Ionic Liquid Catalyzed Per-O-Acetylation and Benzylidene Ring-Opening Reaction
Abstract
1. Introduction
2. Results and Discussion
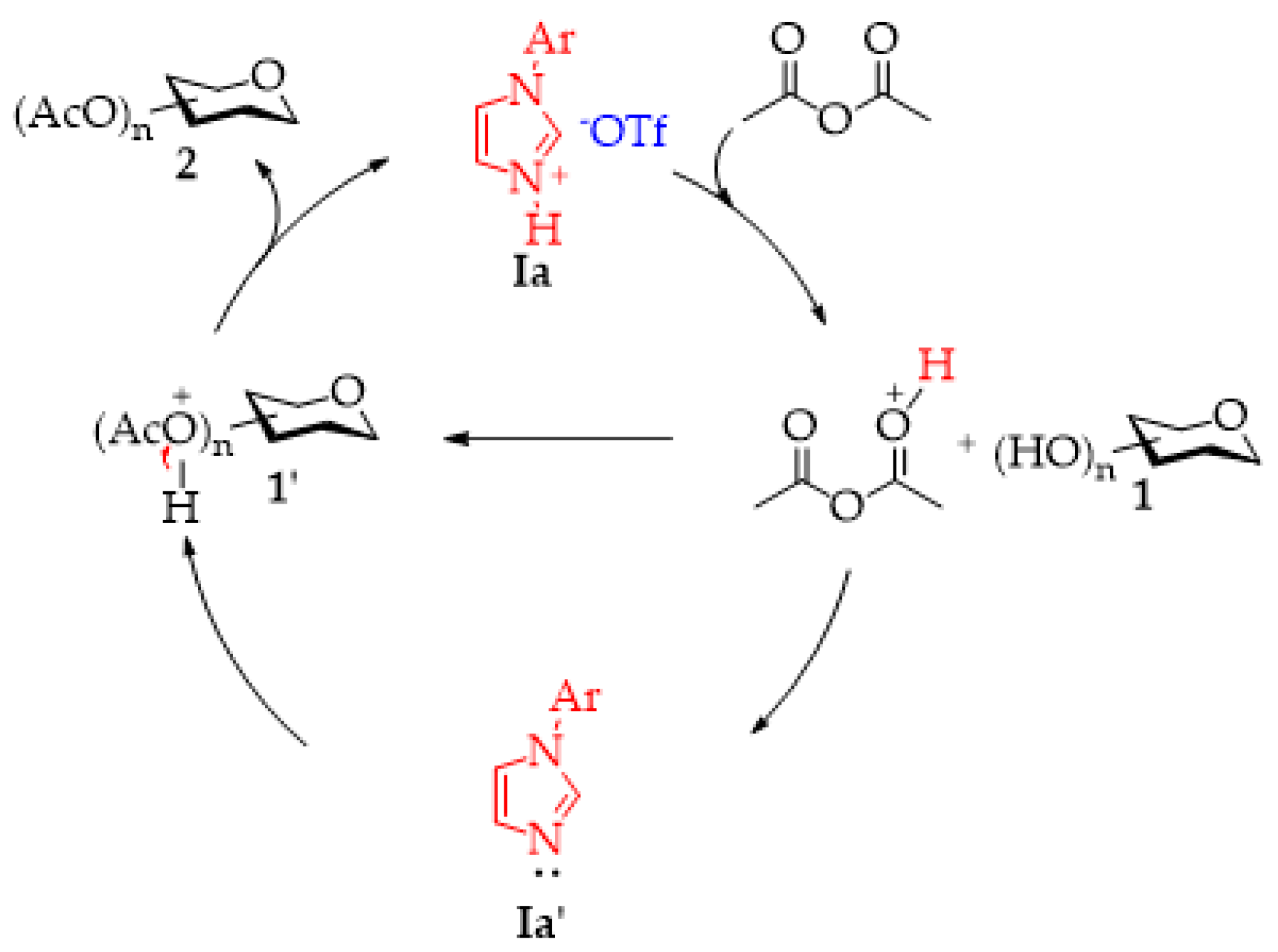
2.1. Per-O-Acetylation
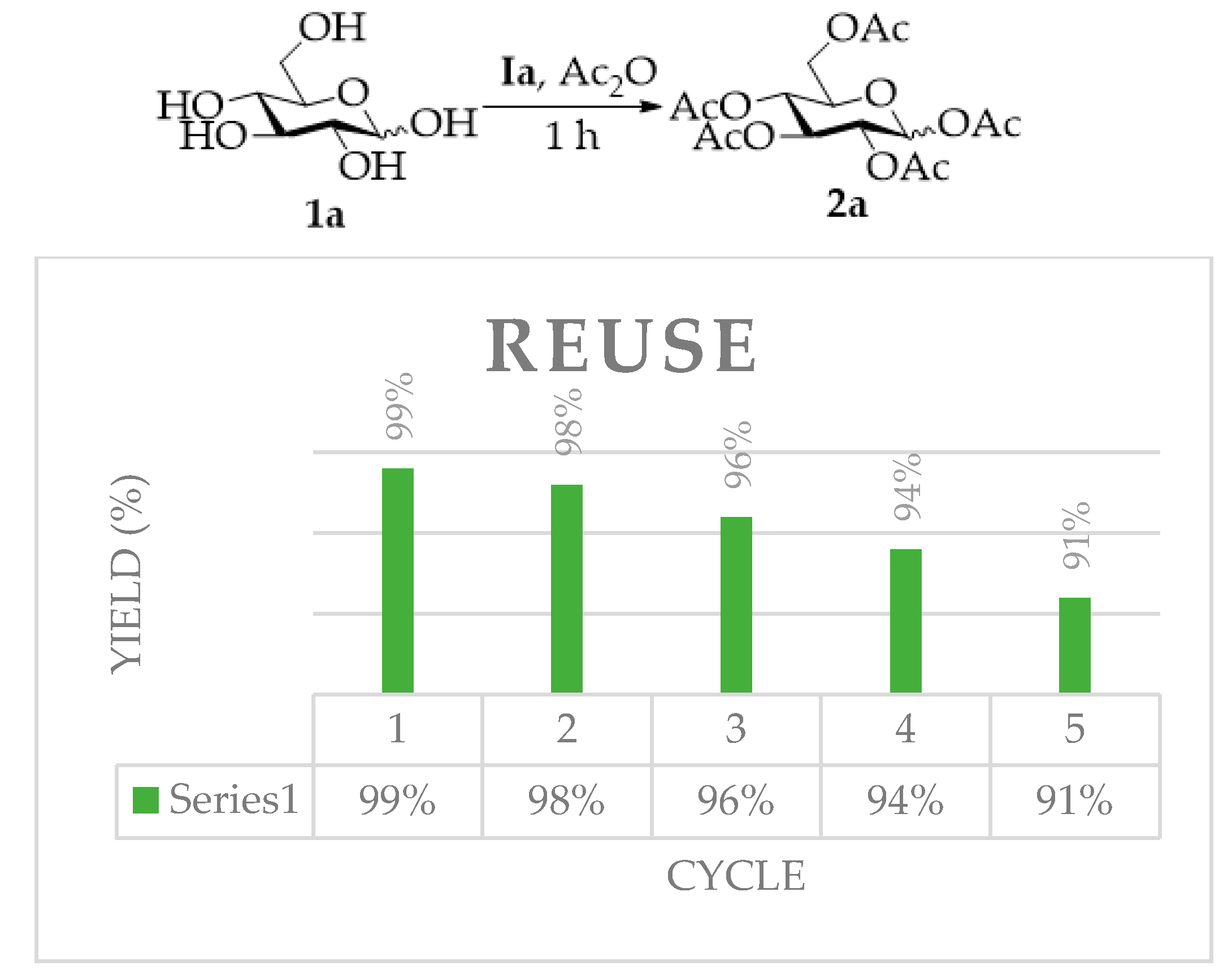
2.2. Reusability of TAIIL for Per-O-Acetylation of d-Glucose
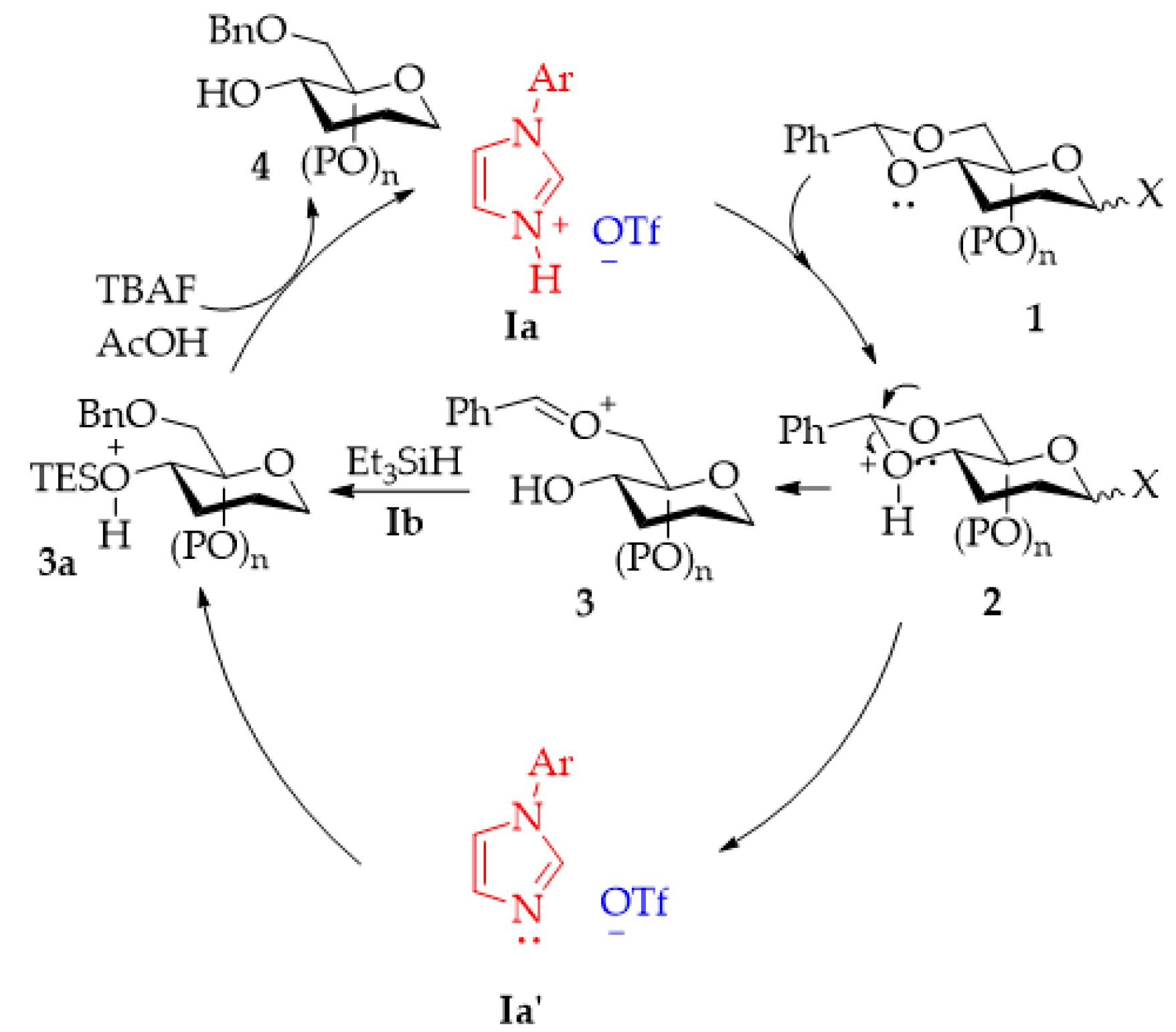
2.3. Reductive Ring Opening of Benzylidene Acetals
3. Materials and Methods
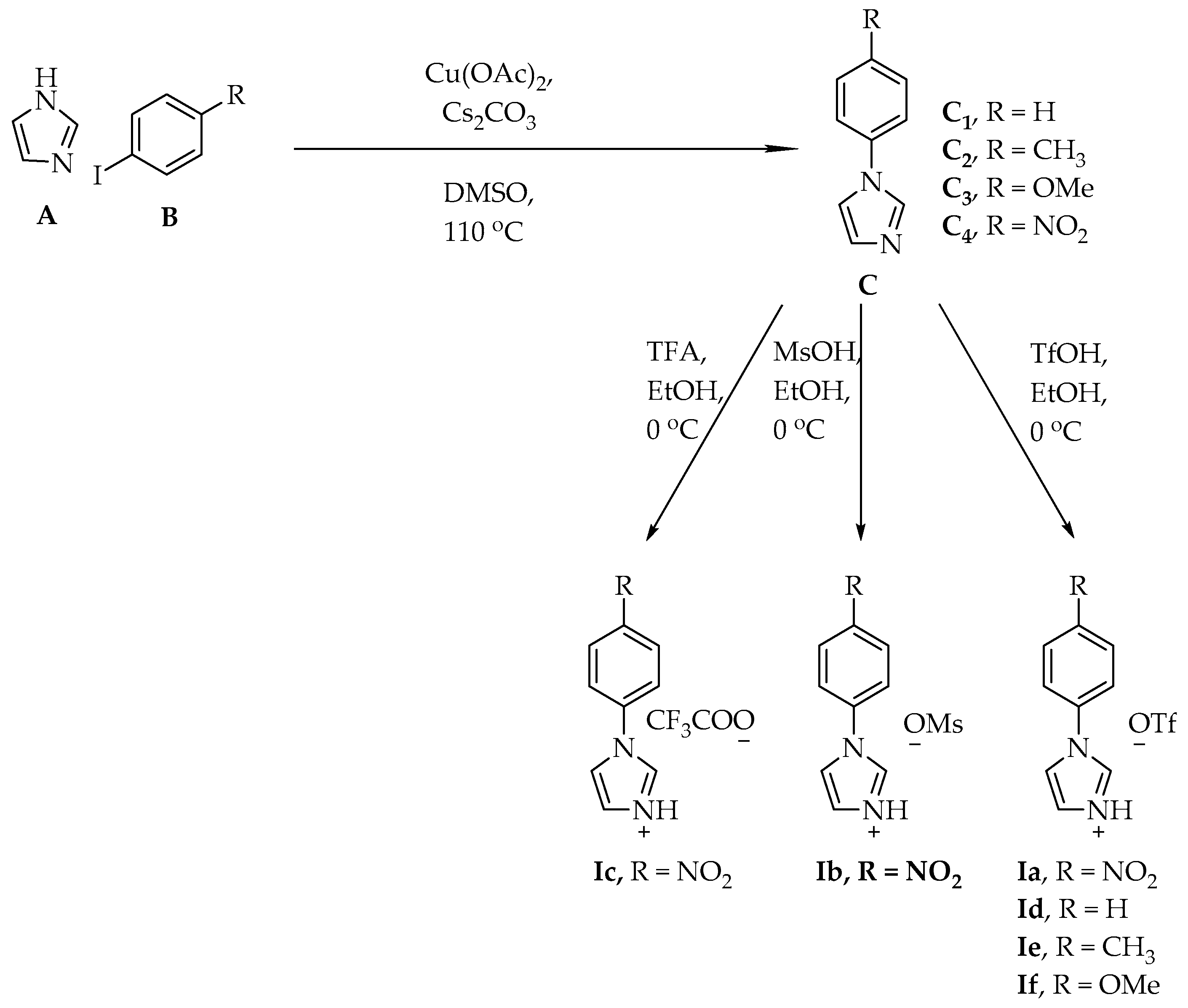
3.1. Synthesis of Ionic Liquid
3.2. General Procedure
3.3. General Information
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Witschi, M.A.; Gervay-Hague, J. Selective acetylation of per-O-TMS-Protected monosaccharides. Org. Lett. 2010, 12, 4312–4315. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.M.; Ferrier, R.J. Monosaccharides, Their Chemistry and Their Roles in Natural Products; John Wiley & Sons: New York, NY, USA, 1995; pp. 360–369. [Google Scholar]
- Jiang, L.; Chan, T.-H. Borane/Bu2BOTf: A mild reagent for the regioselective reductive ring opening of benzylidene acetals in carbohydrates. Tetrahedron Lett. 1998, 37, 355–358. [Google Scholar] [CrossRef]
