Accelerating Kinetic Parameter Identification by Extracting Information from Transient Data: A Hydroprocessing Study Case
Abstract
1. Introduction
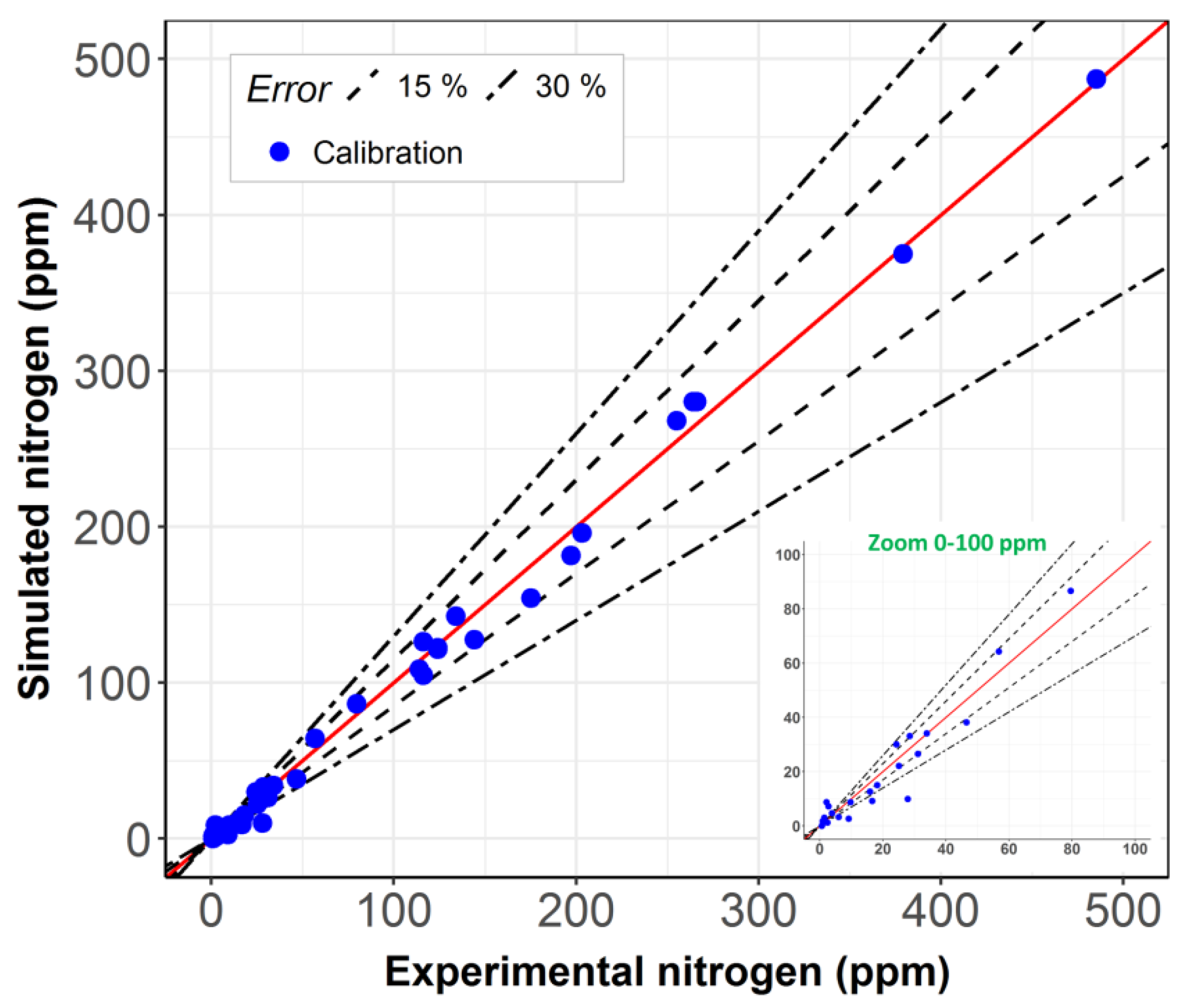
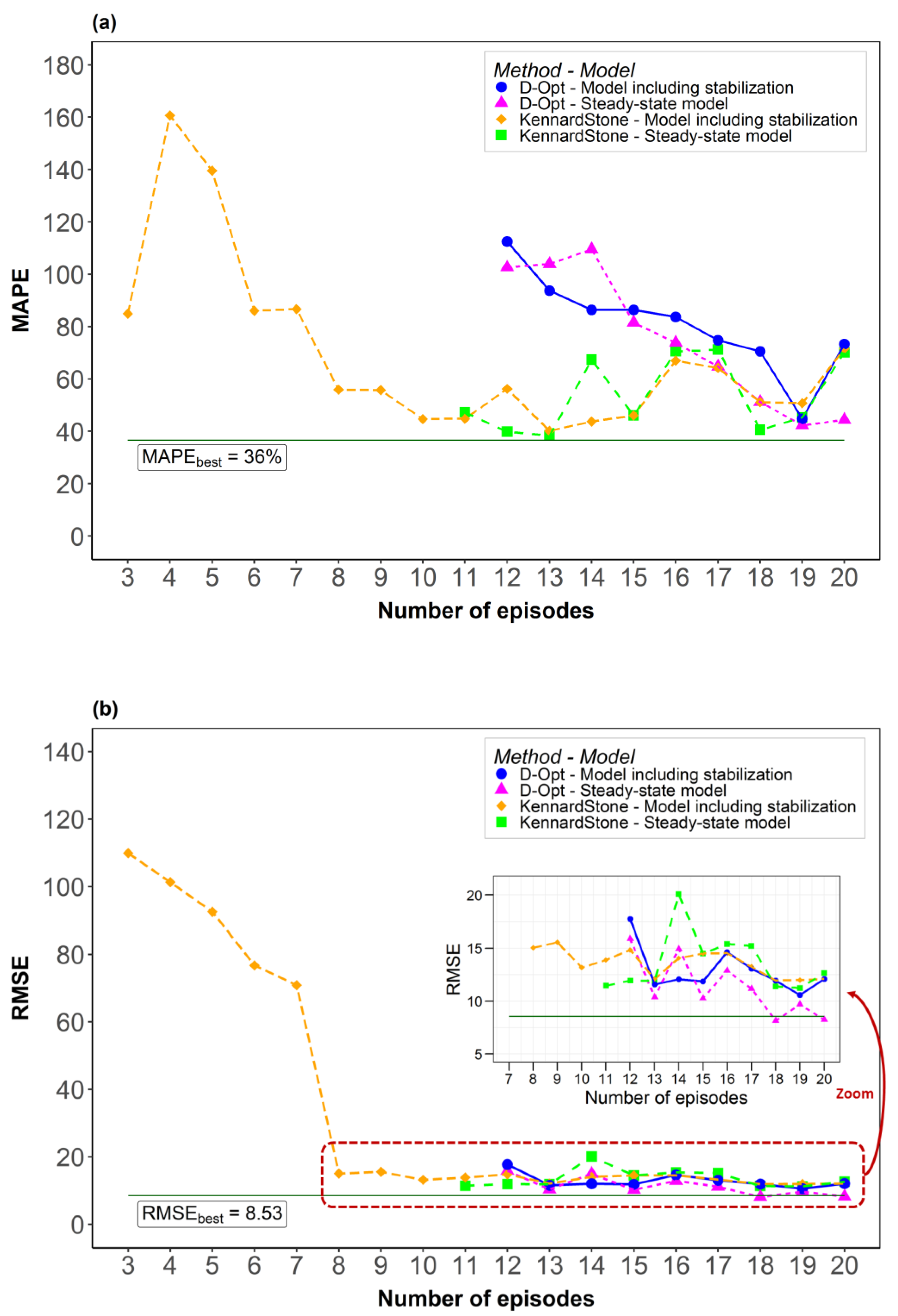
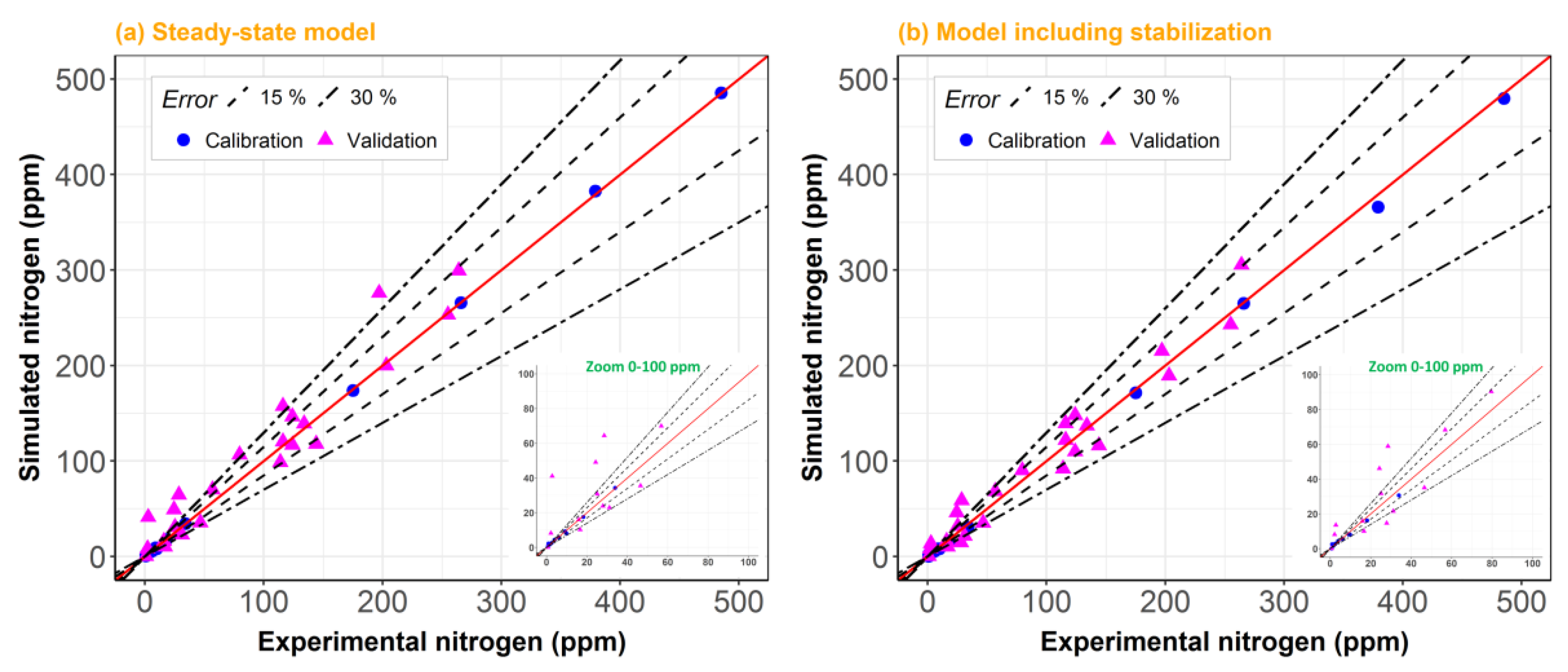
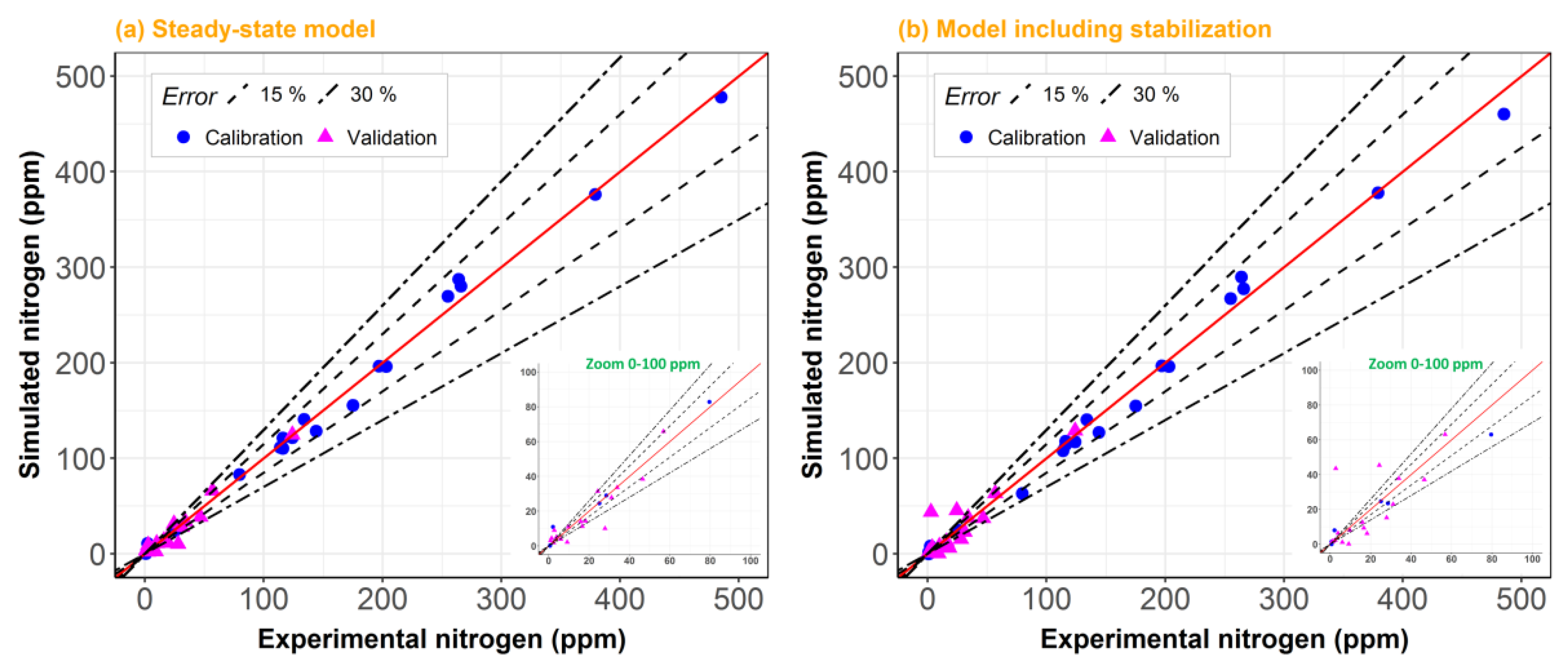
2. Results and Discussion
2.1. Model Including Stabilization vs. Steady-State Model
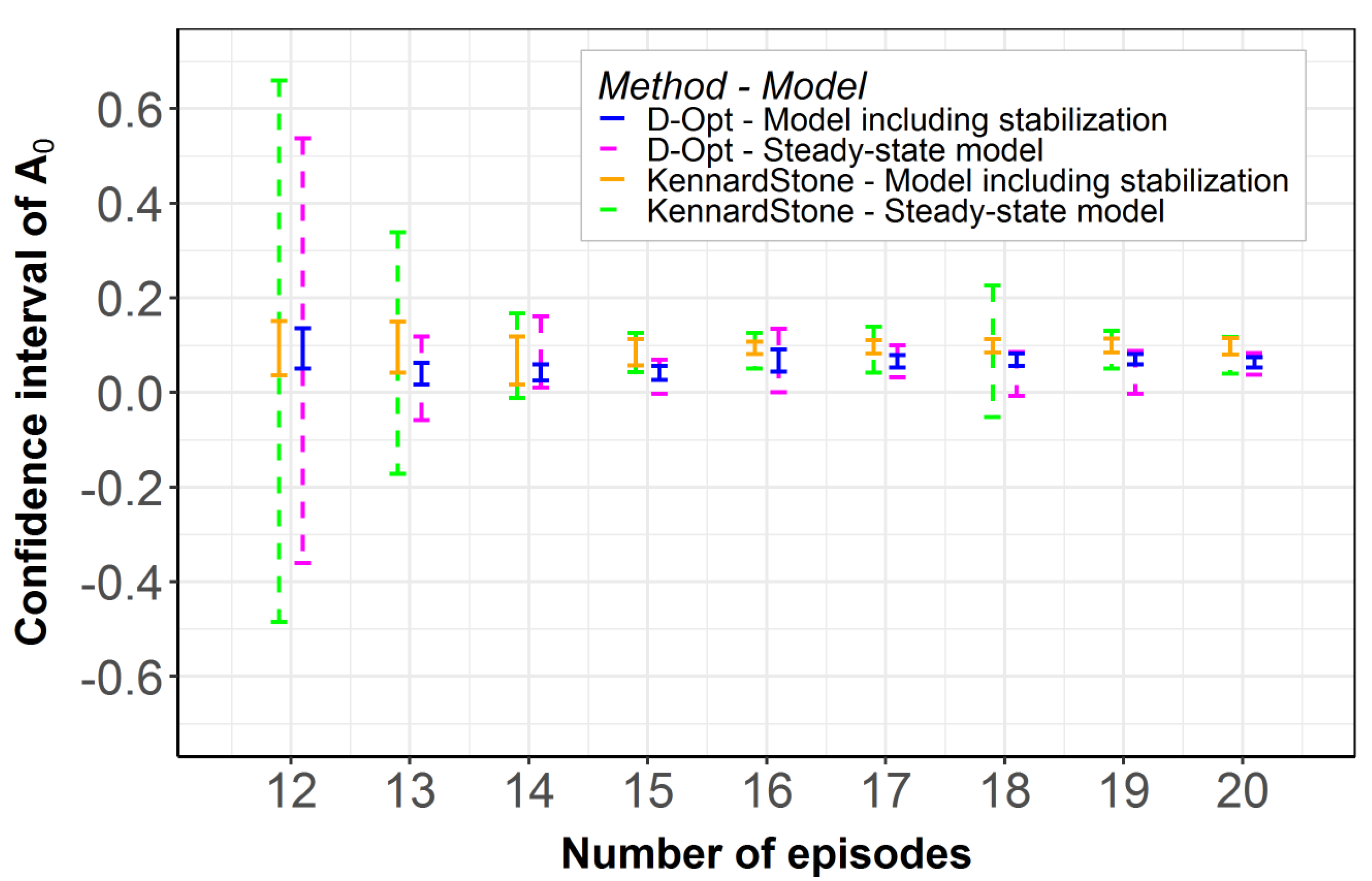
2.2. Model Robustness
3. Materials and Methods
3.1. Pilot Plant
3.2. Operating Conditions and Feedstocks
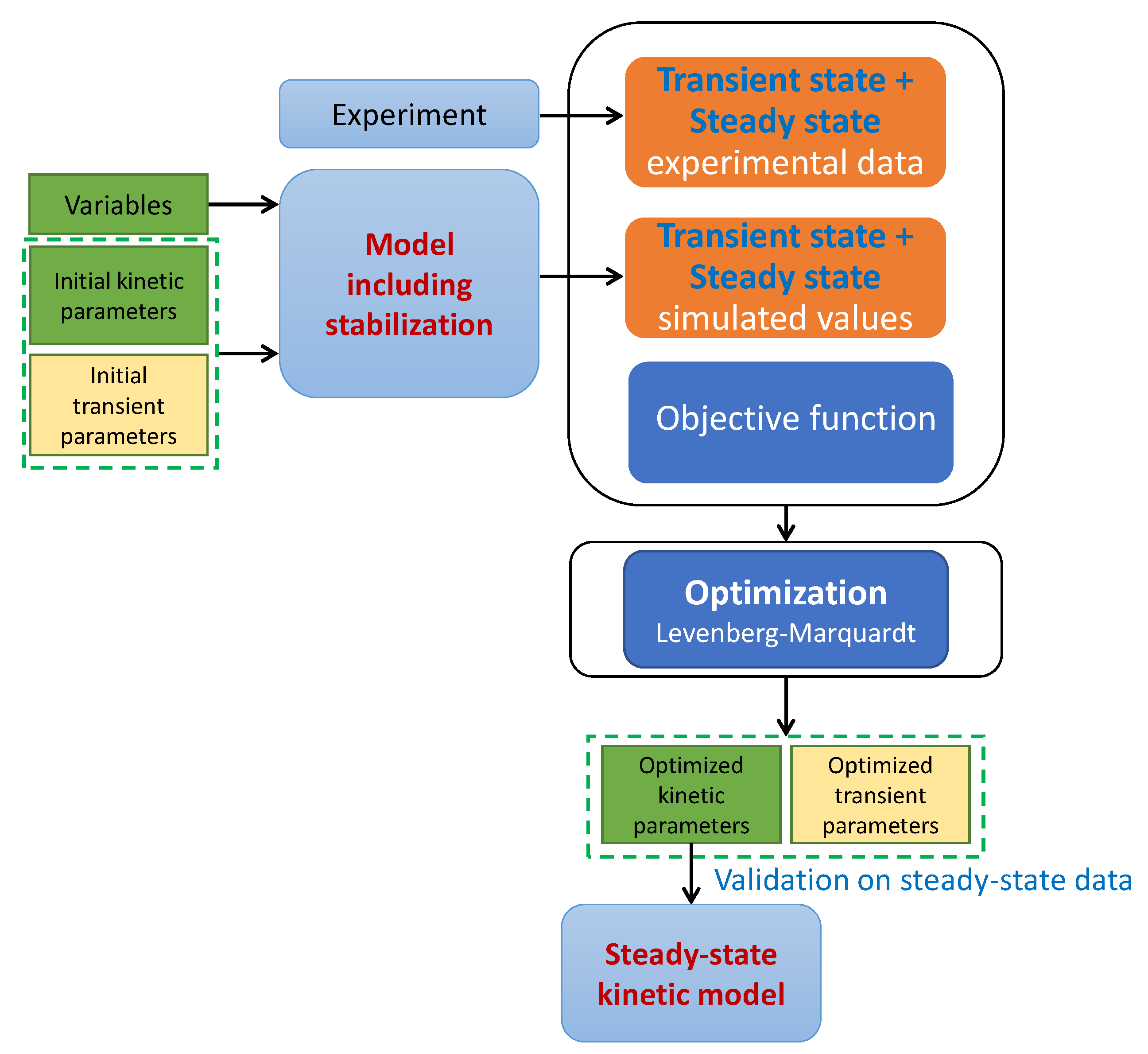
3.3. Modeling
3.3.1. Kinetic Model
3.3.2. Parameter Estimation
3.3.3. Comparison Strategy
- D-criterion: maximize the determinant of the information matrix, which means minimizing the volume of the ellipsoid
- A-criterion: minimize the sum of eigenvalues that correspond to the trace of the variance-covariance matrix
