New Trends in the Conversion of CO2 to Cyclic Carbonates
Abstract
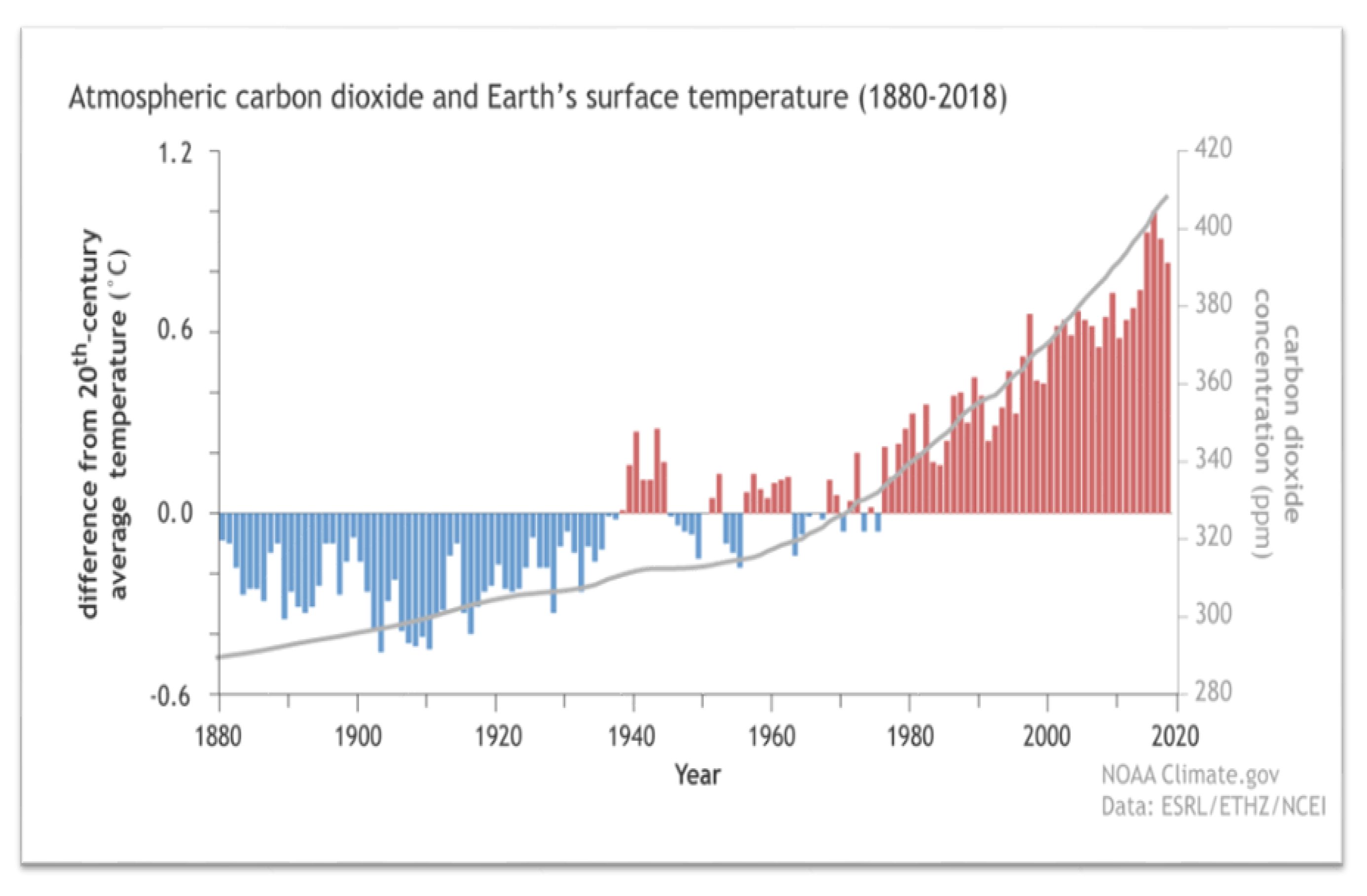
1. Introduction
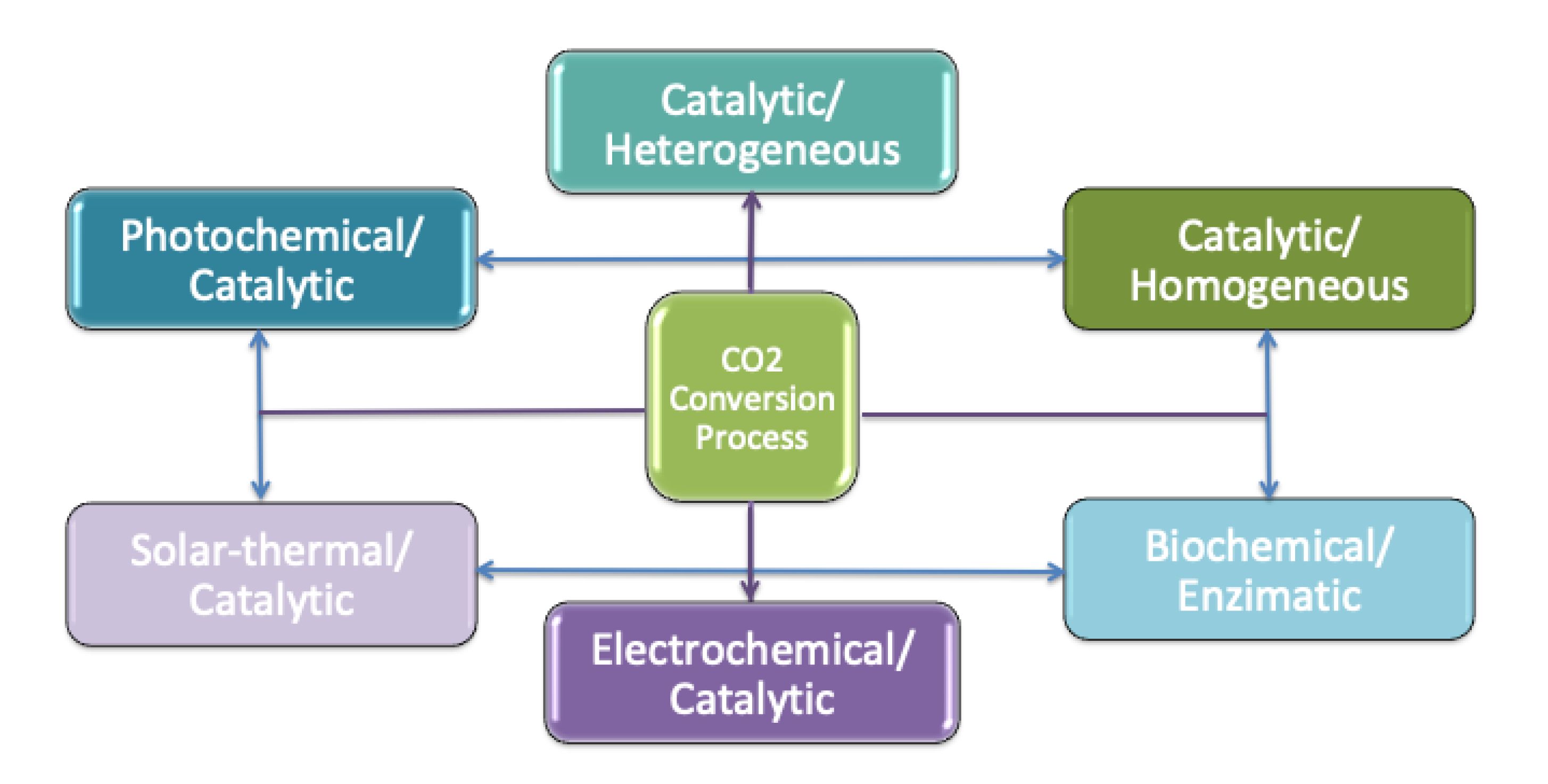
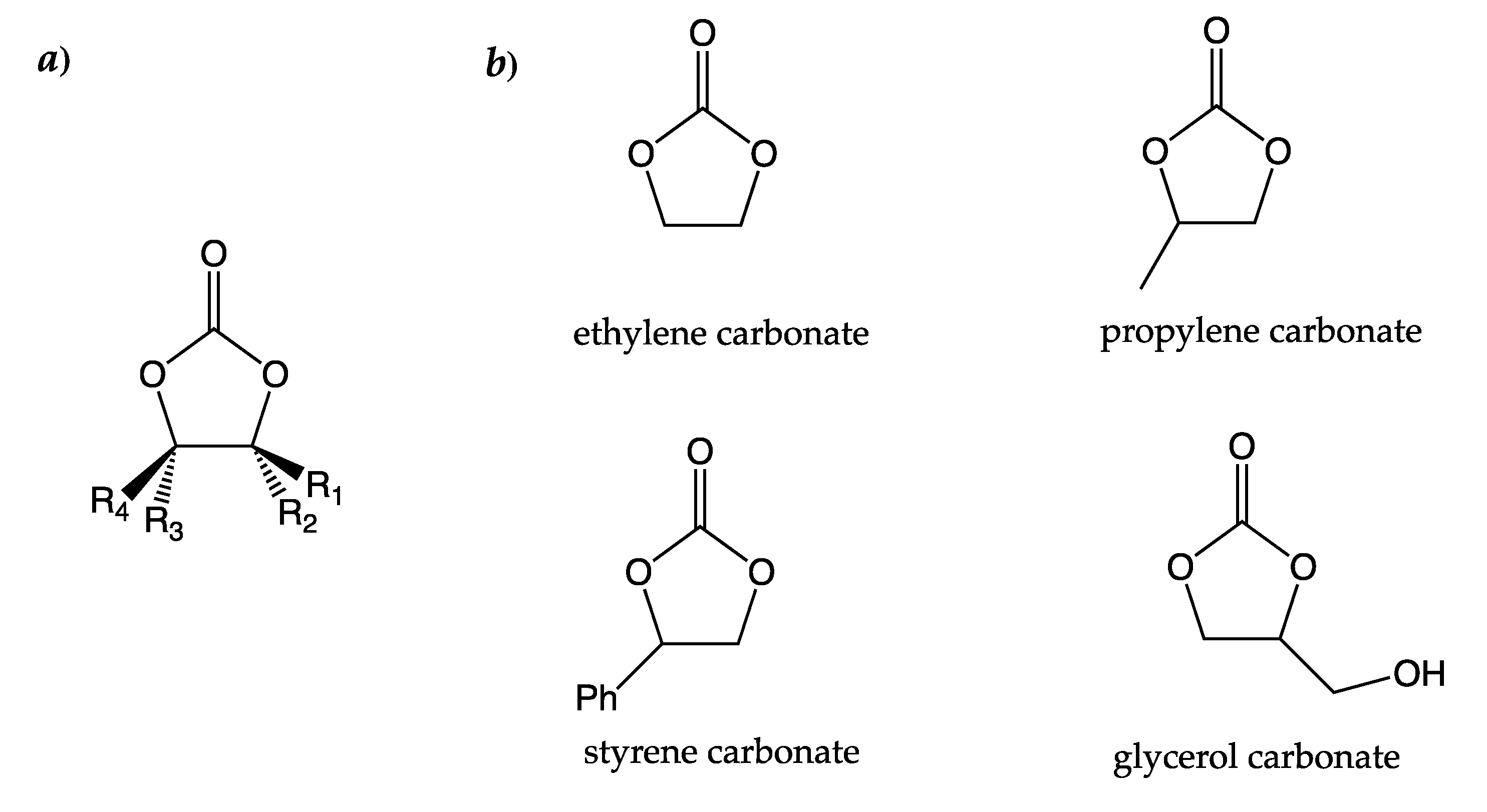
2. Carbon Dioxide to Carbonates
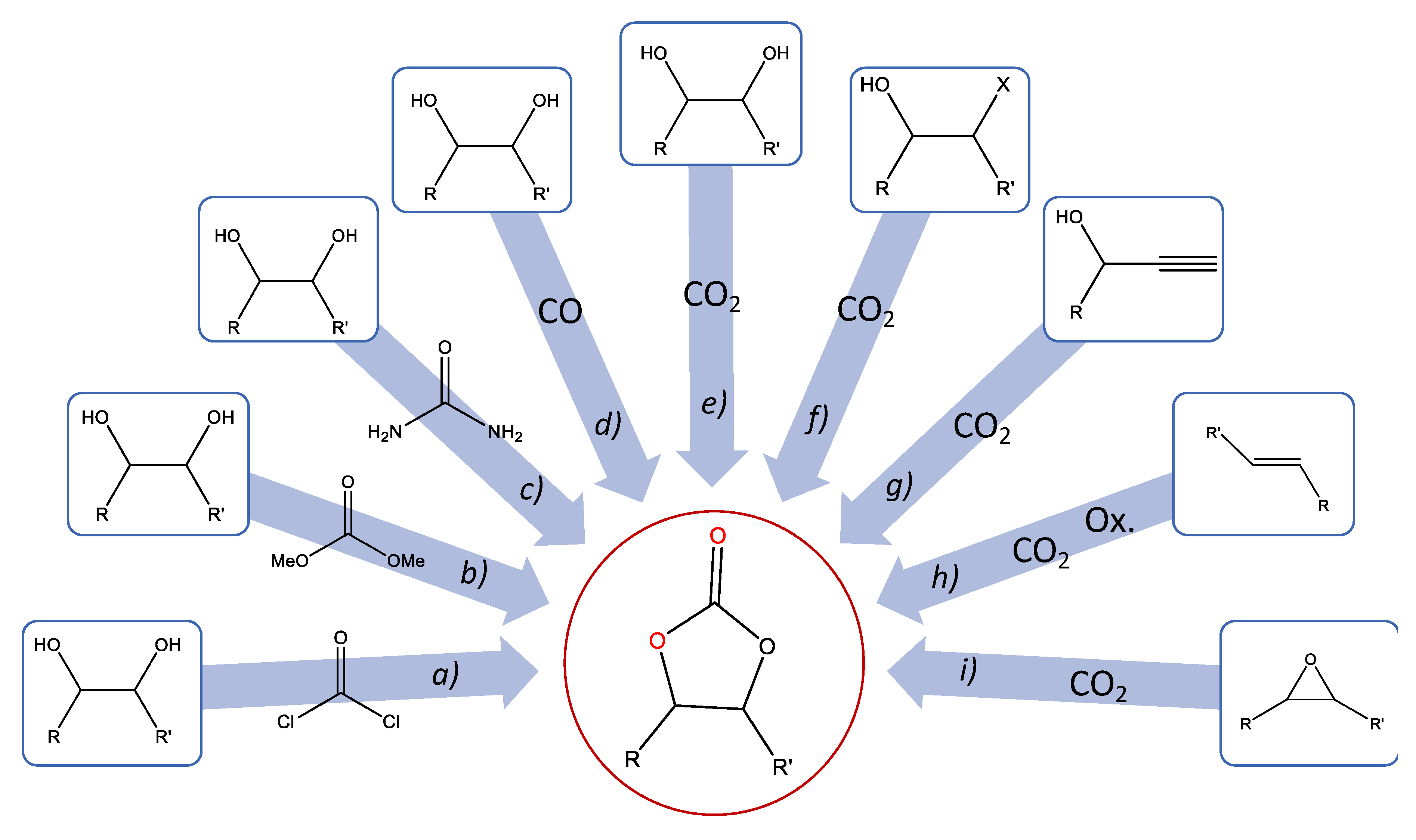
3. Carbonates from Diols
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nunez, C. Global Warming Solutions, Explained. Available online: https://www.nationalgeographic.com (accessed on 7 March 2020).
- Everything You Wanted to Know about Our Changing Climate but Were too Afraid to Ask. Available online: https://www.nrdc.org/stories/global-warming-101 (accessed on 5 March 2020).
- Global Climate Change. Available online: https://www.climate.nasa.gov (accessed on 4 March 2020).
- Lindsey, R. If Carbon Dioxide Hits a New High Every Year, Why isn’t Every Year Hotter than the Last? Available online: https://www.climate.gov (accessed on 7 March 2020).
