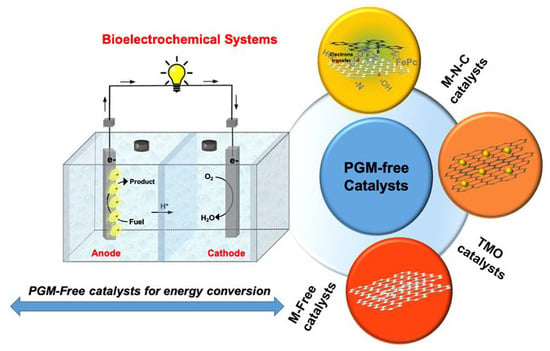
Platinum Group Metal-Free Catalysts for Oxygen Reduction Reaction: Applications in Microbial Fuel Cells
Abstract
1. Introduction
2. Main Types of Bioelectrochemical Systems
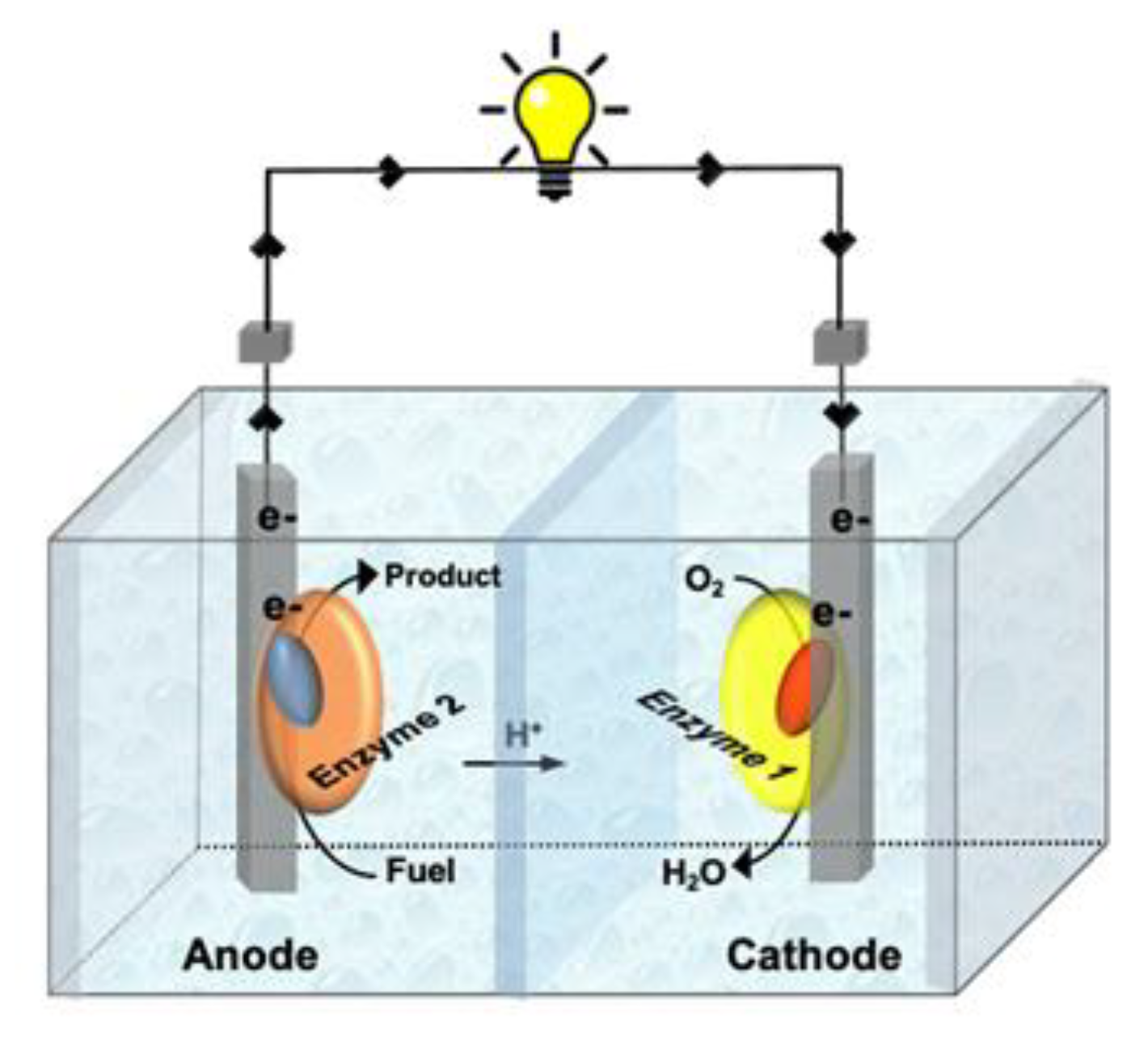
2.1. Enzymatic Fuel Cells
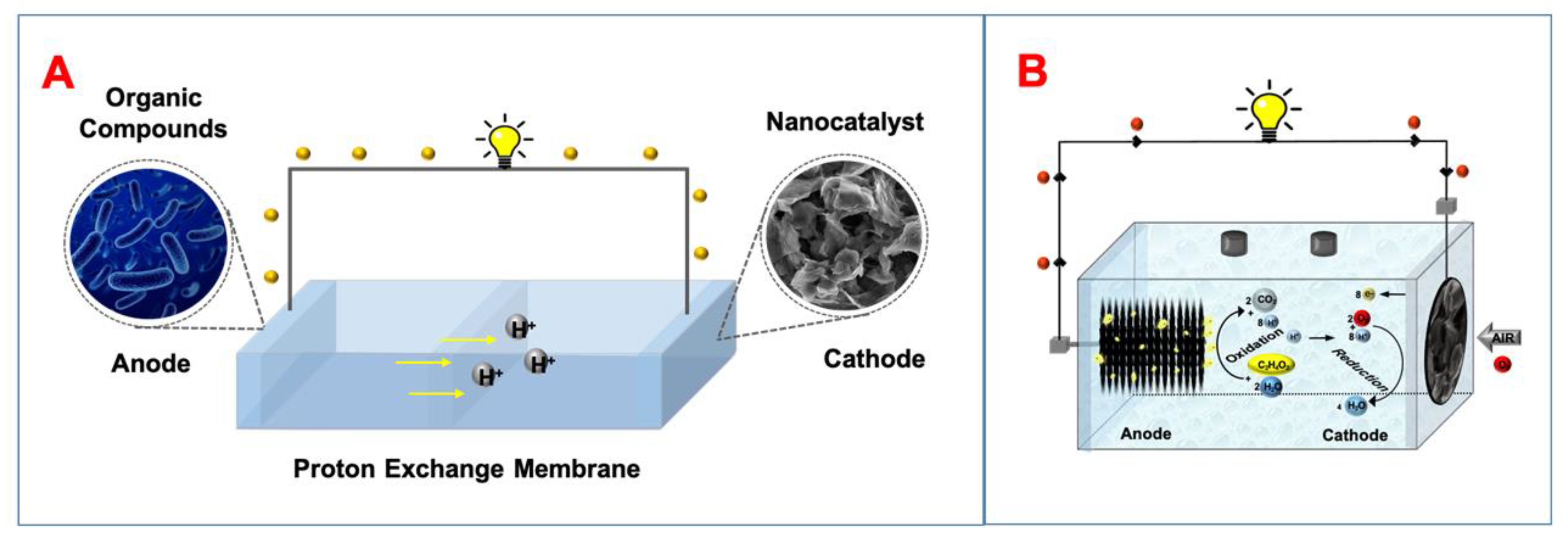
2.2. Microbial Fuel Cells (MFCs)
3. Oxygen Reduction Reaction at the Cathode Side of MFCs: Electrode Kinetics and Electrocatalysis
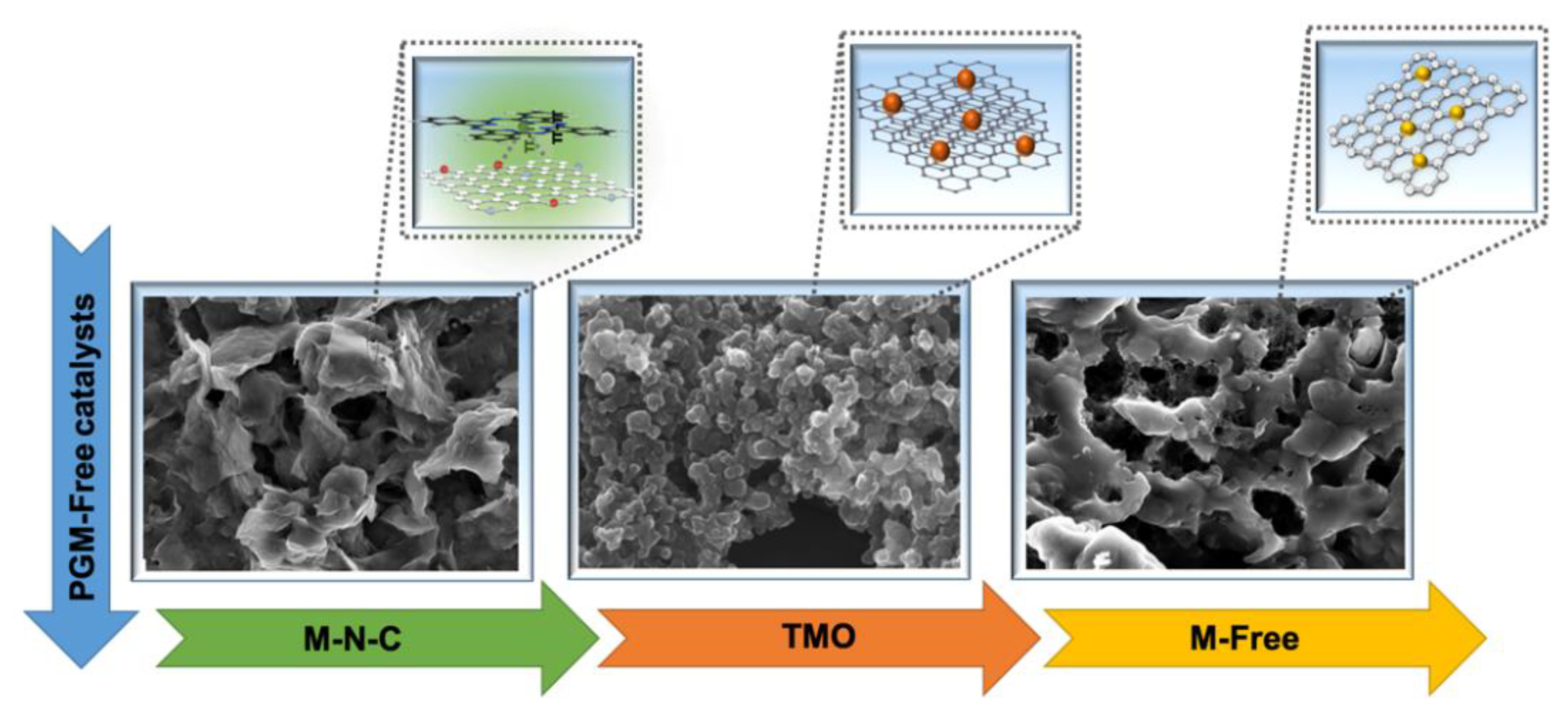
4. Oxygen Reducing Catalysts Based on Platinum Group Metal-Free Materials
4.1. Transition Metal-Nitrogen-Carbon (M-N-C) Catalysts
4.1.1. Pyrolyzed Catalysts
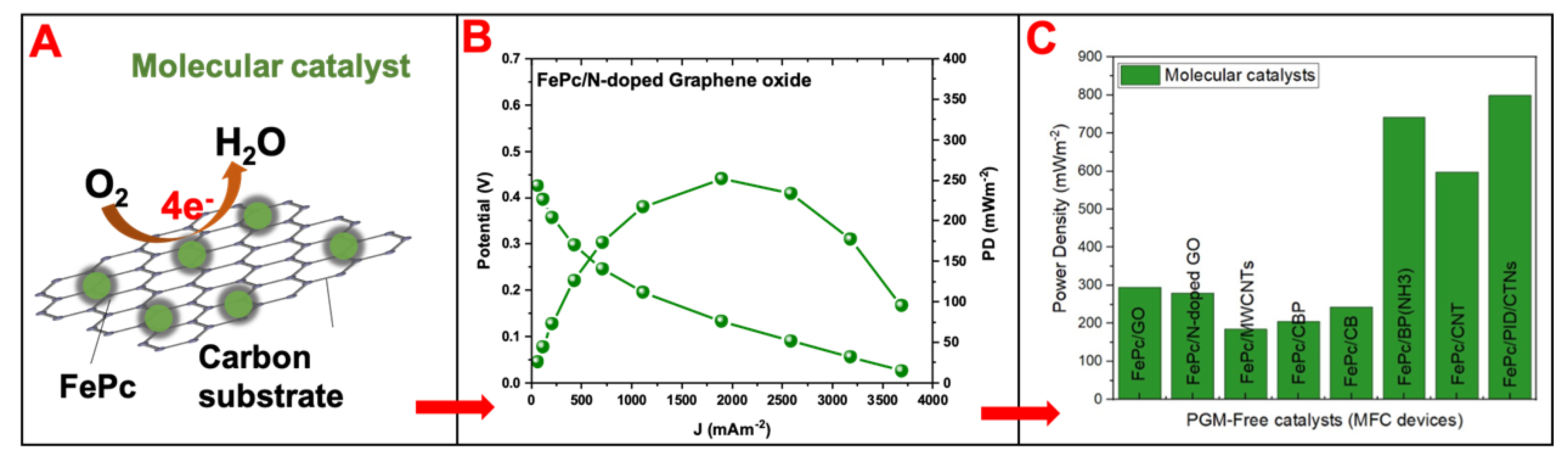
4.1.2. Molecular Catalysts
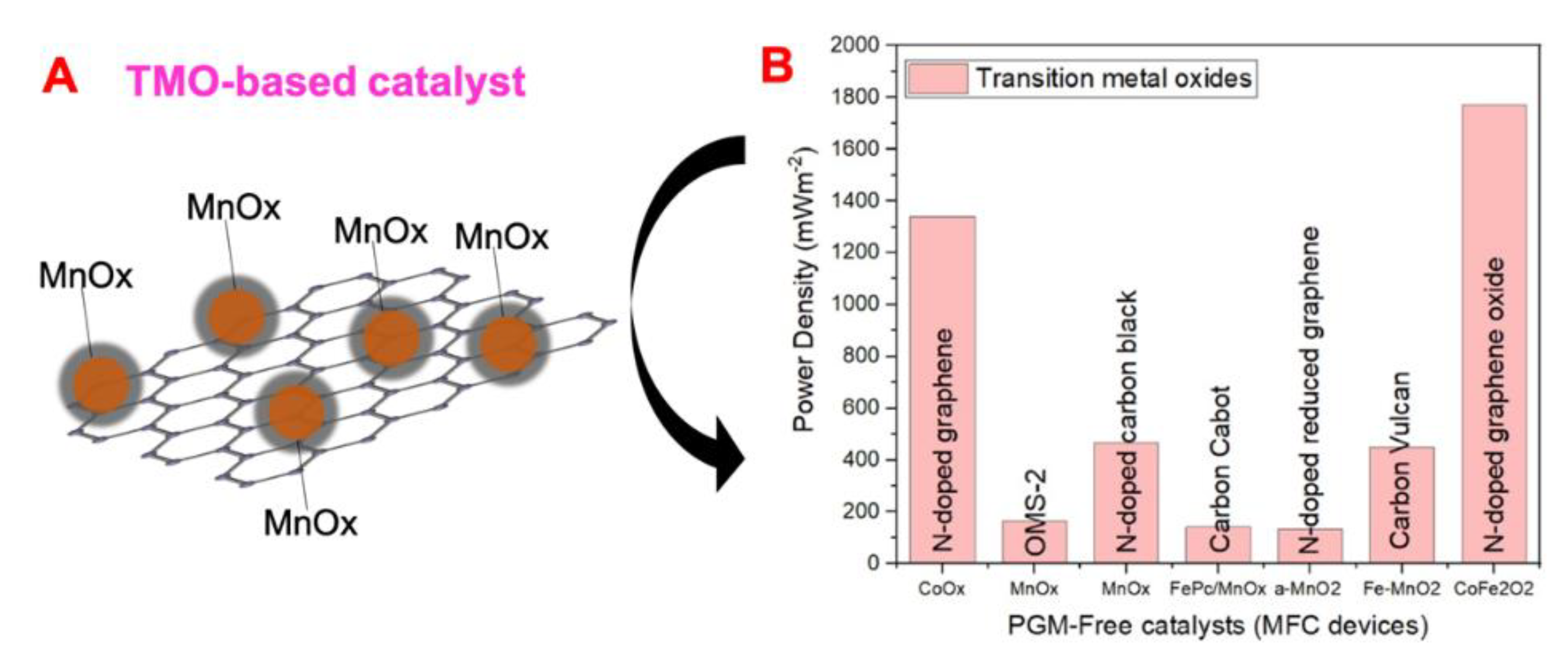
4.2. Transition Metal Oxides (TMOs)
4.3. Metal-Free Catalysts
5. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Jung, S.; Lee, J.; Park, Y.K.; Kwon, E.E. Bioelectrochemical systems for a circular bioeconomy. Bioresour. Technol. 2020, 300, 122748. [Google Scholar] [CrossRef]
- Ivase, T.J.P.; Nyakuma, B.B.; Oladokun, O.; Abu, P.T.; Hassan, M.N. Review of the principal mechanisms, prospects, and challenges of bioelectrochemical systems. Environ. Prog. Sustain. Energy 2020, 39, e13298. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.; Zhao, X.; Li, Y. Factors affecting the efficiency of a bioelectrochemical system: A review. RSC Adv. 2019, 9, 19748–19761. [Google Scholar] [CrossRef]
- Rinaldi, A.; Mecheri, B.; Garavaglia, V.; Licoccia, S.; Di Nardo, P.; Traversa, E. Engineering materials and biology to boost performance of microbial fuel cells: A critical review. Energy Environ. Sci. 2008, 1, 417–429. [Google Scholar] [CrossRef]
- Katz, E. Biofuel Cells with Switchable Power Output. Electroanalysis 2010, 22, 744–756. [Google Scholar] [CrossRef]
- Yuan, Y.; Yuan, T.; Wang, D.; Tang, J.; Zhou, S. Sewage sludge biochar as an efficient catalyst for oxygen reduction reaction in a microbial fuel cell. Biosour. Technol. 2013, 144, 115–120. [Google Scholar] [CrossRef]
- Vries, S.C.; van de Ven, G.W.J.; van Ittersum, M.K.; Giller, K.E. Resource use efficiency and environmental performance of nine major biofuel crops, processed by first-generation conversion techniques. Biomass Bioenergy 2010, 34, 588–601. [Google Scholar] [CrossRef]
- Cecconet, D.; Callegari, A.; Capodaglio, A.G. Bioelectrochemical systems for removal of selected metals and perchlorate from groundwater: A review. Energies 2018, 11, 2643. [Google Scholar] [CrossRef]
- Fang, C.; Achal, V. The potential of microbial fuel cells for remediation of heavy metals from soil and water—Review of application. Microorganisms 2019, 7, 697. [Google Scholar] [CrossRef]
- Włodarczyk, P.P.; Włodarczyk, B. Microbial fuel cell with Ni-Co cathode powered with yeast wastewater. Energies 2018, 11, 3194. [Google Scholar] [CrossRef]
- Sanchez, D.V.P.; Jacobs, D.; Gregory, K.; Huang, J.; Hu, Y.; Vidic, R.; Yun, M. Changes in carbon electrode morphology affect microbial fuel cell performance with Shewanella oneidensis MR-1. Energies 2015, 8, 1817–1829. [Google Scholar] [CrossRef]
- Włodarczyk, P.P.; Włodarczyk, B. Preparation and analysis of ni–co catalyst use for electricity production and COD reduction in microbial fuel cells. Catalysts 2019, 9, 1042. [Google Scholar] [CrossRef]
- Kabutey, F.T.; Zhao, Q.; Wei, L.; Ding, J.; Antwi, P.; Quashie, F.K.; Wang, W. An overview of plant microbial fuel cells (PMFCs): Configurations and applications. Renew. Sustain. Energy Rev. 2019, 110, 402–414. [Google Scholar] [CrossRef]
- Christwardana, M.; Frattini, D.; Accardo, G.; Yoon, S.P.; Kwon, Y. Effects of methylene blue and methyl red mediators on performance of yeast based microbial fuel cells adopting polyethylenimine coated carbon felt as anode. J. Power Sources 2018, 396, 1–11. [Google Scholar] [CrossRef]
- Jannelli, N.; Anna Nastro, R.; Cigolotti, V.; Minutillo, M.; Falcucci, G. Low pH, high salinity: Too much for microbial fuel cells? Appl. Energy 2017, 192, 543–550. [Google Scholar] [CrossRef]
- Addi, H.; Mateo-Ramírez, F.; Ortiz-Martínez, V.M.; Salar-García, M.J.; Hernández-Fernández, F.J.; de los Ríos, A.P.; Godínez, C.; Lotfi, E.M.; Mahi, M.E.; Blanco, L.J.L. Treatment of mineral oil refinery wastewater in microbial fuel cells using ionic liquid based separators. Appl. Sci. 2018, 8, 438. [Google Scholar] [CrossRef]
- Santoro, C.; Arbizzani, C.; Erable, B.; Ieropoulos, I. Microbial fuel cells: From fundamentals to applications. A review. J. Power Sources 2017, 356, 225–244. [Google Scholar] [CrossRef]
- Do, M.H.; Ngo, H.H.; Guo, W.S.; Liu, Y.; Chang, S.W.; Nguyen, D.D.; Nghiem, L.D.; Ni, B.J. Challenges in the application of microbial fuel cells to wastewater treatment and energy production: A mini review. Sci. Total Environ. 2018, 639, 910–920. [Google Scholar] [CrossRef]
- Santoro, S.; Kodali, M.; Herrera, S.; Serov, A.; Ieropoulos, I.; Atanassov, P. Power generation in microbial fuel cells using platinum group metal-free cathode catalyst: Effect of the catalyst loading on performance and costs. J. Power Sources 2018, 378, 169–175. [Google Scholar] [CrossRef]