- Chen, W.; Gu, L.; Zhang, W.; Motari, E.; Cai, L.; Styslinger, T.J.; Wang, P.G. L-rhamnose antigen: A promising alternative to α-Gal for cancer immunotherapies. ACS Chem. Biol. 2011, 6, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Twibanire, J.-A.K.; Omran, R.P.; Grindley, T.B. Facile synthesis of a library of Lyme disease glycolipid antigens. Org. Lett. 2012, 14, 3909–3911. [Google Scholar] [CrossRef] [PubMed]
- Hanessian, S. Total Synthesis of Natural Products: The ‘Chiron’ Approach; Pergamon Press: Oxford, UK, 1983. [Google Scholar]
- Inch, T.D. Formation of convenient chiral intermediates from carbohydrates and their use in synthesis. Tetrahedron 1984, 40, 3161–3213. [Google Scholar] [CrossRef]
- Hernandez-Torres, J.M.; Achkar, J.; Wei, A. Temperature-controlled regioselectivity in the reductive cleavage of p-Methoxybenzylidene acetals. J. Org. Chem. 2004, 69, 7206–7211. [Google Scholar] [CrossRef]
- Wang, C.-C.; Luo, S.-Y.; Shie, C.-R.; Hung, S.-C. Metal trifluoromethanesulfonate-catalyzed regioselective borane-reductive ring opening of benzylidene acetals. Org. Lett. 2002, 4, 847–849. [Google Scholar] [CrossRef] [PubMed]
- Soderquist, J.A.; Kock, I.; Estrella, M.E. Reductive cleavage of acetals and ketals with 9-Borabicyclo [3.3.1] nonane. Org. Process. Res. Dev. 2006, 10, 1076–1079. [Google Scholar] [CrossRef]
