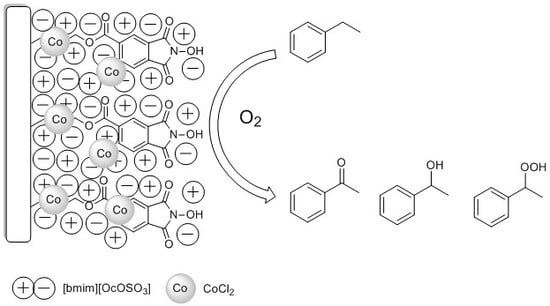
N-Hydroxyphthalimide Supported on Silica Coated with Ionic Liquids Containing CoCl2 (SCILLs) as New Catalytic System for Solvent-Free Ethylbenzene Oxidation
Abstract
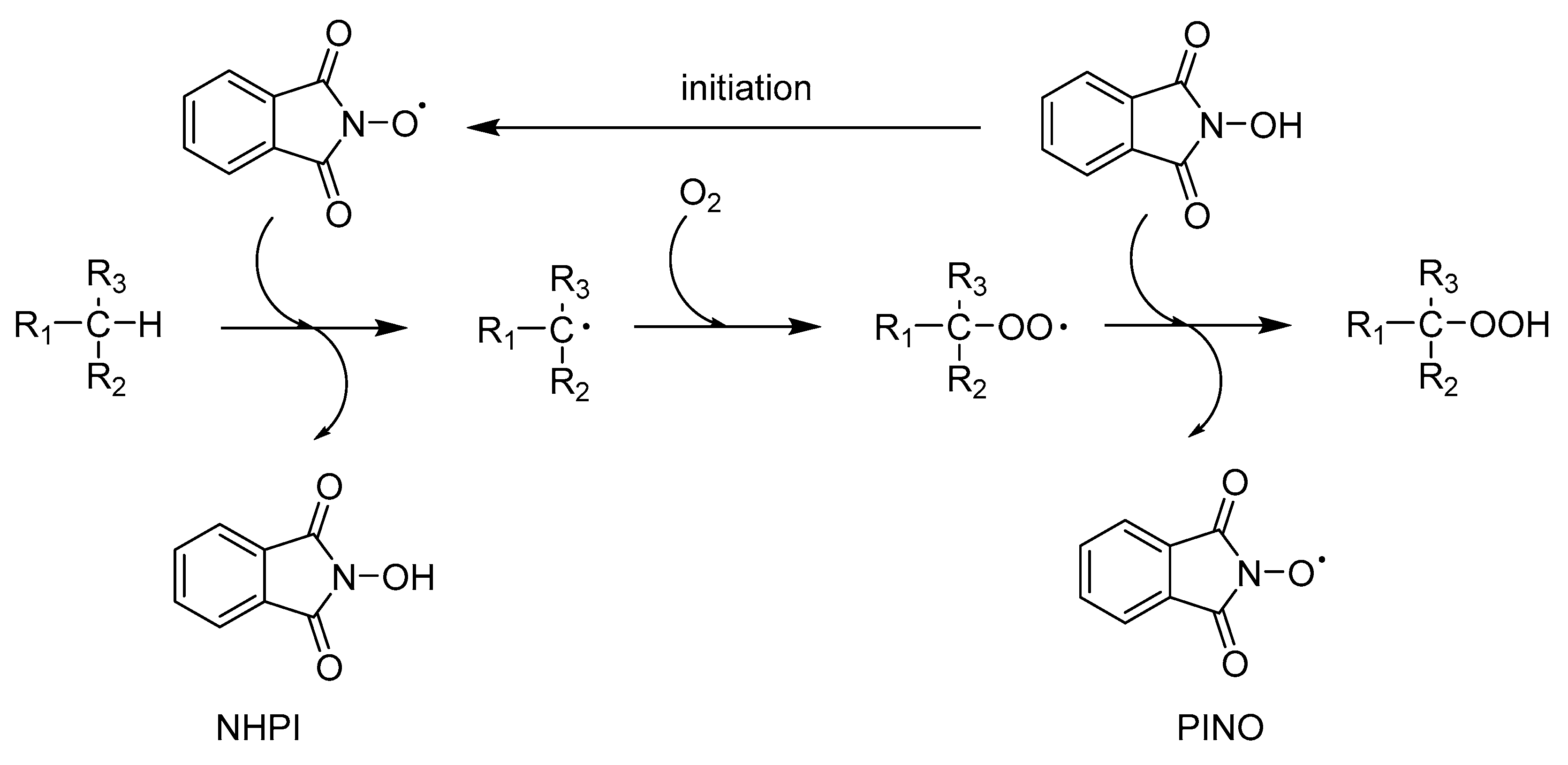