- Zevenhoven, R.; Eloneva, S.; Teir, S. Chemical fixation of CO2 in carbonates: Routes to valuable products and long-term storage. Catal. Today 2006, 115, 73–79. [Google Scholar] [CrossRef]
- Alper, E.; Orhan, O.Y. CO2 utilization: Developments in conversion processes. Petroleum 2017, 3, 109–126. [Google Scholar] [CrossRef]
- Cornils, B.; Herrmann, W.A.; Beller, M.; Paciello, R. Applied Homogeneous Catalysis with Organometallic Compounds: A Comprehensive Handbook in Four Volumes, 3rd ed.; Wiley-VCH: Weinheim, Germany, 2017. [Google Scholar]
- Tamura, M.; Honda, M.; Nakagawa, Y.; Tomishige, K. Direct conversion of CO2 with diols, aminoalcohols and diamines to cyclic carbonates, cyclic carbamates and cyclic ureas using heterogeneous catalysts. J. Chem. Techn. Biotech. 2014, 89, 19–33. [Google Scholar] [CrossRef]
- Buttner, H.; Longwitz, L.; Steinbauer, J.; Wulf, C.; Werner, T. Recent Developments in the Synthesis of Cyclic Carbonates from Epoxides and CO2. Top Curr. Chem. 2017, 375, 50–106. [Google Scholar] [CrossRef]
- Leitner, W. The coordination chemistry of carbon dioxide and its relevance for catalysis: A critical study. Coord. Chem. Rev. 1996, 155, 247–284. [Google Scholar] [CrossRef]
- Tundo, P.; Selva, M. The chemistry of dimethyl carbonate. Acc. Chem. Res. 2002, 35, 706–716. [Google Scholar] [CrossRef]
- McGhee, W.D.; Pan, Y.; Riley, D.P. Highly selective generation of urethanes from amines, carbon-dioxide and alkyl chlorides. J. Chem. Soc. Chem. Com. 1994, 699–700. [Google Scholar] [CrossRef]
- Leitner, W. Carbon-dioxide as a raw-material-the synthesis of formic acid and its derivatives from CO2. Angew. Chem. Int. Ed. Engl. 1995, 34, 2207–2221. [Google Scholar] [CrossRef]
- Ribeiro, A.P.C.; Martins, L.M.D.R.S.; Pombeiro, A.J.L. Carbon dioxide-to-methanol single-pot conversion using a C-scorpionate iron(II) catalyst. Green Chem. 2017, 19, 4801–4962. [Google Scholar] [CrossRef]
- Montoya, C.A.; Gómez, C.F.; Paninho, A.B.; Nunes, A.V.M.; Mahmudov, K.T.; Najdanovic-Visak, V.; Martins, L.M.D.R.S.; Guedes da Silva, M.F.C.; Nunes da Ponte, M.; Pombeiro, A.J.L. Cyclic carbonate synthesis from CO2 and epoxides using zinc(II) complexes of arylhydrazones of β-diketones. J. Catal. 2016, 335, 135–140. [Google Scholar] [CrossRef]
- Chen, Z.; Hadjichristidis, N.; Feng, X.; Gnanou, Y. Poly(urethane–carbonate) from Carbon Dioxide. Macromolecules 2017, 50, 2320–2328. [Google Scholar] [CrossRef]
- Parker, H.L.; Sherwood, J.; Hunt, A.J.; Clark, J.H. Cyclic Carbonates as Green Alternative Solvents for the Heck Reaction. ACS Sust. Chem. Eng. 2014, 2, 1739–1742. [Google Scholar] [CrossRef]
- Beattie, C.; North, M.; Villuendas, P. Proline-Catalysed Amination Reactions in Cyclic Carbonate Solvents. Molecules 2011, 16, 3420–3432. [Google Scholar] [CrossRef] [PubMed]
