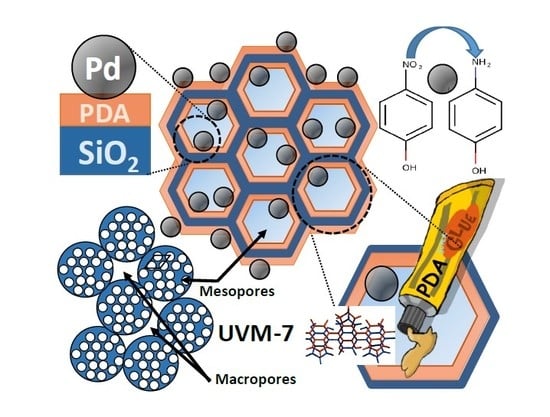
Highly Active Hydrogenation Catalysts Based on Pd Nanoparticles Dispersed along Hierarchical Porous Silica Covered with Polydopamine as Interfacial Glue
Abstract
1. Introduction
2. Results and Discussion
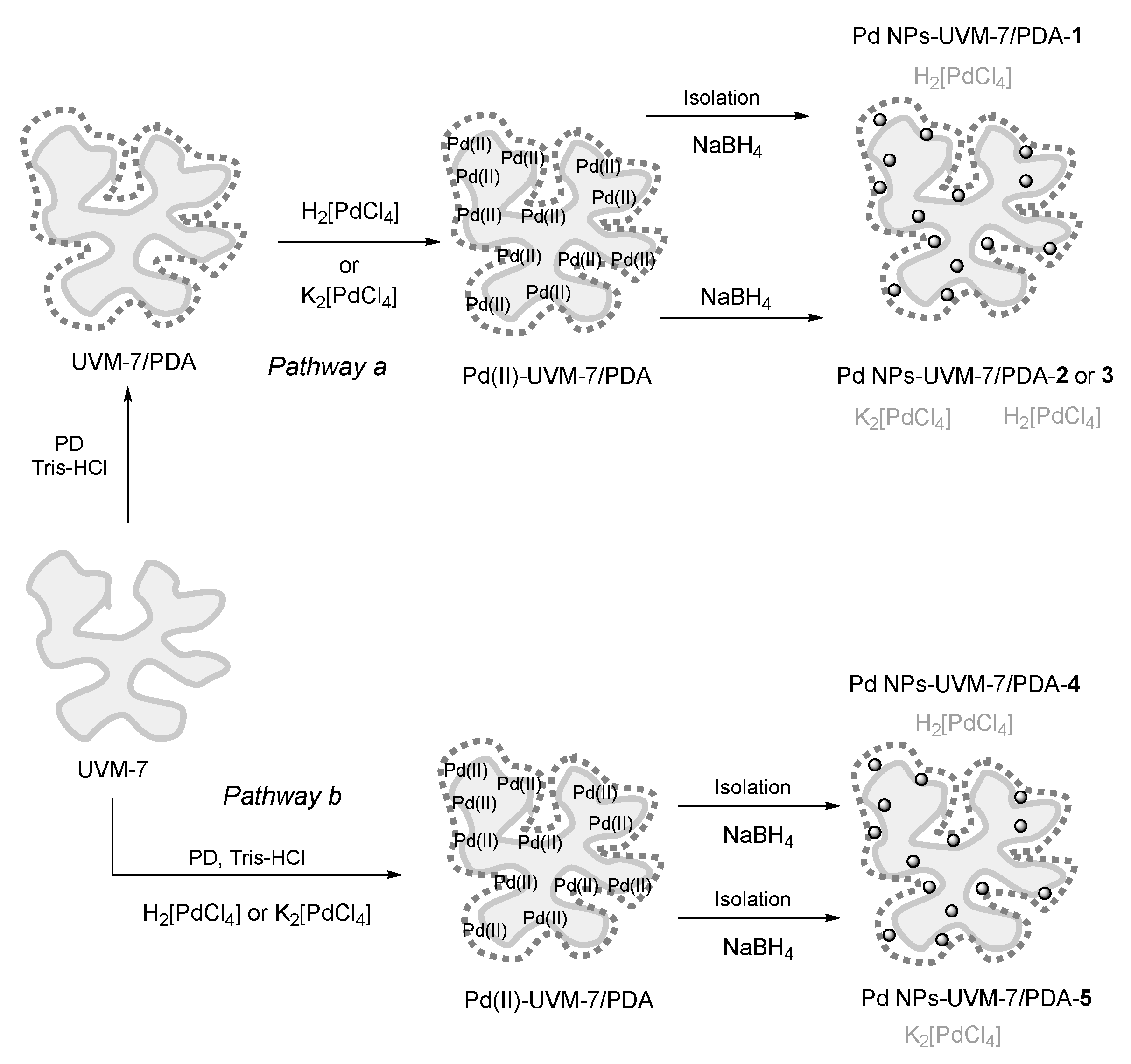
2.1. Synthesis Design
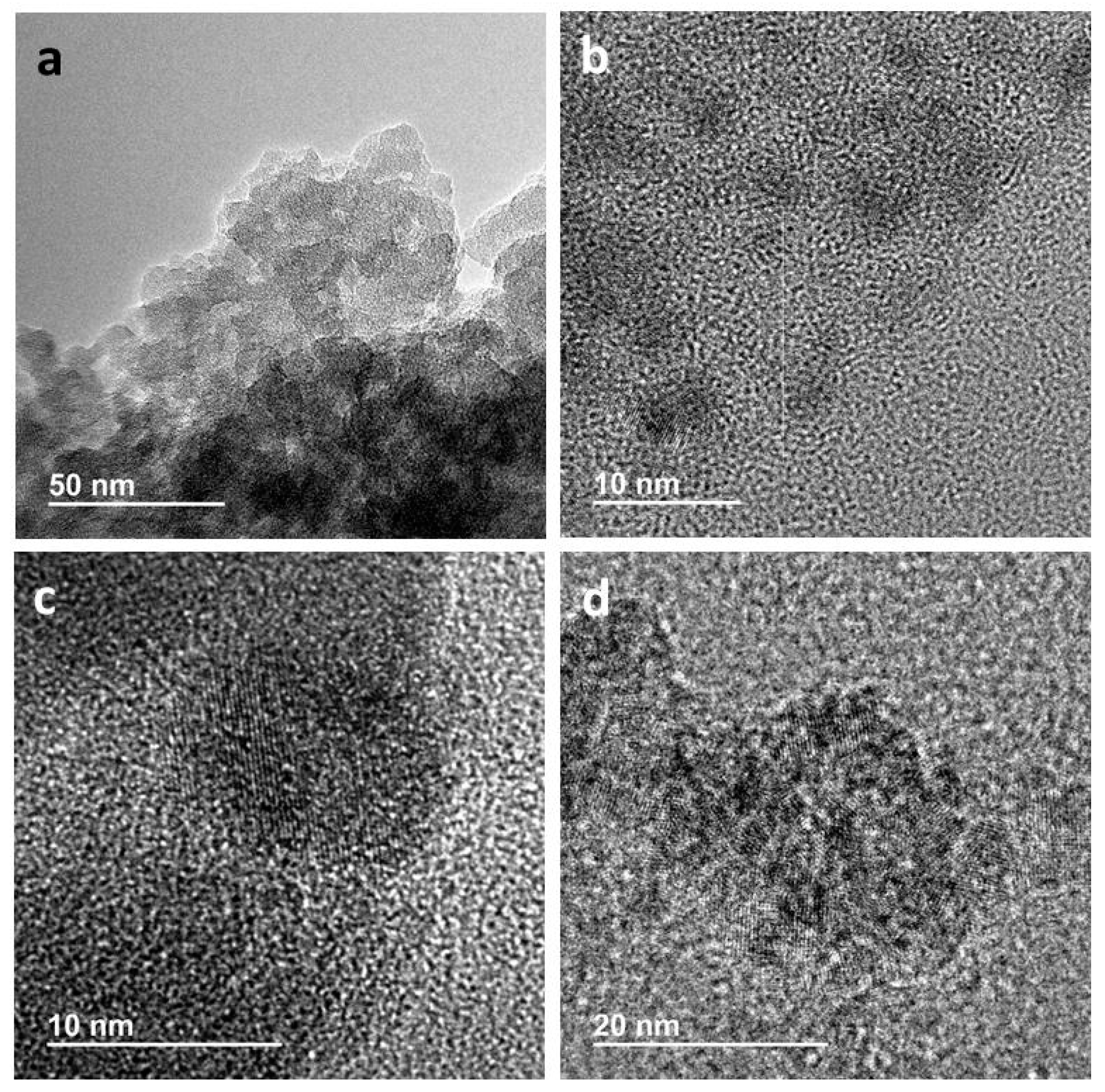
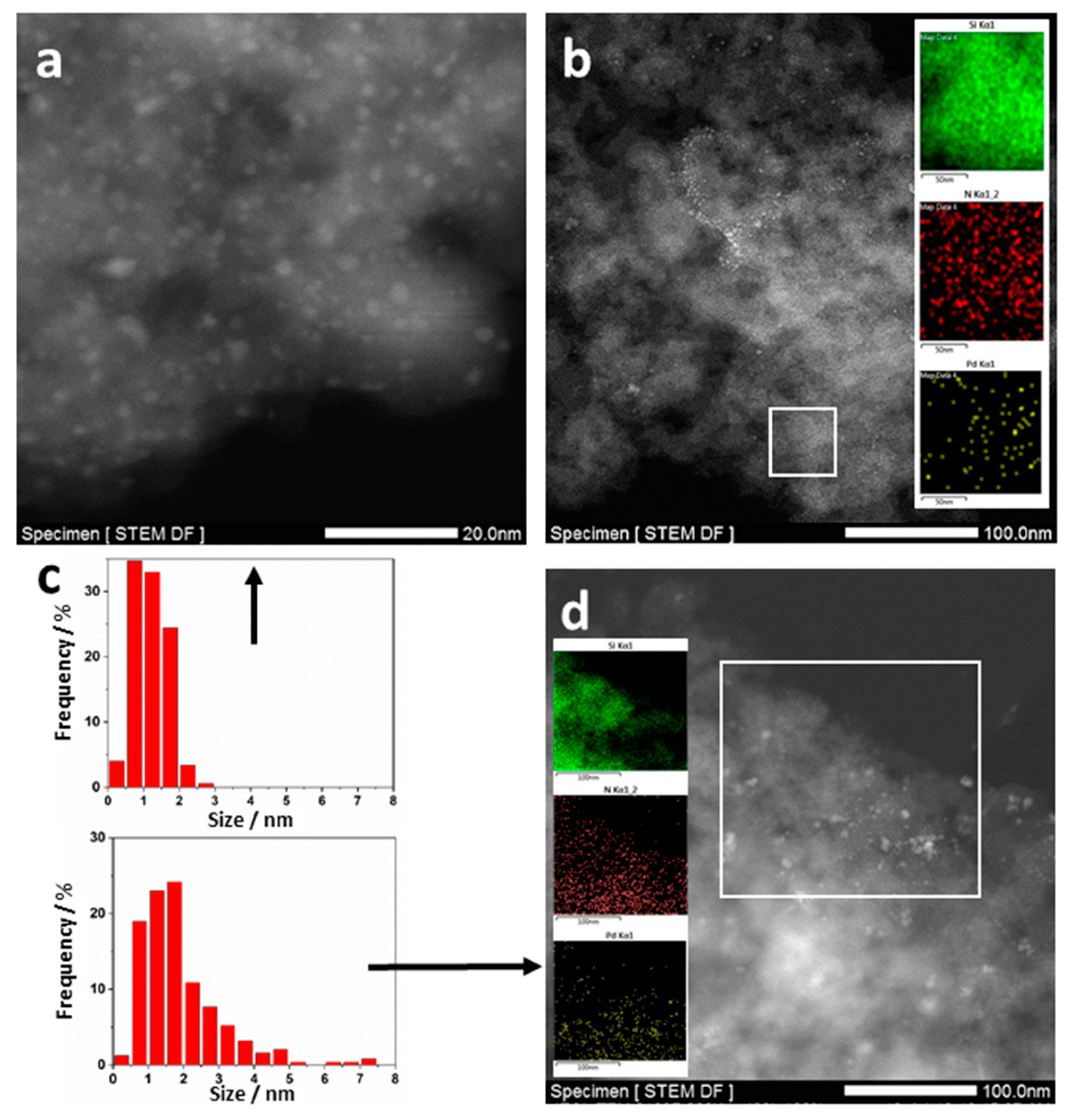
2.2. Catalysts Characterization
2.3. Catalyst Activity
3. Materials and Methods
3.1. Reagents and Materials
3.2. Catalyst Synthesis: Coating the UVM-7 with Polidopamine (UVM-7/PDA)
3.3. Catalyst Synthesis: Immobilization of Pd NPs on UVM-7/PDA
3.4. Characterization Techniques
3.5. Kinetic Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Albiñana, P.A.; El Haskouri, J.; Marcos, M.D.; Estevan, F.; Amorós, P.; Úbeda, M.Á.; Pérez-Pla, F. A new efficient, highly dispersed, Pd nanoparticulate silica supported catalyst synthesized from an organometallic precursor. Study of the homogeneous vs. heterogeneous activity in the Suzuki-Miyaura reaction. J. Catal. 2018, 367, 283–295. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, G.; Sun, X.; Jiang, Y.; Zhang, X. General promoting effect of polydopamine on supported noble metal catalysts. J. Mater. Chem. A 2017, 5, 20789–20796. [Google Scholar] [CrossRef]
- Liu, Y.; Li, G.; Qin, R.; Chen, D. Surface-Engineered Polidopamine Particles as an Efficient Support for Catalytic Applications. Langmuir 2016, 32, 13675–13686. [Google Scholar]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-Inspired Surface Chemistry for Multifunctional Coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef]
- Ryu, J.H.; Meserssmith, P.B.; Lee, H. Polidopamine Surface Chemistry: A Decade of Discovery. ACS Appl. Mater. Interfaces 2018, 10, 7523–7540. [Google Scholar]
- Liu, Y.; Ai, K.; Lu, L. Polydopamine and Its Derivative Materials: Synthesis and Promising Applications in Energy, Environmental, and Biomedical Fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef]
- Qu, R.; Zhang, W.; Liu, N.; Zhang, Q.; Liu, Y.; Li, X.; Wei, Y.; Feng, L. Antioil Ag3PO4 Nanoparticle/Polydopamine/Al2O3 Sandwich Structure for Complex Wastewater Treatment: Dynamic Catalysis under Natural Light. ACS Sustain. Chem. Eng. 2018, 6, 8019–8028. [Google Scholar] [CrossRef]