1. Introduction
2. Results and Discussion
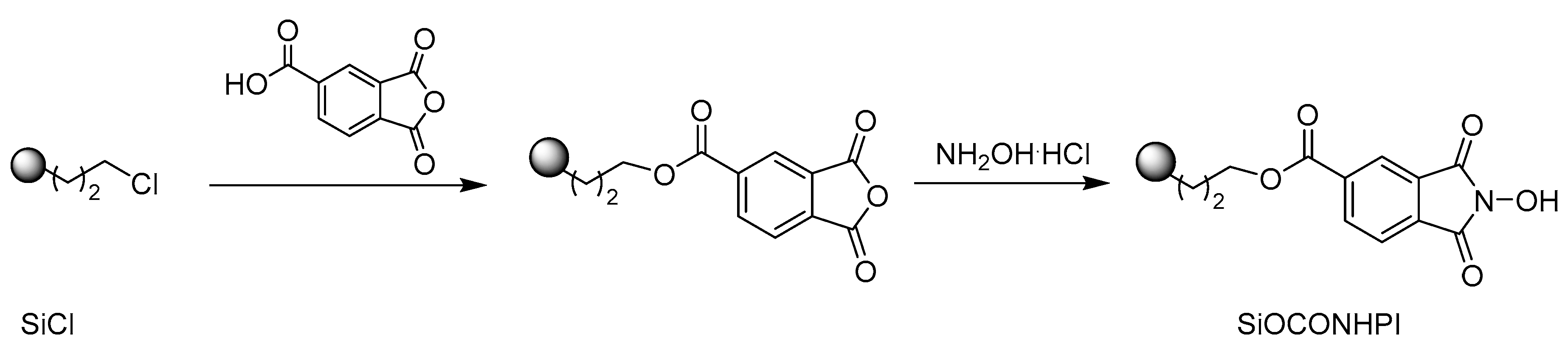
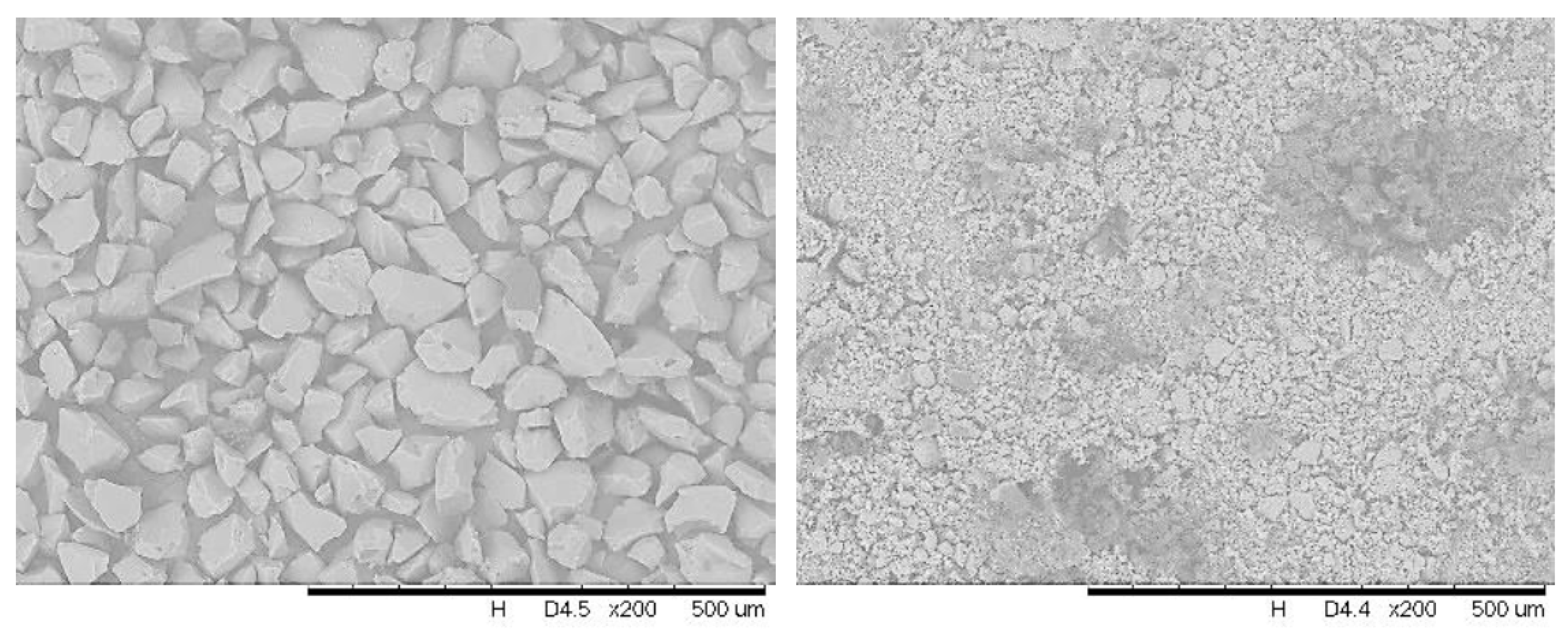
2.1. Preparation of Catalyst
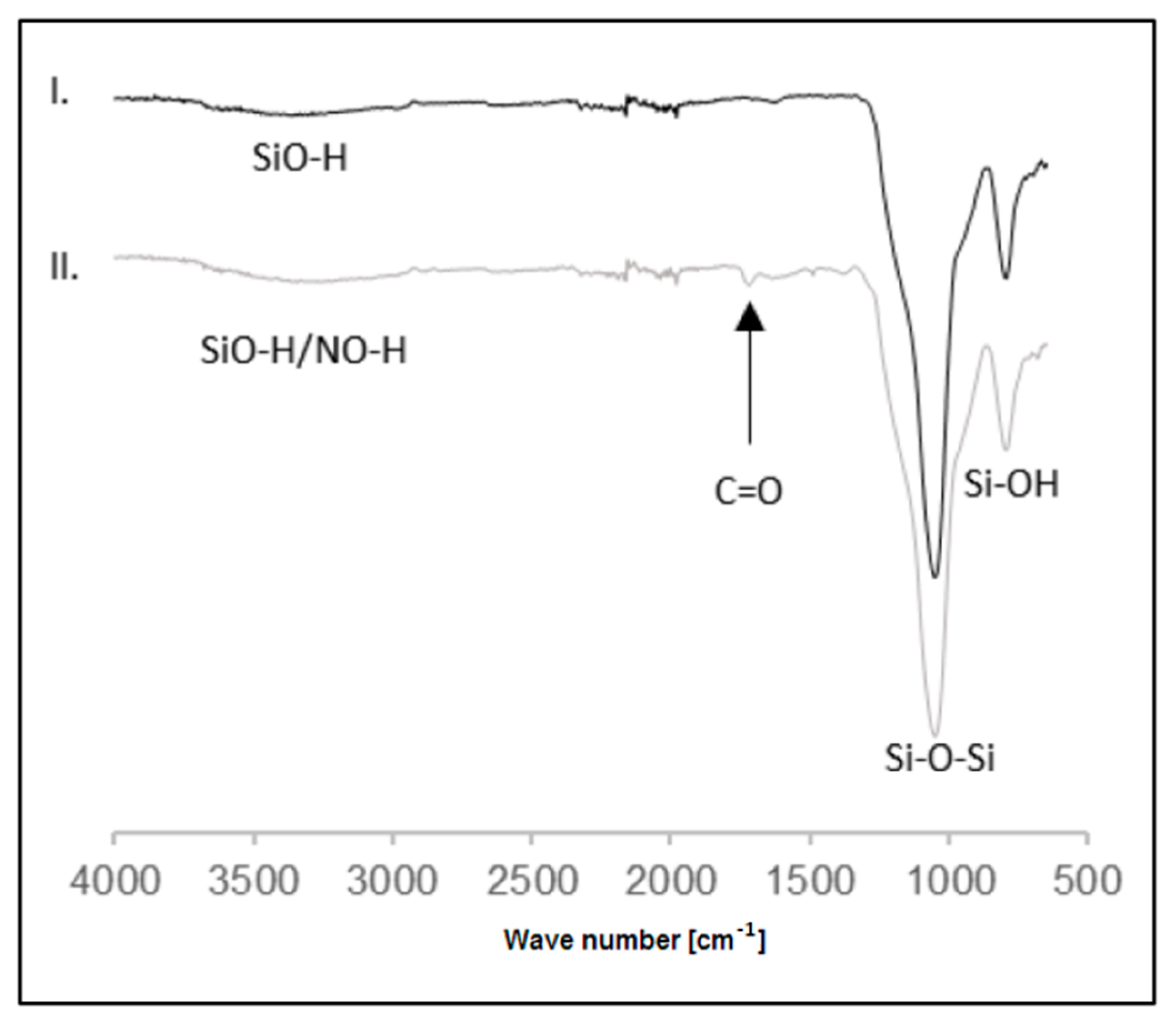
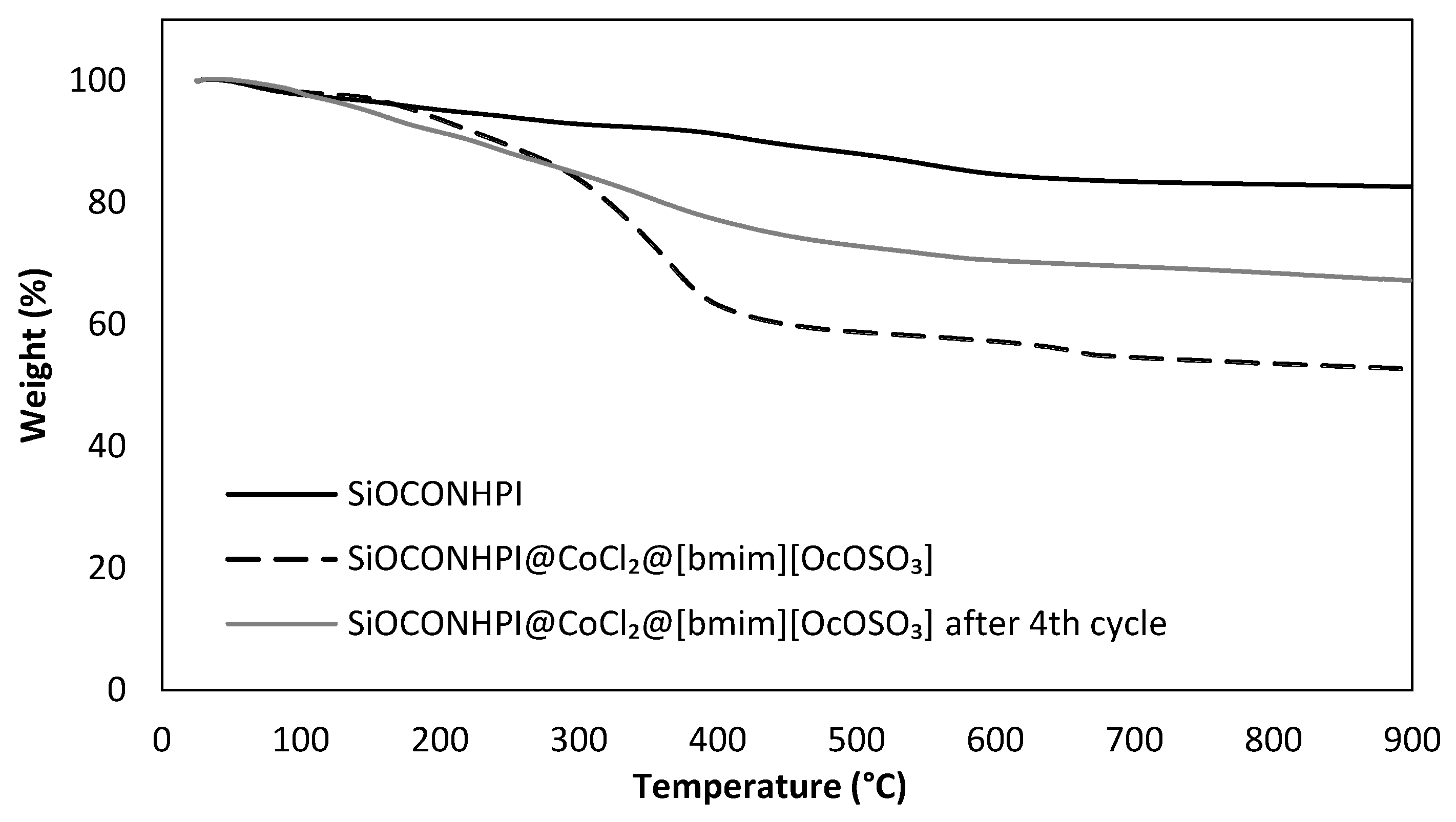
