Biodiesel Production from Melia azedarach and Ricinus communis Oil by Transesterification Process
Abstract
1. Introduction
2. Results and Discussion
2.1. Biochemical Composition of Samples
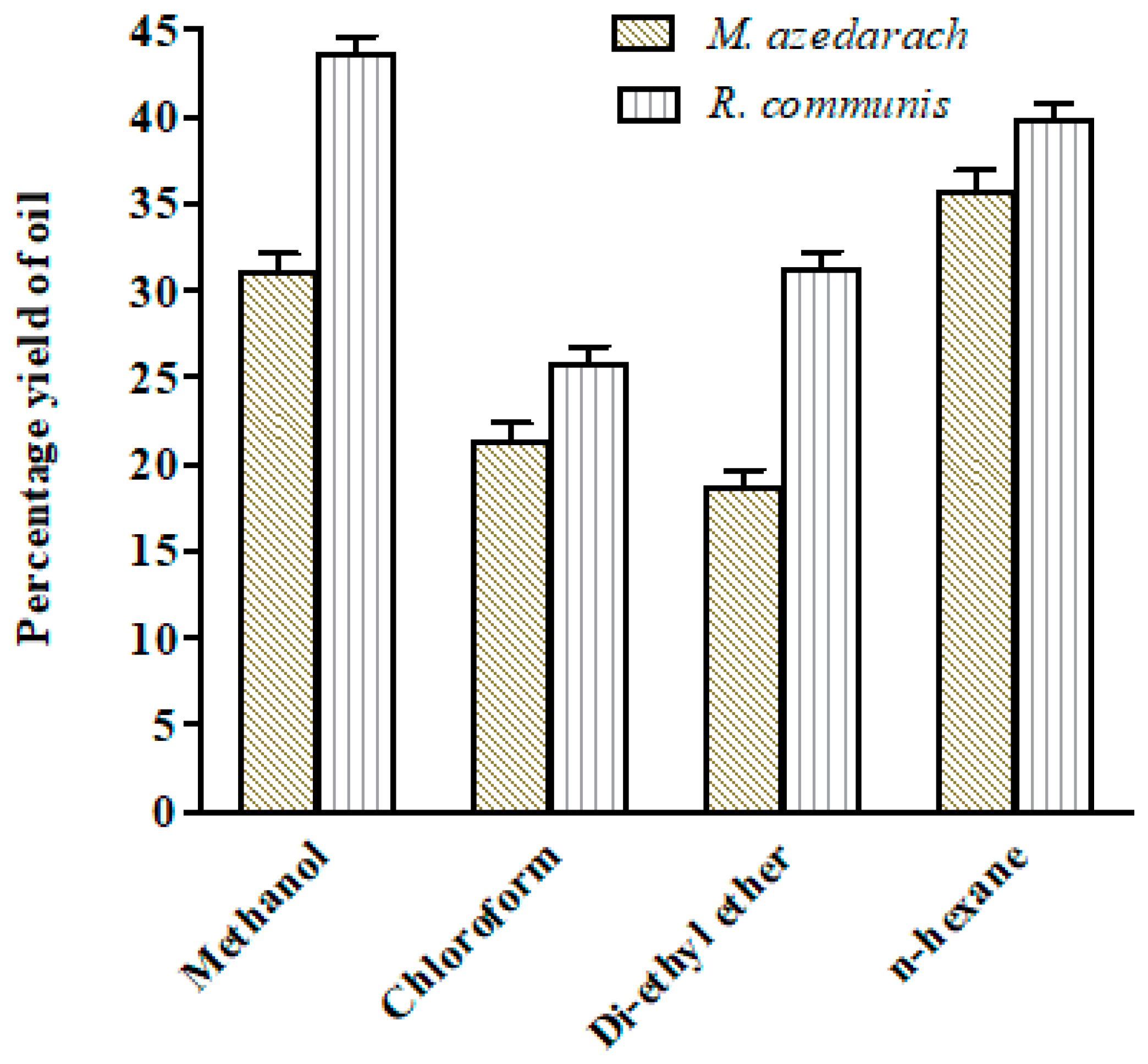
2.2. Oil Extraction from Biomass Samples by Solvent Extraction Method
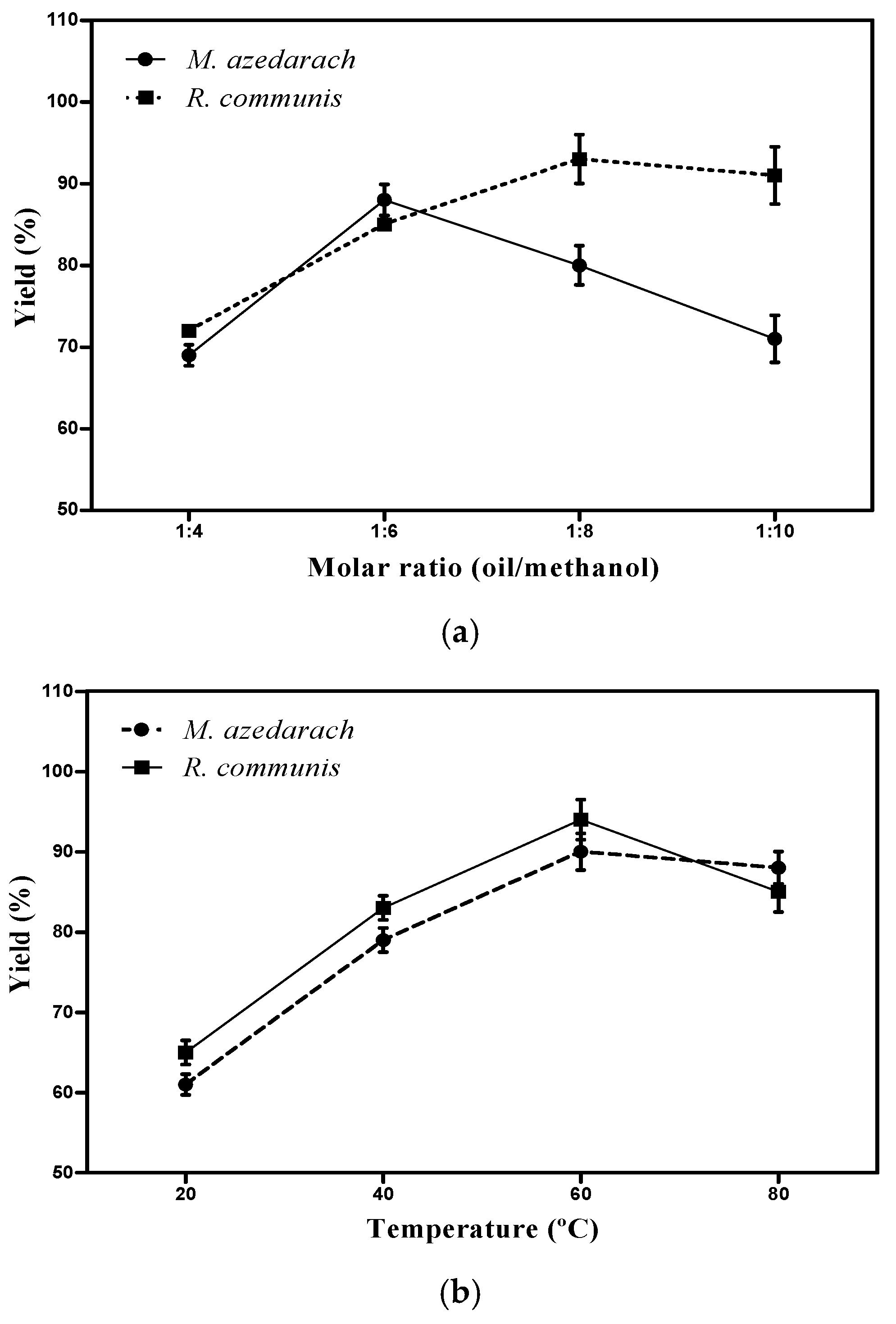
2.3. Base Catalyzed Transesterification of Extracted Oil into Biodiesel
2.4. Characterization of Oil and Biodiesel
2.4.1. Pysico-Chemical Characteristics
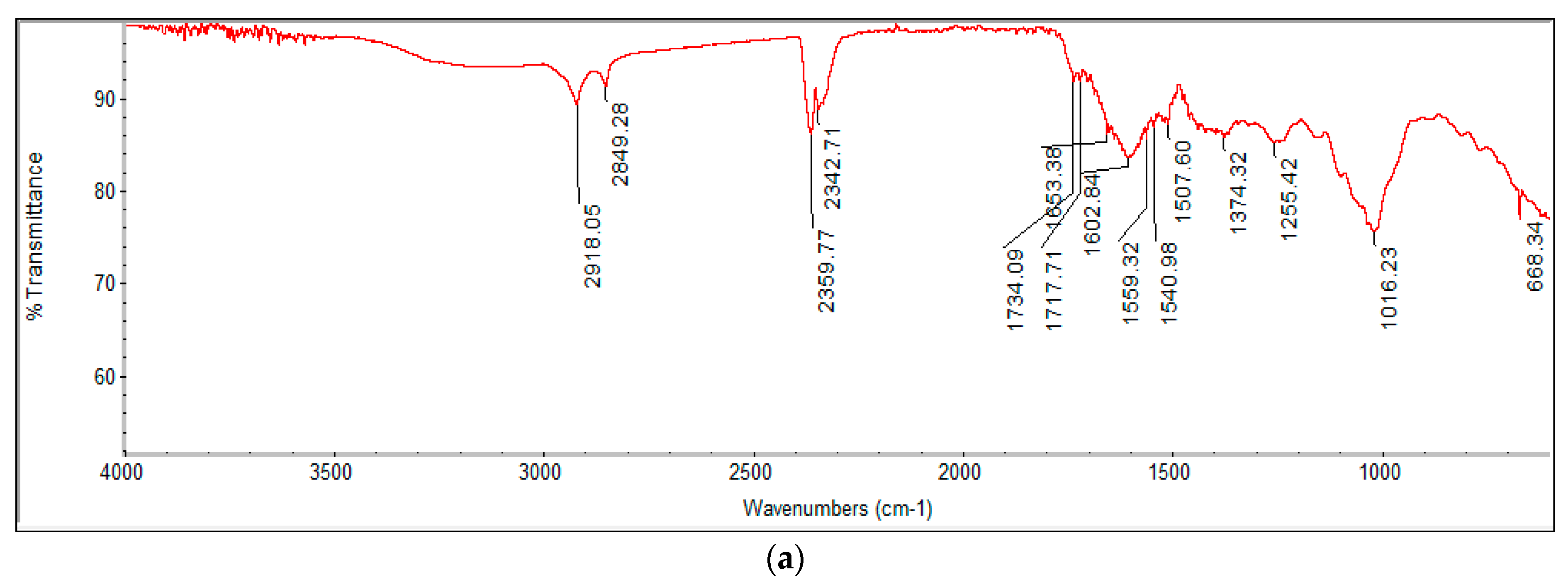
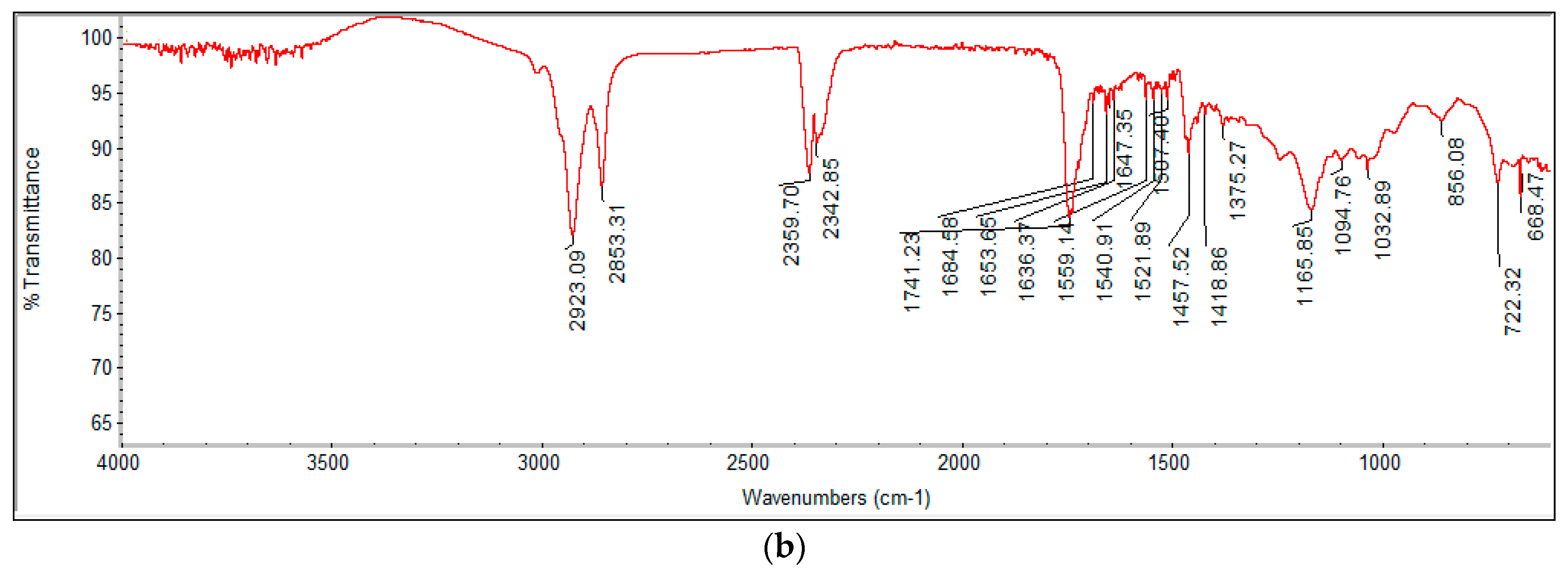
2.4.2. FTIR Analysis and Fatty Acid Profiling by GC
3. Materials and Methods
3.1. Collection and Preparation of Samples
3.2. Extraction of Oil by Soxhlet Apparatus
3.3. Base Catalyzed Transesterification
3.4. Analytical Methods
3.5. Fatty Acid Analysis by GC/MS
3.6. FTIR Analysis of Biodiesel
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cai, J.; Liu, R.; Deng, C. An assessment of biomass resources availability in Shanghai: 2005 analysis. Renew. Sustain. Energy Rev. 2008, 12, 1997–2004. [Google Scholar] [CrossRef]
- Abu-Khader, M.M. Recent progress in CO2 capture/sequestration: A review. Energy Sources Part A 2006, 28, 1261–1279. [Google Scholar] [CrossRef]
- Yang, X.; Liu, P.; Hao, Z.; Shi, J.; Zhang, S. Characterization and identification of freshwater microalgal strains toward biofuel production. BioResources 2011, 7, 686–695. [Google Scholar]