- E-criterion: minimize the largest eigenvalues that minimizes the size of the larger axis of the confidence region, also denoted as the shape criterion.
3.3.4. Robustness
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Nomenclature | ||
| a | Order associated with hydrogen partial pressure in thermodynamic term | - |
| A0 | Resin adsorption coefficient | - |
| b | Parameter in thermodynamic term | - |
| C0 | Coefficient of the ratio nitrogen/sulfur in feedstock | - |
| CN | Organic nitrogen concentration in liquid output stream | ppm m/m |
| CN,0 | Organic nitrogen concentration in feed | ppm m/m |
| CS,0 | Organic sulfur concentration in feed | % m/m |
| E | Activation energy | J.mol−1 |
| fi | Transfer function f of episode i | - |
| gi | Transfer function g of episode i | - |
| k0 | Rate constant at T0 | - |
| LHSV | Liquid hourly space velocity | h−1 |
| LHSVapp | Apparent liquid hourly space velocity of episode i | h−1 |
| LHSVi | Liquid hourly space velocity of episode i | h−1 |
| LHSVi−1 | Liquid hourly space velocity of episode i − 1 | h−1 |
| m | Order of ppH2 | - |
| n | Order of concentration of organic nitrogen | - |
| ppH2 | Hydrogen partial pressure | bar |
| ppH2,ref | Reference H2 partial pressure | bar |
| R | Ideal gas constant | J·mol−1·K−1 |
| res0 | Feed resin | % m/m |
| t | Residence time | h |
| T | Reactor temperature | K |
| T0 | Reference temperature | K |
| Tapp | Apparent temperature of episode i | K |
| Ti | Temperature of episode i | K |
| Ti−1 | Temperature of episode i − 1 | K |
| TMP | Weighted average temperature of simulated distillation by gas chromatography of feed | °C |
| TMPref | Reference weighted average temperature of simulated distillation by gas chromatography | °C |
| TOS | Time on stream | h |
| TOSi−1 | Time on stream of the last point of episode i − 1 | h |
| TOSinit_i | Time on stream when the episode i starts | h |
| tt | Order of concentration of organic nitrogen in thermodynamic term | - |
| u | factor in thermodynamic term | - |
| v | Order associated with the heaviness of feed | - |
| τi | Stabilization time at episode i | h |
References
- Speight, J.G. Hydrocracking. In The Refinery of the Future, 1st ed.; Speight, J.G., Ed.; Gulf Professional: London, UK, 2011; pp. 275–313. ISBN 9780815520412. [Google Scholar]
- Bricker, M.; Thakkar, V.; Petri, J. Hydrocracking in Petroleum Processing. In Handbook of Petroleum Processing; Treese, S.A., Pujadó, P.R., Jones, D.S.J., Eds.; Springer: Cham, Switzerland, 2015; pp. 317–359. ISBN 978-3-319-14528-0. [Google Scholar]
- Stratas Advisors. 13 Countries Move Up in Top 100 Ranking on Diesel Sulfur Limits | Stratas Advisors. Available online: https://stratasadvisors.com/Insights/2019/022819-Top-100-Diesel-Sulfur-Ranking (accessed on 15 January 2020).
- IMO. Sulphur 2020—Cutting Sulphur Oxide Emissions. Available online: http://www.imo.org/en/mediacentre/hottopics/pages/sulphur-2020.aspx (accessed on 15 January 2020).
