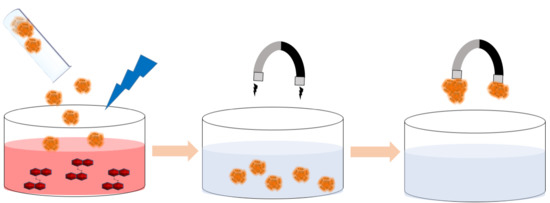
Flavin-Conjugated Iron Oxide Nanoparticles as Enzyme-Inspired Photocatalysts for Azo Dye Degradation
Abstract
1. Introduction
2. Results and Discussion
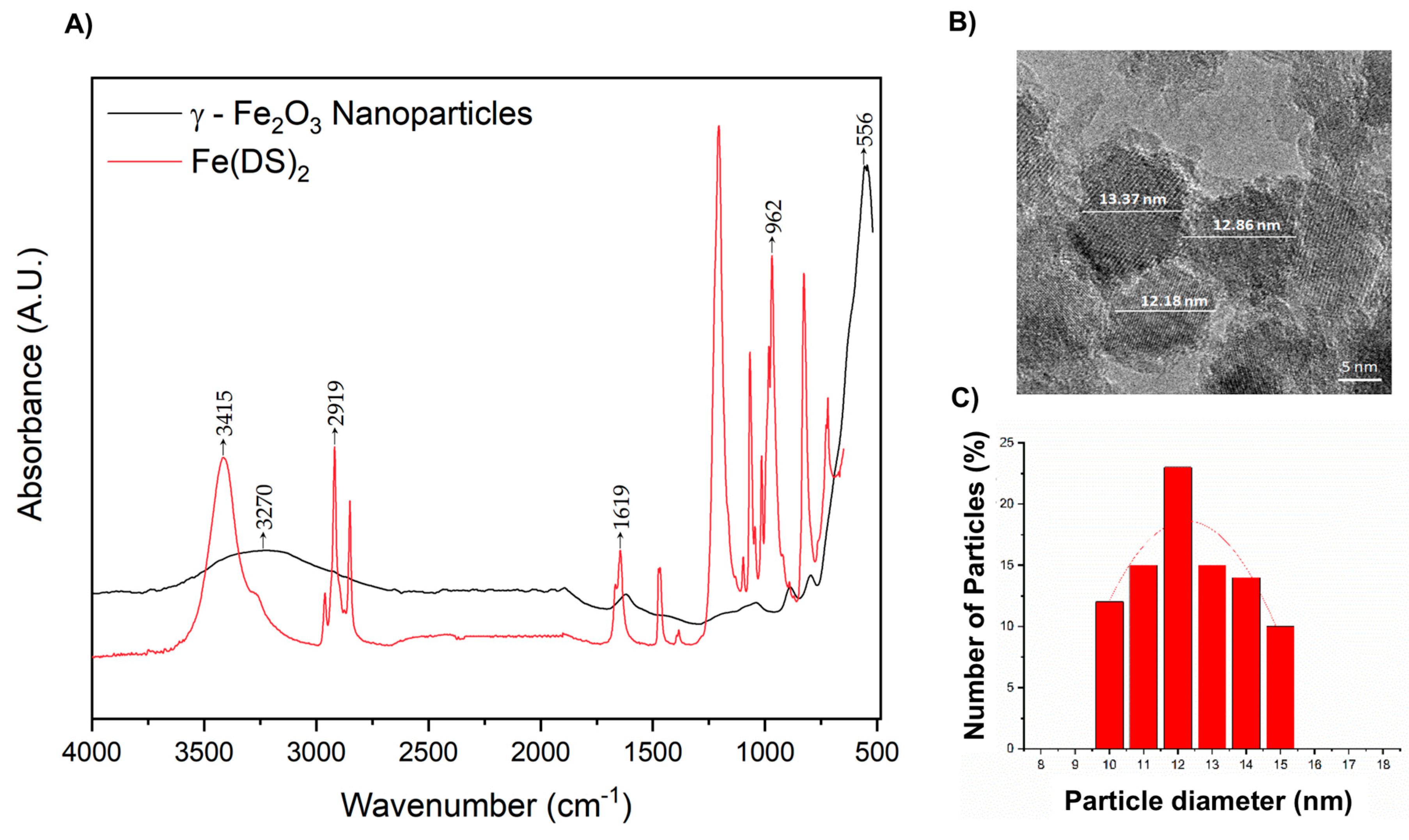
2.1. Synthesis of γ-Fe2O3 Maghemite Iron Oxide Nanoparticles (IONPs)
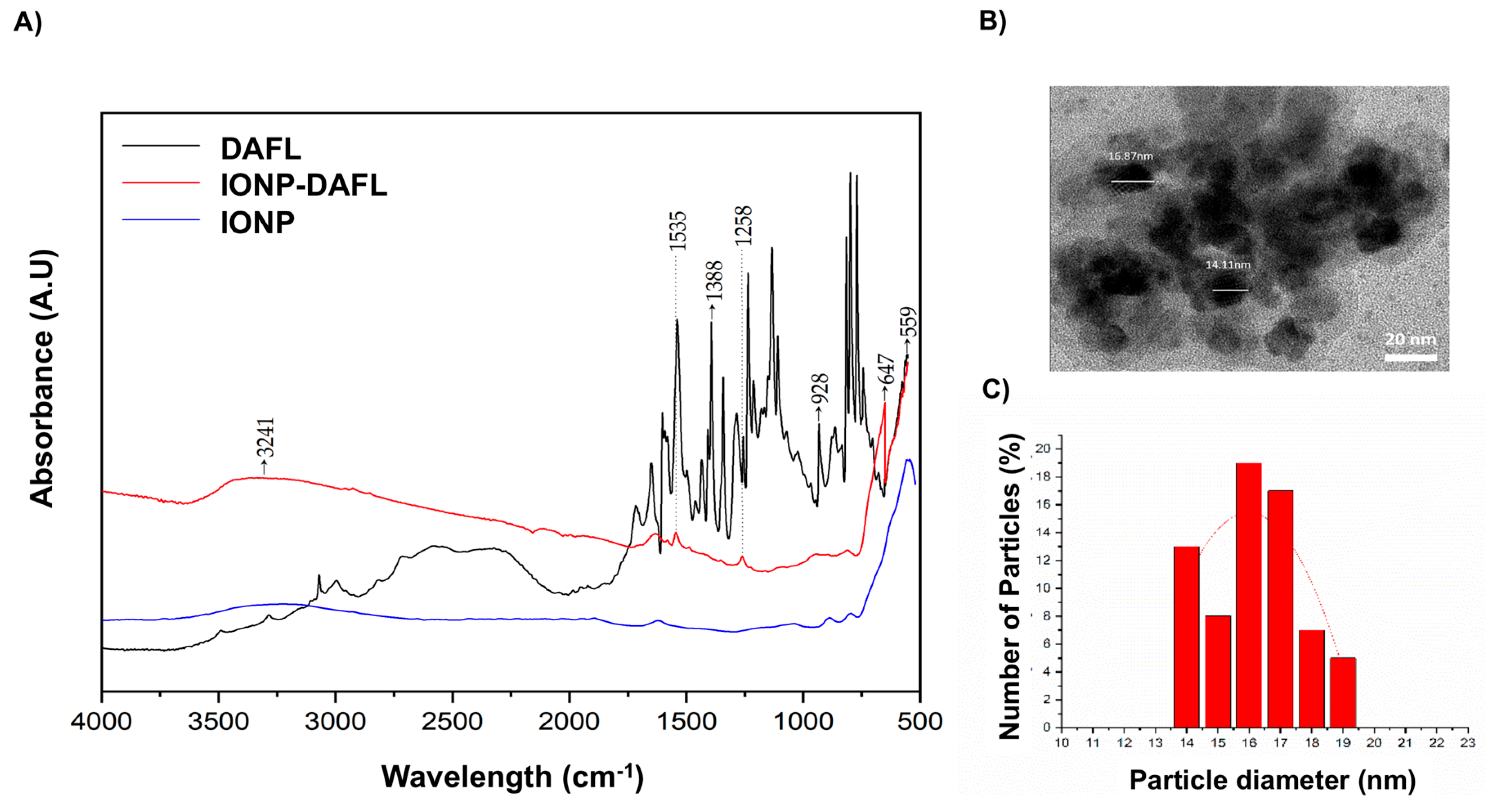
2.2. Surface Modification of γ-Fe2O3 Nanoparticles
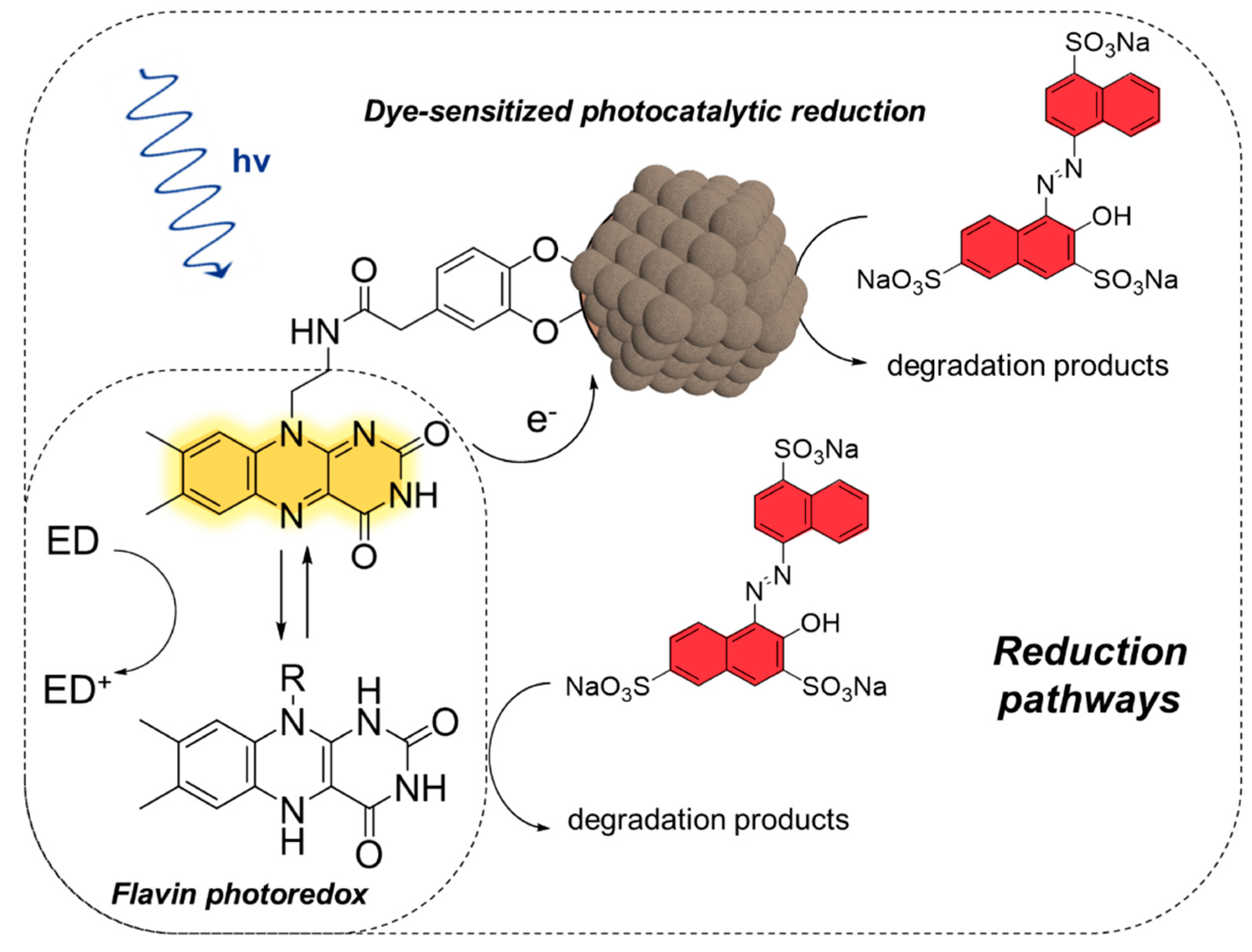
2.3. Amaranth Photodegradation
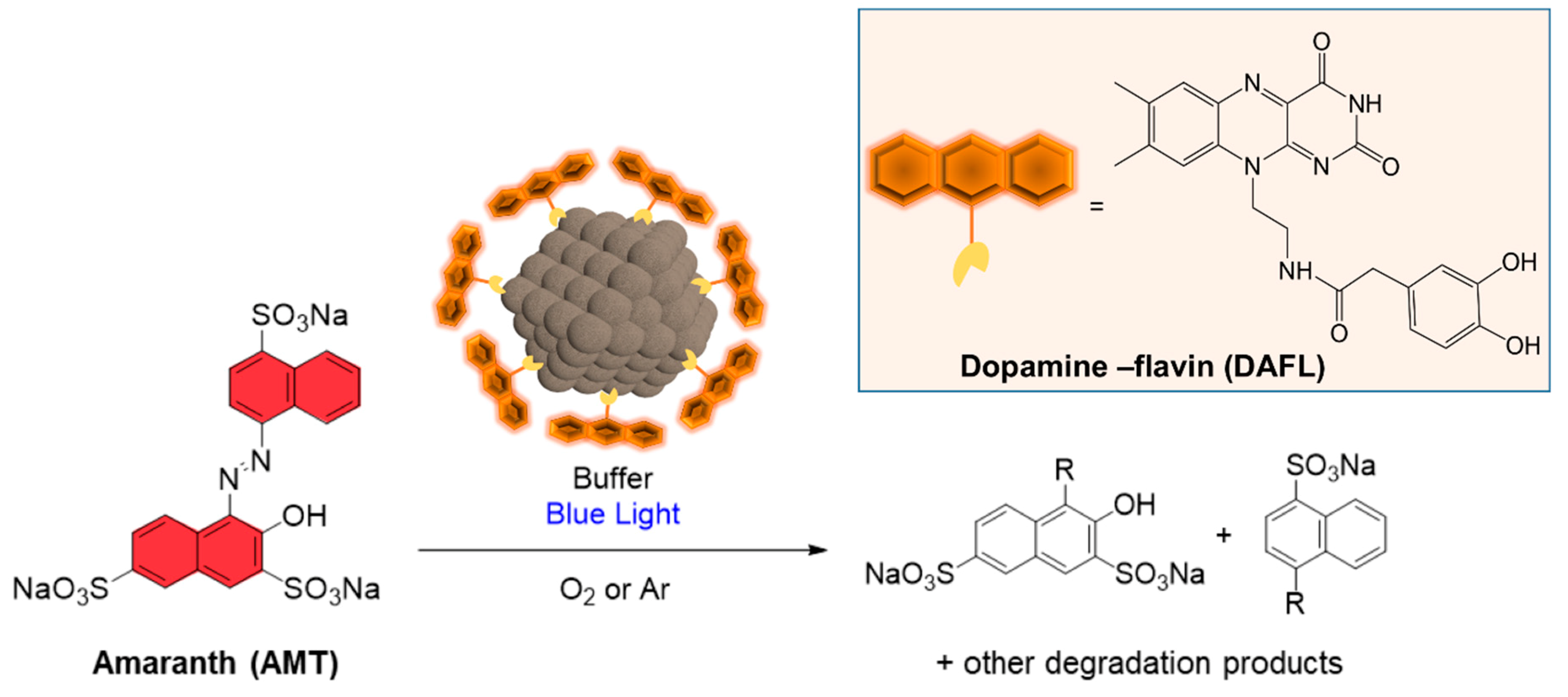
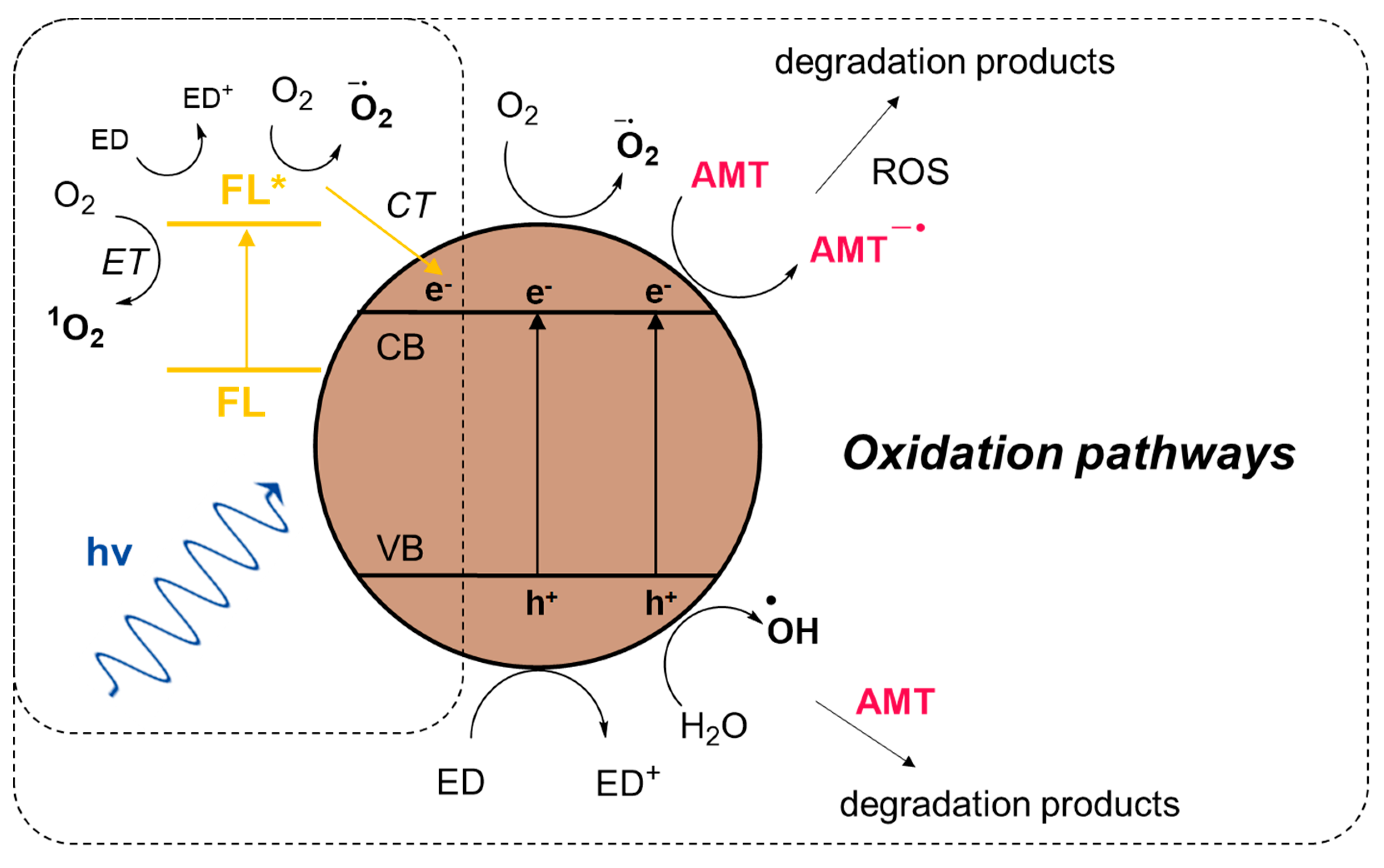
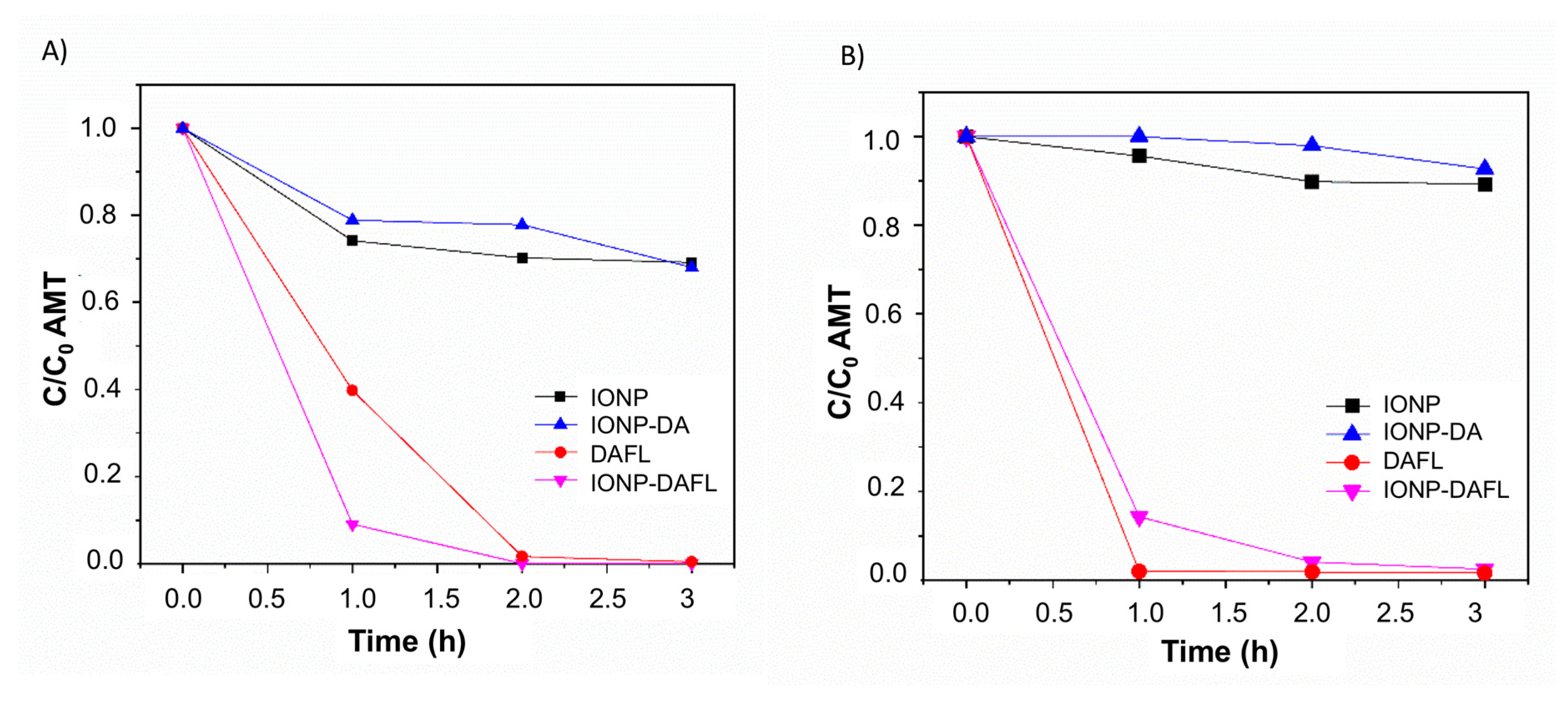
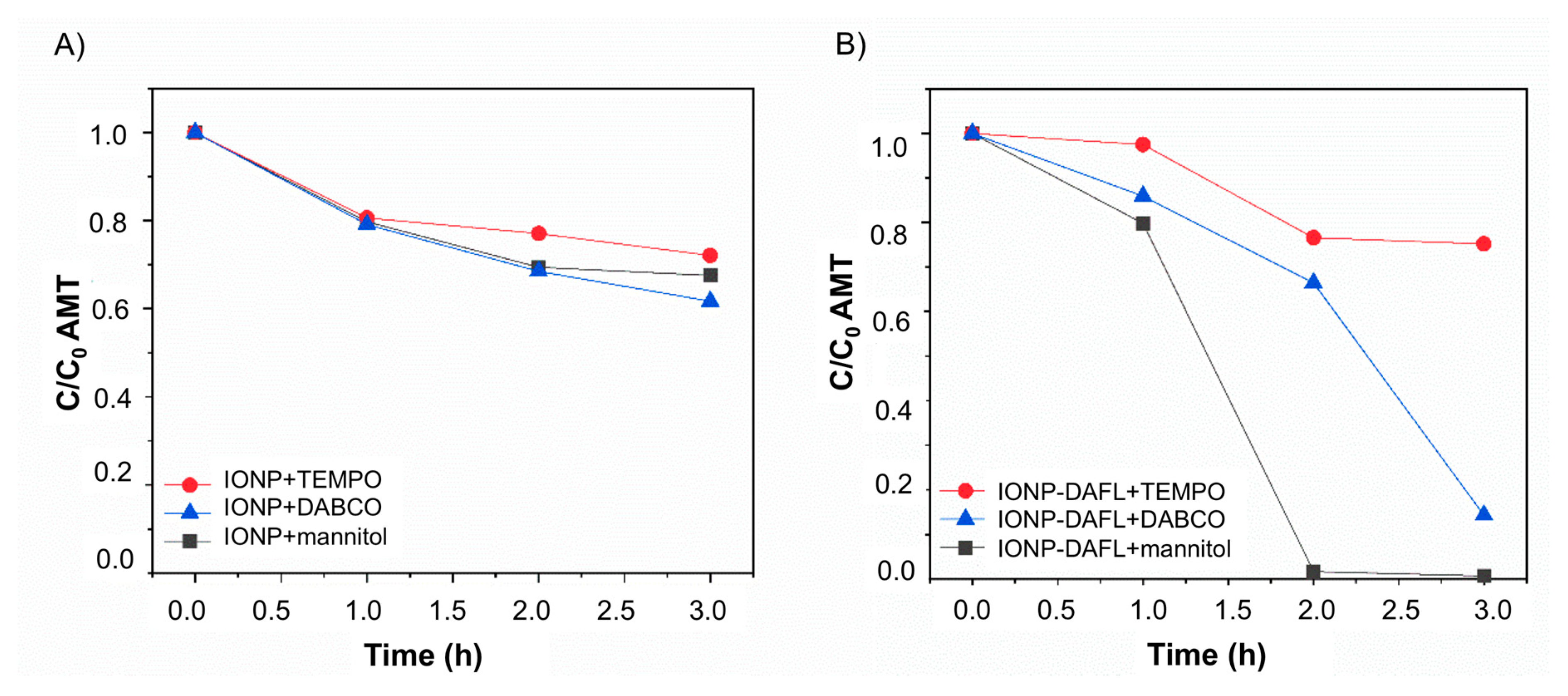
2.3.1. Amaranth Photooxidation (Aerobic Activity)
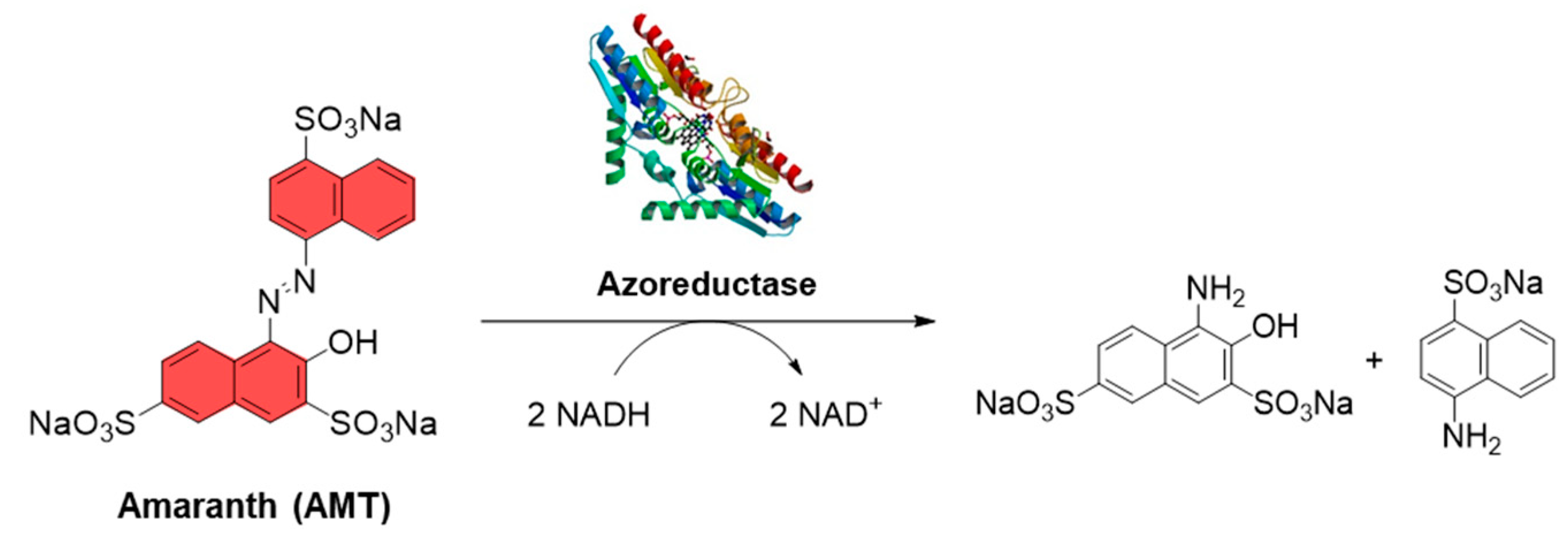
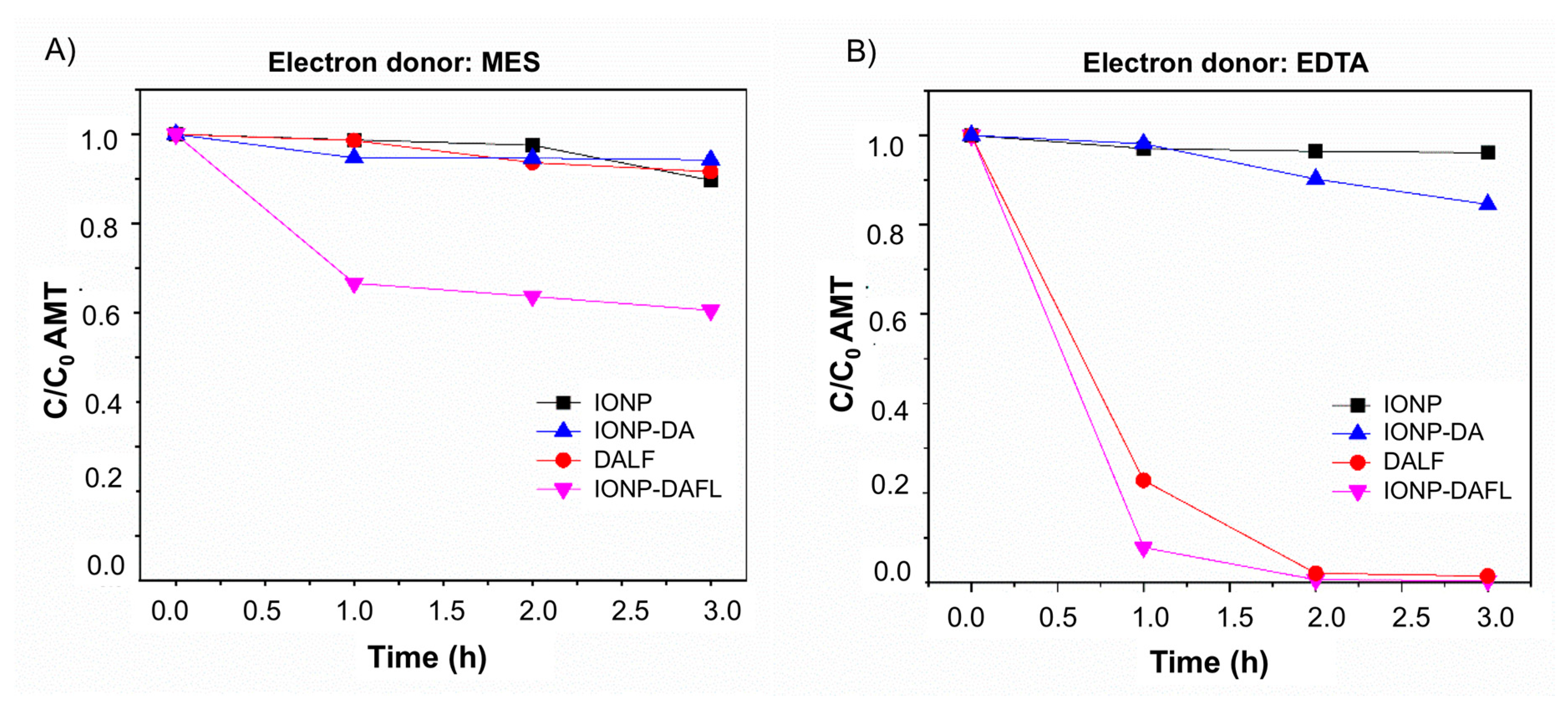
2.3.2. Amaranth Photoreduction (Anaerobic Activity)
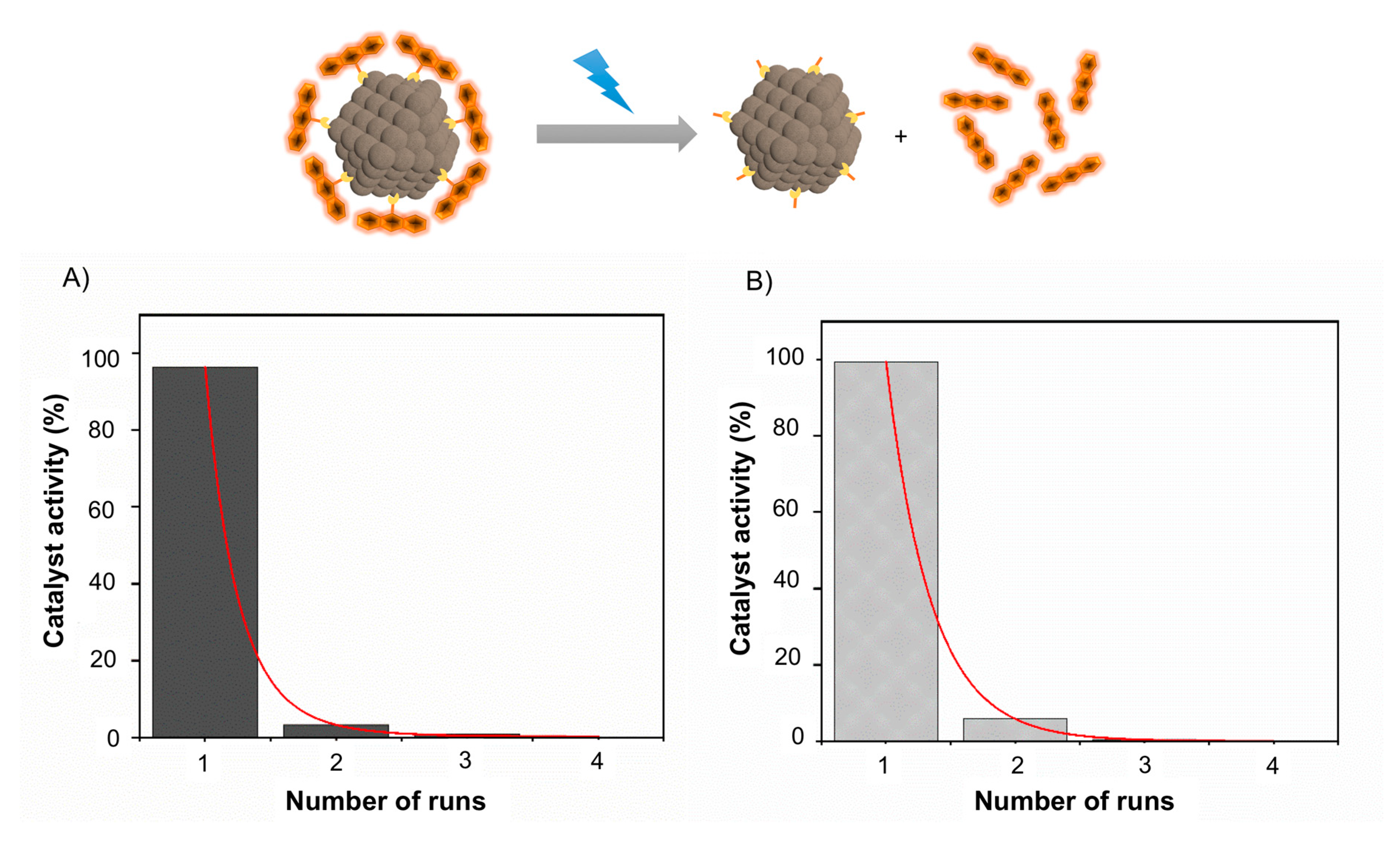
2.3.3. Reusability of the Catalyst
3. Materials and Methods
3.1. General
3.2. Synthesis of Ferrous dodecyl Sulfate (Fe(DS)2)
3.3. Synthesis of γ-Fe2O3 Maghemite Iron Oxide Nanoparticles (IONPs)
3.4. Synthesis of Dopamine-Flavin (DAFL)
3.5. Surface Modification of γ-Fe2O3 Nanoparticles
3.6. General Procedure for Photoreduction and Photooxidation of Amaranth
3.7. General Procedure for ROS Scavenging Experiments
3.8. General Procedure for Recyclability Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowldgments
Conflicts of Interest
References
- Han, F.; Kambala, V.S.R.; Srinivasan, M.; Rajarathnam, D.; Naidu, R. Tailored titanium dioxide photocatalysts for the degradation of organic dyes in wastewater treatment: A review. Appl. Catal. A Gen. 2009, 359, 25–40. [Google Scholar] [CrossRef]
- Fernández, C.; Larrechi, M.S.; Callao, M.P. An analytical overview of processes for removing organic dyes from wastewater effluents. TrAC Trends Anal. Chem. 2010, 29, 1202–1211. [Google Scholar] [CrossRef]
- Jin, C.; Liu, D.; Hu, J.; Wang, Y.; Zhang, Q.; Lv, L.; Zhuge, F. The role of microstructure in piezocatalytic degradation of organic dye pollutants in wastewater. Nano Energy 2019, 59, 372–379. [Google Scholar] [CrossRef]
