Abstract
BaClxFy as well as BaF2 and BaClF catalysts were prepared by solid-state reaction at room temperature with Ba(OH)2 as the precursor and NH4F/NH4Cl as the F and Cl sources. The catalysts were applied for the dehydrochlorination of 1-chloro-1,1-difluoroethane to vinylidene fluoride at 350 °C. The industrial manufacture of vinylidene fluoride (VDF) is carried out at 600–700 °C, whereas the BaClxFy catalysts provided a promising pathway to produce VDF at much lower temperatures. Unfortunately, the selectivity of VDF over BaF2 decreased from 94% to 84% along with the deactivation of the BaF2 catalyst monotonically. In the presence of small amounts of Cl in BaF2, stabilized selectivity was achieved. Over BaCl0.05F0.95, BaCl0.1F0.9 and BaCl0.25F0.75, no decrease in VDF selectivity was observed. Clearly, the presence of small amounts Cl during solid-state preparation inhibited the growth of BaF2 crystalline significantly. Far smaller particles were achieved. The particle size, or more precisely, the crystal size of the barium catalyst played a major role in the catalytic performance. In addition to the crystal growth, the presence of small amounts of Cl during catalyst preparation changed the chemical state of Ba, and therefore the adsorption and activation of the C–Cl bond for HCFC-142b were altered.
1. Introduction
1,1-difluoroethylene (VDF) is one of the major fluoromonomers, which are the feedstock for the production of various resins, rubbers membrane and paints [1]. The polymers derived from VDF (PVDF) or co-polymers with unique chemical resistance, stability at elevated temperatures, oxidation resistance, weatherability, piezoelectricity, dielectric and thermoelectricity, find wide applications in areas including petrochemical, electronic and electrical, and fluorocarbon coating [2]. VDF is the second largest product among fluorocarbons with an annual production capacity of above 53,000 tons. The demand for VDF is increasing rapidly. At present, in industry, VDF is usually produced via the dehydrochlorination of 1,1-difluoro-1-chloroethane (HCFC-142b) at reaction temperatures above 650 °C [3,4]. Dehydrochlorination is an efficient route for the preparation of 1, 1-dichloroethylene (VDC), vinyl chloride monomer (VCM), 2,3,3,3-tetrafluoropropene (HFO-1234yf), and ethylene oxychlorination [5,6,7,8]. As the dehydrochlorination of HCFC-142b is a highly endothermic reaction, very long reaction tubes are adopted to supply the reaction heat. Unfortunately, this also leads to the generation of carbon deposition during the reaction process at elevated temperatures. Consequently, the reactor needs to be cut off to remove the coke after a period of reaction, which significantly reduces the efficiency of continuous production.
We have reported that the dehydrochlorination of HCFC-142b is promoted by catalysts such as N-doped activated carbon [9], N-containing mesoporous carbon [10], and metal fluorides [3,4]. The presence of catalysts reduces the reaction temperature from 650 °C to lower than 350 °C, facilitating saving energy consumption and avoiding the formation of coke during reaction. Although N-doped activated carbon and mesoporous carbon materials exhibit a moderate conversion of HCFC-142b and high selectivity to VDF, they are difficult to recover following deactivation by coking. SrF2 shows a high activity for the pyrolysis of HCFC-142b to VDF under moderate conditions. However, its selectivity and stability are rather low [4]. Although the preparation of SrF2 microparticles with cubic structures improves the performance, the procedure is complicated and it is difficult to scale-up.
Due to the formation of highly corrosive byproducts, HCl and HF, during pyrolysis of HCFC-142b, there are very rare choices for the exploration of proper materials as the catalysts. Therefore, carbon materials and metal fluorides which are HF-corrosion-resistant are the potential catalysts. In addition to carbon materials, metal fluorides were also systematically investigated for the catalytic pyrolysis of HCFC-142b [11]. Among the various metal fluorides, barium fluoride shows relatively high activity in this reaction, with selectivity to VDF of 95% [11]. Other metal fluorides, such as MgF2, AlF3 and fluorinated Cr2O3 were found to be catalysts for dehydrofluorination reactions [12,13,14]. Similar to the chlorination of AlF3 to aluminum chlorofluoride (ACF), BaF2 also tends to be chlorinated to BaClF by Cl species under reaction conditions [15]. Consequently, a rapid decrease in the conversion of HCFC-142b and selectivity to VDF was observed. In a similar reaction system for dehydrofluorination, which was catalyzed by AlF3, strong Lewis acid promoted both dehydrofluorination and coke deposition. As a result, a fast deactivation was seen. It was reported that the pre-deposition of carbon prior to reaction leads to the partial coverage of strong Lewis acid sites and improves the stability of catalysts [16]. As reported previously, BaF2 converts to BaClF via reaction with HCl at reaction temperatures, resulting in the deactivation of the catalyst [11,17]. Clearly, the inhibition of chlorination of the BaF2 catalyst by Cl species is one of the key challenges for the catalytic pyrolysis of HCFC-142b.
In this study, barium fluoride was pre-chlorinated to barium chlorofluoride during the preparation of the catalyst. BaClxFy catalysts were prepared by solid-state reaction at room temperature with various molar ratios of Cl and F. The catalysts were applied for the pyrolysis of 1-chloro-1,1-difluoroethane to vinylidene fluoride. The effect of Cl composition on the performance of the BaF2 catalyst for catalytic pyrolysis was investigated.
