Abstract
Mesoporous LaFeO3/g-C3N4 Z-scheme heterojunctions (LFC) were synthesized via the incorporation of LaFeO3 nanoparticles and porous g-C3N4 ultrathin nanosheets. The as prepared LFC were characterized by transmission electron microscopy, scanning electron microscopy, atomic force microscopy, X-ray photoelectron spectroscopy, powder X-ray diffraction, Raman spectra and N2 adsorption analysis. The structural analysis indicated that the reheating process and the addition of NH4Cl in the thermal polymerization were the key factors to get porous g-C3N4 ultrathin nanosheets and to obtain high specific surface areas of LFC. It remarkably enhanced the adsorption capacity and photocatalytic degradation of LFC for removal of oxytetracycline (OTC). The effect of the mass percentage of LaFeO3 in LFC, pH and temperature on the OTC adsorption was investigated. The LaFeO3/g-C3N4 heterojunction with 2 wt % LaFeO3 (2-LFC) exhibited highest saturated adsorption capacity (101.67 mg g−1) and largest photocatalytic degradation rate constant (1.35 L g−1 min−1), which was about 9 and 5 times higher than that of bulk g-C3N4 (CN), respectively. This work provided a facile method to prepare mesoporous LaFeO3/g-C3N4 heterojunctions with especially well adsorption and photocatalytic activities for OTC, which can facilitate its practical applications in pollution control.
1. Introduction
Environmental pollution is one of the most formidable challenges that human being currently confronts [1]. Antibiotic is a kind of persistent pollutant. Various antibiotics have been found in rivers, soils and groundwater. The increasing antibiotic resistance caused by the release of drugs and specific chemicals into the environment has become the most worrying health threats at present [2,3]. Up to now, several methods, such as biodegradation [4], adsorption [5] and photocatalytic degradation, have been utilized to remove antibiotic pollutants in water. The photocatalysis can be satisfactory applied for decontamination of natural samples through the photocatalytic degradation of toxic pollutants from complex matrices like river waters and waste waters [6]. Oxytetracycline (OTC) is a broad-spectrum antibiotic commonly applied to aquaculture and veterinary medicine, which is mainly excreted in unchanged and active forms in effluents of surface water [7,8]. Most of OTC cannot be eliminated by biological and traditional treatment processes due to its high stability. Therefore, the exploration of a highly efficient strategy to treat the environmental antibiotics pollution is still a research focus in this period [9,10,11]. Semiconductor-based photocatalytic system is regarded as a renewable and economical technology for pollutant removal using solar energy as the power source. A large amount of semiconductor-based photocatalysts have been developed recently to eliminate the antibiotic residues, including the degradation of OTC [12,13,14,15]. However, most of them are ultraviolet light response photocatalysts, which means inefficient utilization of solar energy. The development of visible light driven photocatalysts has thus aroused widespread concern [1,3,16,17]. A large number of visible light responsive photocatalysts have been investigated including oxides (such as Cu2O [18], BiVO4 [19], Bi2WO6 [20]), sulphides (e.g. Zn-Cu-In-S [21], CdS [22]) and BiOX [23,24].
Recently, graphitic carbon nitride (g-C3N4) has attracted extensive attention of researchers. It has high chemical stability and appropriate energy band gap (about 2.7 eV), which made it as the next generation of visible-light responsive photocatalysts for photocatalytic water splitting, organic pollutant photodegradation and CO2 reduction [25,26,27,28]. However, the high recombination rate of photoinduced electron hole pair and poor adsorption capability restricted its wider practical application [29]. The design and construction of heterojunction photocatalysts based on g-C3N4 and narrow band gap semiconductor has been demonstrated an effective approach to significantly enhance the photocatalytic activities due to the improvement of separation efficiency of photoinduced carriers [30]. Designing Z-scheme heterojunctions was one of the most attractive methods to obtain photocatalysts with high quantum efficiency [31]. Compared with the single components, narrow band gap, and visible light responsive photocatalysts, the Z-scheme heterojunctions have more negative conduction band (CB) and more positive valence band (VB). Therefore, it has stronger redox capability and meanwhile retained its respective visible light absorption feature. Many g-C3N4 based Z-scheme heterojunctions have been reported (such as BiVO4/g-C3N4 [32], MoS2/g-C3N4 [33], BiOCl/g-C3N4 [34], Ag3PO4/g-C3N4 [35], WO3/g-C3N4 [36], and so on). LaFeO3 (LF) possessed the bandgap of ∼2.36 eV, corresponding the absorption wavelength of about 525 nm [37]. It was a promising photocatalysts with visible light response. Nowadays, LaFeO3/g-C3N4 Z-scheme heterojunctions have been fabricated to promote the separation efficiency of photoinduced carriers and to enhance the photocatalytic activities [38]. The photocatalytic activities, including degradation performance and hydrogen evolution rate, of LaFeO3/g-C3N4 heterojunctions were much higher than that of pure LaFeO3 and g-C3N4 under visible light irradiation [39,40,41,42,43].