- Fernando, S.; Hall, C.; Jha, S. NO x reduction from biodiesel fuels. Energy Fuels 2006, 20, 376–382. [Google Scholar] [CrossRef]
- Ghobadian, B.; Rahimi, H.; Nikbakht, A.M.; Najafi, G.; Yusaf, T.F. Diesel engine performance and exhaust emission analysis using waste cooking biodiesel fuel with an artificial neural network. Renew. Energy 2009, 34, 976–982. [Google Scholar] [CrossRef]
- Leung, D.Y.; Wu, X.; Leung, M.K.H. A review on biodiesel production using catalyzed transesterification. Appl. Energy 2010, 87, 1083–1095. [Google Scholar] [CrossRef]
- Endut, A.; Abdullah, S.H.Y.S.; Hanapi, N.H.M.; Hamid, S.H.A.; Lananan, F.; Kamarudin, M.K.A.; Umar, R.; Juahir, H.; Khatoon, H. Optimization of biodiesel production by solid acid catalyst derived from coconut shell via response surface methodology. Int. Biodeterior. Biodegrad. 2017, 124, 250–257. [Google Scholar] [CrossRef]
- Chouhan, A.S.; Sarma, A.K. Modern heterogeneous catalysts for biodiesel production: A comprehensive review. Renew. Sustain. Energy Rev. 2011, 15, 4378–4399. [Google Scholar] [CrossRef]
- Carvalho, J.; Ribeiro, A.; Castro, J.D.F.D.; Vilarinho, C.; Castro, F. Biodiesel production by microalgae and macroalgae from north littoral portuguese coast. In Proceedings of the 1st International Conference WASTES: Solutions, Treatments and Opportunities, Centro para a Valorização de Resíduos (CVR), Guimarães, Portugal, 12–14 September 2011; pp. 694–699. [Google Scholar]
