Comparative Study on Enzyme Immobilization Using Natural Hydrogel Matrices—Experimental Studies Supported by Molecular Models Analysis
Abstract
1. Introduction
2. Results
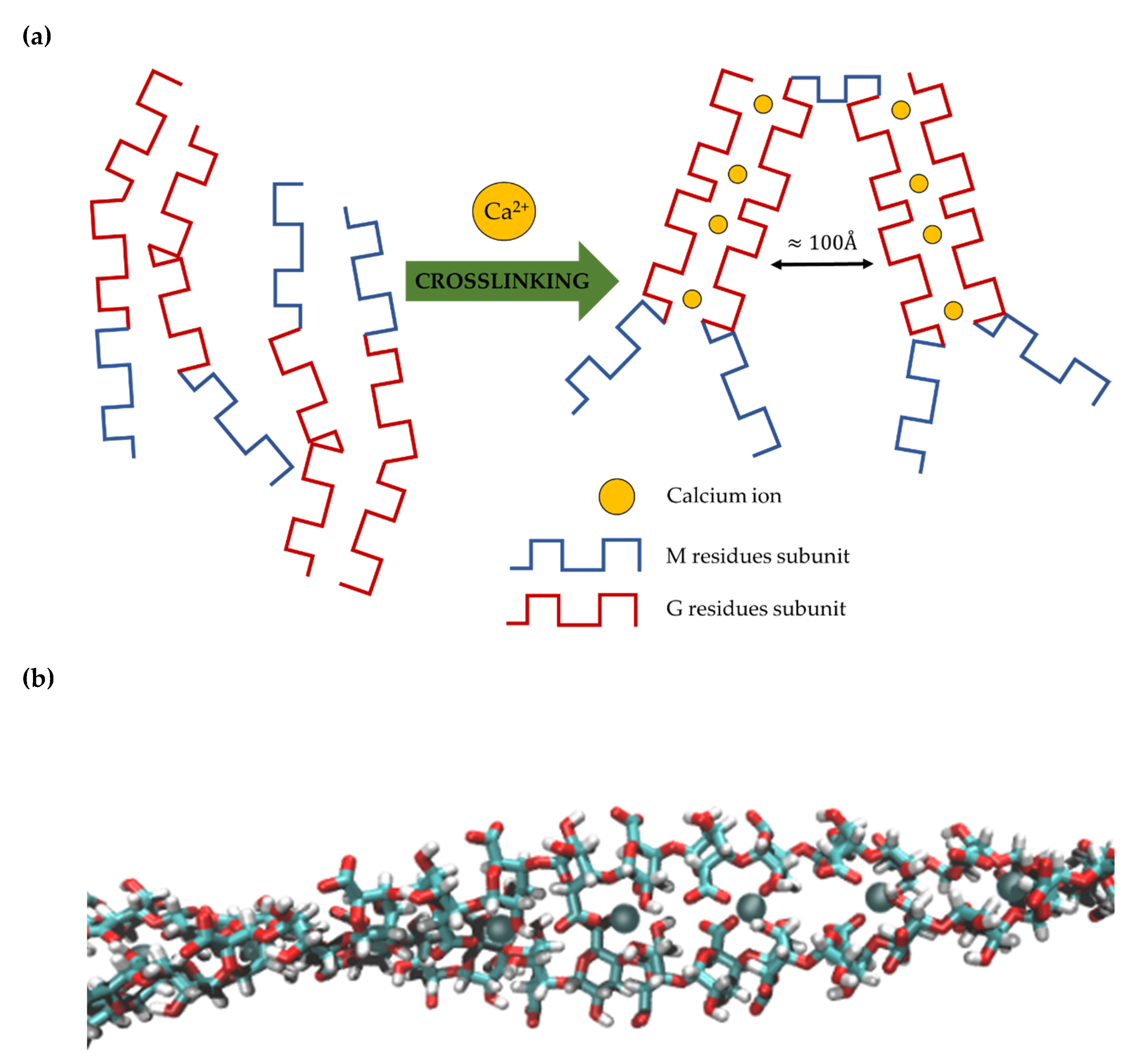
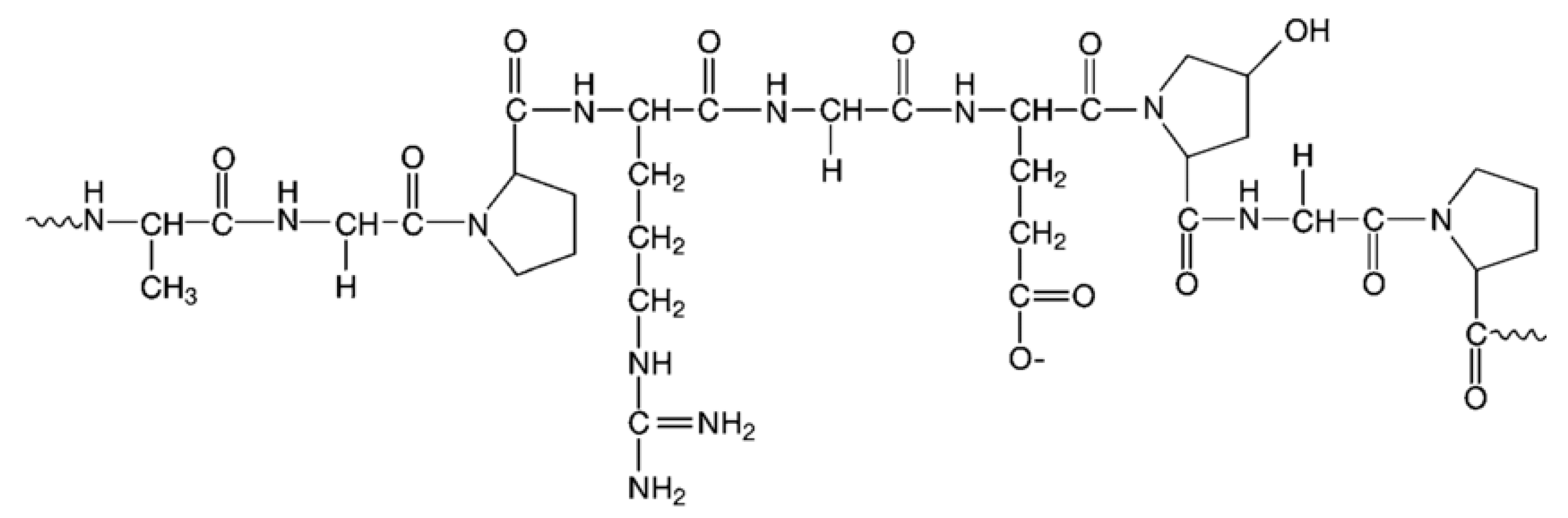
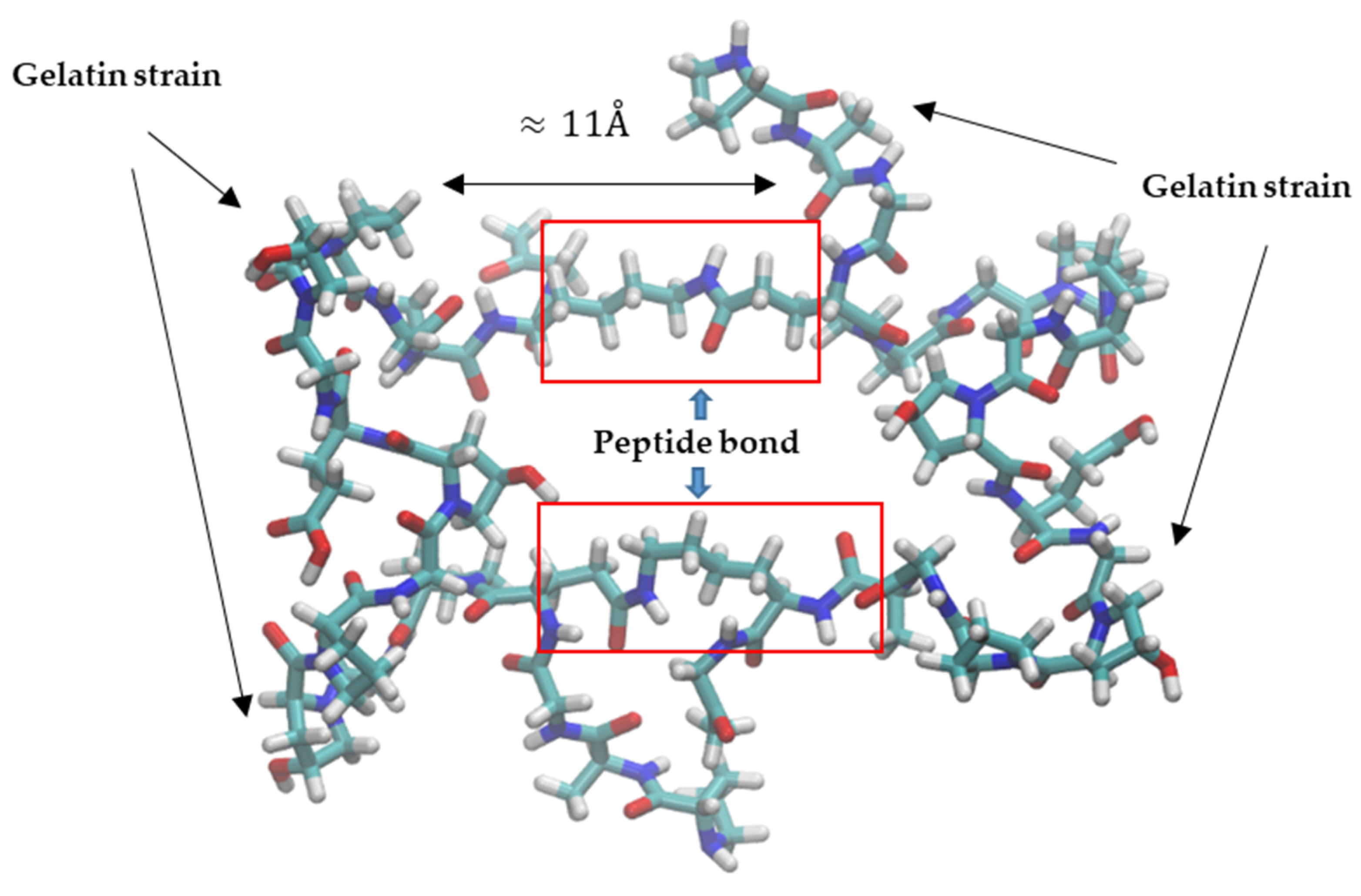
2.1. Molecular Insight into Structural Characteristics of Alginate and Gelatin-Based Materials
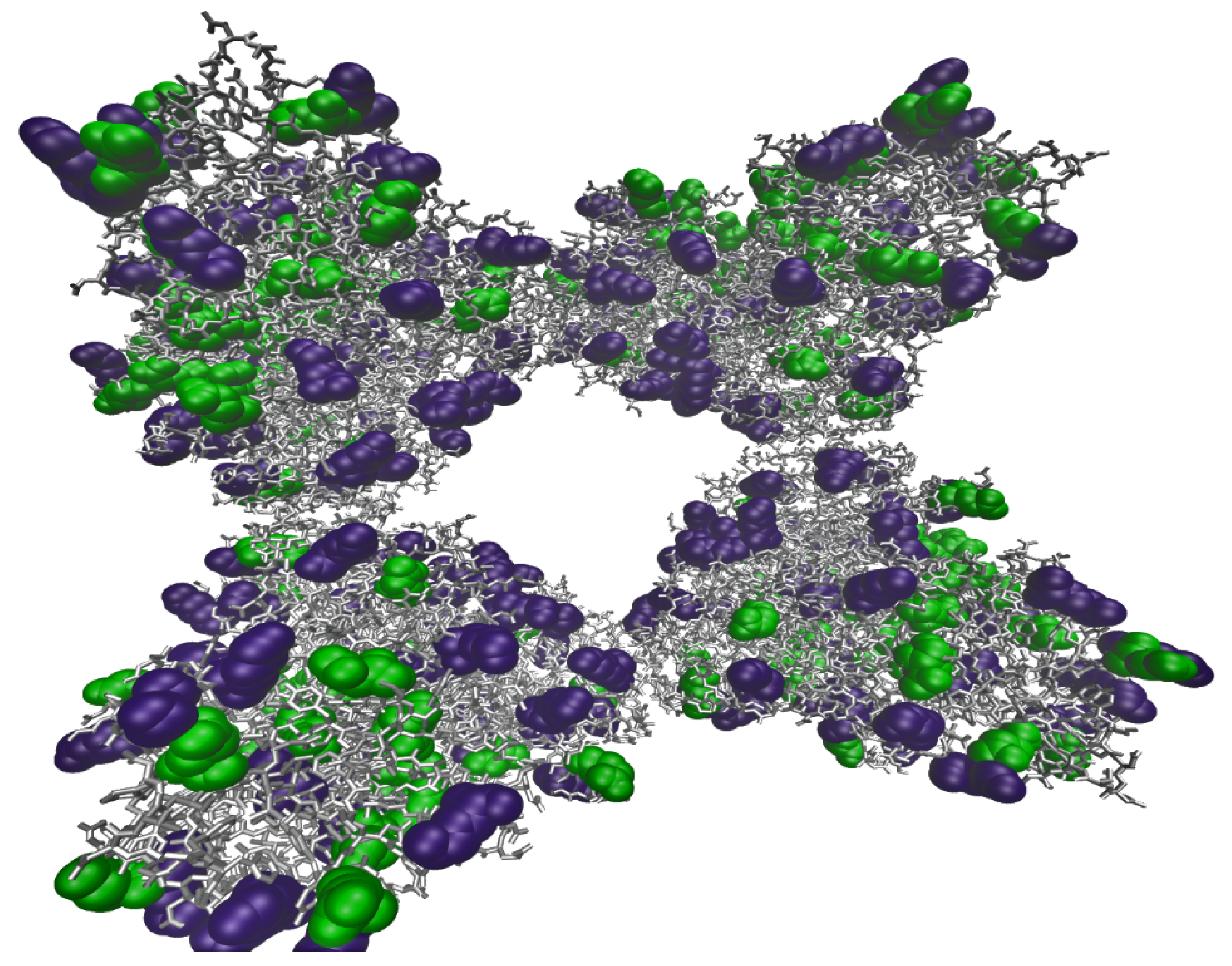
2.1.1. Analysis of Molecular Models of Alginate and Gelatin-Based Hydrogels
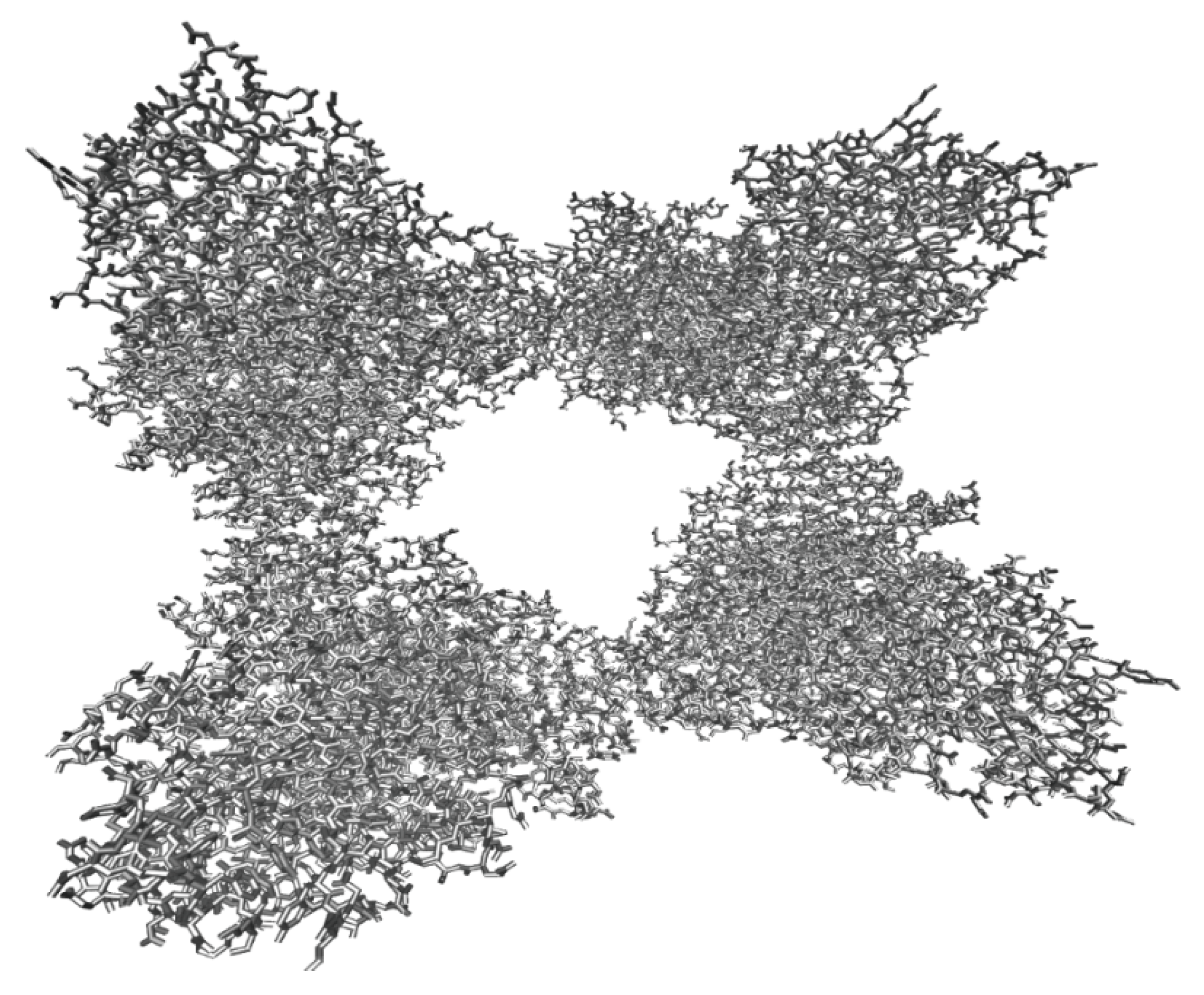
2.1.2. Analysis of Molecular Model of Invertase from Saccharomyces cerevisiae
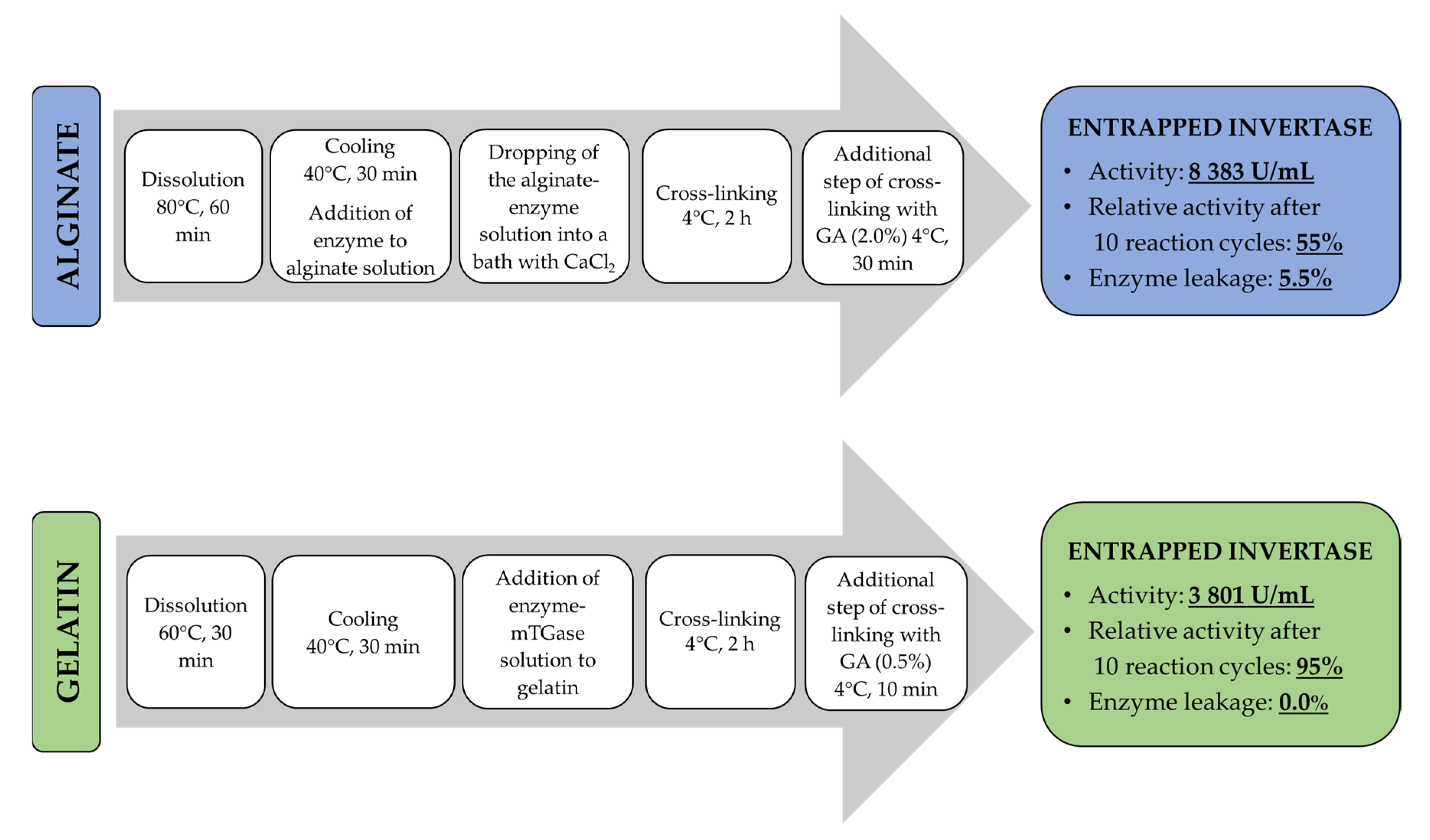
2.2. Experimental Studies on the Process of Invertase Immobilization
2.2.1. Ca2+–Alginate-Based Hydrogel
2.2.2. Gelatin-Based Hydrogel
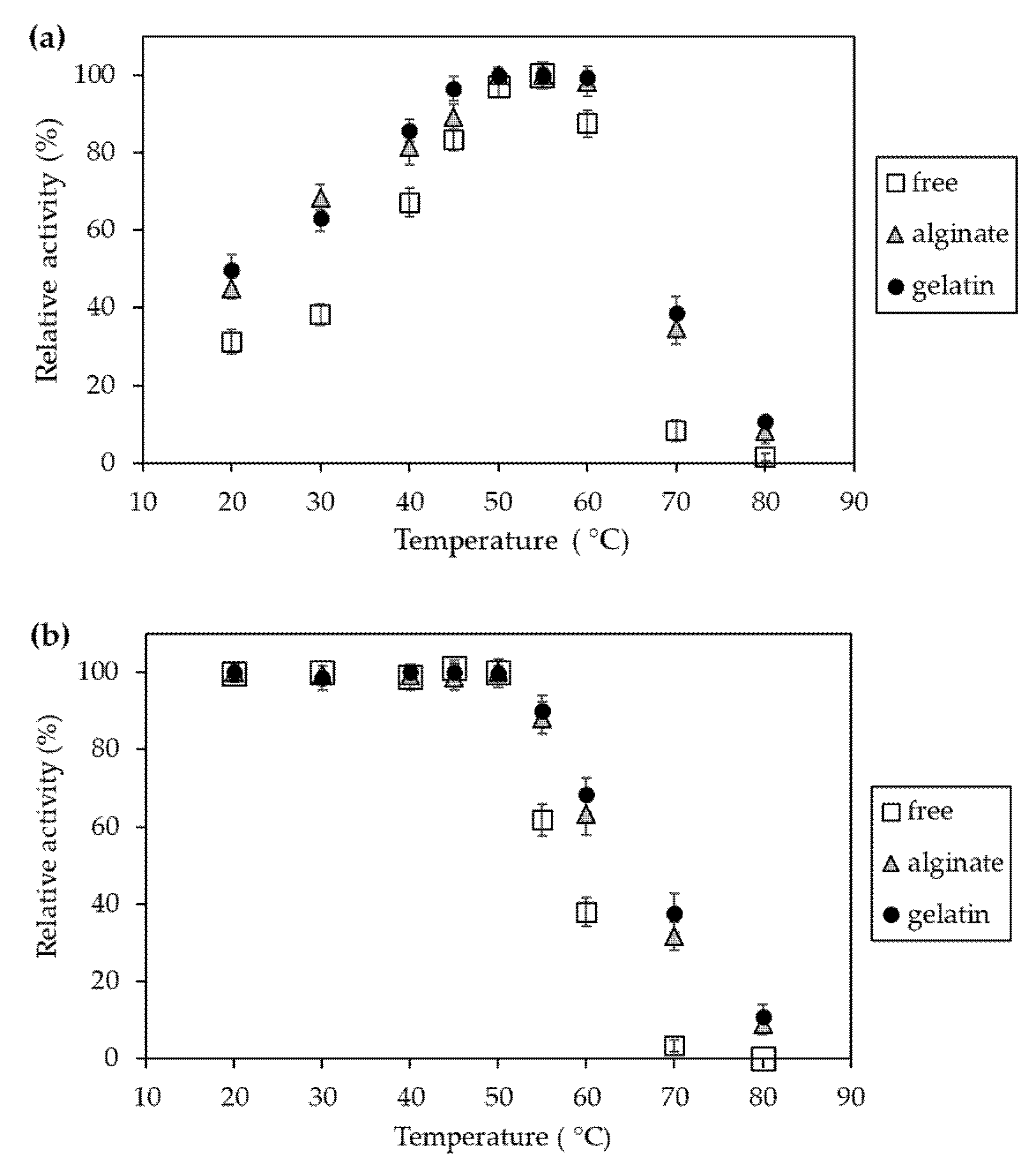
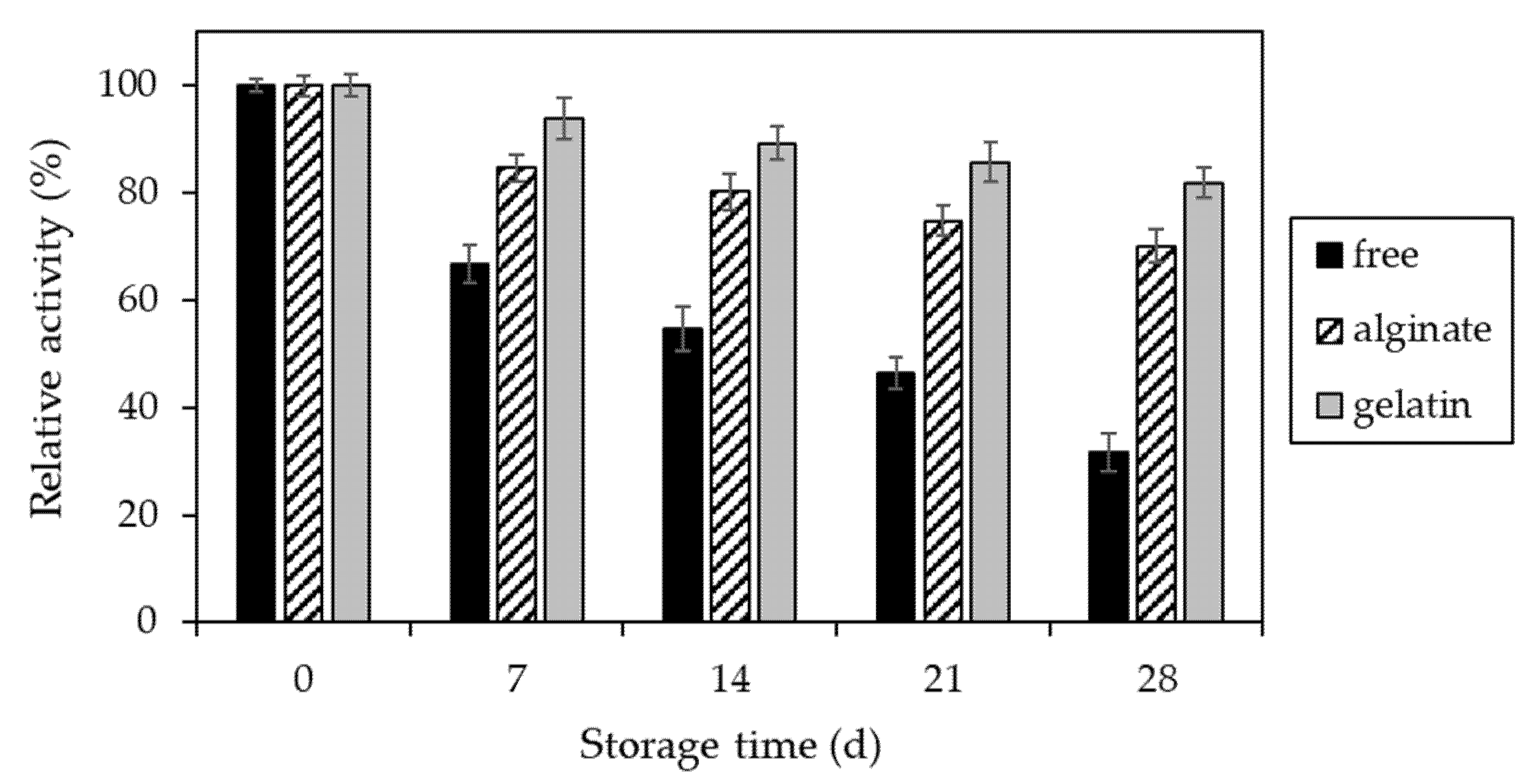
2.3. Characteristics of Invertase Immobilized in Ca2+–Alginate and Gelatin-Based Hydrogels
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Preparation of Molecular Models of Hydrogel Structures
4.2.2. Preparation of Virtual Model of Invertase
4.2.3. Preparation of Hydrogel Matrices Containing Immobilized Invertase
4.2.4. Analytical Test for Determination of Protein Content
4.2.5. Analytical Test for Determination of Glucose Concentration
4.2.6. Determination of Catalytic Activity of Native and Immobilized Invertase
4.2.7. Determination of Enzyme Leakage from Hydrogel Matrices
4.2.8. Influence of the Concentration of the Primary Cross-Linking Agent on the Efficiency of Invertase Immobilization in Hydrogel Matrices
4.2.9. Influence of Additional Step of Cross-Linking with Glutaraldehyde on the Efficiency of Invertase Immobilization in Hydrogel Matrices
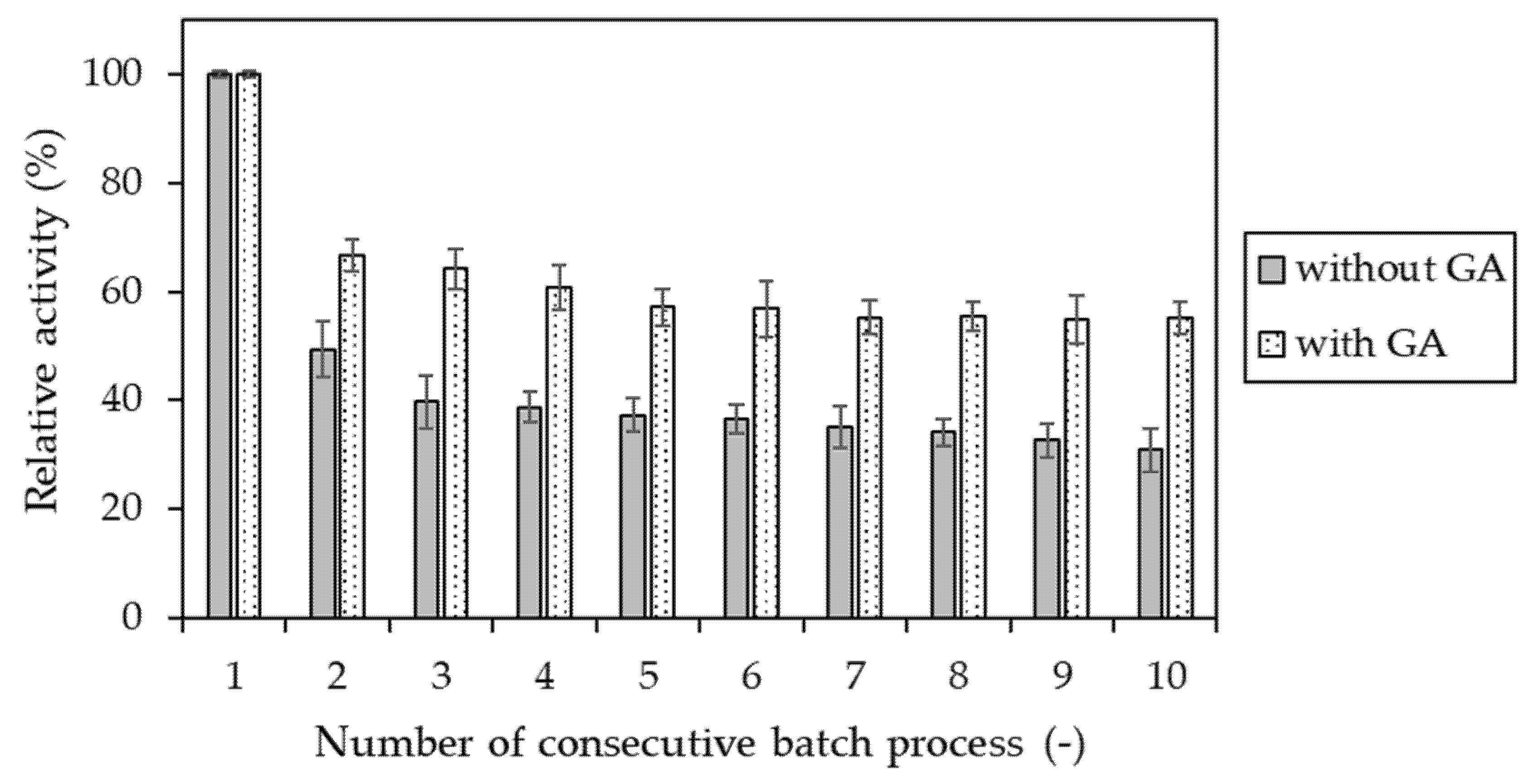
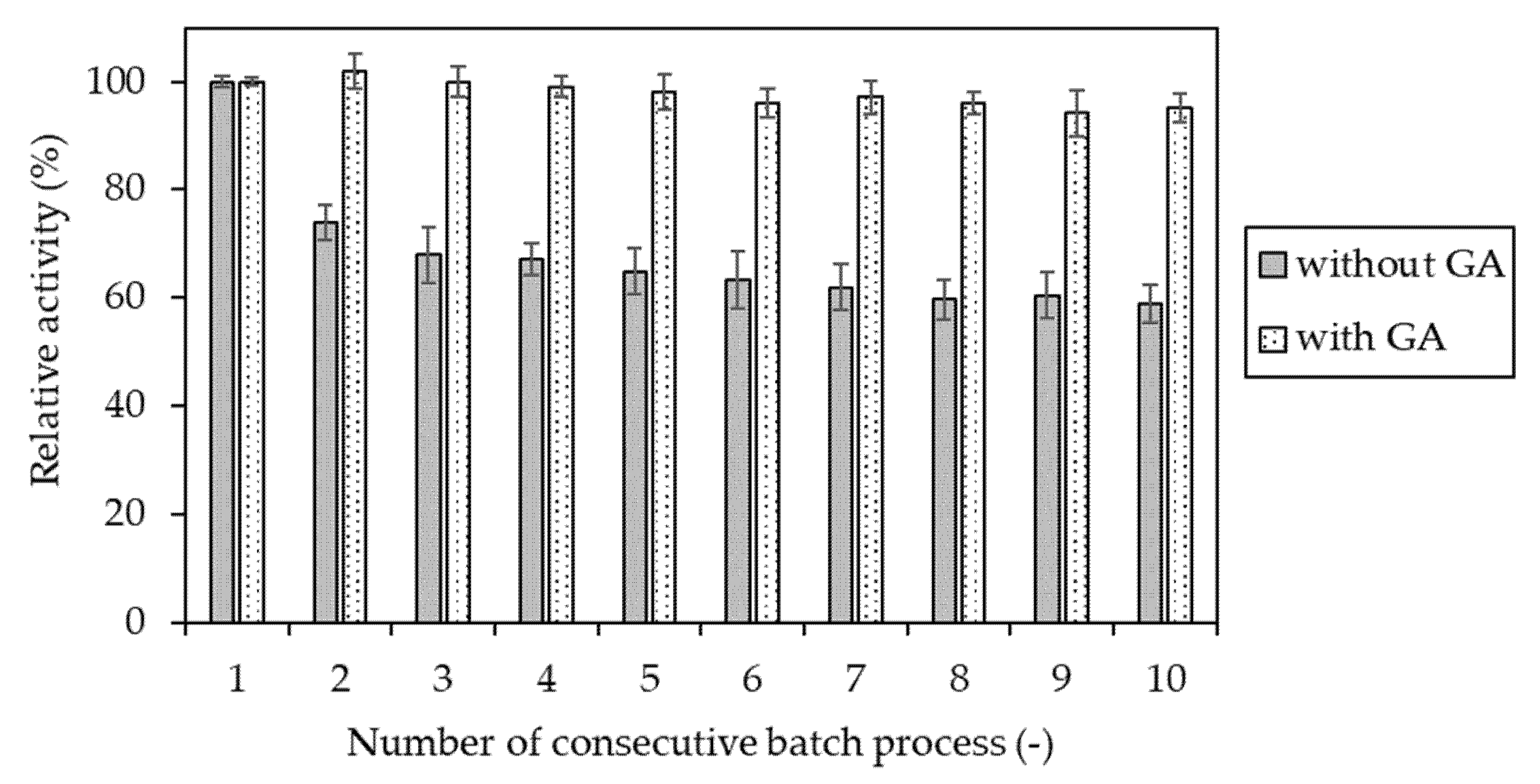
4.2.10. Determination of Operational Stability of Invertase Immobilized in Hydrogel Matrices
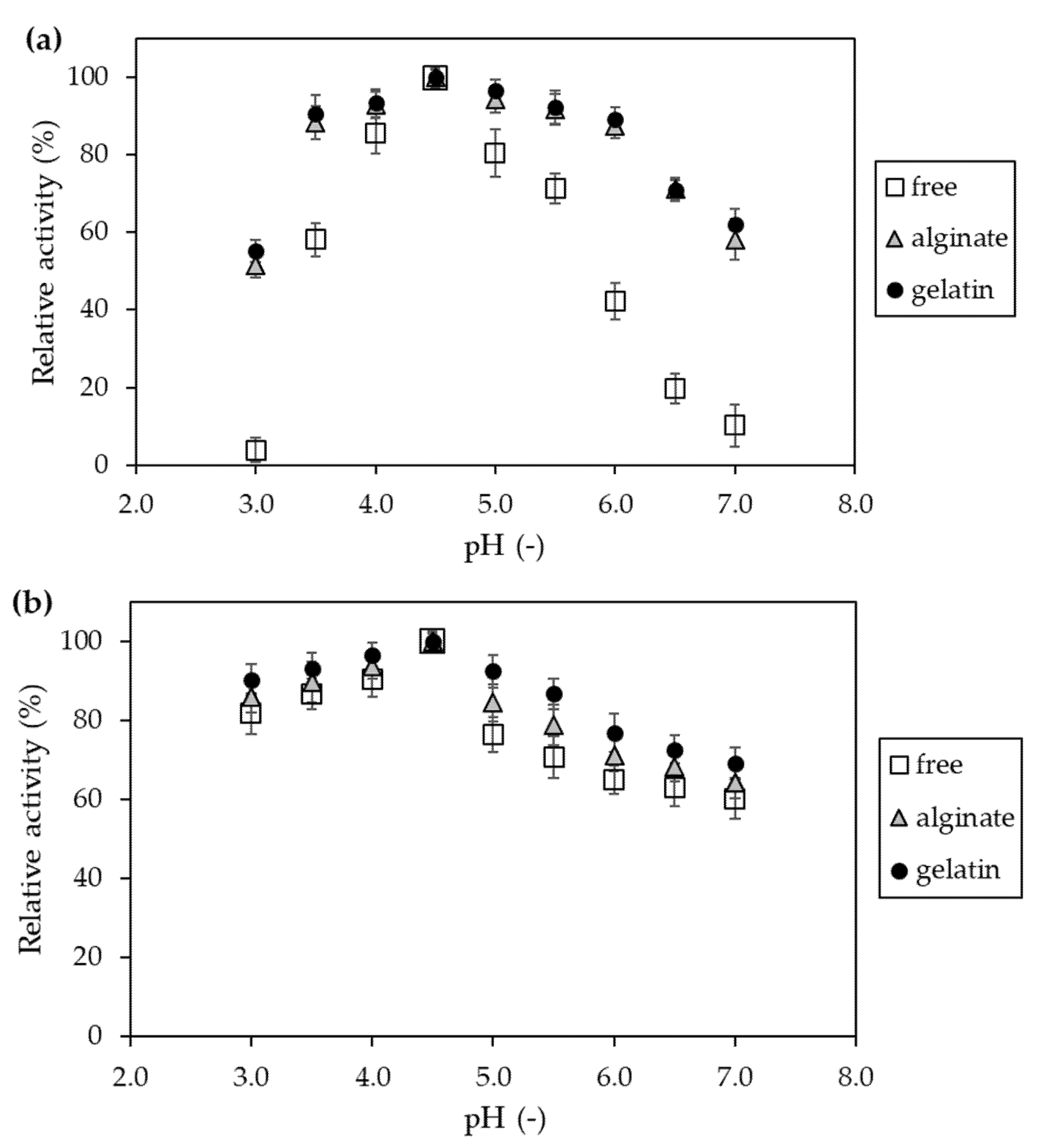
4.2.11. Determination of the Effect of pH on Activity and Stability of Invertase Immobilized in Hydrogel Matrices
4.2.12. Determination of the Effect of Temperature on Activity and Stability of Invertase Immobilized in Hydrogel Matrices
4.2.13. Determination of Storage Stability of Invertase Immobilized in Hydrogel Matrices
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kirk, O.; Borchert, T.V.; Fuglsang, C.C. Industrial enzyme applications. Curr. Opin. Biotechnol. 2002, 13, 345–351. [Google Scholar] [CrossRef]