Adsorption has been widely used in the treatment of antibiotic wastewater owing to the advantages of convenient operation, low cost, high efficiency and no high toxic by-products. However, adsorption just transfers hazardous organic substances to another phase, which cannot be fully mineralized to non-polluting small molecules like carbon dioxide and water [29,44]. The antibiotic pollutants consequently cannot be removed actually by utilizing the adsorption method separately. In addition, the adsorbents can generally be recycled and reused difficultly for long and complicated desorption process of adsorbate. To photocatalytic degradation reaction, the adsorption of organic pollutants on the surface of photocatalysts was the prerequisite for mineralization of the pollutants. So the combination of adsorption with photocatalysis should fabricate a class of materials with higher efficiency. Many reports have been devoted to the development of the heterojunction photocatalysts based on g-C3N4 with the synergetic effect of adsorption and photocatalytic degradation capability [44,45,46,47,48]. The construction of these heterojunction boosted the separation efficiency of photoinduced carriers under visible-light illumination and enhanced the adsorption capability. Herein, we proposed a mesoporous LaFeO3/g-C3N4 Z-scheme heterojunction for removal of oxytetracycline hydrochloride. The synergistic effect of adsorption and photocatalytic degradation of OTC over mesoporous LaFeO3/g-C3N4 heterojunction was evaluated in contrast with pure LaFeO3 and g-C3N4. The adsorption kinetics and adsorption isotherm of OTC on LaFeO3/g-C3N4 were investigated and further analyzed with different models. It was observed that as-prepared mesoporous LaFeO3/g-C3N4 heterojunction possessed more excellent adsorption capability and higher separation efficiency of photoinduced carriers compared with pristine LaFeO3 and g-C3N4.
2. Results and Discussion
2.1. Characterization of Prepared Mesoporous LaFeO3/g-C3N4 Heterojunction
The AFM image of LaFeO3/g-C3N4 heterojunction was shown in Figure 1. The thickness of a single g-C3N4 flake was about 3.5 nm, which indicated that it appeared nanosheet feature. It was also observed that bulk g-C3N4 was formed by multiple nanosheet structures stacked loosely together, suggesting it had larger specific surface area for improving the adsorption capability of OTC. Some LaFeO3 spherical particles were dispersed and attached on the g-C3N4 nanosheets.
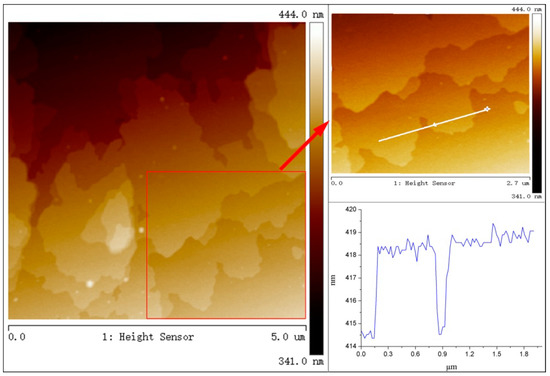
Figure 1.
AFM image of 2-LFC.
The morphology and composition of 2-LFC was confirmed by SEM and EDS and shown in Figure 2a,d. The g-C3N4 nanosheets were aggregated and formed large irregular loose layered structure. The composition of 2-LFC and the amount of La, Fe, C, N and O was measured by EDS spectrum (Figure 2d). The EDS results proved that the weight percent of LaFeO3 was around 2 wt %. It was consistent with the initial dosage of metal nitrates in reaction precursors. Similar LaFeO3 content was obtained by XPS analysis. In addition, the gold signals in the EDS spectrum of 2-LFC originated from the sputtered Au layer before SEM observation.

Figure 2.
(a) SEM, (b) TEM, (c) HRTEM images, and (d) EDS spectrum of 2-LFC.