2.1.1. SiOCONHPI Synthesis
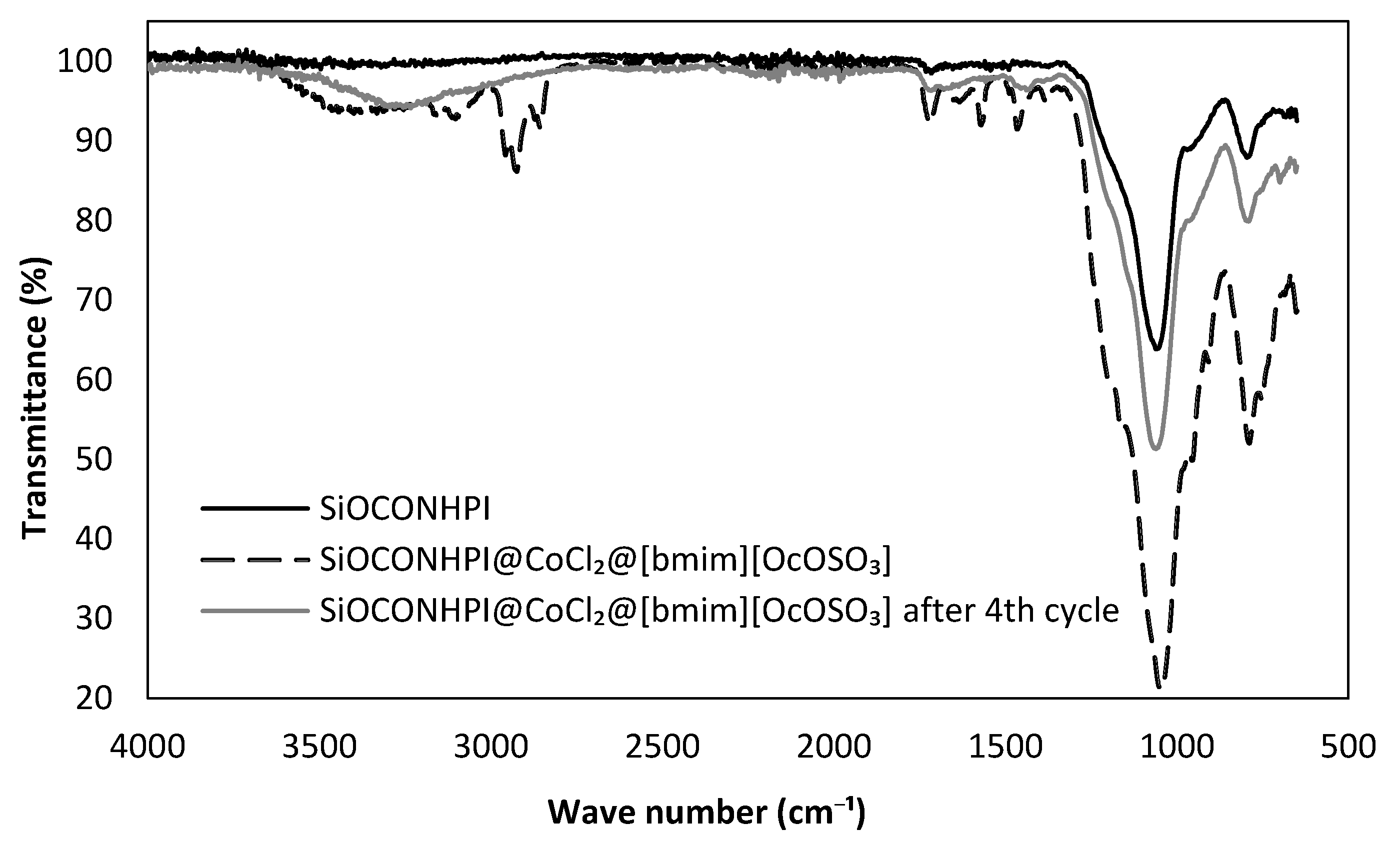
2.1.2. SiOCONHPI@CoCl2@IL Preparation
2.2. Study on Ethylbenzene Oxidation Reaction
2.2.1. Ethylbenzene Oxidation with Oxygen using SiOCONHPI or SiOCONHPI/CoCl2 System