- Monsef, R.; Ghiyasiyan-Arani, M.; Amiri, O.; Salavati-Niasari, M. Sonochemical synthesis, characterization and application of PrVO4 nanostructures as an effective photocatalyst for discoloration of organic dye contaminants in wastewater. Ultrason. Sonochem. 2020, 61, 104822. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Banerjee, A.; Halder, U.; Biswas, R.; Bandopadhyay, R. Degradation of Synthetic Azo Dyes of Textile Industry: A Sustainable Approach Using Microbial Enzymes. Water Conserv. Sci. Eng. 2017, 2, 121–131. [Google Scholar] [CrossRef]
- Sudha, M.; Saranya, A.; Selvakumar, G.; Sivakumar, N. Microbial degradation of Azo Dyes: A review. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 670–690. [Google Scholar]
- Chung, K.-T. Azo dyes and human health: A review. J. Environ. Sci. Health Part C Environ. Carcinog. Ecotoxicol. Rev. 2016, 34, 233–261. [Google Scholar] [CrossRef]
- Shahmoradi, B.; Maleki, A.; Byrappa, K. Photocatalytic degradation of Amaranth and Brilliant Blue FCF dyes using in situ modified tungsten doped TiO2 hybrid nanoparticles. Catal. Sci. Technol. 2011, 1, 1216–1223. [Google Scholar] [CrossRef]
- Saratale, R.G.; Saratale, G.D.; Chang, J.-S.; Govindwar, S.P. Bacterial decolorization and degradation of azo dyes: A review. J. Taiwan Inst. Chem. Eng. 2011, 42, 138–157. [Google Scholar] [CrossRef]
- Delclos, K.B.; Tarpley, W.G.; Miller, E.C.; Miller, J.A. 4-Aminoazobenzene and N,N-Dimethyl-4-Aminoazobenzene as Equipotent Hepatic Carcinogens in Male C57BL/6 x C3H/He F1 Mice and Characterization of N-(Deoxyguanosin-8-yl)-4-Aminoazobenzene as the Major Persistent Hepatic DNA-Bound Dye in These Mice. Cancer Res. 1984, 44, 2540–2550. [Google Scholar]
- Singh, R.L.; Singh, P.K.; Singh, R.P. Enzymatic decolorization and degradation of azo dyes—A review. Int. Biodeterior. Biodegrad. 2015, 104, 21–31. [Google Scholar] [CrossRef]
- Kumar, J.; Bansal, A. Photodegradation of amaranth in aqueous solution catalyzed by immobilized nanoparticles of titanium dioxide. Int. J. Environ. Sci. Technol. 2012, 9, 479–484. [Google Scholar] [CrossRef][Green Version]
- Rafii, F.; Franklin, W.; Cerniglia, C.E. Azoreductase activity of anaerobic bacteria isolated from human intestinal microflora. Appl. Environ. Microbiol. 1990, 56, 2146–2151. [Google Scholar] [CrossRef]