- Kralj, S.; Longobardo, F.; Iglesias, D.; Bevilacqua, M.; Tavagnacco, C.; Criado, A.; Jaen, J.J.D.; Makovec, D.; Marchesan, S.; Melchionna, M.; et al. Ex-Solution Synthesis of Sub-5-nm FeOx Nanoparticles on Mesoporous Hollow N,O-Doped Carbon Nanoshells for Electrocatalytic Oxygen Reduction. ACS Appl. Nano Mater. 2019, 2, 6092–6097. [Google Scholar] [CrossRef]
- Liu, R.; Guo, Y.; Odusote, G.; Qu, F.; Priestley, R. Core-Shell Fe3O4 Polidopamine Nanoparticles Serve Multipurpose as Drug Carrier, Catalyst Support and Carbon Adsorbent. ACS Appl. Mater. Interfaces. 2013, 5, 9167–9171. [Google Scholar]
- Huang, H.; He, Z.; Lin, X.; Ruan, W.; Liu, Y.; Yang, Z. Ultradispersed platinum nanoclusters on polydopamine-functionalized carbon nanotubes as an excellent catalyst for methanol oxidation reaction. Appl. Catal. A Gen. 2015, 490, 65–70. [Google Scholar] [CrossRef]
- Yang, Y.; Reber, A.C.; Gilliland, S.E.; Castano, C.; Gupton, B.F.; Khanna, S.N. More than just a support: Graphene as a solid-state ligand for palladium-catalyzed cross-coupling reactions. J. Catal. 2018, 360, 20–26. [Google Scholar] [CrossRef]
- Hussain, M.A.; Yang, M.; Lee, T.J.; Kim, J.W.; Choi, B.G. High density decoration of noble metal nanoparticles on polydopamine-functionalized molybdenum disulphide. J. Colloid Interface Sci. 2015, 451, 216–220. [Google Scholar] [CrossRef]
- Ma, J.-X.; Yang, H.; Zhang, X.; Li, S.; Ren, R. Well-dispersed graphene-polydopamine-Pd hybrid with enhanced catalytic performance. RSC Adv. 2015, 5, 97520–97527. [Google Scholar] [CrossRef]
- Ye, W.; Yu, J.; Zhou, Y.; Gao, D.; Wang, D.; Wang, C.; Xue, D. Green synthesis of Pt–Au dendrimer-like nanoparticles supported on polydopamine-functionalized graphene and their high performance toward 4- nitrophenol reduction. Appl. Catal. B Environ. 2016, 181, 371–378. [Google Scholar] [CrossRef]
- Ren, F.; Zhai, C.; Zhu, M.; Wang, C.; Wang, H.; Bin, D.; Guo, J.; Yang, P.; Du, Y. Facile synthesis of PtAu nanoparticles supported on polydopamine reduced and modified graphene oxide as a highly active catalyst for methanol oxidation. Electrochimica Acta 2015, 153, 175–183. [Google Scholar] [CrossRef]
- Zhou, J.; Duan, B.; Fang, Z.; Song, J.; Wang, C.; Messersmith, P.B.; Duan, H. Interfacial assembly of Mussel-Inspired Au@Ag@Polydopamine Core-Shell Nanoparticles for Recyclable Nanocatalyts. Adv. Mater. 2014, 26, 701–705. [Google Scholar]
- Sadjadi, S.; Lazzara, G.; Malmir, M.; Heravi, M.M. Pd nanoparticles immobilized on the poly-dopamine decorated hallosyte nanotubes hybridized with N-doped porous carbon monolayer: A versatile catalyst for promoting Pd catalysed reactions. J. Catal. 2018, 366, 245–257. [Google Scholar]
