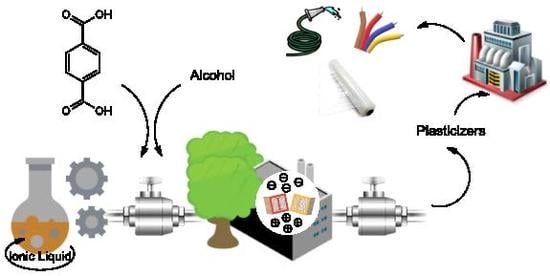
Sustainable Method for the Synthesis of Alternative Bis(2-Ethylhexyl) Terephthalate Plasticizer in the Presence of Protic Ionic Liquids
Abstract
1. Introduction
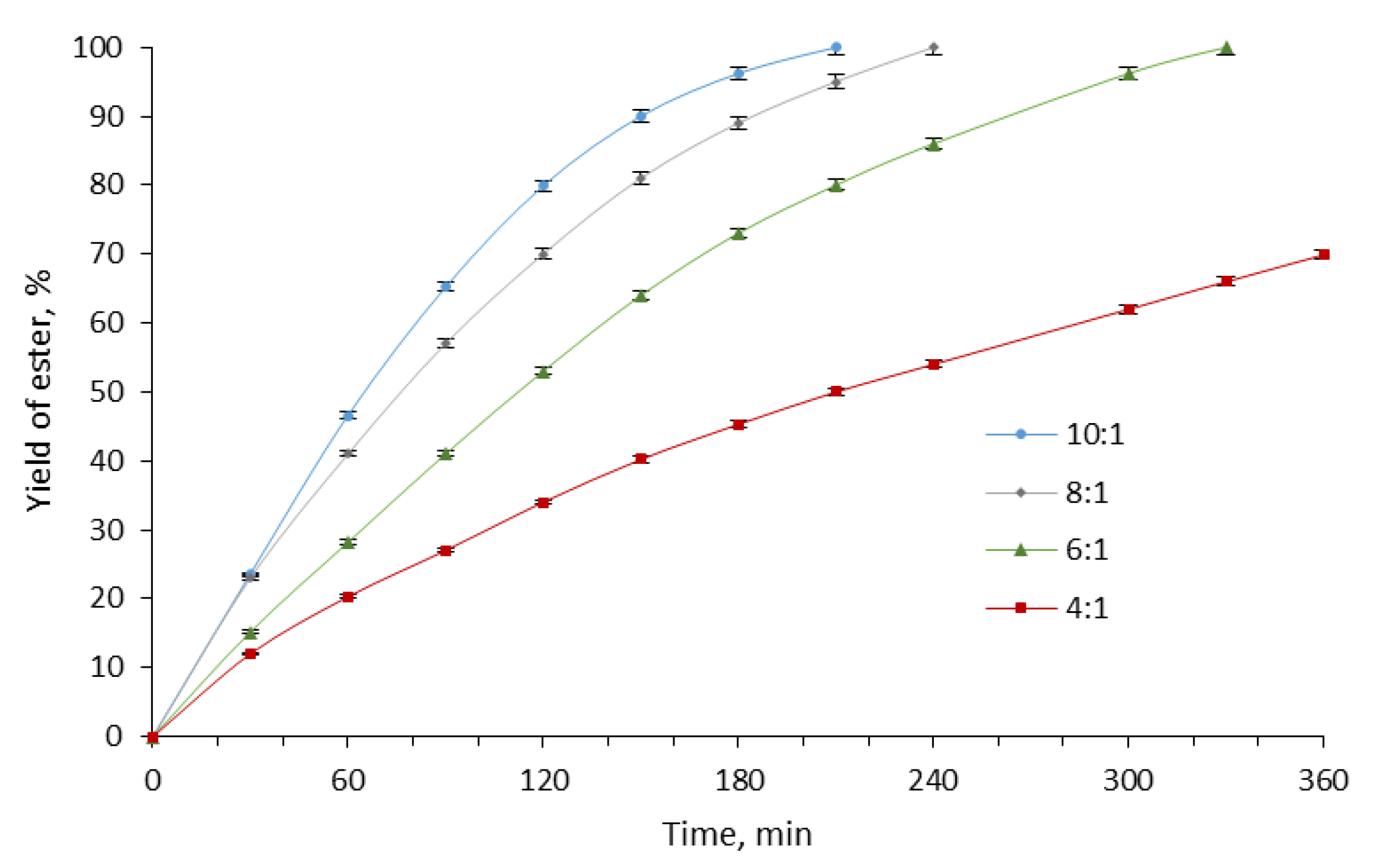
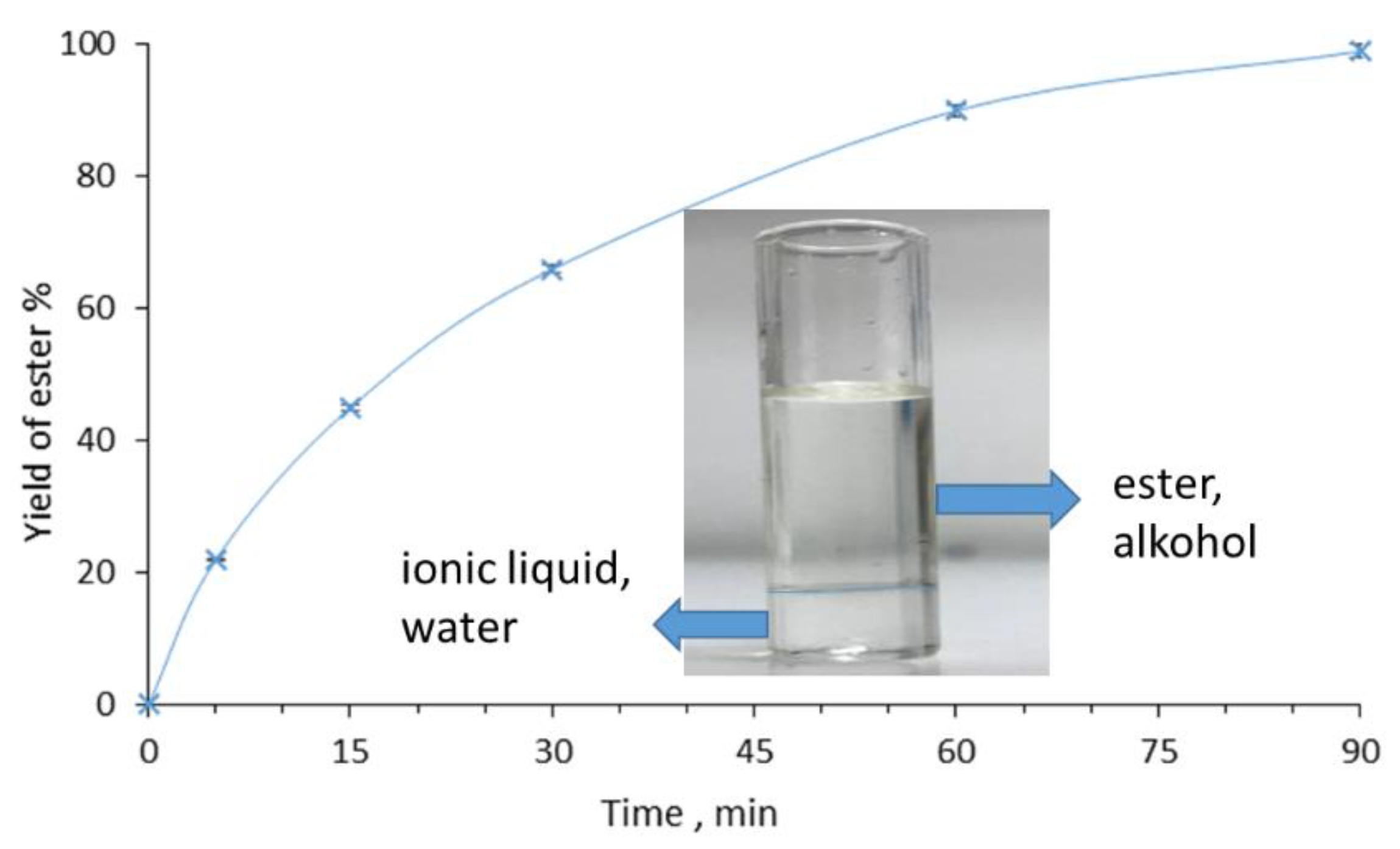
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Methods
3.3. Synthesis
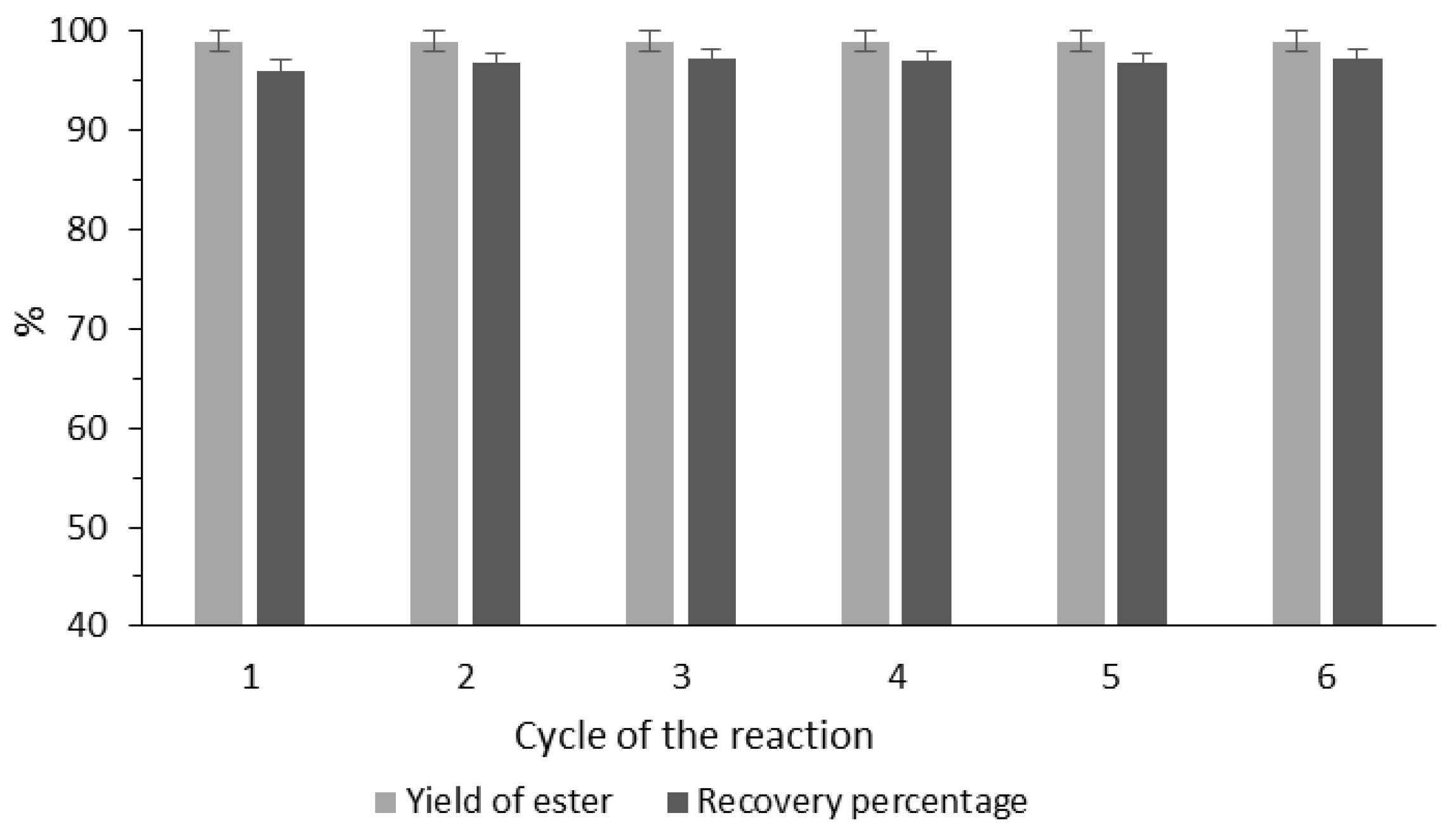
3.4. Recycling Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jamarani, R.; Erythropel, H.C.; Nicell, J.A.; Leask, R.L.; Maric, M. How green is your plasticizer? Polymers 2018, 10, 834. [Google Scholar] [CrossRef] [PubMed]
- Jia, P.; Xia, H.; Tang, K.; Zhou, Y. Plasticizers derived from biomass resources: A short review. Polymers 2018, 10, 1303. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S. Recent developments of biobased plasticizers and their effect on mechanical and thermal properties of Poly(vinyl chloride): A review. Ind. Eng. Chem. Res. 2019, 58, 11659–11672. [Google Scholar] [CrossRef]
- Ball, G.L.; McLellan, C.J.; Bhat, V.S. Toxicological review and oral risk assessment of terephthalic acid (TPA) and its esters: A category approach. Crit Rev. Toxicol. 2012, 42, 28–67. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, I.; Takai, M. Method for Producing Terephthalic Acid Diester. JP 2004300078, 28 October 2004. Available online: https://patents.google.com/patent/JP2004300078A/en (accessed on 1 April 2020).