- Hadibarata, T.; Kristanti, R.A. Fate and cometabolic degradation of benzo[a]pyrene by white-rot fungus Armillaria sp. F022. Bioresour. Technol. 2012, 107, 314–318. [Google Scholar] [CrossRef]
- McMullan, G.; Meehan, C.; Conneely, A.; Kirby, N.; Robinson, T.; Nigam, P.; Banat, I.M.; Marchant, R.; Smyth, W.F. Microbial decolourisation and degradation of textile dyes. Appl. Microbiol. Biotechnol. 2001, 56, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Robinson, T.; McMullan, G.; Marchant, R.; Nigam, P. Remediation of dyes in textile effluent: A critical review on current treatment technologies with a proposed alternative. Bioresour. Technol. 2001, 77, 247–255. [Google Scholar] [CrossRef]
- Misal, S.A.; Gawai, K.R. Azoreductase: A key player of xenobiotic metabolism. Bioresour. Bioprocess. 2018, 5. [Google Scholar] [CrossRef]
- Liu, G.; Zhou, J.; Fu, Q.S.; Wang, J. The Escherichia coli azoreductase AzoR is involved in resistance to thiol-specific stress caused by electrophilic quinones. J. Bacteriol. 2009, 191, 6394–6400. [Google Scholar] [CrossRef] [PubMed]
- König, B.; Kümmel, S.; Svobodová, E.; Cibulka, R. Flavin photocatalysis. Phys. Sci. Rev. 2018, 3. [Google Scholar] [CrossRef]
- Crocker, L.; Fruk, L. Flavin conjugated polydopamine nanoparticles displaying light-driven monooxygenase activity. Front. Chem. 2019, 7, 278. [Google Scholar] [CrossRef] [PubMed]
- Crocker, L.; Koehler, P.; Bernhard, P.; Kerbs, A.; Euser, T.; Fruk, L. Enzyme-inspired flavin-polydopamine as a biocompatible nanoparticle photocatalyst. Nanoscale Horizons 2019, 4, 1318–1325. [Google Scholar] [CrossRef]
- Sakthivel, S.; Neppolian, B.; Shankar, M.V.; Arabindoo, B.; Palanichamy, M.; Murugesan, V. Solar photocatalytic degradation of azo dye: Comparison of photocatalytic efficiency of ZnO and TiO2. Sol. Energy Mater. Sol. Cells 2003, 77, 65–82. [Google Scholar] [CrossRef]
- Cerrón-Calle, G.A.; Aranda-Aguirre, A.J.; Luyo, C.; Garcia-Segura, S.; Alarcón, H. Photoelectrocatalytic decolorization of azo dyes with nano-composite oxide layers of ZnO nanorods decorated with Ag nanoparticles. Chemosphere 2019, 219, 296–304. [Google Scholar] [CrossRef]
