1. Introduction
Though the world is moving towards low-carbon energy sources, its dependence on crude oil to meet its energy needs will continue to linger during this transition period. As the world’s supply of light oils continues to dwindle, the demand for transportation fuels continues to rise, and so there is a need to supplement fossil fuel production through the exploitation of heavy oil and bitumen. These reserves are mostly domicile in Canada, Venezuela and the United States of America [
1] and require specially developed extraction techniques and additional upgrading to make their properties similar to lighter oils, with characteristics that meet existing refinery infrastructures. The two main properties of crude oil influencing their price are American Petroleum Institute (API) gravity and sweetness, which is related to sulfur content, with ‘sweet’ crude defined as containing less than 0.5% sulfur [
2]. Compared to light oil, heavy oil sells at a far lower price because it is much more viscous, the API gravity is less than 20°, it is classified as sour crude, has high content of heteroatoms such as sulfur or nitrogen, and high content of metals such as nickel, vanadium and iron. Poor mobility under reservoir condition due to its very high viscosity makes it more difficult and expensive to extract from the reservoir and also processing costs are higher [
3]. Hence, heat, often in the form of in situ combustion or steam, is commonly deployed to raise the temperature of the oil formation, lowering its viscosity and enhancing recovery. Alternative heating technologies such as electromagnetic techniques could be used to supplement conventional heating. Toe-to-Heel Air Injection (THAI) combined with catalytic upgrading process in situ (CAPRI) has been demonstrated to concurrently recover and upgrade heavy oil underground in a more energy efficient way than commercial Steam-Assisted Gravity Drainage (SAGD) [
4], since it does not require the injection of externally generated steam [
3,
5].
The prospect of the THAI–CAPRI process to produce partially upgraded oil has been proven in simulation, laboratory and field trials [
3,
4,
5,
6,
7,
8,
9,
10,
11,
12,
13,
14]. The comparison of Kerrobert field THAI and SAGD projects showed that, though the THAI peak oil production rate (0.06 m
3 day
−1 m
−1) was lower than SAGD (0.3 m
3 day
−1 m
−1), the produced oil with THAI was moderately upgraded by 5° points API gravity increase, whereas that of SAGD showed negligible upgrading [
4]. THAI laboratory combustion experiments have also shown improved API gravity by more than 7° points increase, remarkable viscosity reduction (>70%) and high oil recovery, at about 80% [
6,
12], compared to the crude feed oil. It was found from the Kerrobert THAI field project by Petrobank Energy and Resources LTD (later Touchstone Exploration Inc.) in Saskatchewan, Canada, that the mobilized hot oil drained by gravity over the catalyst packing around the horizontal well possesses non-uniform temperatures ranging from 100 to 300 °C [
4,
14]. This deficit in temperatures has also been confirmed in a simulation study by Rabiu et al. [
15]. These ranges of temperatures are inadequate to produce significant catalytic upgrading in the hot oil as it flows over the catalyst packing. On the contrary, a temperature of 425 °C has proven to be the optimum from laboratory-scale investigations [
6,
8,
9]. This study therefore focusses on the incorporation of an induction heating coil to the horizontal production well of the THAI–CAPRI process. The aim is to provide additional heating to achieve sufficient and uniform temperature (425 °C or more) for in situ catalytic upgrading and ensure substantial upgrading as the mobilized hot oil flows over the packed catalyst layer. Concurrently, at 425 °C temperature, high coke deposition has been reported, which rapidly deactivates the catalyst and plugs the bed over time [
8,
9]. Therefore, this study addresses two-fold challenge of augmenting the temperature of the CAPRI process, the catalytic version of THAI: firstly, the application of induction heating to alleviate the shortfall in temperatures around the CAPRI section; and secondly, controlling and reducing the rapid catalyst deactivation due to coke deposition.
Incorporating induction heating to the horizontal production well causes heat generation directly within the catalyst bed. This heating method allows the oil and the catalyst to be heated through convention and conduction, due to intimate contact with either steel balls as a susceptor or composite catalyst with conducting component. This can swiftly and effectively convert electromagnetic field into heat and transfer the heat rapidly, as compared to conventional heating [
16,
17]. Henkel et al. [
16] reported the pyrolysis of cane bagasse and Chinese tallow wood biomasses packed compactly in a stainless-steel reactor tube and inductively heated to produce bio-oil, using nitrogen as a carrier gas to move the pyrolysis vapour. The configuration allowed the biomass to be heated via surface-to-surface conduction from the wall of the reactor tube. Furthermore, Abu-Laban et al. [
18] studied induction heating of pellets of Pt/Al
2O
3 catalyst mixed with steel balls (5.5 mm) inside an alumina tube to 234 °C. Lower coke formation and partial deoxygenation of sawdust pyrolysis oil were reported, relative to conventional heating by using a trace heating cable, in which rapid coke fouling and negligible deoxygenation were observed. Induction heating offers appealing benefits for its use in downhole catalytic upgrading of heavy oil, such as a target-specific, rapid-heating rate, uniform temperature, avoidance of heat transfer loss and the precise control of temperature [
19,
20]. This has the advantage of heating a conducting catalyst, directly leading to increased longevity, promoted by desorption of molecules from the catalyst surface, and limited deposition of coke and foulants. This approach establishes a thin temperature gradient across the catalyst surface and drives heat flow from interior to exterior and to the walls of the reactor, whereas conventional heating drives heat flow from exterior to interior, creating a large temperature gradient across the catalyst surface [
17]. In this light, mass transfer is believed to flow in the same direction as heat, stimulating desorption of molecules from the catalyst surface and deterring polymerization and condensation of molecules from forming coke precursors.
