NaBiS2 as a Novel Indirect Bandgap Full Spectrum Photocatalyst: Synthesis and Application
Abstract
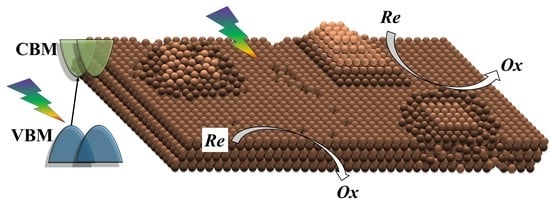
1. Introduction
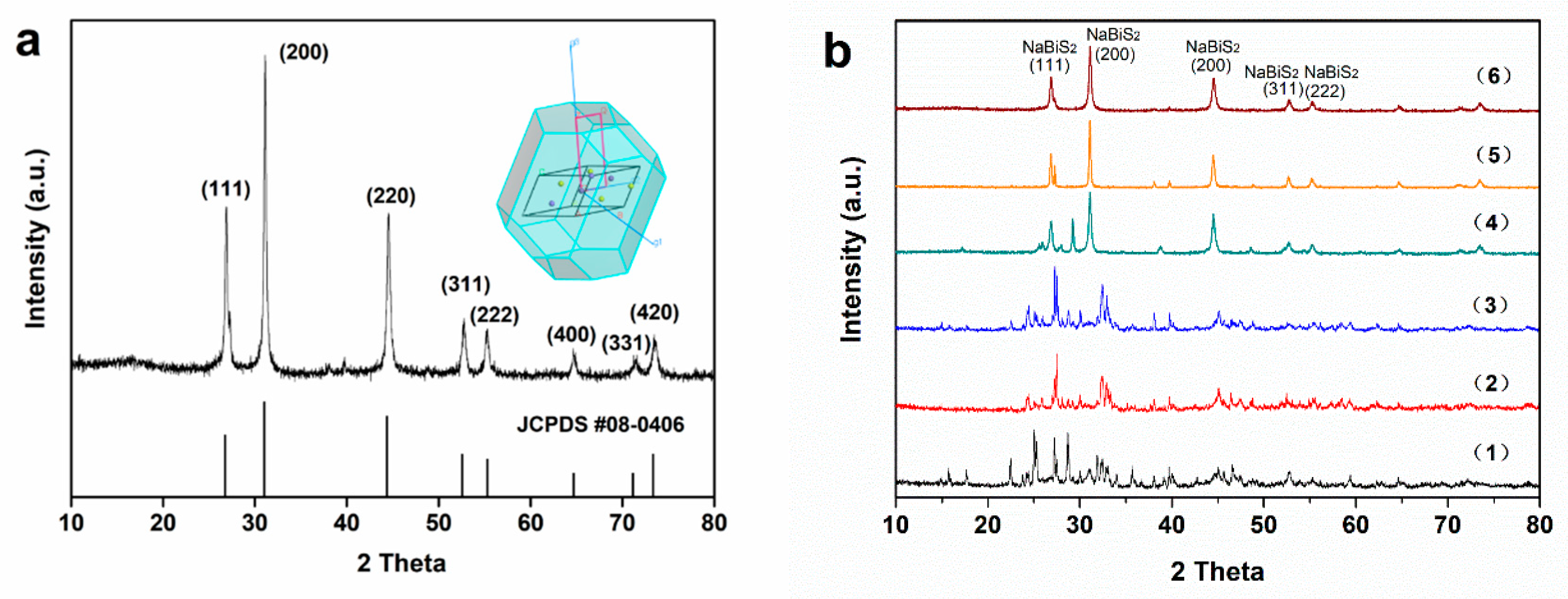
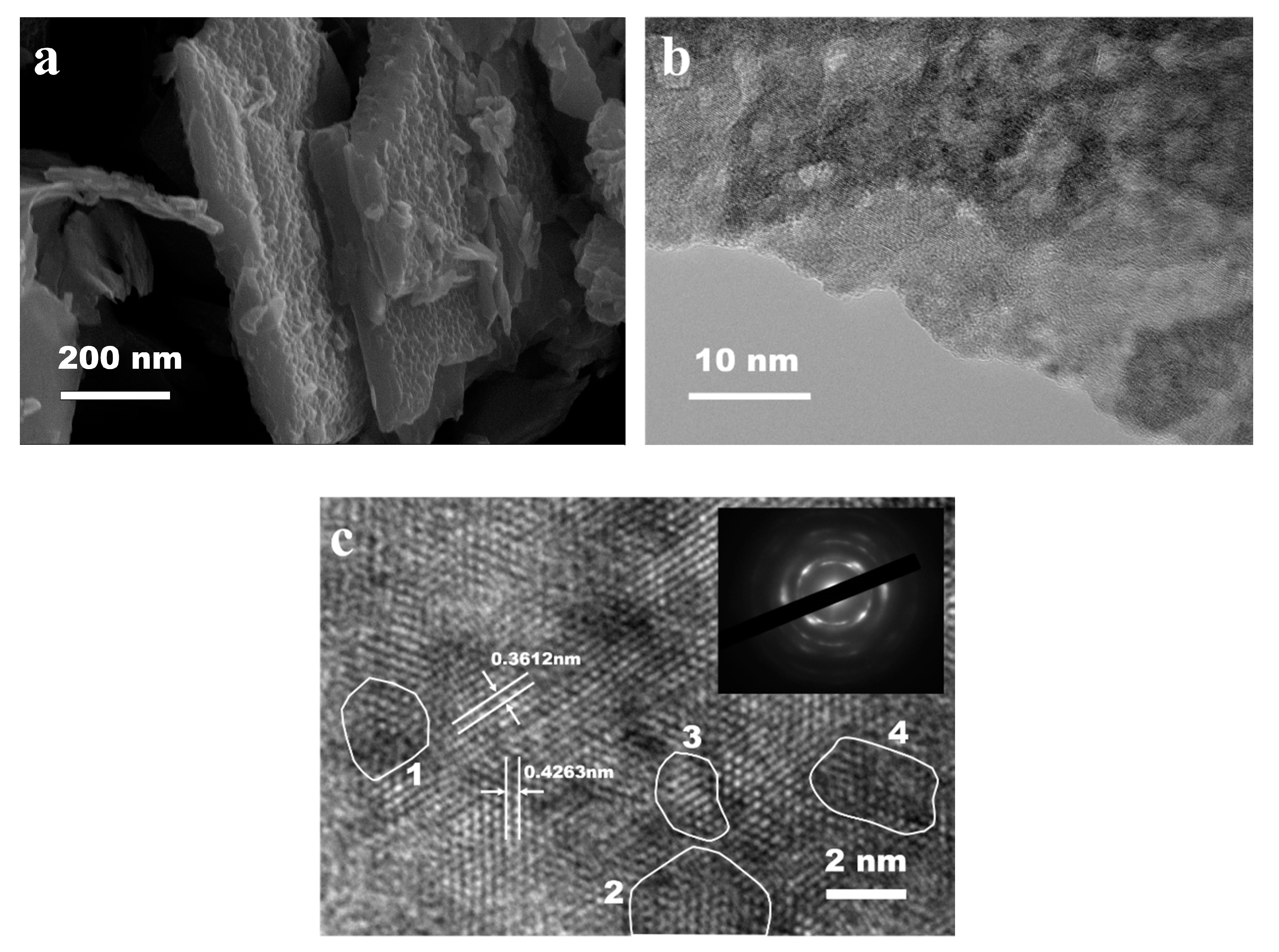
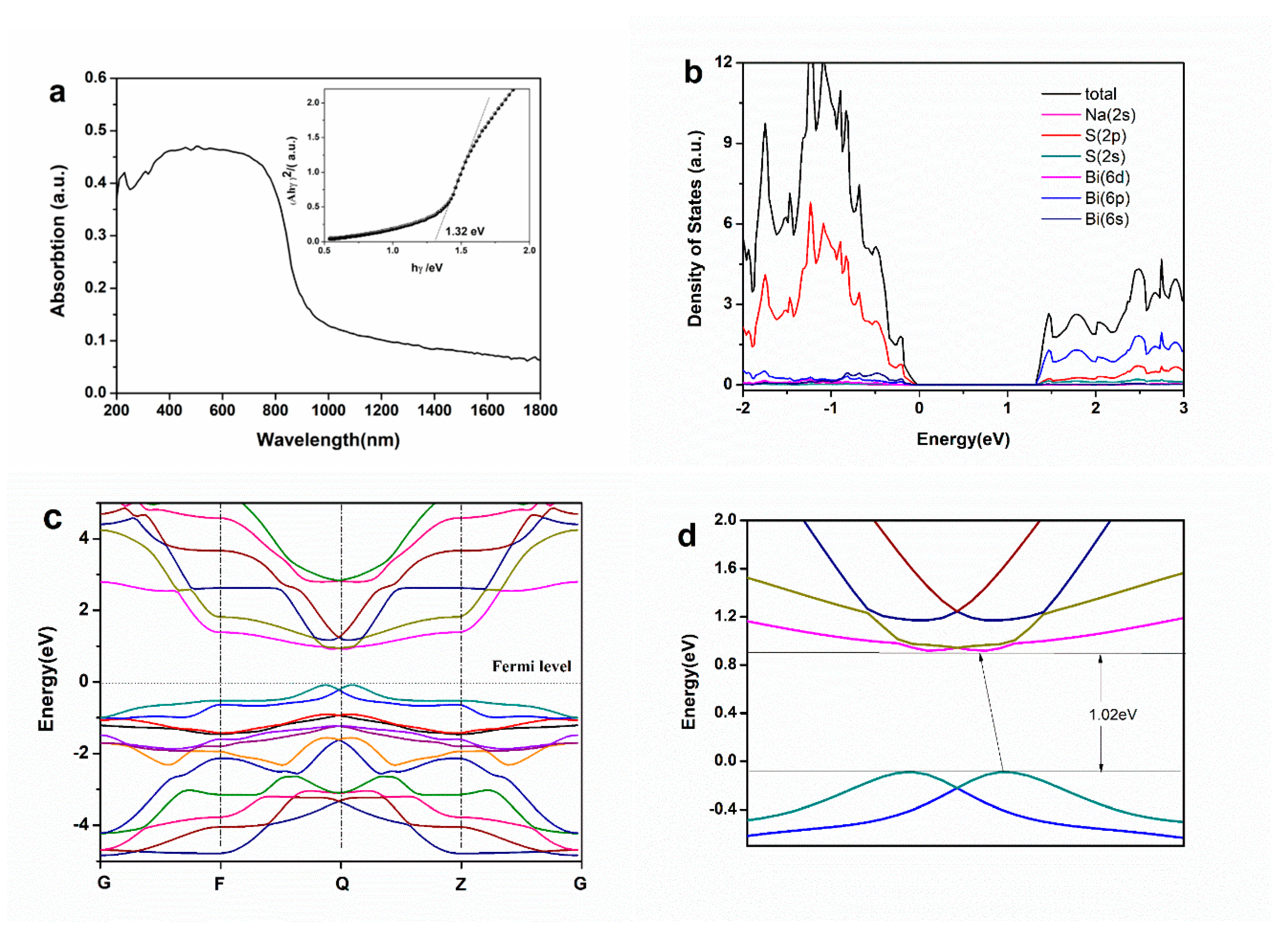
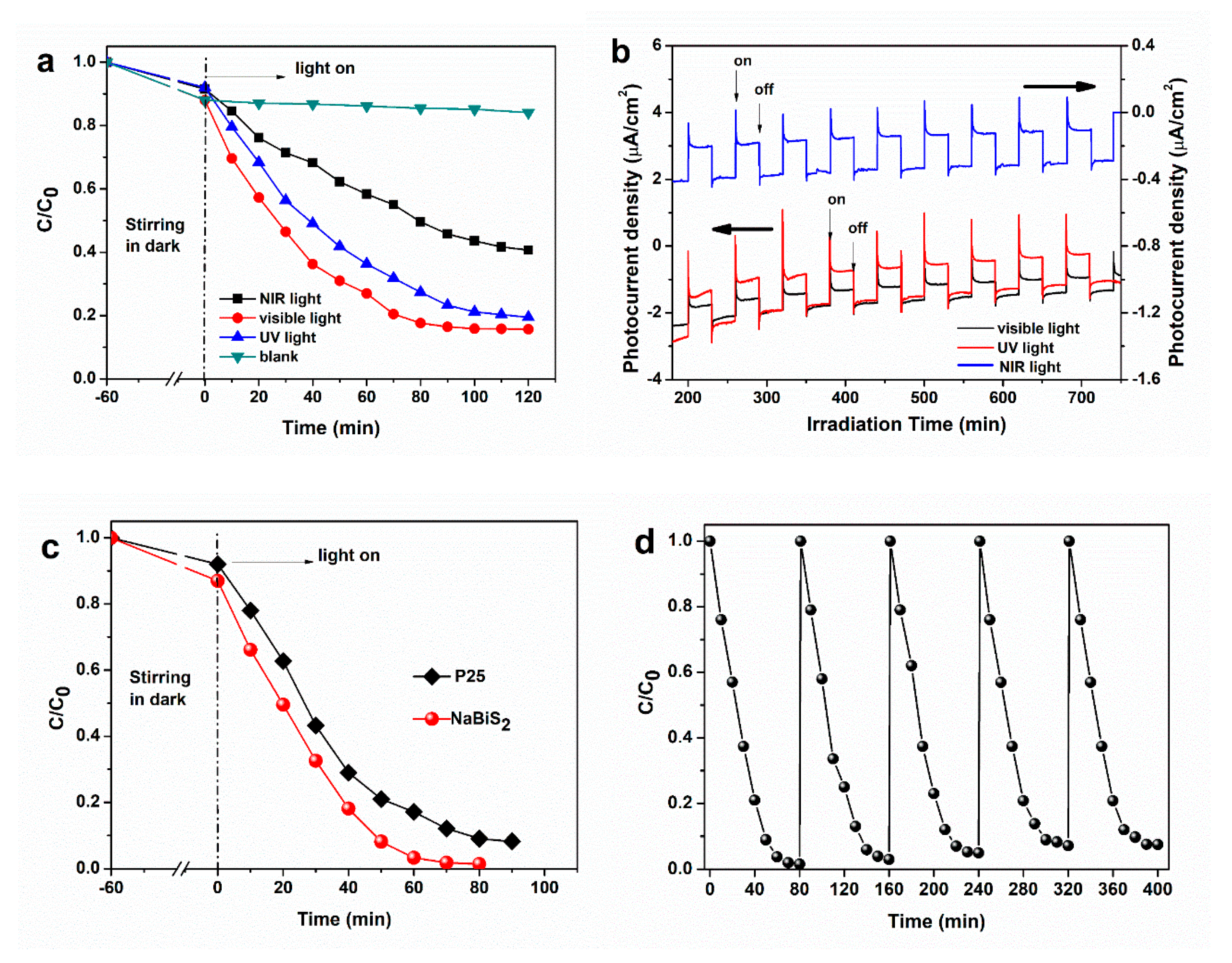
2. Results and Discussion
3. Experimental Section
3.1. Preparation and Characterization of Photocatalyst
3.2. Photocatalytic Activity and Photochemical Measurements
3.3. Theoretical Calculation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Data Availability Statement
Conflicts of Interest
References
- Kubacka, A.; Fernandez-Garcia, M.; Colon, G. Advanced nanoarchitectures for solar photocatalytic applications. Chem. Rev. 2012, 112, 1555–1614. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Ouyang, S.X.; Bi, Y.P.; Umezawa, N.; Oshikiri, M.; Ye, J.H. Nano-photocatalytic materials: Possibilities and challenges. Adv. Mater. 2012, 24, 229–251. [Google Scholar] [CrossRef] [PubMed]
- Kisch, H. Semiconductor photocatalysis--mechanistic and synthetic aspects. Angew. Chem. Int. Ed. 2013, 52, 812–847. [Google Scholar] [CrossRef] [PubMed]
- Zuo, C.T.; Bolink, H.J.; Han, H.W.; Huang, J.S.; Cahen, D.; Ding, L.M. Advances in perovskite solar cells. Adv. Sci. 2016, 3, 1500324.1–1500324.16. [Google Scholar] [CrossRef]