- Sajjad, A.K.L.; Shamaila, S.; Tian, B.; Chen, F.; Zhang, J. Comparative studies of operational parameters of degradation of azo dyes in visible light by highly efficient WOx/TiO2. J. Hazard. Mater. 2010, 177, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Yang, X.; Ma, F.; Li, K.; Xu, L.; Yuan, X.; Guo, Y. Additive-free controllable fabrication of bismuth vanadates and their photocatalytic activity toward dye degradation. Appl. Surf. Sci. 2010, 256, 2215–2222. [Google Scholar] [CrossRef]
- Noman, M.; Shahid, M.; Ahmed, T.; Niazi, M.B.K.; Hussain, S.; Song, F.; Manzoor, I. Use of biogenic copper nanoparticles synthesized from a native Escherichia sp. as photocatalysts for azo dye degradation and treatment of textile effluents. Environ. Pollut. 2019, 257, 113514. [Google Scholar] [CrossRef]
- Feng, Z.; Yu, J.; Kong, J.; Wang, T. A novel porous Al2O3 layer/AgNPs-Hemin composite for degradation of azo dyes under visible and UV irradiation. Chem. Eng. J. 2016, 294, 236–245. [Google Scholar] [CrossRef]
- Ozmen, N.; Erdemoglu, S.; Gungordu, A.; Asilturk, M.; Turhan, D.O.; Akgeyik, E.; Harper, S.L.; Ozmen, M. Photocatalytic degradation of azo dye using core@shell nano-TiO2 particles to reduce toxicity. Environ. Sci. Pollut. Res. 2018, 25, 29493–29504. [Google Scholar] [CrossRef]
- Mishra, M.; Chun, D.-M. α-Fe2O3 as a photocatalytic material: A review. Appl. Catal. A Gen. 2015, 498, 126–141. [Google Scholar] [CrossRef]
- Sundaramurthy, J.; Kumar, P.S.; Kalaivani, M.; Thavasi, V.; Mhaisalkar, S.G.; Ramakrishna, S. Superior photocatalytic behaviour of novel 1D nanobraid and nanoporous α-Fe2O3 structures. RSC Adv. 2012, 2, 8201–8208. [Google Scholar] [CrossRef]
- Ling, D.; Hyeon, T. Chemical design of biocompatible iron oxide nanoparticles for medical applications. Small 2013, 9, 1450–1466. [Google Scholar] [CrossRef] [PubMed]
- Chirita, M.; Grozescu, I. Fe2O3—Nanoparticles, Physical Properties and Their Photochemical and Photoelectrochemical Applications. Chem. Bull. Politech. Univ. Timsisoara 2009, 54, 1–8. [Google Scholar]