- Oliveira, M.A.C.; Mecheri, B.; D’Epifanio, A.; Placidi, E.; Arciprete, F.; Valentini, F.; Perandini, A.; Valentini, V.; Licoccia, S. Graphene oxide nanoplatforms to enhance catalytic performance of iron phthalocyanine for oxygen reduction reaction in bioelectrochemical systems. J. Power Sources 2017, 356, 381–388. [Google Scholar] [CrossRef]
- Santoro, C.; Serov, A.; Stariha, L.; Kodali, M.; Gordon, J.; Babanova, S.; Bretschger, O.; Artyushkova, K.; Atanassov, P. Iron based catalysts from novel low-cost organic precursors for enhanced oxygen reduction reaction in neutral media microbial fuel cells. Energy Environ. Sci. 2016, 9, 2346–2353. [Google Scholar] [CrossRef]
- Rojas-Carbonell, S.; Santoro, C.; Serov, A.; Atanassov, P. Transition metal-nitrogen-carbon catalysts for oxygen reduction reaction in neutral electrolyte. Electrochem. Commun. 2017, 75, 38–42. [Google Scholar] [CrossRef]
- Bajracharya, S.; Sharma, M.; Mohanakrishna, G.; Benneton, X.D.; Strik, D.P.B.T.B.; Sarma, P.M.; Pant, D. An overview on emerging bioelectrochemical systems (BESs): Technology for sustainable electricity, waste remediation, resource recovery, chemical production and beyond. Renew. Energy 2016, 98, 153–170. [Google Scholar] [CrossRef]
- Xiao, X.; Xia, H.Q.; Wu, R.; Bai, L.; Yan, L.; Magner, E.; Cosnier, S.; Lojou, E.; Zhu, Z.; Liu, A. Tackling the challenges of enzymatic (bio) fuel cells. Chem. Rev. 2019, 119, 9509–9958. [Google Scholar] [CrossRef] [PubMed]
- Osman, M.H.; Shah, A.A.; Walsh, F.C. Biosensors and Bioelectronics Recent progress and continuing challenges in bio-fuel cells. Part I: Enzymatic cells. Biosens. Bioelectron. 2011, 26, 3087–3102. [Google Scholar] [CrossRef] [PubMed]
- Willner, I.; Katz, E. Integration of layered redox proteins and conductive supports for bioelectronic applications. Angew. Chem. Int. Ed. 2000, 39, 1180–1218. [Google Scholar] [CrossRef]
- Mecheri, B.; De Porcellinis, D.; Campana, P.T.; Rainer, A.; Trombetta, M.; Marletta, A.; Oliveira, N.O., Jr.; Licoccia, S. Tuning structural changes in glucose oxidase for enzyme fuel cell applications. ACS Appl. Mater. Interfaces 2015, 7, 28311–28318. [Google Scholar] [CrossRef]
- Mano, N.; De Poulpiquet, A. O2 reduction in enzymatic biofuel cells. Chem. Rev. 2018, 118, 2392–2468. [Google Scholar] [CrossRef]
- Shleev, S.; Tkac, J.; Christenson, A.; Ruzgas, T.; Yaropolov, A.I.; Whittaker, J.W.; Gorton, L. Direct electron transfer between copper-containing proteins and electrodes. Biosens. Bioelectron. 2005, 20, 2517–2554. [Google Scholar] [CrossRef]
- Kamitaka, Y.; Tsujimura, S.; Kataoka, S.; Sakurai, T.; Ikeda, T.; Kano, K. Effects of axial ligand mutation of the type I copper site in bilirubin oxidase on direct electron transfer-type bioelectrocatalytic reduction of dioxygen. J. Electroanal. Chem. 2007, 601, 119–124. [Google Scholar] [CrossRef]
- Miura, Y.; Tsujimura, S.; Kurose, S.; Kamitaka, Y.; Kataoka, K.; Sakurai, T.; Kano, K. Direct electrochemistry of CueO and its mutants at residues to and near type I Cu for oxygen-reducing biocathode. Fuel Cells 2009, 9, 70–78. [Google Scholar] [CrossRef]
- Gooding, J.J.; Wibowo, R.; Liu, J.; Yang, W.; Losic, D.; Orbons, S.; Mearns, F.J.; Shapter, J.G.; Hibbert, D.B. Protein electrochemistry using aligned carbon nanotube arrays. J. Am. Chem. Soc. 2003, 125, 9006–9007. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Li, Q.; Su, L.; Yan, Y.; Zhang, J.; Mao, L. Direct electrochemistry of multi-copper oxidases at carbon nanotubes noncovalently functionalized with cellulose derivatives. Electroanalysis 2006, 18, 587–594. [Google Scholar] [CrossRef]
- Cass, A.E.G.; Davis, G.; Francis, G.D.; Hill, H.A.O.; Aston, W.J.; Higgins, I.J.; Plotkin, E.V.; Scott, L.D.L.; Turner, A.P.F. Ferrocene-mediated enzyme electrode for amperometric determination of glucose. Anal. Chem. 1984, 56, 667–671. [Google Scholar] [CrossRef]
- Tsujimura, S.; Tatsumi, H.; Ogawa, J.; Shimizu, S.; Kano, K.; Ikeda, T. Bioelectrocatalytic reduction of dioxygen to water at neutral pH using bilirubin oxidase as an enzyme and 2,2-azinobis (3-ethylbenzothiazolin-6-sulfonate) as an electron transfer mediator. J. Electroanal. Chem. 2001, 496, 69–75. [Google Scholar] [CrossRef]
- Patolsky, F.; Lichtenstein, A.; Willner, I. Electronic transduction of DNA sensing processes on surfaces: Amplification of DNA detection and analysis of single-base mismatches by tagged liposomes. J. Am. Chem. Soc. 2001, 123, 5194–5205. [Google Scholar] [CrossRef] [PubMed]
- Ohnuki, H.; Wako, T.; Mecheri, B.; Wu, H.; Tsuya, D.; Endo, H. Self-powered hydrogen peroxide sensor and its application as a biosensor. Jpn. J. Appl. Phys. 2019, 58, SBBG16. [Google Scholar] [CrossRef]
- Ohnuki, H.; Saiki, T.; Kusakari, A.; Endo, H.; Ichihara, M.; Izumi, M. Incorporation of glucose oxidase into langmuir-blodgett films based on prussian blue applied to amperometric glucose biosensor. Langmuir 2007, 23, 4675–4681. [Google Scholar] [CrossRef]
- Mano, N.; Mao, F.; Heller, A. On the parameters affecting the characteristics of the ‘wired’ glucose oxidase anode. J. Electroanal. Chem. 2005, 574, 347–357. [Google Scholar] [CrossRef]
- Heller, A. Electron-conducting redox hydrogels: Design, characteristics and synthesis. Curr. Opin. Chem. Biol. 2006, 10, 664–672. [Google Scholar] [CrossRef]