TEM and HRTEM images of 2-LFC was displayed in Figure 2b,c. In the TEM image, LaFeO3/g-C3N4 heterojunction revealed a thin and porous lamellar structure with wrinkles on the particle edge. The pore size was inhomogeneous. The pore diameter was from about 10 to 40 nm. The porous structure of 2-LFC and the pore diameters could be clearly observed in Figure 2b. The inhomogeneous pores originated from the thermal decomposition of ammonium chloride, which resembled the results in the literature [49]. LaFeO3 nanoparticles were attached on the surface of mesoporous g-C3N4 nanosheets with some aggregation. The particle sizes of LaFeO3 were estimated to be around 15 to 30 nm. The multiple nanosheets stacked structures and porous lamellar morphology was exhibited distinctly in the HRTEM image (Figure 2c). It also demonstrated that LaFeO3 particles attached on the surface of g-C3N4 nanosheets. The intimate contact of LaFeO3 and mesoporous g-C3N4 nanosheets was the requirement for the formation of the heterostructure and consequently boosting the separation efficiency of photoinduced carriers.
The elemental mapping images of 2-LFC were presented in Figure 3. C, N, O, La and Fe appeared nearly uniform distribution in the field of view. It was the evidence indicating that LaFeO3 particles were homogeneously distributed on the surface of g-C3N4 nanosheets rather than aggregating on the part of g-C3N4, which was conducive to the formation of more heterostructures and further promoting the separation of photogenerated carries.

Figure 3.
The elemental mapping images of 2-LFC.
XRD patterns of the synthesized LF, CN, 2-LFC and 5-LFC heterostructures were shown in Figure 4a. Both CN and LFC heterostructures exhibited two distinct diffraction peaks at 13.0 and 27.6°, which could be indexed to the (110) and (022) planes of CN and be corresponded to the in-plane structural packing motif and the interlayer stacking reflection of aromatic systems, respectively [44]. The XRD pattern of pure LF could be assigned to orthorhombic phase (PDF#37-1493). No obvious diffraction peaks related to LF were observed in 2-LFC heterostructures. It was attributed to the small amount of LaFeO3 in the heterostructures, which could not change the bulk structure and chemical skeleton of g-C3N4 [50,51]. In fact, the elemental mapping, HRTEM and XPS results demonstrated the existence of LaFeO3 and EDS results proved the content of LaFeO3 in 2-LFC heterostructures. With the increase of LaFeO3 content in the heterostructures, the patterns of 5-LFC were composed of diffraction peaks of both LaFeO3 and g-C3N4. It suggested that LaFeO3 has been introduced successfully onto the g-C3N4 nanosheets. In addition, the broaden peaks of 5-LFC revealed small crystals of LaFeO3 in the heterostructures.

Figure 4.
(a) XRD patterns of LF, CN, 2-LFC and 5-LFC, (b) Raman spectra of CN and 2-LFC.
The Raman spectra of CN and 2-LFC were shown in Figure 4b. Raman spectroscopy was considered to be a standard nondestructive and effective technique to characterize the chemical structures, especially the defects and the disorder of sp2 carbon phase in CN and 2-LFC [52,53,54]. There were three characteristic sharp peaks located at 1345, 1590 and 2690 cm−1 for 2-LFC and CN. The peak at 1345 cm−1 corresponded to the D band, usually originated from K-point phonons of A1g symmetry. The peak at 1580 cm−1 assigned to the G band, which associated with zone center phonons of E2g symmetry [52]. The peak of 2690 cm−1 was the 2D band related to the double resonance Raman process [53]. The feature of these peaks was consistent with that of graphene-based materials [54], where the D peak was regarded as a disorder peak derived from the breathing motion of sp2 carbon rings, the G peak was considered as a graphite peak arose from the stretching vibration of sp2 carbon atoms in both rings and chains, and the 2D peak was a typical peak closely interrelated with the single layer grapheme. The Raman spectra results demonstrated the graphene like structure of CN in LaFeO3/g-C3N4 heterojunctions. ID/IG (the D/G peak intensity ratio) was calculated for the comparison of the defects presented in CN and 2-LFC heterojunctions. ID/IG was 1.03 for CN and 1.04 for 2-LFC, which almost kept constant for these samples. It indicated that CN and 2-LFC exhibited plenty of defects originated from the doped nitrogen atoms in the graphite carbon nitride structure [55].