- Ancheyta, J.; Sánchez, S.; Rodríguez, M.A. Kinetic modeling of hydrocracking of heavy oil fractions: A review. Catal. Today 2005, 109, 76–92. [Google Scholar] [CrossRef]
- Jarullah, A.T.; Mujtaba, I.M.; Wood, A.S. Kinetic model development and simulation of simultaneous hydrodenitrogenation and hydrodemetallization of crude oil in trickle bed reactor. Fuel 2011, 90, 2165–2181. [Google Scholar] [CrossRef]
- Becker, P.J.; Celse, B.; Guillaume, D.; Dulot, H.; Costa, V. Hydrotreatment modeling for a variety of VGO feedstocks: A continuous lumping approach. Fuel 2015, 139, 133–143. [Google Scholar] [CrossRef]
- Esmaeel, S.A.; Gheni, S.A.; Jarullah, A.T. 5-Lumps kinetic modeling, simulation and optimization for hydrotreating of atmospheric crude oil residue. Appl. Petrochem. Res. 2016, 6, 117–133. [Google Scholar] [CrossRef]
- Lababidi, H.M.S.; AlHumaidan, F.S. Modeling the hydrocracking kinetics of atmospheric residue in hydrotreating processes by the continuous lumping approach. Energy Fuels 2011, 25, 1939–1949. [Google Scholar] [CrossRef]
- Tang, X.; Li, S.; Yue, C.; He, J.; Hou, J. Lumping kinetics of hydrodesulfurization and hydrodenitrogenation of the middle distillate from chinese shale oil. Oil Shale 2013, 30, 517. [Google Scholar] [CrossRef]
- Bonnardot, J. Kinetic Modelling of Hydro-Treatment Reactions by Study of Different Chemical Groups. Ph.D. Thesis, Claude Bernard University, Lyon, France, 1998. [Google Scholar]
- López García, C.; Hudebine, D.; Schweitzer, J.-M.; Verstraete, J.J.; Ferré, D. In-depth modeling of gas oil hydrotreating: From feedstock reconstruction to reactor stability analysis. Catal. Today 2010, 150, 279–299. [Google Scholar] [CrossRef]
- Oyekunle, L.O.; Edafe, O.A. Kinetic modeling of hydrodenitrogenation of pyridine. Pet. Sci. Technol. 2009, 27, 557–567. [Google Scholar] [CrossRef]
- Raghuveer, C.S.; Thybaut, J.W.; Marin, G.B. Pyridine hydrodenitrogenation kinetics over a sulphided NiMo/γ-Al2O3 catalyst. Fuel 2016, 171, 253–262. [Google Scholar] [CrossRef]
- Nguyen, M.-T.; Tayakout-Fayolle, M.; Pirngruber, G.D.; Chainet, F.; Geantet, C. Kinetic modeling of quinoline hydrodenitrogenation over a NiMo(P)/Al2O3 catalyst in a batch reactor. Ind. Eng. Chem. Res. 2015, 54, 9278–9288. [Google Scholar] [CrossRef]