- Johnsson, R.; Olsson, D.; Ellervik, U. Reductive openings of acetals: Explanation of regioselectivity in borane reductions by mechanistic studies. J. Org. Chem. 2008, 73, 5226–5232. [Google Scholar] [CrossRef]
- Sakagami, M.; Hamana, H. A selective ring-opening reaction of 4,6-O-benzylidene acetals in carbohydrates using trialkyl silane derivatives. Tetrahedron Lett. 2000, 41, 5547–5551. [Google Scholar] [CrossRef]
- Bhattacharjee, S.S.; Gorin, P.A.J. Hydrogenolysis of carbohydrate acetals, ketals, and cyclic orthoesters with lithium aluminium hydride – aluminium trichloride. Can. J. Chem. 1969, 47, 1195–1206. [Google Scholar] [CrossRef]
- Galas, J. The reactivity of cyclic acetals of aldoses and aldosides. Adv. Carbohydr. Chem. Biochem. 1981, 39, 71–156. [Google Scholar]
- Garreg, P.J. Some aspects of regio-, stereo-, and chemoselective reactions in carbohydrate chemistry. Pure Appl. Chem. 1984, 56, 845–858. [Google Scholar] [CrossRef]
- Johansson, R.; Samuelsson, B. Regioselective reductive ring-opening of 4-methoxybenzylidene acetals of hexopyranosides. Access to a novel protecting-group strategy. Part 1. J. Chem. Soc. Perkin Trans. I 1984, 2371–2374. [Google Scholar] [CrossRef]
- Ek, M.; Garegg, P.J.; Hultberg, H.; Oscarson, S. Reductive ring openings of carbohydrate benzylidene acetals using borane-trimethylamine and aluminium chloride. Regioselectivity and solvent dependence. J. Carbohyd. Chem. 1983, 2, 305–311. [Google Scholar] [CrossRef]