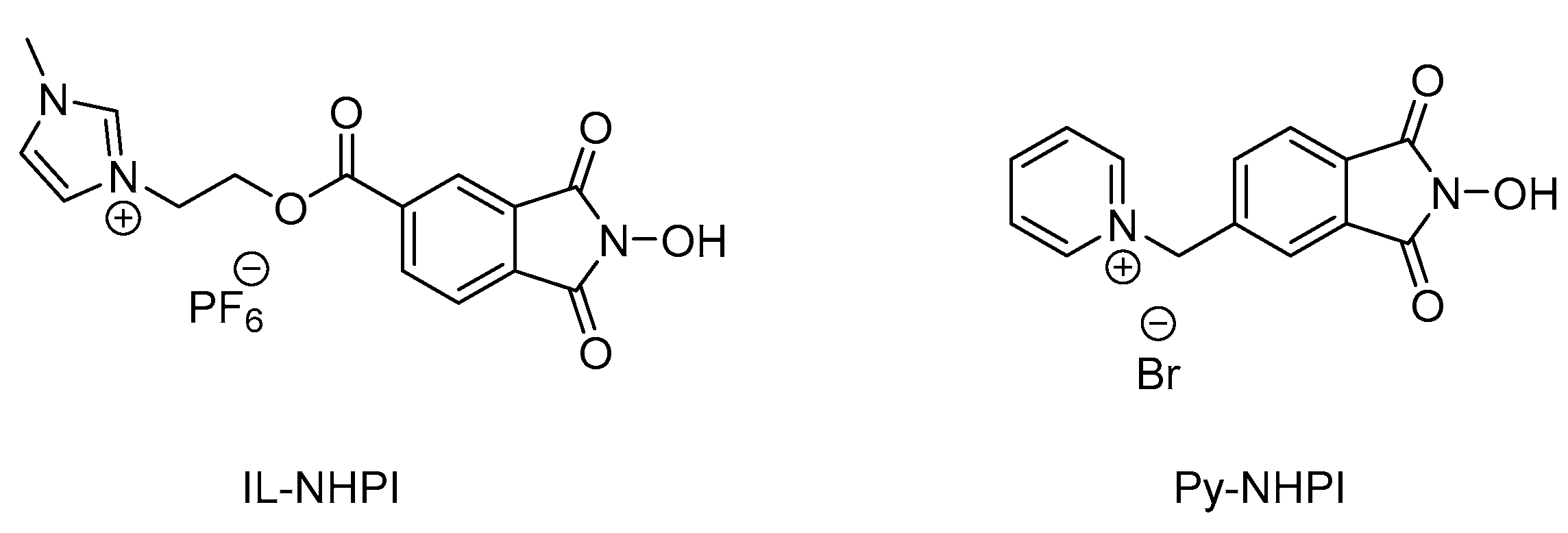
2.2.2. Ethylbenzene Oxidation with Oxygen using SiOCONHPI@CoCl2@[bmim][X] System
2.2.3. Influence of the Structure of Alkyl Substituent in Cation or Anion of Ionic Liquid on Catalytic Activity of SiOCONHPI@CoCl2@IL