2. Results
The catalytic performance of barium chlorofluoride for the pyrolysis of 1-chloro-1,1-difluoroethane (CH3CClF2, HCFC-142b) to vinylidene fluoride (VDF, CH2=CF2) as a function of time-on-stream is shown in Figure 1. The reactions were carried out at atmospheric pressure, a gas hourly space velocity (GHSV) of 1200 h-1, and a reaction temperature of 350 °C. As expected, BaCl2 is rather inactive for the pyrolysis of HCFC-142b. However, consistent with our previous study, BaF2 is efficient for the promotion of pyrolysis [11]. In addition to VDF, vinylidene chlorofluoride (CH2=CClF, VCF) via dehydrofluorination was detected as the major carbon-containing by-product. As indicated in Figure 1a, the conversion of HCFC-142b over BaClF is only around 10%, which is significantly lower than that of BaF2 (40%–60%). Hence, it is reasonable for the gradual deactivation of BaF2 catalysts following the chlorination to BaClF or BaCl2.
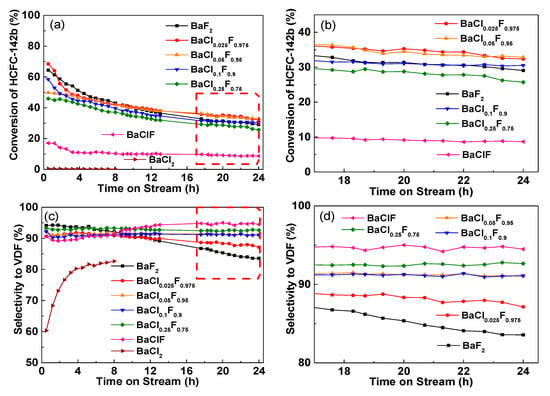
Figure 1.
Catalytic performance of BaClxFy catalysts for the pyrolysis of HCFC-142b as a function of time on stream at atmospheric pressure, a gas hourly space velocity (GHSV) of 1200 h−1, and a reaction temperature of 350 °C. (a) Conversion of HCFC-142b, (b) Highlighted conversion of HCFC-142b for the time on stream between 14 and 24 h, (c) Selectivity to vinylidene fluoride (VDF) and (d) Highlighted selectivity to VDF for the time on stream between 14 and 24 h.
Different from the catalysts of BaF2, BaCl2 and BaClF, doping of small amounts of Cl to BaF2 forming BaClxFy changes the conversion of HCFC-142b slightly. Compared with BaF2, with a doping amount of less than 10%, the conversion levels are improved, while, with increased amounts, a similar or even lower conversion results (Figure 1b).
Unexpectedly, the doping of small amounts of Cl to BaF2 significantly enhances the selectivity to VDF (Figure 1c). For the BaF2 catalyst, the selectivity of VDF decreases from 94% to 84% along with the deactivation of BaF2 catalyst monotonically (Figure 1c). In the presence of small amounts of Cl in BaF2, stabilized selectivity is achieved. Over BaCl0.05F0.95, BaCl0.1F0.9 and BaCl0.25F0.75, no decrease in VDF selectivity is observed (Figure 1d). Although the activity is rather low, it is noted that VDF selectivity over BaClF is high and stable. All these differences are not attributed to the change in specific surface area. Determined by N2 adsorption and desorption, the specific surface areas of all catalyst samples are rather low (˂ 2 m2/g). We suggest that the specific surface play a minor role in the performance difference of catalysts.
As argued previously, for both BaF2 or BaClxFy, the reason for deactivation during the dehydrochlorination reaction is that the catalyst reacts with the HCl generated in the reaction, and then F elements on the catalyst are further replaced by Cl [11]. This is the major contributor to deactivation, in addition to the coke deposition. Clearly, both BaClxFy and BaF2 tend to be further chlorinated during the reaction. However, BaClxFy catalysts exhibit an improved performance, while BaF2 deactivated relatively rapidly. This may indicate that the BaClxFy formed during preparation by solid-state reaction at room temperature is not exactly the same as the BaClxFy formed in the reaction, or after adding Cl in the preparation, it can significantly slow down the speed of catalyst chlorination during the reaction. If the catalyst is quickly chlorinated to BaCl2, a decrease in activity is inevitable as the BaCl2 phase is almost inactive. Aluminum chlorofluoride was confirmed to be an excellent catalyst for C-F bond activation and F/Cl exchange reaction [18,19]. Similarly, barium chlorofluorides are proper catalysts for the dehydrochlorination of HCFC-142b.
The above discussion is confirmed by the results of XRD. As indicated in Figure 2, BaF2 and BaClF phase structures were prepared by the simple solid-state reaction between Ba(OH)2 and NH4F/NH4Cl at room temperature. The XRD patterns of BaF2 and BaClF agree well with the standard patterns (ICSD-85-1341 for BaF2 and ICSD-70-1183 for BaClF). No other impurities are detected by XRD. Clearly, solid-state reaction at room temperature is an effective way for the synthesis of BaF2 and BaClF [20].
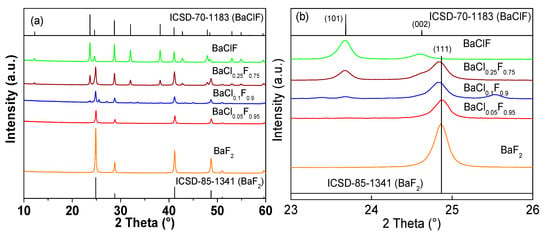
Figure 2.
(a) XRD patterns of BaF2 and barium chlorofluoride catalysts before reactions. (b) Highlighted XRD patterns with 2θ between 23° and 26°.
