Microwave-Assisted Degradation of Biomass with the Use of Acid Catalysis
Abstract
:1. Introduction
2. Results and Discussion
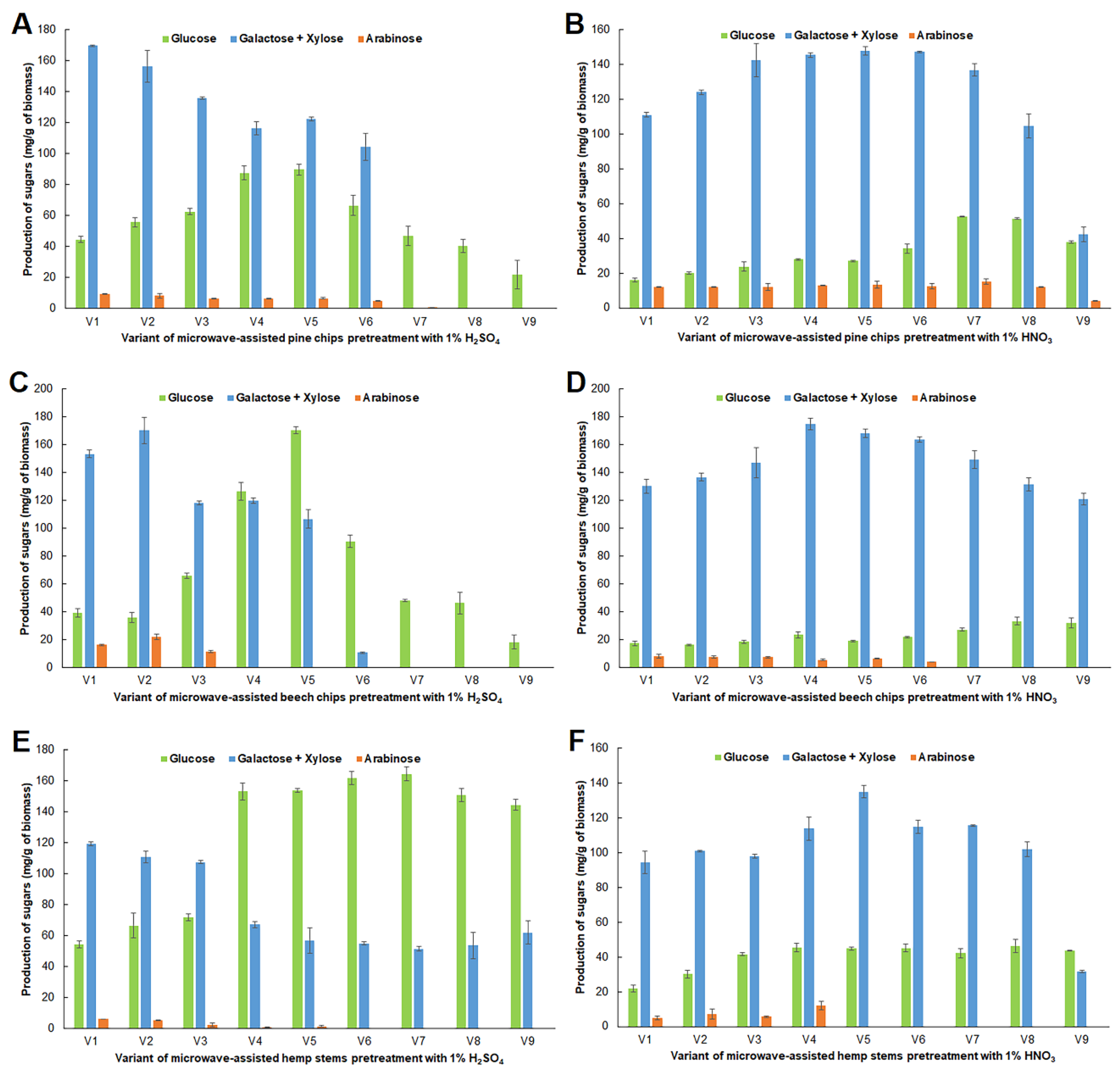
2.1. Impact of Microwave Pretreatment Conditions on the Amount of Carbohydrates Obtained from Analyzed Raw Materials
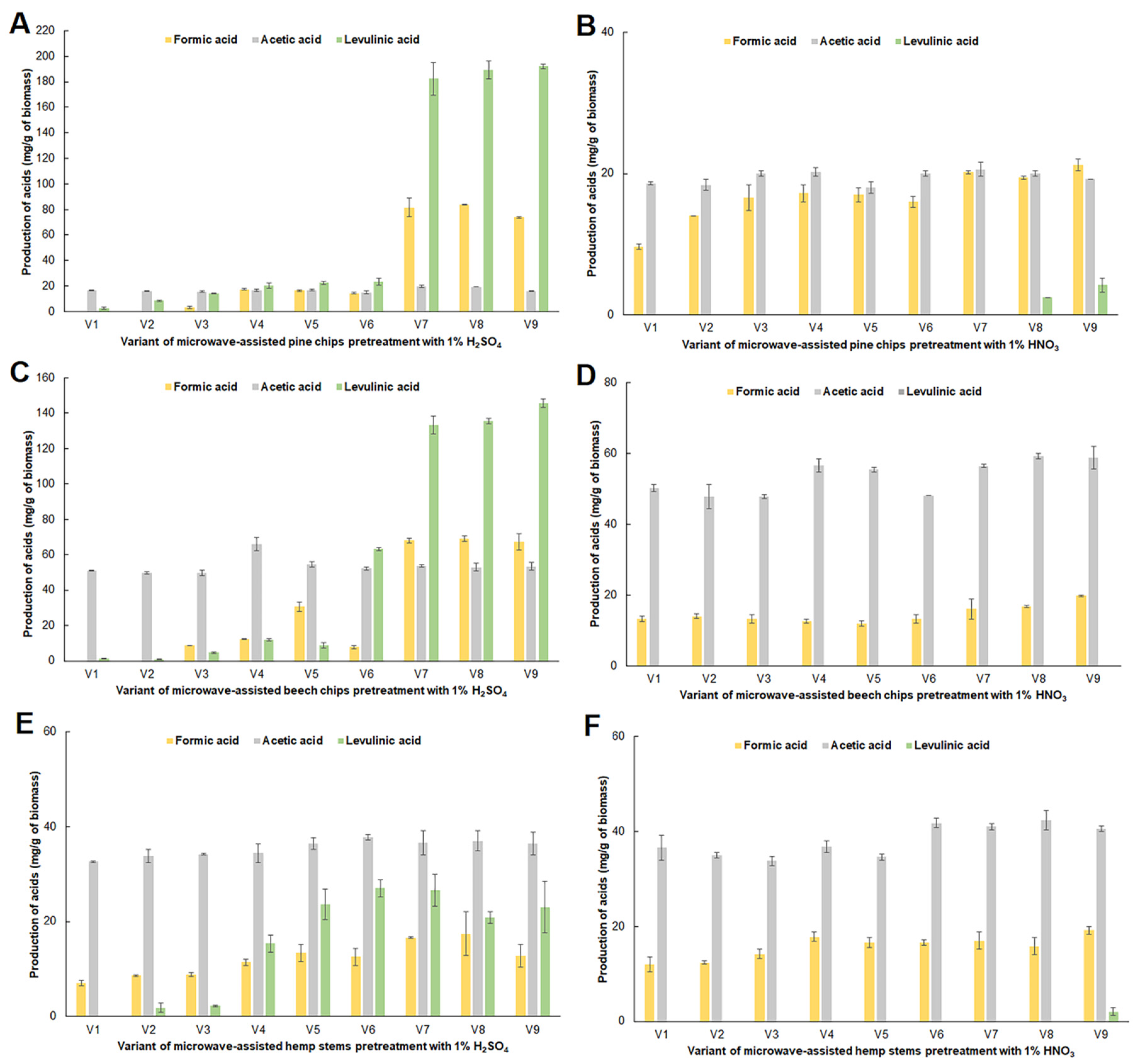
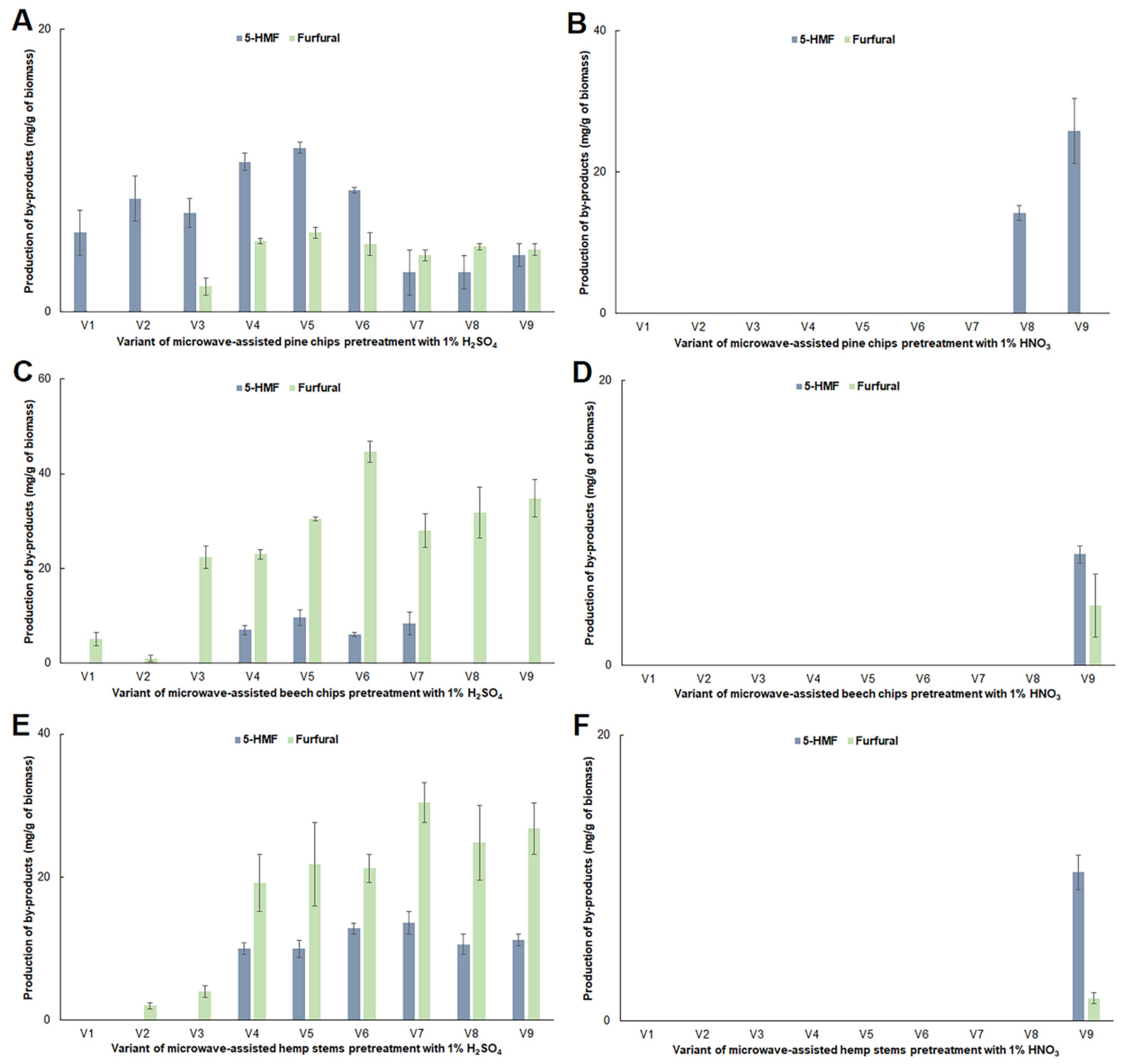
2.2. Impact of Microwave Pretreatment Conditions on the Amount of Organic Acids, 5-HMF and Furfural Obtained from the Tested Plant Biomass
3. Materials and Methods
3.1. Characteristics of Raw Materials
3.2. Chemical Reagents
3.3. Selection of Conditions for Microwave Degradation of Plant Biomass
3.4. Analytical Methods
3.4.1. Biomass Characterization
3.4.2. Determination of Carbohydrates and Organic Acid after Microwave-Assisted Pretreatment
3.4.3. Determination of 5-HMF and Furfural
3.5. Statistics
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sarkar, N.; Ghosh, S.K.; Bannerjee, S.; Aikat, K. Bioethanol production from agricultural wastes: An overview. Renew. Energ. 2012, 37, 19–27. [Google Scholar] [CrossRef]
- Balat, M. Production of bioethanol from lignocellulosic materials via the biochemical pathway: A review. Energ. Convers. Manag. 2011, 52, 858–875. [Google Scholar] [CrossRef]
- Wilkie, A.C.; Riedesel, K.J.; Owens, J.M. Stillage characterization and anaerobic treatment of ethanol stillage from conventional and cellulosic feedstocks. Biomass Bioenerg. 2000, 19, 63–102. [Google Scholar] [CrossRef]
- Kan, X.; Zhang, J.; Tong, Y.W.; Wang, C.-H. Overall evaluation of microwave-assisted alkali pretreatment for enhancement of biomethane production from brewers’ spent grain. Energ. Convers. Manag. 2018, 158, 315–326. [Google Scholar] [CrossRef]