- Shi, Z.J.; Guo, J.; Chen, Y.H.; Li, Q.; Huang, W. Lead-free organic-inorganic hybrid perovskites for photovoltaic applications: Recent advances and perspectives. Adv. Mater. 2017, 29, 1605005.1–1605005.28. [Google Scholar] [CrossRef]
- Chen, B.; Zheng, X.; Bai, Y.; Padture, N.P.; Huang, J. Progress in tandem solar cells based on hybrid organic-inorganic perovskites. Adv. Energy Mater. 2017, 7, 1602400.1–1602400.19. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, Y.; Deng, J.; Wei, J.; Le, T.H.; Chandran, B.K.; Dong, Z.; Chen, Z.; Chen, X.D. Mechanical force-driven growth of elongated bending TiO2 -based nanotubular materials for ultrafast rechargeable lithium ion batteries. Adv. Mater. 2014, 26, 6111–6118. [Google Scholar] [CrossRef]
- Peng, Y.; Chen, Q.G.; Wang, D.; Zhou, H.Y.; Xu, A.W. Synthesis of one-dimensional WO3–Bi2WO6 heterojunctions with enhanced photocatalytic activity. CrystEngComm 2015, 17, 569–576. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, W.; Zhou, L.; Xu, H. Bi2WO6 nano- and microstructures: Shape control and associated visible-light-driven photocatalytic activities. Small 2007, 3, 1618–1625. [Google Scholar] [CrossRef]
- Hou, Y.; Wen, Z.H.; Cui, S.M.; Guo, X.R.; Chen, J.H. Constructing 2D porous graphitic C3N4 nanosheets/nitrogen-doped graphene/layered MoS2 ternary nanojunction with enhanced photoelectrochemical activity. Adv. Mater. 2013, 25, 6291–6297. [Google Scholar] [CrossRef]
