Influence of TiO2 Morphology and Crystallinity on Visible-Light Photocatalytic Activity of TiO2-Bi2O3 Composite in AOPs
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of Synthesized Photocatalysts
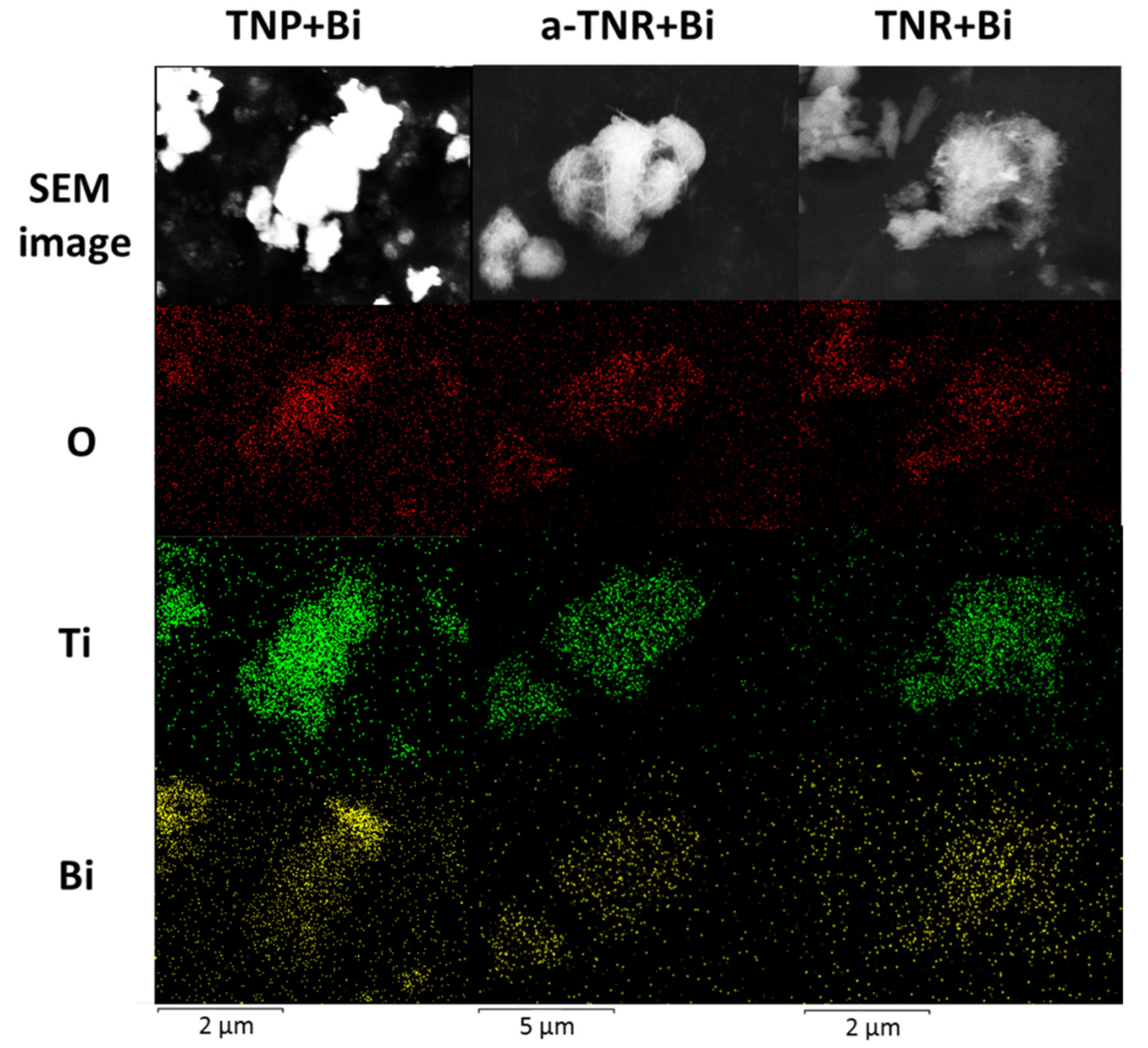
2.1.1. SEM-EDX and Nitrogen Adsorption-Desorption Analysis
2.1.2. XRD Analysis
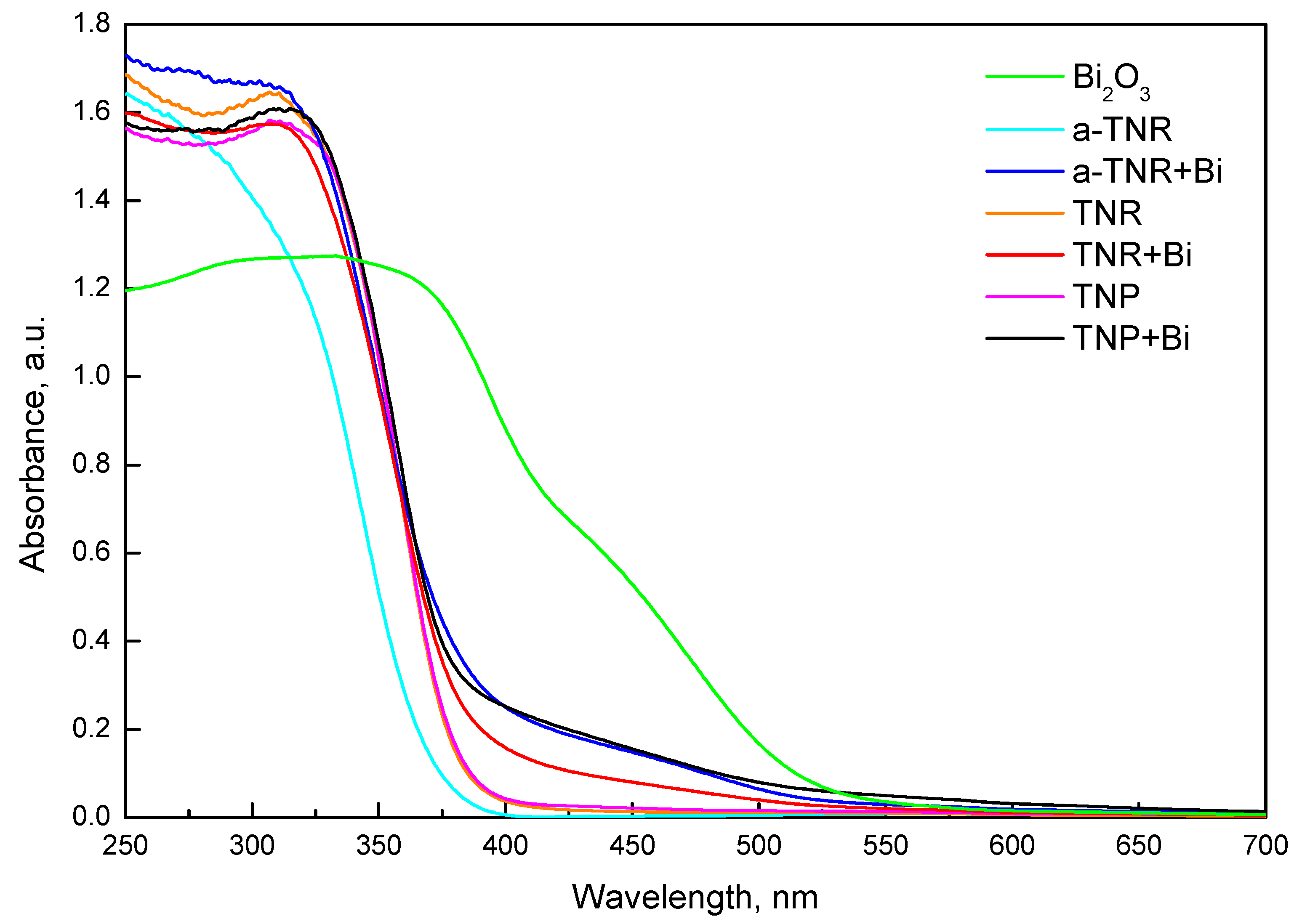
2.1.3. UV-Vis Diffuse Reflectance (UV-Vis DR) Analysis
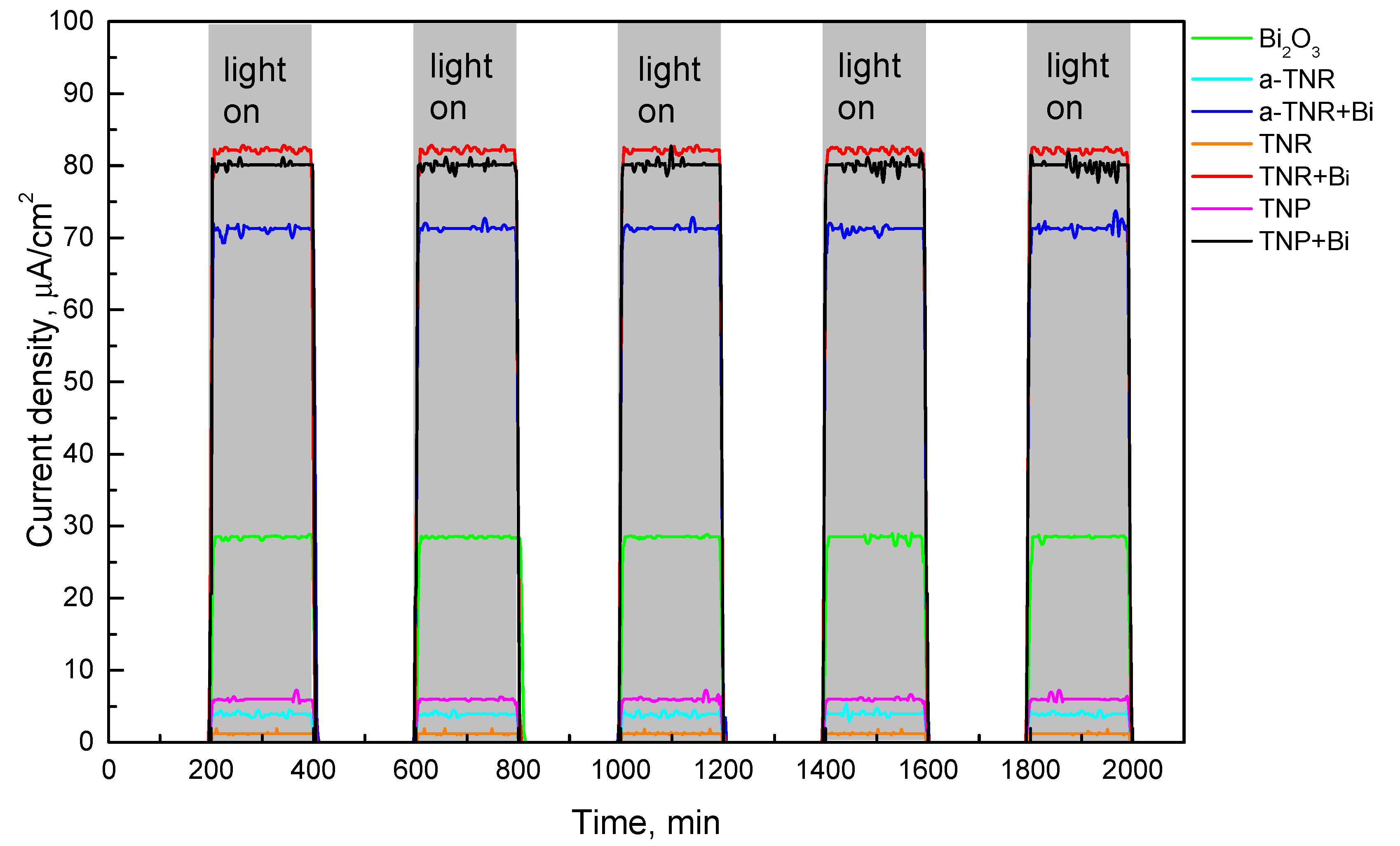
2.1.4. Photo-Electrochemical Measurements
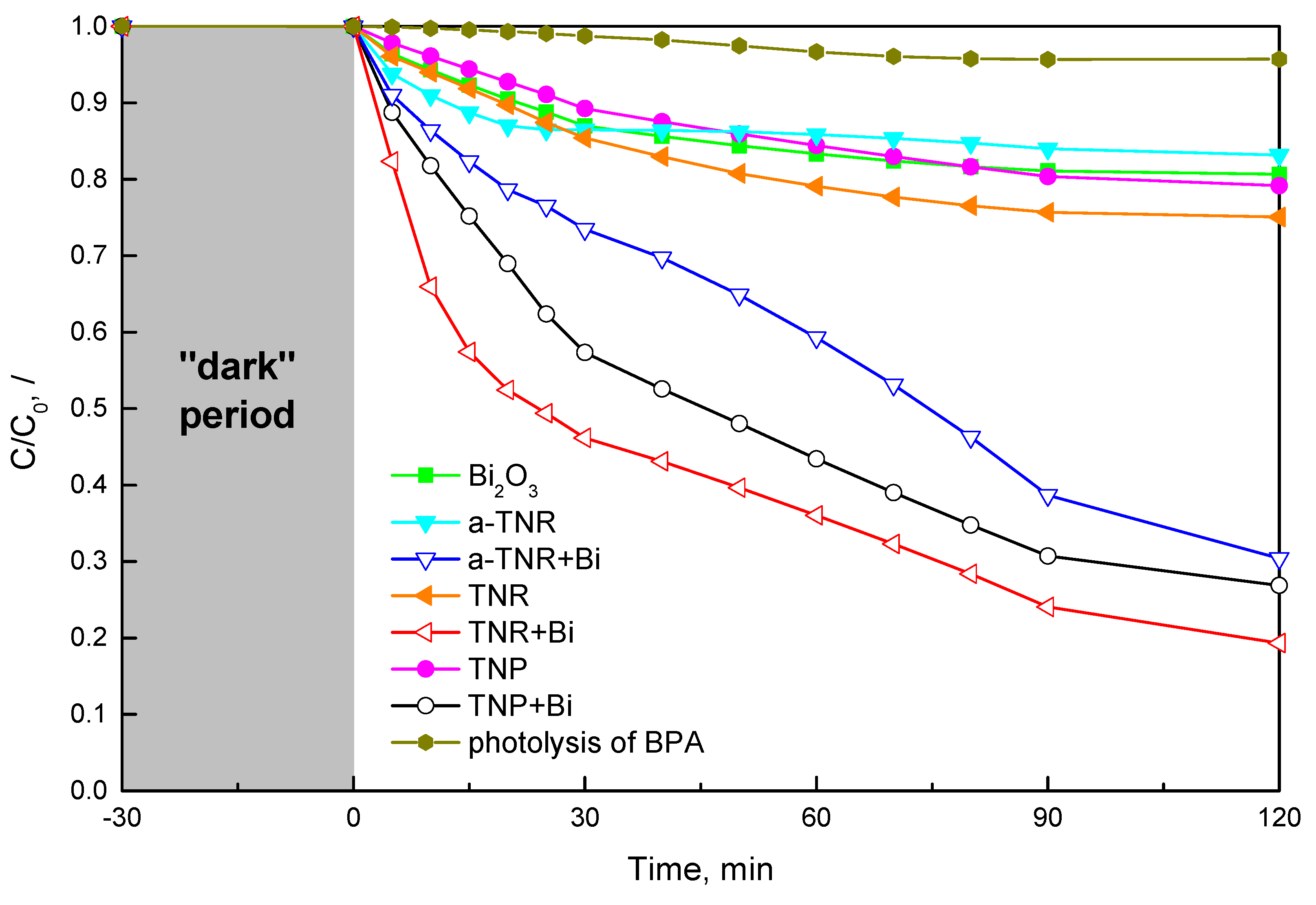
2.2. Photocatalytic BPA Oxidation
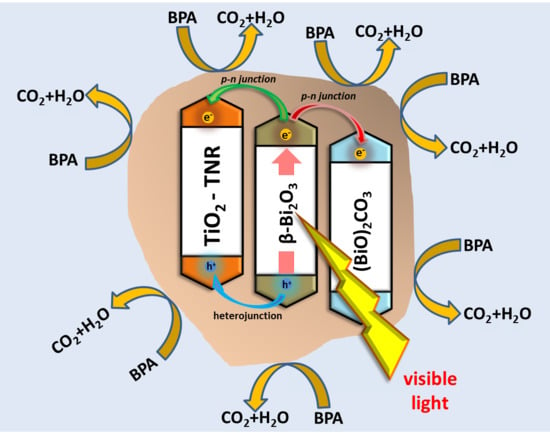
2.3. Proposed Charge Carrier Migration Cascade
3. Materials and Methods
3.1. Catalyst Preparation
3.1.1. TiO2 Support Preparation
3.1.2. Bi2O3 and TiO2–Bi2O3 Composite Preparation
3.2. Characterization Methods
3.3. Catalyst Activity Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhao, J.C.; Wu, K.Q.; Oikawa, K.; Hidaka, H.; Serpone, N. Photoassisted Degradation of Dye Pollutants. 3. Degradation of the Cationic Dye Rhodamine B in Aqueous Anionic Surfactant/TiO2 Dispersions under Visible Light Irradiation: Evidence for the Need of Substrate Adsorption on TiO2 Particles. Environ. Sci. Technol. 1998, 32, 2394–2400. [Google Scholar] [CrossRef]
- Andreozzi, R.; Caprio, V.; Insola, A.; Marotta, R. Advanced oxidation processes (AOP) for water purification and recovery. Catal. Today 1999, 53, 51–59. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, R. Advanced Oxidation Processes (AOPs) in Wastewater Treatment. Curr. Pollut. Rep. 2015, 1, 167–176. [Google Scholar] [CrossRef]
- Hashimoto, K.; Irie, H.; Fujishima, A. TiO2 photocatalysis: A historical overview and future prospects. Jpn. J. Appl. Phys. 2005, 12, 8269–8285. [Google Scholar] [CrossRef]
