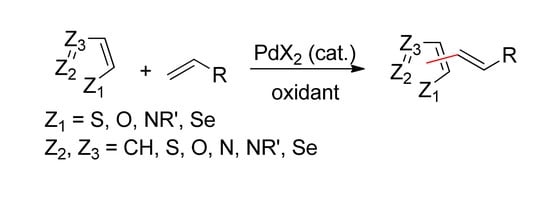
Pd-Catalyzed Intermolecular Dehydrogenative Heck Reactions of Five-Membered Heteroarenes
Abstract
1. Introduction
2. S-Arenes
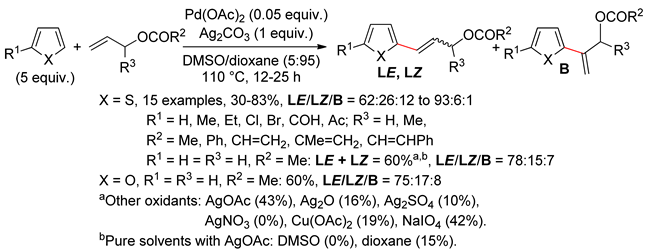
2.1. Thiophenes
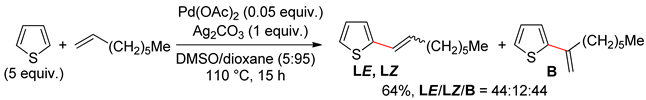
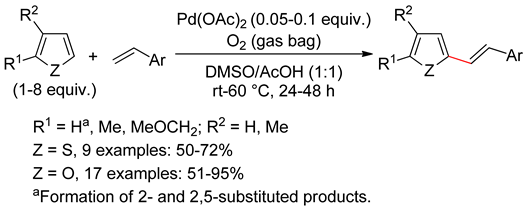
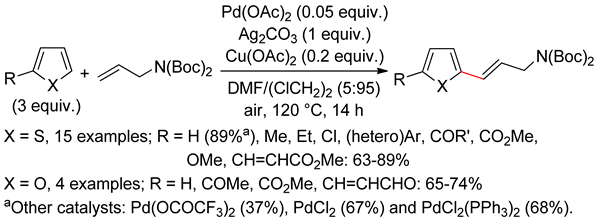
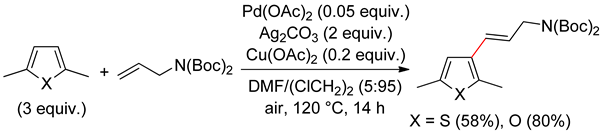
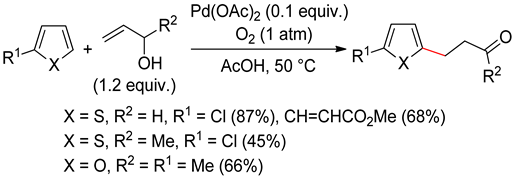
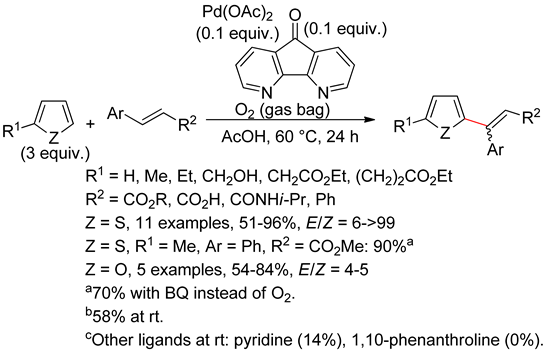
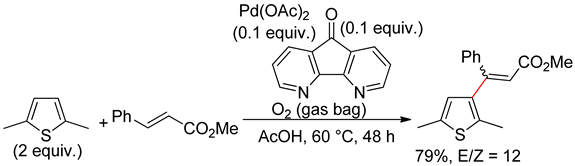
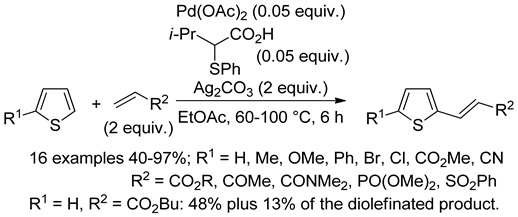
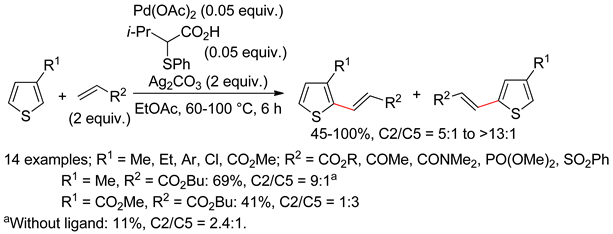
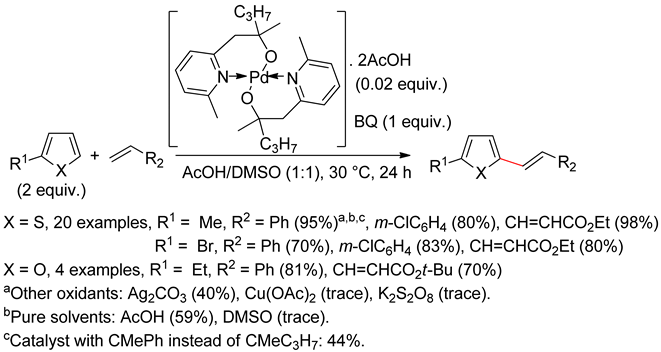
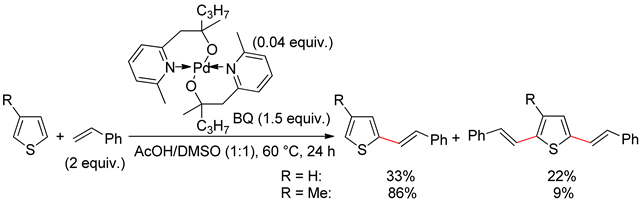
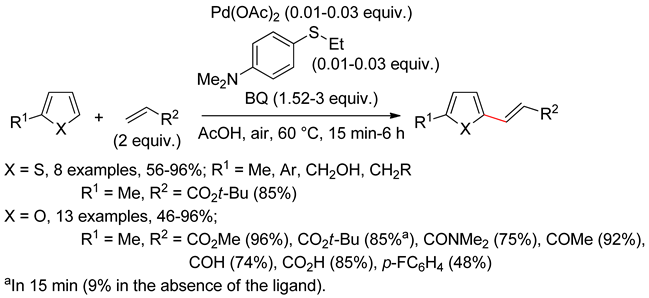
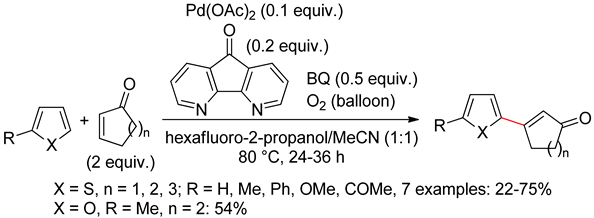
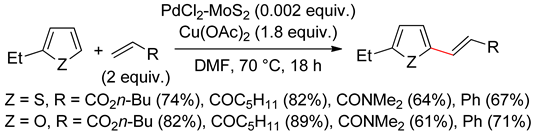

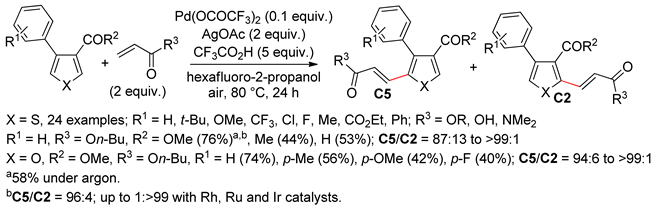
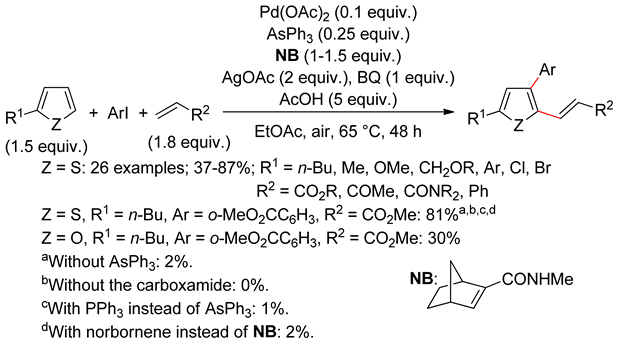
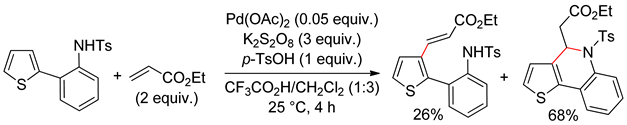
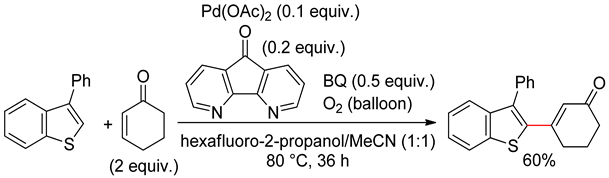
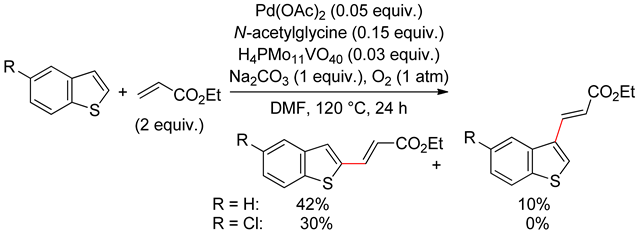
2.2. Benzothiophenes
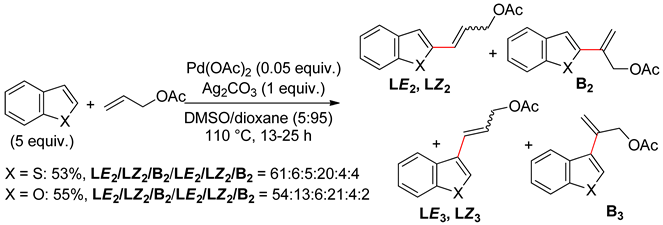
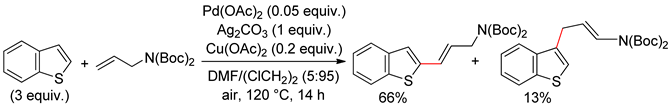
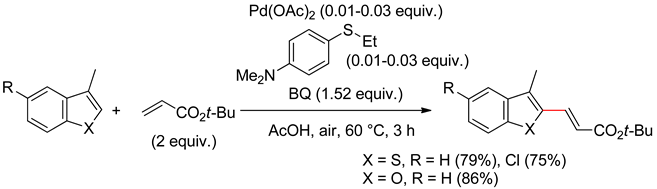
2.3. Thieno[3,2-b]thiophene
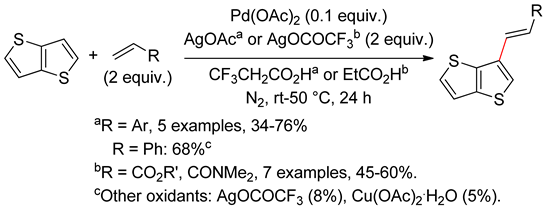
2.4. Thieno[3,2-b]furan
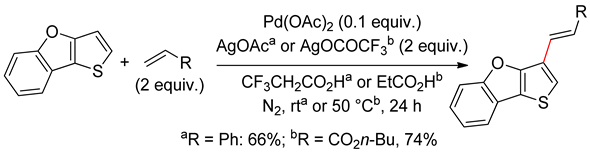
2.5. Sub-Conclusion
3. O-Arenes
3.1. Furans
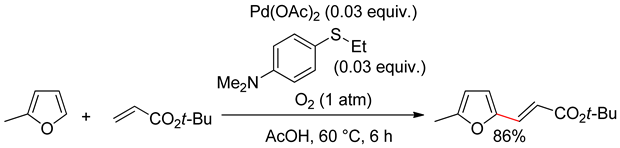

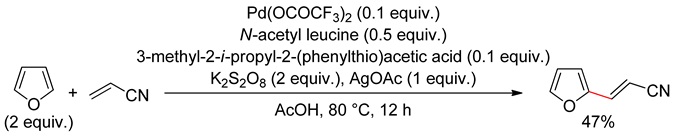
3.2. Benzofurans
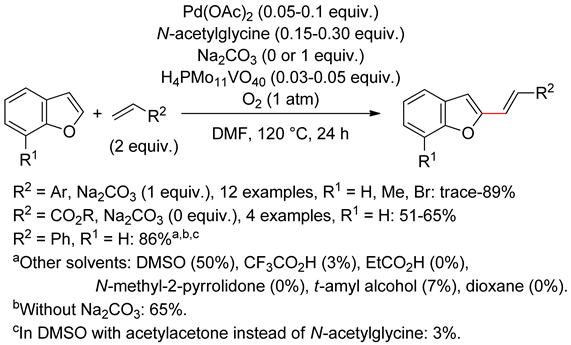
3.3. Sub-Conclusion
4. N-Arenes
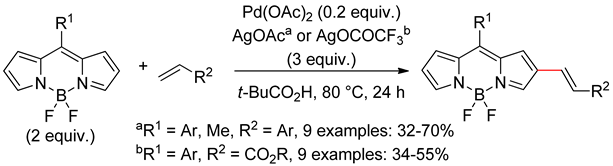
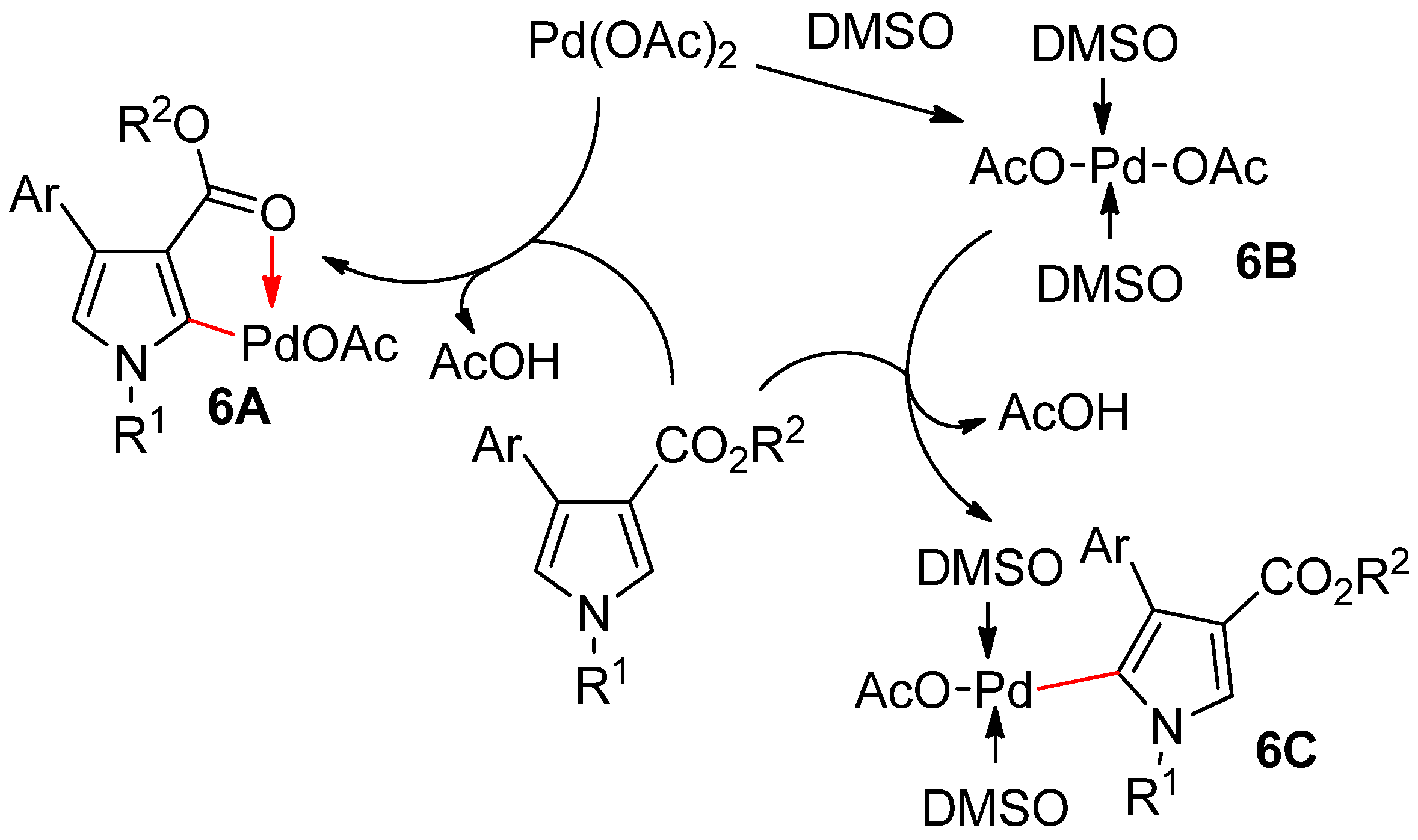
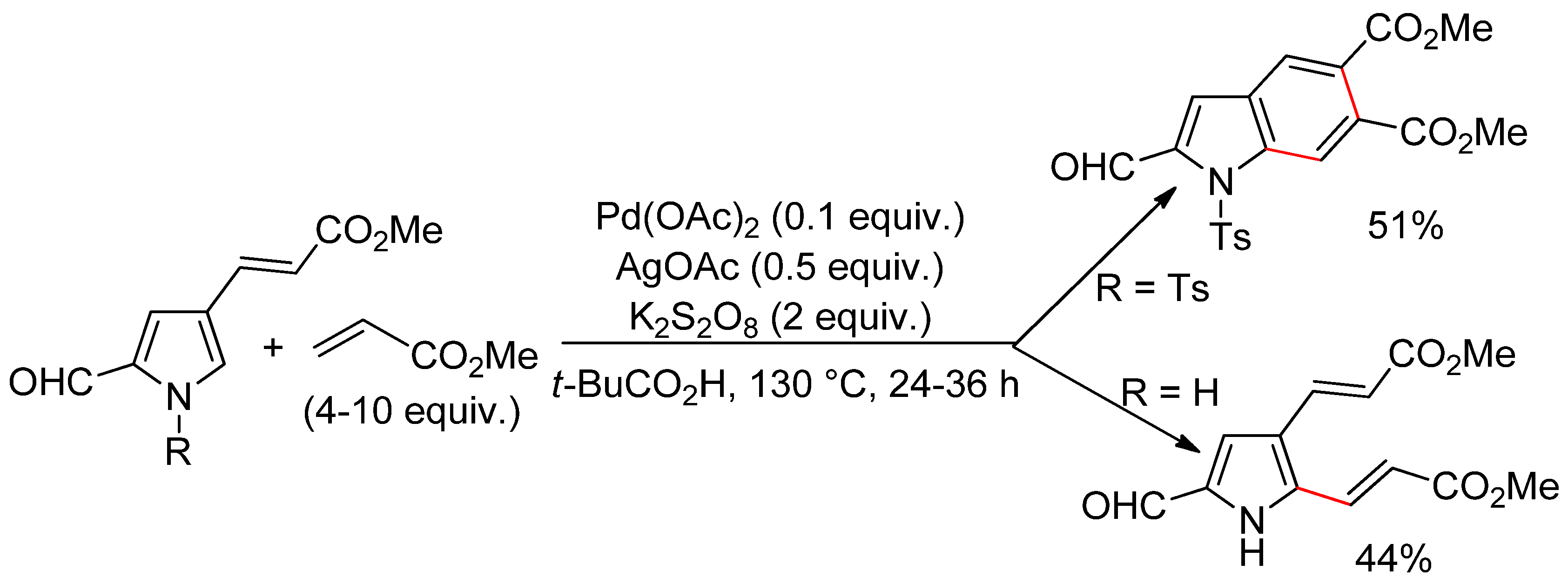
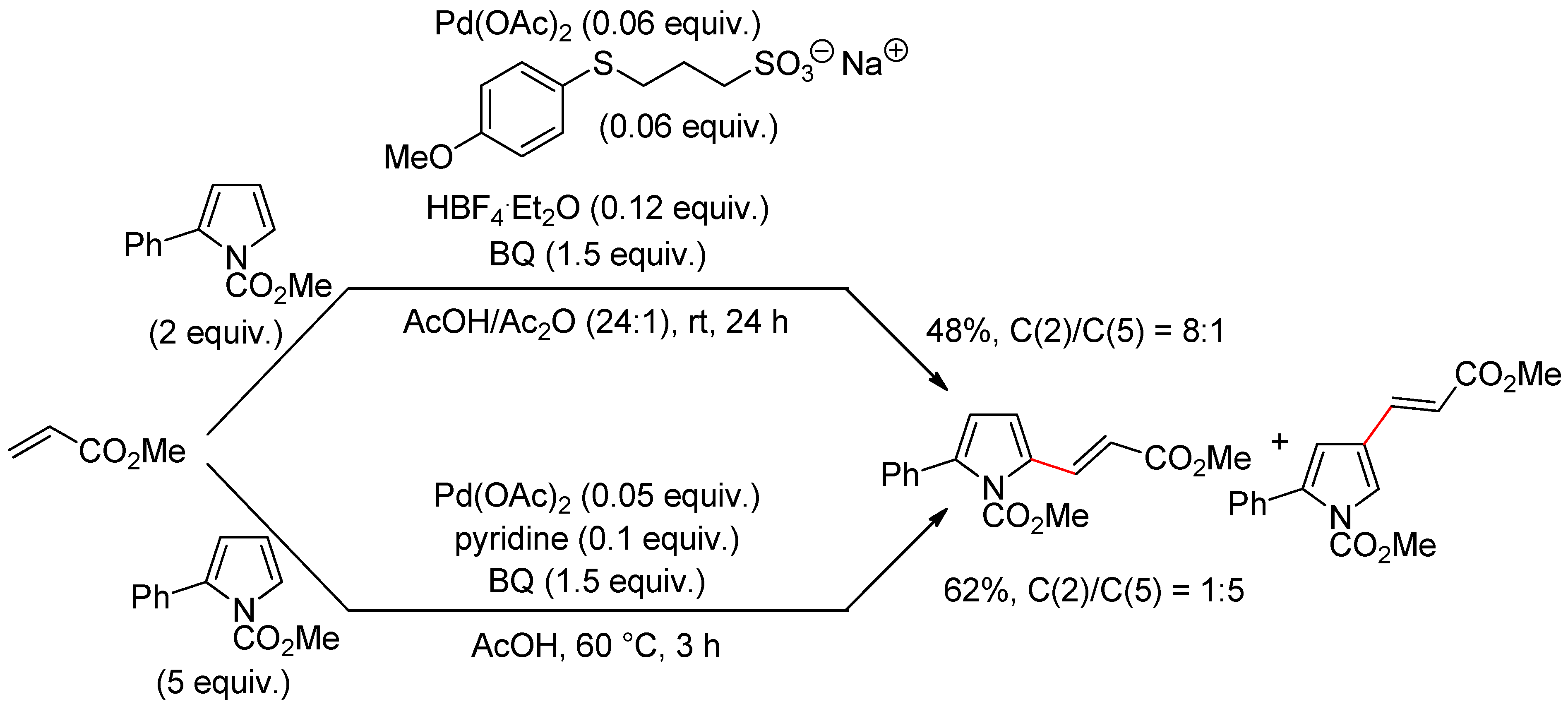
4.1. Pyrroles
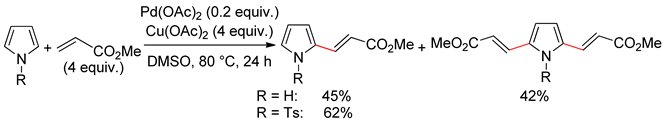
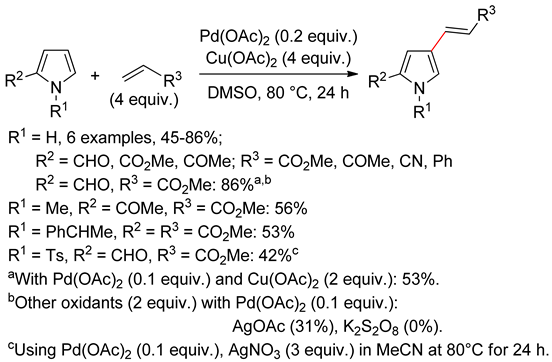
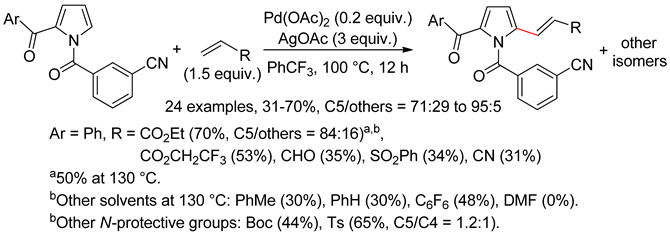
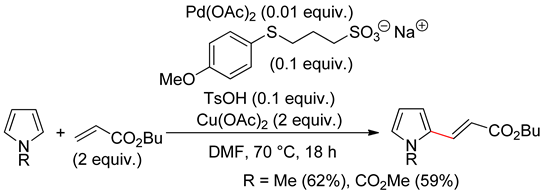
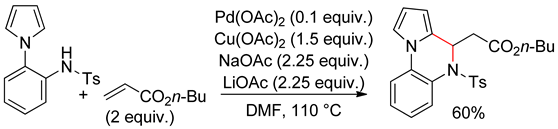
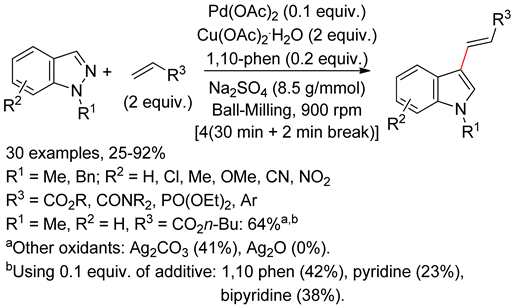
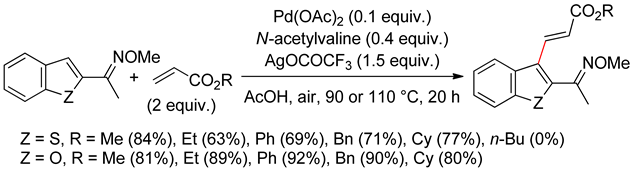
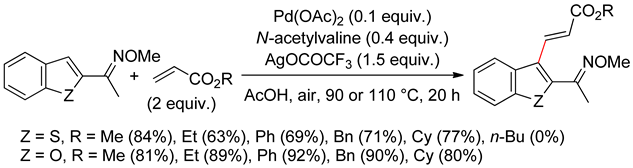
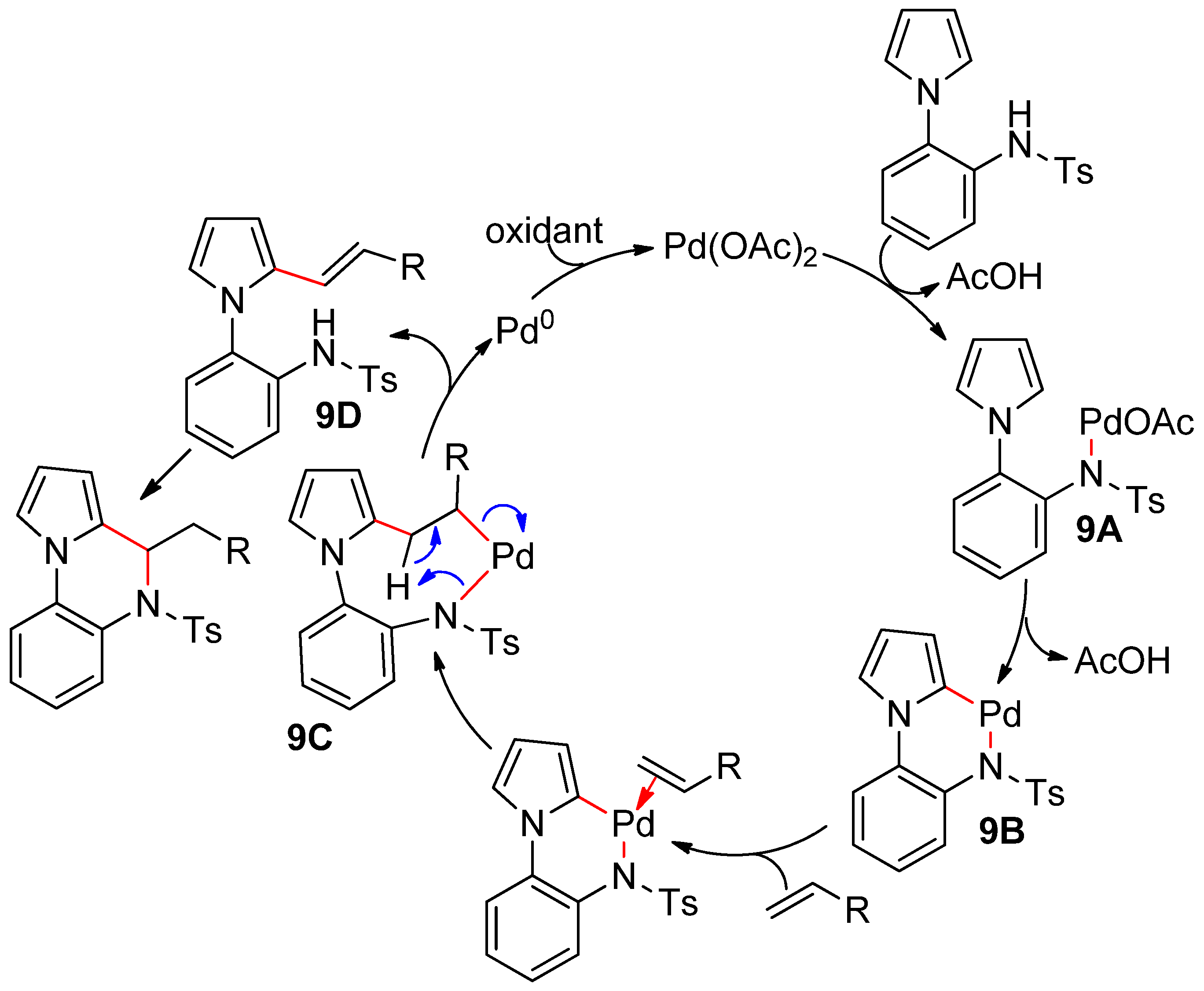
4.2. Indoles
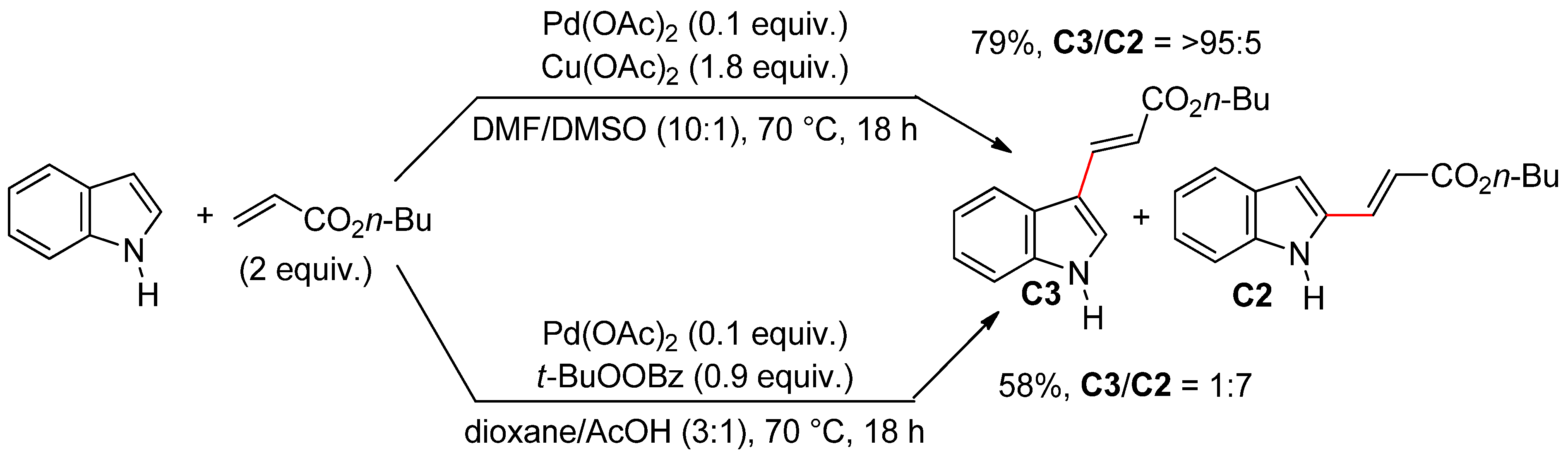
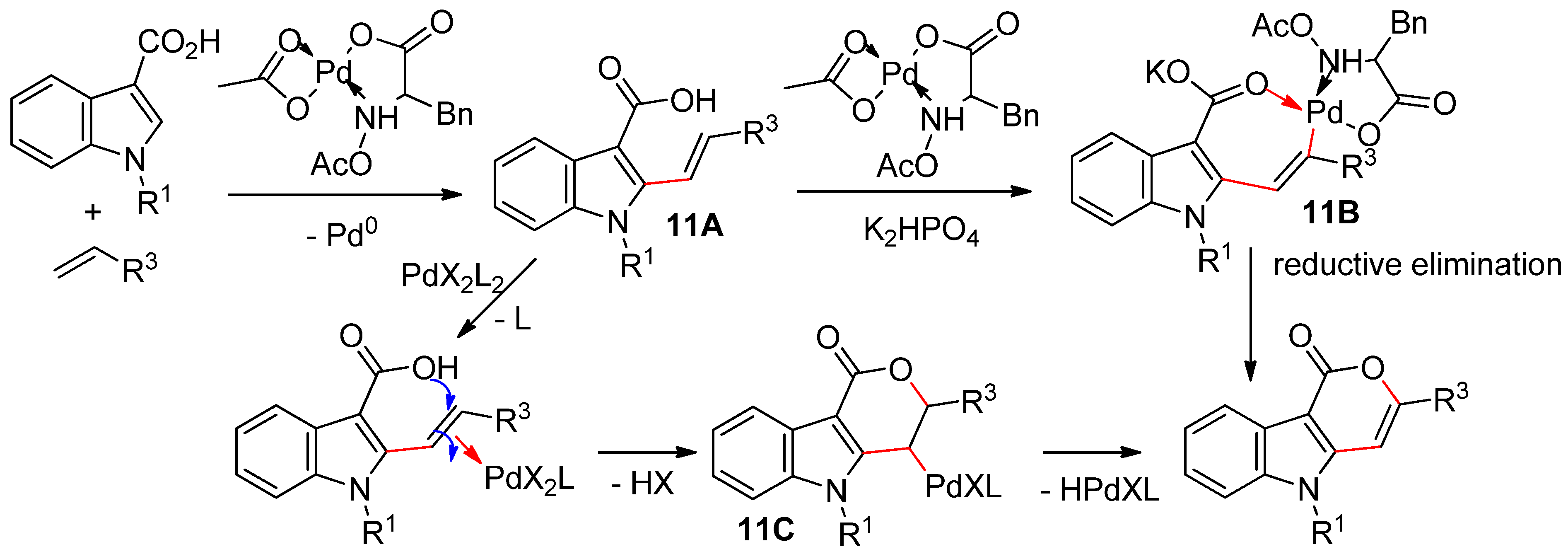
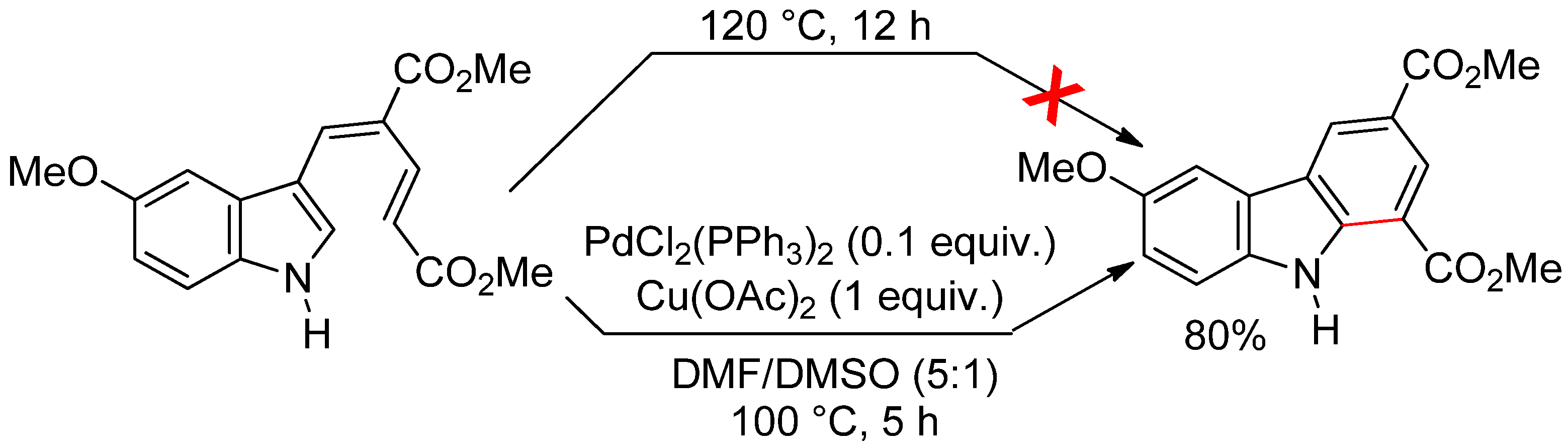
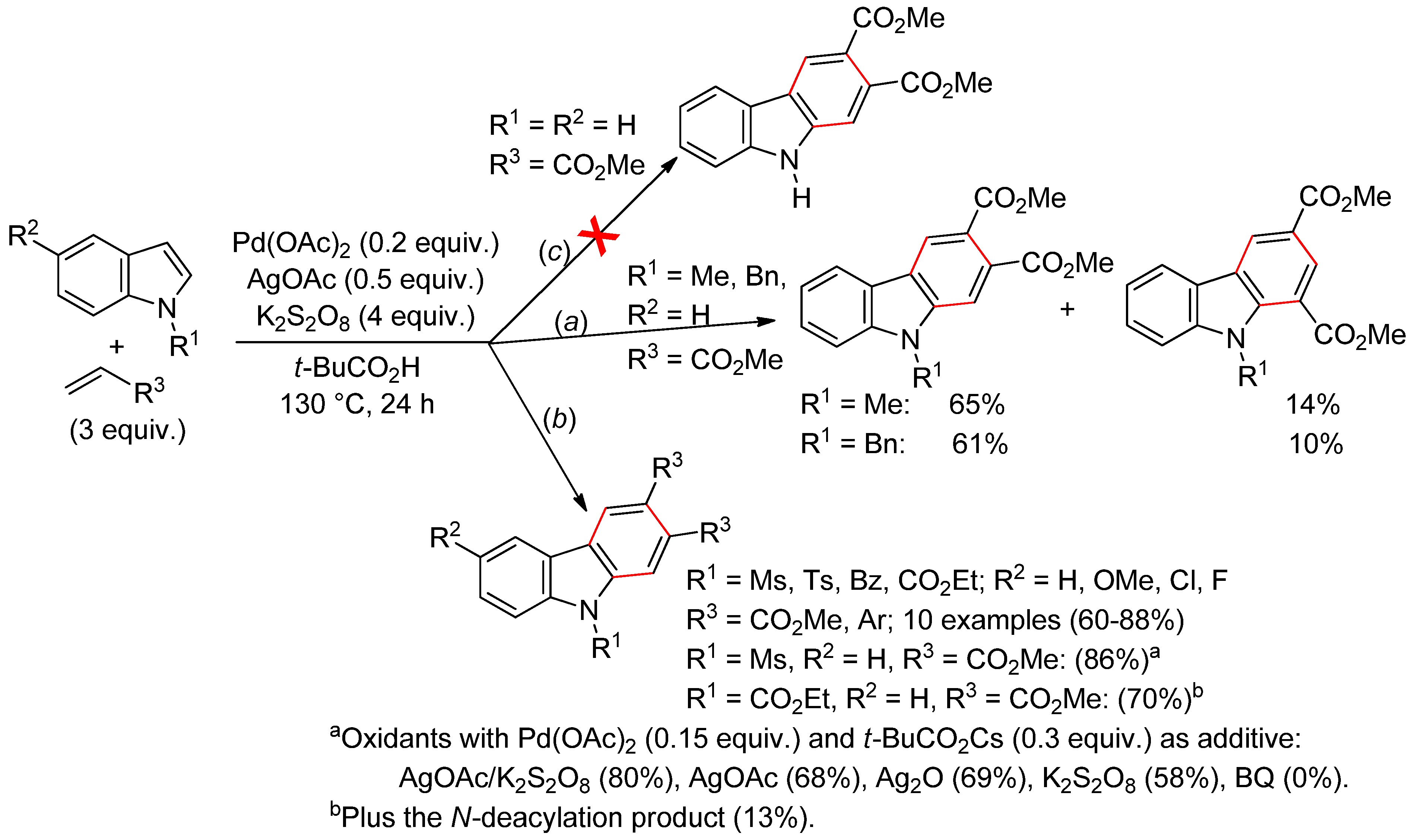
4.2.1. C3 Alkenylations
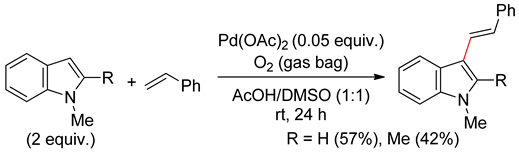
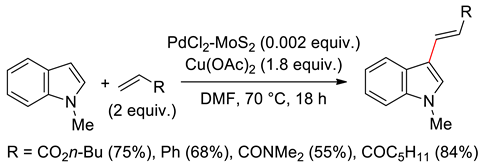

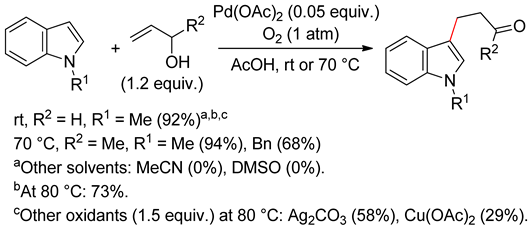
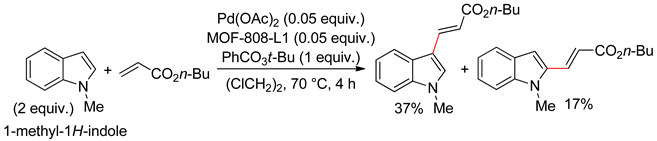
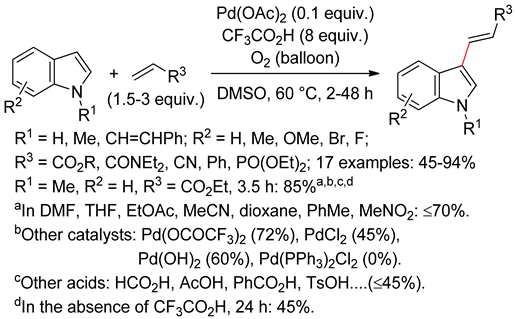
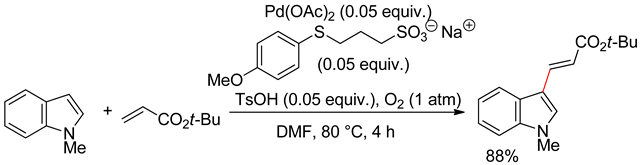
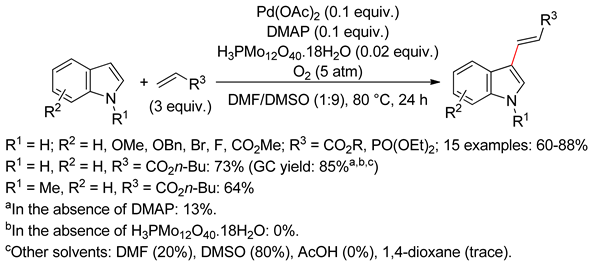

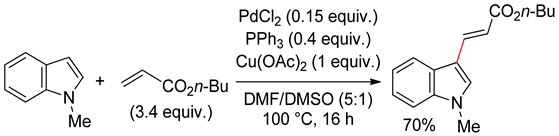
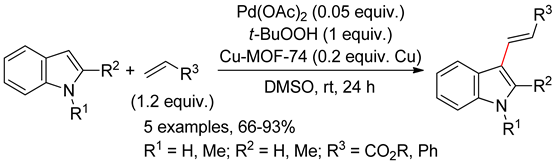
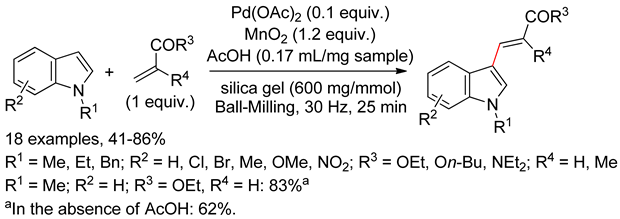
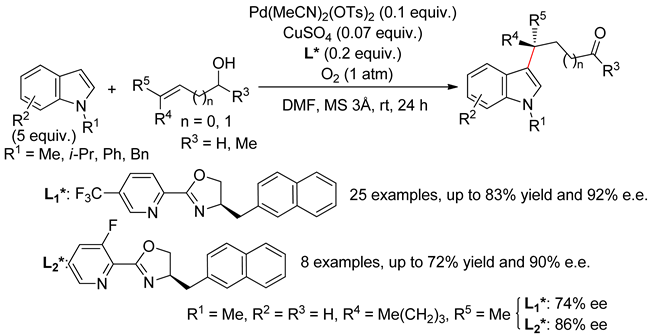
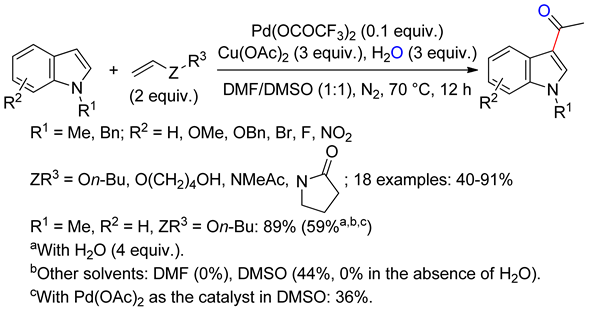

4.2.2. C2 Alkenylations



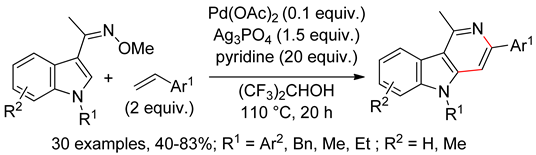
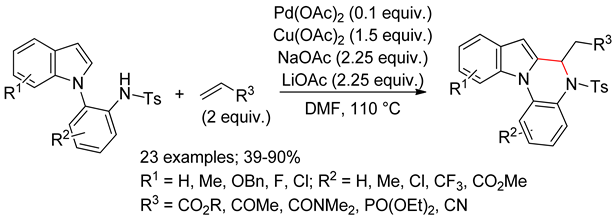
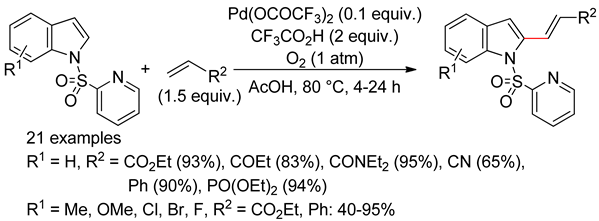
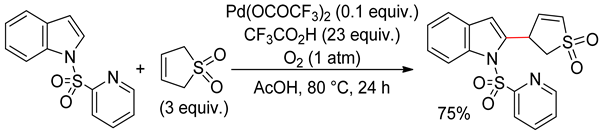
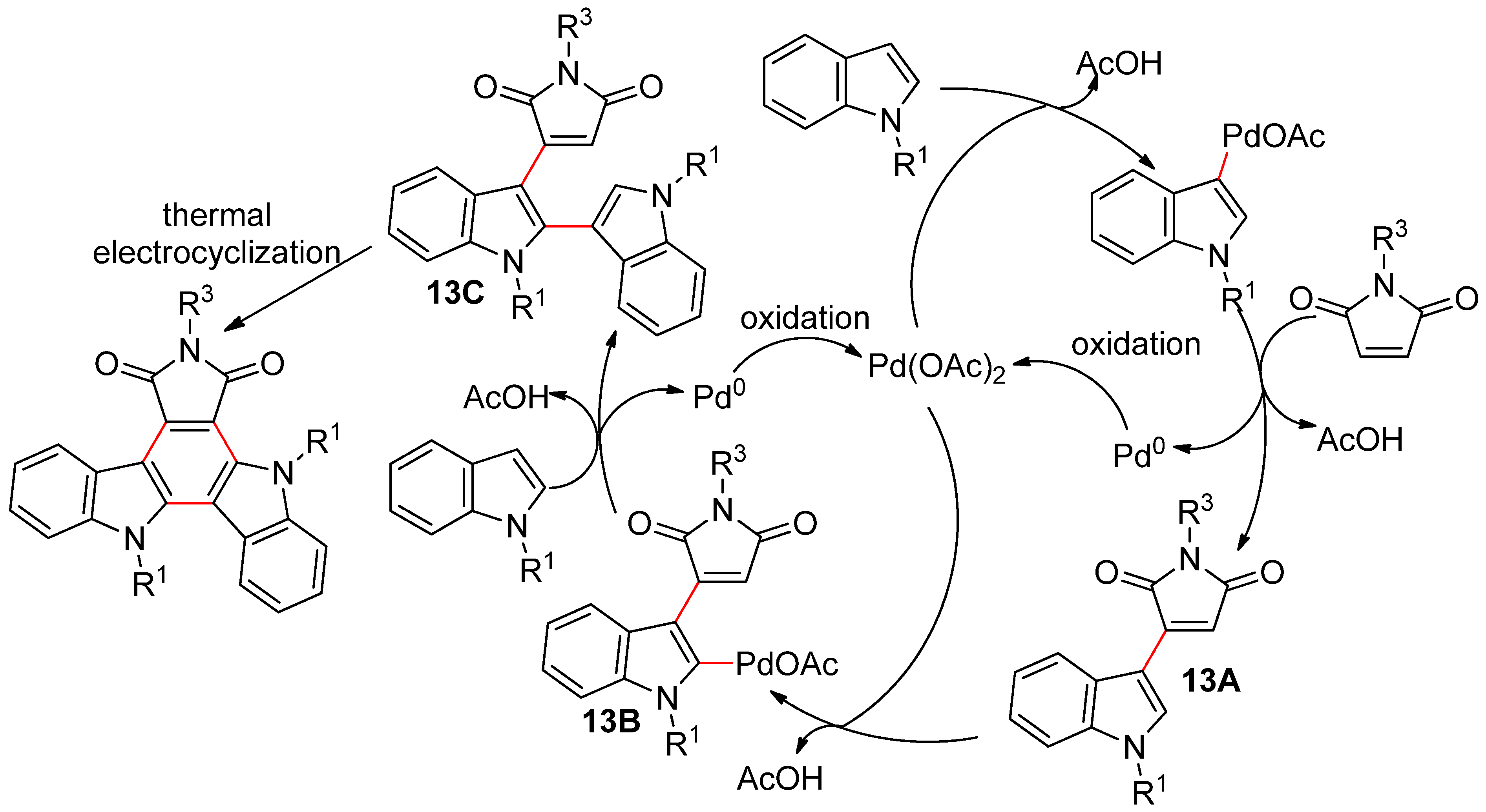
4.2.3. Domino Reactions
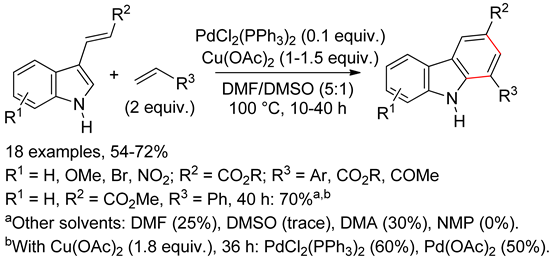
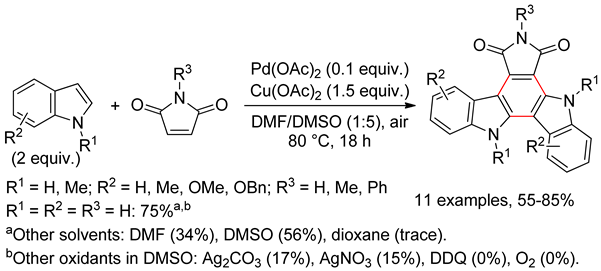

4.3. 7-Azaindoles
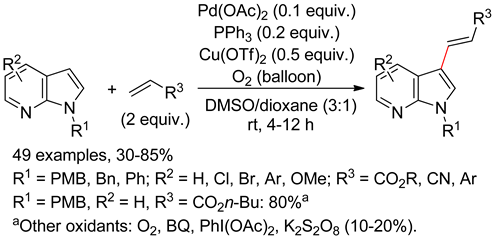
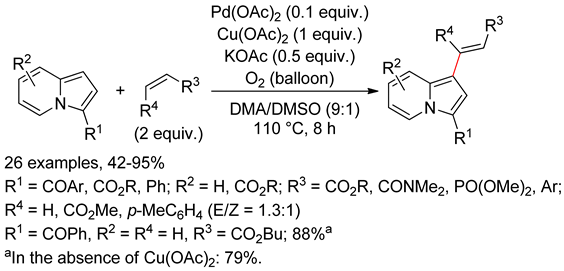
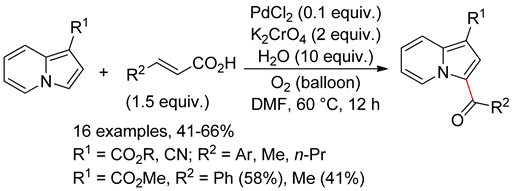
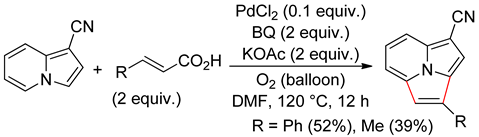
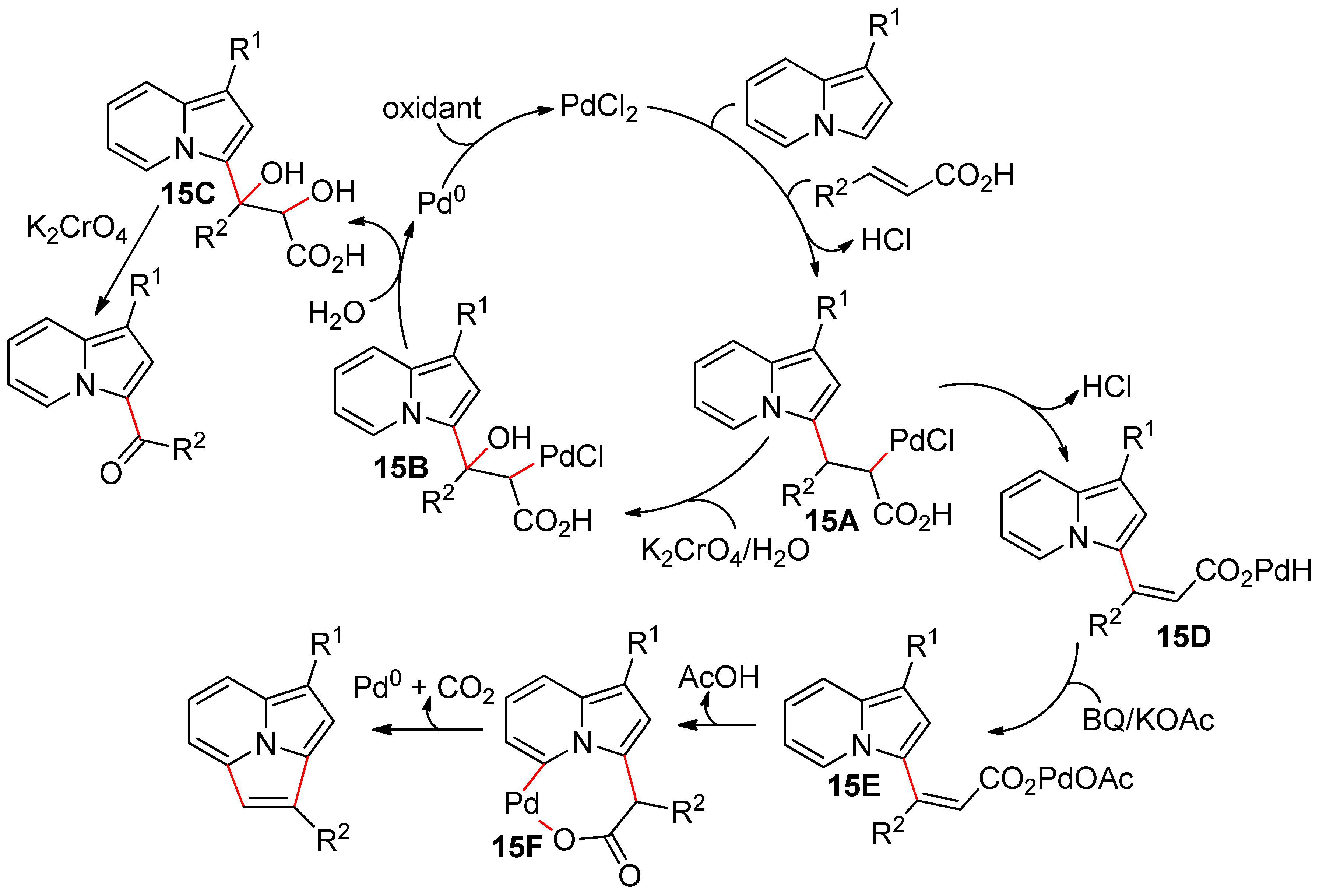
4.4. Indolizines
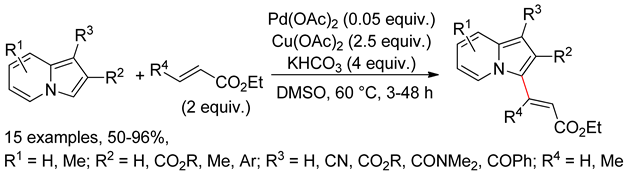
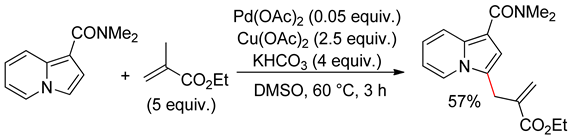
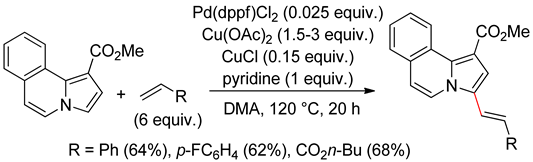
4.5. Sub-Conclusion
5. Se-Arenes
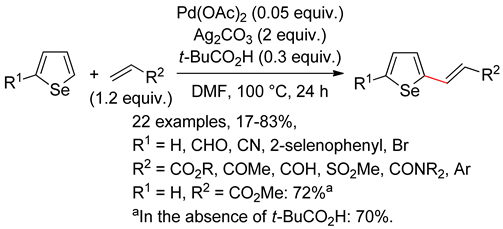
5.1. Selenophenes
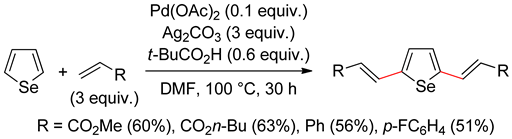
5.2. Benzoselenophene
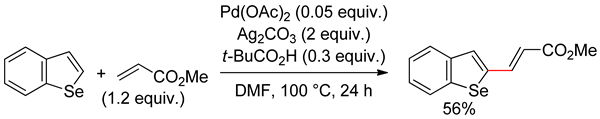
6. S,N-Arenes
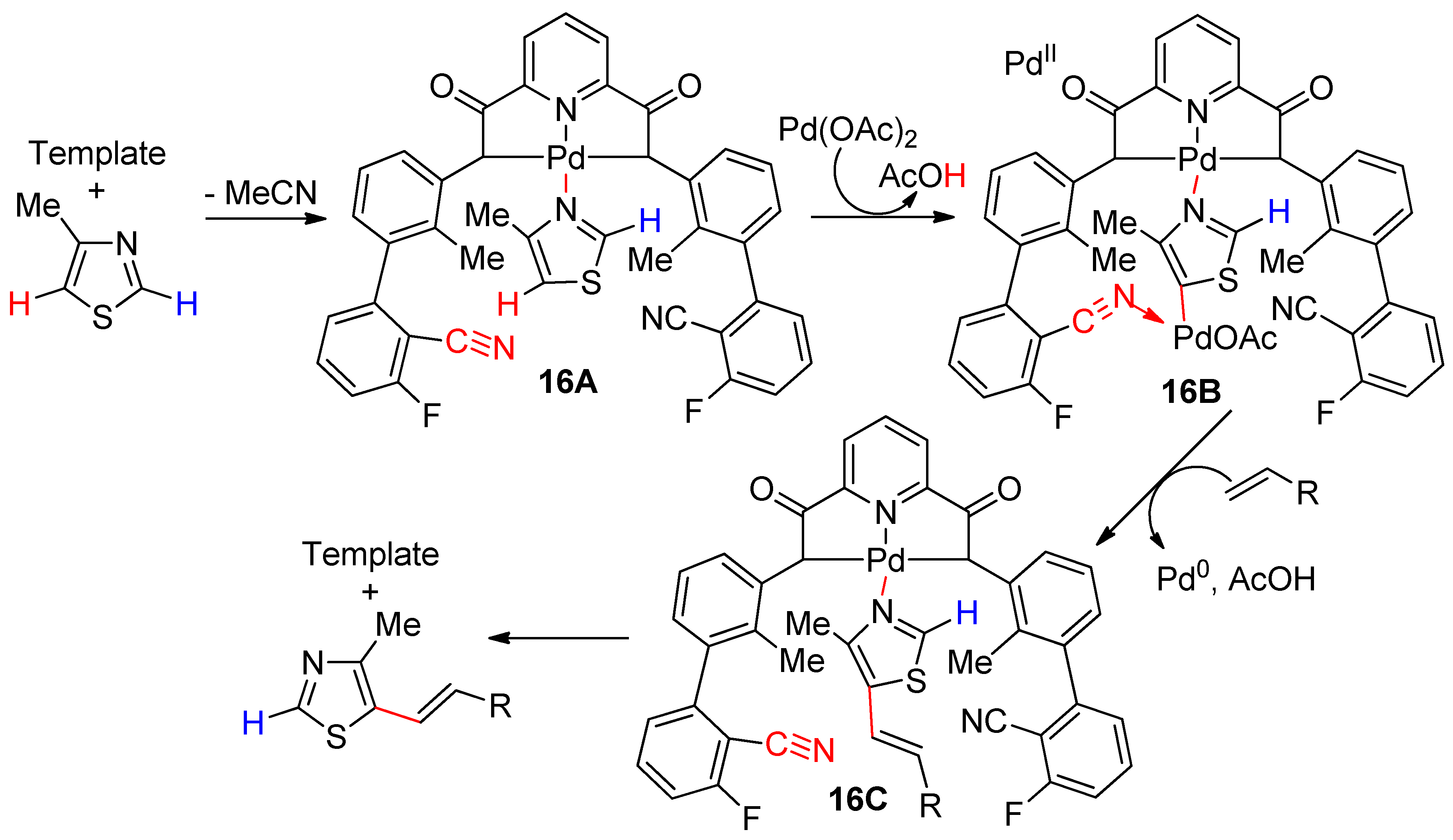
6.1. Thiazoles
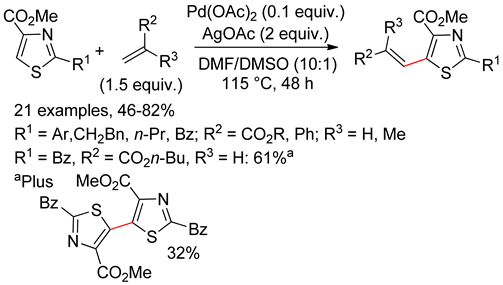
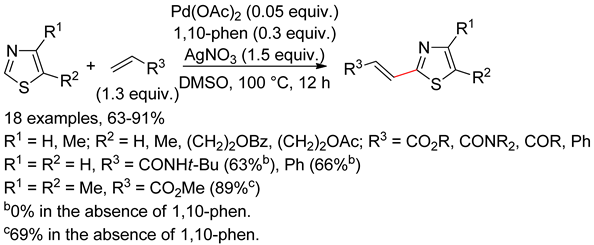
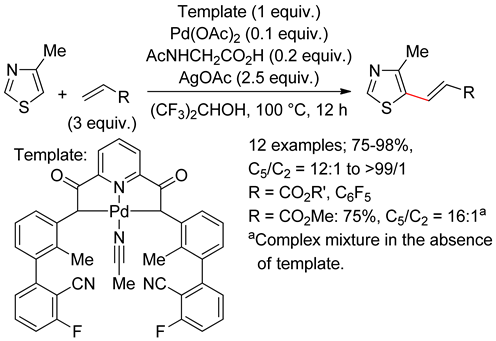
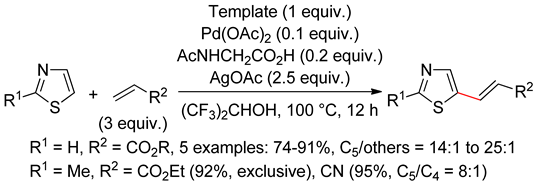
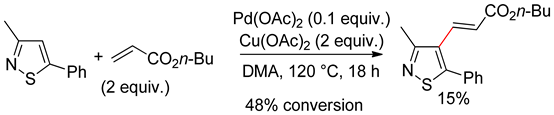
6.2. Isothiazoles
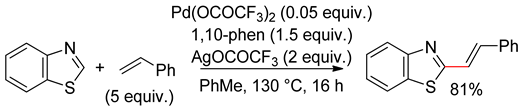
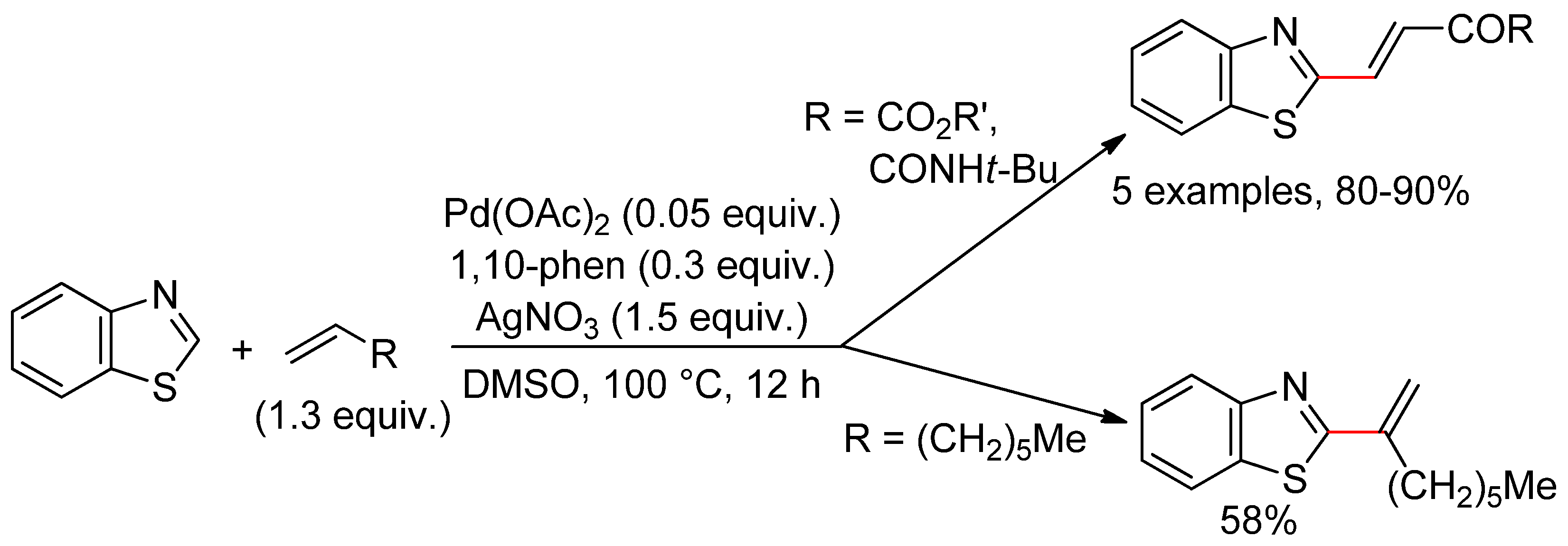
6.3. Benzothiazoles
6.4. Thiazolotriazoles
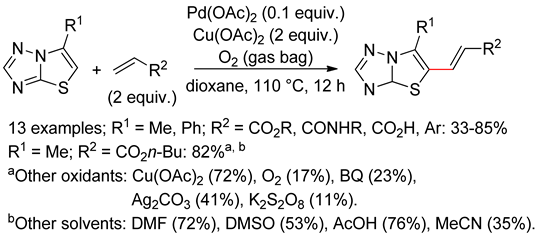
6.5. Imidazo[2,1-b]thiazoles
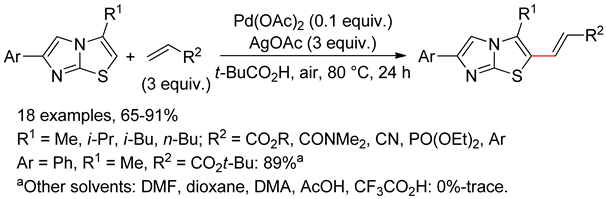
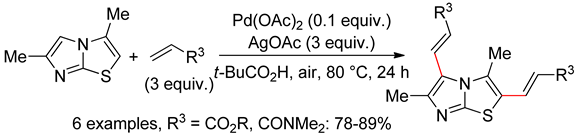
7. O,N-Arenes
7.1. Oxazoles
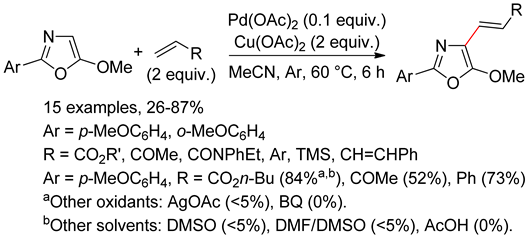
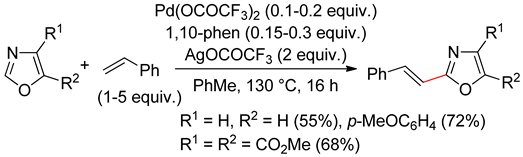
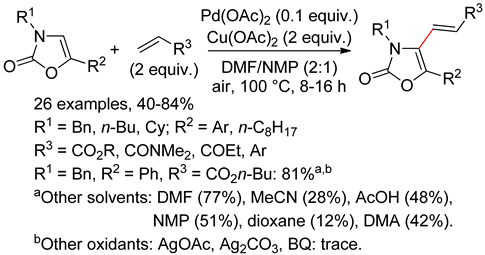
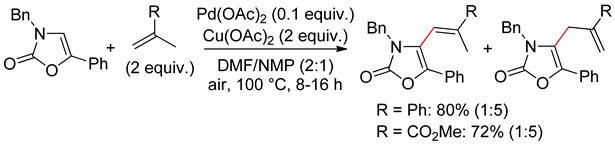
7.2. Oxazolones
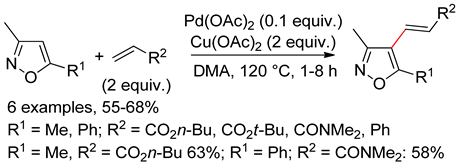
7.3. Isoxazoles
7.4. Benzoxazoles

8. N,N-Arenes
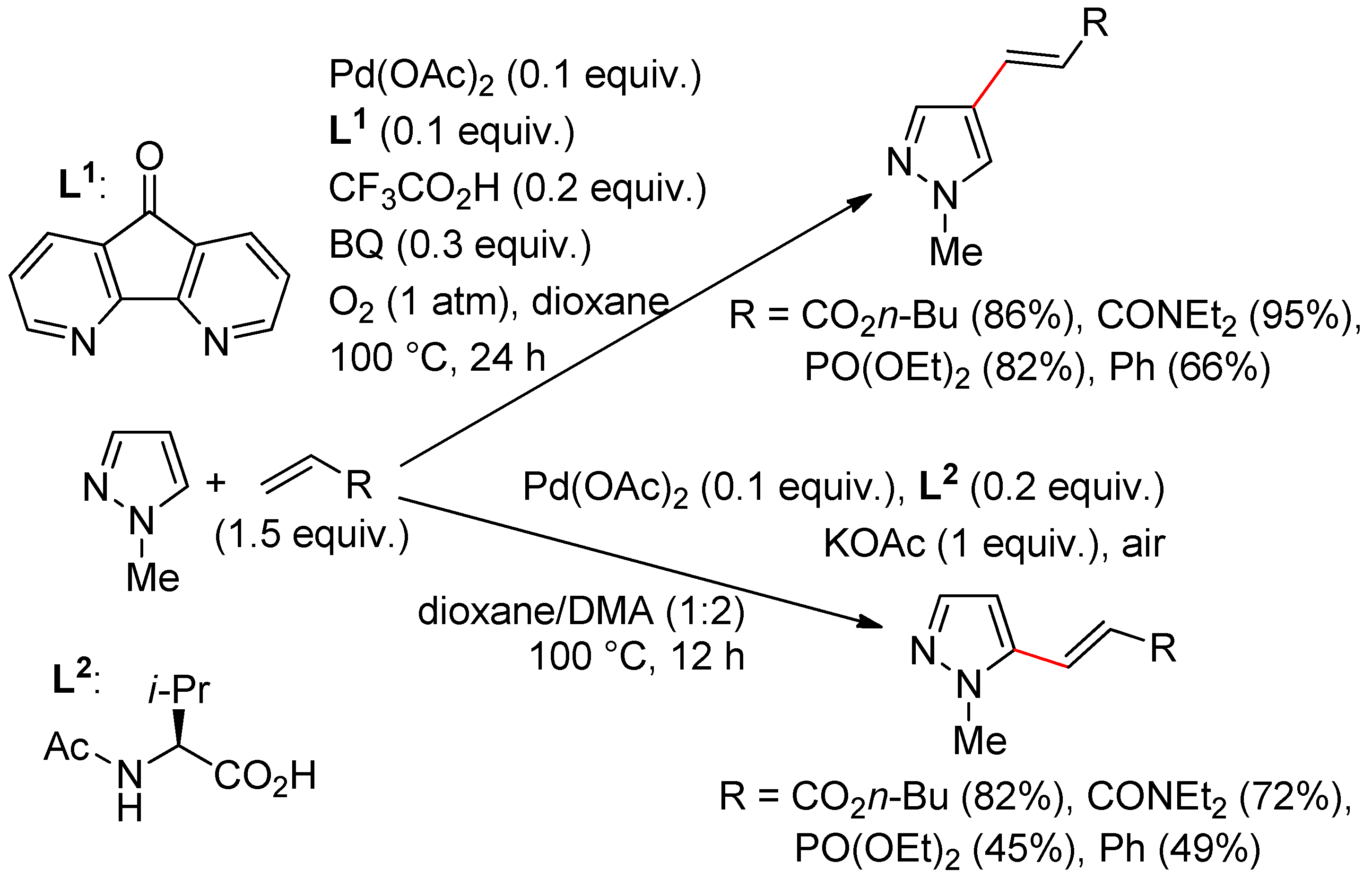
8.1. Pyrazoles