- Wang, S.P.; Li, C.J.; Wang, T.; Zhang, P.; Li, A.; Gong, J.L. Controllable synthesis of nanotube-type graphitic C3N4 and their visible-light photocatalytic and fluorescent properties. J. Mater. Chem. A 2014, 2, 2885–2890. [Google Scholar] [CrossRef]
- Wang, G.; Huang, B.; Ma, X.; Wang, Z.; Qin, X.; Zhang, X.; Dai, Y.; Whangbo, M.H. Cu2(OH)PO4, a near-infrared-activated photocatalyst. Angew. Chem. Int. Ed. 2013, 52, 4810–4813. [Google Scholar] [CrossRef] [PubMed]
- Sang, Y.; Zhao, Z.; Zhao, M.; Hao, P.; Leng, Y.; Liu, H. From UV to near-infrared, WS2 nanosheet: A novel photocatalyst for full solar light spectrum photodegradation. Adv. Mater. 2015, 27, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, X.; Pan, W.; Zhang, G.; Chen, H. Vacancy-rich monolayer BiO2-x as a highly efficient UV, visible, and near-infrared responsive photocatalyst. Angew. Chem. Int. Ed. 2018, 57, 491–495. [Google Scholar] [CrossRef]
- Gao, W.W.; Liu, W.X.; Leng, Y.H.; Wang, X.M.; Wang, X.Q.; Hu, B.; Yu, D.H.; Sang, Y.H.; Liu, H. In2S3 nanomaterial as a broadband spectrum photocatalyst to display significant activity. Appl. Catal. B Environ. 2015, 176, 83–90. [Google Scholar] [CrossRef]
- Cui, G.W.; Wang, W.; Ma, M.Y.; Xie, J.F.; Shi, X.F.; Deng, N.; Xin, J.P.; Tang, B. IR-driven photocatalytic water splitting with WO2-NaxWO3 hybrid conductor material. Nano Lett. 2015, 15, 7199–7203. [Google Scholar] [CrossRef]