- Choi, J.M.; Han, S.S.; Kim, H.S. Industrial applications of enzyme biocatalysis: Current status and future aspects. Biotechnol. Adv. 2015, 33, 1443–1454. [Google Scholar] [CrossRef] [PubMed]
- Basso, A.; Serban, S. Industrial applications of immobilized enzymes—A review. Mol. Catal. 2019, 479, 110607. [Google Scholar] [CrossRef]
- Chapman, J.; Ismail, A.; Dinu, C. Industrial Applications of Enzymes: Recent Advances, Techniques, and Outlooks. Catalysts 2018, 8, 238. [Google Scholar] [CrossRef]
- Miller, A.; Nordenberg, T. Food and Drug Administration. In Encyclopedia of Food Sciences and Nutrition; Caballero, B., Toldra, F., Finglas, P., Eds.; Academic Press: Cambridge, MA, USA, 2003; pp. 2593–2599. [Google Scholar]
- Singh, R.K.; Tiwari, M.K.; Singh, R.; Lee, J.K. From protein engineering to immobilization: Promising strategies for the upgrade of industrial enzymes. Int. J. Mol. Sci. 2013, 14, 1232–1277. [Google Scholar] [CrossRef]
- Bilal, M.; Zhao, Y.; Noreen, S.; Shah, S.Z.H.; Bharagava, R.N.; Iqbal, H.M.N. Modifying bio-catalytic properties of enzymes for efficient biocatalysis: A review from immobilization strategies viewpoint. Biocatal. Biotransform. 2019, 37, 159–182. [Google Scholar] [CrossRef]
- Sheldon, R.A. Enzyme immobilization: The quest for optimum performance. Adv. Synth. Catal. 2007, 349, 1289–1307. [Google Scholar] [CrossRef]
- Homaei, A.A.; Sariri, R.; Vianello, F.; Stevanato, R. Enzyme immobilization: An update. J. Chem. Biol. 2013, 6, 185–205. [Google Scholar] [CrossRef]
- Ó’Fágáin, C. Enzyme stabilization—Recent experimental progress. Enzyme Microb. Technol. 2003, 33, 137–149. [Google Scholar] [CrossRef]
- Liu, Q.; Xun, G.; Feng, Y. The state-of-the-art strategies of protein engineering for enzyme stabilization. Biotechnol. Adv. 2019, 37, 530–537. [Google Scholar] [CrossRef]
- Iyer, P.V.; Ananthanarayan, L. Enzyme stability and stabilization—Aqueous and non-aqueous environment. Process Biochem. 2008, 43, 1019–1032. [Google Scholar] [CrossRef]
- Guzik, U.; Hupert-Kocurek, K.; Wojcieszynska, D. Immobilization as a strategy for improving enzyme properties- Application to oxidoreductases. Molecules 2014, 19, 8995–9018. [Google Scholar] [CrossRef] [PubMed]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Noritomi, H.; Sasanuma, A.; Kato, S.; Nagahama, K. Catalytic properties of cross-linked enzyme crystals in organic media. Biochem. Eng. J. 2007, 33, 228–231. [Google Scholar] [CrossRef]