- Jørgensen, H.; Kristensen, J.B.; Felby, C. Enzymatic conversion of lignocellulose into fermentable sugars: Challenges and opportunities. Biofuels Bioprod. Bioref. 2007, 1, 119–134. [Google Scholar] [CrossRef]
- Ye, S.; Cheng, J. Hydrolysis of lignocellulosic materials for ethanol production: A review. Bioresource Technol. 2002, 83, 1–11. [Google Scholar]
- Mood, S.H.; Golfeshan, A.H.; Tabatabaei, M.; Jouzani, G.S.; Najafi, G.H.; Gholami, M.; Ardjmand, M. Lignocellulosic biomass to bioethanol, a comprehensive review with a focus on pretreatment. Renew. Sust. Energ. Rev. 2013, 27, 77–93. [Google Scholar] [CrossRef]
- Bundhoo, Z.M.A. Microwave-assisted conversion of biomass and waste materials to biofuels. Renew. Sust. Energ. Rev. 2018, 82, 1149–1177. [Google Scholar] [CrossRef]
- Budarin, V.L.; Clark, J.H.; Lanigan, B.A.; Shuttleworth, P.; Macquarrie, D.J. Microwave assisted decomposition of cellulose: A new thermochemical route. Bioresource Technol. 2010, 101, 3776–3779. [Google Scholar] [CrossRef] [PubMed]
- Kłosowski, G.; Mikulski, D.; Menka, A. Microwave-assisted one-step conversion of wood wastes into levulinic acid. Catalysts 2019, 9, 753. [Google Scholar] [CrossRef] [Green Version]
- Monteil-Rivera, F.; Huang, G.H.; Paquet, L.; Deschamps, S.; Beaulieu, C.; Hawari, J. Microwave-assisted extraction of lignin from triticale straw: Optimization and microwave effects. Bioresource Technol. 2012, 104, 775–782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikulski, D.; Kłosowski, G.; Menka, A.; Koim-Puchowska, B. Microwave-assisted pretreatment of maize distillery stillage with the use of dilute sulfuric acid in the production of cellulosic ethanol. Bioresource Technol. 2019, 278, 318–328. [Google Scholar] [CrossRef]