- Movahed, S.K.; Lehi, N.F.; Dabiri, M. Palladium nanoparticles supported on core-shell and York-shell Fe3O4@nitrogen doped carbon cubes as highly efficient, magnetically separable catalyst for the reduction of nitroarenes and the oxidation of alcohols. J. Catal. 2018, 364, 69–79. [Google Scholar]
- Kadam, H.; Tilve, S.G. Advancement in methodologies for reduction of nitroarenes. RSC Adv. 2015, 5, 83391–83407. [Google Scholar] [CrossRef]
- Lara, P.; Philippot, K. The hydrogenation of nitrosoarenes mediated by platinum nanoparticles: An overview. Catal. Sci. Technol. 2014, 4, 2445–2465. [Google Scholar]
- Corma, A. Chemoselective Hydrogenation of Nitro Compounds with Supported Gold Catalysts. Science 2006, 313, 332–334. [Google Scholar] [CrossRef] [PubMed]
- Formenti, D.; Ferretti, F.; Scharnagl, F.K.; Beller, M. Reduction of Nitro Compounds Using 3d-Non-Noble Metal Catalysts. Chem. Rev. 2018, 119, 2611–2680. [Google Scholar] [CrossRef] [PubMed]
- Doherty, S.; Knight, J.G.; Backhouse, T.; Bradfors, A.; Saunders, F.; Bourne, R.A.; Chamberlain, T.W.; Stones, R.; Clayton, A.; Lovelock, K. Hightly efficient aqueous phase reduction of nitroarenes catalysed by phosphine-decorated polymer immobilized ionic liquid stabilized PdNPs. Catal. Sci. Technol. 2018, 8, 1454–1467. [Google Scholar] [CrossRef]
- Doherty, S.; Knight, J.G.; Backhouse, T.; Summers, R.J.; Abood, E.; Simpson, W.; Paget, W.; Bourne, R.A.; Chamberlain, T.W.; Stones, R.; et al. Highly Selective and Solvent-Dependent Reduction of Nitrobenzene to N-Phenylhydroxylamine, Azoxybenzene, and Aniline Catalyzed by Phosphino-Modified Polymer Immobilized Ionic Liquid-Stabilized AuNPs. ACS Catal. 2019, 9, 4777–4791. [Google Scholar] [CrossRef]
- Orlandi, M.; Brenna, D.; Harms, R.; Jost, S.; Benaglia, M. Recent Developments in the Reduction of Aromatic and Aliphatic Nitro Compounds to Amines. Org. Process. Res. Dev. 2016, 22, 430–445. [Google Scholar] [CrossRef]
- Wunder, S.; Lu, Y.; Albrecht, M.; Ballauff, M. Catalytic Activity of Faceted Gold Nanoparticles Studied by a Model Reaction: Evidence for Substrate-Induced Surface Restructuring. ACS Catal. 2011, 1, 908–916. [Google Scholar] [CrossRef]
- Zhao, P.; Feng, X.; Huang, D.; Yang, G.; Astruc, D. Basic concepts and recent advances in nitrophenol reduction by gold- and other transition metal nanoparticles. Co-ord. Chem. Rev. 2015, 287, 114–136. [Google Scholar] [CrossRef]