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photoch. Photobiol. C 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Ge, M.; Cao, C.; Huang, J.; Li, S.; Chen, Z.; Zhang, K.-Q.; Al-Deyab, S.S.; Ali, Y. A review of one-dimensional TiO2 nanostructured materials for environmental and energy applications. J. Mater. Chem. A 2016, 4, 6772–6801. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, Q.; Feng, Z.; Li, M.; Li, C. Importance of the Relationship between Surface Phases and Photocatalytic Activity of TiO2. Angew. Chem. 2008, 9, 1766–1769. [Google Scholar] [CrossRef]
- Magalhães, P.; Andrade, L.; Nunes, O.C.; Mendes, A. Titanium Dioxide Photocatalysis: Fundamentals and Application on Photoinactivation. Rev. Adv. Mater. Sci. 2017, 51, 91–129. [Google Scholar]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Jiang, H.Y.; Cheng, K.; Lin, J. Crystalline metallic Au nanoparticle-loaded α-Bi2O3 microrods for improved photocatalysis. Phys. Chem. Chem. Phys. 2012, 14, 12114–12121. [Google Scholar] [CrossRef]
- Hameed, A.; Montini, T.; Gombac, V.; Fornasiero, P. Surface Phases and Photocatalytic Activity Correlation of Bi2O3/Bi2O4-x Nanocomposite. J. Am. Chem. Soc. 2008, 130, 9658–9659. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Huang, X.; Hu, T.; Wang, J.; Zhang, W.; Zhang, J. Synthesis of three-dimensionally ordered macroporous composite Ag/Bi2O3–TiO2 by dual templates and its photocatalytic activities for degradation of organic pollutants under multiple modes. New J. Chem. 2014, 38, 5293–5302. [Google Scholar] [CrossRef]
- Wu, Y.; Lu, G.; Li, S. The Doping Effect of Bi on TiO2 for Photocatalytic Hydrogen Generation and Photodecolorization of Rhodamine B. J. Phys. Chem. C 2009, 113, 9950–9955. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, H.J.; Qu, J.H. Photoelectrocatalytic degradation of organic contaminants at Bi2O3/TiO2 nanotube array electrode. Appl. Surf. Sci. 2011, 257, 4621–4624. [Google Scholar] [CrossRef]