- Scrosati, B.; Hassoun, J.; Sun, Y.K. Lithium-ion batteries. A look into the future. Energy Environ. Sci. 2011, 4, 3287–3295. [Google Scholar] [CrossRef]
- Guerin, W.; Diallo, A.K.; Kirillov, E.; Helou, M.; Slawinski, M.J.; Brusson, M.; Carpentier, J.F.; Guillaume, S.M. Enantiopure Isotactic PCHC Synthesized by Ring-Opening Polymerization of Cyclohexene Carbonate. Macromolecules 2014, 47, 4230–4235. [Google Scholar] [CrossRef]
- Wang, P.; Liu, S.; Zhou, F.; Yang, B.; Alshammari, A.S.; Lu, L.; Deng, Y. Two-step synthesis of dimethyl carbonate from urea, ethylene glycol and methanol using acid–base bifunctional zinc-yttrium oxides. Fuel Process. Technol. 2014, 126, 359–365. [Google Scholar] [CrossRef]
- Selva, M.; Caretto, A.; Noe, M.; Perosa, A. Carbonate phosphonium salts as catalysts for the transesterification of dialkyl carbonates with diols. The competition between cyclic carbonates and linear dicarbonate products. Org. Biomol. Chem. 2014, 12, 4143–4156. [Google Scholar] [CrossRef]
- Khusnutdinov, R.I.; Shchadneva, N.A.; Mayakova, Y.Y. Reactions of diols with dimethyl carbonate in the presence of W(CO)6 and Co2(CO)8. Russ. J. Org. Chem. 2014, 50, 948–952. [Google Scholar] [CrossRef]
- Indran, V.P.; Saud, A.S.H.; Maniam, G.P.; Yusoff, M.M.; Taufiq-Yap, Y.H.; Rahim, M.H.A. Versatile boiler ash containing potassium silicate for the synthesis of organic carbonates. RSC Adv. 2016, 6, 34877–34884. [Google Scholar] [CrossRef]
- Kuznetsov, V.A.; Pervova, M.G.; Pestov, A.V. Synthesis of alkylene carbonates in ionic liquid. Russ. J. Org. Chem. 2013, 49, 1859–1860. [Google Scholar] [CrossRef]
- Peña-López, M.; Neumann, H.; Beller, M. Iron-Catalyzed Synthesis of Five-Membered Cyclic Carbonates from Vicinal Diols: Urea as Sustainable Carbonylation Agent. Eur. J. Org. Chem. 2016, 3721–3727. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, W.; Zhao, N.; Wei, W.; Sun, Y. Synthesis of cyclic carbonates from urea and diols over metal oxides. Catal. Today 2006, 115, 111–116. [Google Scholar] [CrossRef]
- Castro-Osma, A.J.; North, M.; Wu, X. Synthesis of Cyclic Carbonates Catalysed by Chromium and Aluminium Salphen Complexes. Chem. A Eur. J. 2016, 22, 2100–2107. [Google Scholar] [CrossRef] [PubMed]
- Doro, F.; Winnertz, P.; Leitner, W.; Prokofieva, A.; Muller, T.E. Adapting a Wacker-type catalyst system to the palladium-catalyzed oxidative carbonylation of aliphatic polyols. Green Chem. 2011, 13, 292–295. [Google Scholar] [CrossRef]
- Gabriele, B.; Mancuso, R.; Salerno, G.; Veltri, L.; Costa, M.; Dibenedetto, A. A General and Expedient Synthesis of 5- and 6-Membered Cyclic Carbonates by Palladium-Catalyzed Oxidative Carbonylation of 1,2- and 1,3-Diols. ChemSusChem 2011, 4, 1778–1786. [Google Scholar] [CrossRef]
- Pearson, D.M.; Conley, N.R.; Waymouth, R.M. Palladium-Catalyzed Carbonylation of Diols to Cyclic Carbonates. Adv. Synth. Catal. 2011, 353, 3007–3013. [Google Scholar] [CrossRef]