- Voběrková, S.; Solčány, V.; Vršanská, M.; Adam, V. Immobilization of ligninolytic enzymes from white-rot fungi in cross-linked aggregates. Chemosphere 2018, 202, 694–707. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, D.; Yin, L.; Wang, F. Preparation, activity and structure of cross-linked enzyme aggregates (CLEAs) with nanoparticle. Enzyme Microb. Technol. 2017, 107, 22–31. [Google Scholar] [CrossRef]
- Abraham, T.E.; Joseph, J.R.; Bindhu, L.B.V.; Jayakumar, K.K. Crosslinked enzyme crystals of glucoamylase as a potent catalyst for biotransformations. Carbohydr. Res. 2004, 339, 1099–1104. [Google Scholar] [CrossRef]
- Zdarta, J.; Meyer, A.S.; Jesionowski, T.; Pinelo, M. A general overview of support materials for enzyme immobilization: Characteristics, properties, practical utility. Catalysts 2018, 8, 92. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M.N. Naturally-derived biopolymers: Potential platforms for enzyme immobilization. Int. J. Biol. Macromol. 2019, 130, 462–482. [Google Scholar] [CrossRef]
- Chou, C.; Syu, S.; Chang, J.H.; Aimar, P.; Chang, Y. Bioinspired Pseudozwitterionic Hydrogels with Bioactive Enzyme Immobilization via pH-Responsive Regulation. Langmuir 2019, 35, 1909–1918. [Google Scholar] [CrossRef]
- Elnashar, M.M.M. The Art of Immobilization Using Biopolymers, Biomaterials and Nanobiotechnology. In Biotechnology of Biopolymers; InTech: London, UK, 2011; ISBN 978-953-307-179-4. [Google Scholar]
- Trusek-Holownia, A.; Noworyta, A. Efficient utilisation of hydrogel preparations with encapsulated enzymes—A case study on catalase and hydrogen peroxide degradation. Biotechnol. Rep. 2015, 6, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Labus, K.; Drozd, A.; Trusek-Holownia, A. Preparation and characterisation of gelatine hydrogels predisposed to use as matrices for effective immobilisation of biocatalystst. Chem. Pap. 2016, 70, 523–530. [Google Scholar]
- Czyzewska, K.; Trusek, A. Encapsulated catalase from Serratia genus for H2O2 decomposition in food applications. Polish J. Chem. Technol. 2018, 20, 39–43. [Google Scholar] [CrossRef]
- Batista, R.A.; Espitia, P.J.P.; Quintans, J.S.S.; Freitas, M.M.; Cerqueira, M.Â.; Teixeira, J.A.; Cardoso, J.C. Hydrogel as an alternative structure for food packaging systems. Carbohydr. Polym. 2019, 205, 106–116. [Google Scholar] [CrossRef]
- Joye, I.J. Cereal biopolymers for nano- and microtechnology: A myriad of opportunities for novel (functional) food applications. Trends Food Sci. Technol. 2019, 83, 1–11. [Google Scholar] [CrossRef]
