Effect of Support and Chelating Ligand on the Synthesis of Ni Catalysts with High Activity and Stability for CO2 Methanation
Abstract
1. Introduction
2. Results and Discussion
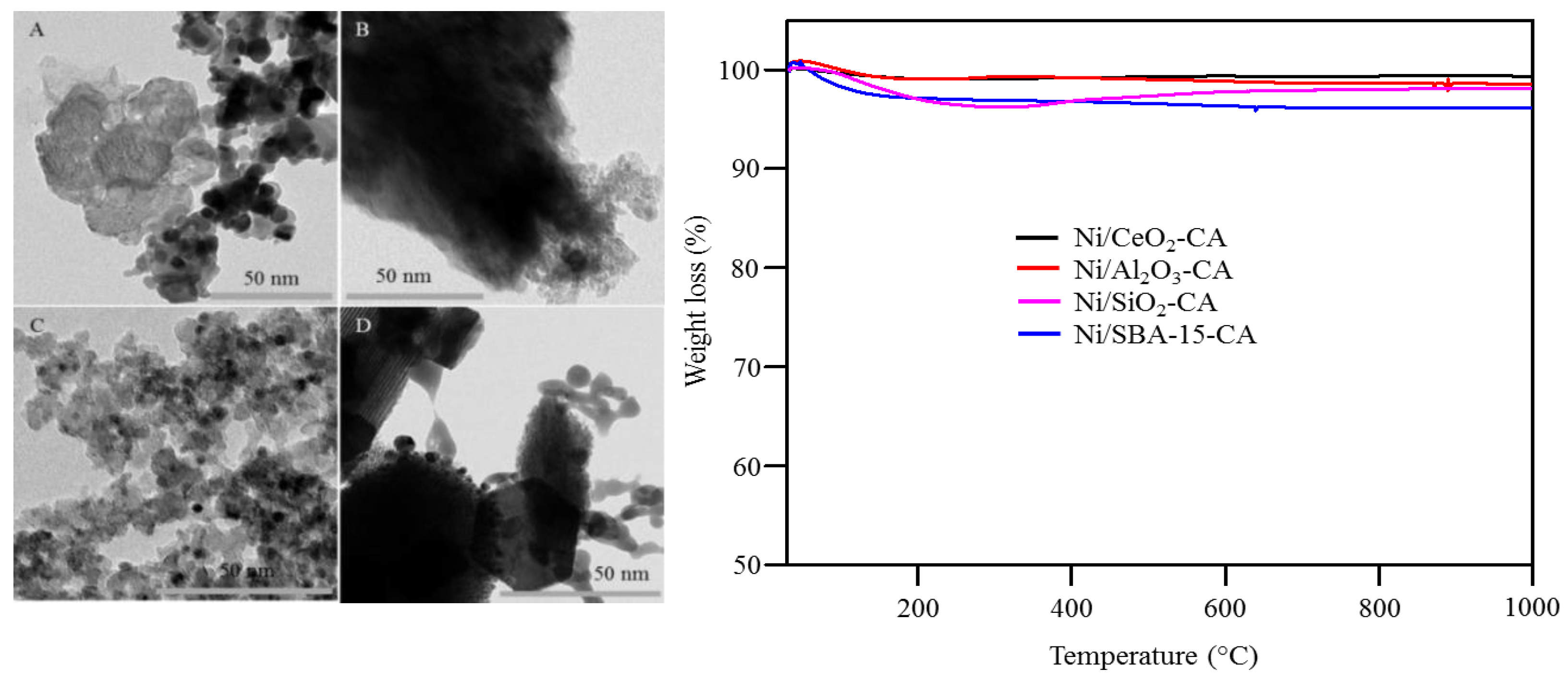
2.1. Catalysts Characterization
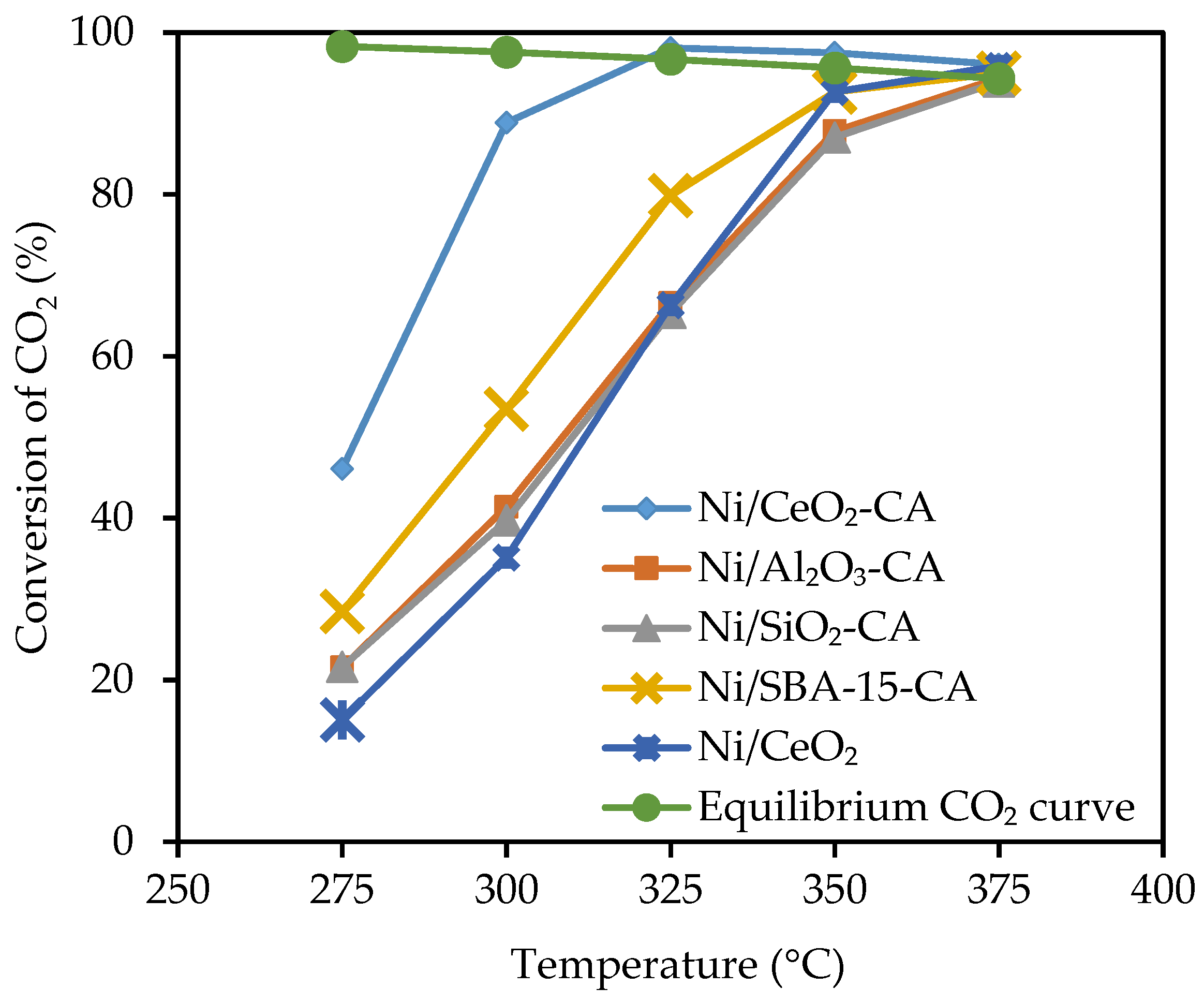
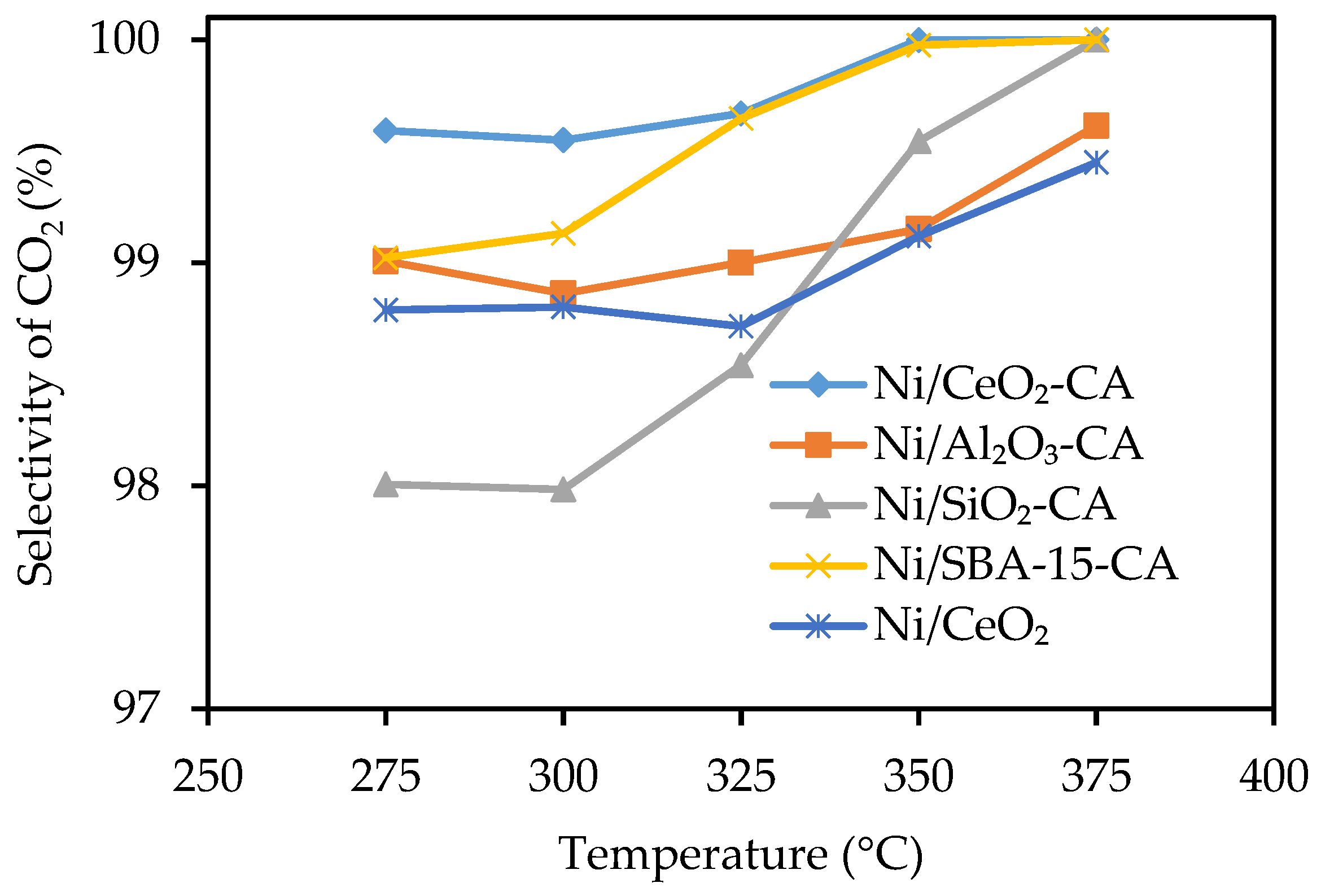
2.2. Catalytic Performance Test
3. Materials and Methods
3.1. Catalysts Preparation
3.2. Catalysts Characterization
3.3. Catalytic Activity Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ocampo, F.; Louis, B.; Kiwi-Minsker, L.; Roger, A.C. Effect of Ce/Zr composition and noble metal promotion on nickel based CexZr1−xO2 catalysts for carbon dioxide methanation. Appl. Catal. A Gen. 2011, 392, 36–44. [Google Scholar] [CrossRef]
- Wu, Y.; Chan, C.W. A data analysis decision support system for the carbon dioxide capture process. Expert Syst. Appl. 2009, 36, 9949–9960. [Google Scholar] [CrossRef]
- Walspurger, S.; Boels, L.; Cobden, P.D.; Eizinga, G.D.; Haije, W.G.; Van den Brink, R.W. The crucial role of the K+–aluminium oxide interaction in K+-promoted alumina- and hydrotalcite-based materials for CO2 sorption at high temperatures. ChemSusChem 2008, 1, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Song, C. Global challenges and strategies for control, conversion and utilization of CO2 for sustainable development involving energy, catalysis, adsorption and chemical processing. Catal. Today 2006, 115, 2–32. [Google Scholar] [CrossRef]
- Omae, I. Aspects of carbon dioxide utilization. Catal. Today 2006, 115, 33–52. [Google Scholar] [CrossRef]
- Weatherbee, G.D.; Bartholomew, C.H. Hydrogenation of CO2 on group VIII metals: I. Specific activity of NiSiO2. J. Catal. 1981, 68, 67–76. [Google Scholar] [CrossRef]
- Peebles, D.E.; Goodman, J.M.; White, J.M. Methanation of carbon dioxide on nickel (100) and the effects of surface modifiers. J. Phys. Chem. 1983, 87, 4378–4387. [Google Scholar] [CrossRef]