- Pandiri, M.; Hossain, M.S.; Foss, F.W.; Rajeshwar, K.; Paz, Y. Enhanced photocatalytic activity of a self-stabilized synthetic flavin anchored on a TiO2 surface. Phys. Chem. Chem. Phys. 2016, 18, 18575–18583. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rochkind, M.; Pandiri, M.; Hossain, M.S.; Foss, F.W.; Rajeshwar, K.; Paz, Y. Enhancement of Photoinduced Visible Light Degradation of Salicylic Acid by Covalently Attached Synthetic Flavins on BiOCl Semiconductor Particle Surfaces. J. Phys. Chem. C 2016, 120, 16069–16079. [Google Scholar] [CrossRef]
- Dongare, P.; MacKenzie, I.; Wang, D.; Nicewicz, D.A.; Meyer, T.J. Oxidation of alkyl benzenes by a flavin photooxidation catalyst on nanostructured metal-oxide films. Proc. Natl. Acad. Sci. USA 2017, 114, 9279–9283. [Google Scholar] [CrossRef]
- Benyettou, F.; Guenin, E.; Lalatonne, Y.; Motte, L. Microwave assisted nanoparticle surface functionalization. Nanotechnology 2011, 22. [Google Scholar] [CrossRef]
- Bhardwaj, V.; Sharma, P.; Chauhan, M.S.; Chauhan, S. Thermodynamic, FTIR, 1H-NMR, and acoustic studies of butylated hydroxyanisole and sodium dodecyl sulfate in ethanol, water rich and ethanol rich solutions. J. Mol. Liq. 2013, 180, 192–199. [Google Scholar] [CrossRef]
- Karimzadeh, I.; Aghazadeh, M.; Ganjali, M.R.; Norouzi, P.; Shirvani-Arani, S.; Doroudi, T.; Kolivand, P.H.; Marashi, S.A.; Gharailou, D. A novel method for preparation of bare and poly(vinylpyrrolidone) coated superparamagnetic iron oxide nanoparticles for biomedical applications. Mater. Lett. 2016, 179, 5–8. [Google Scholar] [CrossRef]
- Hematyar, M.; Es-Haghi, A.; Soleimani, M.; Mokarram, A.R. Determination of trace amounts of nystatin in water and vaccine samples using sodium dodecyl sulfate-coated iron oxide magnetic nanoparticles followed by high-performance liquid chromatography–ultraviolet detection. J. Iran. Chem. Soc. 2017, 14, 2665–2671. [Google Scholar] [CrossRef]
- Liu, C.; Guo, J.; Yang, W.; Hu, J.; Wang, C.; Fu, S. Magnetic mesoporous silica microspheres with thermo-sensitive polymer shell for controlled drug release. J. Mater. Chem. 2009, 19, 4764–4770. [Google Scholar] [CrossRef]
