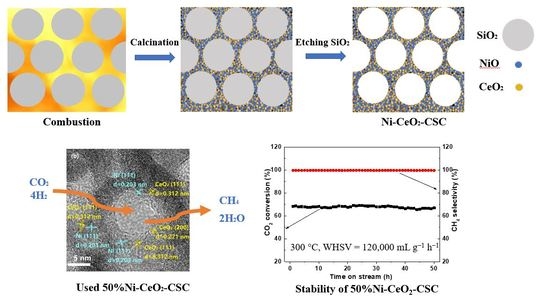
Three-Dimensional Mesoporous Ni-CeO2 Catalysts with Ni Embedded in the Pore Walls for CO2 Methanation
Abstract
1. Introduction
2. Results and Discussion
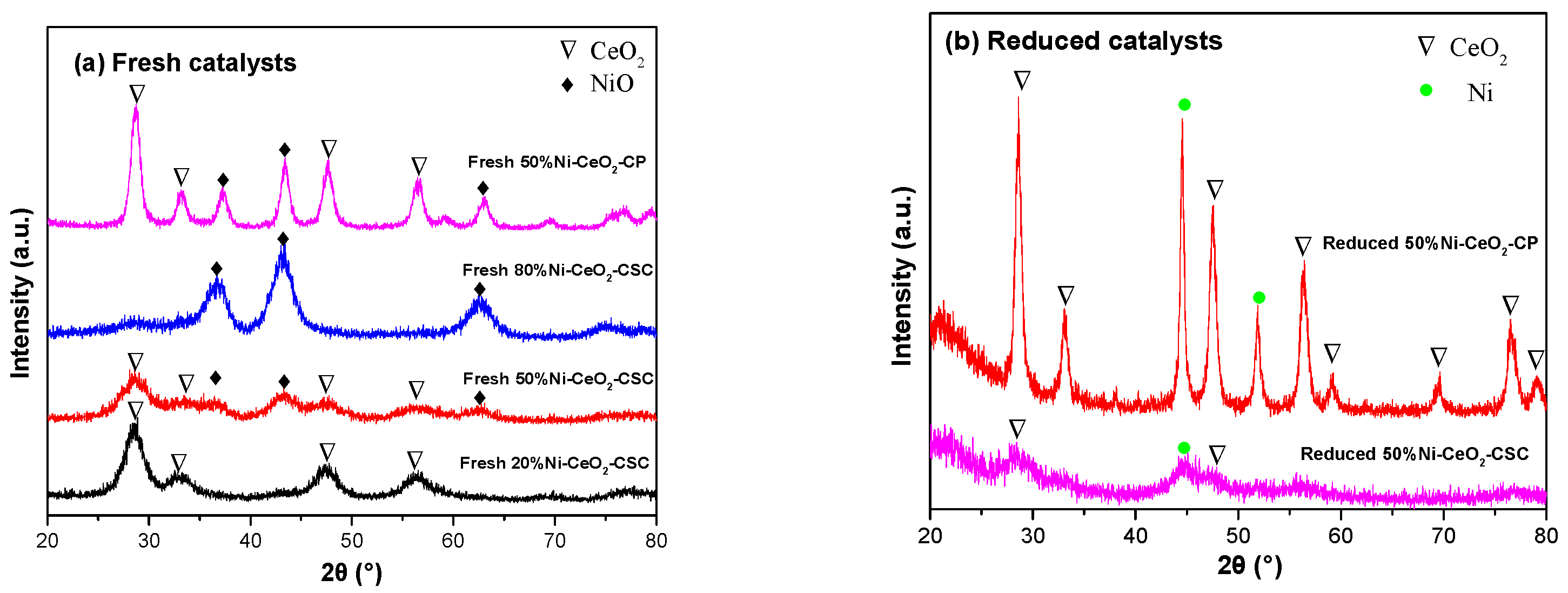
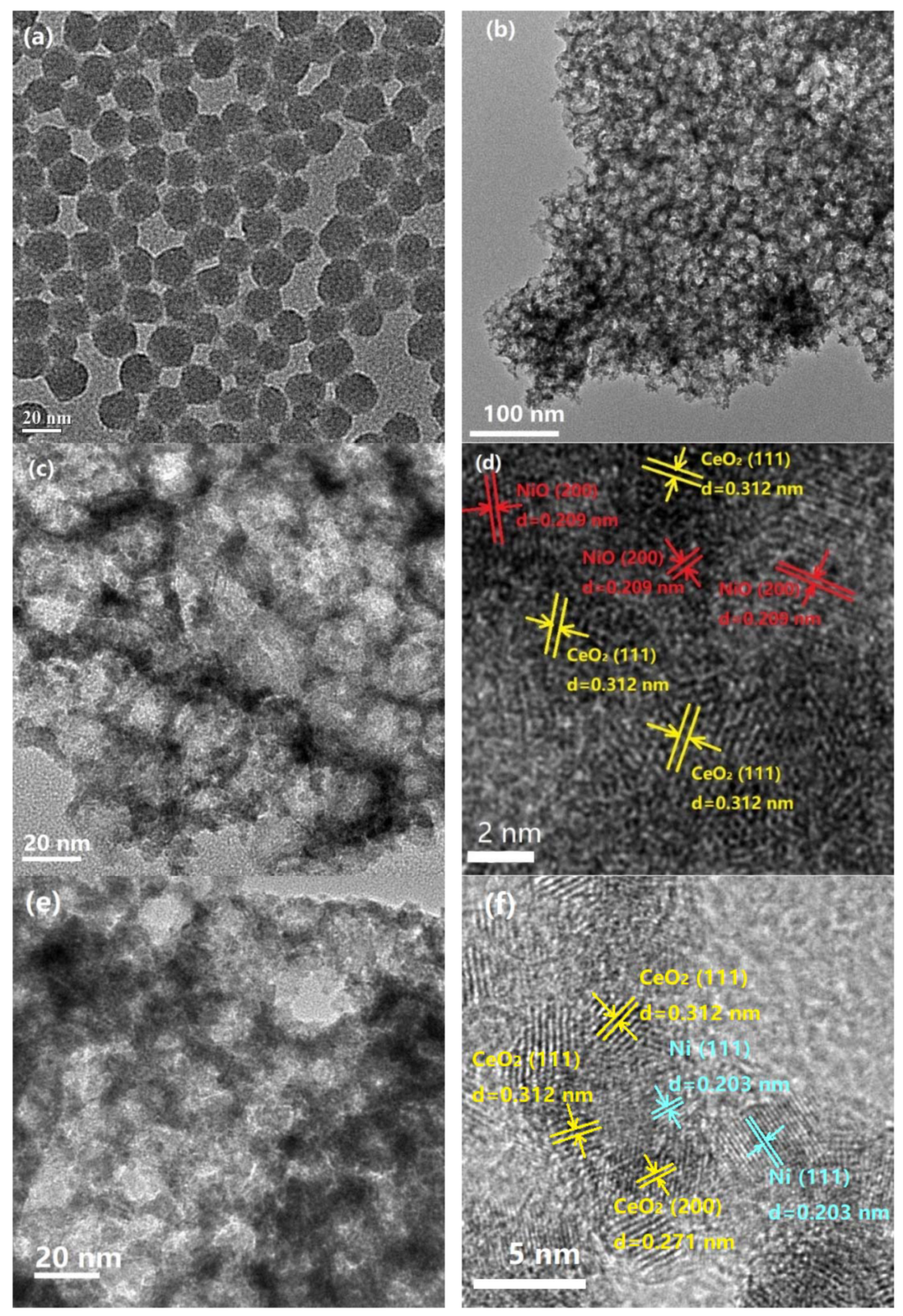
2.1. Characterization of Fresh and Reduced Catalysts
2.2. Catalytic Performance
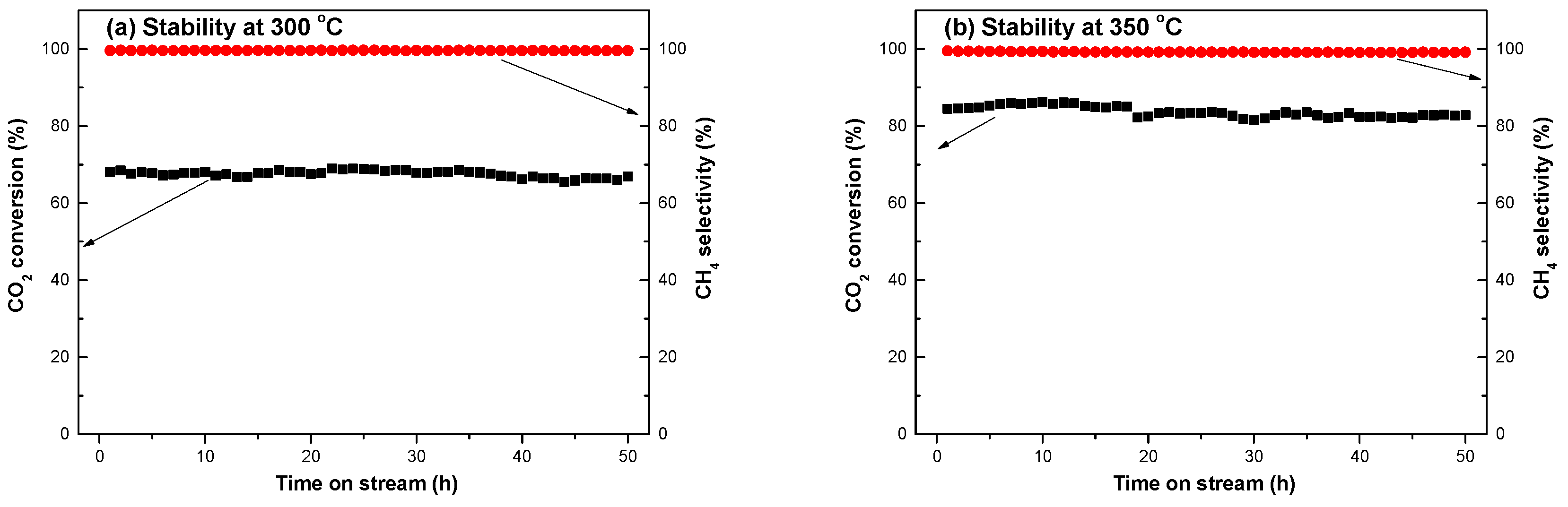
2.3. Characterization of the Used Catalyst
3. Experimental
3.1. Synthesis of Catalysts
3.2. Characterization of Catalysts
3.3. Catalytic Performance
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ma, J.; Sun, N.; Zhang, X.; Zhao, N.; Xiao, F.; Wei, W.; Sun, Y. A short review of catalysis for CO2 conversion. Catal. Today 2009, 148, 221–231. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, W.; Li, Y.; Chen, J.; Yu, B.; Wang, J.; Zhang, L.; Zhang, J. Energy related CO2 conversion and utilization: Advanced materials/nanomaterials, reaction mechanisms and technologies. Nano Energy 2017, 40, 512–539. [Google Scholar] [CrossRef]
- Frontera, P.; Macario, A.; Ferraro, M.; Antonucci, P. Supported Catalysts for CO2 Methanation: A Review. Catalysts 2017, 7, 59. [Google Scholar] [CrossRef]
- Lee, W.J.; Li, C.; Prajitno, H.; Yoo, J.; Patel, J.; Yang, Y.; Lim, S. Recent trend in thermal catalytic low temperature CO2 methanation: A critical review. Catal. Today 2020, in press. [Google Scholar] [CrossRef]
- Martin, N.M.; Hemmingsson, F.; Schaefer, A.; Ek, M.; Merte, L.R.; Hejral, U.; Gustafson, J.; Skoglundh, M.; Dippel, A.-C.; Gutowski, O.; et al. Structure–function relationship for CO2 methanation over ceria supported Rh and Ni catalysts under atmospheric pressure conditions. Catal. Sci. Technol. 2019, 9, 1644–1653. [Google Scholar] [CrossRef]