2.2.4. Attempts to Reuse SiOCONHPI@CoCl2@[bmim][X] System.
3. Materials and Methods
3.1. Materials
3.2. SiOCONHPI Synthesis
3.3. SiOCONHPI@Co(II)@IL Preparation
3.4. Ethylbenzene Oxidation
3.5. Analytic Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Franz, G.; Sheldon, R.A. Ullmann’s Encyclopedia of Industrial Chemistry. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2012; pp. 1–28. [Google Scholar]
- Weissermel, K.; Arpe, H.J. Industrial Organic Chemistry; VCH: Weinheim, Germany, 1997. [Google Scholar]
- Recupero, F.; Punta, C. Free Radical Functionalization of Organic Compounds Catalyzed by N-Hydroxyphthalimide. Chem. Rev. 2007, 107, 3800–3842. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zhang, P.; Wang, Y.; Li, H. Metal-Free Allylic/Benzylic Oxidation Strategies with Molecular Oxygen: Recent Advances and Future Prospects. Green Chem. 2014, 16, 2344–2374. [Google Scholar] [CrossRef]
- Coseri, S. Phthalimide-N-Oxyl (PINO) Radical, a Powerful Catalytic Agent: Its Generation and Versatility towards Various Organic Substrates. Catal. Rev. Sci. Eng. 2009, 51, 218–292. [Google Scholar] [CrossRef]
- Melone, L.; Punta, C. Metal-Free Aerobic Oxidations Mediated by N-Hydroxyphthalimide: A Concise Review. Beilstein J. Org. Chem. 2013, 9, 1296–1310. [Google Scholar] [CrossRef] [PubMed]
- Ishii, Y.; Iwahama, T.; Sakaguchi, S.; Nakayama, K.; Nishiyama, Y. Alkane Oxidation with Molecular Oxygen Using a New Efficient Catalytic System: N-Hydroxyphthalimide (NHPI) Combined with Co(acac)n (n = 2 or 3). J. Org. Chem. 1996, 61, 4520–4526. [Google Scholar] [CrossRef]
- Orlińska, B.; Zawadiak, J. Aerobic Oxidation of Isopropylaromatic Hydrocarbons to Hydroperoxides Catalyzed by N-Hydroxyphthalimide. React. Kinet. Mech. Catal. 2013, 110, 15–30. [Google Scholar] [CrossRef]
- Ishii, Y.; Nakayama, K.; Takeno, M.; Sakaguchi, S.; Iwahama, T.; Nishiyama, Y. A Novel Catalysis of N-Hydroxyphthalimide in the Oxidation of Organic Substrates by Molecular Oxygen. J. Org. Chem. 1995, 60, 3934–3935. [Google Scholar] [CrossRef]
- Lisicki, D.; Orlińska, B. Oxidation of Cycloalkanes Catalysed by N-Hydroxyimides in Supercritical Carbon Dioxide. Chem. Pap. 2020, 74, 711–716. [Google Scholar] [CrossRef]
- Dobras, G.; Lisicki, D.; Pyszny, D.; Orlińska, B. Badania Reakcji Utleniającego Rozszczepienia α-Metylostyrenu Tlenem Wobec N-Hydroksyftalimidu w Alternatywnych Rozpuszczalnikach. Przem. Chem. 2019, 1, 124–129. [Google Scholar] [CrossRef]
- Yu, K.M.K.; Abutaki, A.; Zhou, Y.; Yue, B.; He, H.Y.; Tsang, S.C. Selective Oxidation of Cyclohexane in Supercritical Carbon Dioxide. Catal. Lett. 2007, 113, 115–119. [Google Scholar]
- Yavari, I.; Karimi, E. N-Hydroxyphthalimide-Catalyzed Oxidative Production of Phthalic Acids from Xylenes Using O2/HNO3 in an Ionic Liquid. Synth. Commun. 2009, 39, 3420–3427. [Google Scholar] [CrossRef]
- Dobras, G.; Orlińska, B. Aerobic Oxidation of Alkylaromatic Hydrocarbons to Hydroperoxides Catalysed by N-Hydroxyimides in Ionic Liquids as Solvents. Appl. Catal. A Gen. 2018, 561, 59–67. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, T. PEG1000-DAIL Enhanced Catalysis Activity: Oxidation of Ethylbenzene and Its Derivatives by N-Hydroxyphthalimide and Oxime in 1000-Based Dicationic Acidic Ionic Liquid. Chiang Mai J. Sci. 2014, 41, 138–147. [Google Scholar]
- Lu, T.; Lu, M.; Yu, W.; Liu, Z. Remarkable Effect of PEG-1000-Based Dicationic Ionic Liquid for N-Hydroxyphthalimide-Catalyzed Aerobic Selective Oxidation of Alkylaromatics. Croat. Chem. Acta 2012, 85, 277–282. [Google Scholar] [CrossRef]