- Aditya, T.; Pal, A.; Pal, T. Nitroarene reduction: A trusted model reaction to test nanoparticle catalysts. Chem. Commun. 2015, 51, 9410–9431. [Google Scholar] [CrossRef]
- Gu, S.; Wunder, S.; Lu, Y.; Ballauff, M. Kinetic Analysis of the Catalytic Reduction of 4-Nitrophenosl by Metallic Nanoparticles. J. Phys. Chem.C. 2014, 118, 18618–18625. [Google Scholar] [CrossRef]
- El Haskouri, J.; Ortiz de Zárate, D.; Guillem, C.; Latorre, J.; Caldés, M.; Beltrán, A.; Beltrán, D.; Descalzo, A.B.; Rodríguez-López, G.; Martínez-Máñez, R.; et al. Silica-based powders and monolits with bimodal pore systems. Chem.Comm. 2002, 330–331. [Google Scholar] [CrossRef]
- El Haskouri, J.; Morales, J.M.; De Zárate, D.O.; Fernández, L.; Latorre, J.; Guillem, C.; Beltrán, A.; Beltrán, D.; Amorós, P. Nanoparticulated Silicas with Bimodal Porosity: Chemical Control of the Pore Sizes. Inorg. Chem. 2008, 47, 8267–8277. [Google Scholar] [CrossRef] [PubMed]
- Huerta, L.; Guillem, C.; Latorre, J.; Beltrán, A.; Martínez-Máñez, R.; Marcos, M.D.; Beltran, D.; Amorós, P. Bases for the synthesis of nanoparticulated silicas with bimodal hierarchical porosity. Solid State Sci. 2006, 8, 940–951. [Google Scholar] [CrossRef]
- Pérez-Cabero, M.; Hungria, A.B.; Morales, J.M.; Tortajada, M.; Ramón, D.; Moragues, A.; El Haskouri, J.; Beltrán, A.; Beltran, D.; Amorós, P.; et al. Interconnected mesopores and high accessibility in UVM-7-like silicas. J. Nanoparticle Res. 2012, 14, 14. [Google Scholar] [CrossRef]
- Luo, H.; Gu, C.; Zheng, W.; Dai, F.; Wang, X. Facile synthesis of novel size-controlled antibacterial hybrid spheres using silver nanoparticles loaded with poly-dopamine spheres. RSC Adv. 2015, 5, 13470–13477. [Google Scholar] [CrossRef]
- Cheng, W.; Fan, F.; Zhang, Y.; Pei, Z.; Wang, W.; Pei, Y. A Facile Approach for Fabrication of Core-Shell Magnetic Molecularly Imprinted Nanospheres towards Hypericin. Polymers 2017, 9, 135. [Google Scholar] [CrossRef]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.; Beck, J.S.; Kresge, M.E.L.C.T. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Habber, F.Z. Gradual Electrolytic Reduction of Nitrobenzane with Limited Cathode Potential. Electrochem. Angew. Phys. Chem. 1898, 22, 506–514. [Google Scholar]
- Corma, A.; Concepción, P.; Serna, P. Different Reaction Pathway for the Reduction of Aromatic Nitro Compounds on Gol Catalysts. Angew. Chem. Int. Ed. 2007, 46, 7266–7269. [Google Scholar] [CrossRef]