- Sang, Y.; Liu, H.; Umar, A. Photocatalysis from UV/vis to near-infrared light: Towards full solar-light spectrum activity. ChemCatChem 2015, 7, 559–573. [Google Scholar] [CrossRef]
- Yuan, X.Z.; Wang, H.; Wang, J.J.; Zeng, G.M.; Chen, X.H.; Wu, Z.B.; Jiang, L.B.; Jin, T.X.; Zhang, J.; Wang, H. Near-infrared-driven Cr(vi) reduction in aqueous solution based on a MoS2/Sb2S3 photocatalyst. Catal. Sci. Technol. 2018, 8, 1545–1554. [Google Scholar] [CrossRef]
- Zhu, M.S.; Zhai, C.Y.; Fujitsuka, M.; Majima, T. Noble metal-free near-infrared-driven photocatalyst for hydrogen production based on 2D hybrid of black Phosphorus/WS2. Appl. Catal. B Environ. 2018, 221, 645–651. [Google Scholar] [CrossRef]
- Zhi, L.; Zhang, H.; Yang, Z.; Liu, W.; Wang, B. Interface coassembly of mesoporous MoS2 based-frameworks for enhanced near-infrared light driven photocatalysis. Chem. Commun. 2016, 52, 6431–6434. [Google Scholar] [CrossRef]
- Wu, J.; Liu, B.; Ren, Z.; Ni, M.; Li, C.; Gong, Y.; Qin, W.; Huang, Y.; Sun, C.Q.; Liu, X. CuS/RGO hybrid photocatalyst for full solar spectrum photoreduction from UV/vis to near-infrared light. J. Colloid Interface Sci. 2018, 517, 80–85. [Google Scholar] [CrossRef]
- Ren, Z.X.; Lei, L.; Liu, B.B.; Liu, X.J.; Li, Z.; Lei, X.; Li, C.; Gong, Y.Y.; Niu, L.Y.; Pan, l.K. Cr(VI) reduction in presence of ZnS/RGO photocatalyst under full solar spectrum radiation from UV/vis to near-infrared light. Catal. Today 2018, 315, 46–51. [Google Scholar] [CrossRef]