- Kaczmarek, B.; Nadolna, K.; Owczarek, A. The physical and chemical properties of hydrogels based on natural polymers. In Hydrogels Based on Natural Polymers; Chen, Y., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 151–172. ISBN 9780128164211. [Google Scholar]
- Bao, Z.; Xian, C.; Yuan, Q.; Liu, G.; Wu, J. Natural Polymer-Based Hydrogels with Enhanced Mechanical Performances: Preparation, Structure, and Property. Adv. Healthc. Mater. 2019, 8, 1900670. [Google Scholar] [CrossRef]
- Ha, T.L.B.; Quan, T.M.; Vu, D.N.; Si, D.M. Chapter 11: Naturally Derived Biomaterials: Preparation and Application. In Regenerative Medicine and Tissue Engineering; InTech: London, UK, 2013; pp. 247–272. [Google Scholar]
- Koch-Schmidt, A. Gel-Entrapment of Enzymes. In Biomedical Applications of Immobilized Enzymes and Proteins; Chang, T.M.S., Ed.; Springer: Boston, MA, USA, 1977; pp. 47–67. [Google Scholar]
- Radosinski, L.; Labus, K.; Zemojtel, P.; Wojciechowski, J.W. Development and validation of a virtual gelatin model using molecular modeling computational tools. Molecules 2019, 24, 3365. [Google Scholar] [CrossRef]
- Košovan, P.; Richter, T.; Holm, C. Molecular Simulations of Hydrogels. In Intelligent Hydrogels; Springer Nature: Basel, Switzerland, 2013; pp. 205–220. ISBN 9783319016832. [Google Scholar]
- Saunders, J.R.; Abu-Salih, S.; Khaleque, T.; Hanula, S.; Moussa, W. Modeling theories of intelligent hydrogel polymers. J. Comput. Theor. Nanosci. 2008, 5, 1942–1960. [Google Scholar] [CrossRef]
- Casalini, T.; Perale, G. From Microscale to Macroscale: Nine Orders of Magnitude for a Comprehensive Modeling of Hydrogels for Controlled Drug Delivery. Gels 2019, 5, 28. [Google Scholar] [CrossRef]
- Braccini, I.; Pe, S. Molecular Basis of Ca2+-Induced Gelation in Alginates and Pectins: The Egg-Box Model Revisited. Biomacromolecules 2001, 2, 1089–1096. [Google Scholar] [CrossRef]
- Li, X.; Jia, J.; Mei, Y.; Latour, R.A. Molecular modeling to predict peptide accessibility for peptide-functionalized hydrogels. Biointerphases 2017, 12, 031008. [Google Scholar] [CrossRef] [PubMed]
- Ou, X.; Han, Q.; Dai, H.H.; Wang, J. Molecular dynamic simulations of the water absorbency of hydrogels. J. Mol. Model. 2015, 21, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Eichenbaum, G.M.; Kiser, P.F.; Shah, D.; Simon, S.A.; Needham, D. Investigation of the swelling response and drug loading of ionic microgels: The dependence on functional group composition. Macromolecules 1999, 32, 8996–9006. [Google Scholar] [CrossRef]
- Kamerlin, N.; Elvingson, C. Tracer diffusion in a polymer gel: Simulations of static and dynamic 3D networks using spherical boundary conditions. J. Phys. Condens. Matter 2016, 28. [Google Scholar] [CrossRef]