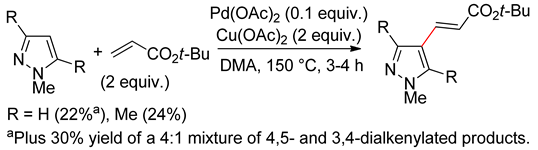
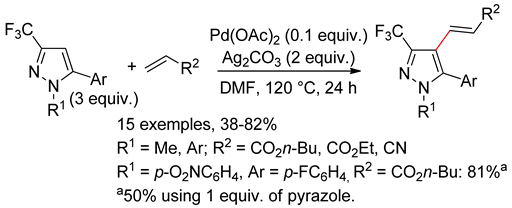
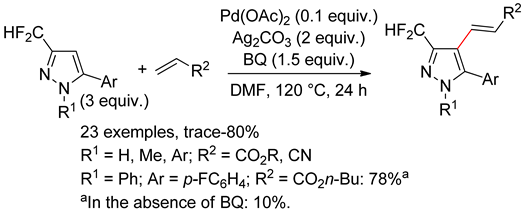
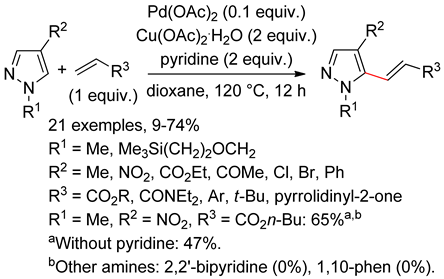
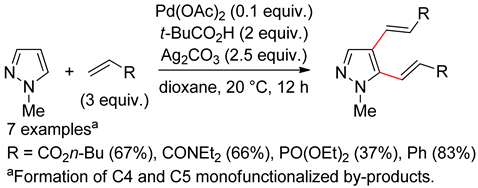
8.2. Pyrazolones
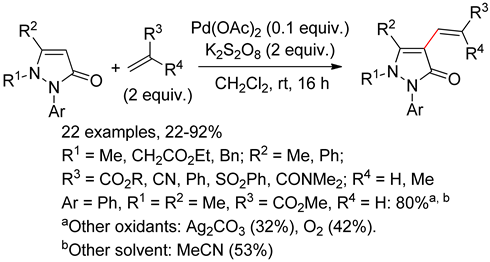
8.3. Indazoles
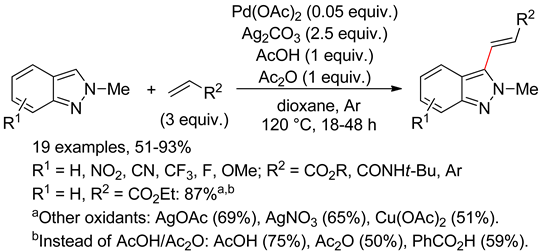
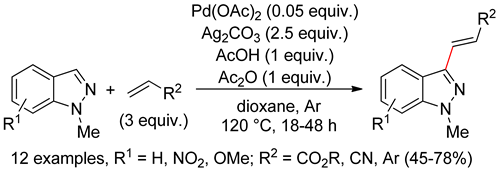
8.4. Imidazoles
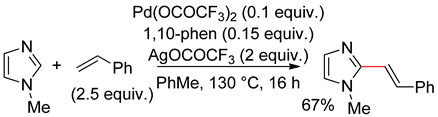
8.5. Benzimidazole
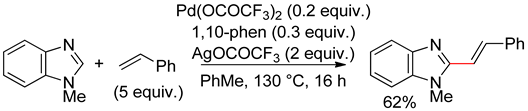
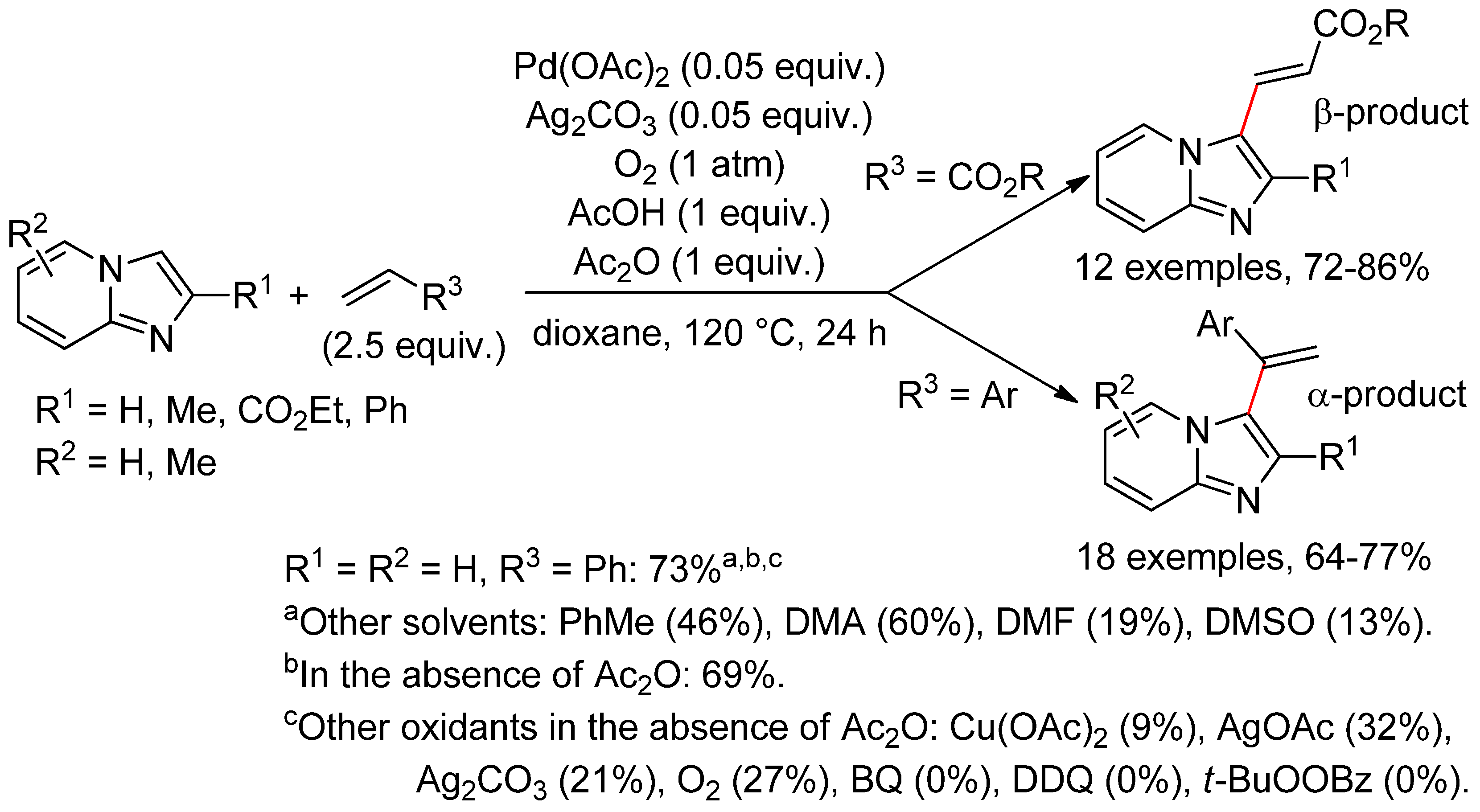
8.6. Imidazo[1,2-a]pyridines
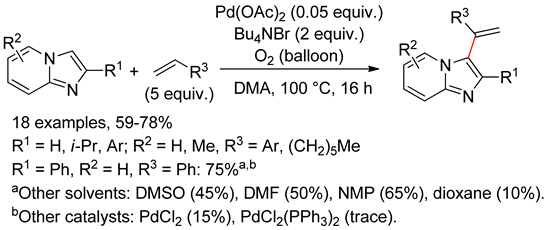
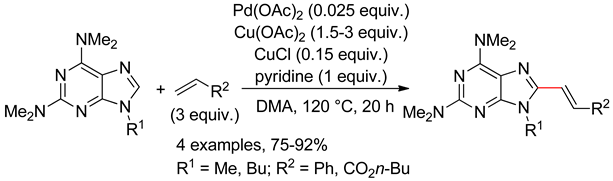
8.7. Purines
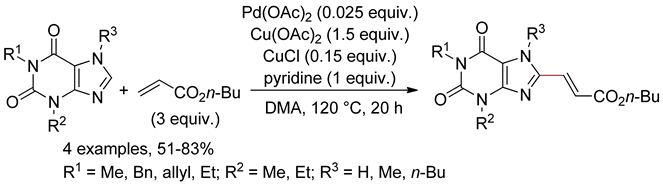
9. O,N,N-Arenes
9.1. 1,3,4-Oxadiazoles

9.2. Sydnones
10. N,N,N-Arenes
10.1. 1,2,4-Triazoles
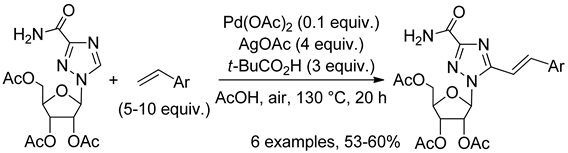
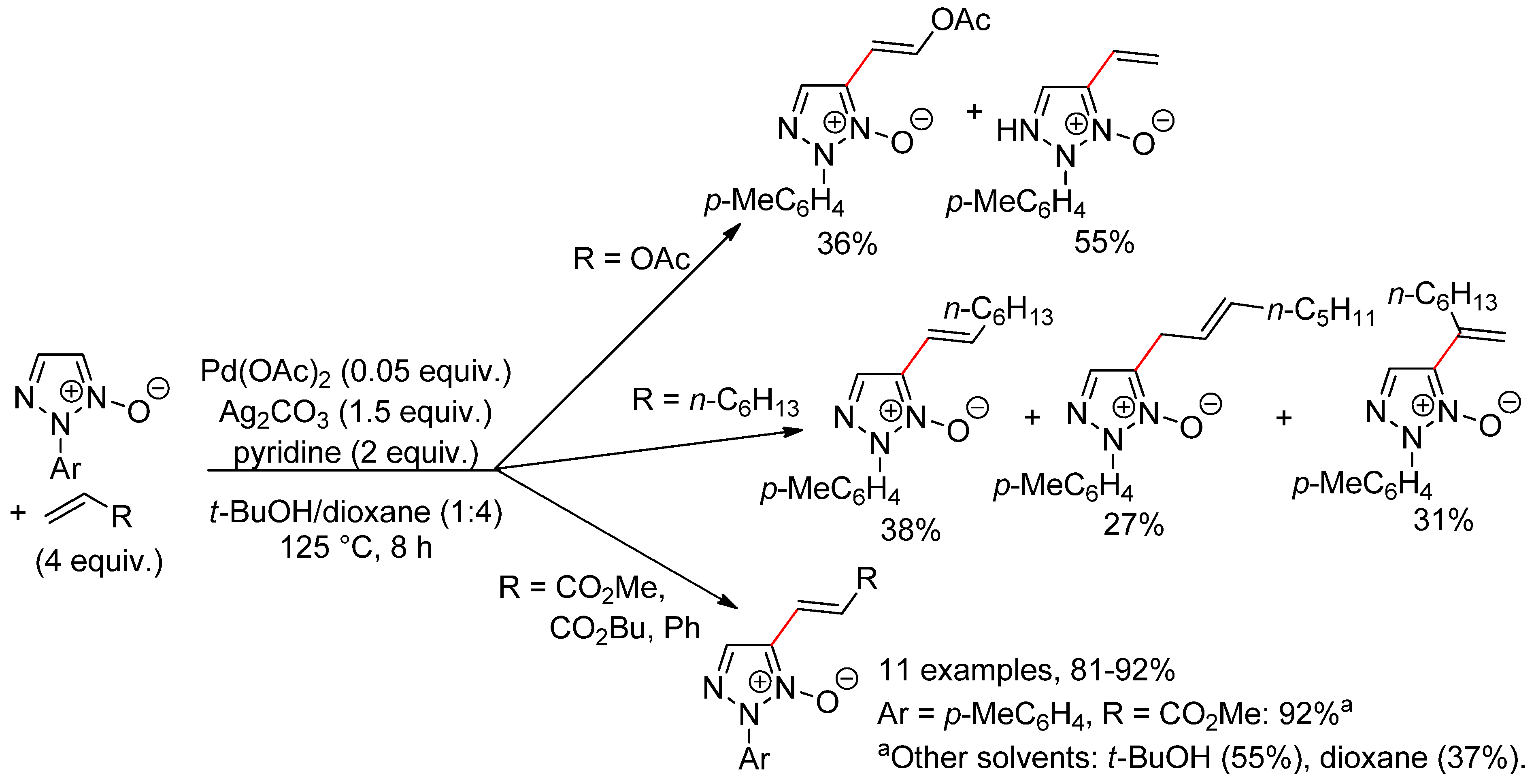
10.2. 1,2,3-Triazole N-Oxides
11. Conclusions and Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Heck, R.F. Palladium Reagents in Organic Syntheses; Academic Press: London, UK, 1985; pp. 260–268. [Google Scholar]
- Tsuji, J. Palladium Reagents and Catalysts, Innovations in Organic Synthesis; Wiley: Chichester, UK, 1995; pp. 55–59. [Google Scholar]
- Tsuji, J. Palladium Reagents and Catalysts, New Perspectives for the 21st Century; Wiley: Chichester, UK, 2004. [Google Scholar]
- The Mizoroki-Heck Reaction; Oestreich, M., Ed.; Wiley: Chichester, UK, 2009. [Google Scholar]
- Moritani, I.; Fujiwara, Y. Aromatic substitution of styrene-palladium chloride complex. Tetrahedron Lett. 1967, 8, 1119–1122. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Moritani, I.; Matsuda, M.; Teranishi, S. Aromatic substitution of olefins. IV Reaction with palladium metal and silver acetate. Tetrahedron Lett. 1968, 9, 3863–3865. [Google Scholar] [CrossRef]
- Mizoroki, T.; Mori, K.; Ozaki, A. Arylation of olefin with aryl iodide catalyzed by palladium. Bull. Chem. Soc. Jpn. 1971, 44, 581. [Google Scholar] [CrossRef]
- Julia, M.; Duteil, M. Condensation of aromatic halides with olefins catalyzed by palladium (0). Bull. Soc. Chim. Fr. 1973, 9–10, 2790. [Google Scholar]
- Heck, R.F.; Nolley, J.P., Jr. Palladium-catalyzed vinylic hydrogen substitution reactions with aryl, benzyl, and styryl halides. J. Org. Chem. 1972, 37, 2320–2322. [Google Scholar] [CrossRef]
- Le Bras, J.; Muzart, J. Intermolecular dehydrogenative Heck reactions. Chem. Rev. 2011, 111, 1170–1214. [Google Scholar] [CrossRef]
- Weng, J.; Yu, Z.; Zhang, G. Direct oxidative coupling between unfunctionalized arene and olefin. Prog. Chem. 2012, 24, 523–544. [Google Scholar]
- Wu, Y.; Wang, J.; Mao, F.; Kwong, F.Y. Palladium-catalyzed cross-dehydrogenative functionalization of C (sp2)-H bonds. Chem. Asian J. 2014, 9, 26–47. [Google Scholar] [CrossRef]
- Maes, J.; Maes, B.U.W. A journey through metal-catalyzed C–H functionalization of heterocycles: Insights and trends. Adv. Heterocycl. Chem. 2016, 120, 137–194. [Google Scholar]
- Ping, L.; Chung, D.S.; Bouffard, J.; Lee, S.-G. Transition metal-catalyzed site- and regio-divergent C–H bond functionalization. Chem. Soc. Rev. 2017, 46, 4299–4328. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Kang, H.; Li, J.; Li, C.-J. En route to intermolecular cross-dehydrogenative coupling reactions. J. Org. Chem. 2019, 84, 12705–12721. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, Q.; Ma, Y.; Jia, Y. Direct olefination at the C-4 position of tryptophan via C–H activation: Application to biomimetic synthesis of clavicipitic acid. Org. Lett. 2013, 15, 4528–4531. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Shao, L.-Y.; Yu, K.-K.; Hu, Y.-H.; Liu, H.-W.; Liao, D.-H.; Ji, Y.-F. Palladium-catalyzed site-selective direct olefination of 6-electron-withdrawing group substituted 3-arylbenzo[d]isoxazoles. Org. Chem. Front. 2017, 4, 1962–1966. [Google Scholar] [CrossRef]
- Leitch, J.A.; Bhonoah, Y.; Frost, C.G. Beyond C2 and C3: Transition-metal-catalyzed C–H functionalization of indole. ACS Catal. 2017, 7, 5618–5627. [Google Scholar] [CrossRef]
- Broggini, G.; Beccalli, E.M.; Fasana, A.; Gazzola, S. Palladium-catalyzed dual C–H or N–H functionalization of unfunctionalized indole derivatives with alkenes and arenes. Beilstein J. Org. Chem. 2012, 8, 1730–1746. [Google Scholar] [CrossRef]
- Thiery, E.; Harakat, D.; Le Bras, J.; Muzart, J. Pd-catalyzed oxidative coupling of alkylfurans with olefins through C-H activation: Synthesis of difurylalkanes. Organometallics 2008, 27, 3996–4004. [Google Scholar] [CrossRef]
