scholar] [CrossRef]
- Groger, H.; Hummel, W.; Buchholz, S.; Drauz, K.; Nguyen, T.V.; Rollmann, C.; Husken, H.; Abokitse, K. Practical asymmetric enzymatic reduction through discovery of a dehydrogenase-compatible biphasic reaction media. Org. Lett. 2003, 5, 173–176. [Google Scholar] [CrossRef] [PubMed]
- De Gonzalo, G.; Lavandera, I.; Faber, K.; Kroutil, W. Enzymatic reduction of ketones in "micro-aqueous" media catalyzed by ADH-A from Rhodococcus ruber. Org. Lett. 2007, 9, 2163–2166. [Google Scholar] [CrossRef]
- Boratynski, F.; Smuga, M.; Wawrzenczyk, C. Lactones 42. Stereoselective enzymatic/microbial synthesis of optically active isomers of whisky lactone. Food Chem. 2013, 141, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Kulig, J.; Simon, R.C.; Rose, C.A.; Husain, S.M.; Häckh, M.; Lüdeke, S.; Zeitler, K.; Kroutil, W.; Pohl, M.; Rother, D. Stereoselective synthesis of bulky 1,2-diols with alcohol dehydrogenases. Catal. Sci. Technol. 2012, 2, 1580–1589. [Google Scholar] [CrossRef]
- O’Reilly, E.; Iglesias, C.; Ghislieri, D.; Hopwood, J.; Galman, J.L.; Lloyd, R.C.; Turner, N.J. A regio- and stereoselective omega-transaminase/monoamine oxidase cascade for the synthesis of chiral 2,5-disubstituted pyrrolidines. Angew. Chem. Int. Ed. Engl. 2014, 53, 2447–2450. [Google Scholar] [CrossRef]
- Willistein, M.; Haas, J.; Fuchs, J.; Estelmann, S.; Ferlaino, S.; Muller, M.; Ludeke, S.; Boll, M. Enantioselective Enzymatic Naphthoyl Ring Reduction. Chemistry 2018, 24, 12505–12508. [Google Scholar] [CrossRef] [PubMed]
- Oura, T.; Kajiwara, S. Substrate specificity and regioselectivity of delta12 and omega3 fatty acid desaturases from Saccharomyces kluyveri. Biosci. Biotechnol. Biochem. 2008, 72, 3174–3179. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.W.; Meinhold, P.; Glieder, A.; Arnold, F.H. Regio- and enantioselective alkane hydroxylation with engineered cytochromes P450 BM-3. J. Am. Chem. Soc. 2003, 125, 13442–13450. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Nguyen, D.; Gonzalez, M.; Cortez, A.; Mullen, S.E.; Cheruzel, L.E. Regio- and stereoselective hydroxylation of 10-undecenoic acid with a light-driven P450 BM3 biocatalyst yielding a valuable synthon for natural product synthesis. Bioorg. Med. Chem. 2014, 22, 5687–5691. [Google Scholar] [CrossRef]
- Hu, S.; Huang, J.; Mei, L.; Yu, Q.; Yao, S.; Jin, Z. Altering the regioselectivity of cytochrome P450 BM-3 by saturation mutagenesis for the biosynthesis of indirubin. J. Mol. Catal. B Enzym. 2010, 67, 29–35. [Google Scholar] [CrossRef]
- Landwehr, M.; Hochrein, L.; Otey, C.R.; Kasrayan, A.; Backvall, J.E.; Arnold, F.H. Enantioselective alpha-hydroxylation of 2-arylacetic acid derivatives and buspirone catalyzed by engineered cytochrome P450 BM-3. J. Am. Chem. Soc. 2006, 128, 6058–6059. [Google Scholar] [CrossRef] [PubMed]
- Negretti, S.; Narayan, A.R.; Chiou, K.C.; Kells, P.M.; Stachowski, J.L.; Hansen, D.A.; Podust, L.M.; Montgomery, J.; Sherman, D.H. Directing group-controlled regioselectivity in an enzymatic C-H bond oxygenation. J. Am. Chem. Soc. 2014, 136, 4901–4904. [Google Scholar] [CrossRef]
- Kubo, T.; Peters, M.W.; Meinhold, P.; Arnold, F.H. Enantioselective epoxidation of terminal alkenes to (R)- and (S)-epoxides by engineered cytochromes P450 BM-3. Chemistry 2006, 12, 1216–1220. [Google Scholar] [CrossRef] [PubMed]