- Xu, J.; Lin, Q.; Su, X.; Duan, H.; Geng, H.; Huang, Y. CO2 methanation over TiO2–Al2O3 binary oxides supported Ru catalysts. Chin. J. Chem. Eng. 2016, 24, 140–145. [Google Scholar] [CrossRef]
- Kim, A.; Debecker, D.P.; Devred, F.; Dubois, V.; Sanchez, C.; Sassoye, C. CO2 methanation on Ru/TiO2 catalysts: On the effect of mixing anatase and rutile TiO2 supports. Appl. Catal. B Environ. 2018, 220, 615–625. [Google Scholar] [CrossRef]
- Jiang, H.; Gao, Q.; Wang, S.; Chen, Y.; Zhang, M. The synergistic effect of Pd NPs and UiO-66 for enhanced activity of carbon dioxide methanation. J. CO2 Util. 2019, 31, 167–172. [Google Scholar] [CrossRef]
- Jia, X.; Zhang, X.; Rui, N.; Hu, X.; Liu, C.-J. Structural effect of Ni/ZrO2 catalyst on CO2 methanation with enhanced activity. Appl. Catal. B Environ. 2019, 244, 159–169. [Google Scholar] [CrossRef]
- Vrijburg, W.L.; Garbarino, G.; Chen, W.; Parastaev, A.; Longo, A.; Pidko, E.A.; Hensen, E.J.M. Ni-Mn catalysts on silica-modified alumina for CO2 methanation. J. Catal. 2020, 382, 358–371. [Google Scholar] [CrossRef]
- Tan, J.; Wang, J.; Zhang, Z.; Ma, Z.; Wang, L.; Liu, Y. Highly dispersed and stable Ni nanoparticles confined by MgO on ZrO2 for CO2 methanation. Appl. Surf. Sci. 2019, 481, 1538–1548. [Google Scholar] [CrossRef]
- Tang, G.; Gong, D.; Liu, H.; Wang, L. Highly Loaded Mesoporous Ni–La2O3 Catalyst Prepared by Colloidal Solution Combustion Method for CO2 Methanation. Catalysts 2019, 9, 442. [Google Scholar] [CrossRef]