As disclosed in Figure 2a, BaF2 has a sharp diffraction peak indicating the complete crystallinity. Following the doping of small amounts of Cl, the crystallinity of BaF2 is weakened significantly. Clearly, during preparation, in addition to the Cl source, the introduction of NH4Cl also functions as a template during the formation of BaF2 particles. Actually, during the process of low-temperature solid-state synthesis, both the particle size and morphology of solid products can be controlled in the presence of proper templates [21,22,23]. A very weak diffraction of BaClF is observed for BaCl0.1F0.9. With the further increase in Cl content, the XRD pattern gradually changes from BaF2 to BaClF, which indicates that the crystal phase of the BaClF is formed continuously after adding Cl, which is more obvious when the Cl content is higher than the BaCl0.25F0.75, because half of the BaF2 is transformed into BaClF. In addition to the transformation of the phase structure, the 2θ of BaF2 shifts following the addition of Cl (Figure 2b). Clearly, the presence of small amounts Cl during solid-state preparation disturbs the formation of BaF2 crystalline significantly [24,25].
The changes in the crystal structure and surface of the catalysts are further reinforced by TEM images (Figure 3). In the absence of Cl, lattice diffraction fringe (0.362 nm) corresponding to (111) of BaF2 is detected [26]. In addition to BaF2, the BaClF crystal structure with a facet of (101), with a lattice diffraction fringe of 0.376 nm, corresponding to BaClF, is also found for BaCl0.25F0.75. Over BaClF, only the lattice diffraction fringe spacing of 0.376 nm is identified, indicating the exposure of the (101) plane. This is consistent with the structure evolution revealed in XRD patterns.
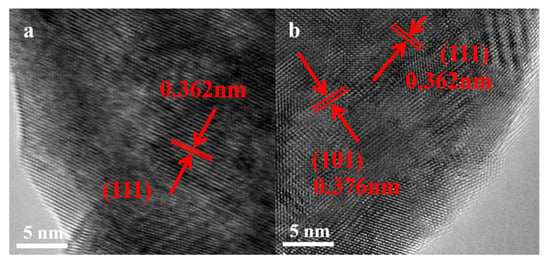
Figure 3.
High-resolution TEM images of (a) BaF2, (b) BaCl0.25F0.75.
Following reaction (time on stream of 24 h), no formation of BaCl2 was detected by XRD (Figure S1). By contrast, the patterns of spent catalysts match with BaClF perfectly. This indicates that the BaClF structure is relatively stable under reaction conditions (pyrolysis of HCFC-142b 350 °C) after 24 hours of reaction. This shows that the deactivation rate was significantly slowed down and the selectivity to VDF was retained (Figure 1). As BaClF phases are obtained for all the spent catalysts, the same activity and selectivity should be achieved. Actually, slight differences in these catalysts are still noticed. Therefore, chlorination of catalysts is not the sole factor for the performance of catalysts.
As shown in Figure 4, the contents of F and Cl of the BaClxFy catalyst before and after reaction were obtained by EDS. The molar ratio of F/Cl after reaction and the calculated ratio of chlorination of the catalyst after reaction are related to the amount of Cl added during the preparation of catalyst. Clearly, the more Cl was added during the preparation, the less F in catalysts was chlorinated. After time on stream of 24 h, over 70% of F was replaced by Cl during the reaction for the BaF2 catalyst (Figure 4a). Although no diffraction peak of BaCl2 was detected by XRD over the spent catalysts, more Cl than F was quantified by EDS over the spent BaF2 catalysts. As illustrated in Figure 4b, the ratio of F/Cl is lower than 0.4, implying the formation of BaCl2 species. We suggest that these BaCl2 structures are highly dispersed in the catalyst. As a result, these small crystalline particles are not detected by XRD. It is well accepted that the detection limit of XRD is 2–3 nm [27]. Once again, the EDS results confirm that BaClF is relatively stable under reaction system. Only small amounts of F were chlorinated during reaction.
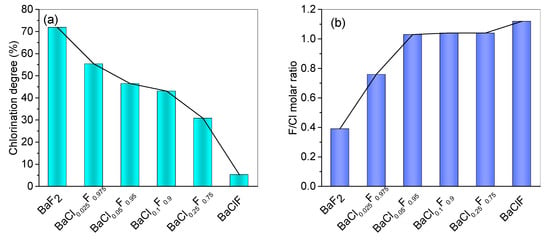
Figure 4.
Chlorination of catalysts during the pyrolysis of HCFC-142b at 350 °C. (a) Chlorination degree of catalysts after time on stream of 24 h. The chlorination degree is calculated according to the molar ratio of F amount replaced by Cl in the reaction to the amount of F in the fresh catalysts. (b) F/Cl molar ratio in the spent catalysts. The elemental amounts of all the catalysts were determined by EDS technique.
With the doping of small amounts of Cl during the catalyst preparation, the chlorination of catalysts during the reaction is significantly inhibited. In addition to BaCl0.025F0.975, the catalysts are maintained at BaClF with an F/Cl of close to 1 (Figure 4b). Different from BaF2 catalyst, addition of NH4Cl during catalyst preparation prohibits the catalysts from being further chlorinated to BaCl2 following reaction.
As presented in Figure 1, BaClF exhibits rather low activity for the conversion of HCFC-142b, although it shows high selectivity to VDF. As BaClxFy catalysts are ultimately chlorinated to BaClF, similar activities to BaClF should be obtained. However, much higher activities of these catalysts were obtained (Figure 1). Consequently, SEM experiments were carried out for the elucidation of these difference.
As displayed in Figure 5, following solid-state reaction between Ba(OH)2 and NH4F at room temperature, irregular particles were obtained. The particle size ranges from 50 to 300 nm. Although low-temperature solid-state reaction is an effective way to prepare nanomaterials, large BaF2 particles were still formed, probably due to the fast fluorination of Ba(OH)2 by F ions [28]. However, with the doping of small amounts of NH4Cl, unexpectedly, much smaller and more uniform particles were achieved for BaCl0.025F0.975. Similarly, in the presence of NaCl, ZnS nanoparticles with a size ranging from 40 to 80 nm were prepared between the solid-state reaction of ZnCl2 and Na2S [29]. Clearly, both NH4Cl and NaCl can function as templates during the formation of particles via solid state reactions [30]. This result is consistent with the observation of XRD patterns. Following the doping of Cl, the crystal growth of BaF2 was inhibited significantly.