Figure 5a depicted the X-ray photoelectron spectroscopy (XPS) survey spectra of CN and 2-LFC heterojunctions. It revealed the as-prepared 2-LFC heterojunctions contained La, Fe, O, C and N. The 2-LFC possessed weaker C1s and N1s peaks because of the lower content of g-C3N4 in the 2-LFC heterojunctions. The O1s peak intensity was stronger than that of CN due to the increase of LaFeO3 mass ratio in the heterojunctions. The high resolution XPS spectra of C1s, N1s and O1s were exhibited in Figure 5b,c and d, respectively. Two peaks were observed for C1s spectra in Figure 5b. They were located at 288.07 and 284.60 eV for CN, and at 287.97 and 284.57 eV for 2-LFC. The peaks at about 288 eV were originated from sp2 bonded C (N-C=N) in triazine cycles of the graphitic C3N4 structure [38]. The peaks at approximately 284.6 eV were allocated to contaminated carbon. It was found that the peaks of CN at about 288 eV shifted toward lower binding energy after it combined with LaFeO3. Furthermore, for N1s spectra in Figure 5c, the peak at 398.50 eV for CN moved to 398.14 eV while it integrated with LaFeO3. This peak was assigned to sp2 hybridized nitrogen atoms. Meanwhile, the O1s peak at 532.13 eV of CN changed to 531.42 eV when LaFeO3 was incorporated into the CN. The C1s, N1s and O1s peaks of CN shifted toward lower binding energy indicated the effect of chemical interaction between LaFeO3 and g-C3N4, and suggested the successful formation of LaFeO3/g-C3N4 heterojunctions [56].
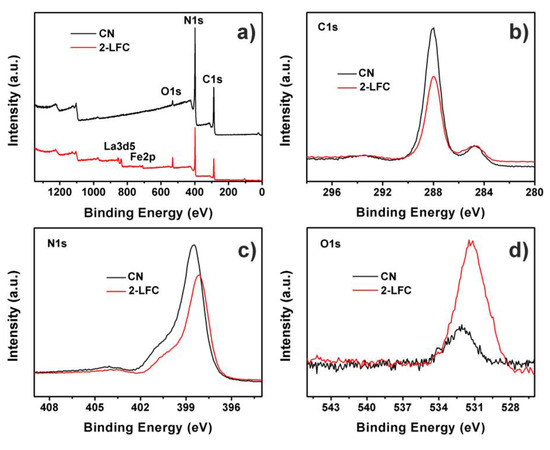
Figure 5.
(a) XPS survey spectra and high resolution XPS spectra of (b) C1s, (c) N1s and (d) O1s of CN and 2-LFC heterojunctions.
The porous structures and BET surface areas of CN and 2-LFC were acquired by the N2 adsorption-desorption experiments implemented at 77 K. The results were displayed in Figure 6. The N2 adsorption-desorption isotherms exhibited type IV feature with high adsorption at high relative pressures. It indicated the formation of mesoporous structures of CN and 2-LFC [29]. The BET surface area of 2-LFC was 79.65 m2 g−1. It was over 5 times than that of CN (14.67 m2 g−1). The BJH pore size distribution curves of CN and 2-LFC (Figure 6b) revealed that these samples had a pore size distribution in the range of 20–60 nm, which was well identical with the pore diameter observed in TEM images (Figure 2c). Moreover, a sharp peak of 2-LFC was located between 12 to 17 nm, which could be attributed to the formation of porous ultra-thin g-C3N4 nanosheets. In our synthetic approach, 2-LFC was prepared by secondary heating proccess, which resulted in thermal etching of bulk CN. It was one of the effective way to manufacture ultra-thin g-C3N4 nanosheets. Consequently, 2-LFC could possesse higher specific surface area and broader pore size distribution, which was conducive to obtain more edge structures and reactive sites [29]. And then, it led to higher adsorption capacity and photocatalytic activity of 2-LFC.
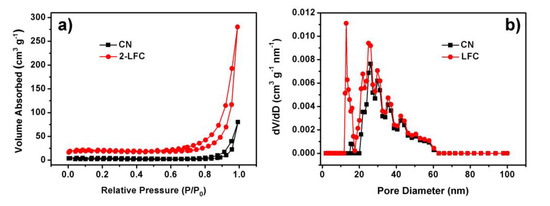
Figure 6.
(a) The N2 adsorption desorption isotherms and (b) the corresponding BJH pore size distribution curves of CN and 2-LFC.