- Olah, G.A.; Goeppert, A.; Prkash, G.K.S. Chemical recycling of carbon dioxide to methanol and dimethyl ether: From greenhouse gas to renewable, environmentally carbon neutral fuels and synthetic hydrocarbons. J. Org. Chem. 2009, 74, 487–498. [Google Scholar] [CrossRef]
- Kilo, M.; Weigel, J.; Wokaun, A.; Koeppel, R.A.; Stoeckli, A.; Baiker, A. Effect of the addition of chromium- and manganese oxides on structural and catalytic properties of copper/zirconia catalysts for the synthesis of methanol from carbon dioxide. J. Mol. Catal. A 1997, 126, 169–184. [Google Scholar] [CrossRef]
- Melian-Cabrera, I.M.; Lopez Granados, M.; Fierro, J.L.G. Reverse topotactic transformation of a Cu–Zn–Al catalyst during wet Pd impregnation: Relevance for the performance in methanol synthesis from CO2/H2 mixtures. J. Catal. 2002, 210, 273–284. [Google Scholar] [CrossRef]
- Olah, G.A.; Goeppert, A.; Prakash, G.K.S. Beyond Oil and Gas: The Methanol Economy; Wiley-VCH, Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006. [Google Scholar]
- Centi, G.; Perathoner, S. Opportunities and prospects in the chemical recycling of carbon dioxide to fuels. Catal. Today 2009, 148, 191–205. [Google Scholar] [CrossRef]
- Ritter, S.K. What can we do with carbon dioxide. Chem. Eng. News 2007, 85, 11–17. [Google Scholar]
- Ashok, J.; Ang, M.L.; Kawi, S. Enhanced activity of CO2 methanation over Ni/CeO2-ZrO2 catalysts: Influence of preparation methods. Catal. Today 2017, 281, 304–311. [Google Scholar] [CrossRef]
- Rahmani, S.; Rezaei, M.; Meshkani, F. Preparation of highly active nickel catalysts supported on mesoporous nanocrystalline g-Al2O3 for CO2 methanation. J. Ind. Eng. Chem. 2014, 20, 346–1352. [Google Scholar] [CrossRef]
- Neuberg, S.; Pennemann, H.; Shanmugam, V.; Thiermann, R.; Zapf, R.; Gac, W.; Greluk, M.; Zawadski, W.; Kolb, G. CO2 methanation in microstructured reactors—Catalyst development and process design. Chem. Eng. Tech. 2019, 42, 2076–2084. [Google Scholar] [CrossRef]