- Ma, F.; Hanna, M.A. Biodiesel production: A review. Bioresour. Technol. 1999, 70, 1–15. [Google Scholar] [CrossRef]
- Swarp, R. Biofuels: Breathing new fire. Biotechnol. News. 2007, 11, 14. [Google Scholar]
- Sruthi, K.; Kumar, R.; Shirisha, G. Determination of physico-chemical properties of Castor Biodiesel: A potential alternate to Conventional Diesel. Int. J. Adv. Res. Eng. Technol. IJARET 2013, 4, 101–107. [Google Scholar]
- Basumatary, S. Transesterification with heterogeneous catalyst in production of biodiesel: A Review. J. Chem. Pharm. Res. 2013, 5, 1–7. [Google Scholar]
- Stavarache, C.E.; Morris, J.; Maeda, Y.; Oyane, I.; Vinatoru, M.I.R.C.E.A. Syringa (Melia azedarach L.) berries oil: A potential source for biodiesel fuel. Rev. Chim. 2008, 59, 672–677. [Google Scholar] [CrossRef]
- Demirbas, A. Biofuels sources, biofuel policy, biofuel economy and global biofuel projections. Energy Convers. Manag. 2008, 49, 2106–2116. [Google Scholar] [CrossRef]
- Dastagir, G.; Hussain, F.; Khattak, K.F. Proximate analysis of plants of family Zygophyllaceae and Euphorbiaceae during winter. Sarhad J. Agric. (Pak.) 2014, 29, 395–400. [Google Scholar]
- Viola, A.O.; Anekwe, G.E. Amino acids and other Biochemical components of Ricinus communis (Variety minor), an anti-conceptive seed. Pak. J. Biol. Sci. 2001, 4, 866–868. [Google Scholar]