- Das, S.K.; Reddy, K.A.; Krovvidi, V.L.N.R.; Mukkanti, K. InCl3 as a powerful catalyst for the acetylation of carbohydrate alcohols under microwave irradiation. Carbohydr. Res. 2005, 340, 1387–1392. [Google Scholar] [CrossRef] [PubMed]
- Bizier, N.P.; Atkins, S.R.; Helland, L.C.; Colvin, S.F.; Twitchell, J.R.; Clon-inger, M.J. Indium triflate catalyzed peracetylation of carbohydrates. Carbohydr. Res. 2008, 343, 1814. [Google Scholar] [CrossRef]
- Limousin, C.; Cleophax, J.; Petit, A.; Loupy, A.; Lukacs, G. Solvent-free synthesis of decyl d-Glycopyranosides under focused microwave irradiation. J. Carbohydr. Chem. 1997, 16, 327. [Google Scholar] [CrossRef]
- Lee, J.C.; Tai, C.A.; Hung, S.C. Sc(OTf)3-catalyzed acetolysis of 1,6-anhydro-β-hexopyranoses and solvent-free per-acetylation of hexoses. Tetrahedron Lett. 2002, 43, 851–855. [Google Scholar] [CrossRef]
- Procopiou, P.A.; Baugh, S.P.D.; Flack, S.S.; Inglis, G.G.A. An extremely powerful acylation reaction of alcohols with acid anhydrides catalyzed by trimethylsilyl trifluoromethanesulfonate. J. Org. Chem. 1998, 63, 2342–2347. [Google Scholar] [CrossRef]