- Fujiyabu, T.; Yoshikawa, Y.; Chung, U.I.; Sakai, T. Structure-property relationship of a model network containing solvent. Sci. Technol. Adv. Mater. 2019, 20, 608–621. [Google Scholar] [CrossRef]
- Chan, L.W.; Jin, Y.; Heng, P.W.S. Cross-linking mechanisms of calcium and zinc in production of alginate microspheres. Int. J. Pharm. 2002, 242, 255–258. [Google Scholar] [CrossRef]
- Haug, A.; Smidsrød, O.; Högdahl, B.; Øye, H.A.; Rasmussen, S.E.; Sunde, E.; Sørensen, N.A. Selectivity of Some Anionic Polymers for Divalent Metal Ions. Acta Chem. Scand. 1970, 24, 843–854. [Google Scholar] [CrossRef]
- Russo, R.; Malinconico, M.; Santagata, G. Effect of cross-linking with calcium ions on the physical properties of alginate films. Biomacromolecules 2007, 8, 3193–3197. [Google Scholar] [CrossRef]
- Borgogna, M.; Skjåk-bræk, G.; Paoletti, S.; Donati, I. On the Initial Binding of Alginate by Calcium Ions. The Tilted Egg-Box Hypothesis. J. Phys. Chem. B 2013, 117, 7277–7282. [Google Scholar] [CrossRef]
- Brus, J.; Urbanova, M.; Czernek, J.; Pavelkova, M.; Kubova, K.; Vyslouzil, J.; Abbrent, S.; Konefal, R.; Horsky, J.; Vetchy, D.; et al. Structure and Dynamics of Alginate Gels Cross-Linked by Polyvalent Ions Probed via Solid State NMR Spectroscopy. Biomacromolecules 2017, 18, 2478–2488. [Google Scholar] [CrossRef]
- Orban, J.M.; Wilson, L.B.; Kofroth, J.A.; El-kurdi, M.S.; Maul, T.M.; Vorp, D.A. P14986330.pdf. J. Biomed. Mater. Res. A 2004, 68, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Haiyan, L.; Kunlong, M.; Zhenghua, X.; Xiaomei, R.; Gang, Y. Preparation and characteristics of gelatin sponges crosslinked by microbial transglutaminase. PeerJ 2017, 5, e3665. [Google Scholar]
- Dardelle, G.; Subramaniam, A.; Normand, V. Determination of covalent cross-linker efficacy of gelatin strands using calorimetric analyses of the gel state. Soft Matter 2011, 23, 3315–3322. [Google Scholar] [CrossRef]
- Yung, C.W.; Wu, L.Q.; Ullman, J.A.; Payne, G.F.; Bentley, W.E.; Barbari, T.A. Transglutaminase crosslinked gelatin as a tissue engineering scaffold. J. Biomed. Mater. Res. A 2007, 83, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Kurosawa, H.; Kokufuta, E.; Veliky, I.A. Preparation of immobilized glucomylase using ca-alginate gel coated with partially quaterized poly(ethleneimine). Biotechnol. Bioeng. 1984, 26, 1393–1394. [Google Scholar] [CrossRef]