- Hwang, S.; Lee, J.; Gi Hong, U.; Gil Seo, J.; Chul Jung, J.; Jun Koh, D.; Lim, H.; Byun, C.; Kyu Song, I. Methane production from carbon monoxide and hydrogen over nickel–alumina xerogel catalyst: Effect of nickel content. J. Ind. Eng. Chem. 2011, 17, 154–157. [Google Scholar] [CrossRef]
- Yamasaki, M.; Komori, M.; Akiyama, E.; Habazaki, H.; Kawashima, A.; Asami, K.; Hashimoto, K. CO2 methanation catalysts prepared from amorphous Ni–Zr–Sm and Ni–Zr–misch metal alloy precursors. Mater. Sci. Eng. A 1999, 267, 220–226. [Google Scholar] [CrossRef]
- Sharma, S.; Hu, Z.; Zhang, P.; McFarland, E.W.; Metiu, H. CO2 methanation on Ru-doped ceria. J. Catal. 2011, 278, 297–309. [Google Scholar] [CrossRef]
- Eckle, S.; Anfang, H.-G.; Behm, R.J. Reaction intermediates and side products in the methanation of CO and CO2 over supported Ru catalysts in H2-rich reformate gases. J. Phys. Chem. C 2011, 115, 1361–1367. [Google Scholar] [CrossRef]
- Karelovic, A.; Ruiz, P. CO2 hydrogenation at low temperature over Rh/γ-Al2O3 catalysts: Effect of the metal particle size on catalytic performances and reaction mechanism. Appl. Catal. B Environ. 2012, 113–114, 237–249. [Google Scholar] [CrossRef]
- Beuls, A.; Swalus, C.; Jacquemin, M.; Heyen, G.; Karelovic, A.; Ruiz, P. Methanation of CO2: Further insight into the mechanism over Rh/γ-Al2O3 catalyst. Appl. Catal. B Environ. 2012, 113–114, 2–10. [Google Scholar] [CrossRef]
- Swalus, C.; Jacquemin, M.; Poleunis, C.; Bertrand, P.; Ruiz, P. CO2 methanation on Rh/γ-Al2O3 catalyst at low temperature: “In situ” supply of hydrogen by Ni/activated carbon catalyst. Appl. Catal. B Environ. 2012, 125, 41–50. [Google Scholar] [CrossRef]
- Park, J.-N.; McFarland, E.W. A highly dispersed Pd–Mg/SiO2 catalyst active for methanation of CO2. J. Catal. 2009, 266, 92–97. [Google Scholar] [CrossRef]
- Yu, K.-P.; Yu, W.-Y.; Kuo, M.-C.; Liou, Y.-C.; Chien, S.-H. Pt/titania-nanotube: A potential catalyst for CO2 adsorption and hydrogenation. Appl. Catal. B Environ. 2008, 84, 12–118. [Google Scholar] [CrossRef]