- Nguyen, M.-T.; Pirngruber, G.D.; Chainet, F.; Tayakout-Fayolle, M.; Geantet, C. Indole hydrodenitrogenation over alumina and silica—Alumina-supported sulfide catalysts—Comparison with quinoline. Ind. Eng. Chem. Res. 2017, 56, 11088–11099. [Google Scholar] [CrossRef]
- Doukeh, R.; Bombos, M.; Trifoi, A.; Mihai, O.; Popovici, D.; Bolocan, I.; Bombos, D. Kinetics of thiophene hydrodesulfurization over a supported Mo-Co-Ni catalyst. Comptes Rendus Chimie 2018, 21, 277–287. [Google Scholar] [CrossRef]
- Charon-Revellin, N.; Dulot, H.; López-García, C.; Jose, J. Kinetic modeling of vacuum gas oil hydrotreatment using a molecular reconstruction approach. Oil Gas Sci. Technol. Rev. IFP Energies Nouv. 2011, 66, 479–490. [Google Scholar] [CrossRef][Green Version]
- Schweitzer, J.-M.; Galtier, P.; Schweich, D. A single events kinetic model for the hydrocracking of paraffins in a three-phase reactor. Chem. Eng. Sci. 1999, 54, 2441–2452. [Google Scholar] [CrossRef]
- Elkamel, A.; Al-Ajmi, A.; Fahim, M. Modeling the hydrocracking process using artificial neural networks. Pet. Sci. Technol. 1999, 17, 931–954. [Google Scholar] [CrossRef]
- Bahmani, M.; Sharifi, K.; Shirvani, M. Product Yields Prediction of Tehran Refinery Hydrocracking Unit Using Artificial Neural Networks. Iran. J. Chem. Eng. 2010, 7, 50–63. [Google Scholar]
- Sadighi, S.; Reza Zahedi, G. Comparison of kinetic-based and artificial neural network modeling methods for a pilot scale vacuum gas oil hydrocracking reactor. Bull. Chem. React. Eng. Catal. 2013, 8. [Google Scholar] [CrossRef]
- Sadighi, S.; Mohaddecy, S.R.S. Evaluating the ability of R for modeling a commercial scale VGO hydrocracking plant using Artificial Neural Network (ANN). Pet. Coal 2018, 60, 358–364. [Google Scholar]
- de Oliveira, L.P.; Hudebine, D.; Guillaume, D.; Verstraete, J.J.; Joly, J.F. A Review of Kinetic Modeling Methodologies for Complex Processes. Oil Gas Sci. Technol. Rev. IFP Energ. Nouv. 2016, 71, 45. [Google Scholar] [CrossRef]
- Yang, S.H.; Satterfield, C.N. Some effects of sulfiding of a NiMoAl2O3 catalyst on its activity for hydrodenitrogenation of quinoline. J. Catal. 1983, 81, 168–178. [Google Scholar] [CrossRef]
- Sau, M.; Basak, K.; Manna, U.; Santra, M.; Verma, R.P. Effects of organic nitrogen compounds on hydrotreating and hydrocracking reactions. Catal. Today 2005, 109, 112–119. [Google Scholar] [CrossRef]