Heavy oil is known to have a low H/C ratio compared to light oil. Therefore, the essence of upgrading is to either increase its hydrogen or decrease carbon, in addition to reducing heteroatoms (S, O, N, etc.) and metals contents. Excessive carbon rejection causes catalyst coking leading to deactivation. On the issue of catalyst deactivation due to coke deposition, one of the ways of suppressing coke formation in oil upgrading has been through the addition of hydrogen gas, whose role is to cap and halt free radical polymerization reaction, as well as decrease hydrogen abstraction from adsorbed hydrocarbon molecules [
11]. Additionally, the presence of hydrogen gas promotes hydroprocessing, hydrodesulfurization (HDS) and hydrodemetallization (HDM) reactions responsible for the removal of sulfur and heavy metals, such as vanadium and nickel, in the heavy oil. It is challenging and hazardous transporting hydrogen gas itself into the reservoir. The risk associated with hydrogen gas has shifted attention to hydrogen carriers such as polycyclic organic compounds, which are known to store, transport and liberate hydrogen in a safer and cost-effective means [
21]. Moreover, it is well-known that asphaltene is soluble in aromatic solvents, so the presence of polycyclic organic solvent in the catalytic upgrading medium would keep the asphaltene solubilized, while undergoing cracking into smaller fragments which are readily hydrogenated by the liberated hydrogen [
22]. On the other hand, the injection of steam has been reported as an alternative technique to supply hydrogen in situ through the water–gas shift (WGS) reaction and can also help to suppress coke formation [
10,
23]. However, to produce appreciable hydrogen, the WGS reaction needs to be conducted at high temperatures, i.e., 400–750 °C [
24], and at a temperature above 425 °C, the catalytic upgrading of heavy oil has been shown to suffer more coke deposition, and as a consequence, severe catalyst deactivation and bed plugging can occur [
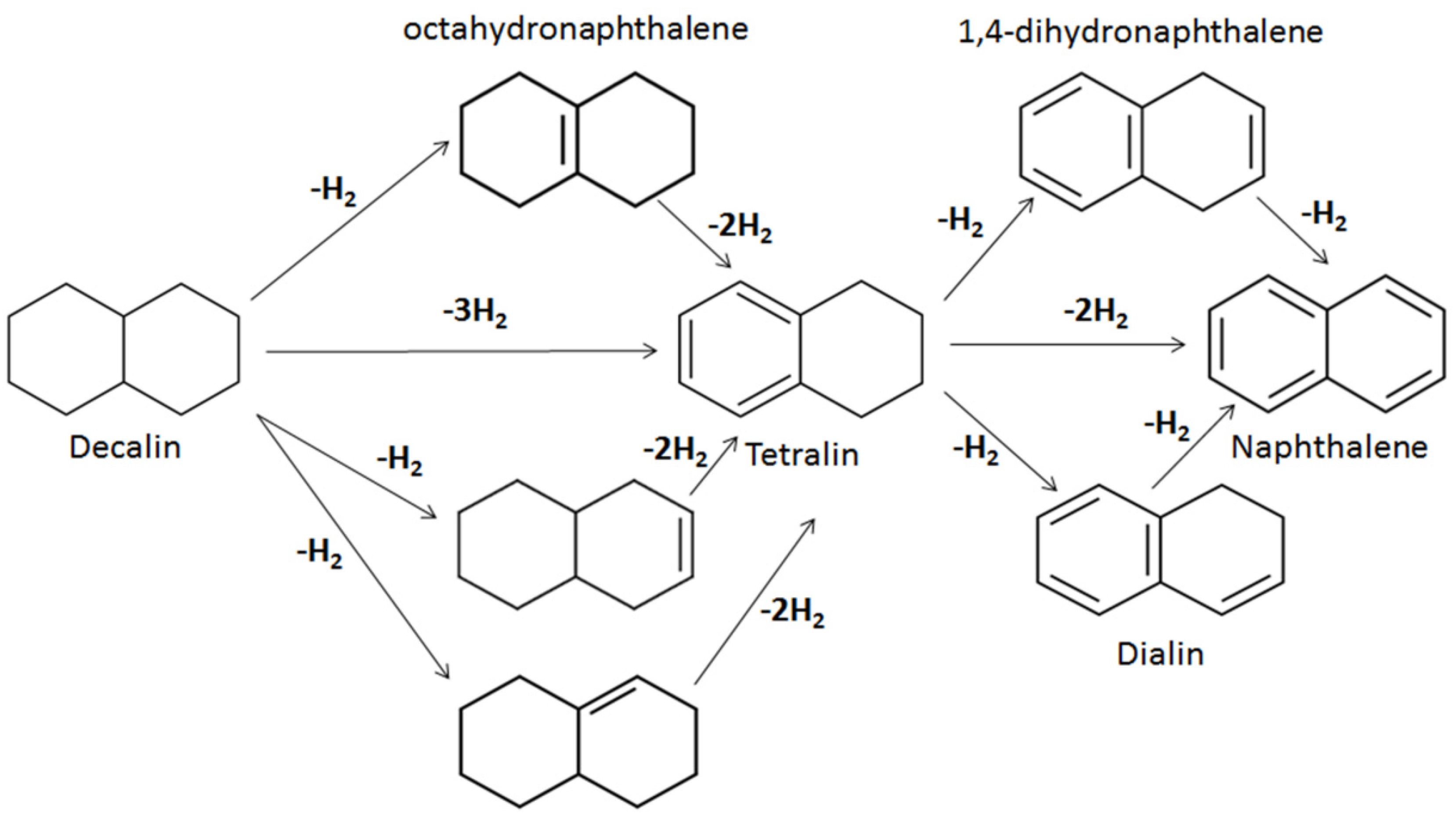
8]. Unlike WGS reaction, where carbon monoxide and steam must be present as reagents, polycyclic organic compounds such as cyclohexane, decalin and tetralin liberate significant amounts of hydrogen in the temperature range 250–425 °C, via dehydrogenation [
17,
22]. Herein, the effect of tetralin and decalin polycyclic organic solvents as H-donors in an inductively heated catalytic upgrading of heavy oil is reported. The objective is to combine it with the horizontal production well of the THAI process, with the hypothesis that it would provide sufficient temperature for catalysis, improve upgrading level and extend catalyst longevity by lowering coke deposition, in comparison to conventional heating. The role of hydrogen in coke reduction and upgraded oil quality improvement was studied. We also investigated coke deposition within the pelleted catalyst (NiMo/Al
2O
3) microstructure that leads to deactivation, using X-ray nano Computed Tomography (X-ray nano-CT). The NiMo/Al
2O