- Du, G.; Lim, S.; Yang, Y.; Wang, C.; Pfefferle, L.; Haller, G.L. Methanation of carbon dioxide on Ni-incorporated MCM-41 catalysts: The influence of catalyst pre-treatment and study of steady-state reaction. J. Catal. 2007, 249, 370–379. [Google Scholar] [CrossRef]
- Lu, B.; Kawamoto, K. Preparation of the highly loaded and well-dispersed NiO/SBA-15 for methanation of producer gas. Fuel 2013, 103, 699–704. [Google Scholar] [CrossRef]
- Yamasaki, M.; Habazaki, H.; Asami, K.; Izumiya, K.; Hashimoto, K. Effect of tetragonal ZrO2 on the catalytic activity of Ni/ZrO2 catalyst prepared from amorphous Ni–Zr alloys. Catal. Commun. 2006, 7, 24–28. [Google Scholar] [CrossRef]
- Tada, S.; Shimizu, T.; Kameyama, H.; Haneda, T.; Kikuchi, R. Ni/CeO2 catalysts with high CO2 methanation activity and high CH4 selectivity at low temperatures. Int. J. Hydrog. Energy 2012, 37, 5527–5531. [Google Scholar] [CrossRef]
- Jwa, E.; Lee, S.B.; Lee, H.W.; Mok, Y.S. Plasma-assisted catalytic methanation of CO and CO2 over Ni–zeolite catalysts. Fuel Process Technol. 2013, 108, 89–93. [Google Scholar] [CrossRef]
- Aziz, M.A.A.; Jalil, A.A.; Triwahyono, S.; Mukti, R.R.; Taufiq-Yap, Y.H.; Sazegar, M.R. Highly active Ni-promoted mesostructured silica nanoparticles for CO2 methanation. Appl. Catal. B Environ. 2014, 147, 359–368. [Google Scholar] [CrossRef]
- Zhou, G.; Liu, H.; Cui, K.; Jia, A.; Hu, G.; Jiao, Z.; Liu, Y.; Zhang, X. Role of surface Ni and Ce species of Ni/CeO2 catalyst in CO2 methanation. Appl. Surf. Sci. 2016, 383, 248–252. [Google Scholar] [CrossRef]
- Takano, H.; Kirihata, Y.; Izumiya, K.; Kumagai, N.; Habazaki, H.; Hashimoto, K. Highly active Ni/Y-doped ZrO2 catalysts for CO2 methanation. Appl. Sur. Sci. 2016, 388, 653–663. [Google Scholar] [CrossRef]
- Ocampo, F.; Louis, B.; Roger, A. Methanation of carbon dioxide over nickel-based Ce0.72Zr0.28O2 mixed oxide catalysts prepared by sol–gel method. Appl. Catal. A Gen 2009, 369, 90–96. [Google Scholar] [CrossRef]