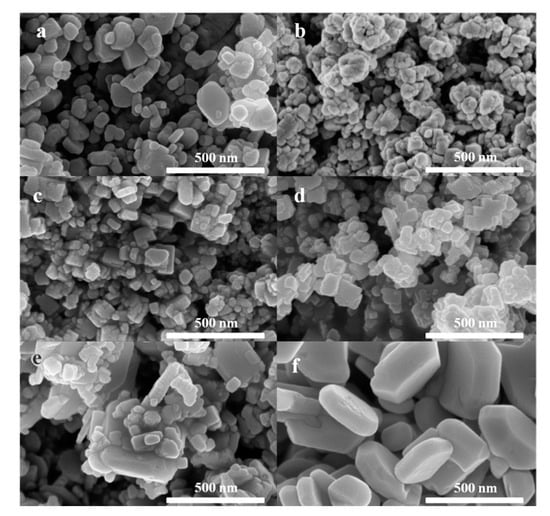
Figure 5.
SEM images of (a) BaF2, (b) BaCl0.025F0.975, (c) BaCl0.05F0.95, (d) BaCl0.1F0.9, (e) BaCl0.25F0.75 and (f) BaClF.
With the further doping of NH4Cl during catalyst preparation, a slight increase in particle size was observed. As presented in Figure 5, the particle size increases with Cl content monotonically. It is worth noting that very large particles for BaClF were obtained, with sizes ranging from 200 to 500 nm. Therefore, the particle size of BaClF is significantly larger than that of BaF2 and BaClxFy. We suggest that this mainly contributes to the low activity of the BaClF catalyst.
The morphology of spent catalysts after a time on stream of 24 h is demonstrated in Figure S2. Compared with the SEM images of fresh catalysts (Figure 5), clear sintering is noticed for all the catalysts. Despite the formation of BaClF after reaction, the particle size of the catalyst is still far smaller than that of BaClF and the spent BaClF. Hence, the activity of the BaClxFy catalysts with low Cl content is still much higher than that of the BaClF after reaction.
To verify the effect of crystal size on the catalytic performance, BaClF catalysts were calcined at 300, 400, 500 and 600 °C in air atmosphere. Generally, metal fluorides tend to sinter significantly at elevated temperatures [31,32]. Hence, in the present study, BaClF catalysts were calcined at high temperatures to obtain various crystal sizes. Interestingly, as illustrated in Figure 6, the diffraction of BaClF first decreases with calcination temperature followed by increasing again at temperatures above 400 °C. Consequently, the crystal sizes were determined by the Scherrer equation based on the XRD patterns [33,34]. Prior to the calcination, the crystal size of BaClF was estimated to be around 130.5 nm. Following calcination at 300 °C, the size decreases to 71.5 nm. Following further increases in temperature, the sizes of 52, 74 and 83 nm were obtained for calcination temperatures of 400, 500 and 600 °C respectively. Clearly, relatively low calcination temperatures facilitate the disorder of BaClF structures. As suggested by Kesavamoorthy et al., the interplanar distance of Ba and F planes drops with temperature. By contrast, the Ba and Cl plane is enhanced by elevated temperatures [35,36]. Nevertheless, varied crystal sizes of BaClF were achieved via calcination at different temperatures.
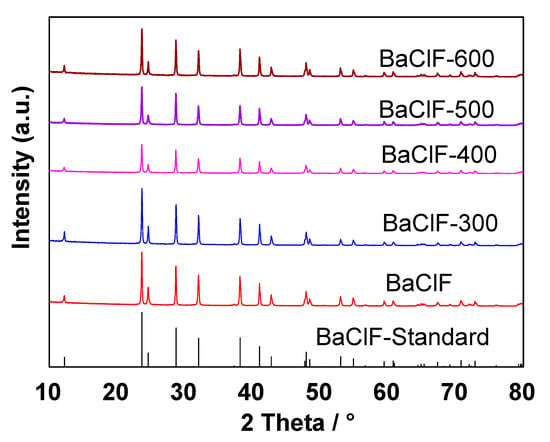
Figure 6.
XRD patterns BaClF catalysts calcined at temperatures between 300 °C and 600 °C.
Following XRD experiments, the calcined BaClF catalysts were further evaluated for the catalytic pyrolysis of HCFC-142b. The results are displayed in Figure 7. After calcination, the conversion levels of HCFC-142b over BaClF are significantly improved (Figure 7a). Generally, the conversion agrees with the crystal sizes obtained by XRD patterns. BaClF calcined at 400 °C exhibits a relatively high conversion with a small crystal size. Further increases in calcination temperature lead to the sintering of BaClF. Correspondingly, the conversion drops with temperature. As small crystalline particles were obtained following calcination, stable selectivity to VDF is also achieved (Figure 7b).
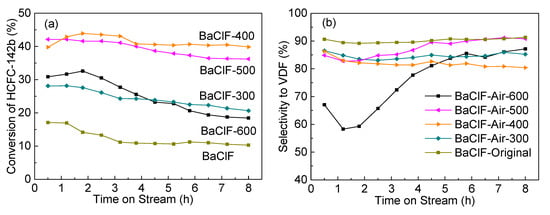
Figure 7.
Effect of calcination on the conversion of (a) HCFC-142b and (b) selectivity to VDF for BaClF catalysts.