3 catalyst used in this work is a bifunctional hydrotreating catalyst widely used in the petroleum-refining industry for HDS and HDM reactions, which involve sulfur and metal removal from gas oil. Consequently, it has been proven that the molybdenum (Mo) active metal is suitable for catalyzing heavy oil upgrading, while the nickel (Ni) promoter enhances desulfurization reaction [
8].
3. Materials and Methods
3.1. Materials
Commercial hydrotreating (HDT) catalyst NiMo/Al
2O
3 with dimensions (quadrilobed-shaped: diameter (a,b) 1.4 × 1.2 mm and length 4.6 ± 2.2 mm) was used in this work. The catalyst comprises approximately 7% nickel promoter and 21% molybdenum active metal in their oxide form. Detailed physicochemical properties and characteristics of the HDT catalyst have been reported elsewhere [
17]. Touchstone Exploration Inc., Canada, supplied the heavy oil used in this work, which is a mixture of oils produced from eight different reservoirs, using THAI process at Kerrobert field, Saskatchewan, Canada.
Table 2 displays the heavy oil properties.
The solvents and gases used include tetralin, 99.5% purity (1,2,3,4-tetahydronaphthalene, CAS Number: 119-64-2, Sigma Aldrich, Gillingham, UK); decalin, 99.6% purity (decahydronaphthalene, CAS Number: 91-17-8, Sigma Aldrich, Gillingham, UK); nitrogen gas (BOC Group, Birmingham, UK); and hydrogen gas, 99.95% purity (BOC Group, Birmingham, UK). Tetralin and decalin solvents were chosen to evaluate their hydrogen-donor capabilities in comparison to catalytic upgrading achieved by using a hydrogen gas environment, whilst nitrogen gas was used to simulate catalytic upgrading via carbon-rejection and dehydrogenation in addition to being cheaper than argon and helium.
3.2. Experimental Setup
The induction heating unit was built by Inductelec, UK, while the catalytic reactor system was assembled by C-Tech Innovation, UK, and commissioned at the School of Chemical Engineering, University of Birmingham, UK.
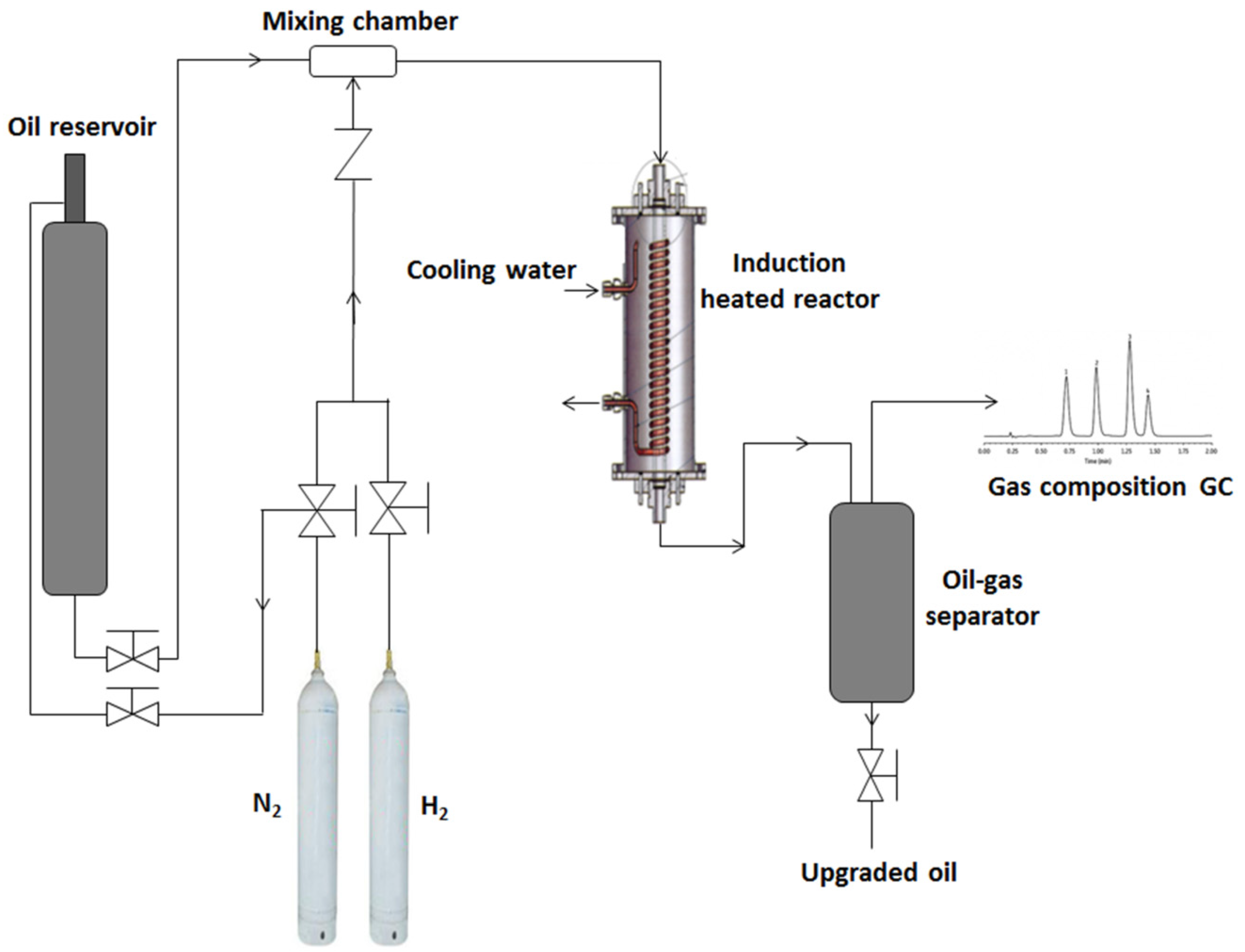
Figure 7 shows the schematics of the induction heating catalytic reactor and the experimental setup. The induction coil used was a sixty-loop-coated copper coil with an inner diameter 44 mm and loop length of 230 mm. The area covered by the copper coils represents the actual reaction zone where the heat is generated, and the catalyst and steel balls (3 mm) were packed within this zone inside the quartz glass tube (20 mm i.d., 40 mm o.d. × 440 mm). The packed bed within the quartz glass tube represents the thickness of catalyst layer wrapped around the horizontal production well of the THAI process. The temperature of the catalytic bed was measured with installed thermocouple (k-type) from the bottom of the reactor, which was validated with a secondary thermocouple inserted into the packed bed; immediately the induction heating was turned off once 425 °C was reached, eliminating the interference of magnetic field. A detailed procedure has been reported elsewhere [
17].
First, a composite catalyst comprising 40% (v/v) iron filings to alumina binder ratio was designed, and also 3 mm wire gauze constructed to potentially convert the magnetic field into heat. These two designs were tested, and error message was displayed on the Inductelec liquid crystal display (LCD), indicating insufficient susceptibility effect. This can be attributed to the thickness of the non-conducting quartz glass tube (10 mm) impeding their interaction with the magnetic field generated inside the tube, due to the wrapped induction coil (