- Kang, S.; Hong, Y.; Jeon, Y. A facile synthesis and characterization of sodium bismuth sulfide (NaBiS2) under hydrothermal condition. Bull. Korean Chem. Soc. 2014, 35, 1887–1890. [Google Scholar] [CrossRef]
- Guo, J.; Ge, Z.H.; Hu, M.Y.; Qin, P.; Feng, J. Facile synthesis of NaBiS2 nanoribbons as a promising visible light-driven photocatalyst. Phys. Status Solidi-Rapid Res. Lett. 2018, 12, 1800135.1–1800135.4. [Google Scholar] [CrossRef]
- Liu, Z.P.; Liang, J.B.; Li, S.; Peng, S.; Qian, Y.T. Synthesis and growth mechanism of Bi2S3 nanoribbons. Chemistry 2004, 10, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Peng, S.; Xie, Q.; Hu, Z.; Yang, Y.; Zhang, S.; Qian, Y. Large scale synthesis of ultralong Bi2S3 nanoribbon via a solvothermal process. Adv. Mater. 2003, 15, 936–940. [Google Scholar] [CrossRef]
- Sheets, W.C.; Stampler, E.S.; Kabbour, H.; Bertoni, M.I.; Cario, L.; Mason, T.O.; Marks, T.J.; Poeppelmeier, K.R. Facile synthesis of BiCuOS by hydrothermal methods. Inorg. Chem. 2007, 46, 10741–10748. [Google Scholar] [CrossRef]
- Wang, H.; Ding, J.; Xu, H.; Qiao, L.; Wang, X.; Lin, Y. One-pot synthesis of BiCuSO nanosheets under ambient atmosphere as broadband spectrum photocatalyst. Nanomaterials 2019, 9, 540. [Google Scholar] [CrossRef]