- Yang, M.; Kuittinen, S.; Zhang, J.; Keinänen, M.; Pappinen, A. Effect of dilute acid pretreatment on the conversion of barley straw with grains to fermentable sugars. Bioresource Technol. 2013, 146, 44–450. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Lee, C.; Yu, C.; Cheng, Y.-S.; Zhang, R.; Jenkins, B.M.; VanderGheynst, J.S. Dilute acid pretreatment and fermentation of sugar beet pulp to ethanol. Appl. Energ. 2013, 105, 1–7. [Google Scholar] [CrossRef]
- Hsu, T.-C.; Guo, G.-L.; Chen, W.-H.; Hwang, W.-S. Effect of dilute acid pretreatment of rice straw on structural properties and enzymatic hydrolysis. Bioresource Technol. 2010, 101, 4907–4913. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shao, S.; Bao, J. Long term storage of dilute acid pretreated corn stover feedstock and ethanol fermentability evaluation. Bioresource Technol. 2016, 201, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Gong, G.; Liu, D.; Huang, Y. Microwave-assisted organic acid pretreatment for enzymatic hydrolysis of rice straw. Biosyst. Eng. 2010, 107, 67–73. [Google Scholar] [CrossRef]
- Ma, H.; Liu, W.-W.; Chen, X.; Wu, Y.-J.; Yu, Z.-L. Enhanced enzymatic saccharification of rice straw by microwave pretreatment. Bioresource Technol. 2009, 100, 1279–1284. [Google Scholar] [CrossRef]
- Zhu, S.; Wu, Y.; Yu, Z.; Zhang, X.; Wang, C.; Yu, F.; Jin, S. Production of ethanol from microwave-assisted alkali pretreated wheat straw. Process Biochem. 2006, 41, 869–873. [Google Scholar] [CrossRef]
- Xu, J.; Chen, H.; Kádár, Z.; Thomsen, A.B.; Schmidt, J.E.; Peng, H. Optimization of microwave pretreatment on wheat straw for ethanol production. Biomass Bioenerg. 2011, 35, 3859–3864. [Google Scholar] [CrossRef]
- Orozco, A.; Ahmad, M.; Rooney, D.; Walker, G. Dilute acid hydrolysis of cellulose and cellulosic bio-waste using a microwave reactor system. Process Saf. Environ. 2007, 85, 446–449. [Google Scholar] [CrossRef]