Figure 7). Hence, a mixed bed system of catalyst and steel balls (3 mm) was investigated. The catalyst-to-steel-balls ratio used was 66% (v/v), of which 28 g was catalyst and the steel balls converted the magnetic field into heat, thereby heating the entire packed bed (NiMo/Al
2O
3 and steel balls). The top and bottom of the packed bed was occupied by glass beads (5 mm), to eliminate interaction with magnetic field and ensure feed oil distribution and adequate catalyst bed wetting. The reactor was operated in the downward flow mode in order to simulate the gravity drainage of the mobilized hot oil ahead of the combustion front in the THAI process, as well as complete catalyst wetting. The THAI feed oil was fed through lines heated to 290 °C by trace heating rope; this simulates typical temperature of the mobilized hot ahead of the combustion front in the THAI process prior to passing over the catalyst layer in the CAPRI process, as well as lowers the heating load in the catalytic reactor. The THAI oil reservoir was pressurized by nitrogen to 25 barg, to push the oil through the line, as shown in
Figure 7. The operating conditions of temperature 425 °C (the optimum reported by Shah et al. [
8]) and pressure 15 barg were established at steady state. Then the oil-flow metering valve was manually opened to initiate oil flow from the THAI oil reservoir through the trace cable heated line (290 °C) into the induction-heated catalytic reactor, at an adjusted flowrate of 1 ± 0.3 mL/min. The reaction medium was either nitrogen or hydrogen gas, depending on the experiment at 400 mL/min, as shown in
Figure 1. The height of the mixed bed of catalyst and steel balls was 230 mm, while the bed porosity was 0.38. The pressure inside the reactor was controlled by back pressure regulator (Swagelok, Manchester, UK), through the regulation of gas holdup inside the reactor. The ratio of H-donor solvent to oil used was 0.045 (g/g), which is within the range of the optimum reported by Hart et al. [
22]. The addition of the H-donor solvent caused negligible impact on the API gravity of the THAI feed oil, while the resultant effect on viscosity due to thinning was less than a 10% decrease. The upgraded oil and gas flowed into the oil–gas separator, where the upgraded oil was collected from the bottom and the flashed-off gas was either sent to gas chromatograph (GC) configured as a refinery gas analyzer (RGA) or vented. The upgraded oil came out foamy, signifying that the oil and gas were well mixed in the mixing chamber and the reactor. Before the start of each experiment, the 28 g NiMo/Al
2O
3 catalyst in the mixed bed was reduced in situ, using hydrogen gas flow of 50 mL/min and pressure of 5 bar at 300 °C for 30 min. This procedure converts the nickel and molybdenum components of the catalyst from their oxide state to active metals. Moreover, since the sulfur content of the THAI feed oil is high (
Table 2), it is believed that in situ sulfidation also occurred during the catalytic upgrading, converting the nickel and molybdenum into their respective sulfide state [
9,
11,
26].