- Denard, C.A.; Bartlett, M.J.; Wang, Y.; Lu, L.; Hartwig, J.F.; Zhao, H. Development of a One-Pot Tandem Reaction Combining Ruthenium-Catalyzed Alkene Metathesis and Enantioselective Enzymatic Oxidation To Produce Aryl Epoxides. ACS Catal. 2015, 5, 3817–3822. [Google Scholar] [CrossRef]
- Loskot, S.A.; Romney, D.K.; Arnold, F.H.; Stoltz, B.M. Enantioselective Total Synthesis of Nigelladine A via Late-Stage C-H Oxidation Enabled by an Engineered P450 Enzyme. J. Am. Chem. Soc. 2017, 139, 10196–10199. [Google Scholar] [CrossRef]
- Farwell, C.C.; McIntosh, J.A.; Hyster, T.K.; Wang, Z.J.; Arnold, F.H. Enantioselective imidation of sulfides via enzyme-catalyzed intermolecular nitrogen-atom transfer. J. Am. Chem. Soc. 2014, 136, 8766–8771. [Google Scholar] [CrossRef]
- Brandenberg, O.F.; Prier, C.K.; Chen, K.; Knight, A.M.; Wu, Z.; Arnold, F.H. Stereoselective Enzymatic Synthesis of Heteroatom-Substituted Cyclopropanes. ACS Catal. 2018, 8, 2629–2634. [Google Scholar] [CrossRef]
- Tang, M.C.; Fu, C.Y.; Tang, G.L. Characterization of SfmD as a Heme peroxidase that catalyzes the regioselective hydroxylation of 3-methyltyrosine to 3-hydroxy-5-methyltyrosine in saframycin A biosynthesis. J. Biol. Chem. 2012, 287, 5112–5121. [Google Scholar] [CrossRef]
- Cho, I.; Prier, C.K.; Jia, Z.J.; Zhang, R.K.; Gorbe, T.; Arnold, F.H. Enantioselective Aminohydroxylation of Styrenyl Olefins Catalyzed by an Engineered Hemoprotein. Angew. Chem. Int. Ed. Engl. 2019, 58, 3138–3142. [Google Scholar] [CrossRef]
- Sello, G.; Orsini, F.; Bernasconi, S.; Gennaro, P.D. Synthesis of enantiopure 2-amino-1-phenyl and 2-amino-2-phenyl ethanols using enantioselective enzymatic epoxidation and regio- and diastereoselective chemical aminolysis. Tetrahedron Asymmetry 2006, 17, 372–376. [Google Scholar] [CrossRef]
- Enoki, J.; Meisborn, J.; Muller, A.C.; Kourist, R. A Multi-Enzymatic Cascade Reaction for the Stereoselective Production of gamma-Oxyfunctionalyzed Amino Acids. Front. Microbiol. 2016, 7, 425. [Google Scholar] [CrossRef] [PubMed]
- Sib, A.; Gulder, T.A.M. Stereoselective Total Synthesis of Bisorbicillinoid Natural Products by Enzymatic Oxidative Dearomatization/Dimerization. Angew. Chem. Int. Ed. Engl. 2017, 56, 12888–12891. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, P.; Sreenilayam, G.; Tyagi, V.; Fasan, R. Gram-Scale Synthesis of Chiral Cyclopropane-Containing Drugs and Drug Precursors with Engineered Myoglobin Catalysts Featuring Complementary Stereoselectivity. Angew. Chem. Int. Ed. Engl. 2016, 55, 16110–16114. [Google Scholar] [CrossRef] [PubMed]
- Itoh, N.; Iwata, C.; Toda, H. Molecular cloning and characterization of a flavonoid-O-methyltransferase with broad substrate specificity and regioselectivity from Citrus depressa. BMC Plant Biol. 2016, 16, 180. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, X.; Zhang, R.K.; Arnold, F.H. Enantiodivergent alpha-Amino C-H Fluoroalkylation Catalyzed by Engineered Cytochrome P450s. J. Am. Chem. Soc. 2019, 141, 9798–9802. [Google Scholar] [CrossRef]