- Wang, H.; Ohnuki, H.; Endo, H.; Izumi, M. Impedimetric and amperometric bifunctional glucose biosensor based on hybrid organic-inorganic thin films. Bioelectrochemistry 2005, 101, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.M.; Akers, N.L.; Hill, A.D.; Johnson, Z.C.; Minteer, S.D. Improving the environment for immobilized dehydrogenase enzymes by modifying nafion with tetraalkylammonium bromides. Biomacromolecules 2004, 5, 1241–1247. [Google Scholar] [CrossRef] [PubMed]
- Beneyton, T.; El Harrak, A.; Griffiths, A.D.; Hellwig, P.; Taly, V. Immobilization of CotA, an extremophilic laccase from Bacillus subtilis, on glassy carbon electrodes for biofuel cell applications. Electrochem. Commun. 2011, 13, 24–27. [Google Scholar] [CrossRef]
- Nimje, V.R.; Chen, C.C.; Chen, H.R.; Chen, C.Y.; Tseng, M.J.; Cheng, K.C.; Shih, R.C.; Chang, Y.F. A single-chamber microbial fuel cell without an air cathode. Int. J. Mol. Sci. 2012, 13, 3933–3948. [Google Scholar] [CrossRef]
- Cheng, S.; Liu, H.; Logan, B.E. Increased performance of single-chamber microbial fuel cells using an improved cathode structure. Electrochem. Commun. 2006, 8, 489–494. [Google Scholar] [CrossRef]
- Logan, B.E.; Cheng, S.; Watson, V.; Estadt, G. Graphite fiber brush anodes for increased power production in air-cathode microbial fuel cells. Environ. Sci. Technol. 2007, 9, 3341–3346. [Google Scholar] [CrossRef]
- Rozendal, R.A.; Hamelers, H.V.M.; Rabaey, K.; Keller, J.; Buisman, C.J.N. Towards practical implementation of bioelectrochemical wastewater treatment. Trends Biotechnol. 2008, 26, 450–459. [Google Scholar] [CrossRef]
- Rismani-Yazdi, S.M.H.; Carver, A.D.; Christy, O.H. Cathodic limitations in microbial fuel cells: An overview. J. Power Sources 2008, 180, 683–694. [Google Scholar] [CrossRef]
- Wang, Z.; Mahadevan, G.D.; Wu, Y.; Zhao, F. Progress of air-breathing cathode in microbial fuel cells. J. Power Sources 2017, 356, 245–255. [Google Scholar] [CrossRef]
- Li, Y.; Li, Q.; Wang, H.; Zhang, L.; Wilkinson, D.P.; Zhang, J. Recent progresses in oxygen reduction reaction electrocatalysts for electrochemical energy applications. Electrochem. Energy Rev. 2019, 2, 518–538. [Google Scholar] [CrossRef]
- Choi, C.H.; Kwon, H.C.; Yook, S.; Shin, H.; Kim, H.; Choi, M. Hydrogen peroxide synthesis via enhanced two-electron oxygen reduction pathway on carbon-coated Pt surface. J. Phys. Chem. C 2014, 118, 30063–30070. [Google Scholar] [CrossRef]
- Ma, R.; Lin, G.; Zhou, Y.; Liu, Q.; Zhang, T.; Shan, G.; Yang, M.; Wang, J. A review of oxygen reduction mechanisms for metal-free carbon-based electrocatalysts. npj Comput. Mater. 2019, 5, 1–15. [Google Scholar] [CrossRef]
- Zhou, R.; Zheng, Y.; Jaroniec, M.; Qiao, S.-Z. Determination of the electron transfer number for the oxygen reduction reaction: From theory to experiment. ACS Catal. 2016, 6, 4720–4728. [Google Scholar] [CrossRef]
- Yang, T.; Wang, M.; Ju, X.; Zhao, J.; Fu, C. The efficient oxygen reduction catalysts based on the non-noble metal and conducting polymers. Int. J. Electrochem. Sci. 2017, 12, 12125–12139. [Google Scholar] [CrossRef]
- Ramaswamy, N.; Mukerjee, S. Fundamental mechanistic understanding of electrocatalysis of oxygen reduction on Pt and non-Pt surfaces: Acid versus Alkaline Media. Adv. Phys. Chem. 2012, 491604, 1–17. [Google Scholar] [CrossRef]
- Wu, S.-H.; Li, P.-C. Electron transfer number control of the oxygen reduction reaction on nitrogen-doped reduced graphene oxides for the air electrodes of zinc-air batteries and organic degradation. Mater. Chem. Phys. 2016, 183, 551–560. [Google Scholar] [CrossRef]
- Brocato, S.; Serov, A.; Atanassov, P. PH dependence of catalytic activity for ORR of the non-PGM catalyst derived from heat-treated Fe-phenanthroline. Electrochim. Acta 2013, 87, 361–365. [Google Scholar] [CrossRef]
- Xie, J.; Li, S.; Zhang, X.; Zhang, J.; Wang, R.; Zhang, H.; Pan, B.; Xie, Y. Atomically thin molybdenum nitride nanosheets with exposed active surface sites for efficient hydrogen evolution. Chem. Sci. 2014, 5, 4615–4620. [Google Scholar] [CrossRef]
- Chen, W.; Fan, Z.; Gu, L.; Bao, X.; Wang, C. Enhanced capacitance of manganese oxide via confinement inside carbon nanotubes. Chem. Commun. 2010, 46, 3905–3907. [Google Scholar] [CrossRef]
- Madkikar, P.; Menga, D.; Harzer, G.S.; Mittermeier, T.; Siebel, A.; Wagner, F.E.; Merz, M.; Schuppler, S.; Nagel, P.; Muñoz-García, A.B.; et al. Nanometric Fe-substituted ZrO2 on carbon black as PGM-free ORR catalyst for PEMFCs. J. Electrochem. Soc. 2019, 166, F3032–F3043. [Google Scholar] [CrossRef]
- Mecheri, B.; Ficca, V.C.A.; Oliveira, M.A.C.; Placidi, E.; Arciprete, F.; D’Epifanio, A.; Licoccia, S. Facile synthesis of graphene-phthalocyanine composites as oxygen reduction electrocatalysts in microbial fuel cells. Appl. Catal. B-Environ. 2018, 237, 699–707. [Google Scholar] [CrossRef]
- Oliveira, M.A.C.; Mecheri, B.; Zurlo, F.; D’Epifanio, A.; Licoccia, S. Optimization of PGM-free cathodes for oxygen reduction in microbial fuel cells. Electrochim. Acta 2020, 334, 135650. [Google Scholar] [CrossRef]