- Ro, H.S.; Kim, H.S. Continuous production of gluconic acid and sorbitol from sucrose using invertase and an oxidoreductase of Zymomonas mobilis. Enzyme Microb. Technol. 1991, 13, 920–924. [Google Scholar] [CrossRef]
- Sainz-Polo, M.A.; Ramírez-Escudero, M.; Lafraya, A.; González, B.; Marín-Navarro, J.; Polaina, J.; Sanz-Aparicio, J. Three-dimensional structure of Saccharomyces invertase: Role of a non-catalytic domain in oligomerization and substrate specificity. J. Biol. Chem. 2013, 288, 9755–9766. [Google Scholar] [CrossRef]
- Stewart, W.W.; Swaisgood, H.E. Characterization of calcium alginate pore diameter by size-exclusion chromatography using protein standards. Enzyme Microb. Technol. 1993, 15, 922–927. [Google Scholar] [CrossRef]
- Schrieber, R.; Gareis, H. Gelatine Handbook: Theory and Industrial Practice; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2007; ISBN 9783527315482. [Google Scholar]
- Barnett, J.A. Beginnings of microbiology and biochemistry: The contribution of yeast research. Microbiology 2003, 149, 557–567. [Google Scholar] [CrossRef]
- Cantarel, B.I.; Coutinho, P.M.; Rancurel, C.; Bernard, T.; Lombard, V.; Henrissat, B. The Carbohydrate-Active EnZymes database (CAZy): An expert resource for glycogenomics. Nucleic Acids Res. 2009, 37, 233–238. [Google Scholar] [CrossRef]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Glutaraldehyde in bio-catalysts design: A useful crosslinker and a versatile tool in enzyme immobilization. RSC Adv. 2014, 4, 1583–1600. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M.N. Sustainable bioconversion of food waste into high-value products by immobilized enzymes to meet bio-economy challenges and opportunities—A review. Food Res. Int. 2019, 123, 226–240. [Google Scholar] [CrossRef] [PubMed]
- Alcántara, A.R. Biocatalysis and Pharmaceuticals: A Smart Tool for Sustainable Development. Catalysts 2019, 9, 792. [Google Scholar] [CrossRef]
- Schmieg, B.; Döbber, J.; Kirschhöfer, F.; Pohl, M.; Franzreb, M. Advantages of hydrogel-based 3D-printed enzyme reactors and their limitations for biocatalysis. Front. Bioeng. Biotechnol. 2019, 6, 1–12. [Google Scholar] [CrossRef]
- Bagal-Kestwal, D.; Kestwal, R.M.; Chiang, B.H.; Karve, M.S. Development of dip-strip sucrose sensors: Application of plant invertase immobilized in chitosan-guar gum, gelatin and poly-acrylamide films. Sens. Actuators B Chem. 2011, 160, 1026–1033. [Google Scholar] [CrossRef]
- Emregul, E.; Sungur, S.; Akbulut, U. Polyacrylamide-gelatine carrier system used for invertase immobilization. Food Chem. 2006, 97, 591–597. [Google Scholar] [CrossRef]