- Vasseur, A.; Harakat, D.; Muzart, J.; Le Bras, J. ESI-MS studies of the dehydrogenative Heck reaction of furans with acrylates using benzoquinone as the reoxidant and DMSO as the solvent. J. Org. Chem. 2012, 77, 5751–5758. [Google Scholar] [CrossRef]
- Vasseur, A.; Harakat, D.; Muzart, J.; Le Bras, J. Aerobic dehydrogenative Heck reactions of heterocycles with styrenes: A negative effect of metallic co-oxidants. Adv. Synth. Catal. 2013, 355, 59–67. [Google Scholar] [CrossRef]
- Rauf, W.; Brown, J.M. Reactive intermediates in catalytic alkenylation; pathways for Mizoroki-Heck, oxidative Heck and Fujiwara-Moritani reactions. Chem. Commun. 2013, 49, 8430–8440. [Google Scholar] [CrossRef]
- Vasseur, A.; Laugel, C.; Harakat, D.; Muzart, J.; Le Bras, J. Ligand-promoted reactivity of alkenes in dehydrogenative Heck reactions of furans and thiophenes. Eur. J. Org. Chem. 2015, 2015, 944–948. [Google Scholar] [CrossRef]
- Jia, K.-Y.; Yu, J.-B.; Jiang, Z.-J.; Su, W.-K. Mechanochemically activated oxidative coupling of indoles with acrylates through C-H activation: Synthesis of 3-vinylindoles and β, β-diindolyl propionates and study of the mechanism. J. Org. Chem. 2016, 81, 6049–6055. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.C. Controlling reactivity in the Fujiwara–Moritani reaction: Examining solvent effects and the addition of 1,3-dicarbonyl ligands on the oxidative coupling of electron rich arenes and acrylates. Tetrahedron Lett. 2020, 61, 151471. [Google Scholar] [CrossRef]
- Hu, H.; Liu, Y.; Zhong, H.; Zhu, Y.; Wang, C.; Ji, M. Heck-type cross-dehydrogenative coupling reactions of indolizines at the 3-position with electron-deficient alkenes through palladium-catalyzed C-H activation. Chem. Asian J. 2012, 7, 884–888. [Google Scholar] [CrossRef] [PubMed]
- Petit, A.; Flygare, J.; Miller, A.T.; Winkel, G.; Ess, D.H. Transition-state metal aryl bond stability determines regioselectivity in palladium acetate mediated C-H bond activation of heteroarenes. Org. Lett. 2012, 14, 3680–3683. [Google Scholar] [CrossRef]
- Asano, R.; Moritani, I.; Fujiwara, Y.; Teranishi, S. Aromatic substitution of olefins. XIX. Reaction of five-membered heterocyclic aromatic compounds with styrene. Bull. Chem. Soc. Jpn. 1973, 46, 663. [Google Scholar] [CrossRef]
- Kozhevnikov, I.V. Vinylation of 5-membered heterocycles by ethylene in the presence of palladium (II). React. Kinet. Catal. Lett. 1976, 5, 439–443. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Maruyama, O.; Yoshidomi, M.; Taniguchi, H. Palladium-catalyzed alkenylation of aromatic heterocycles with olefins. Synthesis of functionalized aromatic heterocycles. J. Org. Chem. 1981, 46, 851–855. [Google Scholar] [CrossRef]
- Patra, P.; Ray, J.K.; Kar, G.K. Catalyst- and steric-controlled alkenylation via chemoselective C–H activation and C–Br activation in Heck reaction of methyl 1-(2-bromoaryl)-3-(2-furyl/thienyl)-5-oxopyrrolidine-2-carboxylates and diethyl 1-(2-bromoaryl)-3-(2-furyl/thienyl)-5-oxopyrrolidine-2,2-dicarboxylate derivatives. Tetrahedron Lett. 2010, 51, 3580–3582. [Google Scholar]
- Taraban’ko, V.E.; Kozhevnikov, I.V.; Matveev, K.I. Palladium (II)-catalyzed arylation of ethylene in the presence of phosphorus-molybdenum-vanadium heteropoly acids. Kinet. Katal. 1978, 19, 1160–1166. [Google Scholar]
- Tani, M.; Sakaguchi, S.; Ishii, Y. Pd (OAc)2-catalyzed oxidative coupling reaction of benzenes with olefins in the presence of molybdovanadophosphoric acid under atmospheric dioxygen and air. J. Org. Chem. 2004, 69, 1221–1226. [Google Scholar] [CrossRef]
- Zhao, J.; Huang, L.; Cheng, K.; Zhang, Y. Palladium-catalyzed alkenation of thiophenes and furans by regioselective C–H bond functionalization. Tetrahedron Lett. 2009, 50, 2758–2761. [Google Scholar] [CrossRef]
- Le Bras, J.; Muzart, J. β-Elimination competitions leading to C=C bonds from alkylpalladium intermediates. Tetrahedron 2012, 68, 10065–10113. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Liu, Z.-Q. Pd-catalyzed olefination of furans and thiophenes with allyl esters. Org. Lett. 2012, 14, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Larock, R.C.; Hightower, T.R. Synthesis of unsaturated lactones via palladium-catalyzed cyclization of alkenoic acids. J. Org. Chem. 1993, 58, 5298–5300. [Google Scholar] [CrossRef]
- Muzart, J. Oxidation adjacent to oxygen of alcohols catalyzed by palladium/dimethyl sulfoxide. In Comprehensive Organic Synthesis, 2nd ed.; Molander, G.A., Knochel, P., Eds.; Oxford: Oxford, UK; Elsevier: Amsterdam, The Netherlands, 2014; Volume 7, pp. 295–301. [Google Scholar]