- Bi, Y.; Hu, H.; Ouyang, S.; Lu, G.; Cao, J.; Ye, J. Photocatalytic and photoelectric properties of cubic Ag3PO4 sub-microcrystals with sharp corners and edges. Chem. Commun. 2012, 48, 3748–3750. [Google Scholar] [CrossRef]
- Liu, C.; Kong, D.; Hsu, PC.; Yuan, H.; Lee, HW.; Liu, Y.; Wang, H.; Wang, S.; Yan, K.; Lin, D.; et al. Rapid water disinfection using vertically aligned MoS2 nanofilms and visible light. Nat. Nanotechnol. 2016, 11, 1098–1104. [Google Scholar] [CrossRef]
- Chang, C.; Teng, F.; Liu, Z. Fully understanding the photochemical properties of Bi2O2(CO3)1-xSx nanosheets. Langmuir 2016, 32, 3811–3819. [Google Scholar] [CrossRef] [PubMed]
- BaQais, A.; Nina, T.; Tangui, L.B.; Kazuhiro, T. Optoelectronic structure and photocatalytic applications of Na(Bi,La)S2 solid solutions with tunable band gaps. Chem. Mater. 2019, 31, 3211–3220. [Google Scholar] [CrossRef]
- Gabrel’yan, B.V.; Lavrentiev, A.A.; Nikiforov, I.Y.; Sobolev, V.V. Electronic energy structure of MBiS2 (M = Li, Na, K) calculated with allowance for the difference between the M-S and Bi-S bond lengths. J. Struct. Chem. 2008, 49, 788–794. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, J.X.; Wang, W.N.; Van de Walle, C.G. First-principles analysis of radiative recombination in lead-halide perovskites. ACS Energy Lett. 2018, 3, 2329–2334. [Google Scholar] [CrossRef]