- Koguchi, S.; Kitazume, T. Synthetic Utilities of Ionic Liquid-Supported NHPI Complex. Tetrahedron Lett. 2006, 47, 2797–2801. [Google Scholar] [CrossRef]
- Wang, J.R.; Liu, L.; Wang, Y.F.; Zhang, Y.; Deng, W.; Guo, Q.X. Aerobic Oxidation with N-Hydroxyphthalimide Catalysts in Ionic Liquid. Tetrahedron Lett. 2005, 46, 4647–4651. [Google Scholar] [CrossRef]
- Melone, L.; Prosperini, S.; Ercole, G.; Pastori, N.; Punta, C. Is It Possible to Implement N-Hydroxyphthalimide Homogeneous Catalysis for Industrial Applications? A Case Study of Cumene Aerobic Oxidation. J. Chem. Technol. Biotechnol. 2014, 89, 1370–1378. [Google Scholar] [CrossRef]
- Hermans, I.; Van Deun, J.; Houthoofd, K.; Peeters, J.; Jacobs, P.A. Silica-Immobilized N-Hydroxyphthalimide: An Efficient Heterogeneous Autoxidation Catalyst. J. Catal. 2007, 251, 204–212. [Google Scholar] [CrossRef]
- Rajabi, F.; Clark, J.H.; Karimi, B.; Macquarrie, D.J. The Selective Aerobic Oxidation of Methylaromatics to Benzaldehydes Using a Unique Combination of Two Heterogeneous Catalysts. Org. Biomol. Chem. 2005, 3, 725–726. [Google Scholar] [CrossRef]
- Kasperczyk, K.; Orlinska, B.; Witek, E.; Łątka, P.; Zawadiak, J.; Proniewicz, L. Polymer-Supported N-Hydroxyphthalimide as Catalyst for Toluene and p-Methoxytoluene Aerobic Oxidation. Catal. Lett. 2015, 145, 1856–1867. [Google Scholar] [CrossRef]
- Łątka, P.; Kasperczyk, K.; Orlińska, B.; Drozdek, M.; Skorupska, B.; Witek, E. N-Hydroxyphthalimide Immobilized on Poly(HEA-Co-DVB) as Catalyst for Aerobic Oxidation Processes. Catal. Lett. 2016, 146, 1991–2000. [Google Scholar] [CrossRef]
- Łątka, P.; Berniak, T.; Drozdek, M.; Witek, E.; Kuśtrowski, P. Formation of N-Hydroxyphthalimide Species in Poly(Vinyl-Diisopropyl-Phtalate Ester-Co-Styrene-Co-Divinylbenzene) and Its Application in Aerobic Oxidation of p-Methoxytoluene. Catal. Commun. 2018, 115, 73–77. [Google Scholar] [CrossRef]
- Gao, B.; Meng, S.; Yang, X. Synchronously Synthesizing and Immobilizing N-Hydroxyphthalimide on Polymer Microspheres and Catalytic Performance of Solid Catalyst in Oxidation of Ethylbenzene by Molecular Oxygen. Org. Process Res. Dev. 2015, 19, 1374–1382. [Google Scholar] [CrossRef]
- Hosseinzadeh, R.; Mavvaji, M.; Tajbakhsh, M.; Lasemi, Z. Synthesis and Characterization of N-Hydroxyphthalimide Immobilized on NaY Nano-Zeolite as a Novel and Efficient Catalyst for the Selective Oxidation of Hydrocarbons and Benzyl Alcohols. React. Kinet. Mech. Catal. 2018, 124, 839–855. [Google Scholar] [CrossRef]
- Sawant, A.D.; Raut, D.G.; Darvatkar, N.B.; Salunkhe, M.M. Recent Developments of Task-Specific Ionic Liquids in Organic Synthesis. Green Chem. Lett. Rev. 2011, 4, 41–54. [Google Scholar] [CrossRef]
- Korth, W.; Jess, A. Solid Catalysts with Ionic Liquid Layer (SCILL). In Supported Ionic Liquids: Fundamentals and Applications; Wiley-VCH: Weinheim, Germany, 2014; pp. 279–306. [Google Scholar]
- Gu, Y.; Li, G. Ionic Liquids-Based Catalysis with Solids: State of the Art. Adv. Synth. Catal. 2009, 351, 817–847. [Google Scholar] [CrossRef]
- Mehnert, C.P.; Cook, R.A.; Dispenziere, N.C.; Afeworki, M. Supported Ionic Liquid Catalysis—A New Concept for Homogeneous Hydroformylation Catalysis. J. Am. Chem. Soc. 2002, 124, 12932–12933. [Google Scholar] [CrossRef]
- Riisager, A.; Wasserscheid, P.; Van Hal, R.; Fehrmann, R. Continuous Fixed-Bed Gas-Phase Hydroformylation Using Supported Ionic Liquid-Phase (SILP) Rh Catalysts. J. Catal. 2003, 219, 452–455. [Google Scholar] [CrossRef]