- Jiang, Z.; Zhang, L.; Dong, C.; Cai, Z.; Tang, W.; Li, H.; Xu, L.; Xiao, J. Palladium-catalyzed highly regioselective arylation of allylamines with thiophenes and furans. Adv. Synth. Catal. 2012, 354, 3225–3230. [Google Scholar] [CrossRef]
- Huang, L.; Qi, J.; Wu, X.; Wu, W.; Jiang, H. Aerobic oxidative coupling between carbon nucleophiles and allylic alcohols: A strategy to construct β-(hetero) aryl ketones and aldehydes through hydrogen migration. Chem. Eur. J. 2013, 19, 15462–15466. [Google Scholar] [CrossRef]
- Muzart, J. Palladium-catalysed reactions of alcohols, Part B: Formation of C-C and C-N bonds from unsaturated alcohols. Tetrahedron 2005, 61, 4179–4212. [Google Scholar] [CrossRef]
- Álvarez-Casao, Y.; Fernández-Ibáñez, M.Á. S,O-Ligand-promoted Pd-catalyzed C–H olefination of thiophenes. Eur. J. Org. Chem. 2019, 2019, 1842–1845. [Google Scholar] [CrossRef]
- Li, Y.; Shen, Z.; Huang, M.; Zhang, J.; Kim, J.K.; Wu, Y. Highly catalytic activity of bis(alkoxo)-palladium complexes for Fujiwara-Moritani. Chin. J. Org. Chem. 2018, 38, 200–207. [Google Scholar] [CrossRef]
- Gorsline, B.J.; Wang, L.; Ren, P.; Carrow, B.P. C–H alkenylation of heteroarenes: Mechanism, rate, and selectivity changes enabled by thioether ligands. J. Am. Chem. Soc. 2017, 139, 9605–9614. [Google Scholar] [CrossRef]
- Wen, Z.-K.; Zhao, Z.-K.; Wang, N.-J.; Chen, Z.-L.; Chao, J.-B.; Feng, L.-H. Palladium-catalyzed controllable reductive/oxidative Heck coupling between cyclic enones and thiophenes via C-H activation. Org. Lett. 2019, 21, 9545–9549. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Deng, G.; Gu, J.; Hua, W.; Jia, X.; Xi, K. In site preparation of Pd (II)-MoS2 complex: A new high-efficiency catalyst for alkenylation of heteroaromatics by direct C-H bond activation. Appl. Catal. A Gen. 2015, 508, 80–85. [Google Scholar] [CrossRef]
- Van Velthoven, N.; Henrion, M.; Dallenes, J.; Krajnc, A.; Bugaev, A.L.; Liu, P.; Bals, S.; Soldatov, A.V.; Mali, G.; De Vos, D.E. S,O-functionalized metal-organic frameworks as heterogeneous single-site catalysts for the oxidative alkenylation of arenes via C−H activation. ACS Catal. 2020, 10, 5077–5085. [Google Scholar] [CrossRef]
- Gao, S.; Wu, Z.; Wu, F.; Lin, A.; Yao, H. Catalyst-controlled regiodivergent dehydrogenative Heck reaction of 4-arylthiophene/furan-3-carboxylates. Adv. Synth. Catal. 2016, 358, 4129–4135. [Google Scholar] [CrossRef]
- Della Ca’, N.; Fontana, M.; Motti, E.; Catellani, M. Pd/norbornene: A winning combination for selective aromatic functionalization via C−H bond activation. Acc. Chem. Res. 2016, 49, 1389–1400. [Google Scholar] [CrossRef]
- Cheng, H.-G.; Chen, S.; Chen, R.; Zhou, Q. Palladium (II)-initiated Catellani-type reactions. Angew. Chem. Int. Ed. 2019, 58, 5832–5844. [Google Scholar] [CrossRef]
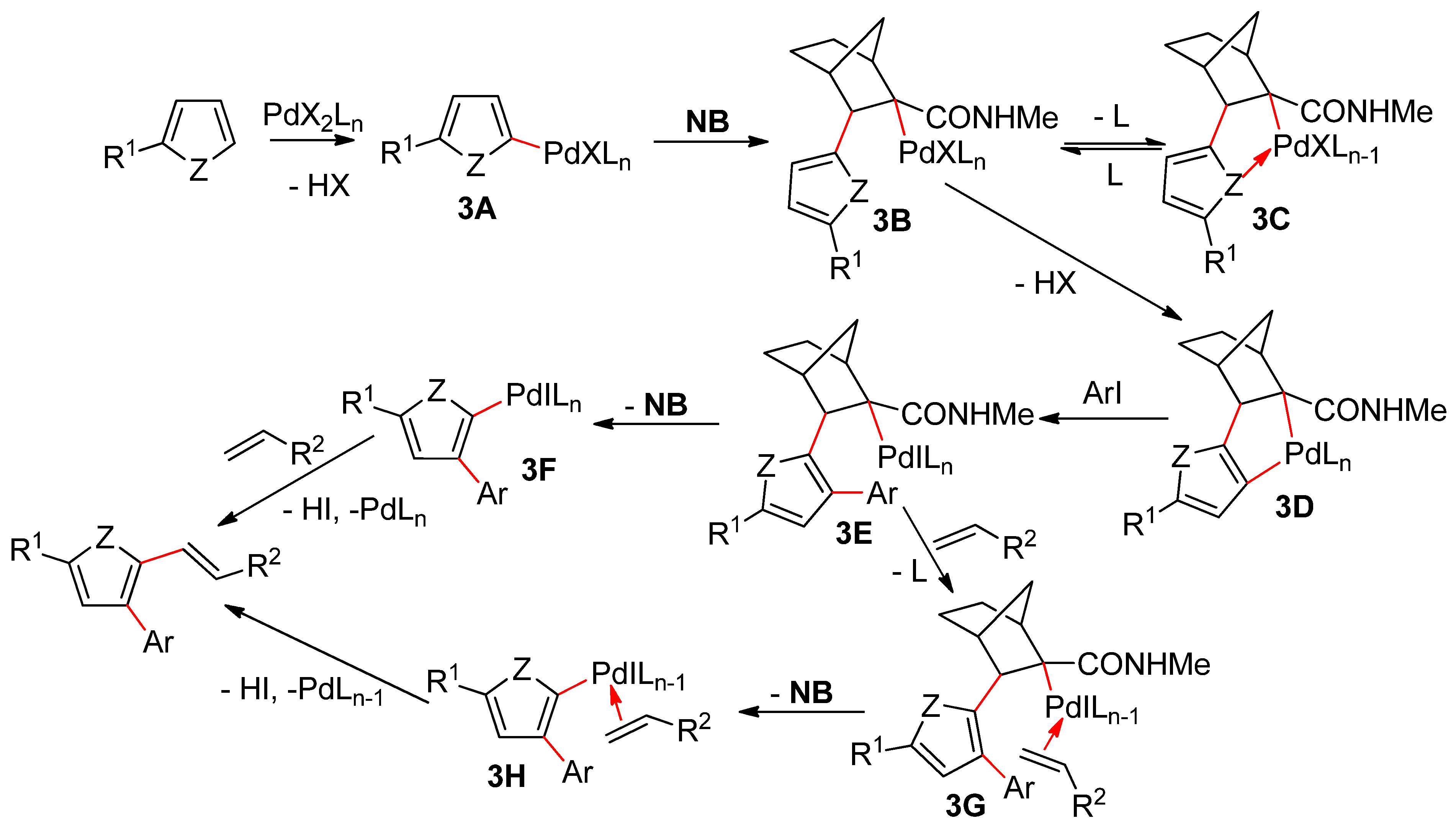
- Wang, J.; Dong, G. Palladium/norbornene cooperative catalysis. Chem. Rev. 2019, 119, 7478–7528. [Google Scholar] [CrossRef]
- Li, R.; Zhou, Y.; Xu, X.; Dong, G. Direct vicinal difunctionalization of thiophenes enabled by the palladium/norbornene cooperative catalysis. J. Am. Chem. Soc. 2019, 141, 18958–18963. [Google Scholar] [CrossRef]
- Catellani, M.; Fagnola, M.C. Palladacycles as intermediates for selective dialkylation of arenes and subsequent fragmentation. Angew. Chem. Int. Ed. Engl. 1995, 33, 2421–2422. [Google Scholar] [CrossRef]
- Kim, B.S.; Lee, S.Y.; Youn, S.-W. Pd-catalyzed sequential C-C and C-N bond formations for the synthesis of N-heterocycles: Exploiting protecting group-directed C-H activation under modified reaction conditions. Chem. Asian J. 2011, 6, 1952–1957. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, D.-H.; Engle, K.M.; Yu, J.-Q. Pd (II)-catalyzed hydroxyl-directed C-H olefination enabled by monoprotected amino acid ligands. J. Am. Chem. Soc. 2010, 132, 5916–5921. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Leow, D.; Wan, L.; Yu, J.-Q. Ether-directed ortho-C–H olefination with a palladium (II)/monoprotected amino acid catalyst. Angew. Chem. Int. Ed. 2013, 52, 1245–1247. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Ke, S.; Qiu, L.; Zhang, X.; Lin, S. Palladium (II)/polyoxometalate-catalyzed direct alkenylation of benzofurans under atmospheric dioxygen. Chem. Cat. Chem. 2014, 6, 1531–1534. [Google Scholar] [CrossRef]
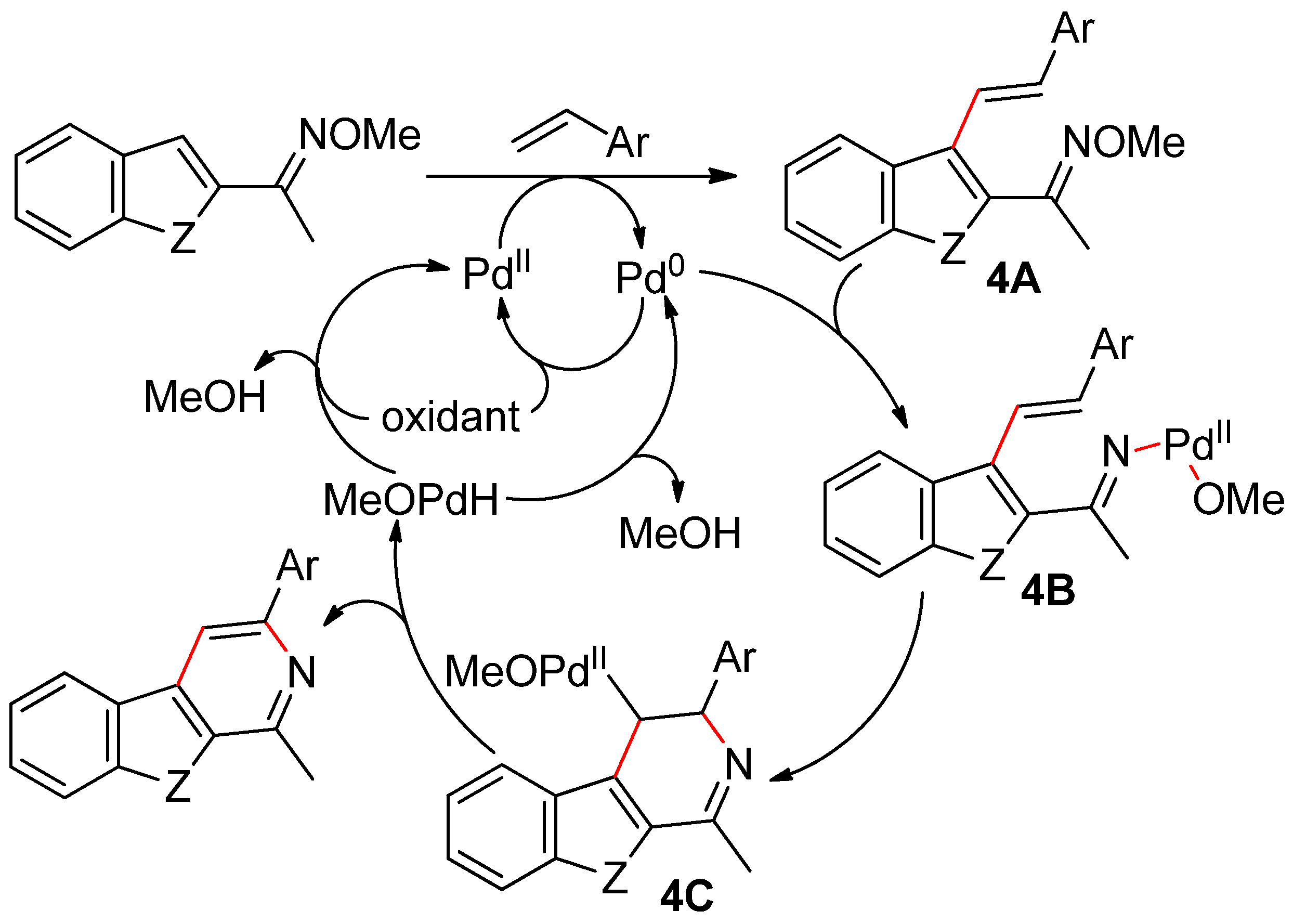