- Li, J.; Zhang, L.; Li, Y.; Yu, Y. Synthesis and internal electric field dependent photoreactivity of Bi3O4Cl single-crystalline nanosheets with high {001} facet exposure percentages. Nanoscale 2014, 6, 167–171. [Google Scholar] [CrossRef]
- Di, J.; Xia, J.X.; Ge, Y.P.; Li, H.P.; Ji, H.Y.; Zhang, Q.; Li, H.M.; Li, M.N. Novel visible-light-driven CQDs/Bi2WO6 hybrid materials with enhanced photocatalytic activity toward organic pollutants degradation and mechanism insight. Appl. Catal. B Environ. 2015, 168–169, 51–61. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Xie, Z.; Wang, X.; Jia, Y. NaBiS2 as a Novel Indirect Bandgap Full Spectrum Photocatalyst: Synthesis and Application. Catalysts 2020, 10, 413. https://doi.org/10.3390/catal10040413
Wang H, Xie Z, Wang X, Jia Y. NaBiS2 as a Novel Indirect Bandgap Full Spectrum Photocatalyst: Synthesis and Application. Catalysts. 2020; 10(4):413. https://doi.org/10.3390/catal10040413
Chicago/Turabian StyleWang, Huanchun, Zheng Xie, Xuanjun Wang, and Ying Jia. 2020. "NaBiS2 as a Novel Indirect Bandgap Full Spectrum Photocatalyst: Synthesis and Application" Catalysts 10, no. 4: 413. https://doi.org/10.3390/catal10040413
APA StyleWang, H., Xie, Z., Wang, X., & Jia, Y. (2020). NaBiS2 as a Novel Indirect Bandgap Full Spectrum Photocatalyst: Synthesis and Application. Catalysts, 10(4), 413. https://doi.org/10.3390/catal10040413