- Vujčić, Z.; Miloradović, Z.; Milovanović, A.; Božić, N. Cell wall invertase immobilisation within gelatin gel. Food Chem. 2011, 126, 236–240. [Google Scholar] [CrossRef]
- Kotwal, S.M.; Shankar, V. Immobilized invertase. Biotechnol. Adv. 2009, 27, 311–322. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar]
| Amino Acid | Percentage (%) | Amino Acid | Percentage (%) |
|---|---|---|---|
| Glycine (Gly) | 32.9 | Threonine (Thr) | 1.80 |
| Proline (Pro) | 13.2 | Phenylalanine (Phe) | 1.4 |
| Alanine (Ala) | 11.2 | Isoleucine (Ile) | 1.0 |
| Hydroxyproline (HPro) | 9.1 | Hydroxylysine (HLys) | 0.6 |
| Glutamic acid (Glu) | 4.8 | Asparagine (Asn) | 2.9 |
| Arginine (Arg) | 4.9 | Histidine (His) | 0.6 |
| Aspartic acid (Asp) | 2.9 | Tyrosine (Tyr) | 0.3 |
| Serine (Ser) | 3.5 | Methionine (Met) | 0.4 |
| Lysine (Lys) | 2.7 | Glutamine (Gln) | 2.5 |
| Leucine (Leu) | 2.3 | Cysteine (Cys) | 0.0 |
| Amino Acid | Percentage (%) | Amino Acid | Percentage (%) |
|---|---|---|---|
| Glycine (Gly) | 5.8 | Threonine (Thr) | 7.6 |
| Proline (Pro) | 5.1 | Phenylalanine (Phe) | 7.0 |
| Alanine (Ala) | 5.7 | Isoleucine (Ile) | 3.5 |
| Valine (Val) | 5.7 | Tryptophan (Trp) | 3.1 |
| Glutamic acid (Glu) | 5.8 | Asparagine (Asn) | 8.6 |
| Arginine (Arg) | 2.5 | Histidine (His) | 0.8 |
| Aspartic acid (Asp) | 6.2 | Tyrosine (Tyr) | 6.0 |
| Serine (Ser) | 9.0 | Methionine (Met) | 2.0 |
| Lysine (Lys) | 4.9 | Glutamine (Gln) | 3.5 |
| Leucine (Leu) | 6.6 | Cysteine (Cys) | 0.4 |
| CaCl2 Concentration (% w/v) | Activity of Bound Enzyme (U mL−1) | Immobilization Yield (%) | Enzyme Leakage (%) |
|---|---|---|---|
| 2 | 7570 | 38.0 | 20.7 |
| 4 | 9210 | 46.2 | 17.9 |
| 10 | 10,300 | 51.7 | 16.2 |
| 15 | 12,202 | 61.2 | 10.4 |
| 20 | 11,513 | 57.7 | 11.0 |
| 30 | 4012 | 20.1 | 10.7 |
| GA Concentration (% v/v) | Activity of Bound Enzyme (U mL−1) | Immobilization Yield (%) | Relative Activity of Bound Enzyme (%) | Enzyme Leakage (%) |
|---|---|---|---|---|
| 0.0 | 11,975 | 60.8 | 100 | 11.2 |
| 0.5 | 9292 | 47.2 | 77.6 | 8.6 |
| 1.0 | 8924 | 45.3 | 74.5 | 8.5 |
| 2.0 | 8620 | 43.7 | 72.0 | 7.4 |
| 4.0 | 6900 | 35.0 | 57.6 | 7.3 |
| Cross-Linking Time (min) | Activity of Bound Enzyme (U mL−1) | Immobilization Yield (%) | Relative Activity of Bound Enzyme (%) | Enzyme Leakage (%) |
|---|---|---|---|---|
| 0 | 11,471 | 59.1 | 100 | 11.0 |
| 5 | 9693 | 49.9 | 84.5 | 9.2 |
| 10 | 8631 | 44.5 | 75.2 | 7.9 |
| 20 | 8493 | 43.8 | 74.0 | 7.0 |
| 30 | 8383 | 43.2 | 73.1 | 5.5 |
| 45 | 8300 | 42.8 | 72.4 | 5.3 |
| 60 | 8245 | 42.5 | 71.9 | 5.4 |