- Fu, X.; Yang, J.; Deng, K.; Shao, L.; Xia, C.; Ji, Y. Tandem C–C/C–N formation via palladium-catalyzed C–H activation/styrenation and sequential annulation of O-methylketoxime with styrenes. Org. Lett. 2019, 21, 3505–3509. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, T.; Shimo, N.; Maeda, K.; Sonoda, A.; Murahashi, S.I. Palladium-induced pyridine synthesis from unsaturated ketoximes. Tetrahedron Lett. 1976, 17, 383–386. [Google Scholar] [CrossRef]
- Kitamura, M.; Narasaka, K. Synthesis of aza-heterocycles from oximes by amino-Heck reaction. Chem. Rec. 2002, 2, 268–277. [Google Scholar] [CrossRef]
- Morita, T.; Satoh, T.; Miura, M. Palladium (II)-catalyzed direct C–H alkenylation of thienothiophene and related fused heteroarenes. Org. Lett. 2015, 17, 4384–4387. [Google Scholar] [CrossRef]
- Kasahara, A.; Izumi, T.; Yodono, M.; Saito, R.; Takeda, T.; Sugawara, T. Arylation and vinylation reactions of benzo[b]furan via organopalladium intermediates. Bull. Chem. Soc. Jpn. 1973, 46, 1220. [Google Scholar] [CrossRef]
- Maehara, A.; Satoh, T.; Miura, M. Palladium-catalyzed direct oxidative vinylation of thiophenes and furans under weakly basic conditions. Tetrahedron 2008, 64, 5982–5986. [Google Scholar] [CrossRef]
- Aouf, C.; Thiery, E.; Le Bras, J.; Muzart, J. Palladium-catalyzed dehydrogenative coupling of furans with styrenes. Org. Lett. 2009, 11, 4096–4099. [Google Scholar] [CrossRef]
- Jia, C.; Lu, W.; Kitamura, T.; Fujiwara, Y. Highly efficient Pd-catalyzed coupling of arenes with olefins in the presence of tert-butyl hydroperoxide as oxidant. Org. Lett. 1999, 1, 2097–2100. [Google Scholar] [CrossRef]
- Tsuji, J.; Nagashima, H. Palladium-catalyzed oxidative coupling of aromatic compounds with olefins using t-butyl perbenzoate as hydrogen acceptor. Tetrahedron 1984, 40, 2699–2702. [Google Scholar] [CrossRef]
- Yin, B.; Fu, M.; Wang, L.; Liu, J.; Zhu, Q. Dual ligand-promoted palladium-catalyzed nondirected C–H alkenylation of aryl ethers. Chem. Commun. 2020, 56, 3293–3296. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Zhou, H.; Chen, J.; Xu, J.; Wu, X.; Lin, A.; Yao, H. Solvent-controlled switchable C–H alkenylation of 4-aryl-1H-pyrrole-3-carboxylates: Application to the total synthesis of (±)-rhazinilam. Org. Lett. 2014, 16, 4884–4887. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Gao, S.; Huang, Y.; Lin, A.; Yao, H. Solvent-controlled C2/C5-regiodivergent alkenylation of pyrroles. Chem. Eur. J. 2015, 21, 15820–15825. [Google Scholar] [CrossRef]
- Zierkiewicz, W.; Privalov, T. A theoretical study of the essential role of DMSO as a solvent/ligand in the Pd (OAc)2/DMSO catalyst system for aerobic oxidation. Organometallics 2005, 24, 6019–6028. [Google Scholar] [CrossRef]
- Laha, J.K.; Bhimpuria, R.A.; Mule, G.B. Site-selective oxidative C4 alkenylation of (NH)-pyrroles bearing an electron-withdrawing C2 group. Chem. Cat. Chem. 2017, 9, 1092–1096. [Google Scholar] [CrossRef]
- Duan, J.-H.; Mi, R.-J.; Sun, J.; Zhou, M.-D. Regioselective C5 alkenylation of 2-acylpyrroles via Pd (II)-catalyzed C–H bond activation. Org. Chem. Front. 2018, 5, 162–165. [Google Scholar] [CrossRef]
- Wang, L.; Guo, W.; Zhang, X.-X.; Xia, X.-D.; Xiao, W.J. Synthesis of indolo [1,2-a]quinoxalines via a Pd-catalyzed regioselective C–H olefination/cyclization sequence. Org. Lett. 2012, 14, 740–743. [Google Scholar] [CrossRef]
- Wang, J.; Wu, O.; Gong, O.; Cheng, K.; Liu, O.; Yu, C.; Hao, E.; Jiao, L. Direct β-selective styrylation of BODIPY dyes via palladium(II)-catalyzed C−H functionalization. Adv. Synth. Catal. 2019, 361, 769–777. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Gong, Q.; Wang, H.; Hao, E.; Lo, P.-C.; Jiao, L. β-AlkenylBODIPY dyes: Regioselective synthesis via oxidative C–H olefination, photophysical properties, and bioimaging studies. J. Org. Chem. 2019, 84, 5078–5090. [Google Scholar] [CrossRef] [PubMed]
- Petrini, M. Regioselective direct C-alkenylation of indoles. Chem. Eur. J. 2017, 23, 16115–16151. [Google Scholar] [CrossRef] [PubMed]
- Sandtorv, A.H. Transition metal-catalyzed C-H activation of indoles. Adv. Synth. Catal. 2015, 357, 2403–2435. [Google Scholar] [CrossRef]
- Koubachi, J.; Brahmi, N.E.; Guillaumet, G.; Kazzouli, S.E. Oxidative alkenylation of fused bicyclic heterocycles. Eur. J. Org. Chem. 2019, 2019, 2568–2586. [Google Scholar] [CrossRef]
- Grimster, N.P.; Gauntlett, C.; Godfrey, C.R.A.; Gaunt, M.J. Palladium-catalyzed intermolecular alkenylation of indoles by solvent-controlled regioselective C-H functionalization. Angew. Chem. Int. Ed. 2005, 44, 3125–3129. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-L.; Gao, Y.-R.; Mao, S.; Zhang, Y.-L.; Wang, Y.-F.; Wang, Y.-Q. Palladium-catalyzed intermolecular C3 alkenylation of indoles using oxygen as the oxidant. Org. Lett. 2012, 14, 5920–5923. [Google Scholar] [CrossRef]