- Amani, H.; Ahmad, Z.; Asif, M.; Hameed, B.H. Transesterification of waste cooking palm oil by MnZr with supported alumina as a potential heterogeneous catalyst. J. Ind. Eng. Chem. 2014, 20, 4437–4442. [Google Scholar] [CrossRef]
- Farooq, M.; Ramli, A.; Subbarao, D. Biodiesel production from waste cooking oil using bifunctional heterogeneous solid catalysts. J. Clean. Prod. 2013, 59, 131–140. [Google Scholar] [CrossRef]
- Predojević, Z.J. The production of biodiesel from waste frying oils: A comparison of different purification steps. Fuel 2008, 87, 3522–3528. [Google Scholar] [CrossRef]
- Knothe, G.; Steidley, K.R. Kinematic viscosity of biodiesel fuel components and related compounds. Influence of compound structure and comparison to petrodiesel fuel components. Fuel 2005, 84, 1059–1065. [Google Scholar] [CrossRef]
- Oliveira, J.S.; Montalvão, R.; Daher, L.; Suarez, P.A.; Rubim, J.C. Determination of methyl ester contents in biodiesel blends by FTIR-ATR and FTNIR spectroscopies. Talanta 2006, 69, 1278–1284. [Google Scholar] [CrossRef] [PubMed]
- Atabani, A.E.; Silitonga, A.S.; Ong, H.C.; Mahlia, T.M.I.; Masjuki, H.H.; Badruddin, I.A.; Fayaz, H. Non-edible vegetable oils: A critical evaluation of oil extraction, fatty acid compositions, biodiesel production, characteristics, engine performance and emissions production. Renew. Sustain. Energy Rev. 2013, 18, 211–245. [Google Scholar] [CrossRef]
- Moser, B.R. Biodiesel production, properties, and feedstocks. Cell. Dev. Biol.-Plant 2009, 45, 229–266. [Google Scholar] [CrossRef]