- Chrobok, A.; Baj, S.; Pudło, W.; Jarzębski, A. Supported Hydrogensulfate Ionic Liquid Catalysis in Baeyer-Villiger Reaction. Appl. Catal. A Gen. 2009, 366, 22–28. [Google Scholar] [CrossRef]
- Arras, J.; Steffan, M.; Shayeghi, Y.; Claus, P. The Promoting Effect of a Dicyanamide Based Ionic Liquid in the Selective Hydrogenation of Citral. Chem. Commun. 2008, 4058–4060. [Google Scholar] [CrossRef]
- Arras, J.; Steffan, M.; Shayeghi, Y.; Ruppert, D.; Claus, P. Regioselective Catalytic Hydrogenation of Citral with Ionic Liquids as Reaction Modifiers. Green Chem. 2009, 11, 716–723. [Google Scholar] [CrossRef]
- Kernchen, U.; Etzold, B.; Korth, W.; Jess, A. Solid Catalyst Ionic Liquid Layer (SCILL)—A New Concept to Improve Selectivity Illustrated by Hydrogenation of Cyclooctadiene. Chem. Eng. Technol. 2007, 30, 985–994. [Google Scholar] [CrossRef]
- Karimi, B.; Badreh, E. SBA-15-Functionalized TEMPO Confined Ionic Liquid: An Efficient Catalyst System for Transition-Metal-Free Aerobic Oxidation of Alcohols with Improved Selectivity. Org. Biomol. Chem. 2011, 9, 4194–4198. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, D.; Xia, Z.; Gao, J.; Zhang, T.; Ma, J.; Zhang, D.; Tong, Z. Supported Ionic-Liquid Layer on Polystyrene-TEMPO Resin: A Highly Efficient Catalyst for Selective Oxidation of Activated Alcohols with Molecular Oxygen. Mon. Chem. 2013, 144, 251–254. [Google Scholar] [CrossRef]
- Dobras, G.; Sitko, M.; Petroselli, M.; Caruso, M.; Cametti, M.; Punta, C.; Orlińska, B. Solvent-Free Aerobic Oxidation of Ethylbenzene Promoted by NHPI/Co(II) Catalytic System: The Key Role of Ionic Liquids. ChemCatChem 2020, 12, 259–266. [Google Scholar] [CrossRef]
- Kasperczyk, K.; Orlińska, B.; Zawadiak, J. Aerobic Oxidation of Cumene Catalysed by 4-Alkyloxycarbonyl-N-Hydroxyphthalimide. Cent. Eur. J. Chem. 2014, 12, 1176–1182. [Google Scholar] [CrossRef]
- Zawadiak, J.; Gilner, D.; Kulicki, Z.; Baj, S. Concurrent Iodimetric Determination of Cumene Hydroperoxide and Dicumenyl Peroxide Used for Reaction Control in Dicumenyl Peroxide Synthesis. Analyst 1993, 118, 1081–1083. [Google Scholar] [CrossRef]
- Denney, D.B.; Goodyear, W.F.; Goldstein, B. Concerning the Mechanism of the Reduction of Hydroperoxides by Trisubstituted Phosphines and Trisubstituted Phosphites. J. Am. Chem. Soc. 1960, 82, 1393–1395. [Google Scholar] [CrossRef]
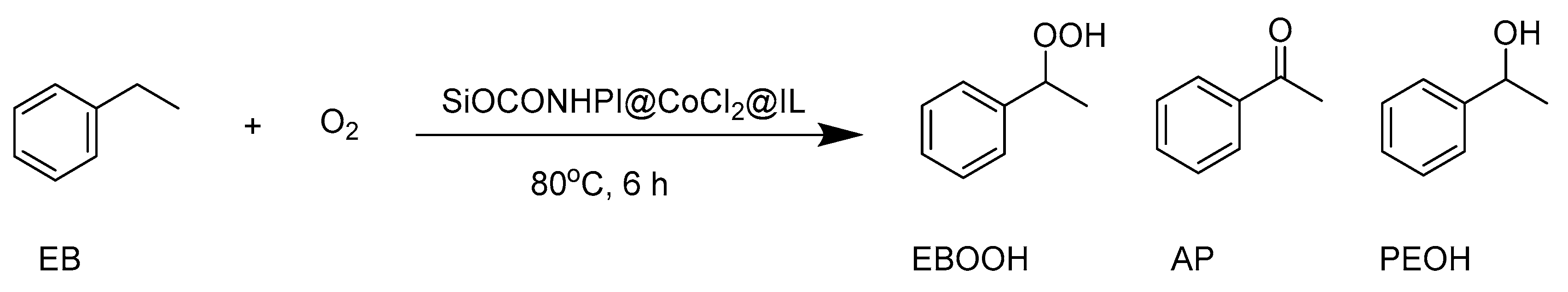
| Entry | Catalyst | α (%) | SEBOOH (%) | SAP (%) | SPEOH (%) |
|---|---|---|---|---|---|
| 1 | - | 4.7 | 67.1 | 12.3 | 20.6 |
| 2 a | CoCl2 | 2.8 | 81.4 | - | - |
| 3 b | SiOCONHPI | 8.6 | 51.0 | 19.4 | 29.6 |
| 4 c | SiOCONHPI/CoCl2 | 8.2 | 20.0 | 52.9 | 26.2 |
| 5 d | SiOCONHPI/[bmim][OcOSO3] | 6.4 | 71.2 | 19.0 | 9.8 |
| 6 e | SiOCONHPI/CoCl2/[bmim][OcOSO3] | 11.3 | 25.4 | 50.0 | 24.6 |
| 7 f | SiOCONHPI@[bmim][OcOSO3] | 6.1 | 70.0 | 19.1 | 10.9 |
| 8 g | SiOCONHPI@CoCl2@[bmim][OcOSO3] | 12.1 | 23.0 | 50.6 | 26.3 |
| 9 g | SiOCONHPI@CoCl2@[bmim][Cl] | 10.2 | 26.8 | 55.0 | 17.9 |
| 10 g | SiOCONHPI@CoCl2@[bmim][CF3SO3] | 9.0 | 19.8 | 54.3 | 25.9 |
| 11 g | SiOCONHPI@CoCl2@[bmim][BF4] | 8.4 | 36.5 | 49.1 | 14.4 |
| 12 g | SiOCONHPI@CoCl2@[bmim][CH3COO] | 8.4 | 21.1 | 55.6 | 23.3 |
| 13 g | SiOCONHPI@CoCl2@[bmim][PF6] | 7.3 | 23.0 | 69.7 | 7.7 |
| 14 g | SiOCONHPI@CoCl2@[bmim][Br] | 6.9 | 29.9 | 51.7 | 18.4 |
| 15 g | SiOCONHPI@CoCl2@[bmim][NTf2] | 6.6 | 23.5 | 50.2 | 26.3 |
| Entry | Catalyst | α (%) | SEBOOH (%) | SAP (%) | SPEOH (%) |
|---|---|---|---|---|---|