In comparison, BaF2 catalysts were also calcined under the same temperatures. As disclosed in Figure S3a, different from BaClF, a strengthening in the diffraction of BaF2 is observed at calcination between 300 and 600 °C. According to the calculation, the crystal size of BaF2 increases from 42 to 51 and 55 nm after calcination at 400 and 600 °C respectively. Although these catalysts were finally chlorinated to BaClF after reaction (Figure S3b), they still show different catalytic performances. As shown in Figure S4a, the calcination of BaF2 leads to a significant decrease in the conversion of HCFC-142b. When calcined at 700 °C, BaF2 is almost totally deactivated. However, no clear difference was found for VDF selectivity (Figure S4b).
Based on the above discussion, XRD revealed that a smaller crystal size was achieved following Cl doping (Figure 2). In addition, as confirmed by SEM images (Figure 5), Cl doping also lead to the decrease in the particle size. Hence, we suggest that the particle size or crystal size of a barium catalyst plays a major role in the catalytic performance. Similar results were also confirmed for Cr2O3 catalysts for the dehydrofluorination of 1,1-difluoroethane [37].
The catalysts were further investigated by XPS characterization and the results are shown in Figure 8. The binding energy of Ba in BaF2 was determined to be 795.2 eV, which is consistent with the standard [38,39]. Upon the addition of Cl element to BaF2, a clear shift in Ba 3d3/2 towards a higher binding energy is noticed (Figure 8a). Clearly, small amounts of Cl in BaF2 not only inhibit the growth of BaF2 and BaClF crystalline, but also affect the chemical state of the Ba element. Due to the different interactions of Ba with Cl and F, the fast growth of crystalline is inhibited. In addition to the crystal growth, these differences may also contribute to the improved conversion and selectivity. Due to the change in Ba-binding energy, the adsorption and activation of the C-Cl bond for HCFC-142b are altered. A similar trend is also found for the F 1s spectrum (Figure 8b). Consistent with XRD and TEM results, the XPS spectra of spent catalysts confirm the formation of BaClF during the catalytic pyrolysis of HCFC-142b (Figure S5). In addition to the composition of catalysts, reaction temperature [40], reaction pressure and environment [41] play an important role in the catalytic performance of the pyrolysis process. These issues will be addressed in the future studies after the optimization of the catalysts.
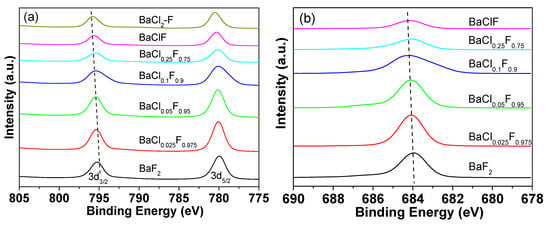
Figure 8.
High resolution XPS of (a) Ba 3d and (b) F 1s spectra of BaF2 and barium chlorofluoride catalysts before reactions.
3. Materials and Methods
3.1. Preparation of Catalysts
Barium chlorofluoride was synthesized by solid-state grinding method with the reaction between Ba(OH)2·8H2O, NH4F and NH4Cl [42,43]. Ba(OH)2·8H2O, NH4F and NH4Cl were purchased from Aladdin Co. (Shanghai, China) with analytical purity (>98.0%) without further purification. During the preparation of barium chlorofluoride, a total of 0.1mol NH4F and NH4Cl with different proportions were mixed and grinded in a mortar rigorously for 0.5 h. Then, 0.1 mol Ba(OH)2·8H2O powder was added to the mixture, followed by grinding for a further 1 h. During the grinding, pungent gas was smelt (emission of NH3). Following the release of crystal water, wet paste was formed gradually. The paste was dried at 120 °C for 5 h, leading to the formation of barium chlorofluoride. For comparison, neat BaF2 and BaCl2 were also prepared following similar procedures. The chemical equation used during preparation is as follows. The conditions of catalyst preparation are listed in Table 1.

Table 1.
Preparation conditions of catalysts for BaF2, barium chlorofluoride and BaCl2.
3.2. Catalytic Activity
The catalytic activity of BaClxFy was evaluated in a tubular fixed-bed reactor. A nickel tube with an inner diameter of 22 mm was adopted as the reactor. Blank experiments confirmed that no reaction was detected in the absence of catalysts. The HCFC-142b (>99.0%, Juhua Co., Zhejiang, China) reaction gas was diluted by equivalent amounts of N2 (>99.9%, Mingxin Gas Co., Hangzhou, China) which were controlled by mass flow rate controllers (D09-11C, Qixing, Beijing, China) respectively. Prior to reaction, a 2 mL catalyst with a particle size ranging from 0.425 to 0.850 mm (20–40 mesh) was loaded to the isothermal zone of the reactor. A thermal couple was located in the middle the catalyst bed for the detection of reaction temperature. The reactions were carried out at atmospheric pressure, a gas hourly space velocity (GHSV) of 1200 h−1 (HCFC-142b and N2), and a reaction temperature of 350 °C. KOH solution with a concentration of 1 mol/L was used to remove the effluent acid gas (HCl and HF) formed during the reaction. A gas chromatograph (GC-1690 JieDao) equipped with a thermal conductivity detector (TCD) was used to analyze the gas composition.
3.3. Catalyst Characterization
X-ray diffraction (XRD) experiments were carried out for the identification of crystal structures. XRD patterns were obtained over an X’Pert Pro analytical instrument (Almelo, Overijssel, The Netherlands). X-Ray photoelectron spectroscopy (XPS) measurements were performed by Thermo EscaLab 250Xi (Cambridge, Massachusetts, USA), equipped with monochromatized Al Kα X-ray as the excitation source (24.2 W). The binding energy values were corrected for charging effect by referring to the adventitious C1s line at 284.5 eV. Scanning electron microscopy (SEM) studies were carried out on a Helios G4 CX (Cambridg, Massachusetts, USA) equipped with an X-ray energy spectrometer (EDS). Transmission Electron Microscope (TEM) studies were carried out on a Tecnai G2 F30 (Hillsboro, Oregon, USA).