- Bobbink, F.D.; Gruszka, W.; Hulla, M.; Das, S.; Dyson, P.J. Synthesis of cyclic carbonates from diols and CO2 catalyzed by carbenes. Chem. Commun. 2016, 52, 10787–10790. [Google Scholar] [CrossRef]
- Honda, M.; Tamura, M.; Nakao, K.; Suzuki, K.; Nakagawa, Y.; Tomishige, K. Direct Cyclic Carbonate Synthesis from CO2 and Diol over Carboxylation/Hydration Cascade Catalyst of CeO2 with 2-Cyanopyridine. ACS Catal. 2014, 4, 1893–1896. [Google Scholar] [CrossRef]
- Lim, Y.N.; Lee, C.; Jang, H.-Y. Metal-Free Synthesis of Cyclic and Acyclic Carbonates from CO2 and Alcohols. Eur. J. Org. Chem. 2014, 1823–1826. [Google Scholar] [CrossRef]
- de Caro, P.; Bandres, M.; Urrutigoïty, M.; Cecutti, C.; Thiebaud-Roux, S. Recent Progress in Synthesis of Glycerol Carbonate and Evaluation of Its Plasticizing Properties. Front. Chem. 2019, 308, 1–13. [Google Scholar] [CrossRef]
- Hirose, T.M.; Shimizu, S.; Qu, S.; Shitara, H.; Kodama, K.; Wang, L. Economical synthesis of cyclic carbonates from carbon dioxide and halohydrins using K2CO3. RSC Adv. 2016, 6, 69040–69044. [Google Scholar] [CrossRef]
- Chen, K.; Shi, G.; Dao, R.; Mei, K.; Zhou, X.; Li, H.; Wang, C. Tuning the basicity of ionic liquids for efficient synthesis of alkylidene carbonates from CO2 at atmospheric pressure. Chem. Comm. 2016, 52, 7830–7833. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Ma, J.; Zhu, Q.; Qian, Q.; Han, H.; Mei, Q.; Han, B. Zinc(II)-catalyzed reactions of carbon dioxide and propargylic alcohols to carbonates at room temperature. Green Chem. 2016, 18, 382–385. [Google Scholar] [CrossRef]
- Gu, Y.; Shi, F.; Deng, Y. Ionic Liquid as an Efficient Promoting Medium for Fixation of CO2: Clean Synthesis of α-Methylene Cyclic Carbonates from CO2 and Propargyl Alcohols Catalyzed by Metal Salts under Mild Conditions. J. Org. Chem. 2004, 69, 391–394. [Google Scholar] [CrossRef]
- Wu, J.; Kozak, J.A.; Simeon, F.; Hatton, T.A.; Jamison, T.F. Mechanism-guided design of flow systems for multicomponent reactions: Conversion of CO2 and olefins to cyclic carbonates. Chem. Sci. 2014, 5, 1227–1231. [Google Scholar] [CrossRef]
- Huang, S.Y.; Liu, S.G.; Li, J.P.; Wei, W.; Ning, Z.H.A.O.; Wei, W.E.I.; Sun, Y.H. Synthesis of cyclic carbonate from carbon dioxide and diols over metal acetates. J. Fuel Chem. Tech. 2007, 35, 701–705. [Google Scholar] [CrossRef]
- Büttner, H.; Steinbauer, J.; Werner, T. Synthesis of Cyclic Carbonates from Epoxides and Carbon Dioxide by Using Bifunctional One-Component Phosphorus-Based Organocatalysts. ChemSusChem 2015, 16, 2655–2669. [Google Scholar] [CrossRef]
- Tomishige, K.; Yasuda, H.; Yoshida, Y.; Nurunnabi, M.; Li, B.; Kunimori, K. Novel Route to Propylene Carbonate: Selective Synthesis from Propylene Glycol and Carbon Dioxide. Catal. Lett. 2004, 95, 45–49. [Google Scholar] [CrossRef]