| 1 a | SiOCONHPI/CoCl2 | 8.2 | 20.0 | 52.9 | 26.2 |
| 2 | SiOCONHPI@CoCl2@[bmim][Cl] | 10.2 | 26.8 | 55.0 | 17.9 |
| 3 | SiOCONHPI@CoCl2@[hmim][Cl] | 11.1 | 21.8 | 52.6 | 25.4 |
| 4 | SiOCONHPI@CoCl2@[omim][Cl] | 11.3 | 17.5 | 57.0 | 25.2 |
| 5 | SiOCONHPI@CoCl2@[C10mim][Cl] | 11.9 | 20.0 | 57.1 | 22.6 |
| 6 | SiOCONHPI@CoCl2@[bmim][CH3OSO3] | 10.2 | 36.2 | 38.7 | 25.1 |
| 7 | SiOCONHPI@CoCl2@[bmim][OcOSO3] | 12.1 | 23.0 | 50.6 | 26.3 |
| 8 | SiOCONHPI@CoCl2@[bmim][C12H25OSO3] | 14.3 | 15.8 | 58.3 | 25.9 |
| 9 | SiOCONHPI@CoCl2@[emim][OcOSO3] | 12.8 | 28.8 | 44.6 | 26.6 |
| Entry | Cycle | Catalyst | α (%) | SEBOOH (%) | SAP (%) | SPEOH (%) |
|---|---|---|---|---|---|---|
| 1 a | 1 | SiOCONHPI/CoCl2 | 8.2 | 20.0 | 52.9 | 26.2 |
| 2 | 2 | 8.9 | 28.5 | 48.1 | 23.3 | |
| 3 | 3 | 8.4 | 30.7 | 46.3 | 23.0 | |
| 4 | 4 | 8.1 | 43.4 | 34.2 | 22.3 | |
| 5 b | 1 | SiOCONHPI@CoCl2@[bmim][OcOSO3] | 12.1 | 23.0 | 50.6 | 26.3 |
| 6 | 2 | 12.4 | 24.3 | 43.8 | 31.8 | |
| 7 | 3 | 11.3 | 30.5 | 36.2 | 33.3 | |
| 8 | 4 | 8.0 | 44.1 | 37.1 | 18.6 | |
| 9 b | 1 | SiOCONHPI@CoCl2@[bmim][Cl] | 10.2 | 26.8 | 55.0 | 17.9 |
| 10 | 2 | 10.1 | 42.5 | 40.2 | 17.3 | |
| 11 | 3 | 7.4 | 59.0 | 25.2 | 15.8 | |
| 12 | 4 | 7.7 | 61.6 | 24.0 | 14.4 | |
| 13 b | 1 | SiOCONHPI@CoCl2@[bmim][CF3SO3] | 9.0 | 19.8 | 54.3 | 25.9 |
| 14 | 2 | 9.5 | 26.8 | 48.4 | 24.5 | |
| 15 | 3 | 9.0 | 38.3 | 40.6 | 20.7 | |
| 16 | 4 | 7.4 | 57.1 | 32.8 | 10.1 |
| Entry | Cycle | Catalyst | α (%) | SEBOOH (%) | SAP (%) | SPEOH (%) |
|---|---|---|---|---|---|---|
| 1 a | 1 | SiOCONHPI@CoCl2@[bmim][CH3OSO3] | 10.2 | 36.2 | 38.7 | 25.1 |
| 2 | 2 | 9.3 | 47.7 | 30.0 | 22.3 | |
| 3 | 3 | 8.2 | 58.8 | 25.5 | 15.7 | |
| 4 | 4 | 6.8 | 61.4 | 23.2 | 14.5 | |
| 5 a | 1 | SiOCONHPI@CoCl2@[bmim][OcOSO3] | 12.1 | 23.0 | 50.6 | 26.3 |
| 6 | 2 | 12.4 | 24.3 | 43.8 | 31.8 | |
| 7 | 3 | 11.3 | 30.5 | 36.2 | 33.3 | |
| 8 | 4 | 8.0 | 44.1 | 37.1 | 18.6 | |
| 9 a | 1 | SiOCONHPI@CoCl2@[bmim][C12H25OSO3] | 14.3 | 15.8 | 58.3 | 25.9 |
| 10 | 2 | 11.6 | 26.3 | 50.5 | 23.2 | |
| 11 | 3 | 9.8 | 37.5 | 36.8 | 25.7 | |
| 12 | 4 | 8.8 | 47.8 | 30.2 | 22.0 | |
| 13 a | 1 | SiOCONHPI@CoCl2@[emim][OcOSO3] | 12.8 | 28.8 | 44.6 | 26.6 |
| 14 | 2 | 10.0 | 36.0 | 38.1 | 25.9 | |
| 15 | 3 | 9.9 | 36.4 | 34.6 | 29.0 | |
| 16 | 4 | 9.5 | 44.9 | 27.1 | 28.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobras, G.; Kasperczyk, K.; Jurczyk, S.; Orlińska, B. N-Hydroxyphthalimide Supported on Silica Coated with Ionic Liquids Containing CoCl2 (SCILLs) as New Catalytic System for Solvent-Free Ethylbenzene Oxidation. Catalysts 2020, 10, 252. https://doi.org/10.3390/catal10020252
Dobras G, Kasperczyk K, Jurczyk S, Orlińska B. N-Hydroxyphthalimide Supported on Silica Coated with Ionic Liquids Containing CoCl2 (SCILLs) as New Catalytic System for Solvent-Free Ethylbenzene Oxidation. Catalysts. 2020; 10(2):252. https://doi.org/10.3390/catal10020252
Chicago/Turabian StyleDobras, Gabriela, Kornela Kasperczyk, Sebastian Jurczyk, and Beata Orlińska. 2020. "N-Hydroxyphthalimide Supported on Silica Coated with Ionic Liquids Containing CoCl2 (SCILLs) as New Catalytic System for Solvent-Free Ethylbenzene Oxidation" Catalysts 10, no. 2: 252. https://doi.org/10.3390/catal10020252
APA StyleDobras, G., Kasperczyk, K., Jurczyk, S., & Orlińska, B. (2020). N-Hydroxyphthalimide Supported on Silica Coated with Ionic Liquids Containing CoCl2 (SCILLs) as New Catalytic System for Solvent-Free Ethylbenzene Oxidation. Catalysts, 10(2), 252. https://doi.org/10.3390/catal10020252