- Cao, N.-Y.-P.; Celse, B.; Guillaume, D.; Guibard, I.; Thybaut, J.W. Stabilization time modeling for hydroprocessing: Identification of the dominant factors. Chem. Eng. Sci. 2020, 213. [Google Scholar] [CrossRef]
- Waldron, C.; Pankajakshan, A.; Quaglio, M.; Cao, E.; Galvanin, F.; Gavriilidis, A. An autonomous microreactor platform for the rapid identification of kinetic models. React. Chem. Eng. 2019, 47, 2331. [Google Scholar] [CrossRef]
- Marquardt, D.W. An algorithm for least-squares estimation of nonlinear parameters. J. Soc. Indust. Appl. Math 1963, 11, 431–441. [Google Scholar] [CrossRef]
- More, J.J. The Levenberg-Marquardt Algorithm: Implementation and Theory; Springer: Berlin/Heidelberg, Germany, 1978; ISBN 978-3-540-35972-2. [Google Scholar]
- Kennard, R.W.; Stone, L.A. Computer aided design of experiments. Technometrics 1969, 11, 137–148. [Google Scholar] [CrossRef]
- Atkinson, A.C. Beyond response surfaces: Recent developments in optimum experimental design. Chemom. Intell. Lab. Syst. 1995, 28, 35–47. [Google Scholar] [CrossRef]
- Aguiar, P.F.; Bourguignon, B.; Khots, M.S.; Massart, D.L.; Phan-Than-Luu, R. D-optimal designs. Chemom. Intell. Lab. Syst. 1995, 30, 199–210. [Google Scholar] [CrossRef]
- Pronzato, L.; Pázman, A. Design of Experiments in Nonlinear Models; Springer: New York, NY, USA, 2013. [Google Scholar]
- Saptoro, A.; Tadé, M.O.; Vuthaluru, H. A modified Kennard-Stone algorithm for optimal division of data for developing artificial neural Network Models. Chem. Prod. Process Modeling 2012, 7. [Google Scholar] [CrossRef]
- Celse, B.; Da Costa, J.J.; Costa, V. Experimental design in nonlinear case applied to hydrocracking model: How many points do we need and which ones? Int. J. Chem. Kinet. 2016, 48, 660–670. [Google Scholar] [CrossRef]
- Fedorov, V.V. Theory of Optimal Experiments; Academic Press: New York, NY, USA, 1972; ISBN 9780323162463. [Google Scholar]
- Vandevender, W.H.; Haskell, K.H. The SLATEC Mathematical Subroutine Library. Signum Newsl. 1982, 17, 16–21. [Google Scholar] [CrossRef]
- Fox, P.A.; Hall, A.P.; Schryer, N.L. The PORT Mathematical Subroutine Library. ACM Trans. Math. Softw. 1978, 4, 104–126. [Google Scholar] [CrossRef]
- Fontijn, A.; Sabadell, A.J.; Ronco, R.J. Homogeneous chemiluminescent measurement of nitric oxide with ozone. Implications for continuous selective monitoring of gaseous air pollutants. Anal. Chem. 1970, 42, 575–579. [Google Scholar] [CrossRef]