- Jiang, K.; Back, S.; Akey, A.J.; Xia, C.; Hu, Y.; Liang, W.; Schaak, D.; Stavitski, E.; Nørskov, J.K.; Siahrostami, S.; et al. Highly selective oxygen reduction to hydrogen peroxide on transition metal single atom coordination. Nat. Commun. 2019, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, L.; Zhao, K.; Shi, S.; Shao, S.; Zhang, L.; Sun, X.; Zhao, Y.; Zhang, J. Atomically dispersed metal catalysts for the oxygen reduction reaction: Synthesis, characterization, reaction mechanisms and electrochemical energy applications. Energy Environ. Sci. 2019, 12, 2890–2923. [Google Scholar] [CrossRef]
- Setzler, B.P.; Zhuang, Z.; Wittkop, J.A.; Yan, Y. Activity targets for nanostructured platinum-group-metal-free catalysts in hydroxide exchange membrane fuel cells. Nat. Nanotechnol. 2016, 11, 1020–1025. [Google Scholar] [CrossRef]
- Lefèvre, M.; Proietti, E.; Jaouen, F.; Dodelet, J.P. Iron-based catalysts with improved oxygen reduction activity in polymer electrolyte fuel cells. Science 2009, 324, 71–74. [Google Scholar] [CrossRef]
- Jaouen, F.; Proietti, E.; Lefèvre, M.; Chenitz, R.; Dodelet, J.P.; Wu, G.; Chung, H.T.; Johnston, C.M.; Zelenay, P. Recent advances in non-precious metal catalysis for oxygen-reduction reaction in polymer electrolyte fuel cells. Energy Environ. Sci. 2011, 4, 114–130. [Google Scholar] [CrossRef]
- Artyushkova, K.; Serov, A.; Rojas-Carbonell, S.; Atanassov, P. Chemistry of multitudinous active sites for oxygen reduction reaction in transition metal-nitrogen-carbon electrocatalysts. J. Phys. Chem. C 2015, 119, 25917–25928. [Google Scholar] [CrossRef]
- Mohamed, H.O.; Sayed, E.T.; Obaid, M.; Choi, Y.J.; Park, S.G.; Al-Qaradawi, S.; Chae, K.J. Transition metal nanoparticles doped carbon paper as a cost-effective anode in a microbial fuel cell powered by pure and mixed biocatalyst cultures. Int. J. Hydrog. Energy 2018, 43, 21560–21571. [Google Scholar] [CrossRef]
- Azargohar, R.; Dalai, A.K. Biochar is a precursor of activated carbon. Appl. Biochem. Biotech. 2006, 131, 762–773. [Google Scholar] [CrossRef]
- Mineva, T.; Matanovic, I.; Atanassov, P.; Sougrati, M.-T.; Stievano, L.; Clémancey, M.; Kochem, A.; Latour, J.-M.; Jaouen, F. Understanding active sites in pyrolyzed Fe-N-C catalysts for fuel cell cathodes by bridging density functional theory calculations and 57Fe mössbauer spectroscopy. ACS Catal. 2019, 9, 9359–9371. [Google Scholar] [CrossRef]
- Xu, X.; Xia, Z.; Zhang, X.; Sun, R.; Sun, X.; Li, H.; Wu, C.; Wang, J.; Wang, S.; Sun, G. Atomically dispersed Fe-N-C derived from dual metal-organic frameworks as efficient oxygen reduction electrocatalysts in direct methanol fuel cells. Appl. Catal. B Environ. 2019, 259, 118042. [Google Scholar] [CrossRef]
- Yin, X.; Utetiwabo, W.; Sun, S.; Lian, Y.; Chen, R.; Yang, W. Incorporation of CeF3 on single-atom dispersed Fe/N/C with oxophilic interface as highly durable electrocatalyst for proton exchange membrane fuel cell. J. Catal. 2019, 374, 43–50. [Google Scholar] [CrossRef]
- Ratso, S.; Sougrati, M.T.; Käärik, M.; Merisalu, M.; Rähn, M.; Kisand, V.; Kikas, A.; Paiste, P.; Leis, J.; Sammelselg, V.; et al. Effect of ball-milling on the oxygen reduction reaction activity of iron and nitrogen co-doped carbide-derived carbon catalysts in acid media. ACS Appl. Energy Mater. 2019, 2, 7952–7962. [Google Scholar] [CrossRef]
- Nabae, Y.; Moriya, S.; Matsubayashi, K.; Lyth, S.M.; Malon, M.; Wu, L.; Islam, N.M.; Koshigoe, Y.; Kuroki, S.; Kakimoto, M.A.; et al. The role of Fe species in the pyrolysis of Fe phthalocyanine and phenolic resin for preparation of carbon-based cathode catalysts. Carbon 2010, 48, 2613–2624. [Google Scholar] [CrossRef]
- Miller, H.A.; Bellini, M.; Oberhauser, W.; Deng, X.; Chen, H.; He, Q.; Passaponti, M.; Innocenti, M.; Yang, Y.; Sun, F.; et al. Heat treated carbon supported iron (II) phthalocyanine oxygen reduction catalysts: Elucidation of the structure-activity relationship using X-ray absorption spectroscopy. Phys. Chem. Chem. Phys. 2016, 18, 33142–33151. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chung, H.T.; Cullen, D.A.; Wagner, S.; Kramm, U.I.; More, K.L.; Zelenay, P.; Wu, G. High-performance fuel cell cathodes exclusively containing atomically dispersed iron active sites. Energy Environ. Sci. 2019, 12, 2548–2558. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, J.; Wang, F.; Dai, L. Efficient oxygen reduction reaction (ORR) catalysts based on single iron atoms dispersed on a hierarchically structured porous carbon framework. Angew. Chem. Int. Ed. 2018, 57, 1–7. [Google Scholar]
- Ao, X.; Zhang, W.; Li, Z.; Lv, L.; Ruan, Y.; Wu, H.-H.; Chiang, W.-H.; Wang, C.; Liue, M.; Zeng, X.C. Unraveling the high-activity nature of Fe-N-C electrocatalysts for oxygen reduction reaction: The extraordinary synergy between Fe-N4 and Fe4N. J. Mater. Chem. A 2012, 7, 11792–11801. [Google Scholar] [CrossRef]
- Chung, D.Y.; Kim, M.J.; Kang, N.; Yoo, J.M.; Shin, H.; Kim, O.-H.; Sung, Y.-E. Low-temperature and gram-scale synthesis of two-dimensional Fe-N-C carbon sheets for robust electrochemical oxygen reduction reaction. Chem. Mater. 2017, 29, 2890–2898. [Google Scholar] [CrossRef]
- Chen, M.; He, Y.; Spendelow, J.S.; Wu, G. Atomically dispersed metal catalysts for oxygen reduction. ACS Energy Lett. 2019, 4, 1619–1633. [Google Scholar] [CrossRef]
- Huang, X.; Yang, Z.; Dong, B.; Wang, Y.; Tang, T.; Hou, Y. In-situ Fe2N@N-doped porous carbon hybrids as superior catalysts for oxygen reduction reaction. Nanoscale 2013, 9, 8102–8106. [Google Scholar] [CrossRef] [PubMed]