- Millet, M.-M.; Tarasov, A.V.; Girgsdies, F.; Algara-Siller, G.; Schlögl, R.; Frei, E. Highly Dispersed Ni0/NixMg1–xO Catalysts Derived from Solid Solutions: How Metal and Support Control the CO2 Hydrogenation. ACS Catal. 2019, 9, 8534–8546. [Google Scholar] [CrossRef]
- Zeng, L.; Wang, Y.; Li, Z.; Song, Y.; Zhang, J.; Wang, J.; He, X.; Wang, C.; Lin, W. Highly Dispersed Ni Catalyst on Metal–Organic Framework-Derived Porous Hydrous Zirconia for CO2 Methanation. ACS Appl. Mater. Interfaces 2020. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, S.N.; Chong, C.C.; Teh, L.P.; Vo, D.-V.N.; Ainirazali, N.; Triwahyono, S.; Jalil, A.A.; Setiabudi, H.D. Promising hydrothermal technique for efficient CO2 methanation over Ni/SBA. Int. J. Hydrog. Energy 2019, 44, 20792–20804. [Google Scholar] [CrossRef]
- Bacariza, M.C.; Graça, I.; Bebiano, S.S.; Lopes, J.M.; Henriques, C. Micro- and mesoporous supports for CO2 methanation catalysts: A comparison between SBA-15, MCM-41 and USY zeolite. Chem. Eng. Sci. 2018, 175, 72–83. [Google Scholar] [CrossRef]
- Guo, X.; Traitangwong, A.; Hu, M.; Zuo, C.; Meeyoo, V.; Peng, Z.; Li, C. Carbon Dioxide Methanation over Nickel-Based Catalysts Supported on Various Mesoporous Material. Energy Fuels 2018, 32, 3681–3689. [Google Scholar] [CrossRef]
- Guo, M.; Lu, G. The effect of impregnation strategy on structural characters and CO2 methanation properties over MgO modified Ni/SiO2 catalysts. Catal. Commun. 2014, 54, 55–60. [Google Scholar] [CrossRef]
- Chen, Y.; Qiu, B.; Liu, Y.; Zhang, Y. An active and stable nickel-based catalyst with embedment structure for CO2 methanation. Appl. Catal. B Environ. 2020, 269, 118801. [Google Scholar] [CrossRef]