- Larm, N.E.; Bhawawet, N.; Thon, J.A.; Baker, G.A. Best practices for reporting nanocatalytic performance: Lessons learned from nitroarene reduction as a model reaction. New J. Chem. 2019, 43, 17932–17936. [Google Scholar] [CrossRef]
- Lara, L.R.S.; Zottis, A.D.; Elias, W.C.; Faggion, D.; De Campos, C.E.M.; Acuña, J.J.S.; Domingos, J. The catalytic evaluation of in situ grown Pd nanoparticles on the surface of Fe3O4@dextran particles in the p-nitrophenol reduction reaction. RSC Adv. 2015, 5, 8289–8296. [Google Scholar] [CrossRef]
- Batt, L.; Hague, D.N.; Margerison, D.; Shooter, D.; Wayne, R.P. The Practice of Kinetics. In Comprehensive Chemical Kinetics; Elsevier: Amsterdam, The Netherlands, 1969; Volume 1, p. 343. [Google Scholar]
- Menumerov, E.; Hughes, R.A.; Neretina, S. Catalytic Reduction of 4-Nitrophenol: A Quantitative Assessment of the Role of Dissolved Oxygen in Determining the Induction Time. Nano Lett. 2016, 16, 7791–7797. [Google Scholar] [CrossRef] [PubMed]
- An, M.; Cui, J.; Wang, L. Magnetic Recyclable Nanocomposite Catalysts with Good Dispersibility and High Catalytic Activity. J. Phys. Chem. C 2014, 118, 3062–3068. [Google Scholar] [CrossRef]
- Dong, Z.; Le, X.; Dong, C.; Zhang, W.; Li, X.; Ma, J. Ni@Pd core–shell nanoparticles modified fibrous silica nanospheres as highly efficient and recoverable catalyst for reduction of 4-nitrophenol and hydrodechlorination of 4-chlorophenol. Appl. Catal. B Environ. 2015, 162, 372–380. [Google Scholar] [CrossRef]
- Gu, X.; Qi, W.; Xu, X.; Sun, Z.; Zhang, L.; Liu, W.; Pan, X.; Su, D. Covalently functionalized carbon nanotube supported Pd nanoparticles for catalytic reduction of 4-nitrophenol. Nanoscale 2014, 6, 6609–6616. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.A.; Makis, J.J.; Marvin, K.A.; Rodenbusch, S.E.; Stevenson, K.J. Size-Dependent Hydrogenation of p-Nitrophenol with Pd Nanoparticles Synthesized with Poly(amido)amine Dendrimer Templates. J. Phys. Chem. C 2013, 117, 22644–22651. [Google Scholar] [CrossRef]
- Wu, X.; Lu, C.; Zhang, W.; Yuan, G.; Xiong, R.; Zhang, X. A novel reagentless approach for synthesizing cellulose nanocrystal-supported palladium nanoparticles with enhanced catalytic performance. J. Mater. Chem. A 2013, 1, 8645. [Google Scholar] [CrossRef]