- Steinhoff, B.A.; Fix, S.R.; Stahl, S.S. Mechanistic study of alcohol oxidation by the Pd (OAc)2/O2/DMSO catalyst system and implications for the development of improved aerobic oxidation catalysts. J. Am. Chem. Soc. 2002, 124, 766–767. [Google Scholar] [CrossRef]
- Huang, Q.; Song, Q.; Cai, J.; Zhang, X.; Lin, S. Palladium (II)/polyoxometalate-catalyzed direct C-3 alkenylation of indoles using dioxygen as the terminal oxidant. Adv. Synth. Catal. 2013, 355, 1512–1516. [Google Scholar] [CrossRef]
- An, Y.L.; Yang, Z.H.; Zhang, H.H.; Zhao, S.-Y. Palladium-catalyzed tandem regioselective oxidative coupling from indoles and maleimides: One-pot synthesis of indolopyrrolocarbazoles and related indolylmaleimides. Org. Lett. 2016, 18, 152–155. [Google Scholar] [CrossRef]
- Verma, A.K.; Danodia, A.K.; Saunthwal, R.K.; Patel, M.; Choudhary, D. Palladium-catalyzed triple successive C–H functionalization: Direct synthesis of functionalized carbazoles from indoles. Org. Lett. 2015, 17, 3658–3661. [Google Scholar] [CrossRef]
- Cirujano, F.G.; Leo, P.; Vercammen, J.; Smolders, S.; Orcajo, G.; De Vos, D.E. MOFs extend the lifetime of Pd(II) catalyst for room temperature alkenylation of enamine-like arenes. Adv. Synth. Catal. 2018, 360, 3872–3876. [Google Scholar] [CrossRef]
- Hermann, G.N.; Becker, P.; Bolm, C. Mechanochemical rhodium (III)-catalyzed C-H bond functionalization of acetanilides under solventless conditions in a ball mill. Angew. Chem. Int. Ed. 2015, 54, 7414–7417. [Google Scholar] [CrossRef]
- Mei, T.S.; Werner, E.W.; Burckle, A.J.; Sigman, M.S. Enantioselective redox-relay oxidative Heck arylations of acyclic alkenyl alcohols using boronic acids. J. Am. Chem. Soc. 2013, 135, 6830–6833. [Google Scholar] [CrossRef]
- Mei, T.-S.; Patel, H.H.; Sigman, M.S. Enantioselective construction of remote quaternary stereocentres. Nature 2014, 508, 340–344. [Google Scholar] [CrossRef]
- Zhang, C.; Santiago, C.B.; Crawford, J.M.; Sigman, M.S. Enantioselective dehydrogenative Heck arylations of trisubstituted alkenes with indoles to construct quaternary stereocenters. J. Am. Chem. Soc. 2015, 137, 15668–15671. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xue, D.; Lu, W.; Fan, X.; Wang, C.; Xiao, J. 3-Acylindoles via palladium-catalyzed regioselective arylation of electron-rich olefins with indoles. RSC Adv. 2013, 3, 11463–11466. [Google Scholar] [CrossRef]
- Daves, G.D., Jr.; Hallberg, A. 1.2-Additions to heteroatom-substituted olefins by organopalladium reagents. Chem. Rev. 1989, 89, 1433–1445. [Google Scholar] [CrossRef]
- Cabri, W.; Candiani, I. Recent developments and new perspectives in the Heck reaction. Acc. Chem. Res. 1995, 28, 2–7. [Google Scholar] [CrossRef]
- Mo, J.; Xu, L.; Xiao, J. Ionic liquid-promoted, highly regioselective Heck arylation of electron-rich olefins by aryl halides. J. Am. Chem. Soc. 2005, 127, 751–760. [Google Scholar] [CrossRef]
- Guo, T.L.; Jiang, Q.B.; Huang, F.; Chen, J.P.; Yu, Z.K. Palladium-catalyzed, copper-mediated construction of benzene rings from the reactions of indoles with in situ generated enones. Org. Chem. Front. 2014, 1, 707–711. [Google Scholar] [CrossRef]
- Takeda, D.; Hirano, K.; Satoh, T.; Miura, M. Palladium-catalyzed direct arylation and alkenylation of 3-(indol-3-yl)propionic acids through C–H bond cleavage. Heterocycles 2014, 88, 275–286. [Google Scholar]
- Terrey, M.J.; Holmes, A.; Perry, C.C.; Cross, W.B. C–H olefination of tryptophan residues in peptides: Control of residue selectivity and peptide–amino acid cross-linking. Org. Lett. 2019, 21, 7902–7907. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhou, Z.-X.; Kurmoo, M.; Liu, Y.-J.; Zeng, M.-H. Carboxylate-assisted Pd (II)-catalyzed ortho-C–H and remote C–H activation: Economical synthesis of pyrano [4,3-b]indol-1(5H)-ones. Org. Lett. 2019, 21, 2847–2850. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.-P.; Tang, S.-B.; Yang, J.-Y.; Zhang, L.-L.; Xia, C.-C.; Ji, Y.-F. Cascade reaction for the synthesis of carbolines from O-methylketoximes and styrenes via palladium-catalyzed C–H activation and sequential annulation. Eur. J. Org. Chem. 2019, 5974–5977. [Google Scholar] [CrossRef]
- Albarghouti, G.; Kotikalapudi, R.; Lankri, D.; Valerio, V.; Tsvelikhovsky, D. Cascade Pd (II)-catalyzed Wacker lactonization-Heck reaction: Rapid assembly of spiranoid lactones. Chem. Commun. 2016, 52, 3095–3098. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.-L.; Chen, W.-L.; Gao, Y.-R.; Mao, S.; Zhang, Y.-L.; Wang, Y.-Q. Palladium-catalyzed intermolecular C-2 alkenylation of indoles using oxygen as the oxidant. Adv. Synth. Catal. 2014, 356, 1085–1092. [Google Scholar] [CrossRef]