- Aldana, P.A.U.; Ocampo, F.; Kobl, K.; Louis, B.; Thibault-Starzyk, F.; Daturi, M.; Bazin, P.; Thomas, S.; Roger, A.C. Catalytic CO2 valorization into CH4 on Ni-based ceria-zirconia. Reaction mechanism by operando IR spectroscopy. Catal. Today 2013, 215, 201–207. [Google Scholar] [CrossRef]
- Pan, Q.; Peng, J.; Sun, T.; Wang, S.; Wang, S. Insight into the reaction route of CO2 methanation: Promotion effect of medium basic sites. Catal. Commun. 2014, 45, 74–78. [Google Scholar] [CrossRef]
- Zhu, H.; Razzaq, R.; Li, C.; Muhmmad, Y.; Zhang, S. Catalytic methanation of carbon dioxide by active oxygen material CexZr1−xO2 supported Ni-Co bimetallic nanocatalysts. AiChE J. 2013, 59, 2567–2576. [Google Scholar] [CrossRef]
- Bian, Z.; Xin, Z.; Meng, X.; Tao, M.; Lv, Y.H.; Gu, J. Effect of citric acid on the synthesis of CO methanation catalysts with high activity and excellent stability. Ind. Eng. Chem. Res. 2017, 56, 2383–2392. [Google Scholar] [CrossRef]
- Yu, C.; Hu, J.; Zhou, W.; Fan, Q. Novel Ni/CeO2-Al2O3 composite catalysts synthesized by one-step citric acid complex and their performance in catalytic partial oxidation of methane. J. Energy Chem. 2014, 23, 235–243. [Google Scholar] [CrossRef]
- Suárez-Toriello, V.A.; Santolalla-Vargas, C.E.; de los Reyes, J.A.; Vázquez-Zavala, A.; Vrinat, M.; Geantet, C. Influence of the solution pH in impregnation with citric acid and activity of Ni/W/Al2O3 catalysts. J. Mol. Catal. A Chem. 2015, 404–405, 36–46. [Google Scholar]
- Zhang, Q.; Long, K.; Wang, J.; Zhang, T.; Song, Z.; Lin, Q. A novel promoting effect of chelating ligand on the dispersion of Ni species over Ni/SBA-15 catalyst for dry reforming of methane. Ind. J. Hydrog. Energy 2017, 42, 14103–14114. [Google Scholar] [CrossRef]
- Shanmugam, V.; Neuberg, S.; Zapf, R.; Hessel, V.; Kolb, G. Novel route to control the size, distribution and location of Ni nanoparticles in mesoporous silica for steam reforming of propylene glycol in microchannel reactor. Catal. Commun. 2016, 83, 43–47. [Google Scholar] [CrossRef]