- Wollinsky, B.; Ludwig, L.; Xie, X.; Li, S.M. Breaking the regioselectivity of indole prenyltransferases: Identification of regular C3-prenylated hexahydropyrrolo[2,3-b]indoles as side products of the regular C2-prenyltransferase FtmPT1. Org. Biomol. Chem. 2012, 10, 9262–9270. [Google Scholar] [CrossRef]
- Schätzle, S.; Steffen-Munsberg, F.; Thontowi, A.; Höhne, M.; Robins, K.; Bornscheuer, U.T. Enzymatic Asymmetric Synthesis of Enantiomerically Pure Aliphatic, Aromatic and Arylaliphatic Amines with (R)-Selective Amine Transaminases. Adv. Synth. Catal. 2011, 353, 2439–2445. [Google Scholar] [CrossRef]
- Simon, R.C.; Grischek, B.; Zepeck, F.; Steinreiber, A.; Belaj, F.; Kroutil, W. Regio- and stereoselective monoamination of diketones without protecting groups. Angew. Chem. Int. Ed. Engl. 2012, 51, 6713–6716. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, J.A.; Coelho, P.S.; Farwell, C.C.; Wang, Z.J.; Lewis, J.C.; Brown, T.R.; Arnold, F.H. Enantioselective intramolecular C-H amination catalyzed by engineered cytochrome P450 enzymes in vitro and in vivo. Angew. Chem. Int. Ed. Engl. 2013, 52, 9309–9312. [Google Scholar] [CrossRef]
- Li, J.; Pan, J.; Zhang, J.; Xu, J.-H. Stereoselective synthesis of l-tert-leucine by a newly cloned leucine dehydrogenase from Exiguobacterium sibiricum. J. Mol. Catal. B Enzym. 2014, 105, 11–17. [Google Scholar] [CrossRef]
- Wildberger, P.; Brecker, L.; Nidetzky, B. Chiral resolution through stereoselective transglycosylation by sucrose phosphorylase: Application to the synthesis of a new biomimetic compatible solute, (R)-2-O-alpha-D-glucopyranosyl glyceric acid amide. Chem. Commun. 2014, 50, 436–438. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Milosavić, N.B.; Prodanović, R.M.; Jankov, R.M. A simple and efficient one-step, regioselective, enzymatic glucosylation of arbutin by α-glucosidase. Tetrahedron Lett. 2007, 48, 7222–7224. [Google Scholar] [CrossRef]
- Chen, X.; Xu, L.; Jin, L.; Sun, B.; Gu, G.; Lu, L.; Xiao, M. Efficient and Regioselective Synthesis of beta-GalNAc/GlcNAc-Lactose by a Bifunctional Transglycosylating beta-N-Acetylhexosaminidase from Bifidobacterium bifidum. Appl. Environ. Microbiol. 2016, 82, 5642–5652. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Huang, S.; Chokhawala, H.; Sun, M.; Zheng, H.; Chen, X. Highly efficient chemoenzymatic synthesis of naturally occurring and non-natural alpha-2,6-linked sialosides: A P. damsela alpha-2,6-sialyltransferase with extremely flexible donor-substrate specificity. Angew. Chem. Int. Ed. Engl. 2006, 45, 3938–3944. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Yao, W.; Cheng, J.; Zhang, X.; Jin, L.; Yu, H.; Chen, X.; Wang, F.; Cao, H. Regioselective chemoenzymatic synthesis of ganglioside disialyl tetrasaccharide epitopes. J. Am. Chem. Soc. 2014, 136, 5205–5208. [Google Scholar] [CrossRef] [PubMed]
- Nishihachijo, M.; Hirai, Y.; Kawano, S.; Nishiyama, A.; Minami, H.; Katayama, T.; Yasohara, Y.; Sato, F.; Kumagai, H. Asymmetric synthesis of tetrahydroisoquinolines by enzymatic Pictet-Spengler reaction. Biosci. Biotechnol. Biochem. 2014, 78, 701–707. [Google Scholar] [CrossRef]
- Bonamore, A.; Rovardi, I.; Gasparrini, F.; Baiocco, P.; Barba, M.; Molinaro, C.; Botta, B.; Boffi, A.; Macone, A. An enzymatic, stereoselective synthesis of (S)-norcoclaurine. Green Chem. 2010, 12, 1623–1627. [Google Scholar] [CrossRef]