- Monteverde Videla, A.H.A.; Osmieri, L.; Specchia, S. Non-noble metal (NNM) catalysts for fuel cells: Tuning the activity by a rational step by step single variable evolution. In Electrochemistry of N4 Macrocyclic Metal Complexes; Bedioui, F., Zagal, J.H., Eds.; Springer International Publishing AG: Basel, Switzerland, 2016; pp. 69–101. [Google Scholar]
- Monteverde Videla, A.H.A.; Osmieri, L.; Armandi, M.; Specchia, S. Varying the morphology of Fe-N-C electrocatalysts by templating Iron Phthalocyanine precursor with different porous SiO2 to promote the Oxygen Reduction Reaction. Electrochim. Acta 2014, 177, 43–50. [Google Scholar] [CrossRef]
- Zagal, J.H.; Fethi, B. (Eds.) Electrochemistry of N4 Macrocyclic Metal Complexes; Springer: Berlin/Heidelberg, Germany, 2016; Volume 1: Energy. [Google Scholar]
- Zhang, Z.; Dou, M.; Ji, J.; Wang, F. Phthalocyanine tethered iron phthalocyanine on graphitized carbon black as superior electrocatalyst for oxygen reduction reaction. Nano Energy 2017, 34, 338–343. [Google Scholar] [CrossRef]
- Cui, L.; Lv, G.; Dou, Z.; He, X. Fabrication of iron phthalocyanine/graphene micro/nanocomposite by solvothermally assisted π–π assembling method and its application for oxygen reduction reaction. Electrochim. Acta 2013, 106, 272–278. [Google Scholar] [CrossRef]
- Taniguchi, T.; Tateishi, H.; Miyamoto, S.; Hatakeyama, K.; Ogata, C.; Funatsu, A.; Hayami, A.; Makinose, Y.; Matsushita, N.; Koinuma, M.; et al. A self-assembly route to an iron phthalocyanine/reduced graphene oxide hybrid electrocatalyst affording an ultrafast oxygen reduction reaction. Part. Part. Syst. Charact. 2013, 30, 1063–1070. [Google Scholar] [CrossRef]
- Jiang, Y.; Lu, Y.; Lv, X.; Han, D.; Zhang, Q.; Niu, L.; Chen, W. Enhanced catalytic performance of Pt free iron phthalocyanine by graphene support for efficient oxygen reduction reaction. ACS Catal. 2013, 3, 1263–1271. [Google Scholar] [CrossRef]
- Zhang, C.; Hao, Z.; Yin, H.; Liu, H.; Hou, L. Iron phthalocyanine and nitrogen-doped graphene composite as a novel non-precious catalyst for the oxygen reduction reaction. Nanoscale 2013, 4, 7326–7329. [Google Scholar] [CrossRef]
- Oliveira, M.A.C.; Ficca, V.C.A.; Gokhale, R.; Santoro, C.; Mecheri, B.; D’Epifanio, A.; Licoccia, S.; Atanassov, P. Iron(II) phthalocyanine (FePc) over carbon support for oxygen reduction reaction electrocatalysts operating in alkaline electrolyte. J. Solid State Electrochem. 2020, 1–12. [Google Scholar] [CrossRef]
- Osmieri, L.; Monteverde Videla, A.H.A.; Armandi, M.; Specchia, S. Influence of different transition metals on the properties of Me-N-C (Me = Fe, Co, Cu, Zn) catalysts synthesized using SBA-15 as tubular nano-silica reactor for oxygen reduction reaction. Int. J. Hydrog. Energy 2016, 41, 22570–22588. [Google Scholar] [CrossRef]
- Santoro, C.; Rojas-Carbonell, S.; Awais, R.; Gokhale, R.; Kodali, M.; Serov, A.; Artyushkova, K.; Atanassov, P. Influence of platinum group metal-free catalyst synthesis on microbial fuel cell performance. J. Power Sources 2018, 375, 11–20. [Google Scholar] [CrossRef]
- Chen, Y.; Artyushkova, K.; Rojas-Carbonell, S.; Serov, A.; Matanovic, I.; Santoro, C.; Atanassov, P. Inhibition of surface chemical moieties by tris(hydroxymethyl)aminomethane: A key to understanding oxygen reduction on iron–nitrogen–carbon catalysts. ACS Appl. Energy Mater. 2018, 1, 1942–1949. [Google Scholar] [CrossRef]
- Mecheri, B.; Gokhale, R.; Santoro, C.; Oliveira, M.A.C.; D’Epifanio, A.; Licoccia, S.; Serov, A.; Artyushkova, K.; Atanassov, P. Oxygen reduction reaction electrocatalysts derived from iron salt and benzimidazole and aminobenzimidazole precursors and their application in microbial fuel cell cathode. ACS Appl. Energy Mater. 2018, 1, 5755–5765. [Google Scholar] [CrossRef] [PubMed]
- Birry, L.; Mehta, P.; Jaouen, F.; Dodelet, J.-P.; Guiot, S.R.; Tartakovsky, B. Application of iron-based cathode catalysts in a microbial fuel cell. Electrochim. Acta 2011, 56, 1505–1511. [Google Scholar] [CrossRef]
- Kodali, M.; Santoro, C.; Serov, A.; Kabir, S.; Artyushkova, K.; Matanovic, I.; Atanassov, P. Air breathing cathodes for microbial fuel cell using Mn-, Fe-, Co- and Ni-containing platinum group metal-free catalysts. Electrochim. Acta 2017, 231, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, Q.; Xu, T.; Wei, K.; Yin, M.; Liang, P.; Huang, X.; Zhang, X. One-step ball milling-prepared nano Fe2O3 and nitrogen-doped graphene with high oxygen reduction activity and its application in microbial fuel cells. Front. Environ. Sci. Eng. 2020, 14, 30. [Google Scholar] [CrossRef]
- Yang, W.; Wang, X.; Rossi, R.; Logan, B.E. Low-cost Fe-N-C catalyst derived from Fe (III)–chitosan hydrogel to enhance power production in microbial fuel cells. Chem. Eng. J. 2019, 380, 122522. [Google Scholar] [CrossRef]
- Mahalingam, S.; Ayyaru, S.; Ahn, Y.-H. Enhanced cathode performance of Fe2O3, boron nitride-doped rGO nanosheets for microbial fuel cell applications. Sustain. Energy Fuels 2020, 4, 1454–1468. [Google Scholar] [CrossRef]
- Tang, H.; Cai, S.; Xie, S.; Wang, Z.; Tong, Y.; Pan, M.; Lu, X. Metal-organic-framework-derived dual metal- and nitrogen-doped carbon as efficient and robust oxygen reduction reaction catalysts for microbial fuel cells. Adv. Sci. 2020, 3, 1500265. [Google Scholar] [CrossRef]