- Wei, N.; Cui, H.; Wang, C.; Zhang, G.; Song, Q.; Sun, W.; Song, X.; Sun, M.; Tian, J. Bi2O3 nanoparticles incorporated porous TiO2 films as an effective p-n junction with enhanced photocatalytic activity. J. Am. Ceram. Soc. 2017, 100, 1339–1349. [Google Scholar] [CrossRef]
- Huang, Y.; Wei, Y.; Wang, J.; Luo, D.; Fan, L. Controllable fabrication of Bi2O3/TiO2 heterojunction with excellent visible-light responsive photocatalytic performance. J. Appl. Surf. Sci. 2017, 423, 119–130. [Google Scholar] [CrossRef]
- Reddy, N.L.; Emin, S.; Valant, M.; Shankar, M.V. Nanostructured Bi2O3/TiO2 photocatalyst for enhanced hydrogen production. Int. J. Hydrogen Energy 2017, 42, 6627–6636. [Google Scholar] [CrossRef]
- La, J.; Huang, Y.; Luo, G.; Lai, J.; Liu, C.; Chu, G. Synthesis of bismuth oxide nanoparticles by solution combustion method. Particul. Sci. Technol. 2013, 31, 287–290. [Google Scholar] [CrossRef]
- Anilkumara, M.; Pasricha, R.; Ravic, V. Synthesis of bismuth oxide nanoparticles by citrate gel method. Ceram. Int. 2005, 31, 889–891. [Google Scholar] [CrossRef]
- Mallahi, M.; Shokuhfar, A.; Vaezi, M.R.; Esmaeilirad, A.; Mazinani, V. Synthesis and characterization of Bismuth oxide nanoparticles via sol-gel method. Am. J. Eng. Res. 2014, 3, 162–165. [Google Scholar]
- Wu, C.; Shen, L.; Huang, Q.; Zhang, Y.-C. Hydrothermal synthesis and characterization of Bi2O3 nanowires. Mater. Lett. 2011, 65, 1134–1136. [Google Scholar] [CrossRef]
- Yang, Q.; Li, Y.; Yin, Q.; Wang, P.; Cheng, Y. Hydrothermal synthesis of bismuth oxide needles. Mater. Lett. 2002, 55, 46–49. [Google Scholar] [CrossRef]
- Hernandez-Delgadillo, R.; Velasco-Arias, D.; Martinez-Sanmiguel, J.J.; Diaz, D.; Zumeta-Dube, I.; Arevalo-Niño, K.; Cabral-Romero, C. Bismuth oxide aqueous colloidal nanoparticles inhibit Candida albicans growth and biofilm formation. Int. J. Nanomed. 2013, 8, 1645–1652. [Google Scholar]
- De Sousa, V.C.; Morelli, M.R.; Kiminami, R.H.G. Bismuth oxide aqueous colloidal nanoparticles inhibit Candida albicans growth and biofilm formation. Ceram. Int. 2000, 26, 561–564. [Google Scholar] [CrossRef]