- Teh, Y.Y.; Lee, K.T.; Chen, W.-H.; Lin, S.-C.; Sheen, H.-K. Dilute sulfuric acid hydrolysis of red macroalgae Eucheuma denticulatum with microwave-assisted heating for biochar production and sugar recovery. Bioresource Technol. 2017, 246, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Gandolfi, S.; Ottolina, G.; Consonni, R.; Riva, S.; Patel, I. Fractionation of hemp hurds by organosolv pretreatment and its effect on production of lignin and sugars. ChemSusChem 2014, 7, 1991–1999. [Google Scholar] [CrossRef] [PubMed]
- Bak, J.S.; Ko, J.K.; Han, Y.H.; Lee, B.C.; Choi, I.G.; Kim, K.H. Improved enzymatic hydrolysis yield of rice straw using electron beam irradiation pretreatment. Bioresource Technol. 2009, 100, 1285–1290. [Google Scholar] [CrossRef] [PubMed]
- Duarte, C.L.; Ribeiro, M.A.; Oikawa, H.; Mori, M.N.; Napolitano, C.M.; Galvăo, C.A. Electron beam combined with hydrothermal treatment for enhancing the enzymatic convertibility of sugarcane bagasse. Radiat. Phys. Chem. 2012, 81, 1008–1011. [Google Scholar] [CrossRef]
- Dziekońska-Kubczak, U.; Berłowska, J.; Dziugan, P.; Patelski, P.; Pielech-Przybylska, K.; Balcerek, M. Nitric acid pretreatment of Jerusalem artichoke stalks for enzymatic saccharification and bioethanol production. Energies 2018, 11, 2153. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.; Afzal, W.; Cheng, K.; Liu, N.; Pauly, M.; Bell, A.T.; Liu, Z.; Prausnitz, J.M. Nitric-acid hydrolysis of Miscanthus giganteus to sugars fermented to bioethanol. Biotechnol. Bioproc. Eng. 2015, 20, 304–314. [Google Scholar] [CrossRef] [Green Version]
- Zhang, N.; Li, S.; Xiong, L.; Hong, Y.; Chen, Y. Cellulose-hemicellulose interaction in wood secondary cell wall. Model. Simulat. Mater. Sci. Eng. 2015, 23, 085010. [Google Scholar] [CrossRef]
- Amini, N.; Haritos, V.S.; Tanksale, A. Microwave assisted pretreatment of eucalyptus sawdust enhances enzymatic saccharification and maximizes fermentable sugar yield. Renew. Energ. 2018, 127, 653–660. [Google Scholar] [CrossRef]