- Greenmana, G.J.; Santoro, C.; Serov, A.; Melhuish, C.; Atanassov, P.; Ieropoulos, I.A. Improved power and long- term performance of microbial fuel cell with Fe-N-C catalyst in air-breathing cathode. Energy 2018, 144, 1073–1079. [Google Scholar]
- Erable, B.; Oliot, M.; Lacroix, R.; Bergel, A.; Serov, A.; Kodali, M.; Santoro, C.; Atanassov, P. Iron-nicarbazin derived platinum group metal-free electrocatalyst in scalable-size air-breathing cathodes for microbial fuel cells. Electrochim. Acta 2018, 277, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fan, Y.S. Pyrolysis of iron phthalocyanine on activated carbon as highly efficient non noble metal oxygen reduction catalyst in microbial fuel cells. Chem. Eng. J. 2019, 361, 416–427. [Google Scholar] [CrossRef]
- Jasinski, R. A new fuel cell cathode catalyst. Nature 1964, 201, 1212–1213. [Google Scholar] [CrossRef]
- Zion, N.; Friedman, A.; Levy, N.; Elbaz, L. Bioinspired electrocatalysis of oxygen reduction reaction in fuel cells using molecular catalysts. Adv. Mater. 2018, 30, 1800406. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Liu, C.; Wang, Z.; Zhang, Z.; Zhang, M.; Chang, X.; Zhang, W.; Cao, R. Reactivity and Mechanism Studies of Hydrogen Evolution Catalyzed by Copper Corroles. ACS Catal. 2016, 6, 6429–6437. [Google Scholar] [CrossRef]
- Raggio, M.; Mecheri, B.; Nardis, S.; D’Epifanio, A.; Licoccia, S.; Paolesse, R. Metallo-corroles supported on carbon nanostructures as oxygen reduction electrocatalysts in neutral media. Eur. J. Inorg. Chem. 2019, 44, 4760–4765. [Google Scholar] [CrossRef]
- Shpilman, J.S.; Friedman, A.; Zion, N.; Levy, N.; Major, D.T.; Elbaz, L. Combined experimental and theoretical study of cobalt corroles as catalysts for oxygen reduction reaction. J. Phys. Chem. C 2019, 123, 30129–30136. [Google Scholar] [CrossRef]
- Levy, N.; Shpilman, J.S.; Honig, H.C.; Major, D.T.; Elbaz, L. A surprising substituent effect in corroles on the electrochemical activation of oxygen reduction. Chem. Commun. 2017, 53, 12942–12945. [Google Scholar] [CrossRef]
- Levy, N.; Mahammed, A.; Kosa, M.; Major, D.T.; Gross, Z.; Elbaz, L. Metallocorroles as nonprecious-metal catalysts for oxygen reduction. Angew. Chem. Int. Ed. 2015, 54, 14080–14084. [Google Scholar] [CrossRef] [PubMed]
- Zagal, J.H.; Kruusenberg, I.; Tammeveski, K.; Recio, J.; Muñoz, K.; Venegas, R. Oxygen reduction on carbon-supported metallophthalocyanines and metalloporphyrins. Elsevier Encycl. Interfacial Chem. Electrochem. 2018, 812–819. [Google Scholar] [CrossRef]
- Dong, G.; Huang, M.; Guan, L. Iron phthalocyanine coated on single-walled carbon nanotubes composite for the oxygen reduction reaction in alkaline media. Phys. Chem. Chem. Phys. 2012, 14, 2557–2559. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Long, Y.-T. Superior Catalytic Activity of Electrochemically Reduced Graphene Oxide Supported Iron Phthalocyanines toward Oxygen Reduction Reaction. ACS Appl. Mater. Interfaces 2015, 7, 24063–24068. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Toshimitsu, F.; Yang, Z.; Fujigaya, T.; Nakashima, N. Pristine carbon nanotube/iron phthalocyanine hybrids with a well-defined nanostructure show excellent efficiency and durability for oxygen reduction reaction. J. Mater. Chem. A 2013, 5, 1184–1191. [Google Scholar] [CrossRef]
- Van Veen, J.A.R.; Colijn, H.A.; van Baar, J.F. On the effect of a heat treatment on the structure of carbon-supported metalloporphyrins and phthalocyanines. Electrochim. Acta 1998, 33, 801–804. [Google Scholar] [CrossRef]
- Nguyen, M.-T.; Mecheri, B.; Iannaci, A.; D’Epifanio, A.; Licoccia, S. Iron/polyindole-based electrocatalysts to enhance oxygen reduction in microbial fuel cells. Electrochim. Acta 2016, 190, 388–395. [Google Scholar] [CrossRef]
- Iannaci, A.; Mecheri, B.; D’Epifanio, A.; Elorri, M.J.L.; Liccocia, S. Iron–nitrogen-functionalized carbon as efficient oxygen reduction reaction electrocatalyst in microbial fuel cells. Int. J. Hydrog. Energy 2016, 41, 19637–19644. [Google Scholar] [CrossRef]
- Santoro, C.; Gokhale, A.; Mecheri, B.; D’Epifanio, A.; Licoccia, S.; Serov, A.; Artyushkova, K.; Atanassov, P. Design of iron (II) phthalocyanine-derived oxygen reduction electrocatalysts for high-power-density microbial fuel cells. ChemSusChem 2017, 10, 3243–3251. [Google Scholar] [CrossRef]
- Osgood, H.; Devaguptapu, S.V.; Xu, H.; Cho, J.; Wu, G. Transition metal (Fe, Co, Ni, and Mn) oxides for oxygen reduction and evolution bifunctional catalysts in alkaline media. Nano Today 2016, 11, 601–625. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Wei, Z. Transition-metal-oxide-based catalysts for the oxygen reduction reaction. J. Mater. Chem. A 2018, 6, 8194–8209. [Google Scholar] [CrossRef]
- Goswami, C.; Kashyap Hazarika, K.; Bharali, P. Transition metal oxide nanocatalysts for oxygen reduction reaction. Mater. Sci. Energy Technol. 2018, 1, 117–128. [Google Scholar] [CrossRef]
- Sun, M.; Liu, H.; Liu, Y.; Qu, J.; Li, J. Graphene-based transition metal oxide nanocomposites for the oxygen reduction reaction. Nanoscale 2015, 7, 1250–1269. [Google Scholar] [CrossRef] [PubMed]