| mTgase Concentration (% w/v) | Activity of Bound Enzyme (U mL−1) | Immobilization Yield (%) | Enzyme Leakage (%) |
|---|---|---|---|
| 0.25 | 3932 | 19.7 | 8.4 |
| 0.50 | 4503 | 22.6 | 3.3 |
| 1.00 | 4884 | 24.5 | 0.0 |
| 2.00 | 4820 | 24.2 | 0.0 |
| 3.00 | 4725 | 23.7 | 0.0 |
| 4.00 | 4852 | 24.3 | 0.0 |
| GA Concentration (% v/v) | Activity of Bound Enzyme (U mL−1) | Immobilization Yield (%) | Relative Activity of Bound Enzyme (%) | Enzyme Leakage (%) |
|---|---|---|---|---|
| 0.0 | 4826 | 24.5 | 100 | 0.0 |
| 0.5 | 4338 | 22.0 | 89.9 | 0.0 |
| 1.0 | 3884 | 19.7 | 80.5 | 0.0 |
| 2.0 | 3091 | 15.7 | 64.0 | 0.0 |
| 4.0 | 2352 | 11.9 | 48.7 | 0.0 |
| Cross-Linking Time (min) | Activity of Bound Enzyme (U mL−1) | Immobilization Yield (%) | Relative Activity of Bound Enzyme (%) | Enzyme Leakage (%) |
|---|---|---|---|---|
| 0 | 4249 | 22.4 | 100 | 0.0 |
| 5 | 3832 | 20.2 | 90.2 | 0.0 |
| 10 | 3801 | 20.0 | 89.4 | 0.0 |
| 20 | 3203 | 16.8 | 75.4 | 0.0 |
| 30 | 3076 | 16.2 | 72.4 | 0.0 |
| 45 | 3013 | 15.8 | 70.9 | 0.0 |
| 60 | 3044 | 16.0 | 71.6 | 0.0 |
| No. | Temperature Optimum [°C] | Thermostability* [%] | Operational Stability* after 10 Cycles [%] | Storage Stability* after 1 month [%] | Ref. [-] |
|---|---|---|---|---|---|
| 1 | 55 | 70 (in 60 °C) 40 (in 70 °C) | 95 | 82 | Current study |
| 2 | 45 | 28 (in 65 °C) | 71 | 93 | [62] |
| 3 | 55 | 30 (in 70 °C) | 30–80 | - | [63] |
| 4 | 60 | 75 (in 60 °C) 10 (in 70 °C) | 90 | - | [64] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Labus, K.; Wolanin, K.; Radosiński, Ł. Comparative Study on Enzyme Immobilization Using Natural Hydrogel Matrices—Experimental Studies Supported by Molecular Models Analysis. Catalysts 2020, 10, 489. https://doi.org/10.3390/catal10050489
Labus K, Wolanin K, Radosiński Ł. Comparative Study on Enzyme Immobilization Using Natural Hydrogel Matrices—Experimental Studies Supported by Molecular Models Analysis. Catalysts. 2020; 10(5):489. https://doi.org/10.3390/catal10050489
Chicago/Turabian StyleLabus, Karolina, Kamila Wolanin, and Łukasz Radosiński. 2020. "Comparative Study on Enzyme Immobilization Using Natural Hydrogel Matrices—Experimental Studies Supported by Molecular Models Analysis" Catalysts 10, no. 5: 489. https://doi.org/10.3390/catal10050489
APA StyleLabus, K., Wolanin, K., & Radosiński, Ł. (2020). Comparative Study on Enzyme Immobilization Using Natural Hydrogel Matrices—Experimental Studies Supported by Molecular Models Analysis. Catalysts, 10(5), 489. https://doi.org/10.3390/catal10050489