4. Conclusions
BaF2 and BaClxFy catalysts were successfully prepared via the solid-state reaction method at room temperature. Both catalysts promote the pyrolysis of HCFC-142b (CH3CClF2) to VDF (CH2=CF2) at 350 °C, which is significantly lower than the industrial manufacture temperature (600–700 °C) by the thermal decomposition of HCFC-142b. However, for the BaF2 catalyst, the selectivity of VDF decreases from 94% to 84% along with the deactivation of the BaF2 catalyst monotonically. In the presence of small amounts of Cl in BaF2, stabilized selectivity is achieved. Over BaCl0.05F0.95, BaCl0.1F0.9 and BaCl0.25F0.75, no decrease in VDF selectivity is observed. According to XRD results, the presence of small amounts Cl during solid-state preparation disturb the formation of BaF2 crystalline significantly. Despite the formation of BaClF after reaction, the particle size of the BaClxFy catalysts are still far smaller than that of BaClF and the spent BaClF. Hence, the activity of the BaClxFy catalysts with a low Cl content is still much higher than that of the BaClF after reaction. We suggest that the particle size, or more precisely, the crystal size of the barium catalyst play a major role in the catalytic performance. In addition to the crystal growth, the presence of small amounts of Cl during catalyst preparation changes the chemical state of Ba, and therefore the adsorption and activation of the C-Cl bond for HCFC-142b are altered.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4344/10/4/377/s1, Figure S1: XRD patterns of spent BaF2 and barium chlorofluoride catalysts; Figure S2: XRD patterns BaF2 catalysts calcined at temperatures between 300 °C and 600 °C; Figure S3: XRD patterns BaF2 catalysts calcined at temperatures between 300 °C and 600 °C; Figure S4: Effect of calcination on the conversion of HCFC-142b and selectivity to VDF for BaF2 catalysts; Figure S5: High resolution XPS spectra of SEM images of BaF2 and barium chlorofluoride catalysts following reaction with time on stream of 24 h.
Author Contributions
Conceptualization, W.H.; methodology, W.H. and W.Y.; validation, W.Y., Y.L. (Yongnan Liu), J.L. and H.Y.; formal analysis, W.Y. and B.L.; investigation, W.Y., Y.L. (Yongnan Liu), J.L. and H.Y.; resources, W.H., H.T., A.C. and Y.L. (Ying Li); data curation, W.Y.; writing—original draft preparation, W.Y.; writing—review and editing, W.H.; visualization, W.Y.; supervision, W.H., H.T., A.C. and Y.L. (Ying Li); project administration, W.H.; funding acquisition, W.H. and A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Zhejiang Provincial Natural Science Foundation of China under grant no. LY19B060009 and LY19B050004.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Ameduri, B. From Vinylidene Fluoride (VDF) to the Applications of VDF-Containing Polymers and Copolymers: Recent Developments and Future Trends. Chem. Rev. 2009, 109, 6632–6686. [Google Scholar] [CrossRef] [PubMed]
- Améduri, B.; Boutevin, B.; Kostov, G. Fluoroelastomers: Synthesis, properties and applications. Prog. Polym. Sci. 2001, 26, 105–187. [Google Scholar] [CrossRef]
- Wang, Z.; Han, W.; Tang, H.; Liu, H. CaBaFx composite as robust catalyst for the pyrolysis of 1-chloro-1,1-difluoroethane to vinylidene fluoride. Catal. Commun. 2019, 120, 42–45. [Google Scholar] [CrossRef]
- Wang, Z.; Han, W.; Liu, H. EDTA-assisted hydrothermal synthesis of cubic SrF2 particles and their catalytic performance for the pyrolysis of 1-chloro-1,1-difluoroethane to vinylidene fluoride. Crystengcomm 2019, 21, 1691–1700. [Google Scholar] [CrossRef]
- Zhang, P.Z.; Jiang, Z.B.; Cui, Y.H.; Xie, G.Q.; Jin, Y.Z.; Guo, L.L.; Xu, Y.Q.; Zhang, Q.F.; Li, X.N. Catalytic performance of ionic liquid for dehydrochlorination reaction: Excellent activity and unparalled stability. Appl. Catal. B Environ. 2019, 255, 10. [Google Scholar] [CrossRef]
- Sun, X.; Liu, X.; Qin, Y.C.; Qiang, L.; He, Y.P.; Su, D.S.; Song, L.J.; Sun, Z.L. Direct Conversion of Acetylene and 1,2-Dichloroethane to Vinyl Chloride Monomer over a Supported Carbon Nitride Catalyst: Tunable Activity Controlled by the Synthesis Temperature. Ind. Eng. Chem. Res. 2019, 58, 5404–5413. [Google Scholar] [CrossRef]