- Zywitzki, D.; Jing, H.; Tuysuz, H.; Chan, C.K.J. High surface area, amorphous titania with reactive Ti3+ through a photo-assisted synthesis method for photocatalytic H2 generation. Mater. Chem. A 2017, 5, 10957–10967. [Google Scholar] [CrossRef]
- Ohtani, B.; Ogawa, Y.; Nishimoto, S. Photocatalytic activity of amorphous—Anatase mixture of titanium (IV) oxide particles suspended in aqueous solutions. J. Phys. Chem. B 1997, 100, 3746–3752. [Google Scholar] [CrossRef]
- Tanaka, K.; Capule, M.F.V.; Hisanaga, T. Effect of crystallinity of TiO2 on its photocatalytic action. Chem. Phys. Lett. 1991, 187, 73–76. [Google Scholar] [CrossRef]
- Stone, V.F.; Davis, R.J. Synthesis, characterization, and photocatalytic activity of titania and niobia mesoporous molecular sieves. Chem. Mater. 1998, 10, 1468–1474. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, Q. Effects of amorphous contents and particle size on the photocatalytic properties of TiO2 nanoparticles. Scr. Mater. 2001, 44, 1195–1198. [Google Scholar] [CrossRef]
- Li, J.; Chen, C.; Zhao, J.; Zhu, H.; Orthman, J. Photodegradation of dye pollutants on TiO2 nanoparticles dispersed in silicate under UV–VIS irradiation. J. Appl. Catal. B Environ. 2002, 37, 331–338. [Google Scholar] [CrossRef]
- Randorn, C.; Wongnawa, S.; Boonsin, P. Bleaching of methylene blue by hydrated titanium dioxide. ScienceAsia 2004, 30, 149–156. [Google Scholar] [CrossRef]
- Liu, A.R.; Wang, S.M.; Zhao, Y.R.; Zheng, Z. Low-temperature preparation of nanocrystalline TiO2 photocatalyst with a very large specific surface area. Mater. Chem. Phys. 2006, 99, 131–134. [Google Scholar] [CrossRef]
- Žerjav, G.; Arshad, M.S.; Djinović, P.; Zavašnik, J.; Pintar, A. Electron trapping energy states of TiO2–WO3 composites and their influence on photocatalytic degradation of bisphenol A. Appl. Catal. B Environ. 2017, 2009, 273–284. [Google Scholar] [CrossRef]