- Saunthwal, R.K.; Patel, M.; Kumar, S.; Danodia, A.K.; Verma, A.K. Pd (II)-catalyzed C-H activation of styrylindoles: Short, efficient, and regioselective synthesis of functionalized carbazoles. Chem. Eur. J. 2015, 21, 18601–18605. [Google Scholar] [CrossRef]
- Ozaki, K.; Zhang, H.; Ito, H.; Lei, A.; Itami, K. One-shot indole-to-carbazole π-extension by a Pd–Cu–Ag trimetallic system. Chem. Sci. 2013, 4, 3416–3420. [Google Scholar] [CrossRef]
- Laha, J.K.; Dayal, N. A tandem approach to functionalized carbazoles from indoles via two successive regioselective oxidative Heck reactions followed by thermal electrocyclization. Org. Lett. 2015, 17, 4742–4745. [Google Scholar] [CrossRef]
- Kannaboina, P.; Kumar, K.A.; Das, P. Site-selective intermolecular oxidative C-3 alkenylation of 7-azaindoles at room temperature. Org. Lett. 2016, 18, 900–903. [Google Scholar] [CrossRef]
- Huang, Y.; Song, F.; Wang, Z.; Xi, P.; Wu, N.; Wang, Z.; Lan, J.; You, J. Dehydrogenative Heck coupling of biologically relevant N-heteroarenes with alkenes: Discovery of fluorescent core frameworks. Chem. Commun. 2012, 48, 2864–2866. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Shi, F.; Ji, M.; Kan, Y.; Hu, H. Palladium catalyzed C−H olefination of indolizines at the 1-position with molecular oxygen as the terminal oxidant. Asian J. Chem. 2019, 8, 1555–1560. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, L.; Zhang, Z.; Zhang, Y. Palladium-catalyzed oxidative C-H bond and C=C double bond cleavage: C-3 acylation of indolizines with ±, β-unsaturated carboxylic acids. Org. Lett. 2011, 13, 1342–1345. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-Y.; Sahoo, S.K.; Huang, C.L.; Chan, T.-H.; Cheng, Y.-J. Pd (II)-catalyzed direct dehydrogenative mono- and diolefination of selenophenes. Org. Lett. 2020, 22, 2318–2322. [Google Scholar] [CrossRef]
- Li, Z.; Ma, L.; Tang, C.; Xu, J.; Wu, X.; Yao, H. Palladium (II)-catalyzed oxidative Heck coupling of thiazole-4-carboxylates. Tetrahedron Lett. 2011, 52, 5643–5647. [Google Scholar] [CrossRef]
- Liu, W.; Yu, X.; Kuang, C. Palladium-catalyzed C-2 selective olefination of thiazoles. Org. Lett. 2014, 16, 1798–1801. [Google Scholar] [CrossRef]
- Achar, T.K.; Biswas, J.P.; Porey, S.; Pal, T.; Ramakrishna, K.; Maiti, S.; Maiti, D. Palladium-catalyzed template directed C-5 selective olefination of thiazoles. J. Org. Chem. 2019, 84, 8315–8321. [Google Scholar] [CrossRef]
- Chappell, B.; Dedman, N.; Wheeler, S. Studies on the palladium-catalysed direct alkenylation of 1,2-azoles. Tetrahedron Lett. 2011, 52, 3223–3225. [Google Scholar] [CrossRef]
- Lee, W.-C.; Wang, T.-H.; Ong, T.-G. Ligand promoted Pd-catalyzed dehydrogenative alkenylation of hetereoarenes. Chem. Commun. 2014, 50, 3671–3673. [Google Scholar] [CrossRef]
- Yi, C.; Hua, R. An efficient palladium-catalyzed Heck coupling of aryl chlorides with alkenes. Tetrahedron Lett. 2006, 47, 2573–2576. [Google Scholar] [CrossRef]
- Ruan, J.; Xiao, J. From α-arylation of olefins to acylation with aldehydes: A journey in regiocontrol of the Heck reaction. Acc. Chem. Res. 2011, 44, 614–626. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.H.; Sigman, M.S. Mechanistic approaches to palladium-catalyzed alkene difunctionalization reactions. Org. Biomol. Chem. 2008, 6, 4083–4088. [Google Scholar] [CrossRef] [PubMed]
- Kocovsky, P.; Bäckvall, J.-E. The syn/anti-dichotomy in the palladium-catalyzed addition of nucleophiles to alkenes. Chem. Eur. J. 2015, 21, 36–56. [Google Scholar] [CrossRef] [PubMed]
- White, D.R.; Bornowski, E.C.; Wolfe, J.P. Pd-catalyzed C-C, C-N, and C-O bond-forming difunctionalization reactions of alkenes bearing tethered aryl/alkenyl triflates. Isr. J. Chem. 2020, 60, 259–267. [Google Scholar] [CrossRef]
- Liu, W.; Wang, S.; Zhan, H.; Lin, J.; He, P.; Jiang, Y. Highly regioselective palladium-catalyzed direct alkenylation of thiazolo[3,2-b]-1,2,4-triazoles via C-H activation. Tetrahedron Lett. 2014, 55, 3549–3552. [Google Scholar] [CrossRef]
- Huang, G.; Teng, M.; Liu, B.; Rong, M.; Liu, Y.; Chen, Y. Palladium-catalyzed site-selective C–H alkenylation of imidazo [2,1-b]thiazoles. J. Organomet. Chem. 2016, 818, 163–167. [Google Scholar] [CrossRef]
- Cui, S.; Wojtas, L.; Antilla, J.C. Pd-catalyzed C4-olefination of oxazoles via C–H bond activation: Divergent synthesis of functionalized amino alcohol and amino acid derivatives. Org. Lett. 2011, 13, 5040–5043. [Google Scholar] [CrossRef]