- Mikulski, D.; Kłosowski, G. Microwave-assisted dilute acid pretreatment in bioethanol production from wheat and rye stillages. Biomass Bioenerg. 2020, 136, 105528. [Google Scholar] [CrossRef]
- Zhu, Z.; Liu, Y.; Yang, X.; McQueen-Mason, S.J.; Gomez, L.D.; Macquarrie, D.J. Comparative evaluation of microwave-assisted acid, alkaline, and inorganic salt pretreatments of sugarcane bagasse for sugar recovery. Biomass Convers. Biorefinery 2020. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Alriksson, B.; Nilvebrant, N.O. Bioconversion of lignocellulose: Inhibitors and detoxification. Biotechnol. Biofuels 2013, 6, 1–10. [Google Scholar]
- Lacerda, V.S.; López-Sotelo, J.B.; Correa-Guimarães, A.; Hernández-Navarro, S.; Sánchez-Bascones, M.; Navas-Gracia, L.M.; Martín-Ramos, P.; Pérez-Lebeña, E.; Martín-Gil, J. A kinetic study on microwave-assisted conversion of cellulose and lignocellulosic waste into hydroxymethylfurfural/furfural. Bioresource Technol. 2015, 180, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Tsang, D.C.W.; Shen, D.; Zhou, Y.; Jin, Z.; Zhou, D.; Lu, W.; Long, Y. Acidic seawater improved 5-hydroxymethylfurfural yield from sugarcane bagasse under microwave hydrothermal liquefaction. Environ. Res. 2020, 184, 109340. [Google Scholar] [CrossRef]
- Ruiz, R.; Date, T.E. Determination of carbohydrates in biomass by high performance liquid chromatography. In Laboratory Analytical Procedure No. 002; Citeseer; National Renewable Research Laboratory: Golden, CO, USA, 1996. [Google Scholar]
- Mikulski, D.; Kłosowski, G. Efficiency of dilute sulfuric acid pretreatment of distillery stillage in the production of cellulosic ethanol. Bioresource Technol. 2018, 268, 424–433. [Google Scholar] [CrossRef]