- Santos, M.C.; Seabra, A.B.; Pelegrino, M.T.; Haddad, P.S. Synthesis, characterization and cytotoxicity of glutathione- and PEG-glutathione-superparamagnetic iron oxide nanoparticles for nitric oxide delivery. Appl. Surf. Sci. 2016, 367, 26–35. [Google Scholar] [CrossRef]
- Prodan, A.M.; Iconaru, S.L.; Chifiriuc, C.M.; Bleotu, C.; Ciobanu, C.S.; Motelica-Heino, M.; Sizaret, S.; Predoi, D. Magnetic properties and biological activity evaluation of iron oxide nanoparticles. J. Nanomater. 2013, 2013. [Google Scholar] [CrossRef]
- Wu, W.; Wu, Z.; Yu, T.; Jiang, C.; Kim, W.-S. Recent progress on magnetic iron oxide nanoparticles: Synthesis, surface functional strategies and biomedical applications. Sci. Technol. Adv. Mater. 2015, 16. [Google Scholar] [CrossRef]
- Zhu, N.; Ji, H.; Yu, P.; Niu, J.; Farooq, M.U.; Akram, M.W.; Udego, I.O.; Li, H.; Niu, X. Surface modification of magnetic iron oxide nanoparticles. Nanomaterials 2018, 8, 810. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, J.; Xu, Y.; Lovas, K.; Qin, Y.; Bao, Y. Surface functionalization of dopamine coated iron oxide nanoparticles for various surface functionalities. J. Magn. Magn. Mater. 2017, 427, 220–224. [Google Scholar] [CrossRef]
- Geiseler, B.; Fruk, L. Bifunctional catechol based linkers for modification of TiO2 surfaces. J. Mater. Chem. 2012, 22, 735–741. [Google Scholar] [CrossRef]
- Du, X.; Li, L.; Li, J.; Yang, C.; Frenkel, N.; Welle, A.; Heissler, S.; Nefedov, A.; Grunze, M.; Levkin, P.A. Uv-triggered dopamine polymerization: Control of polymerization, surface coating, and photopatterning. Adv. Mater. 2014, 26, 8029–8033. [Google Scholar] [CrossRef]
- Rieff, B.; Bauer, S.; Mathias, G.; Tavan, P. IR spectra of flavins in solution: DFT/MM description of redox effects. J. Phys. Chem. B 2011, 115, 2117–2123. [Google Scholar] [CrossRef]
- Sonibare, O.O.; Haeger, T.; Foley, S.F. Structural characterization of Nigerian coals by X-ray diffraction, Raman and FTIR spectroscopy. Energy 2010, 35, 5347–5353. [Google Scholar] [CrossRef]
- Radu, T.; Iacovita, C.; Benea, D.; Turcu, R. X-Ray Photoelectron Spectroscopic Characterization of Iron Oxide Nanoparticles. Appl. Surf. Sci. 2017, 405, 337–343. [Google Scholar] [CrossRef]