- Zhen, W.; Li, B.; Lu, G.; Ma, J. Enhancing catalytic activity and stability for CO2 methanation on Ni@MOF-5 via control of active species dispersion. Chem. Commun. 2015, 51, 1728–1731. [Google Scholar] [CrossRef]
- Mihet, M.; Grad, O.; Blanita, G.; Radu, T.; Lazar, M.D. Effective encapsulation of Ni nanoparticles in metal-organic frameworks and their application for CO2 methanation. Int. J. Hydrog. Energy 2019, 44, 13383–13396. [Google Scholar] [CrossRef]
- Lin, X.; Wang, S.; Tu, W.; Hu, Z.; Ding, Z.; Hou, Y.; Xu, R.; Dai, W. MOF-derived hierarchical hollow spheres composed of carbon-confined Ni nanoparticles for efficient CO2 methanation. Catal. Sci. Technol. 2019, 9, 731–738. [Google Scholar] [CrossRef]
- De Rogatis, L.; Cargnello, M.; Gombac, V.; Lorenzut, B.; Montini, T.; Fornasiero, P. Embedded Phases: A Way to Active and Stable Catalysts. ChemSusChem 2010, 3, 24–42. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, H.; Zhao, G. Iron-copper bimetallic nanoparticles embedded within ordered mesoporous carbon as effective and stable heterogeneous Fenton catalyst for the degradation of organic contaminants. Appl. Catal. B Environ. 2015, 164, 396–406. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L.; Peng, H.; You, X.; Peng, C.; Xu, X.; Liu, W.; Fang, X.; Wang, Z.; Zhang, N.; et al. Nickel nanoparticles embedded in mesopores of AlSBA-15 with a perfect peasecod-like structure: A catalyst with superior sintering resistance and hydrothermal stability for methane dry reforming. Appl. Catal. B Environ. 2018, 224, 488–499. [Google Scholar] [CrossRef]