- Ong, H.C.; Silitonga, A.S.; Masjuki, H.H.; Mahlia, T.M.I.; Chong, W.T.; Boosroh, M.H. Production and comparative fuel properties of biodiesel from non-edible oils: Jatropha curcas, Sterculiafoetida and Ceibapentandra. Energy Convers. Manag. 2013, 73, 245–255. [Google Scholar] [CrossRef]
- Pinzi, S.; Garcia, I.L.; Lopez-Gimenez, F.J.; Luque de Castro, M.D.; Dorado, G.; Dorado, M.P. The ideal vegetable oil-based biodiesel composition: A review of social, economical and technical implications. Energy Fuels 2009, 23, 2325–2341. [Google Scholar] [CrossRef]
- Akpan, U.G.; Jimoh, A.; Mohammed, A.D. Extraction, Characterization and Modification of Castor Seed Oil. Leonardo J. Sci. 2006, 8, 43–52. [Google Scholar]
- Anitha, A. Transesterification of used cooking oils catalyzed by CsTPA/SBA15 catalyst system in biodiesel production. Int. J. Eng. Technol. 2012, 4, 34. [Google Scholar] [CrossRef]
- Warra, A.A. Physico-chemical and GC/MS analysis of castor bean (Ricinus communis L.) seed oil. Chem. Mater. Res. 2015, 7. [Google Scholar]
- Matwijczuk, A.; Oniszczuk, T.; Matwijczuk, A.; Chruściel, E.; Kocira, A.; Niemczynowicz, A.; Wójtowicz, A.; Combrzyński, M.; Wiącek, D. Use of FTIR Spectroscopy and Chemometrics with Respect to Storage Conditions of Moldavian Dragonhead Oil. Sustainability 2019, 11, 6414. [Google Scholar] [CrossRef]
| Parameters | Melia azedarach | Ricinus communis |
|---|---|---|
| Dry matter (%) | 94.2 ± 0.1 | 96.9 ± 0.0 |