- Nakajima, I.; Takai, M. Method for Producing Terephthalic Acid Diester. JP 2004339075, 2 December 2004. Available online: https://patents.google.com/patent/JP2004339075A/en?oq=JP+2004339075 (accessed on 1 April 2020).
- Matsumoto, S. Catalyst for Esterification and Transesterification and Process for Producing Ester. U.S. Patent 2005/0176986, 18 April 2005. [Google Scholar]
- Ikeda, H.; Inoue, T.; Takahashi, K. Method for Producing Diester of Terephthalic Acid. JP 2005306759, 4 November 2005. Available online: https://patents.google.com/patent/JP2005306759A/en?oq=JP+2005306759 (accessed on 1 April 2020).
- Matsuo, T.; Nakajima, I.; Takai, M. Method for Producing Terephthalic Acid Diester. JP 2006273799, 12 October 2006. Available online: https://patents.google.com/patent/JP2006273799A/en?oq=JP+2006273799 (accessed on 1 April 2020).
- Osborne, V.H.; Turner, P.W.; Cook, S.L. Production of Terephthalic Acid Di-Esters. WO 2008/094396, 7 August 2008. Available online: https://patents.google.com/patent/WO2008094396A1/en?oq=WO+2008%2f094396 (accessed on 1 April 2020).
- Nakajima, I.; Takai, M. Method for Producing Terephthalic Acid Diester. JP 2009185040, 20 August 2009. Available online: https://patents.google.com/patent/JP2009185040A/en?oq=JP+2009185040 (accessed on 1 April 2020).
- Sutor, E.; Grzybek, R.; Grymel, A.; Janik, L.; Trybuła, J.; Tkacz, B.; Fiszer, R.; Krueger, A.; Jasienkiewicz, J.; Filipiak, B.; et al. Process for the Preparation of Dioctyl Terephthalate. PL 216179, 31 March 2014. Available online: https://patents.google.com/patent/PL216179B1/en?oq=PL+216179 (accessed on 1 April 2020).
- Grass, M. Dialkyl Terephthalates and Their Use. U.S. Patent 20070179229, 2 August 2007. [Google Scholar]
- Matuszek, K.; Pankalla, E.; Grymel, A.; Latos, P.; Chrobok, A. Studies on the Solubility of Terephthalic Acid in Ionic Liquids. Molecules 2020, 25, 80. [Google Scholar] [CrossRef] [PubMed]
- Kaller, A.S.M.; Bronneberg, R.; Stammer, J.; Das, M.; Harnischmacher, G. Method for Producing Diesters of Terephthalic Acid with A Dehydration of Recirculated Alcohol. WO 2016046118A1, 31 March 2016. Available online: https://patents.google.com/patent/WO2016046118A1/en?oq=WO+2016046118A1 (accessed on 1 April 2020).