- Liu, Q.; Tian, Y. One-pot synthesis of NiO/SBA-15 monolith catalyst with a three-dimensional framework for CO2 methanation. Int. J. Hydrog. Energy 2017, 42, 12295–12300. [Google Scholar] [CrossRef]
- Yu, Y.; Cao, C.Y.; Chen, Z.; Liu, H.; Li, P.; Dou, Z.F.; Song, W.G. Au nanoparticles embedded into the inner wall of TiO2 hollow spheres as a nanoreactor with superb thermal stability. Chem. Commun. 2013, 49, 3116–3118. [Google Scholar] [CrossRef]
- Li, M.; Amari, H.; van Veen, A.C. Metal-oxide interaction enhanced CO2 activation in methanation over ceria supported nickel nanocrystallites. Appl. Catal. B Environ. 2018, 239, 27–35. [Google Scholar] [CrossRef]
- Atzori, L.; Cutrufello, M.G.; Meloni, D.; Monaci, R.; Cannas, C.; Gazzoli, D.; Sini, M.F.; Deiana, P.; Rombi, E. CO2 methanation on hard-templated NiO—CeO2 mixed oxides. Int. J. Hydrog. Energy 2017, 42, 20689–20702. [Google Scholar] [CrossRef]
- Zhou, G.; Liu, H.; Cui, K.; Xie, H.; Jiao, Z.; Zhang, G.; Xiong, K.; Zheng, X. Methanation of carbon dioxide over Ni/CeO2 catalysts: Effects of support CeO2 structure. Int. J. Hydrog. Energy 2017, 42, 16108–16117. [Google Scholar] [CrossRef]
- Bian, Z.; Chan, Y.M.; Yu, Y.; Kawi, S. Morphology dependence of catalytic properties of Ni/CeO2 for CO2 methanation: A kinetic and mechanism study. Catal. Today 2018. [Google Scholar] [CrossRef]
- Ye, R.-P.; Li, Q.; Gong, W.; Wang, T.; Razink, J.J.; Lin, L.; Qin, Y.-Y.; Zhou, Z.; Adidharma, H.; Tang, J.; et al. High-performance of nanostructured Ni/CeO2 catalyst on CO2 methanation. Appl. Catal. B Environ. 2020, 268, 118474. [Google Scholar] [CrossRef]