- Cui, K.; Liang, Z.; Zhang, J.; Zhang, Y. Synthesis of Cyclohexene Carbonate Catalyzed by Polymer-Supported Catalysts. Synt. Comm. 2015, 45, 702–713. [Google Scholar] [CrossRef]
- Tomishige, K.; Tamura, M.; Nakagawa, Y. Conversion with Alcohols and Amines into Carbonates, Ureas, and Carbamates over CeO2 Catalyst in the Presence and Absence of 2-Cyanopyridine. Chem. Rec. 2019, 1354–1379. [Google Scholar] [CrossRef]
- Comerford, J.W.; Ingram, I.D.; North, V.M.; Wu, X. Sustainable metal-based catalysts for the synthesis of cyclic carbonates containing five-membered rings. Green Chem. 2015, 17, 1966–1987. [Google Scholar] [CrossRef]
- Bobbink, F.D.; Vasilyev, D.; Hulla, M.; Chamam, S.; Menoud, F.; Laurenczy, G. Intricacies of Cation–Anion Combinations in Imidazolium Salt-Catalyzed Cycloaddition of CO2 Into Epoxides. ACS Catal. 2018, 8, 2589–2594. [Google Scholar] [CrossRef]
- Chang, H.; Li, Q.; Cui, X.; Wang, H.; Bu, Z.; Qiao, C.; Lin, T. Conversion of carbon dioxide into cyclic carbonates using wool powder-KI as catalysts. J. CO2 Util. 2018, 24, 174–179. [Google Scholar] [CrossRef]
- Bobbink, F.D.; Dyson, P.J. Synthesis of carbonates and related compounds incorporating CO2 using ionic liquid-type catalysts: State-of-the-art and beyond. J. Catal. 2016, 343, 52–61. [Google Scholar] [CrossRef]
- Calabrese, C.; Giacalone, F.; Aprile, C. Hybrid Catalysts for CO2 Conversion into Cyclic Carbonates. Catalysts 2019, 9, 325. [Google Scholar] [CrossRef]
- Müller, K.; Mokrushina, L.; Arlt, W. Thermodynamic Constraints for the Utilization of CO2. Chem. Ing. Tech. 2014, 86, 497–503. [Google Scholar] [CrossRef]
- Tomishige, K.; Yasuda, H.; Yoshida, Y.; Nurunnabi, M.; Li, B.; Kunimori, K. Catalytic performance and properties of ceria-based catalysts for cyclic carbonate synthesis from glycol and carbon dioxide. Green Chem. 2004, 6, 206–214. [Google Scholar] [CrossRef]
- Tomishige, K.; Kunimori, K. Catalytic and direct synthesis of dimethyl carbonate starting from carbon dioxide using CeO2-ZrO2 solid solution heterogeneous catalyst: Effect of H2O removal from the reaction system. App. Catal. A Gen. 2002, 237, 103–109. [Google Scholar] [CrossRef]
- McGuire, T.M.; Lopez-Vidal, E.M.; Gregory, G.L.; Buchard, A. Synthesis of 5- to 8-membered cyclic carbonates from diols and CO: A one-step, atmospheric pressure and ambient temperature procedure. J. CO2 Util. 2018, 27, 283–288. [Google Scholar] [CrossRef]
- Kamphuis, A.J.; Picchionia, F.; Pescarmona, P.P. CO2-fixation into cyclic and polymeric carbonates: Principles and applications. Green Chem. 2019, 21, 406–448. [Google Scholar] [CrossRef]
- Katritzky, A.R. Comprehensive Organic Functional Group Transformations: Synthesis: Carbon with One Heteroatom Attached by a Single Bond; Elsevier: Amsterdam, The Netherlands, 1995; Volume 2. [Google Scholar]