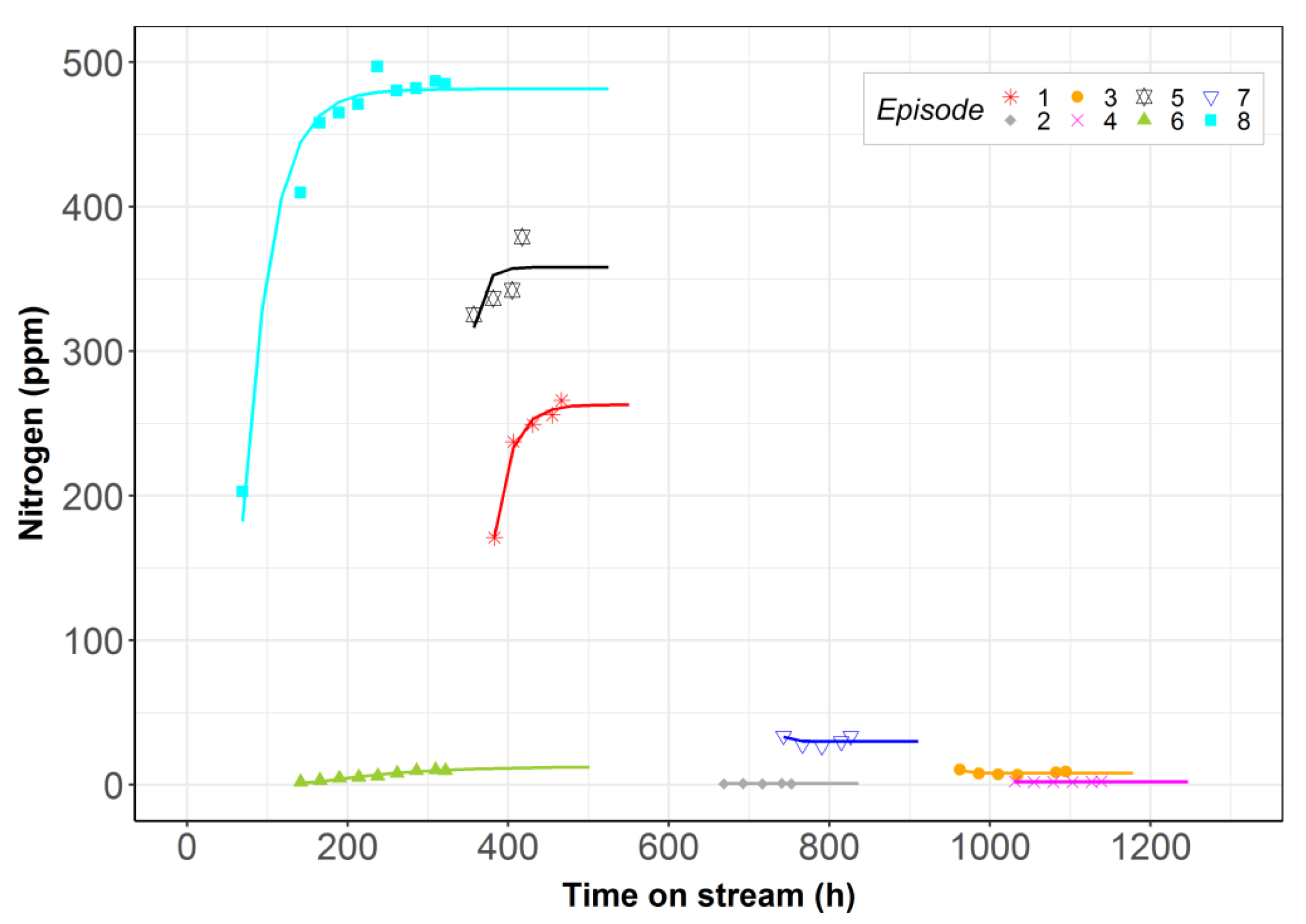

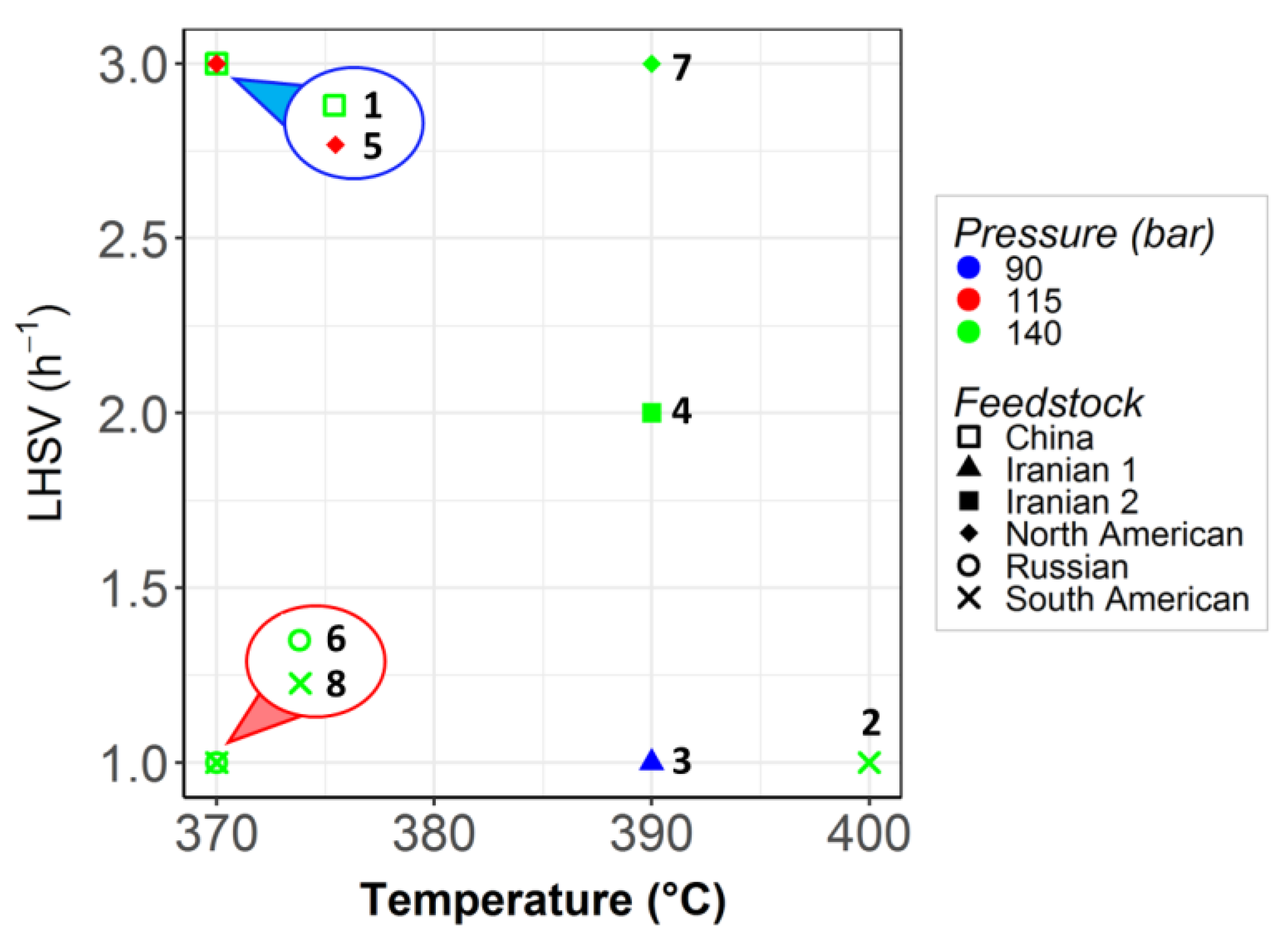

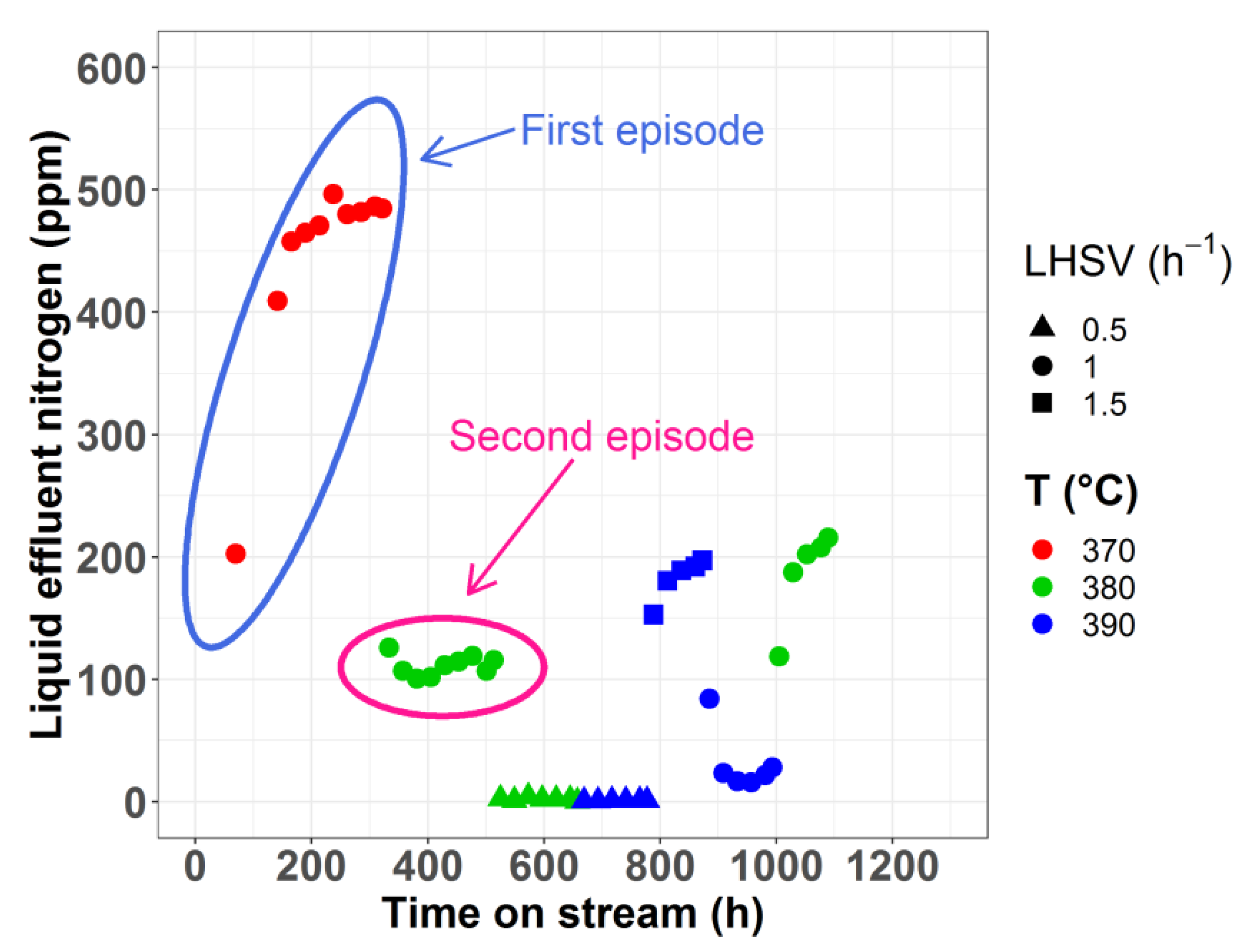
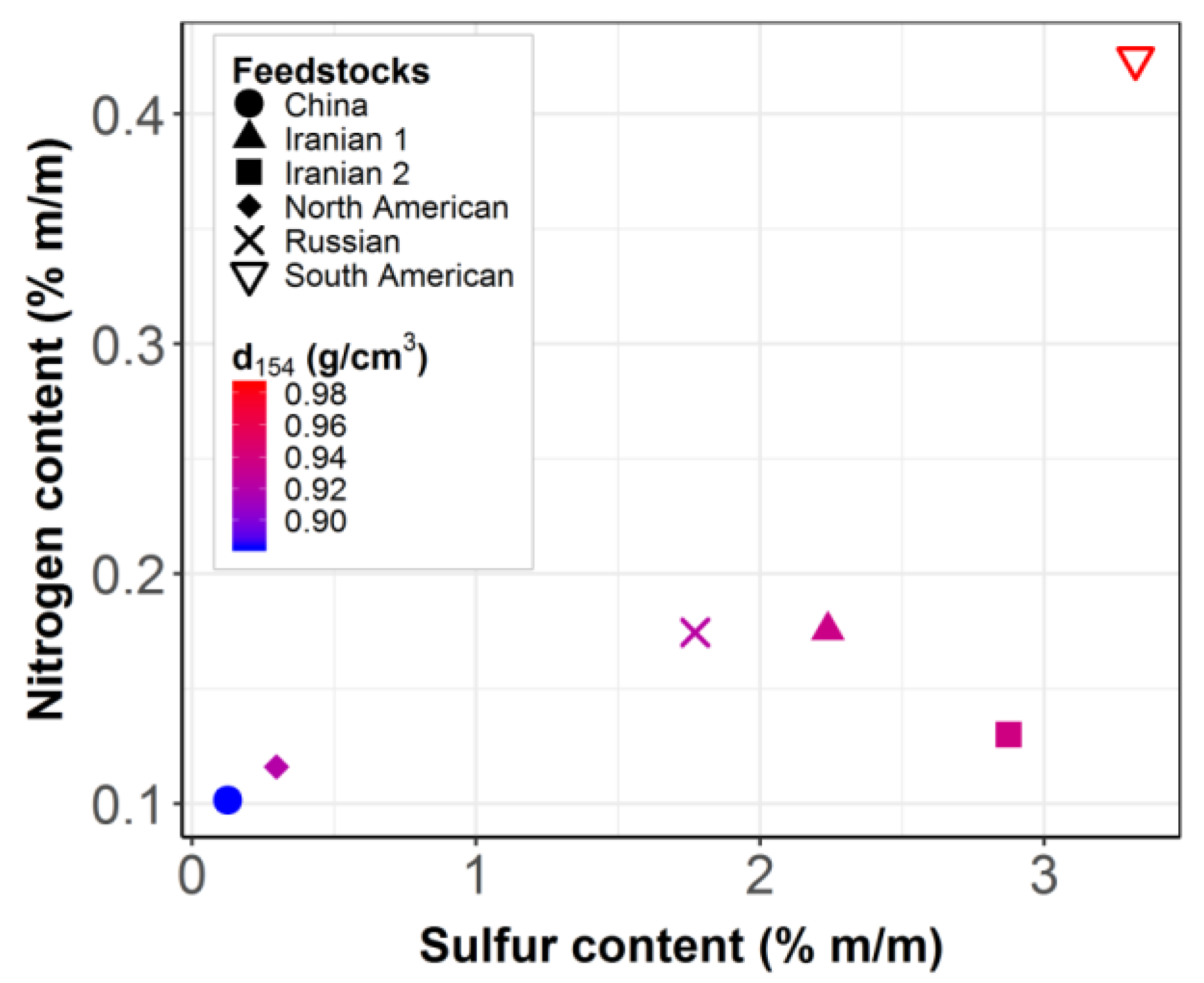
| Episode | LHSV (h−1) | T (°C) | P (bar) | Feedstock |
|---|---|---|---|---|
| 1 | 3 | 370 | 140 | China |
| 2 | 1 | 400 | 140 | South American |
| 3 | 1 | 390 | 90 | Iranian 1 |
| 4 | 2 | 390 | 140 | Iranian 2 |
| 5 | 3 | 370 | 115 | North American |
| 6 | 1 | 370 | 140 | Russian |
| 7 | 3 | 390 | 140 | North American |
| 8 | 1 | 370 | 140 | South American |
| Model | MAPE | RMSE | ||
|---|---|---|---|---|
| μ | σ | μ | σ | |
| Steady-state kinetic model | 83.9 | 43.3 | 29.1 | 12.6 |
| Model including stabilization | 69.4 | 26.9 | 20.5 | 4.0 |
| Steady-State Kinetic Model | Model Including Stabilization | |
|---|---|---|
| Original calibration database | 15 steady-state points | 104 points (89 transient points + 15 steady-state points) |
| Strategy | Select randomly and add noise to 3 steady-state points (corresponding to 20% of the original calibration database) | Select randomly and add noise to 21 points, which can be transient and/or steady-state points (corresponding to 20% of the original calibration database) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, N.-Y.-P.; Celse, B.; Guillaume, D.; Guibard, I.; Thybaut, J.W. Accelerating Kinetic Parameter Identification by Extracting Information from Transient Data: A Hydroprocessing Study Case. Catalysts 2020, 10, 361. https://doi.org/10.3390/catal10040361
Cao N-Y-P, Celse B, Guillaume D, Guibard I, Thybaut JW. Accelerating Kinetic Parameter Identification by Extracting Information from Transient Data: A Hydroprocessing Study Case. Catalysts. 2020; 10(4):361. https://doi.org/10.3390/catal10040361
Chicago/Turabian StyleCao, Ngoc-Yen-Phuong, Benoit Celse, Denis Guillaume, Isabelle Guibard, and Joris W. Thybaut. 2020. "Accelerating Kinetic Parameter Identification by Extracting Information from Transient Data: A Hydroprocessing Study Case" Catalysts 10, no. 4: 361. https://doi.org/10.3390/catal10040361
APA StyleCao, N.-Y.-P., Celse, B., Guillaume, D., Guibard, I., & Thybaut, J. W. (2020). Accelerating Kinetic Parameter Identification by Extracting Information from Transient Data: A Hydroprocessing Study Case. Catalysts, 10(4), 361. https://doi.org/10.3390/catal10040361