- Cho, D.H.; Lee, Y.J.; Um, Y.; Sang, B.-I.; Kim, Y.H. Detoxification of model phenolic compounds in lignocellulosic hydrolysates with peroxidase for butanol production from Clostridium beijerinckii. Appl. Microbiol. Biotechnol. 2009, 83, 1035–1043. [Google Scholar] [CrossRef]
| Components | Pine Chips | Beech Chips | Hemp Stems |
|---|---|---|---|
| Dry weight [%] | 93.07a ± 0.12 | 93.63a ± 0.13 | 91.19b ± 0.07 |
| Content of cellulose [% dry weight] | 49.93a ± 0.87 | 56.24b ± 1.14 | 67.16c ± 1.91 |
| Content of hemicelluloses [% dry weight] | 14.33a ± 2.49 | 22.67b ± 1.45 | 11.83a ± 1.93 |
| Content of lignin [% dry weight] | 26.68a ± 0.27 | 14.67b ± 0.65 | 11.33c ± 0.29 |
| Biomass Pretreatment Variant | Power of the Microwave Generator [W] | Pressure [PSI] | Pretreatment Time [min] |
|---|---|---|---|
| V1 | 300 | 54 | 10 |
| V2 | 300 | 54 | 15 |
| V3 | 300 | 54 | 20 |
| V4 | 300 | 93 | 10 |
| V5 | 300 | 93 | 15 |
| V6 | 300 | 93 | 20 |
| V7 | 300 | 152 | 10 |
| V8 | 300 | 152 | 15 |
| V9 | 300 | 152 | 20 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kłosowski, G.; Mikulski, D.; Lewandowska, N. Microwave-Assisted Degradation of Biomass with the Use of Acid Catalysis. Catalysts 2020, 10, 641. https://doi.org/10.3390/catal10060641
Kłosowski G, Mikulski D, Lewandowska N. Microwave-Assisted Degradation of Biomass with the Use of Acid Catalysis. Catalysts. 2020; 10(6):641. https://doi.org/10.3390/catal10060641
Chicago/Turabian StyleKłosowski, Grzegorz, Dawid Mikulski, and Natalia Lewandowska. 2020. "Microwave-Assisted Degradation of Biomass with the Use of Acid Catalysis" Catalysts 10, no. 6: 641. https://doi.org/10.3390/catal10060641
APA StyleKłosowski, G., Mikulski, D., & Lewandowska, N. (2020). Microwave-Assisted Degradation of Biomass with the Use of Acid Catalysis. Catalysts, 10(6), 641. https://doi.org/10.3390/catal10060641