- Kominami, H.; Oki, K.; Kohno, M.; Onoue, S.I.; Kera, Y.; Ohtani, B. Novel solvothermal synthesis of niobium(V)oxide powders and their photocatalytic activity in aqueous suspensions. J. Mater. Chem. 2001, 11, 604–609. [Google Scholar] [CrossRef]
- Benmami, M.; Chhor, K.; Kanaev, A.V. Supported nanometric titanium oxide sols as a new efficient photocatalyst. J. Phys. Chem. B 2005, 109, 19766–19771. [Google Scholar] [CrossRef]
- Wu, C.; Zhao, X.; Ren, Y.; Yue, Y.; Hua, W.; Cao, Y.; Tang, Y.; Gao, Z. Gas-phase photo-oxidations of organic compounds over different forms of zirconia. J. Mol. Catal. A Chem. 2005, 229, 233–239. [Google Scholar] [CrossRef]
- Zhang, Z.; Maggard, P.A. Investigation of photocatalytically-active hydrated forms of amorphous titania, TiO2 × nH2O. J. Photochem. Photobiol. A 2007, 186, 8–13. [Google Scholar] [CrossRef]
- Li, J.; Liu, S.; He, Y.; Wang, J. Adsorption and degradation of the cationic dyes over Co doped amorphous mesoporous titania–silica catalyst under UV and visible light irradiation. Microporous Mesoporous Mater. 2008, 115, 416–425. [Google Scholar] [CrossRef]
- Li, Y.; Sasaki, T.; Shimizu, Y.; Koshizaki, N. Hexagonal-Close-Packed, Hierarchical Amorphous TiO2 Nanocolumn Arrays: Transferability, Enhanced Photocatalytic Activity, and Superamphiphilicity without UV Irradiation. J. Am. Chem. Soc. 2008, 130, 14755–14762. [Google Scholar] [CrossRef]
- Tueysuez, H.; Chan, C.K. Preparation of amorphous and nanocrystalline sodium tantalum oxide photocatalysts with porous matrix structure for overall water splitting. Nano Energy 2013, 2, 116–123. [Google Scholar] [CrossRef]
- Grewe, T.; Tueysuez, H. Designing photocatalysts for hydrogen evolution: Are complex preparation strategies necessary to produce active catalysts? ChemSusChem 2015, 8, 3084–3091. [Google Scholar] [CrossRef]