- Seonghee, K.; Kato, S.; Ishizaki, T.; Li, O.L.; Kang, J. Transition metal (Fe, Co, Ni) nanoparticles on selective amino-N-doped carbon as high-performance oxygen reduction reaction electrocatalyst. Nanomaterials 2019, 9, 742. [Google Scholar]
- Su, Y.; Zhu, Y.; Yang, X.; Shen, J.; Lu, J.; Zhang, X.; Chen, J.; Li, C. A highly efficient catalyst toward oxygen reduction reaction in neutral media for microbial fuel cells. Ind. Eng. Chem. Res. 2013, 52, 6076–6082. [Google Scholar] [CrossRef]
- Li, X.; Hu, B.; Suib, S.; Lei, Y.; Li, B. Manganese dioxide as a new cathode catalyst in microbial fuel cells. J. Power Sources 2010, 195, 2586–2591. [Google Scholar] [CrossRef]
- Gautam, R.K.; Bhattacharjee, H.; Mohan, S.V.; Verma, A. Nitrogen doped graphene supported α-MnO2 nanorods for efficient ORR in a microbial fuel cell. RSC Adv. 2016, 6, 110091–110101. [Google Scholar] [CrossRef]
- Farahani, F.S.; Mecheri, B.; Majidi, M.R.; Oliveira, M.A.C.; D’Epifanio, A.; Zurlo, F.; Placidi, E.; Arciprete, F.; Licoccia, S. MnOx-based electrocatalysts for enhanced oxygen reduction in microbial fuel cell air cathodes. J. Power Sources 2018, 390, 45–53. [Google Scholar] [CrossRef]
- Farahani, F.S.; Mecheri, B.; Majidi, M.R.; Placidi, E.; D’Epifanio, A. Carbon-supported Fe/Mn-based perovskite-type oxides boost oxygen reduction in bioelectrochemical systems. Carbon 2019, 145, 716–724. [Google Scholar] [CrossRef]
- Huang, Q.; Zhou, P.; Yang, H.; Zhu, L.; Wu, H. In situ generation of inverse spinel CoFe2O4 nanoparticles onto nitrogen-doped activated carbon for an effective cathode electrocatalyst of microbial fuel cells. Chem. Eng. J. 2017, 325, 466–473. [Google Scholar] [CrossRef]
- Burkitt, R.; Whiffen, T.R.; Yu, E.H. Iron phthalocyanine and MnOx composite catalysts for microbial fuel cell applications. Appl. Catal. B Environ. 2016, 181, 279–288. [Google Scholar] [CrossRef]
- Zhang, Y.; Deng, L.; Hu, H.; Qiao, Y.; Yuan, H.; Chen, D.; Chang, M.; Wei, H. Pomelo peel-derived, N-doped biochar microspheres as an efficient and durable metal-free ORR catalyst in microbial fuel cells. Sustain. Energy Fuels 2020, 4, 1642–1653. [Google Scholar] [CrossRef]
- Mao, K.; Zhang, W.; Dai, J.; Zeng, X.C. Carbon fragments as highly active metal-free catalysts for the oxygen reduction reaction: A mechanistic study. Nanoscale 2019, 11, 19422–19428. [Google Scholar] [CrossRef] [PubMed]
- Zhong, K.; Li, M.; Yang, Y.; Zhang, H.; Zhang, B.; Tang, J.; Yan, J.; Su, M.; Yang, Z. Nitrogen-doped biochar derived from watermelon rind as oxygen reduction catalyst in air cathode microbial fuel cells. Appl. Energy 2019, 242, 516–525. [Google Scholar]
- Liu, X.; Zhang, Y.; Li, Z.; Feng, R.; Zhang, Y. Characterization of corncob-derived biochar and pyrolysis kinetics in comparison with corn stalk and sawdust. Bioresour. Technol. 2014, 170, 76–82. [Google Scholar] [CrossRef]
- Yuan, H.; Deng, L.; Qi, Y.; Kobayashi, N.; Tang, J. Nonactivated and activated biochar derived from bananas as alternative cathode catalyst in microbial fuel cells. Sci. World J. 2014, 2014, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Agrafioti, E.; Bouras, G.; Kalderis, D.; Diamadopoulos, E. Biochar production by sewage sludge pyrolysis. J. Anal. Appl. Pyrolysis 2018, 101, 72–78. [Google Scholar] [CrossRef]
- Goldfarb, J.L.; Buessing, L.; Gunn, E.; Lever, M.; Billias, A.; Casoliba, E.; Schievano, A.; Adani, F. Novel integrated biorefinery for olive mill waste management: Utilization of secondary waste for water treatment. ACS Sustain. Chem. Eng. 2017, 5, 876–884. [Google Scholar] [CrossRef]
- Goldfarb, J.L.; Dou, G.; Salari, M.; Grinstaff, M.W. Biomass-based fuels and activated carbon electrode materials: An integrated approach to green energy systems. ACS Sustain. Chem. Eng. 2017, 5, 3046–3054. [Google Scholar] [CrossRef]
- Hagemann, N.; Spokas, K.; Schmidt, H.-P.; Kägi, R.; Böhler, M.A.; Bucheli, T.D. Activated carbon, biochar and charcoal: Linkages and synergies across pyrogenic carbon’s ABCs. Water 2018, 10, 182. [Google Scholar] [CrossRef]
- Dhelipan, A.; Arunchander, A.; Sahu, A.K.; Kalpana, D. Activated carbon from orange peels as supercapacitor electrode and catalyst support for oxygen reduction reaction in proton exchange membrane fuel cell. J. Saudi Chem. Soc. 2017, 21, 487–494. [Google Scholar] [CrossRef]
- Lv, Y.; Gan, L.; Liu, M.; Xiong, W.; Xua, Z.; Zhu, D.; Wright, D.S. A self-template synthesis of hierarchical porous carbon foams based on banana peel for supercapacitor electrodes. J. Power Sources 2012, 209, 152–157. [Google Scholar] [CrossRef]
- Deng, L.-F.; Dong, G.; Cai, X.-X. Biochar derived from the inner membrane of passion fruit as cathode catalyst of microbial fuel cells in neutral solution. J. Fuel Chem. Technol. 2018, 46, 120–128. [Google Scholar]
- Li, M.; Zhang, H.; Xiao, T.; Wang, S.; Zhang, B.; Chen, D.; Su, M.; Tang, J. Low-cost biochar derived from corncob as oxygen reduction catalyst in air cathode microbial fuel cells. Electrochim. Acta 2018, 283, 780–788. [Google Scholar] [CrossRef]
- Chang, H.-C.; Gustave, W.; Yuan, Z.-F.; Xiao, Y.; Chen, Z. One-step fabrication of binder-free air cathode for microbial fuel cells by using balsa wood biochar. Environ. Technol. Innov. 2020, 18, 100615. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, Y.; Zhou, W.; Li, L.; Huang, S.; Chen, S. Biomass-derived heteroatoms-doped mesoporous carbon for efficient oxygen reduction in microbial fuel cells. Biosens. Bioelectron. 2017, 98, 350–356. [Google Scholar] [CrossRef]