- Voskanyan, A.A.; Chan, K.-Y.; Li, C.-Y.V. Colloidal Solution Combustion Synthesis: Toward Mass Production of a Crystalline Uniform Mesoporous CeO2 Catalyst with Tunable Porosity. Chem. Mater. 2016, 28, 2768–2775. [Google Scholar] [CrossRef]
- Voskanyan, A.A.; Ho, C.-K.; Chan, K.Y. 3D δ-MnO2 nanostructure with ultralarge mesopores as high-performance lithium-ion battery anode fabricated via colloidal solution combustion synthesis. J. Power Sources 2019, 421, 162–168. [Google Scholar] [CrossRef]
- Wang, L.; Liu, H. Mesoporous Co-CeO2 catalyst prepared by colloidal solution combustion method for reverse water-gas shift reaction. Catal. Today 2018, 316, 155–161. [Google Scholar] [CrossRef]
- Shan, W.; Luo, M.; Ying, P.; Shen, W.; Li, C. Reduction property and catalytic activity of Ce1−XNiXO2 mixed oxide catalysts for CH4 oxidation. Appl. Catal. A Gen. 2003, 246, 1–9. [Google Scholar] [CrossRef]
- Das, S.; Ashok, J.; Bian, Z.; Dewangan, N.; Wai, M.H.; Du, Y.; Borgna, A.; Hidajat, K.; Kawi, S. Silica–Ceria sandwiched Ni core–shell catalyst for low temperature dry reforming of biogas: Coke resistance and mechanistic insights. Appl. Catal. B Environ. 2018, 230, 220–236. [Google Scholar] [CrossRef]
- Li, S.; Liu, G.; Zhang, S.; An, K.; Ma, Z.; Wang, L.; Liu, Y. Cerium-modified Ni-La2O3/ZrO2 for CO2 methanation. J. Energy Chem. 2020, 43, 155–164. [Google Scholar] [CrossRef]
- Xu, L.; Wang, F.; Chen, M.; Nie, D.; Lian, X.; Lu, Z.; Chen, H.; Zhang, K.; Ge, P. CO2 methanation over rare earth doped Ni based mesoporous catalysts with intensified low-temperature activity. Int. J. Hydrog. Energy 2017, 42, 15523–15539. [Google Scholar] [CrossRef]