- Erjavec, B.; Kaplan, R.; Pintar, A. Effects of heat and peroxide treatment on photocatalytic activity of titanate nanotubes. Catal. Today 2015, 241, 15–24. [Google Scholar] [CrossRef]
- Žerjav, G.; Arshad, M.S.; Djinović, P.; Junkar, I.; Kovač, J.; Zavašnik, J.; Pintar, A. Improved electron–hole separation and migration in anatase TiO2 nanorod/reduced graphene oxide composites and their influence on photocatalytic performance. Nanoscale 2017, 9, 4578–4592. [Google Scholar] [CrossRef] [PubMed]
- Astuti, Y.; Fauziyah, A.; Nurhayati, S.; Wulansari, A.D.; Andianingrum, R.; Hakim, A.R.; Bhaduri, G. Synthesis of α-Bismuth oxide using solution combustion method and its photocatalytic properties. IOP Conf. Ser. Mater. Sci. Eng. 2016, 107, 12006–12013. [Google Scholar] [CrossRef]
- Zhu, G.; Liu, Y.; Hojamberdiev, M.; Han, J.; Rodríguez, J.; Bilmes, S.A.; Liu, P. Thermodecomposition synthesis of porous β-Bi2O3/Bi2O2CO3 heterostructured photocatalysts with improved visible light photocatalytic activity. New J. Chem. 2015, 39, 9557–9568. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, P.; Liu, J.; Yu, J. New understanding of the difference of photocatalytic activity among anatase, rutile and brookite TiO2. Phys. Chem. Chem. Phys. 2014, 16, 20382–20386. [Google Scholar] [CrossRef]
- Dette, C.; Pérez-Osorio, M.A.; Kley, C.S.; Punke, P.; Patrick, C.E.; Jacobson, P.; Giustino, F.; Jung, S.J.; Kern, K. TiO2 Anatase with a Bandgap in the Visible Region. Nano Lett. 2014, 14, 6533–6538. [Google Scholar] [CrossRef]
- Rahman, M.; MacElroy, D.; Dowling, D.P. Influence of the physical, structural and chemical properties on the photoresponse property of magnetron sputtered TiO2 for the application of water splitting. J. Nanosci. Nanotechnol. 2011, 11, 8642–8651. [Google Scholar] [CrossRef]
- Butler, M.A.; Ginley, D.S. Prediction of Flatband Potentials at Semiconductor-Electrolyte Interfaces from Atomic Electronegativities. J. Electrochem. Soc. 1978, 125, 228–232. [Google Scholar] [CrossRef]