- Pandey, D.; Deo, G. Effect of support on the catalytic activity of supported Ni–Fe catalysts for the CO2 methanation reaction. J. Ind. Eng. Chem. 2016, 33, 99–107. [Google Scholar] [CrossRef]
- Hu, D.; Gao, J.; Ping, Y.; Jia, L.; Gunawan, P.; Zhong, Z.; Xu, G.; Gu, F.; Su, F. Enhanced investigation of CO methanation over Ni/Al2O3 Catalysts for synthetic natural gas production. Ind. Eng. Chem. Res. 2012, 51, 4875–4886. [Google Scholar] [CrossRef]
- Muroyama, H.; Tsuda, Y.; Asakoshi, T.; Masitah, H.; Okanishi, T.; Matsui, T.; Eguchi, K. Carbon dioxide methanation over Ni catalysts supported on various metal oxides. J. Catal. 2016, 343, 178–184. [Google Scholar] [CrossRef]
- Lakshmanan, P.; Kim, M.S.; Park, E.D. A highly loaded Ni@ SiO2 core–shell catalyst for CO methanation. Appl. Catal. A Gen. 2016, 513, 98–105. [Google Scholar] [CrossRef]
- Yan, X.; Liu, Y.; Zhao, B.; Wang, Z.; Wang, Y.; Liu, C.J. Methanation over Ni/SiO2: Effect of the catalyst preparation methodologies. Int. J. Hydrog. Energy 2013, 38, 2283–2291. [Google Scholar] [CrossRef]
- Wu, H.C.; Chang, Y.C.; Wu, J.H.; Lin, J.H.; Linc, I.K.; Chen, C.S. Methanation of CO2 and reverse water gas shift reactions on Ni/SiO2 catalysts: The influence of particle size on selectivity and reaction pathway. Catal. Sci. Technol. 2015, 5, 4154–4163. [Google Scholar] [CrossRef]
- Chen, X.; Jin, J.; Sha, G.; Li, C.; Zhang, B.; Su, D.; Williams, C.T.; Liang, C. Silicon–nickel intermetallic compounds supported on silica as a highly efficient catalyst for CO methanation. Catal. Sci. Technol. 2014, 4, 53–61. [Google Scholar] [CrossRef]
- Fukuhara, C.; Hayakawa, K.; Suzuki, Y.; Kawasaki, W.; Watanabe, R. A novel nickel-based structured catalyst for CO2 methanation: A honeycomb-type Ni/CeO2 catalyst to transform greenhouse gas into useful resources. Appl. Catal. A Gen. 2017, 532, 12–18. [Google Scholar] [CrossRef]