- Shie, C.-R.; Tzeng, Z.-H.; Kulkarni, S.S.; Uang, B.-J.; Hsu, C.-Y.; Hung, S.-C. Cu(OTf)2 as an efficient and dual-purpose catalyst in the regioselective reductive ring opening of benzylidene acetals. Angew. Chem. 2005, 117, 1693–1696. [Google Scholar] [CrossRef]
- Thota, N.; Björkling, F.; Kankala, S. Triphenylcarbenium tetrafluoroborate as an efficient catalyst in the regioselective reductive ring opening of benzylidene acetals of carbohydrates. Chem. Select 2019, 4, 7976–7980. [Google Scholar] [CrossRef]
- Wu, H.; Shen, Y.; Fan, L.Y.; Wan, Y.; Shi, D.Q. Solid silica sulfuric acid (SSA) as a novel and efficient catalyst for acetylation of aldehydes and sugars. Tetrahedron 2006, 62, 7995. [Google Scholar] [CrossRef]
- Mukhopadhyay, B.; Russell, D.A.; Field, R.A. One-pot acetalation–acetylation of sugar derivatives employing perchloric acid immobilized on silica. Carbohydr. Res. 2005, 340, 1075–1080. [Google Scholar] [CrossRef] [PubMed]
- Misra, A.K.; Tiwari, P.; Madhusudan, S.K. HClO4–SiO2 catalyzed per-O-acetylation of carbohydrates. Carbohydr. Res. 2005, 340, 325–329. [Google Scholar] [CrossRef]
- Wu, L.Q.; Yin, Z.K. Sulfonic acid-functionalized nano γ-Al2O3 catalyzed per-O-acetylated of carbohydrates. Carbohydr. Res. 2013, 365, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.B.; Zhang, B.; Zhou, J.F.; Li, J.; Shi, C.J.; Huang, T.; Wang, Z.F.; Tang, J. H2SO4-SiO2: The highly efficient and reusable catalyst for per-O-Acetylation of carbohydrates under solvent-free conditions. J. Carbohydr. Chem. 2011, 30, 165–177. [Google Scholar] [CrossRef]
- Wasserscheid, P.; Welton, T. Ionic Liquids in Synthesis; Wiley-VCH Verlag: Weinheim, Germany, 2003. [Google Scholar]
- Welton, T. Ionic liquids in catalysis. Coord. Chem. Rev. 2004, 248, 2459–2477. [Google Scholar] [CrossRef]
- Welton, T. Room-temperature ionic liquids. Solvents for synthesis and catalysis. Chem. Rev. 1999, 99, 2071–2083. [Google Scholar] [CrossRef]
- Wasserscheid, P.; Keim, W. Ionic liquids—New “solutions” for transition metal catalysis. Angew. Chem. Int. Ed. 2000, 39, 3772–3789. [Google Scholar] [CrossRef]
- Forsyth, S.A.; MacFarlane, D.R.; Thomson, R.J.; Itzstein, M.V. Rapid, clean, and mild O-acetylation of alcohols and carbohydrates in an ionic liquid. Chem. Commun. 2002, 714–715. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A.P.; Bell, T.J.; Handa, S.; Stoddar, B. O-Acetylation of cellulose and monosaccharides using a zinc-based ionic liquid. Green Chem. 2005, 7, 705–707. [Google Scholar] [CrossRef]
- Murugesan, S.; Karst, N.; Islam, T.; Wiencek, J.M.; Linhardt, R.J. Dialkyl imidazolium benzoates-room temperature ionic liquids useful in the peracetylation and perbenzoylation of simple and sulfated saccharides. Synlett 2003, 9, 1283–1286. [Google Scholar]