| Moisture (%) | 5.8 ± 0.0 | 3.1 ± 0.1 |
| Crude protein (%) | 14.0 ± 0.3 | 15.3 ± 0.2 |
| Crude fat (%) | 51.9 ± 0.2 | 60.5 ± 0.5 |
| Crude fiber (%) | 12.2 ± 0.1 | 9.6 ± 0.1 |
| Carbohydrate (%) | 8.5 ± 0.1 | 5.8 ± 0.5 |
| Ash (%) | 5.4 ± 0.5 | 2.9 ± 0.4 |
| Paramaeters | Melia azedarach | Ricinus communis | ||
|---|---|---|---|---|
| Oil | Biodiesel | Oil | Biodiesel | |
| Acid value (mg KOH/g oil) | 5.4 ± 0.1 | 0.9 ± 0.0 | 2.0 ± 0.0 | 0.8 ± 0.0 |
| Saponification value (mg KOH/g oil) | 171.8 ± 0.2 | 48.9 ± 0.5 | 174.9 ± 0.7 | 181.3 ± 0.4 |
| Iodine value (g I2 100 g−1) | 127.2 ± 0.3 | 119.0 ± 0.2 | 83.5 ± 0.2 | 81.3 ± 0.3 |
| Cetane number | - | 49.2 ± 0.5 | - | 41.2 ± 0.1 |
| Density15oC (g/cm3) | 0.9 ± 0.0 | 0.9 ± 0.0 | 0.9 ± 0.0 | 0.9 ± 0.0 |
| Specific gravity (g/mL) | 0.95 | 0.89 | 0.94 | 0.87 |
| Kinematic viscosity (mm2/s) | 18.1 ± 0.1 | 3.2 ± 0.0 | 22.1 ± 0.1 | 11.1 ± 0.1 |
| Refractive index (30 °C) | 1.4 ± 0.0 | 1.4 ± 0.0 | 1.5 ± 0.0 | 1.5 ± 0.0 |
| Cloud point | - | < −10 | - | < −10 |
| Pour point | - | −28 | - | −31 |
| Flash point | - | 131.3 ± 0.5 | - | 261.2 ± 1.3 |
| Calorific value (MJ/kg) | - | 33.1 ± 0.4 | - | 37.9 ± 1.8 |
| Cold filter plugging point | - | −30 | - | −35 |
| Sulfur content (%) | - | <0.001 | - | <0.001 |
| Free fatty acid | 0.6 ± 0.0 | 0.3 ± 0.0 | 0.7 ± 0.0 | 0.3 ± 0.0 |
| Oil | Palmitic acid (C16:0) | Palmitoleic acid (C16:1) | Stearic acid (C18:0) | Oleic acid (C18:1) | Ricinoleic acid (C18:1 OH) | Linoleic acid (C18:2) | Linolenic acid (C18:3) |