- Li, H.; Han, L.; Cooper-White, J.; Kim, I. Palladium nanoparticles decorated carbon nanotubes: Facile synthesis and their applications as highly efficient catalysts for the reduction of 4-nitrophenol. Green Chem. 2012, 14, 586. [Google Scholar] [CrossRef]
- Esumi, K.; Isono, R.; Yoshimura, T. Preparation of PAMAM− and PPI−Metal (Silver, Platinum, and Palladium) Nanocomposites and Their Catalytic Activities for Reduction of 4-Nitrophenol. Langmuir 2004, 20, 237–243. [Google Scholar] [CrossRef]
- Arora, S.; Kapoor, P.; Singla, M.L. Catalytic studies of palladium nanoparticles immobilized on alumina synthesized by a simple physical precipitation method. React. Kinet. Mech. Catal. 2010, 99, 157–165. [Google Scholar] [CrossRef]
- Dong, W.; Cheng, S.; Feng, C.; Shang, N.; Gao, S.; Wang, C. Fabrication of highly dispersed Pd nanoparticles supported on reduced graphene oxide for catalytic reduction of 4-nitrophenol. Catal. Commun. 2017, 90, 70–74. [Google Scholar] [CrossRef]
- Mei, Y.; Lu, Y.; Polzer, F.; Ballauff, M.; Drechsler, M. Catalytic Activity of Palladium Nanoparticles Encapsulated in Spherical Polyelectrolyte Brushes and Core−Shell Microgels. Chem. Mater. 2007, 19, 1062–1069. [Google Scholar] [CrossRef]
- Fang, Y.; Wang, E. Simple and direct synthesis of oxygenous carbon supported palladium nanoparticles with high catalytic activity. Nanoscale 2013, 5, 1843. [Google Scholar] [CrossRef] [PubMed]
- Fageria, P.; Uppala, S.; Nazir, R.; Gangopadhyay, S.; Chang, C.-H.; Basu, M.; Pande, S. Synthesis of Monometallic (Au and Pd) and Bimetallic (AuPd) Nanoparticles Using Carbon Nitride (C3N4) Quantum Dots via the Photochemical Route for Nitrophenol Reduction. Langmuir 2016, 32, 10054–10064. [Google Scholar] [CrossRef] [PubMed]
- Le, X.; Dong, Z.; Li, X.; Zhang, W.; Le, M.; Ma, J. Fibrous nano-silica supported palladium nanoparticles: An efficient catalyst for the reduction of 4-nitrophenol and hydrodechlorination of 4-chlorophenol under mild conditions. Catal. Commun. 2015, 59, 21–25. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, H.; Feng, C.; Gao, S.; Shang, N.; Wang, Z. Multifunctional Pd@MOF core–shell nanocomposite as highly active catalyst for p-nitrophenol reduction. Catal. Commun. 2015, 72, 29–32. [Google Scholar] [CrossRef]
- Veisi, H.; Pirhayati, M.; Kakanejadifard, A.; Mohammadi, P.; Abdi, M.R.; Gholami, J.; Hemmati, S. In Situ Green Synthesis of Pd Nanoparticles on Tannic Acid-Modified Magnetite Nanoparticles as a Green Reductant and Stabilizer Agent: Its Application as a Recyclable Nanocatalyst (Fe3O4@TA/Pd) for Reduction of 4-Nitrophenol and Suzuki Reactions. ChemistrySelect 2018, 3, 1820–1826. [Google Scholar] [CrossRef]