- Xu, Y.; Schoonen, M.A.A. The absolute energy positions of conduction and valence bands of selected semiconducting minerals. Am. Mineral. 2000, 85, 543–556. [Google Scholar] [CrossRef]
- Abe, R.; Takami, H.; Murani, N.; Ohtani, B. Pristine simple oxides as visible light driven photocatalysts: Highly efficient decomposition of organic compounds over platinum-loaded tungsten oxide. J. Am. Chem. Soc. 2008, 130, 7780–7781. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wan, L.; Sun, C.; Wang, X.; Chen, Z.; Smith, S.C.; Cheng, H.M.; Lu, G.Q. Band-to-band visible-light photon excitation and photoactivity induced by homogeneous nitrogen doping in layered titanates. Chem. Mater. 2009, 21, 1266–1274. [Google Scholar] [CrossRef]
- Ayekoe, P.Y.; Robert, D.; Gone, D.L. Environ. Preparation of effective TiO2/Bi2O3 photocatalysts for water treatment. Chem. Lett. 2016, 14, 387–393. [Google Scholar] [CrossRef]
- Yi, S.; Yue, X.; Xu, D.; Liu, Z.; Zhao, F.; Wangab, D.; Lin, Y. Study on photogenerated charge transfer properties and enhanced visible-light photocatalytic activity of p-type Bi2O3/n-type ZnO heterojunctions. New J. Chem. 2015, 39, 2917–2924. [Google Scholar] [CrossRef]
- Zhang, Z.; Shao, C.; Li, X.; Wang, C.; Zhang, M.; Liu, Y. Electrospun Nanofibers of p-Type NiO/n-Type ZnO Heterojunctions with Enhanced Photocatalytic Activity. ACS Appl. Mater. Interfaces 2010, 2, 2915–2923. [Google Scholar] [CrossRef]
- Dai, G.; Yu, J.; Liu, G. Synthesis and Enhanced Visible-Light Photoelectrocatalytic Activity of p−n Junction BiOI/TiO2 Nanotube Arrays. J. Phys. Chem. C 2011, 115, 7339–7346. [Google Scholar] [CrossRef]

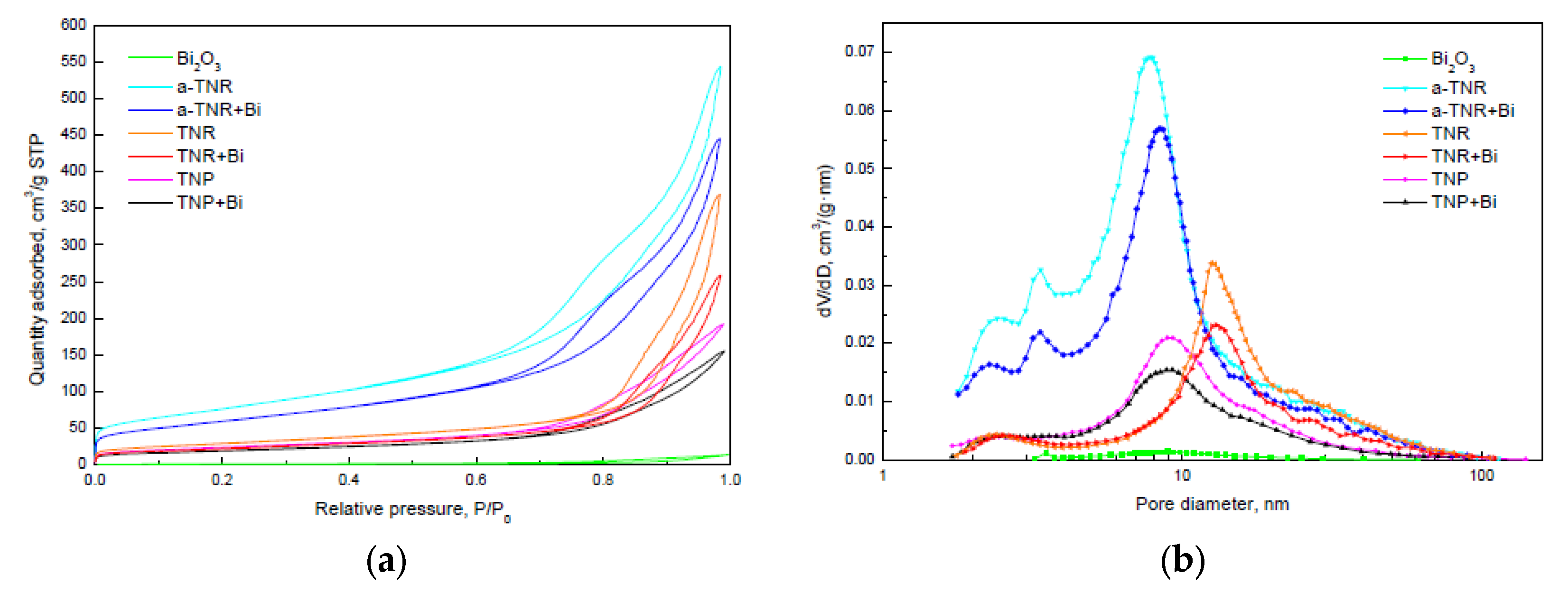
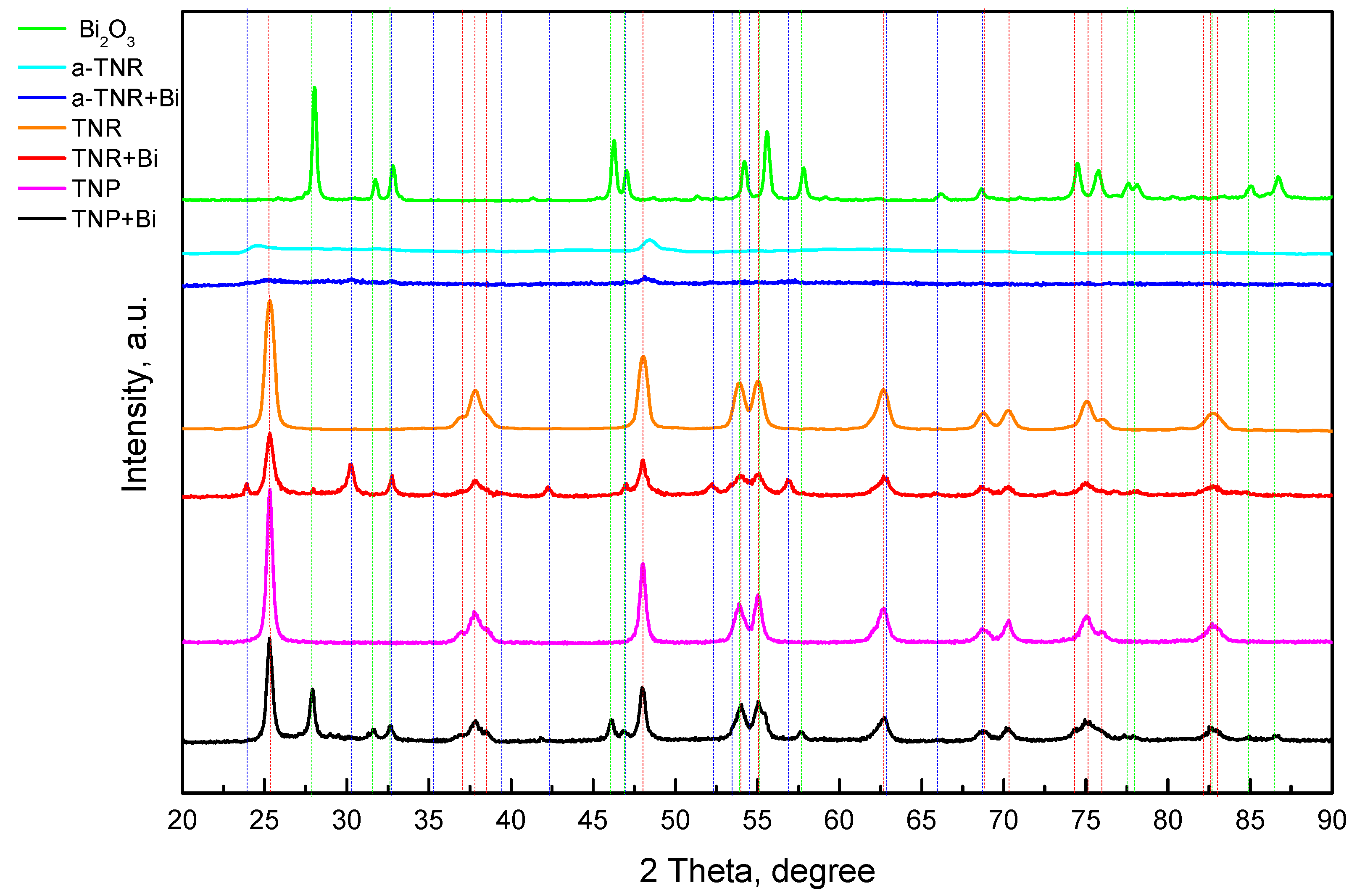
| Sample | SBET (m2/g) | dpore (nm) | Vpore (cm3/g) | Average Crystallite Size (nm) | ||
|---|---|---|---|---|---|---|
| Anatase TiO2 | Tetragonal Β-Bi2O3 | (Bio)2CO3 | ||||