- Grosvenor, A.P.; Kobe, B.A.; Biesinger, M.C.; McIntyre, N.S. Investigation of multiplet splitting of Fe 2p XPS spectra and bonding in iron compounds. Surf. Interface Anal. 2004, 36, 1564–1574. [Google Scholar] [CrossRef]
- Suresh, R.; Giribabu, K.; Manigandan, R.; Stephen, A.; Narayanan, V. Fabrication of Ni-Fe2O3 magnetic nanorods and application to the detection of uric acid. RSC Adv. 2014, 4, 17146–17155. [Google Scholar] [CrossRef]
- Guivar, J.A.R.; Sanches, E.A.; Bruns, F.; Sadrollahi, E.; Morales, M.A.; López, E.O.; Litterst, F.J. Vacancy ordered γ-Fe2O3 nanoparticles functionalized with nanohydroxyapatite: XRD, FTIR, TEM, XPS and Mössbauer studies. Appl. Surf. Sci. 2016, 389, 721–734. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A.Y. Generation and Detection of Reactive Oxygen Species in Photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef]
- Gonçalves, L.C.P.; Mansouri, H.R.; Bastos, E.L.; Abdellah, M.; Fadiga, B.S.; Sá, J.; Rudroff, F.; Mihovilovic, M.D. Morpholine-based buffers activate aerobic photobiocatalysis: Via spin correlated ion pair formation. Catal. Sci. Technol. 2019, 9, 1365–1371. [Google Scholar] [CrossRef]
- Massey, V.; Stankovich, M.; Hemmerich, P. Light-Mediated Reduction of Flavoproteins with Flavins as Catalysts. Biochemistry 1978, 17, 1–8. [Google Scholar] [CrossRef]
- De Castro, S.A.; Ruggiero, E.; Ruiz-De-Angulo, A.; Rezabal, E.; Mareque-Rivas, J.C.; Lopez, X.; López-Gallego, F.; Salassa, L. Riboflavin as a bioorthogonal photocatalyst for the activation of a PtIV prodrug. Chem. Sci. 2017, 8, 4619–4625. [Google Scholar] [CrossRef]
- Orchard, K.L.; Hojo, D.; Sokol, K.P.; Chan, M.-J.; Asao, N.; Adschiri, T.; Reisner, E. Catechol-TiO2 hybrids for photocatalytic H2 production and photocathode assembly. Chem. Commun. 2017, 53, 12638–12641. [Google Scholar] [CrossRef]
- Pellegrin, Y.; Odobel, F. Les donneurs d’électron sacrificiels pour la production de combustible solaire. Comptes Rendus Chim. 2017, 20, 283–295. [Google Scholar] [CrossRef]
- Patel, T.K.; Williamson, J.D. Mannitol in Plants, Fungi, and Plant–Fungal Interactions. Trends Plant Sci. 2016, 21, 486–497. [Google Scholar] [CrossRef]