- Ding, W.; Williams, D.R.; Northcote, P.; Siegel, M.M.; Tsao, R.; Ashcroft, J.; Morton, G.O.; Alluri, M.; Abbanat, D.; Maiese, W.M.; et al. Pyrroindomycins, novel antibiotics produced by Streptomyces rugosporus sp. LL-42D005. I. Isolation and structure determination. J. Antibiot. 1994, 47, 1250–1257. [Google Scholar] [CrossRef]
- Zehner, S.; Kotzsch, A.; Bister, B.; Sussmuth, R.D.; Mendez, C.; Salas, J.A.; van Pee, K.H. A regioselective tryptophan 5-halogenase is involved in pyrroindomycin biosynthesis in Streptomyces rugosporus LL-42D005. Chem. Biol. 2005, 12, 445–452. [Google Scholar] [CrossRef]
- Lin, S.; Van Lanen, S.G.; Shen, B. Regiospecific chlorination of (S)-beta-tyrosyl-S-carrier protein catalyzed by SgcC3 in the biosynthesis of the enediyne antitumor antibiotic C-1027. J. Am. Chem. Soc. 2007, 129, 12432–12438. [Google Scholar] [CrossRef]
- Frese, M.; Guzowska, P.H.; Voss, H.; Sewald, N. Regioselective Enzymatic Halogenation of Substituted Tryptophan Derivatives using the FAD-Dependent Halogenase RebH. ChemCatChem 2014, 6, 1270–1276. [Google Scholar] [CrossRef]
- Silva, M.M.C.; Riva, S.; e Melo, M.L.S. Regioselective enzymatic acylation of vicinal diols of steroids. Tetrahedron 2005, 61, 3065–3073. [Google Scholar] [CrossRef]
- Simeó, Y.; Sinisterra, J.V.; Alcántara, A.R. Regioselective enzymatic acylation of pharmacologically interesting nucleosides in 2-methyltetrahydrofuran, a greener substitute for THF. Green Chem. 2009, 11, 855–862. [Google Scholar] [CrossRef]
- Díaz-Rodríguez, A.; Fernández, S.; Lavandera, I.; Ferrero, M.; Gotor, V. Novel and efficient regioselective enzymatic approach to 3′-, 5′- and 3′,5′-di-O-crotonyl 2′-deoxynucleoside derivatives. Tetrahedron Lett. 2005, 46, 5835–5838. [Google Scholar] [CrossRef]
- Kaar, J.L.; Jesionowski, A.M.; Berberich, J.A.; Moulton, R.; Russell, A.J. Impact of ionic liquid physical properties on lipase activity and stability. J. Am. Chem. Soc. 2003, 125, 4125–4131. [Google Scholar] [CrossRef]
- Azuma, H.; Miyasaka, K.; Yokotani, T.; Tachibana, T.; Kojima-Yuasa, A.; Matsui-Yuasa, I.; Ogino, K. Lipase-catalyzed preparation of optically active 1′-acetoxychavicol acetates and their structure-activity relationships in apoptotic activity against human leukemia HL-60 cells. Bioorg. Med. Chem. 2006, 14, 1811–1818. [Google Scholar] [CrossRef]
- Kołodziejska, R.; Górecki, M.; Frelek, J.; Dramiński, M. Enantioselective enzymatic desymmetrization of the prochiral pyrimidine acyclonucleoside. Tetrahedron Asymmetry 2012, 23, 683–689. [Google Scholar] [CrossRef]
- Manzuna Sapu, C.; Backvall, J.E.; Deska, J. Enantioselective enzymatic desymmetrization of prochiral allenic diols. Angew. Chem. Int. Ed. Engl. 2011, 50, 9731–9734. [Google Scholar] [CrossRef]
- Lim, R.; Mitsunobu, K. Effect of dibutyryl cyclic AMP on nucleic acid and protein synthesis in neuronal and glial tumor cells. Life Sci. II 1972, 11, 1063–1070. [Google Scholar] [CrossRef]
- Nishino, S.F. Direct acridine orange counting of bacteria preserved with acidified lugol iodine. Appl. Environ. Microbiol. 1986, 52, 602–604. [Google Scholar] [CrossRef]