| TNP | 86 | 13.7 | 0.29 | 20 | - | - |
| TNP + Bi | 70 | 13.4 | 0.25 | 20 | 25 | - |
| a-TNR | 278 | 10.5 | 0.85 | - | - | - |
| a-TNR + Bi | 217 | 11.1 | 0.69 | - | a N.D. | a N.D. |
| TNR | 105 | 19.3 | 0.57 | 14 | - | - |
| TNR + Bi | 81 | 17.5 | 0.40 | 15 | N.D. | 18 |
| Bi2O3 | 4.7 | 16.5 | 0.02 | - | 31 | - |
| Sample | Content (wt. %) | Ti:Bi Actual Ratio * | ||
|---|---|---|---|---|
| O | Ti | Bi | ||
| TNP + Bi | 45 | 40 | 15 | 1:0.37 |
| a-TNR + Bi | 40 | 41 | 19 | 1:0.46 |
| TNR + Bi | 44 | 43 | 13 | 1:0.30 |
| Sample | TCfresh | TCspent | TCspent-TCfresh | TOCR | TOCM | TOCA |
|---|---|---|---|---|---|---|
| (%) | ||||||
| TNP | 0.16 | 0.20 | 0.04 | 0 | - | - |
| TNP + Bi | 0.16 | 0.32 | 0.16 | 23.0 | 20.0 | 3.0 |
| a-TNR | 0.86 | 1.6 | 0.74 | 0 | - | - |
| a-TNR + Bi | 0.90 | 1.2 | 0.3 | 21.0 | 16.0 | 5.0 |
| TNR | 0.23 | 0.68 | 0.45 | 11.0 | 4.0 | 7.0 |
| TNR + Bi | 0.36 | 0.87 | 0.51 | 51.0 | 43.0 | 8.0 |
| Bi2O3 | 0.34 | 0.5 | 0.26 | 4.0 | 1.4 | 2.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Žerjav, G.; Pintar, A. Influence of TiO2 Morphology and Crystallinity on Visible-Light Photocatalytic Activity of TiO2-Bi2O3 Composite in AOPs. Catalysts 2020, 10, 395. https://doi.org/10.3390/catal10040395
Žerjav G, Pintar A. Influence of TiO2 Morphology and Crystallinity on Visible-Light Photocatalytic Activity of TiO2-Bi2O3 Composite in AOPs. Catalysts. 2020; 10(4):395. https://doi.org/10.3390/catal10040395
Chicago/Turabian StyleŽerjav, Gregor, and Albin Pintar. 2020. "Influence of TiO2 Morphology and Crystallinity on Visible-Light Photocatalytic Activity of TiO2-Bi2O3 Composite in AOPs" Catalysts 10, no. 4: 395. https://doi.org/10.3390/catal10040395
APA StyleŽerjav, G., & Pintar, A. (2020). Influence of TiO2 Morphology and Crystallinity on Visible-Light Photocatalytic Activity of TiO2-Bi2O3 Composite in AOPs. Catalysts, 10(4), 395. https://doi.org/10.3390/catal10040395