- Qian, H.; He, Q.; Zheng, J.; Li, S.; Zhang, S. Catechol-functionalized microporous organic polymer as supported media for Pd nanoparticles and its high catalytic activity. Polymer 2014, 55, 550–555. [Google Scholar] [CrossRef]
- Farzad, E.; Veisi, H. Fe3O4/SiO2 nanoparticles coated with polydopamine as a novel magnetite reductant and stabilizer sorbent for palladium ions: Synthetic application of Fe3O4/SiO2@PDA/Pd for reduction of 4-nitrophenol and suzuki reactions. J. Ind. Eng. Chem. 2018, 60, 114–124. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nature methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 61, 373–380. [Google Scholar] [CrossRef]


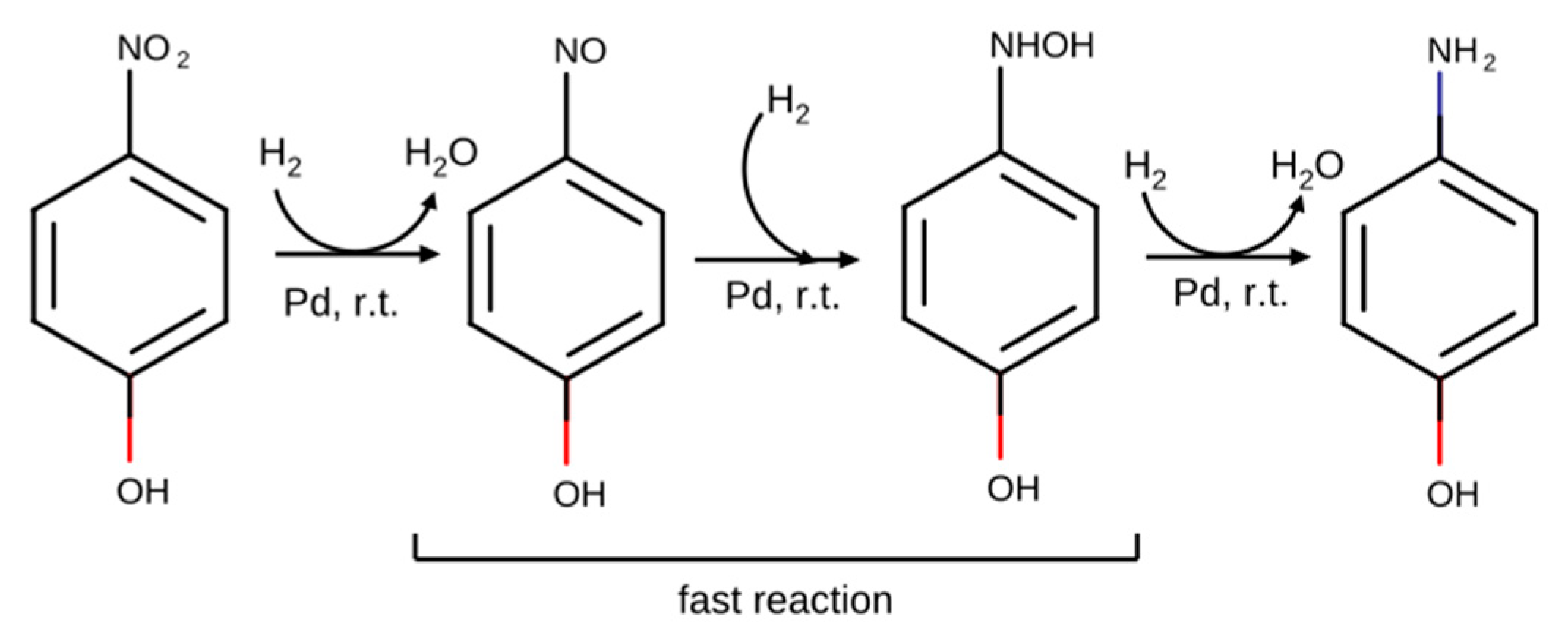


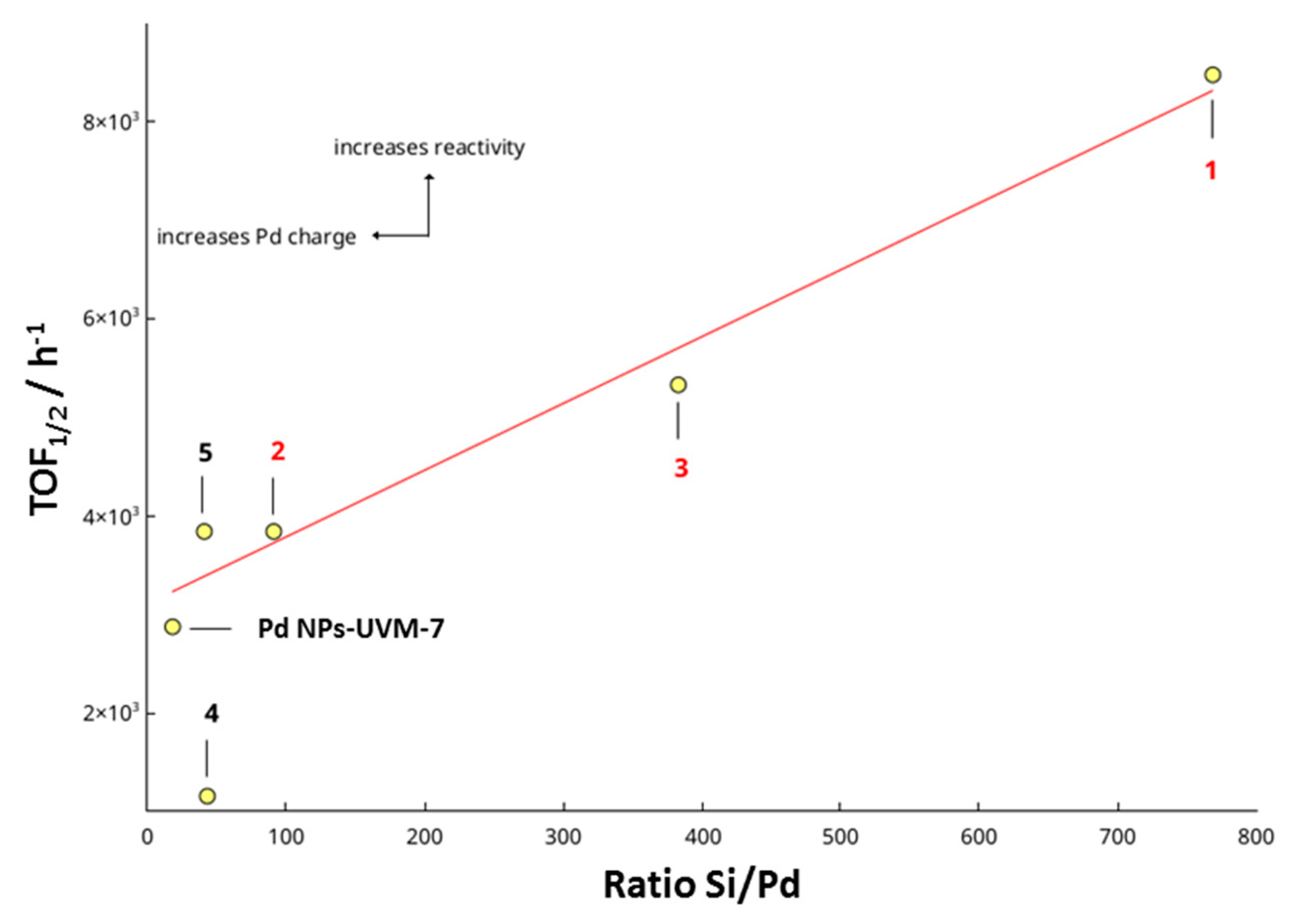
| Catalyst n | Si/Pd 1 Molar Ratio | PDA Content 2 (wt %) | Water Content 2 (wt %) |
|---|---|---|---|
| 1 | 768 | 10 | 2 |
| 2 | 92 | 13 | 3 |
| 3 | 383 | 7 | 2 |
| 4 | 44 | 21 | 3 |
| 5 | 41 | 9 | 2 |
| Catalyst. n | d100 1 nm | Surface Area 2 m2/g | Mesopore Size 3 nm | Mesopore Volume 3 cm3/g | Large Pore Size 4 nm | Large Pore Volume 4 cm3/g |
|---|---|---|---|---|---|---|
| UVM-7 | 4.3 | 1100 | 2.86 | 0.95 | 27.3 | 1.62 |
| 1 | 4.6 | 998 | 2.54 | 0.61 | 13.7 | 1.32 |
| 2 | shoulder | 628 | 2.50 | 0.41 | 16.6 | 1.21 |
| 3 | 4.5 | 604 | 2.60 | 0.42 | 13.6 | 0.88 |
| 4 | shoulder | 376 | 2.66 | 0.27 | 18.2 | 0.60 |
| 5 | 4.5 | 1026 | 2.53 | 0.70 | 10.9 | 0.96 |
| Substrate | 4-Nitrophenolate 1 | |||||
|---|---|---|---|---|---|---|
| Catalyst (n) | 1 | 2 | 3 | 4 | 5 | Pd NPs-UVM-7 |
| t1/2/s | 170 | 375 | 270 | 1250 | 375 | 500 |
| TOF1/2/h−1 | 8470 | 3840 | 5330 | 1152 | 3840 | 2280 |
| Ref. | Pd Support | k_app × 102/s−1 | t1/2 /s | [4NP] × 104/M | [Pd] × 106/M | [BH4−] /M | TOF1/2 /h−1 | TOF1/2/[BH4−] × 10−4 /h−1M−1 |
|---|---|---|---|---|---|---|---|---|
| - | PDA@UVM-7 1 | - | 170 | 1.00 | 0.06 | 0.02 | 8470 | 42.3 |
| [43] | Dextrane Fe3O4/SiO2 | 2.01 | 34 | 0.90 | 0.47 | 0.03 | 11110 | 38.8 |
| [44] | Pd-Ni@KCC-1 | 2.04 | 34 | 0.32 | 0.27 | 0.08 | 17570 | 21.4 |
| [45] | C nanotubes functionalized | 1.05 | 66 | 0.38 | 0.26 | 0.02 | 3445 | 21.4 |
| [46] | Poly(amido)amine dendrimer | 52.8 | 1 | 1.20 | 7.50 | 0.04 | 6954 | 18.3 |
| [47] | Cellulose nanocrystals | 0.57 | 122 | 22.7 | 0.40 | 0.03 | 4437 | 14.8 |