- Liguori, A.; Sindona, G.; Uccella, N. Sequence effect on the slow degradations of dinucleotides by fast atom bombardment tandem mass spectrometry. Biomed. Environ. Mass Spectrom. 1988, 16, 451–454. [Google Scholar] [CrossRef] [PubMed]
- García, J.; Fernández, S.; Ferrero, M.; Sanghvi, Y.S.; Gotor, V. A mild, efficient and regioselective enzymatic procedure for 5′-O-benzoylation of 2′-deoxynucleosides. Tetrahedron Lett. 2004, 45, 1709–1712. [Google Scholar] [CrossRef]
- Xiao, P.; Zhang, S.; Ma, H.; Zhang, A.; Lv, X.; Zheng, L. Stereoselective synthesis of caffeic acid amides via enzyme-catalyzed asymmetric aminolysis reaction. J. Biotechnol. 2013, 168, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Farwell, C.C.; Zhang, R.K.; McIntosh, J.A.; Hyster, T.K.; Arnold, F.H. Enantioselective Enzyme-Catalyzed Aziridination Enabled by Active-Site Evolution of a Cytochrome P450. ACS Cent. Sci. 2015, 1, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-Y.; Li, K.; He, T.; Feng, X.-W.; Wang, N.; Wang, X.-Y.; Yu, X.-Q. A novel enzymatic tandem process: Utilization of biocatalytic promiscuity for high stereoselective synthesis of 5-hydroxyimino-4,5-dihydrofurans. Tetrahedron 2011, 67, 2681–2688. [Google Scholar] [CrossRef]
- Reetz, M.T.; Prasad, S.; Carballeira, J.D.; Gumulya, Y.; Bocola, M. Iterative saturation mutagenesis accelerates laboratory evolution of enzyme stereoselectivity: Rigorous comparison with traditional methods. J. Am. Chem. Soc. 2010, 132, 9144–9152. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, E.E.; Hoff, B.H.; Moen, A.R.; Anthonsen, T. Enatioselective enzymatic preparation of chiral glutaric monocarboxylic acids and amides. J. Mol. Catal. B Enzym. 2003, 21, 55–58. [Google Scholar] [CrossRef]
- Krumlinde, P.; Bogar, K.; Backvall, J.E. Asymmetric synthesis of bicyclic diol derivatives through metal and enzyme catalysis: Application to the formal synthesis of sertraline. Chemistry 2010, 16, 4031–4036. [Google Scholar] [CrossRef]
- Wuensch, C.; Glueck, S.M.; Gross, J.; Koszelewski, D.; Schober, M.; Faber, K. Regioselective enzymatic carboxylation of phenols and hydroxystyrene derivatives. Org. Lett. 2012, 14, 1974–1977. [Google Scholar] [CrossRef]
- Williams, G.J.; Domann, S.; Nelson, A.; Berry, A. Modifying the stereochemistry of an enzyme-catalyzed reaction by directed evolution. Proc. Natl. Acad. Sci. USA 2003, 100, 3143–3148. [Google Scholar] [CrossRef]
- Husain, S.M.; Stillger, T.; Dünkelmann, P.; Lödige, M.; Walter, L.; Breitling, E.; Pohl, M.; Bürchner, M.; Krossing, I.; Müller, M.; et al. Stereoselective Reduction of 2-Hydroxy Ketones towards syn- and anti-1,2-Diols. Adv. Synth. Catal. 2011, 353, 2359–2362. [Google Scholar] [CrossRef]
- Wu, X.; Fei, M.; Chen, Y.; Wang, Z.; Chen, Y. Enzymatic synthesis of L-norephedrine by coupling recombinant pyruvate decarboxylase and omega-transaminase. Appl. Microbiol. Biotechnol. 2014, 98, 7399–7408. [Google Scholar] [CrossRef] [PubMed]
- Wuensch, C.; Pavkov-Keller, T.; Steinkellner, G.; Gross, J.; Fuchs, M.; Hromic, A.; Lyskowski, A.; Fauland, K.; Gruber, K.; Glueck, S.M.; et al. Regioselective Enzymatic beta-Carboxylation of para-Hydroxy-styrene Derivatives Catalyzed by Phenolic Acid Decarboxylases. Adv. Synth. Catal. 2015, 357, 1909–1918. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).