- Ratchahat, S.; Sudoh, M.; Suzuki, Y.; Kawasaki, W.; Watanabe, R.; Fukuhara, C. Development of a powerful CO2 methanation process using a structured Ni/CeO2 catalyst. J. CO2 Utilization 2018, 24, 210–219. [Google Scholar] [CrossRef]
- Atzori, L.; Cutrufello, M.G.; Meloni, D.; Cannas, C.; Gazzoli, D.; Monaci, R.; Sini, M.F.; Rombi, E. Highly active NiO-CeO2 catalysts for synthetic natural gas production by CO2 methanation. Catal. Today 2018, 299, 183–192. [Google Scholar] [CrossRef]
- Le, T.A.; Kim, M.S.; Lee, S.H.; Kim, T.W.; Park, E.D. CO and CO2 methanation over supported Ni catalysts. Catal. Today 2017, 293–294, 89–96. [Google Scholar] [CrossRef]
- Trovarelli, A.; Deleitenburg, C.; Dolcetti, G.; Lorca, J.L. CO2 methanation under transient and steady-state conditions over Rh/CeO2 and CeO2-promoted Rh/SiO2: The role of surface and bulk ceria. J. Catal. 1995, 151, 111–124. [Google Scholar] [CrossRef]
- Zhou, G.; Liu, H.; Cui, K.; Xie, H.; Jiao, Z.; Zhang, G.; Xiong, K.; Zheng, X. Methanation of carbon dioxide over Ni/CeO2 catalysts: Effects of support CeO2 structure. Int. J. Hydrog. Energy 2017, 42, 16108–16117. [Google Scholar] [CrossRef]
- Shanmugam, V.; Zapf, R.; Neuberg, S.; Hessel, V.; Kolb, G. Effect of ceria and zirconia promotors on Ni/SBA-15 catalysts for coking and sintering resistant steam reforming of propylene glycol in microreactors. App. Catal. B Environ. 2017, 203, 859–869. [Google Scholar] [CrossRef]
- Razzaq, R.; Zhu, H.; Jiang, L.; Muhammad, U.; Li, C.; Zhang, S. Catalytic methanation of CO and CO2 in coke oven gas over Ni−Co/ZrO2−CeO2. Ind. Eng. Chem. Res. 2013, 52, 2247–2256. [Google Scholar] [CrossRef]
- Shanmugam, V.; Zapf, R.; Hessel, V.; Pennemann, H.; Kolb, G. Nano-architectured CeO2 supported Rh with remarkably enhanced catalytic activity for propylene glycol reforming reaction in microreactors. Appl. Catal. B Environ. 2018, 226, 403–411. [Google Scholar] [CrossRef]