3.3. Analytical Techniques
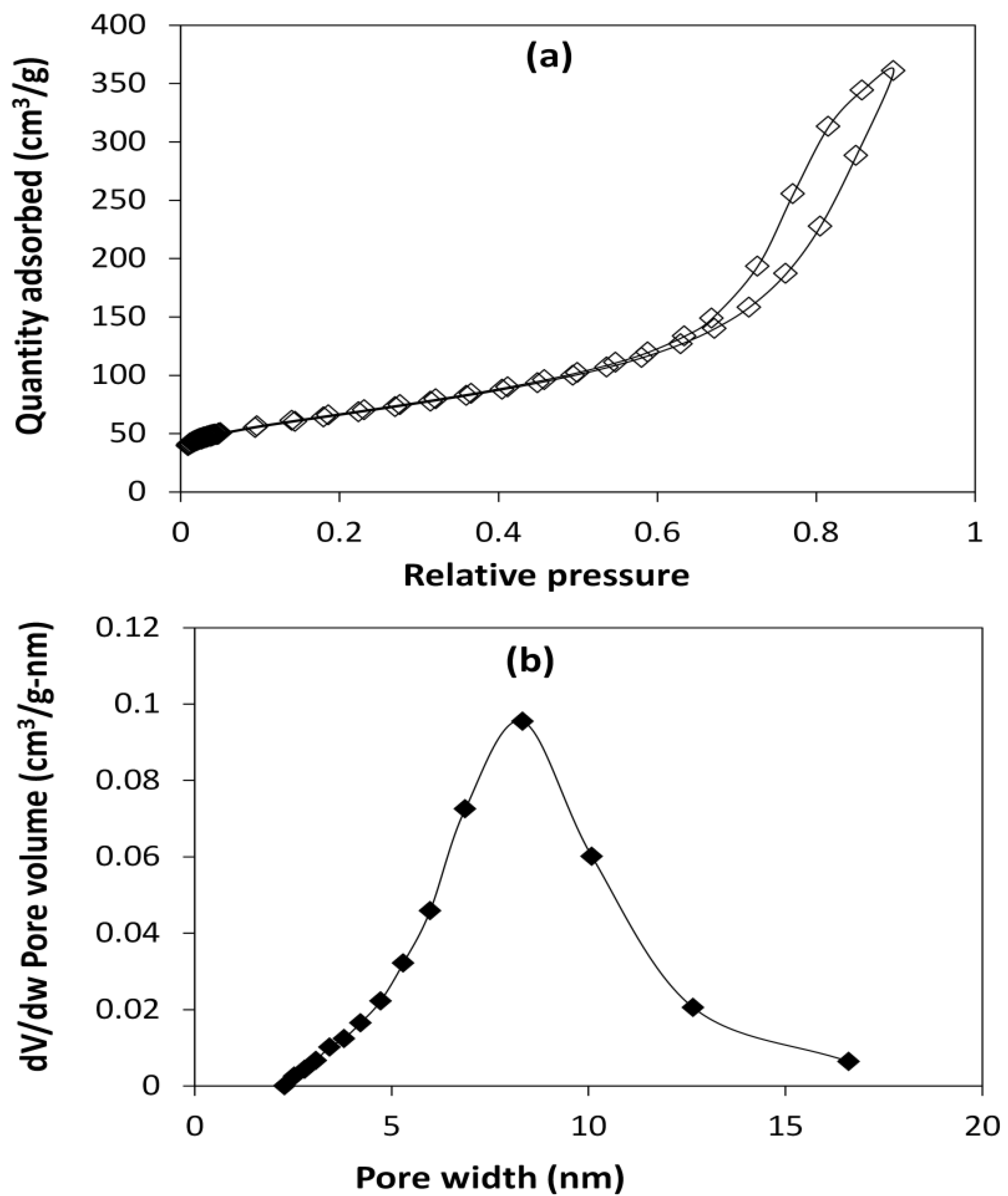
A Micromeritics Analytical Instrument ASAP® 2010 was used to perform nitrogen adsorption–desorption cycles, and catalyst surface area was determined by fitting the Brunauer–Emmett–Teller (BET) model to the data, while the Barrett–Joyner–Halenda (BJH) equation was used to determine the pore size distribution. The number and strength of the acid sites of the NiMo/Al2O3 catalyst used was determined by temperature programmed desorption (TPD), using Micromeritics AutoChem II 2920 analyzer (Micromeritics Instrument Corporation, Norcross, GA, USA), tert-butylamine (CH3)3CNH2 g−1) and 0.2 g of catalyst. A ramp temperature from 25 to 500 °C at 10 °C/min for 60 min under argon gas flow 50 mL/min was used, and it was desorbed with helium gas flow 50 mL/min. A portable density meter Anton Parr DMA 35 (Anton Paar GmbH, Graz, Austria) was used to measure the API gravity of the oil samples. The viscosity of the oil samples was measured with the rheometer Bohlin CVO 50NF (Malvern Instruments Ltd., Worcester, UK) at 20 °C, using parallel plate geometry with 100 μm gap and 100 s−1 constant shear rate. The asphaltene content of the oil samples was precipitated by mixing 1 g of oil to 40 mL n-heptane, and carried out in accordance to American Society for Testing and Material (ASTM) method D3279.