- Mao, W.; Bai, Y.; Wang, W.; Wang, B.; Xu, Q.; Shi, L.; Li, C.; Lu, J. Highly Selective Dehydrochlorination of 1,1,1,2-Tetrafluoro-2-chloropropane to 2,3,3,3-Tetrafluoropropene over Alkali Metal Fluoride Modified MgO Catalysts. ChemCatChem 2017, 9, 824–832. [Google Scholar] [CrossRef]
- Scharfe, M.; Zichittella, G.; Kondratenko, V.A.; Kondratenko, E.V.; Lopez, N.; Perez-Ramirez, J. Mechanistic origin of the diverging selectivity patterns in catalyzed ethane and ethene oxychlorination. J. Catal. 2019, 377, 233–244. [Google Scholar] [CrossRef]
- Wang, Z.; Han, W.; Zhang, C.; Zhou, S.; Wang, H.; Tang, H.; Liu, H. Preparation of N-Doped Activated Carbon for Catalytic Pyrolysis of 1-Chloro-1,1-difluoroethane to Vinylidene Fluoride. Chemistryselect 2018, 3, 1015–1018. [Google Scholar] [CrossRef]
- Wang, Z.; Han, W.; Tang, H.; Li, Y.; Liu, H. Preparation of N-doped ordered mesoporous carbon and catalytic performance for the pyrolysis of 1-chloro-1,1-difluoroethane to vinylidene fluoride. Microporous Mesoporous Mater. 2019, 275, 200–206. [Google Scholar] [CrossRef]
- Han, W.; Liu, B.; Kang, Y.; Wang, Z.; Yu, W.; Yang, H.; Liu, Y.; Lu, J.; Tang, H.; Li, Y.; et al. Experimental and DFT Mechanistic Study of Dehydrohalogenation of 1-Chloro-1,1-difluoroethane over Metal Fluorides. Ind. Eng. Chem. Res. 2019, 58, 18149–18159. [Google Scholar] [CrossRef]
- Han, W.; Zhang, C.; Wang, H.; Zhou, S.; Tang, H.; Yang, L.; Wang, Z. Sub-nano MgF2 embedded in carbon nanofibers and electrospun MgF2 nanofibers by one-step electrospinning as highly efficient catalysts for 1,1,1-trifluoroethane dehydrofluorination. Catal. Sci. Technol. 2017, 7, 6000–6012. [Google Scholar] [CrossRef]
- Han, W.; Wang, H.; Liu, B.; Li, X.; Tang, H.; Li, Y.; Liu, H. PVDF mediated fabrication of freestanding AlF3 sub-microspheres: Facile and controllable synthesis of alpha, beta and theta-AlF3. Mater. Chem. Phys. 2020, 240. [Google Scholar] [CrossRef]
- Liu, B.; Han, W.; Li, X.; Li, L.; Tang, H.; Lu, C.; Li, Y.; Li, X. Quasi metal organic framework with highly concentrated Cr2O3 molecular clusters as the efficient catalyst for dehydrofluorination of 1,1,1,3,3-pentafluoropropane. Appl. Catal. B Environ. 2019, 257. [Google Scholar] [CrossRef]
- Calvo, B.; Marshall, C.P.; Krahl, T.; Krohnert, J.; Trunschke, A.; Scholz, G.; Braun, T.; Kemnitz, E. Comparative study of the strongest solid Lewis acids known: ACF and HS-AlF3. Dalton Trans. 2018, 47, 16461–16473. [Google Scholar] [CrossRef]
- Fang, X.-X.; Wang, Y.; Jia, W.-Z.; Song, J.-D.; Wang, Y.-J.; Luo, M.-F.; Lu, J.-Q. Dehydrofluorination of 1, 1, 1, 3, 3-pentafluoropropane over C-AlF3 composite catalysts: Improved catalyst stability by the presence of pre-deposited carbon. Appl. Catal. Gen. 2019, 576, 39–46. [Google Scholar] [CrossRef]
- Teinz, K.; Wuttke, S.; Boerno, F.; Eicher, J.; Kemnitz, E. Highly selective metal fluoride catalysts for the dehydrohalogenation of 3-chloro-1,1,1,3-tetrafluorobutane. J. Catal. 2011, 282, 175–182. [Google Scholar] [CrossRef]
- Meissner, G.; Dirican, D.; Jager, C.; Braun, T.; Kemnitz, E. Et3GeH versus Et3SiH: Controlling reaction pathways in catalytic C-F bond activations at a nanoscopic aluminum chlorofluoride. Catal. Sci. Technol. 2017, 7, 3348–3354. [Google Scholar] [CrossRef]
- Krahl, T.; Kemnitz, E. The very strong solid Lewis acids aluminium chlorofluoride (ACF) and bromofluoride (ABF)—Synthesis, structure, and Lewis acidity. J. Fluor. Chem. 2006, 127, 663–678. [Google Scholar] [CrossRef]
- Yu, Y.; Jia, D.Z.; Ge, W.W.; Jin, C.F.; Xin, X.Q. Syhthesis of inorganic nano-materials by solid state reaction at low-heating temperatures. Chin. J. Inorg. Chem. 2004, 20, 881–888. [Google Scholar]
- Liu, X.H.; Yu, L. Synthesis of nanosized nickel hydroxide by solid-state reaction at room temperature. Mater. Lett. 2004, 58, 1327–1330. [Google Scholar] [CrossRef]
- Yu, X.H.; Li, F.; Ye, X.R.; Xin, X.Q.; Xue, Z.L. Synthesis of cerium(IV) oxide ultrafine particles by solid-state reactions. J. Am. Ceram. Soc. 2000, 83, 964–966. [Google Scholar] [CrossRef]
- Sun, Z.P.; Liu, L.; Zhang, L.; Jia, D.Z. Rapid synthesis of ZnO nano-rods by one-step, room-temperature, solid-state reaction and their gas-sensing properties. Nanotechnology 2006, 17, 2266–2270. [Google Scholar] [CrossRef]
- Wang, X.L.; Liu, Z.Q.; Stevens-Kalceff, M.A.; Riesen, H. Mechanochemical Preparation of Nanocrystalline BaFCl Doped with Samarium in the 2+Oxidation State. Inorg. Chem. 2014, 53, 8839–8841. [Google Scholar] [CrossRef]