| [48] | Carbon nanotubes/ hyperbranched polymers | 0.50 | 139 | 2.00 | 1.58 | 0.18 | 18693 | 10.3 |
| [49] | Poly(propyleneimine) | 36.4 | 2 | 1.00 | 20.0 | 0.10 | 9445 | 9.44 |
| [50] | Al2O3 | 1.50 | 46 | 1000 | 3.65 | 0.01 | 1067 | 8.07 |
| [51] | Graphene oxide | 1.60 | 43 | 1.00 | 120 | 0.50 | 34625 | 6.92 |
| [52] | Spherical polyelectrolyte brushes | 0.44 | 157 | 16.61 | 2.26 | 0.01 | 508 | 5.08 |
| [53] | C amorphous | 0.88 | 78 | 1.00 | 2.40 | 0.39 | 15896 | 4.11 |
| [54] | C3N4 | 0.02 | 4159 | 1.00 | 0.20 | 0.01 | 216 | 2.16 |
| [40] | Dextrane | 0.10 | 693 | 0.99 | 0.15 | 0.10 | 1727 | 1.73 |
| [55] | Dendritic silica | 0.80 | 87 | 0.77 | 24.1 | 0.02 | 85 | 0.52 |
| [56] | MOF core–shell | 1.50 | 46 | 0.67 | 50.0 | 0.02 | 60 | 0.39 |
| [57] | Fe3O4@TA (Tannic acid) | 1.80 | 39 | 0.97 | 4.27 | 0.20 | 736 | 0.37 |
| [58] | Hydroxyl functionalized polymer | 1.09 | 64 | 0.67 | 144 | 0.01 | 19 | 0.20 |
| [59] | Fe3O4/SiO2 | 7.52 | 9 | 2.00 | 50.0 | 0.15 | 263 | 0.18 |
| [49] | Poly(amidoamine) | 0.36 | 193 | 1.00 | 20.0 | 0.10 | 93 | 0.09 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ródenas, M.; El Haskouri, J.; Ros-Lis, J.V.; Marcos, M.D.; Amorós, P.; Úbeda, M.Á.; Pérez-Pla, F. Highly Active Hydrogenation Catalysts Based on Pd Nanoparticles Dispersed along Hierarchical Porous Silica Covered with Polydopamine as Interfacial Glue. Catalysts 2020, 10, 449. https://doi.org/10.3390/catal10040449
Ródenas M, El Haskouri J, Ros-Lis JV, Marcos MD, Amorós P, Úbeda MÁ, Pérez-Pla F. Highly Active Hydrogenation Catalysts Based on Pd Nanoparticles Dispersed along Hierarchical Porous Silica Covered with Polydopamine as Interfacial Glue. Catalysts. 2020; 10(4):449. https://doi.org/10.3390/catal10040449
Chicago/Turabian StyleRódenas, Miguel, Jamal El Haskouri, José Vicente Ros-Lis, M. Dolores Marcos, Pedro Amorós, M. Ángeles Úbeda, and Francisco Pérez-Pla. 2020. "Highly Active Hydrogenation Catalysts Based on Pd Nanoparticles Dispersed along Hierarchical Porous Silica Covered with Polydopamine as Interfacial Glue" Catalysts 10, no. 4: 449. https://doi.org/10.3390/catal10040449
APA StyleRódenas, M., El Haskouri, J., Ros-Lis, J. V., Marcos, M. D., Amorós, P., Úbeda, M. Á., & Pérez-Pla, F. (2020). Highly Active Hydrogenation Catalysts Based on Pd Nanoparticles Dispersed along Hierarchical Porous Silica Covered with Polydopamine as Interfacial Glue. Catalysts, 10(4), 449. https://doi.org/10.3390/catal10040449