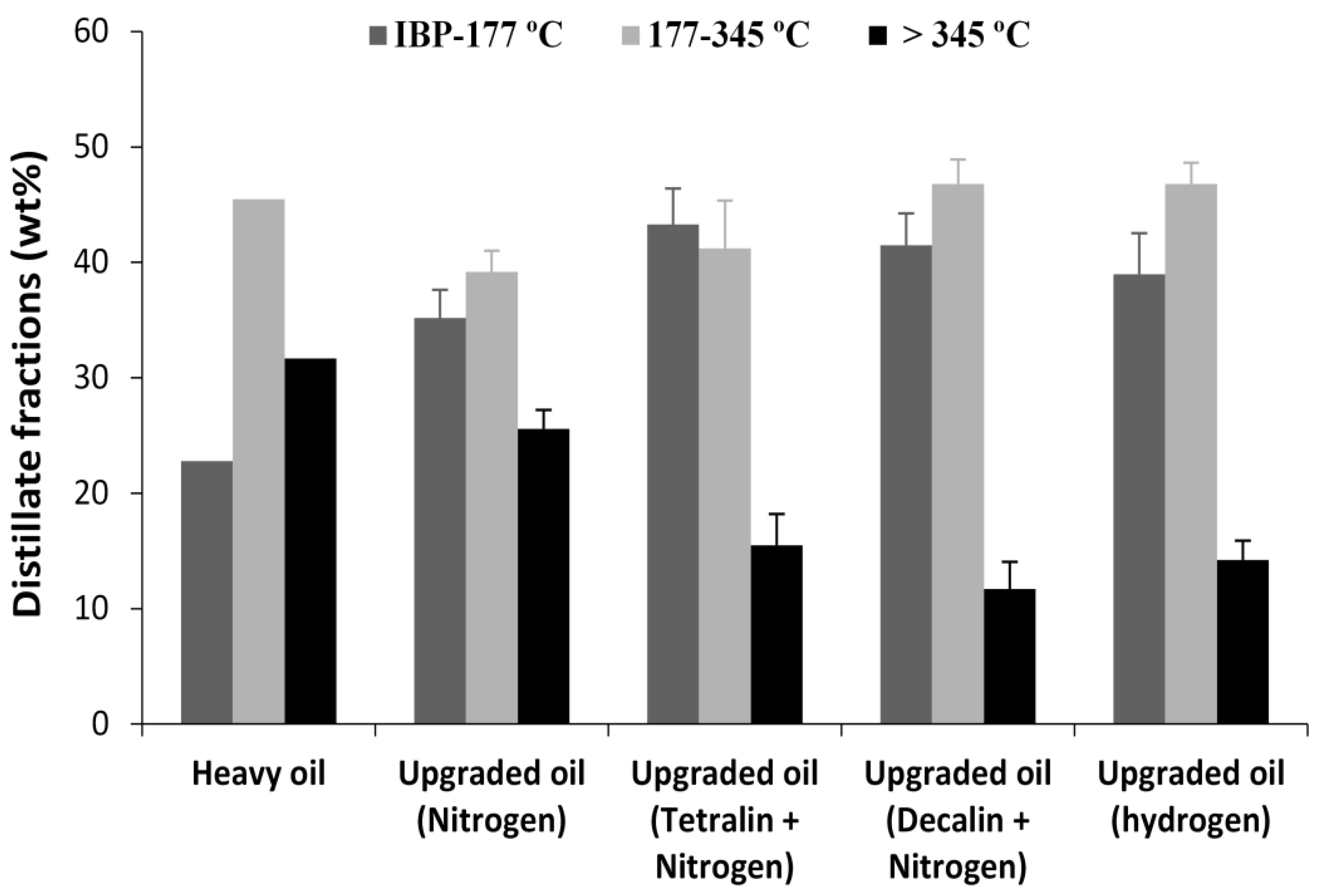
Diaz and Yarranton [
36] reported that calibrated simulated distillation (SimDist) with paraffinic standards, and conventional reference oil is not suitable for heavy oil and bitumen characterization. Hence, the true boiling point (TBP) curves of the oil samples was obtained by using a Thermogravimetric Analyzer, TGA (TG 209 F1 Iris
® instrument, NETZSCH-Geratebau GmbH, Selb, Germany), with the temperature ramped from 25 to 345 °C, at a heating rate of 10 °C/min, and an isothermal 345 °C for 5 min, to ensure complete vaporization of the middle distillate fractions under nitrogen flowrate of 40 mL/min. The measured weight loss due to evaporation of light and middle distillate fractions as the sample is heated in an inert atmosphere (N
2), gives similar data to distillation curve obtained using ASTM method D86 [
28,
37], and also with SimDist using ASTM method D2892 [
28]. However, beyond 350 °C, complex cracking reactions begins to occur [
38]. Hence, to avoid thermal cracking reactions, the TGA was conducted only to the limit of boiling temperature of the middle distillate fractions (345 °C). Nonetheless, to ensure accuracy, the sample size was very small (8 mg), in order to allow a quick exit of the vaporized portion, as well as ensure weight loss matches component boiling point distribution.