- Shanmugam, V.; Neuberg, S.; Zapf, R.; Pennemann, H.; Kolb, G. Hydrogen production over highly active Pt based catalyst coatings by steam reforming of methanol: Effect of support and co-support. Int. J. Hydrog. Energy 2020, 45, 1658–1670. [Google Scholar] [CrossRef]
- Kolb, G.; Zapf, R.; Hessel, V.; Löwe, H. Propane steam reforming in micro-channels—results from catalyst screening and optimisation. Appl. Catal. A Gen. 2004, 277, 155–166. [Google Scholar] [CrossRef]
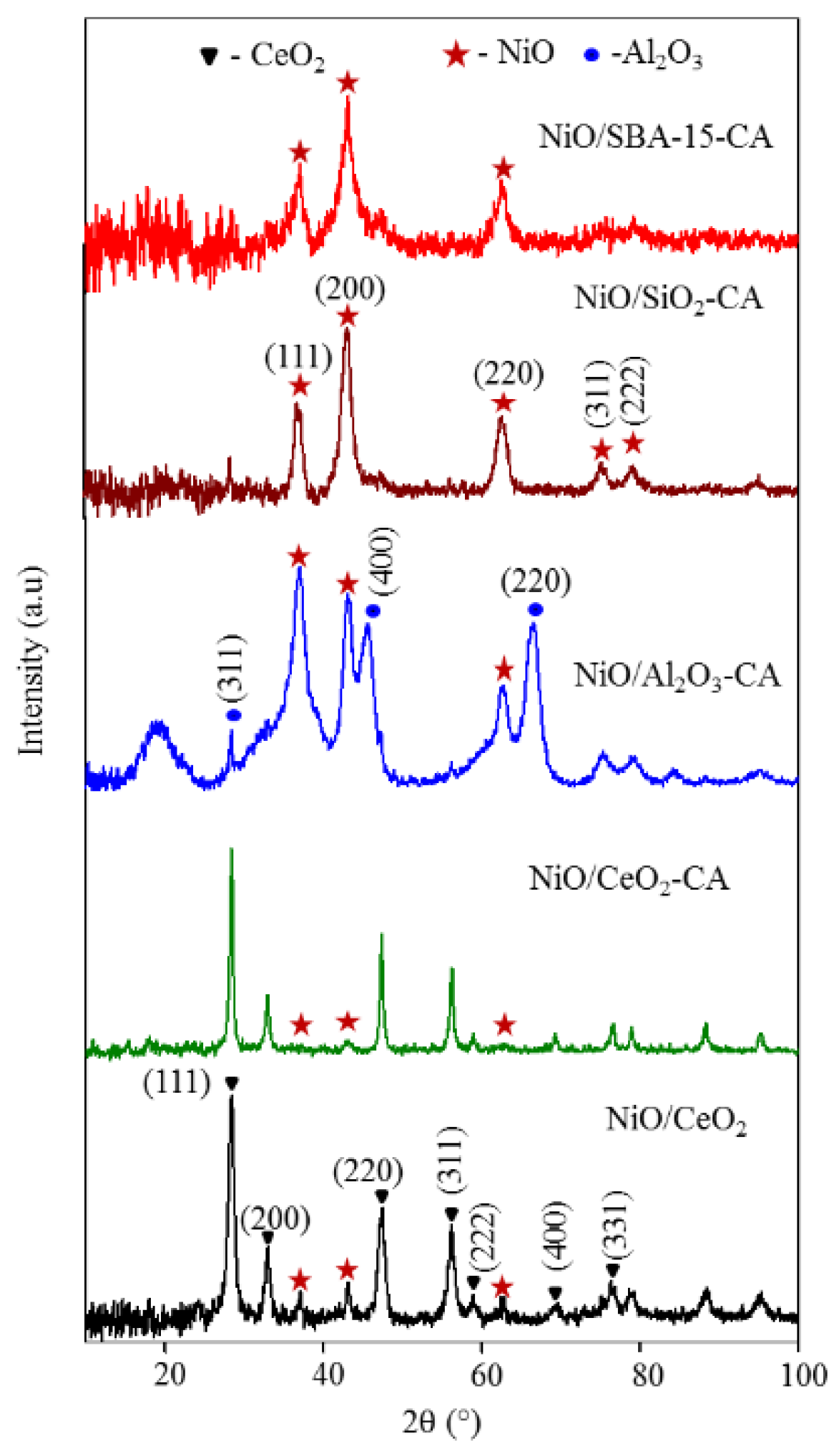
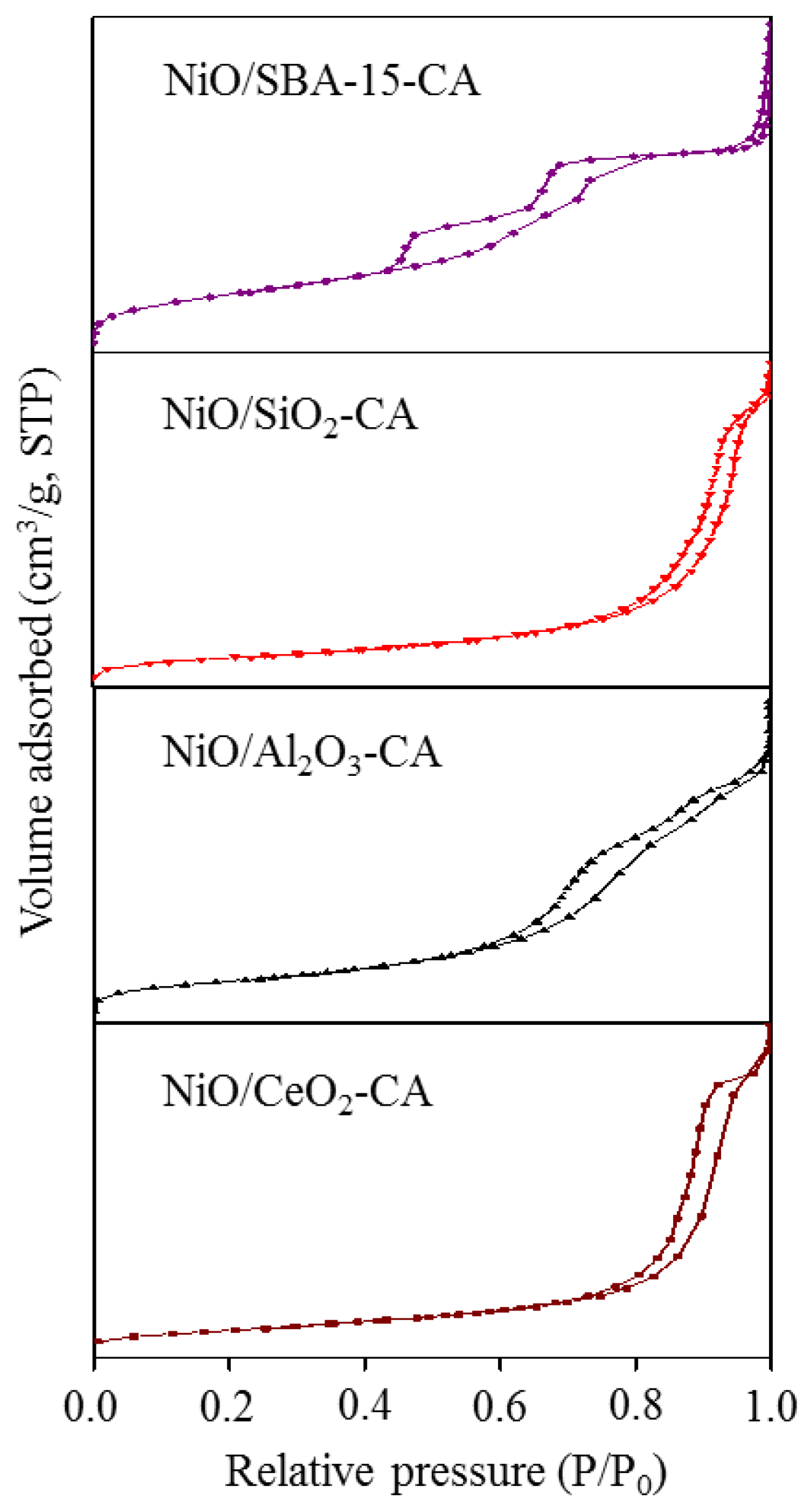
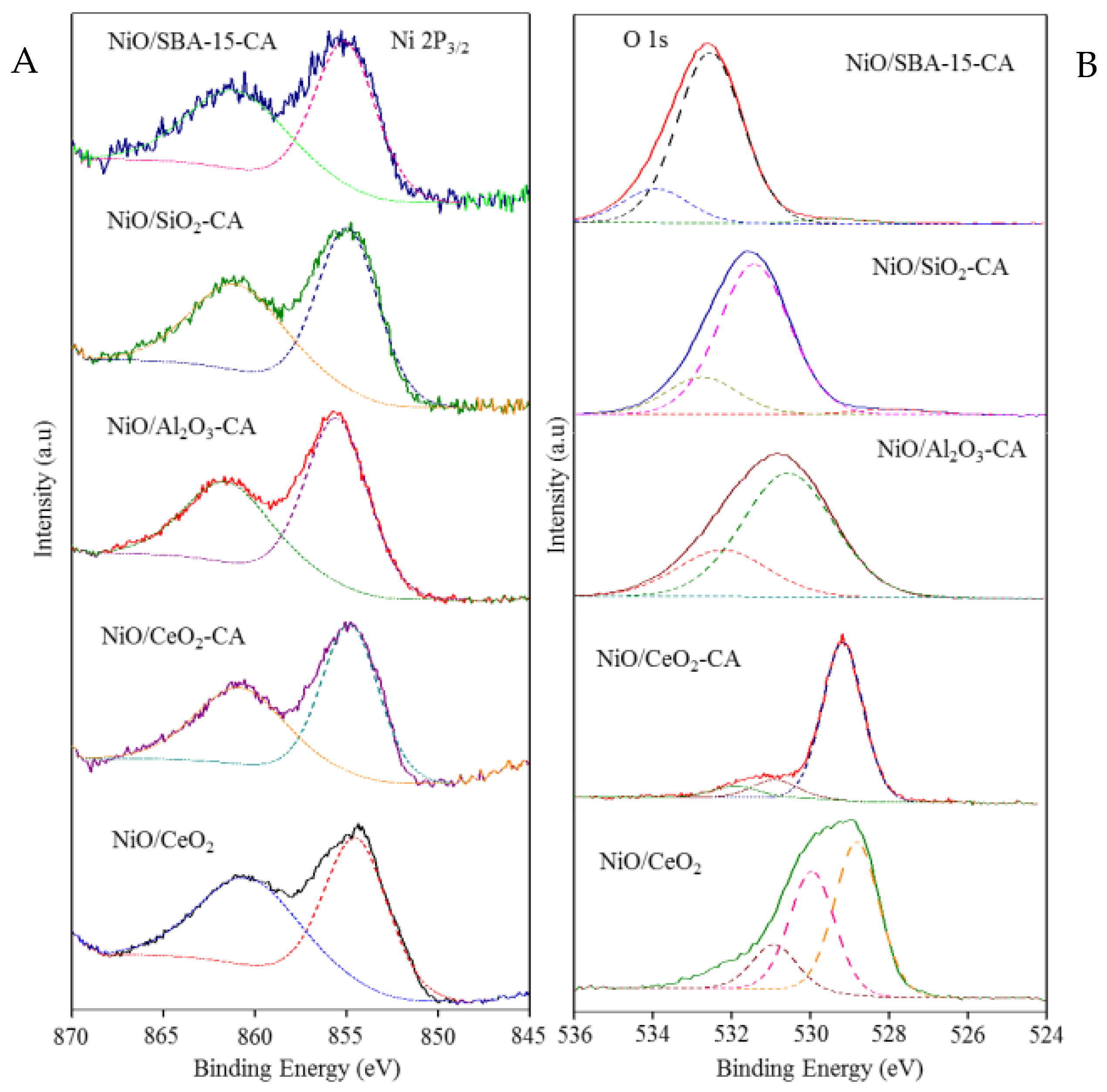

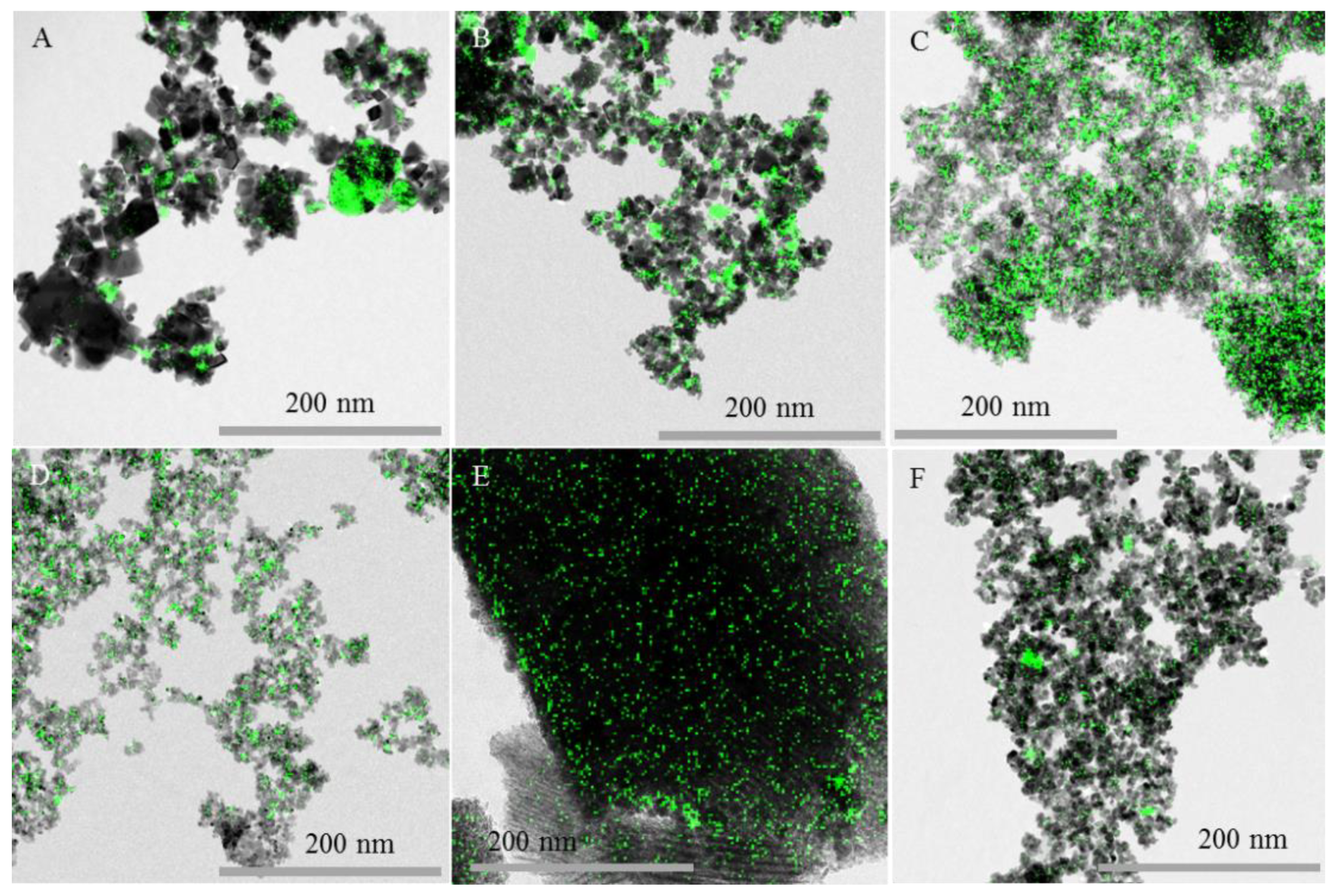



| Catalysts | SBET (m2g−1) | Crystallite Size of NiO from XRD (nm) | Crystallite Size of NiO from TEM (nm) |
|---|---|---|---|
| Ni/CeO2 | 41 | 10.8 | 10 |
| Ni/CeO2-CA | 44 | 5.8 | 6 |
| Ni/Al2O3-CA | 153 | 7.4 | 8 |
| Ni/SiO2-CA | 230 | 6.4 | 7 |
| Ni/SBA-15-CA | 380 | 4.9 | 4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shanmugam, V.; Neuberg, S.; Zapf, R.; Pennemann, H.; Kolb, G. Effect of Support and Chelating Ligand on the Synthesis of Ni Catalysts with High Activity and Stability for CO2 Methanation. Catalysts 2020, 10, 493. https://doi.org/10.3390/catal10050493
Shanmugam V, Neuberg S, Zapf R, Pennemann H, Kolb G. Effect of Support and Chelating Ligand on the Synthesis of Ni Catalysts with High Activity and Stability for CO2 Methanation. Catalysts. 2020; 10(5):493. https://doi.org/10.3390/catal10050493
Chicago/Turabian StyleShanmugam, Vetrivel, Stefan Neuberg, Ralf Zapf, Helmut Pennemann, and Gunther Kolb. 2020. "Effect of Support and Chelating Ligand on the Synthesis of Ni Catalysts with High Activity and Stability for CO2 Methanation" Catalysts 10, no. 5: 493. https://doi.org/10.3390/catal10050493
APA StyleShanmugam, V., Neuberg, S., Zapf, R., Pennemann, H., & Kolb, G. (2020). Effect of Support and Chelating Ligand on the Synthesis of Ni Catalysts with High Activity and Stability for CO2 Methanation. Catalysts, 10(5), 493. https://doi.org/10.3390/catal10050493