- Hu, F.; Tong, S.; Lu, K.; Chen, C.-M.; Su, F.-Y.; Zhou, J.; Lu, Z.-H.; Wang, X.; Feng, G.; Zhang, R. Reduced graphene oxide supported Ni-Ce catalysts for CO2 methanation: The support and ceria promotion effects. J. CO2 Util. 2019, 34, 676–687. [Google Scholar] [CrossRef]
- Daroughegi, R.; Meshkani, F.; Rezaei, M. Enhanced activity of CO2 methanation over mesoporous nanocrystalline Ni–Al2O3 catalysts prepared by ultrasound-assisted co-precipitation method. Int. J. Hydrog. Energy 2017, 42, 15115–15125. [Google Scholar] [CrossRef]
- Zhao, K.; Wang, W.; Li, Z. Highly efficient Ni/ZrO2 catalysts prepared via combustion method for CO2 methanation. J. CO2 Util. 2016, 16, 236–244. [Google Scholar] [CrossRef]
- Ashok, J.; Ang, M.L.; Kawi, S. Enhanced activity of CO2 methanation over Ni/CeO2-ZrO2 catalysts: Influence of preparation methods. Catal. Today 2017, 281, 304–311. [Google Scholar] [CrossRef]
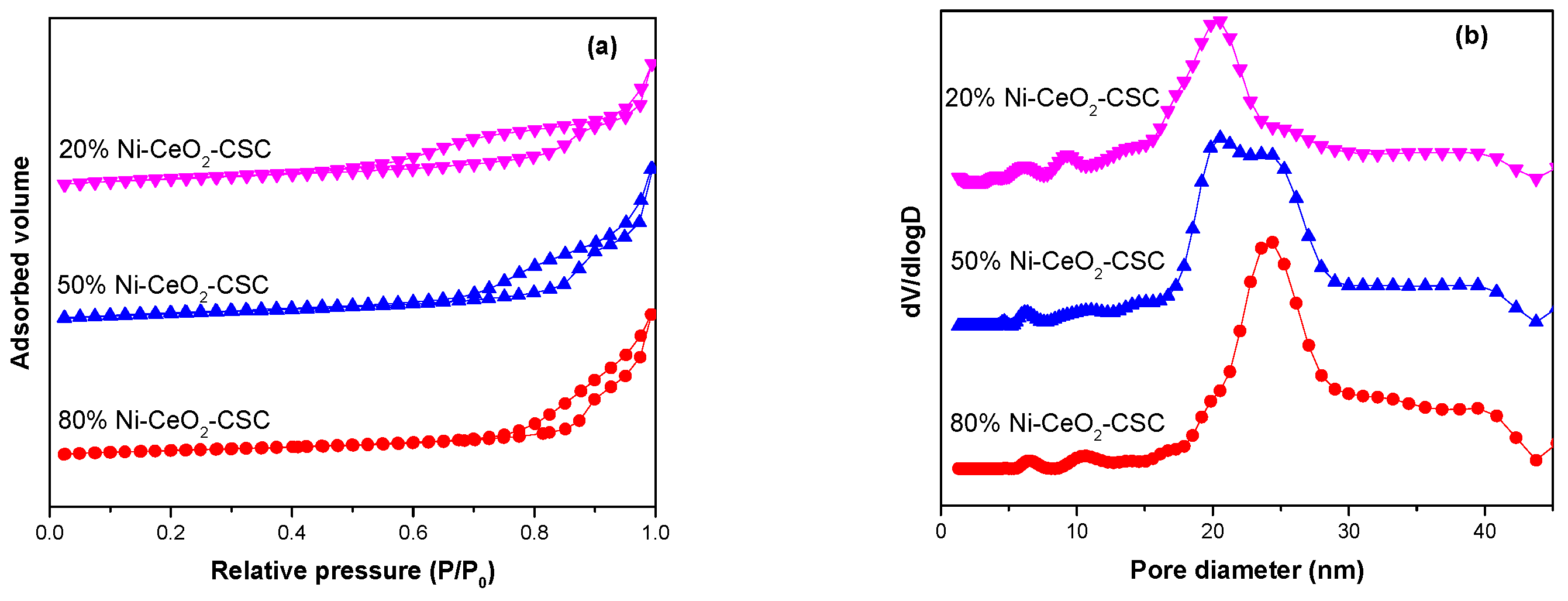
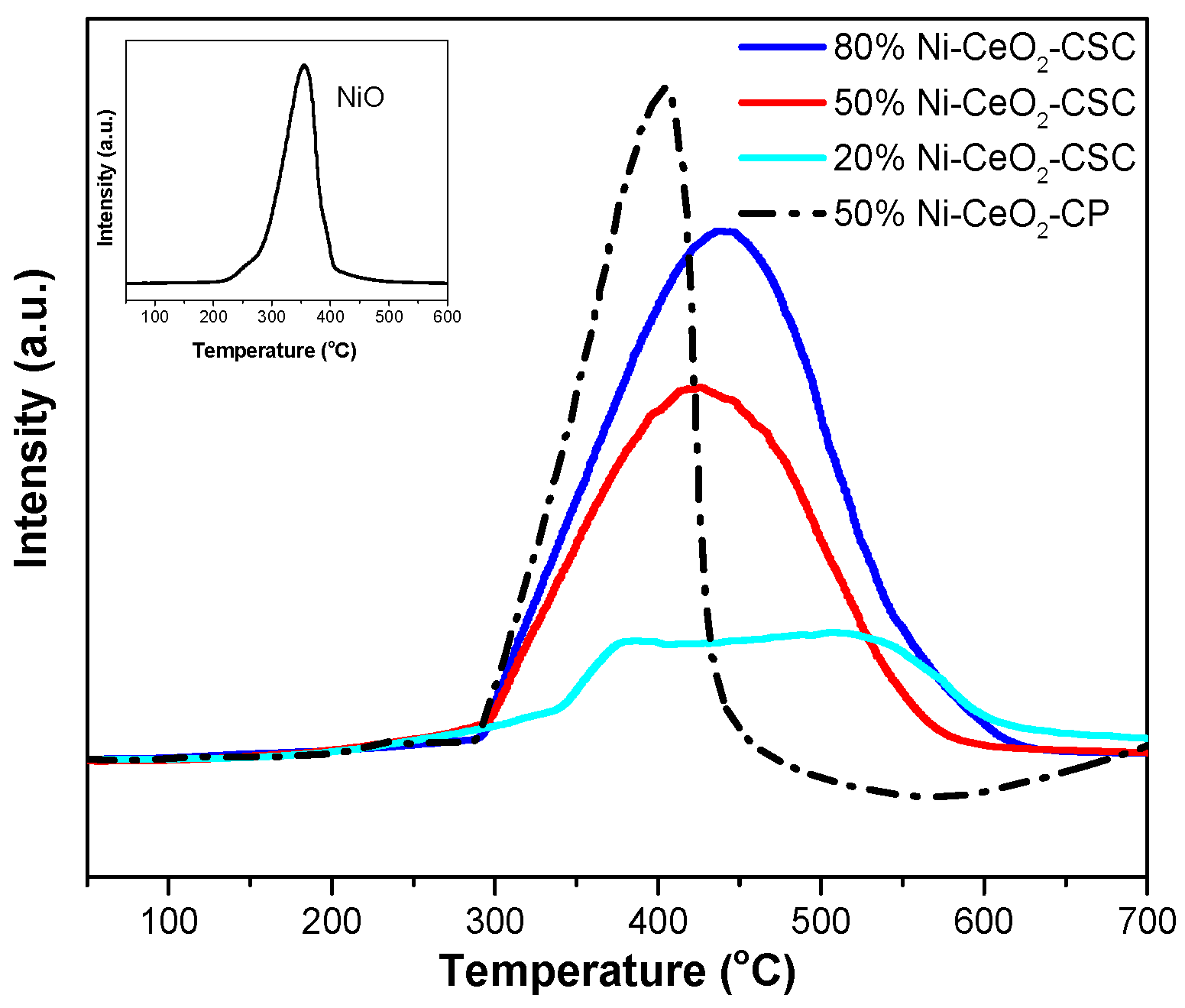
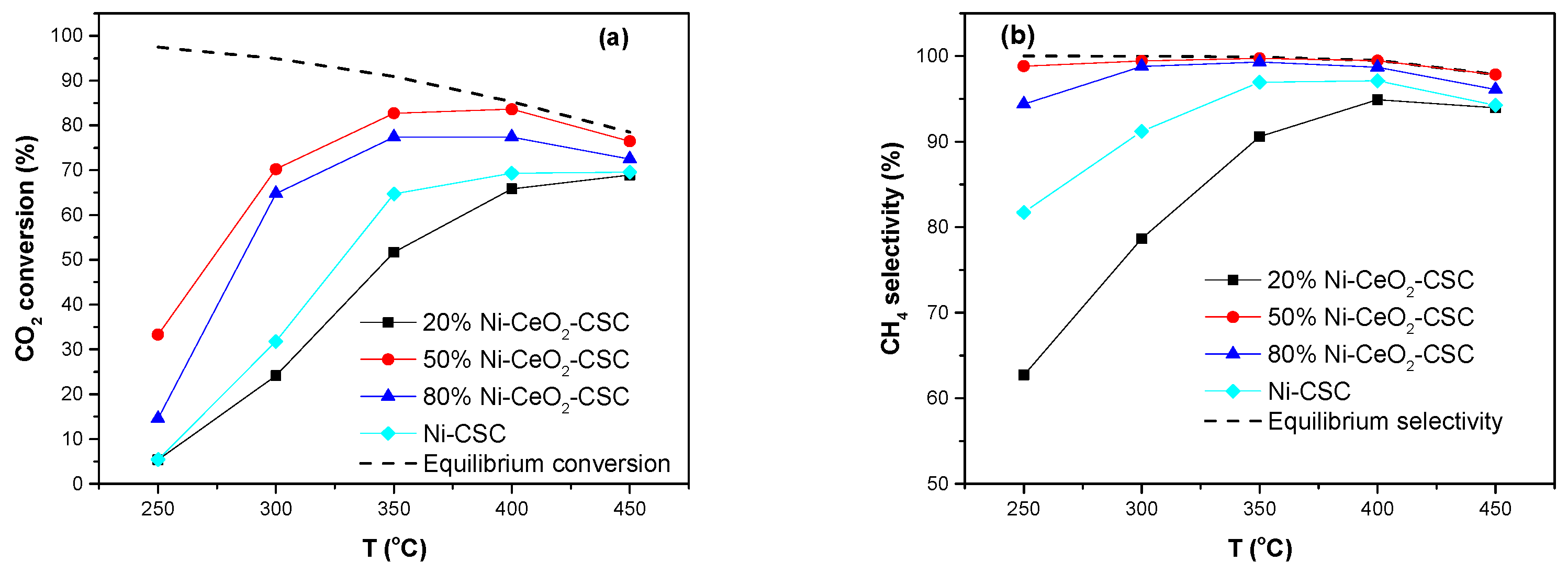
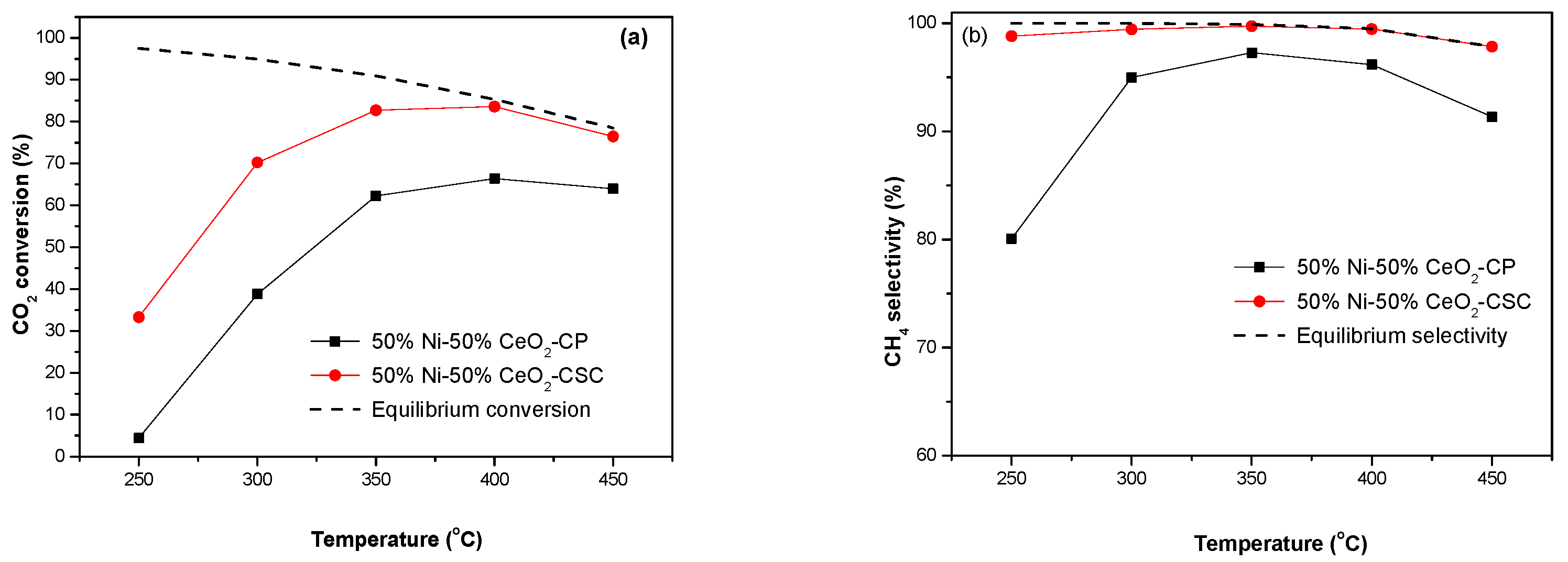
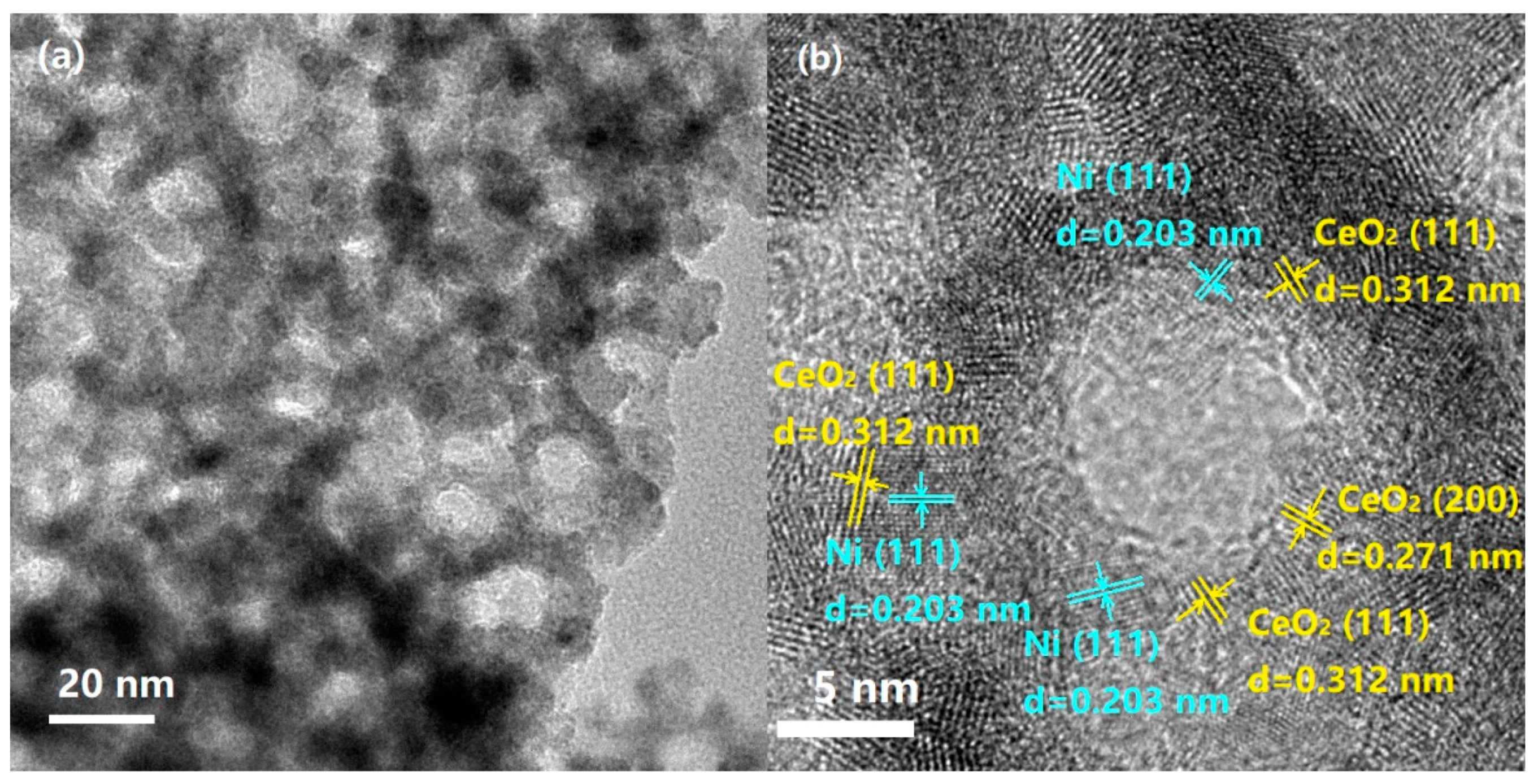
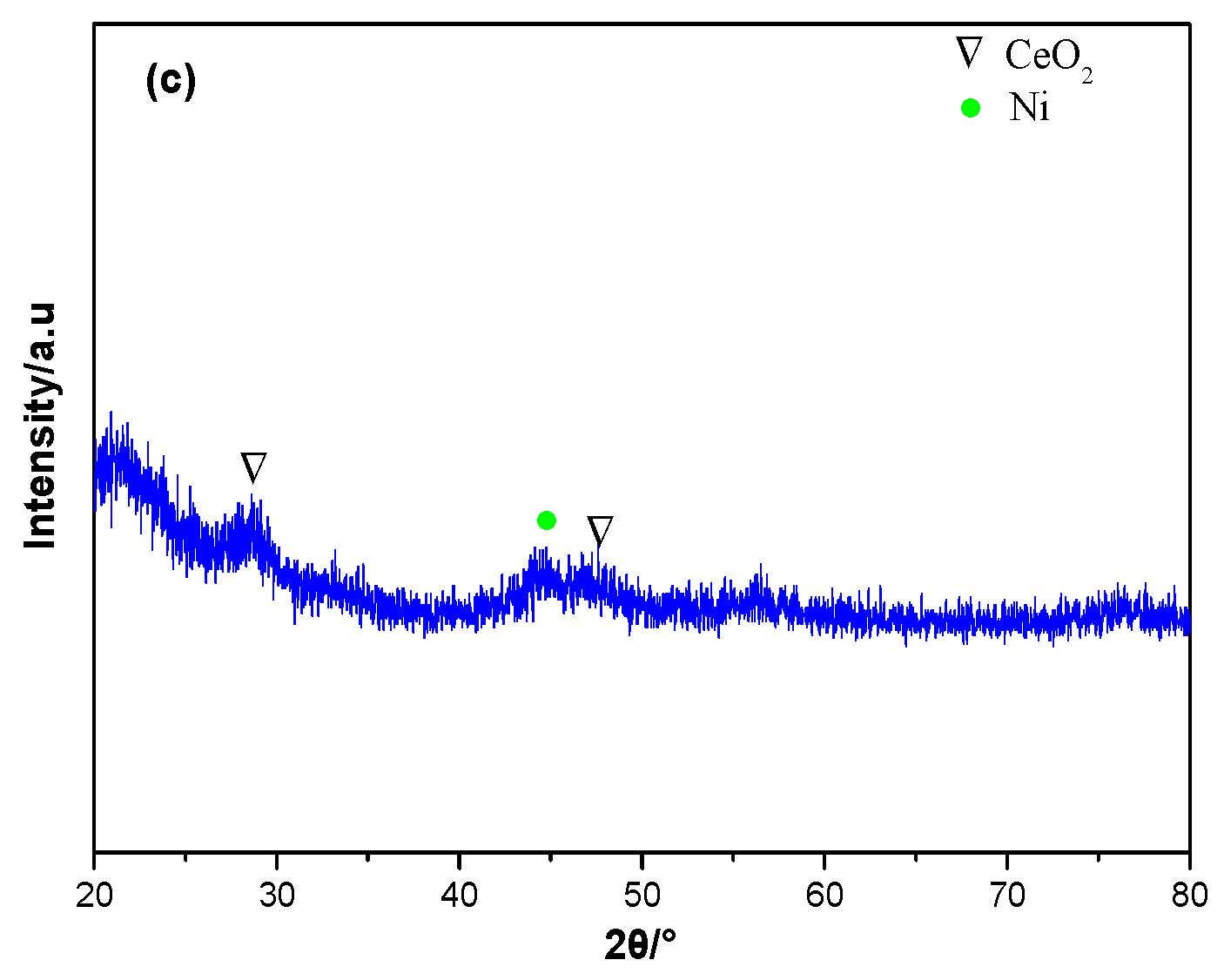
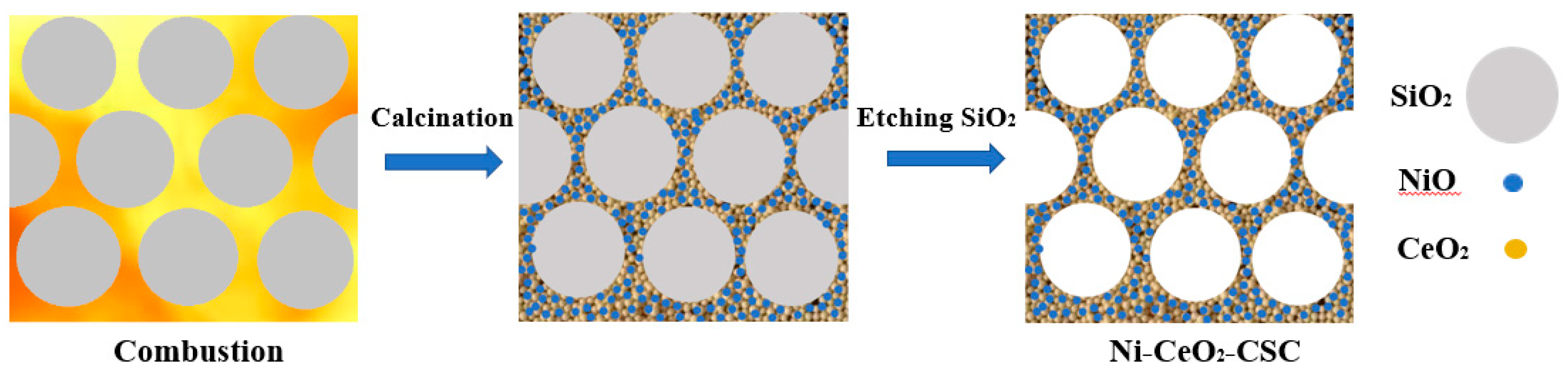
| Samples | SBET (m2/g) | CeO2 (nm) a | NiO (nm) a |
|---|---|---|---|
| 20% Ni-CeO2-CSC | 134.0 | 3.9 | / b |
| 50% Ni-CeO2-CSC | 121.5 | 3.1 | / b |
| 80% Ni-CeO2-CSC | 94.9 | / b | 3.7 |
| 50% Ni-CeO2-CP | 38.7 | 8.2 | 8.9 |
| Catalysts | CeO2 (nm) a | Ni (nm) a |
|---|---|---|
| Reduced 50% Ni-CeO2-CSC | 3.2 | / b |
| Reduced 50% Ni-CeO2-CP | 11.8 | 24.8 |
| Catalyst | WHSV (mL.gcat−1.h−1) | Reaction Temperature (°C) | XCO2 (%) | CO2 Conversion Rate (×10−5 molCO2/gcat/s) | Ref. |