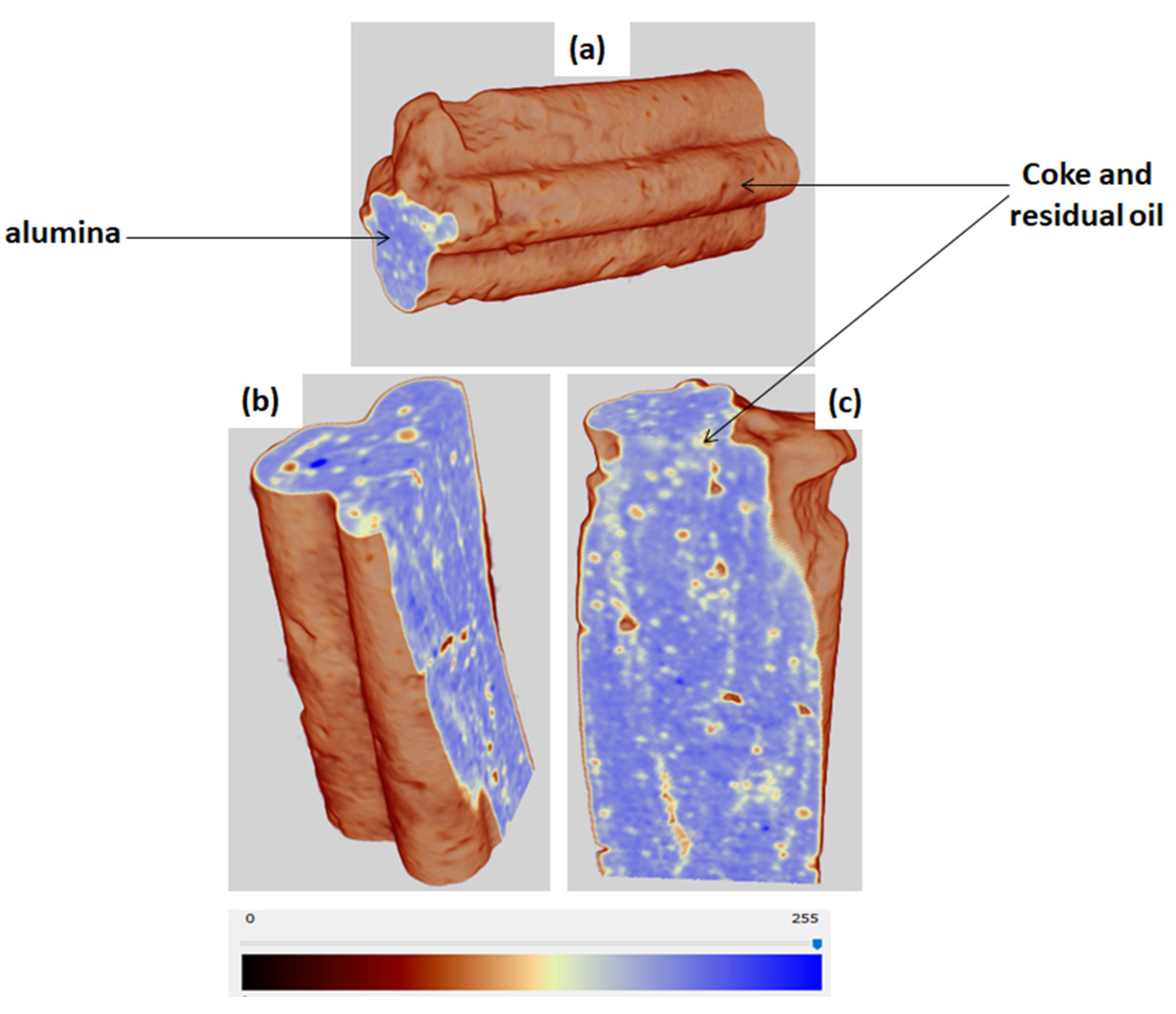
The coke deposited on the catalyst after upgrading experiments was determined by using TGA programmed as follows: a ramped temperature from 25 to 900 °C, at a heating rate of 10 °C/min, and an isothermal temperature of 900 °C for 10 min, under air flowrate of 50 mL/min. The microstructure and density of the spent catalyst pellet were examined using X-ray Computed Nanotomography, X-ray nano-CT (Bruker SkyScan 2211, Billerica, MA, USA).The scan was carried out without any special sample preparation, using microfocus mode, voltage 85 kV, emission current 210 μA, exposure time 130 ms, image pixel size 7 μm and rotation step 0.2°.