- Lin, J.Q.; Fang, G.; Jin, C.; Sun, Y.; Zuo, S.; Qian, C.; Lin, W.; Zang, X.; Liu, L.; Chen, Y. Method for Synthesizing Dioctyl Terephthalate Through Esterification. CN 102001948A, 6 April 2011. Available online: https://patents.google.com/patent/CN102001948A/en?oq=CN102001948A (accessed on 1 April 2020).
- Xuesong, P. Production Method of Dioctyl Terephthalate. CN 102701984A, 3 October 2012. Available online: https://patents.google.com/patent/CN102701984A/en?oq=CN102701984A (accessed on 1 April 2020).
- Hongyun, G.; Maolin, T.; Lingling, L.; Chenxi, L.; Maohua, D. Applications of Acidic Functionalized Ion Liquid in Esterification Reaction. CN 103752340A, 30 April 2014. Available online: https://patents.google.com/patent/CN103752340A/en?oq=CN103752340A (accessed on 1 April 2020).
- Matuszek, K.; Chrobok, A.; Coleman, F.; Seddon, K.R.; Swadźba-Kwaśny, M. Tailoring ionic liquid catalysts: Structure, acidity and catalytic activity of protonic ionic liquids based on anionic clusters, [(HSO4)(H2SO4)x]− (x = 0, 1, or 2). Green Chem. 2014, 16, 3463–3471. [Google Scholar] [CrossRef]
- Dorosz, U.; Barteczko, N.; Latos, P.; Erfurt, K.; Pankalla, E.; Chrobok, A. Highly efficient biphasic system for the synthesis of alkyl lactates in the presence of acidic ionic liquids. Catalysts 2020, 10, 37. [Google Scholar] [CrossRef]
- Chiappe, C.; Rajamani, S.; D’Andrea, F. A dramatic effect of the ionic liquid structure in esterification reactions in protic ionic media. Green Chem. 2013, 15, 137–143. [Google Scholar] [CrossRef]
- George, A.; Brandt, A.; Tran, K.; Zahari, S.M.S.N.S.; Klein-Marcuschamer, D.; Sun, N.; Sathitsuksanoh, N.; Shi, J.; Stavila, V.; Parthasarathi, R.; et al. Design of low-cost ionic liquids for lignocellulosic biomass pretreatment. Green Chem. 2015, 17, 1728–1734. [Google Scholar] [CrossRef]
- Gutmann, V. The Donor-Acceptor Approach to Molecular Interactions; Plenum Press: New York, NY, USA, 1978. [Google Scholar]