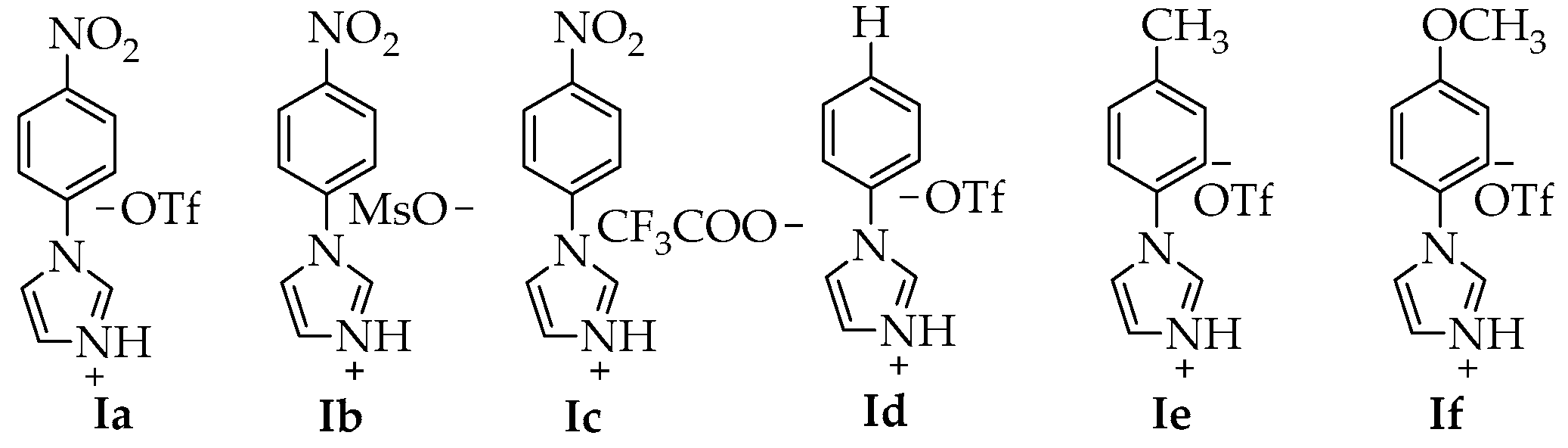
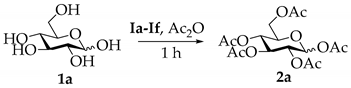
| Entry | IL | T (°C) | t (h) | Ac2O (eq) | P (Yield) a |
|---|---|---|---|---|---|
| 1 | Ia | 25 | 1 h | 10 | 2a (99%) |
| 2 | Ib | 25 | 1 h | 10 | 2a (98%) |
| 3 | Ic | 25 | 1 h | 10 | 2a (29%) |
| 4 | Id | 25 | 1 h | 10 | 2a (70%) |
| 5 | Ie | 25 | 1 h | 10 | 2a (96%) |
| 6 | If | 25 | 1 h | 10 | 2a (86%) |
| 7 | Ia | 25 | 1 h | 7.5 | 2a (99%) |
| 8 | Ia | 25 | 1 h | 6.0 | 2a (99%) |
| 9 | Ia | 25 | 1 h | 5.25 | 2a (95%) |
| 10 | Ia | 25 | 24 h | 6.0 | 2a (94%) |
| 11 | Ia | 80 | 1 h | 6.0 | 2a (92%) |

| Entry | SM | t (h) | Ac2O (eq) | P (Yield) |
|---|---|---|---|---|
| 1 | 1b | 1 h | 6 | 2b (98%) |
| 2 | 1c | 3 h | 6 | 2c (93%) |
| 3 | 1d | 1 h | 4.8 | 2d (99%) |
| 4 | 1e | 1 h | 4.8 | 2e (97%) |
| 5 | 1f | 1 h | 9.6 | 2f (96%) |

| Entry | Solvent | IL (eq) | t (h) | P (Yield) a |
|---|---|---|---|---|
| 1 | DCM | Ia (1.0) | 6 h | 4a (78%) |
| 2 | DCM | Ib (1.0) | 6 h | 4a (4%) |
| 3 | DCM | Ic (1.0) | 6 h | 4a (6%) |
| 4 | DCM | Id (1.0) | 6 h | 4a (8%) |
| 5 | DCM | Ie (1.0) | 6 h | 4a (58%) |
| 6 | DCM | If (1.0) | 6 h | 4a (4%) |
| 7 | ACN | Ia (1.0) | 1 h | 4a (88%) |