|---|---|---|---|---|---|
| 50% Ni-CeO2-CSC | 120,000 | 250 | 33 | 4.91 | This Work |
| 300 | 70 | 10.42 | This Work | ||
| 20% Ni-Ce/RGO | 36,000 | 250 | 20 | 1.79 | [40] |
| 300 | 80 | 7.14 | [40] | ||
| 25% Ni/Al2O3 | 9,000 | 250 | 7 | 0.17 | [41] |
| 300 | 50 | 1.24 | [41] | ||
| 15% Ni/ZrO2 | 48,000 | 250 | 15 | 1.61 | [42] |
| 300 | 60 | 6.43 | [42] | ||
| 10% Ni/CeO2-ZrO2 | 20,000 | 250 | 46 | 0.91 | [43] |
| 300 | 55 | 1.09 | [43] | ||
| 6% Ni/ZrO2 | 15,000 | 250 | 84 | 2.50 | [11] |
| 6% Ni-MgO/ZrO2 | 15,000 | 250 | 90 | 2.68 | [11] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Hu, J.; Liu, H.; Wei, Q.; Gong, D.; Mo, L.; Tao, H.; Zhang, C. Three-Dimensional Mesoporous Ni-CeO2 Catalysts with Ni Embedded in the Pore Walls for CO2 Methanation. Catalysts 2020, 10, 523. https://doi.org/10.3390/catal10050523
Wang L, Hu J, Liu H, Wei Q, Gong D, Mo L, Tao H, Zhang C. Three-Dimensional Mesoporous Ni-CeO2 Catalysts with Ni Embedded in the Pore Walls for CO2 Methanation. Catalysts. 2020; 10(5):523. https://doi.org/10.3390/catal10050523
Chicago/Turabian StyleWang, Luhui, Junang Hu, Hui Liu, Qinhong Wei, Dandan Gong, Liuye Mo, Hengcong Tao, and Chengyang Zhang. 2020. "Three-Dimensional Mesoporous Ni-CeO2 Catalysts with Ni Embedded in the Pore Walls for CO2 Methanation" Catalysts 10, no. 5: 523. https://doi.org/10.3390/catal10050523
APA StyleWang, L., Hu, J., Liu, H., Wei, Q., Gong, D., Mo, L., Tao, H., & Zhang, C. (2020). Three-Dimensional Mesoporous Ni-CeO2 Catalysts with Ni Embedded in the Pore Walls for CO2 Methanation. Catalysts, 10(5), 523. https://doi.org/10.3390/catal10050523