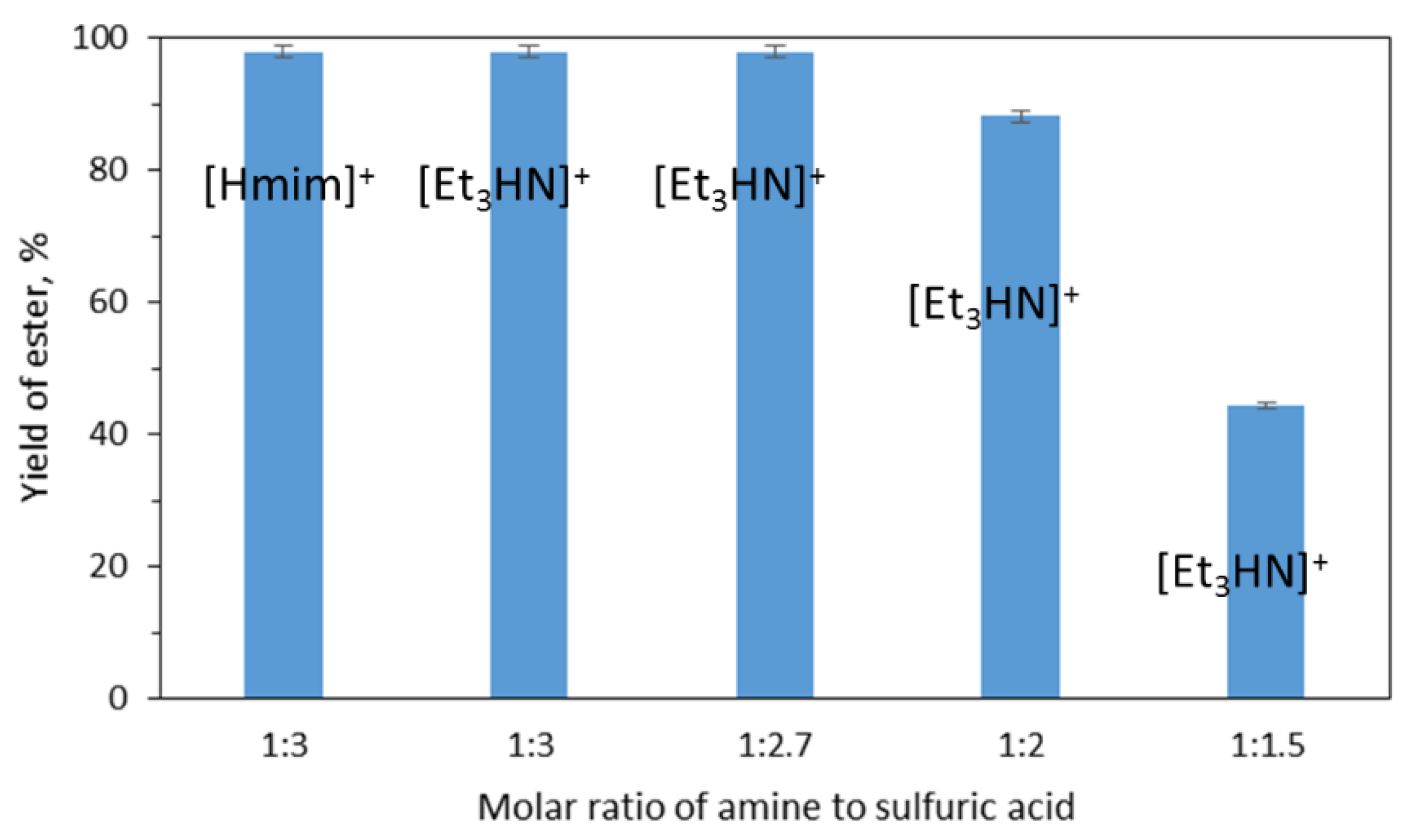

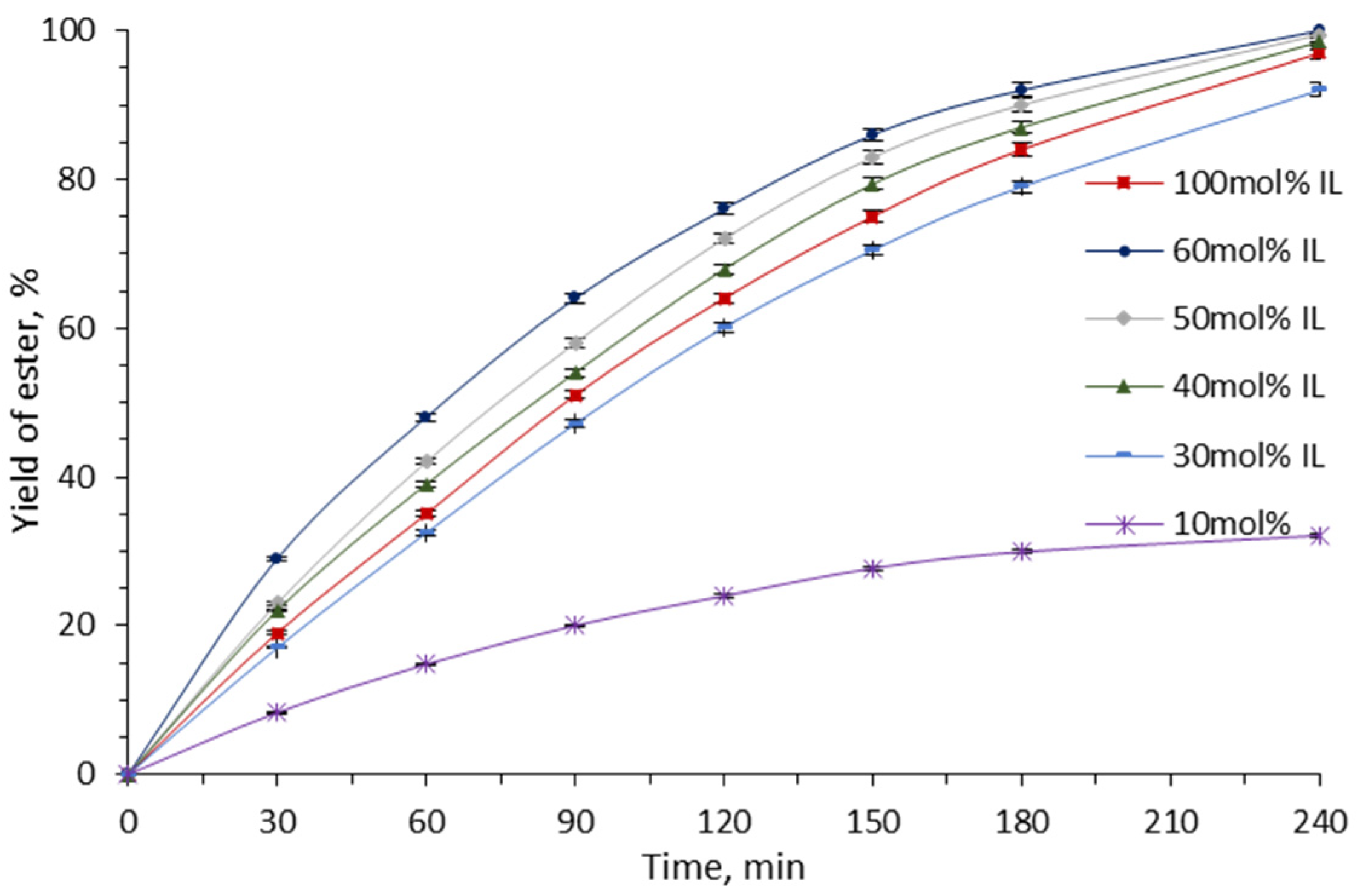
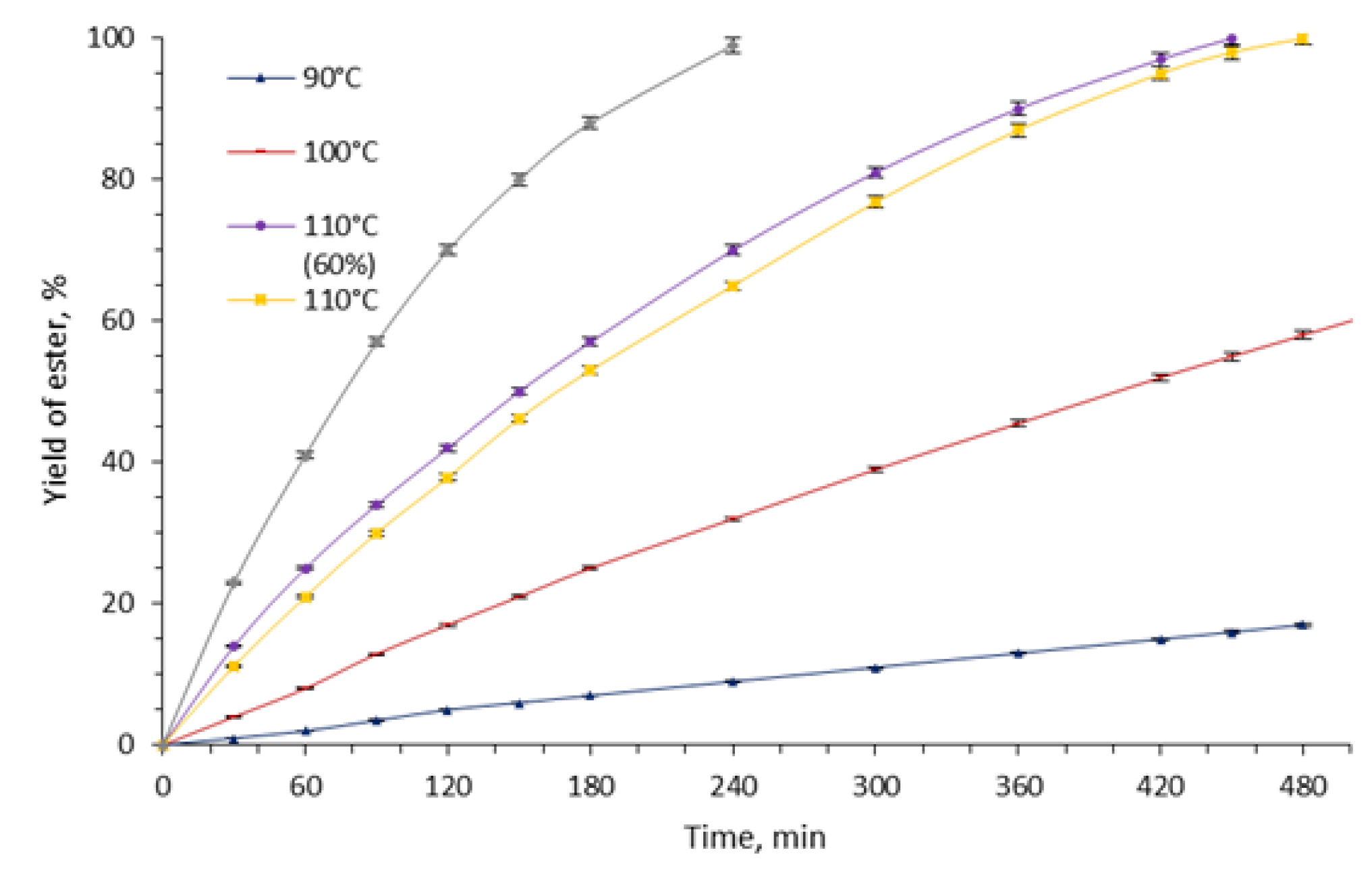

| Molar Ratio Et3N:H2SO4 | 1:3 | 1:2.7 | 1:2 | 1:1.5 |
|---|---|---|---|---|
| Yield of ester, % (time, h) | 99.5 (4) | 99.1 (4) | 99.2 (5) | 99.7 (14) |
| Yield of ether, % (time, h) | 23.1 (4) | 2.5 (4) | 2.2 (4) | 1.7 (4) |
| Temp., °C | Yield of Bis(2-Ethylhexyl) Ether, % Ionic Liquid 1:3 | Yield of Bis(2-Ethylhexyl) Ether, % Ionic Liquid 1:2.7 |
|---|---|---|
| 90 | 0.7 | 0.6 |
| 100 | 5.3 | 2.1 |
| 110 | 5.2 | 2.2 |
| 120 | 23.1 | 2.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grymel, A.; Latos, P.; Matuszek, K.; Erfurt, K.; Barteczko, N.; Pankalla, E.; Chrobok, A. Sustainable Method for the Synthesis of Alternative Bis(2-Ethylhexyl) Terephthalate Plasticizer in the Presence of Protic Ionic Liquids. Catalysts 2020, 10, 457. https://doi.org/10.3390/catal10040457
Grymel A, Latos P, Matuszek K, Erfurt K, Barteczko N, Pankalla E, Chrobok A. Sustainable Method for the Synthesis of Alternative Bis(2-Ethylhexyl) Terephthalate Plasticizer in the Presence of Protic Ionic Liquids. Catalysts. 2020; 10(4):457. https://doi.org/10.3390/catal10040457
Chicago/Turabian StyleGrymel, Aleksander, Piotr Latos, Karolina Matuszek, Karol Erfurt, Natalia Barteczko, Ewa Pankalla, and Anna Chrobok. 2020. "Sustainable Method for the Synthesis of Alternative Bis(2-Ethylhexyl) Terephthalate Plasticizer in the Presence of Protic Ionic Liquids" Catalysts 10, no. 4: 457. https://doi.org/10.3390/catal10040457
APA StyleGrymel, A., Latos, P., Matuszek, K., Erfurt, K., Barteczko, N., Pankalla, E., & Chrobok, A. (2020). Sustainable Method for the Synthesis of Alternative Bis(2-Ethylhexyl) Terephthalate Plasticizer in the Presence of Protic Ionic Liquids. Catalysts, 10(4), 457. https://doi.org/10.3390/catal10040457