- Hagemann, H.; D’Anna, V.; Daku, M.L.; Kubel, F. Crystal Chemistry in the Barium Fluoride Chloride System. Cryst. Growth Des. 2012, 12, 1124–1131. [Google Scholar] [CrossRef]
- Chialanza, M.R.; Schneider, J.F.; Keuchkerian, R.; Romero, M.; Faccio, R.; Olivera, A.; Pereira, H.B. Structural analysis of oxyfluoride borate glass and BaF2 crystallization from phase separation. J. Am. Ceram. Soc. 2020, 103, 3126–3137. [Google Scholar] [CrossRef]
- Saowadee, N.; Agersted, K.; Bowen, J.R. Lattice constant measurement from electron backscatter diffraction patterns. J. Microsc. 2017, 266, 200–210. [Google Scholar] [CrossRef]
- Radmilovic, V.; Ophus, C.; Marquis, E.A.; Rossell, M.D.; Tolley, A.; Gautam, A.; Asta, M.; Dahmen, U. Highly monodisperse core-shell particles created by solid-state reactions. Nat. Mater. 2011, 10, 710–715. [Google Scholar] [CrossRef]
- Lan, C.; Hong, K.Q.; Wang, W.Z.; Wang, G.H. Synthesis of ZnS nanorods by annealing precursor ZnS nanoparticles in NaCl flux. Solid State Commun. 2003, 125, 455–458. [Google Scholar] [CrossRef]
- Li, F.; Yu, X.H.; Pan, H.J.; Wang, M.L.; Xin, X.Q. Syntheses of MO2 (M = Si, Ce, Sn) nanoparticles by solid-state reactions at ambient temperature. Solid State Sci. 2000, 2, 767–772. [Google Scholar] [CrossRef]
- Mao, W.; Bai, Y.; Wang, B.; Wang, W.; Ma, H.; Qin, Y.; Li, C.; Lu, J.; Liu, Z.W. A facile sol-gel synthesis of highly active nano alpha-aluminum fluoride catalyst for dehydrofluorination of hydrofluorocarbons. Appl. Catal. B Environ. 2017, 206, 65–73. [Google Scholar] [CrossRef]
- Jia, Z.H.; Mao, W.; Bai, Y.B.; Wang, B.; Ma, H.; Li, C.; Lu, J. Hollownano-MgF2 supported catalysts: Highly active and stable in gas-phase dehydrofluorination of 1,1,1,3,3-pentafluoropropane. Appl. Catal. B Environ. 2018, 238, 599–608. [Google Scholar] [CrossRef]
- Mozafari, M.; Moztarzadeh, F.; Tahriri, M. Green synthesis and characterisation of spherical PbS luminescent micro- and nanoparticles via wet chemical technique. Adv. Appl. Ceram. 2011, 110, 30–34. [Google Scholar] [CrossRef]
- Drits, V.; Srodon, J.; Eberl, D.D. XRD measurement of mean crystalline thickness of illite and illite/smectite: Reappraisal of the Kubler index and the Scherrer equation. Clay Min. 1997, 45, 461–475. [Google Scholar] [CrossRef]
- Kesavamoorthy, R.; Sundarakkannan, B.; Rao, G.V.N.; Sastry, V.S. Thermal effect on BaFCl: High-temperature X-ray diffraction. Thermochim. Acta 1997, 307, 185–195. [Google Scholar] [CrossRef]
- Sundarakkannan, B.; Kesavamoorthy, R.; Nisha, J.A.; Sridharan, V.; Sivakumar, T. Antiferroelectric-to-paraelectric transition in BaFCl. Phys. Rev. B 1998, 57, 11632–11638. [Google Scholar] [CrossRef]
- Han, W.F.; Wang, Z.K.; Li, X.J.; Tang, H.D.; Xi, M.; Li, Y.; Liu, H.Z. Solution combustion synthesis of nano-chromia as catalyst for the dehydrofluorination of 1,1-difluoroethane. J. Mater. Sci. 2016, 51, 11002–11013. [Google Scholar] [CrossRef]
- Andrade, A.B.; Ferreira, N.S.; Valerio, M.E.G. Particle size effects on structural and optical properties of BaF2 nanoparticles. RSC Adv. 2017, 7, 26839–26848. [Google Scholar] [CrossRef]
- Au, C.T.; Chen, K.D.; Ng, C.F. Characterization of BaX2/Gd2O3 (X = F, Cl, Br) catalysts for the oxidative coupling of methane. Appl. Catal. Gen. 1998, 171, 283–291. [Google Scholar] [CrossRef]
- Laws, P.A.; Hayley, B.D.; Anthony, L.M.; Roscoe, J.M. Kinetic study of the mechanism of the low-temperature pyrolysis of vinyl bromide. J. Phys. Chem. 2001, 105, 1830–1837. [Google Scholar] [CrossRef]
- Kunsagi-Mate, S.; Vegh, E.; Nagy, G.; Kollar, L. Influence of the molecular environment on the three-center versus four-center elimination of HBr from vinyl bromide: A theoretical approach. J. Phys. Chem. 2002, 106, 6319–6324. [Google Scholar] [CrossRef]
- Hu, P.; Cao, Y.; Jia, D.; Li, Q.; Liu, R. Engineering the metathesis and oxidation-reduction reaction in solid state at room temperature for nanosynthesis. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Al-Terkawi, A.A.; Scholz, G.; Emmerling, F.; Kemnitz, E. Mechanochemical Synthesis, Characterization, and Structure Determination of New Alkaline Earth Metal-Tetrafluoroterephthalate Frameworks. Cryst. Growth Des. 2016, 16, 1923–1933. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).