- Wu, L.; Wang, H.; Tu, Z.; Ding, B.; Xiao, Y.; Lu, J. Synthesis of Cyclic Carbonates from CO2 and Diols via Electrogenerated N-Heterocyclic Carbenes. Int. J. Electrochem. Sci. 2012, 7, 11540–11549. [Google Scholar]
- Okoturo, O.O.; VanderNoot, T.J. Temperature dependence of viscosity for room temperature ionic liquids. J. Electroanal. Chem. 2004, 568, 167–181. [Google Scholar] [CrossRef]
- Bobbink, F.D.; Das, S.; Laurenczy, G.; Dyson, P.J. Metal-Free Catalyst for the Chemoselective Methylation of Amines Using Carbon Dioxide as a Carbon Source. Angew. Chem. Int. Ed. 2014, 47, 12876–12879. [Google Scholar]
- Liu, J.; Li, Y.; Zhang, J.; He, D. Glycerol carbonylation with CO2 to glycerol carbonate over CeO2 catalyst and the influence of CeO2 preparation methods and reaction parameters. Appl. Catal. A Gen. 2016, 513, 9–18. [Google Scholar] [CrossRef]
- Zhang, J.; He, D. Synthesis of glycerol carbonate and monoacetin from glycerol and carbon dioxide over Cu catalysts: The role of supports. J. Chem. Technol. Biotechnol. 2015, 90, 1077–1085. [Google Scholar] [CrossRef]
- Morikawa, H.; Yamaguchi, J.; Sugimura, S.; Minamoto, M.; Gorou, Y.; Morinaga, H.; Motokucho, S. Systematic synthetic study of four diastereomerically distinct limonene-1, 2-diols and their corresponding cyclic carbonates. Beilstein J. Org. Chem. 2019, 15, 130–136. [Google Scholar] [CrossRef]
- Błażek, K.; Datta, J. Renewable natural resources as green alternative substrates to obtain bio-based non-isocyanate polyurethanes-review. Critical Rev. Environ. Sci. Tech. 2019, 49, 173–211. [Google Scholar] [CrossRef]
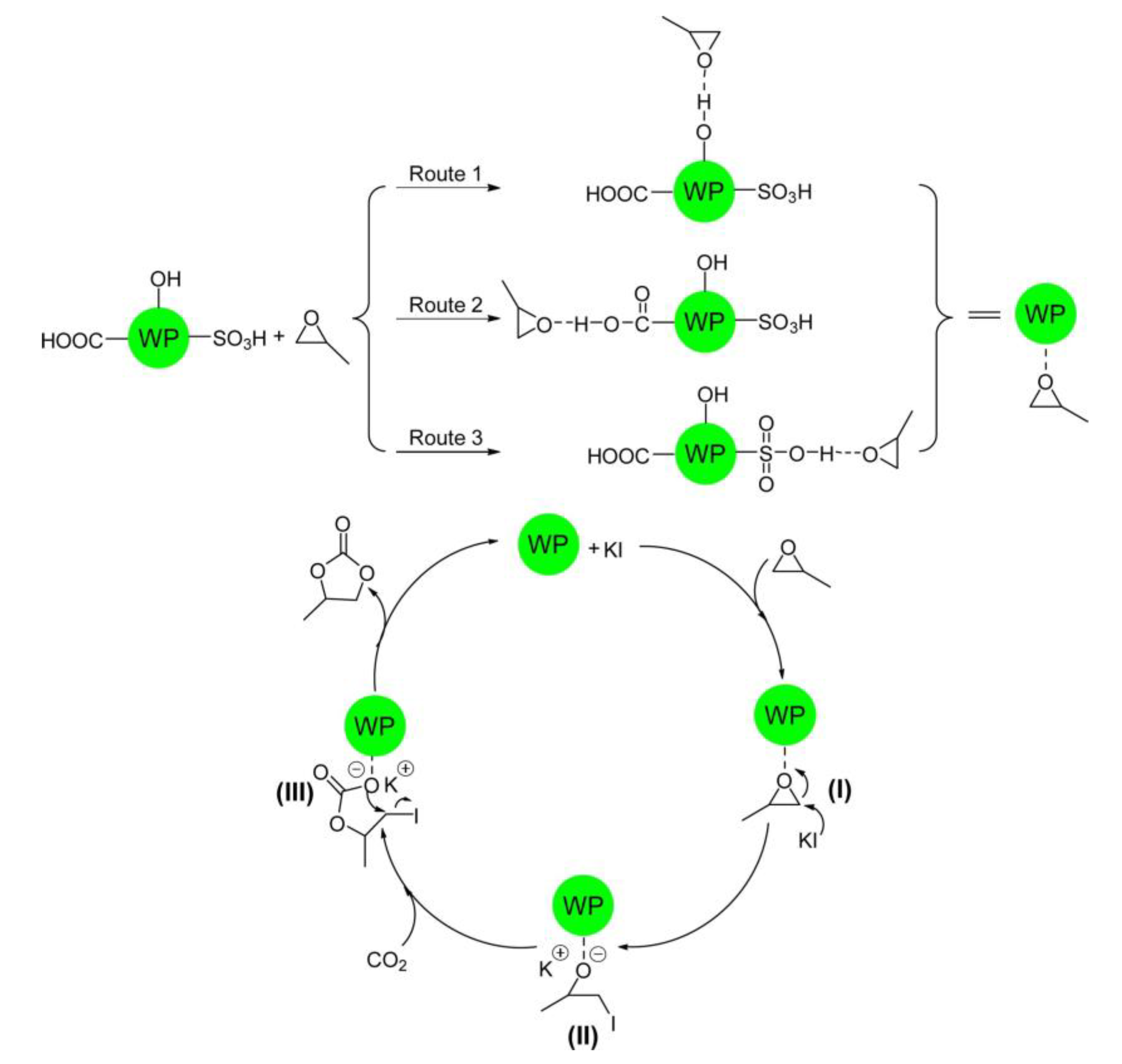
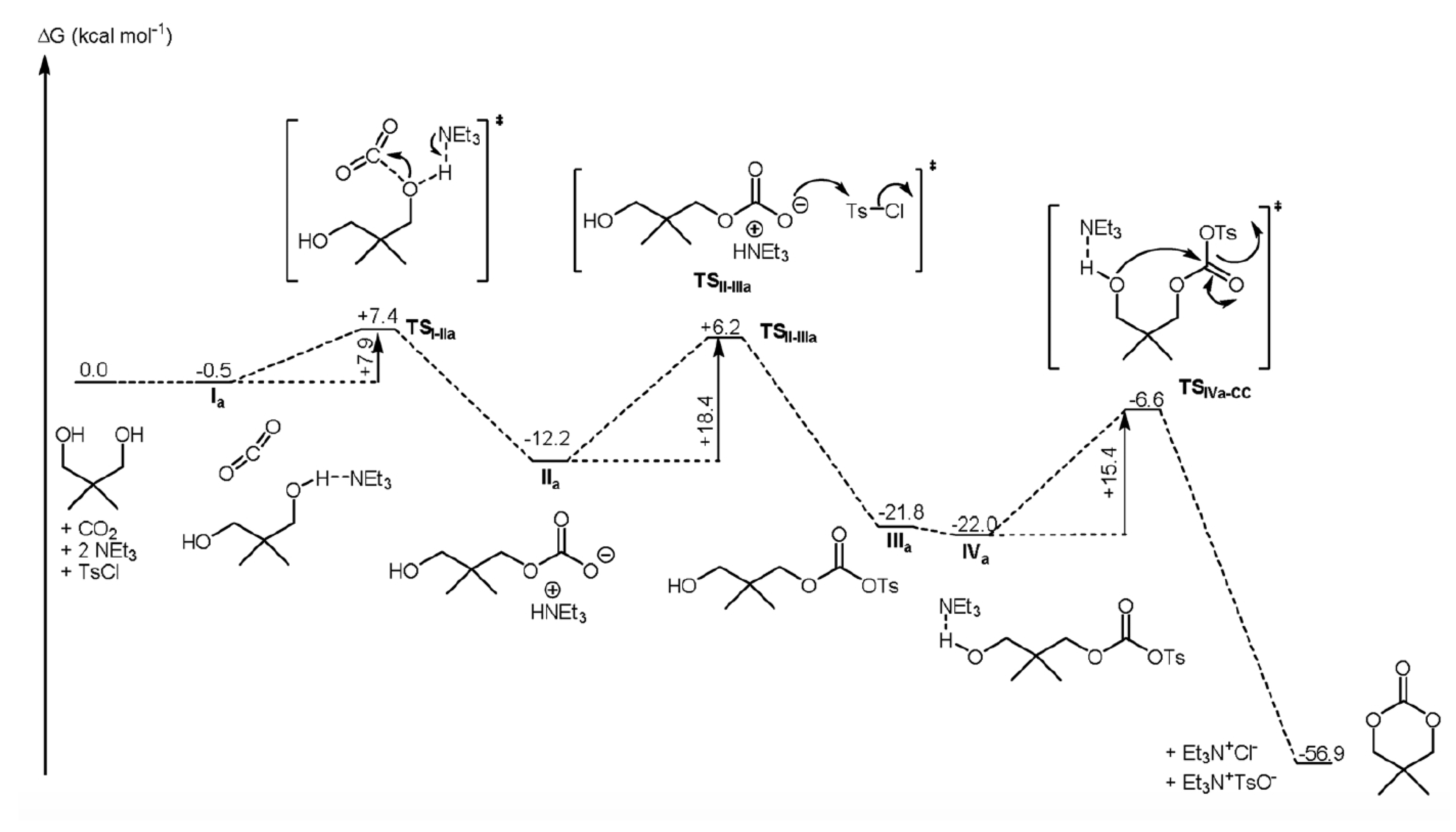


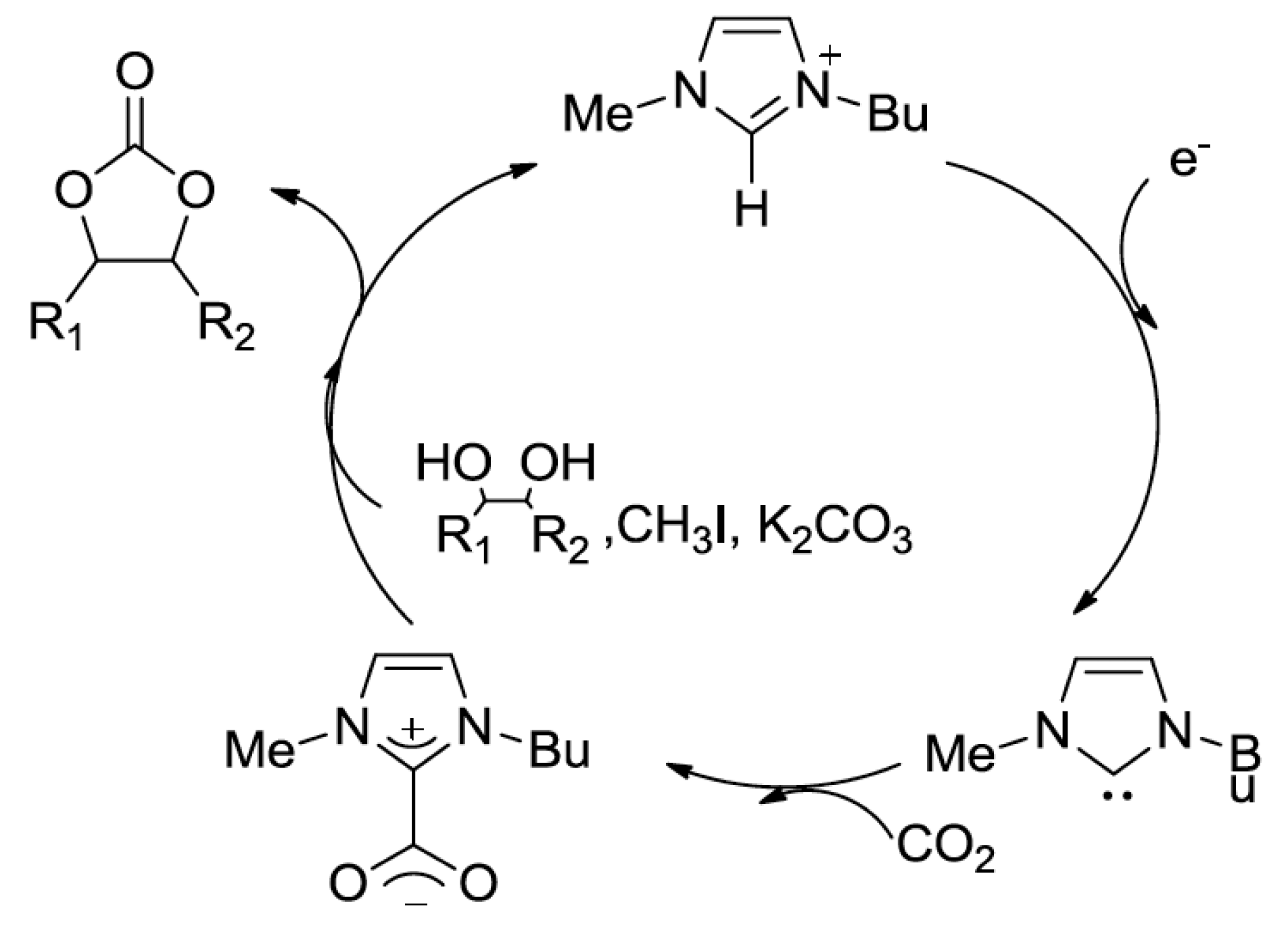
| Entry | Catalyst | SBET (m2 g−1) | Glycol Conversion (%) | Urea Conversion (%) | Selectivity (%) |
|---|---|---|---|---|---|
| 1 | CaO | 9.5 | 43.7 | 79.9 | 82.2 |
| 2 | La2O3 | 21 | 48.3 | 87.4 | 82.4 |
| 3 | MgO | 7.4 | 56.0 | 94.1 | 89.8 |
| 4 | ZnO | 6.7 | 62.6 | 97.8 | 95.2 |
| 5 | ZrO2 | 33.4 | 20.6 | 70.8 | 43.9 |
| 6 | Al2O3 | 136.5 | 19.0 | 71.2 | 40.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes, E.J.C.; Ribeiro, A.P.C.; Martins, L.M.D.R.S. New Trends in the Conversion of CO2 to Cyclic Carbonates. Catalysts 2020, 10, 479. https://doi.org/10.3390/catal10050479
Lopes EJC, Ribeiro APC, Martins LMDRS. New Trends in the Conversion of CO2 to Cyclic Carbonates. Catalysts. 2020; 10(5):479. https://doi.org/10.3390/catal10050479
Chicago/Turabian StyleLopes, Erivaldo J.C., Ana P.C. Ribeiro, and Luísa M.D.R.S. Martins. 2020. "New Trends in the Conversion of CO2 to Cyclic Carbonates" Catalysts 10, no. 5: 479. https://doi.org/10.3390/catal10050479
APA StyleLopes, E. J. C., Ribeiro, A. P. C., & Martins, L. M. D. R. S. (2020). New Trends in the Conversion of CO2 to Cyclic Carbonates. Catalysts, 10(5), 479. https://doi.org/10.3390/catal10050479