4. Conclusions
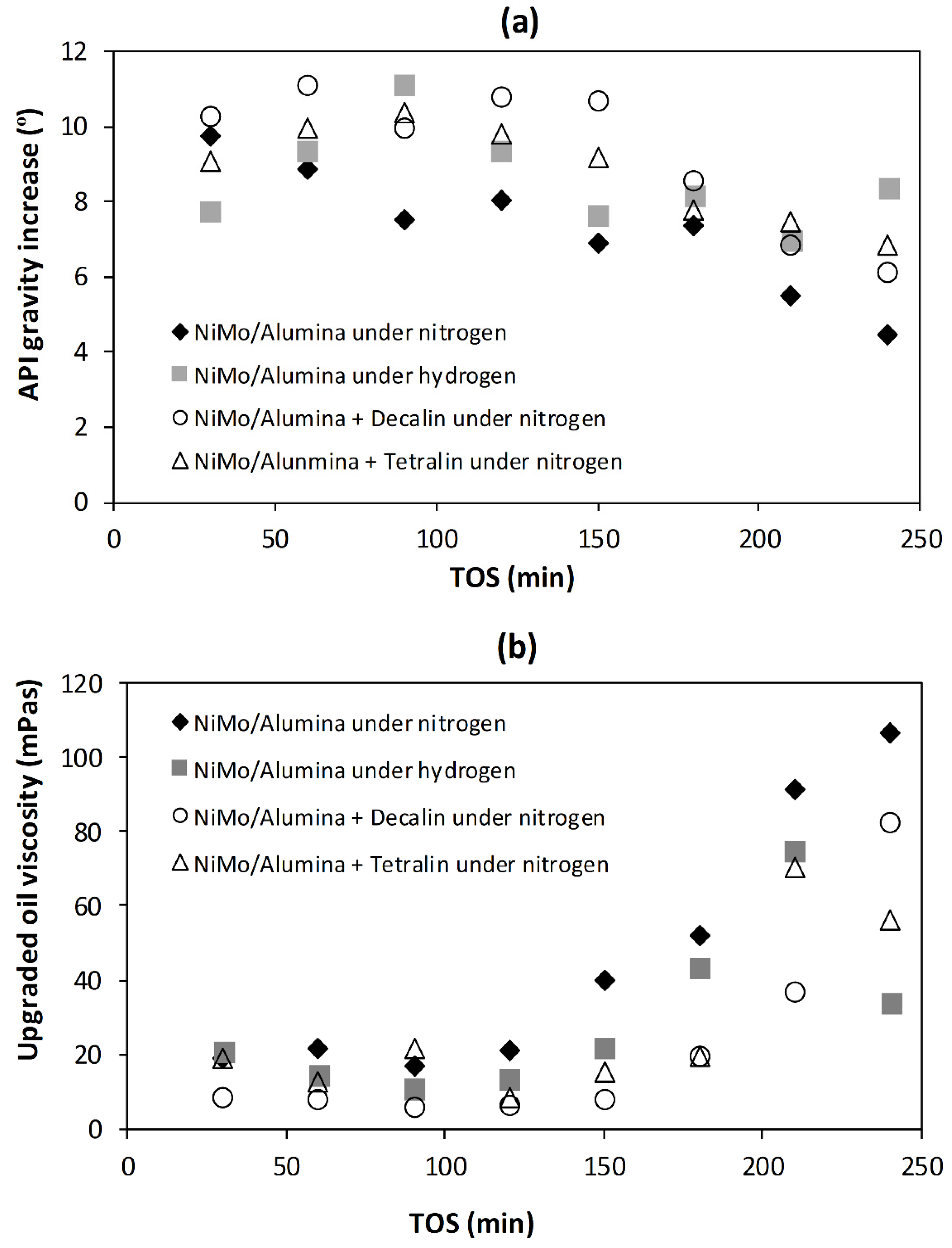
The effects of tetralin and decalin as H-donor solvents in an inductively heated catalytic upgrading of heavy oil were investigated at a temperature 425 °C, pressure 15 bar, catalyst/steel balls 66% (v/v), H-donor/oil 0.045 (g/g) and LHSV 0.75 h−1. The results were evaluated with those obtained under nitrogen gas and hydrogen gas environments, and also against previously reported results for the same THAI oil and experimental conditions with conventional heating under nitrogen gas reaction medium alone. It was found that under nitrogen gas medium alone, inductively heated catalytic bed produced upgraded oils with API gravity increase with TOS in the range of 4.5–9.7°, while conventional heating achieved 2.2–7.3° above 12.8° for the THAI oil. Likewise, viscosity was 45 mPas (induction heating) against 121 mPas (conventional heating) relative to 863 mPas for the THAI heavy oil. The addition of hydrogen gas achieved a 1.4° point increase above 7.3° (nitrogen gas alone), while the incorporation H-donor solvents further increased the API gravity by 1.5° (tetralin) and 2° (decalin), respectively. The formation of coke was suppressed under hydrogen gas and H-donor solvents in the following order: nitrogen gas (22%) < tetralin (17%) < hydrogen gas (14%) < decalin (13.4%). It is clear that decalin suppressed coke formation slightly better than hydrogen gas, and this difference can be attributed to hydrogen gas solubility in the oil under the prevailing pressure and temperature, and the ability of the decalin to solubilized the asphaltene while the liberated hydrogen halt free radicals polymerization to form stable and lower molecular weight hydrocarbon molecules. Additionally, the endothermic nature of the reaction resulting in hydrogen liberation from H-donor solvents, such as tetralin and decalin, and the ability of the induction heating method to heat up the catalytic bed rapidly in response to changes provides a synergistic effect that promotes further hydrocracking and hydrogenation. This reduction of coke formation under a hydrogen-rich environment proves the role of hydrogen in catalyst longevity and quality of produced oil during catalytic upgrading of heavy oil. Conclusively, a pelleted catalyst with micro- and meso-pores suffers pore-mouth plugging, leading to rapid loss of catalytic activity, which is reflected in API gravity decrease within 150 min TOS, suggesting macro-porous catalyst to accommodate species such as resins and asphaltenes, or dispersed nano-sized catalyst to avoid diffusion limitation, pore-mouth blockage and expose the macromolecular weight species to the active sites during catalytic upgrading of heavy oil.