| 8 | ACN | Ia (0.5) | 1 h | 4a (90%) |
| 9 | ACN | Ia (0.25) | 2 h | 4a (77%) |
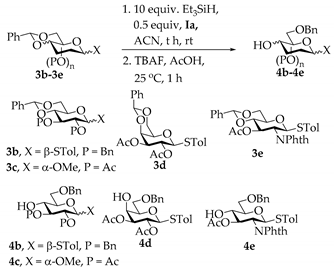
| Entry | SM | t (h) | P (Yield) |
|---|---|---|---|
| 1 | 3b | 24 h | 4b (85%) |
| 2 | 3c | 1 h | 4c (88%) |
| 3 | 3d | 8 h | 4d (72%) |
| 4 | 3e | 1 h | 4e (82%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thul, M.; Wu, Y.-P.; Lin, Y.-J.; Du, S.-L.; Wu, H.-R.; Ho, W.-Y.; Luo, S.-Y. Ionic Liquid Catalyzed Per-O-Acetylation and Benzylidene Ring-Opening Reaction. Catalysts 2020, 10, 642. https://doi.org/10.3390/catal10060642
Thul M, Wu Y-P, Lin Y-J, Du S-L, Wu H-R, Ho W-Y, Luo S-Y. Ionic Liquid Catalyzed Per-O-Acetylation and Benzylidene Ring-Opening Reaction. Catalysts. 2020; 10(6):642. https://doi.org/10.3390/catal10060642
Chicago/Turabian StyleThul, Mayur, Yao-Peng Wu, Yi-Jyun Lin, Shu-Lin Du, Hsin-Ru Wu, Wen-Yueh Ho, and Shun-Yuan Luo. 2020. "Ionic Liquid Catalyzed Per-O-Acetylation and Benzylidene Ring-Opening Reaction" Catalysts 10, no. 6: 642. https://doi.org/10.3390/catal10060642
APA StyleThul, M., Wu, Y.-P., Lin, Y.-J., Du, S.-L., Wu, H.-R., Ho, W.-Y., & Luo, S.-Y. (2020). Ionic Liquid Catalyzed Per-O-Acetylation and Benzylidene Ring-Opening Reaction. Catalysts, 10(6), 642. https://doi.org/10.3390/catal10060642