- Lee, S.H.; Choi, D.S.; Kuk, S.K.; Park, C.B. Photobiocatalysis: Activating Redox Enzymes by Direct or Indirect Transfer of Photoinduced Electrons. Angew. Chemie Int. Ed. 2018, 57, 7958–7985. [Google Scholar] [CrossRef] [PubMed]
- Špačková, J.; Svobodová, E.; Hartman, T.; Stibor, I.; Kopecká, J.; Cibulková, J.; Chudoba, J.; Cibulka, R. Visible Light [2+2] Photocycloaddition Mediated by Flavin Derivative Immobilized on Mesoporous Silica. ChemCatChem 2017, 9, 1177–1181. [Google Scholar] [CrossRef]
- Sheraz, M.A.; Kazi, S.H.; Ahmed, S.; Anwar, Z.; Ahmad, I. Photo, thermal and chemical degradation of riboflavin. Beilstein J. Org. Chem. 2014, 10, 1999–2012. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.A.; Wani, M.Y.; Hashim, M.A. Microemulsion method: A novel route to synthesize organic and inorganic nanomaterials: 1st Nano Update. Arab. J. Chem. 2012, 5, 397–417. [Google Scholar] [CrossRef]
- Chen, H. Recent Advances in Azo Dye Degrading Enzyme Research. Curr. Protein Pept. Sci. 2006, 7, 101–111. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, S.; Quan, X.; Yu, H.; Zhao, H. Integration of microfiltration and visible-light-driven photocatalysis on g-C3N4 nanosheet/reduced graphene oxide membrane for enhanced water treatment. Appl. Catal. B Environ. 2016, 194, 134–140. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nehme, S.I.; Crocker, L.; Fruk, L. Flavin-Conjugated Iron Oxide Nanoparticles as Enzyme-Inspired Photocatalysts for Azo Dye Degradation. Catalysts 2020, 10, 324. https://doi.org/10.3390/catal10030324
Nehme SI, Crocker L, Fruk L. Flavin-Conjugated Iron Oxide Nanoparticles as Enzyme-Inspired Photocatalysts for Azo Dye Degradation. Catalysts. 2020; 10(3):324. https://doi.org/10.3390/catal10030324
Chicago/Turabian StyleNehme, Samer I., Leander Crocker, and Ljiljana Fruk. 2020. "Flavin-Conjugated Iron Oxide Nanoparticles as Enzyme-Inspired Photocatalysts for Azo Dye Degradation" Catalysts 10, no. 3: 324. https://doi.org/10.3390/catal10030324
APA StyleNehme, S. I., Crocker, L., & Fruk, L. (2020). Flavin-Conjugated Iron Oxide Nanoparticles as Enzyme-Inspired Photocatalysts for Azo Dye Degradation. Catalysts, 10(3), 324. https://doi.org/10.3390/catal10030324