- Miran, W.; Nawaz, M.; Jang, J.; Lee, D.S. Conversion of orange peel waste biomass to bioelectricity using a mediator-less microbial fuel cell. Sci. Total Environ. 2016, 547, 197–205. [Google Scholar] [CrossRef]
- Sciarria, T.P.; Tenca, A.; D’Epifanio, A.; Mecheri, B.; Merlino, G.; Barbato, M.; Borin, S.; Licoccia, S.; Garavaglia, V.; Adani, F. Using olive mill wastewater to improve performance in producing electricity from domestic wastewater by using single-chamber microbial fuel cell. Bioresour. Technol. 2013, 147, 246–253. [Google Scholar] [CrossRef]
- Sciarria, T.P.; Costa de Oliveira, M.A.; Mecheri, B.; D’Epifanio, A.; Goldfarb, J.L.; Adani, F. Metal-free activated biochar as an oxygen reduction reaction catalyst in single chamber microbial fuel cells. J. Power Sources 2020, 462, 228183. [Google Scholar] [CrossRef]
- Xue, H.; He, T.; Chabu, J.M.; Liu, J.; Wu, H.; Zheng, J.; Tan, M.; Ma, J.; Shen, R.; Deng, L.; et al. Iron single clusters anchored on N-doped porous carbon as superior trace-metal catalysts toward oxygen reduction. Adv. Mater. Interfaces 2018, 5, 1701345. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, J.; Huang, T. The key roles of trace iron for nitrogen, sulfur dual-doped carbon nanospheres as high efficient oxygen reduction catalyst. J. Mater. Sci. 2018, 53, 1404–1413. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Konkena, B.; Jayaramulu, K.; Schuhmann, W.; Maji, T.K. Synthesis of nano-porous carbon and nitrogen doped carbon dots from an anionic MOF: A trace cobalt metal residue in carbon dots promotes electrocatalytic ORR activity. J. Mater. Chem. A 2017, 5, 13573–13580. [Google Scholar] [CrossRef]
- Ye, R.; Dong, J.; Wang, L.; Mendoza-Cruz, R.; Li, Y.; An, P.-F.; Yacamán, M.J.; Yakobson, B.I.; Chen, D.; Tour, J.M. Manganese deception on graphene and implications in catalysis. Carbon 2018, 132, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Masa, J.; Xia, W.; Muhler, M.; Schuhmann, W. On the role of metals in nitrogen-doped carbon electrocatalysts for oxygen reduction. Angew. Chem. Int. Ed. 2015, 54, 10102–10120. [Google Scholar] [CrossRef] [PubMed]
| 1 | [96] |
| pH | Pathway | Reactions | E0 vs. RHE1 |
|---|---|---|---|
| <7 | Direct four-electron | O2 + 4H+ + 4e− → 2 H2O | 1.230 |
| <7 | two-electron | O2 + 2H+ + 2e− → H2O2 | 0.695 |
| <7 | - | H2O2 + 2H+ + 2e− → 2 H2O | 1.776 |
| >7 | Direct four-electron | O2 + 2 H2O + 4e− → 4 OH− | 1.230 |
| >7 | two-electron | O2 + H2O + 2e− → H2O− + OH− | 0.695 |
| >7 | - | H2O− + H2O + 2e− ⇨ OH− | 1.776 |
| Carbon Source | Iron Source | Nitrogen Source | Pyrolysis T | PD | Ref. |
|---|---|---|---|---|---|
| Benzimidazole, Aminobenzimidazole | Fe(NO3)3 | Benzimidazole, Aminobenzimidazole | 900 °C | 1620 mWm−2 | [95] |
| Ketjen Black | ClFeTMPP, FePc | NH3(g) | 700 °C | 590 mWm−2 | [96] |
| Aminoantipyrine | Fe(NO3)3 | Aminoantipyrine | 950 °C | 2510 mWm−2 | [97] |
| Graphene | Fe2O3 | Pyrrole | 600 °C | 1380 mWm−2 | [98] |
| Activated carbon | FeCl3 | Chitosan | 800 °C | 2400 mWm−2 | [99] |
| Graphene oxide | Fe2O3 | BNNS1s | 550 °C | 1673 mWm−2 | [100] |
| 2-methylimidazole | FeCl2 | 2-methylimidazole | 800 °C | 4335 mWm−2 | [101] |
| Aminoantipyrine | Fe(NO3)3 | Aminoantipyrine | 950 °C | 1300 mWm−2 | [102] |
| Nicarbazin | Fe(NO3)3 | NH3(g), Nicarbazin | 900 °C | 1850 mWm−2 | [103] |
| Activated carbon | Fe(II)Pc | Fe(II)Pc | 900 °C | 1092 mWm−2 | [104] |
| Phenolic resin | Fe(II)Pc | Fe(II)Pc | 600 °C | 330 mWm−2 | [75] |
| Activated carbon | Fe(II)Pc | Fe(II)Pc | 400–1000 °C | 120 mWm−2 | [76] |
| Ricobendazole, niclosamide | Fe(NO3)3 | Ricobendazole, niclosamide | 975 °C | 2510 mWm−2 | [21] |
| Biomass | Pyrolysis T | Power Density | Ref. |
| Sewage sludge | 900 °C | 500 mWm−2 | [6] |
| Banana | 900 °C | 528 mWm−2 | [136] |
| Passion fruit | 900 °C | 1153 mWm−2 | [143] |
| Corncob | 650 °C | 459 mWm−2 | [144] |
| Balsa wood | 800 °C | 200 mWm−2 | [145] |
| Egg | 900 °C | 737 mWm−2 | [146] |
| Orange peel | 50 °C | 359 mWm−2 | [147] |
| Pt/C | - | 704 mWm−2 | [146] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa de Oliveira, M.A.; D’Epifanio, A.; Ohnuki, H.; Mecheri, B. Platinum Group Metal-Free Catalysts for Oxygen Reduction Reaction: Applications in Microbial Fuel Cells. Catalysts 2020, 10, 475. https://doi.org/10.3390/catal10050475
Costa de Oliveira MA, D’Epifanio A, Ohnuki H, Mecheri B. Platinum Group Metal-Free Catalysts for Oxygen Reduction Reaction: Applications in Microbial Fuel Cells. Catalysts. 2020; 10(5):475. https://doi.org/10.3390/catal10050475
Chicago/Turabian StyleCosta de Oliveira, Maida Aysla, Alessandra D’Epifanio, Hitoshi Ohnuki, and Barbara Mecheri. 2020. "Platinum Group Metal-Free Catalysts for Oxygen Reduction Reaction: Applications in Microbial Fuel Cells" Catalysts 10, no. 5: 475. https://doi.org/10.3390/catal10050475
APA StyleCosta de Oliveira, M. A., D’Epifanio, A., Ohnuki, H., & Mecheri, B. (2020). Platinum Group Metal-Free Catalysts for Oxygen Reduction Reaction: Applications in Microbial Fuel Cells. Catalysts, 10(5), 475. https://doi.org/10.3390/catal10050475