|---|---|---|---|---|---|---|---|
| Melia azedarach | 9.1 ± 0.5 | 1.8 ± 0.01 | 3.9 ± 0.1 | 61.5 ± 0.4 | - | 9.2 ± 0.2 | 0.8 ± 0.0 |
| Ricinus communis | 1.5 ± 0.1 | 0.9 ± 0.0 | 1.8 ± 0.1 | 6.0 ± 0.0 | 72.5 ± 0.7 | 5.1 ± 0.0 | 1.7 ± 0.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Awais, M.; Musmar, S.A.; Kabir, F.; Batool, I.; Rasheed, M.A.; Jamil, F.; Khan, S.U.; Tlili, I. Biodiesel Production from Melia azedarach and Ricinus communis Oil by Transesterification Process. Catalysts 2020, 10, 427. https://doi.org/10.3390/catal10040427
Awais M, Musmar SA, Kabir F, Batool I, Rasheed MA, Jamil F, Khan SU, Tlili I. Biodiesel Production from Melia azedarach and Ricinus communis Oil by Transesterification Process. Catalysts. 2020; 10(4):427. https://doi.org/10.3390/catal10040427
Chicago/Turabian StyleAwais, Muhammad, Sa’ed A Musmar, Faryal Kabir, Iram Batool, Muhammad Asif Rasheed, Farrukh Jamil, Sami Ullah Khan, and Iskander Tlili. 2020. "Biodiesel Production from Melia azedarach and Ricinus communis Oil by Transesterification Process" Catalysts 10, no. 4: 427. https://doi.org/10.3390/catal10040427
APA StyleAwais, M., Musmar, S. A., Kabir, F., Batool, I., Rasheed, M. A., Jamil, F., Khan, S. U., & Tlili, I. (2020). Biodiesel Production from Melia azedarach and Ricinus communis Oil by Transesterification Process. Catalysts, 10(4), 427. https://doi.org/10.3390/catal10040427