- Lu, Z.; Luo, F.; Wang, L.; Zhu, G. Palladium-catalyzed direct alkenylation of 2-oxazolones: An entry to 3,4,5-trisubstituted 2-oxazolones. J. Org. Chem. 2013, 78, 10894–10901. [Google Scholar] [CrossRef]
- Wang, X.; Fang, X.; Xiao, H.; Gong, D.; Yang, X.; Wu, F. A new and direct route to 3-fluoromethyl substituted pyrazol-4-acrylates via Pd-catalyzed C–H activation. Tetrahedron 2013, 69, 6993–7000. [Google Scholar] [CrossRef]
- Han, S.J.; Kim, H.T.; Joo, J.M. Direct C–H alkenylation of functionalized pyrazoles. J. Org. Chem. 2016, 81, 689–698. [Google Scholar] [CrossRef]
- Kim, H.T.; Ha, H.; Kang, G.; Kim, O.S.; Ryu, H.; Biswas, A.K.; Lim, S.M.; Baik, M.-H.; Joo, J.M. Ligand-controlled regiodivergent C−H alkenylation of pyrazoles and its application to the synthesis of indazoles. Angew. Chem. Int. Ed. 2017, 56, 16262–16266. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Gong, H.; Kuang, C. Room-temperature direct alkenylation of 5-pyrazolones. Eur. J. Org. Chem. 2013, 2013, 5276–5281. [Google Scholar] [CrossRef]
- Naas, M.; El Kazzouli, S.; Essassi, E.M.; Bousmina, M.; Guillaumet, G. Palladium-catalyzed oxidative direct C3- and C7-alkenylations of indazoles: Application to the synthesis of gamendazol. Org. Lett. 2015, 17, 4320–4323. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Yang, X.; Wu, C.; Su, W. Palladium-catalyzed C-H/C-H cross-coupling by mechanochemistry: Direct alkenylation and heteroarylation of N1-protected 1H-indazoles. J. Org. Chem. 2020, 85, 1009–1021. [Google Scholar] [CrossRef] [PubMed]
- Tashrifi, Z.; Mohammadi-Khanaposhtani, M.; Larijani, B.; Mahdavi, M. C3-Functionalization of imidazo [1,2-a]pyridines. Eur. J. Org. Chem. 2020, 2020, 269–284. [Google Scholar] [CrossRef]
- Cao, H.; Lei, S.; Liao, J.; Huang, J.; Qiu, H.; Chen, Q.; Qiu, S.; Chen, Y. Palladium (II)-catalyzed intermolecular oxidative C-3 alkenylations of imidazo[1,2-a]pyridines by substrate-controlled regioselective C–H functionalization. RSC Adv. 2014, 4, 50137–50140. [Google Scholar] [CrossRef]
- Ghosh, M.; Naskar, A.; Mitra, S.; Hajra, A. Palladium-catalyzed α-selective alkenylation of imidazo [1,2-a]pyridines through aerobic cross-dehydrogenative coupling reaction. Eur. J. Org. Chem. 2015, 2015, 715–718. [Google Scholar] [CrossRef]
- Salvanna, N.; Reddy, P.R.; Das, B. Palladium-catalyzed cross-coupling of 1,3,4-oxadiazoles and styrenes: An efficient method to synthesize 2-alkenyl-1,3,4-oxadiazoles. Synlett 2018, 29, 71–74. [Google Scholar] [CrossRef]
- Yang, Y.; Kuang, C. Room-temperature direct alkenylation of 3-arylsydnones. Eur. J. Org. Chem. 2014, 2014, 7810–7813. [Google Scholar] [CrossRef]
- Heck, R.F. The palladium-catalyzed arylation of enol esters, ethers, and halides. A new synthesis of 2-aryl aldehydes and ketones. J. Am. Chem. Soc. 1968, 90, 5535–5542. [Google Scholar] [CrossRef]
- Kasahara, A.; Izumi, T.; Fukuda, N. The palladium-catalyzed phenylation of enol esters with iodobenzene. Bull. Chem. Soc. Jpn. 1977, 50, 551–552. [Google Scholar] [CrossRef]
- Tang, J.; Cong, M.; Xia, Y.; Quéléver, G.; Fan, Y.; Qu, F.; Peng, L. Pd-catalyzed oxidative C–H alkenylation for synthesizing arylvinyltriazole nucleosides. Org. Biomol. Chem. 2015, 13, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, Y.; Xu, B.; Kuang, C. Palladium-catalyzed olefination and arylation of 2-substituted 1,2,3-triazole N-oxides. Org. Lett. 2013, 15, 2342–2345. [Google Scholar] [CrossRef] [PubMed]
- Trost, B.M. Atom economy–a challenge for organic synthesis: Homogeneous catalysis leads the way. Angew. Chem. Int. Ed. Engl. 1995, 34, 259–281. [Google Scholar] [CrossRef]



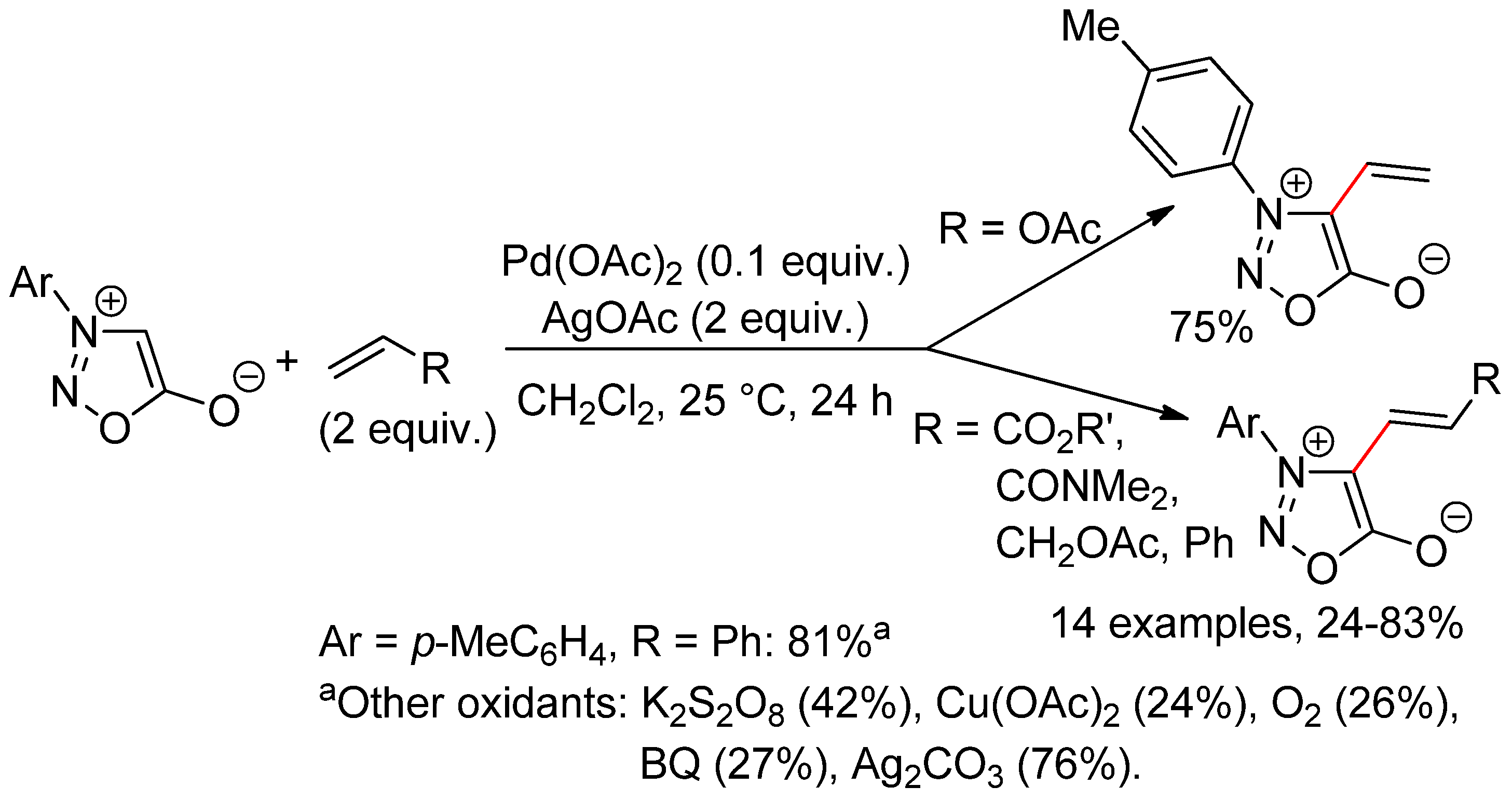

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le Bras, J.; Muzart, J. Pd-Catalyzed Intermolecular Dehydrogenative Heck Reactions of Five-Membered Heteroarenes. Catalysts 2020, 10, 571. https://doi.org/10.3390/catal10050571
Le Bras J, Muzart J. Pd-Catalyzed Intermolecular Dehydrogenative Heck Reactions of Five-Membered Heteroarenes. Catalysts. 2020; 10(5):571. https://doi.org/10.3390/catal10050571
Chicago/Turabian StyleLe Bras, Jean, and Jacques Muzart. 2020. "Pd-Catalyzed Intermolecular Dehydrogenative Heck Reactions of Five-Membered Heteroarenes" Catalysts 10, no. 5: 571. https://doi.org/10.3390/catal10050571
APA StyleLe Bras, J., & Muzart, J. (2020). Pd-Catalyzed Intermolecular Dehydrogenative Heck Reactions of Five-Membered Heteroarenes. Catalysts, 10(5), 571. https://doi.org/10.3390/catal10050571