2.2. Adsorption Kinetics of OTC
The effect of the mass percentage of LaFeO3 in LaFeO3/g-C3N4 heterojunctions, pH and temperature on the OTC adsorption was investigated (Figure 7). A series of LFC heterojunctions with different dosages of LaFeO3 were ultilized as the adsorbents for the adsorption of OTC (Figure 7a). The mass ratio of LaFeO3 in the heterojunctions can significantly affect the adsorption amount of OTC. When the mass percentage of LaFeO3 was between 2% and 10%, corresponding to 2-LFC, 5-LFC and 10-LFC samples, the adsorption capacity was highest. The adsorption capacity decreased slightly with the increase of LaFeO3 content in this mass range. As the comparison, the adsorption capacity of the g-C3N4 bulk material (CN) obtained by only one-time thermal polymerization was far lower than that of the heterojunctions with LaFeO3 mass content of 2–10%. This was because the 2-LFC, 5-LFC and 10-LFC heterojunctions was synthesized by secondary heating approach, which resulted in thermal etching. This was considered to be one of the effective methods reported in the literature to prepare ultra-thin g-C3N4 nanosheets. The formation of ultra-thin g-C3N4 nanosheets made it have higher specific surface area (Figure 6). By contrast, the g-C3N4 bulk material was prepared by one-time thermal polymerization. CN aggregated into micron level particles in this reaction condition and had lower specific surface area. Therefore, CN had poor adsorption performance. This inference was consistent with the above BET results. And the porous ultra-thin structure of g-C3N4 nanosheets had been confirmed by AFM and HRTEM images. As the mass percentage of LaFeO3 was higher than 10%, the adsorption capacity of heterojunctions decreased sharply. It was probably because too many LaFeO3 gathered on the surface of g-C3N4 nanosheets, which reduced the adsorption active sites and then hindered the adsorption of OTC on g-C3N4 nanosheets.
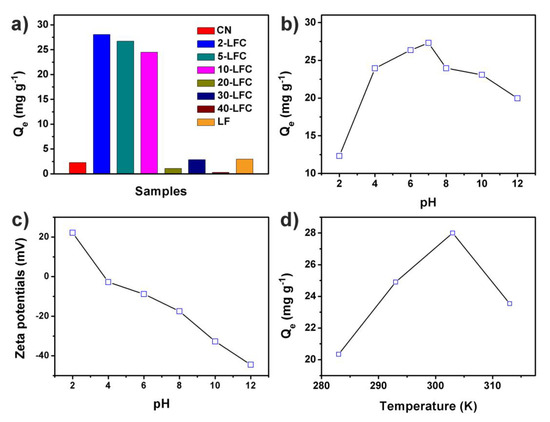
Figure 7.
(a) Adsorption of OTC over CN, LF and LFC heterojunctions with different mass ratio of LaFeO3 and g-C3N4, (b) the pH effect of 2-FLC on OTC adsorption, (c) zeta potentials of 2-FLC at different solution pHs, and (d) the temperature effect of 2-FLC on OTC adsorption.
The effect of solution pH on OTC adsorption on 2-LFC at 303 K were shown in Figure 7b for the investigation of the electrostatic interaction during the adsorption process. According to Figure 7b, the neutral pH condition is more favorable for the adsorption of oxytetracycline. The adsorption capacity increased linearly with the increasing of pH value of solution in the pH range of 2 to 7, and reached the maximum when pH was 7. The adsorption capacity decreased as raising pH value since it exceeded 7. These results were associated with the surface properties of 2-LFC and the relationship between the molecular species of OTC and the solution pH. Oxytetracycline had different molecular species under different pH values [8]. OTC was positive charged as solution pH < 3.53 and negative charged as pH > 9.58. It was zwitterion in the pH range of 3.53 to 9.58. Furthermore, zeta potentials of 2-LFC at different solution pHs were measured and displayed in Figure 7c. negative charged 2-LFC was found in the pH range 4–12. When the solution pH was lower than 4, the adsorption capacity was low due to the electrostatic repulsion between OTC and positive charged 2-LFC. As the solution pH was between 4 and 7, the proportion of neutral OTC molecules increased. 2-LFC had a larger adsorption capacity owing to the complexation. With the further increasd of pH, OTC was negatively charged and adsorption was inhibited.
The effect of temperature on OTC adsorption was investigated at 283, 293, 303 and 313 K (Figure 7d). The adsorption capacity increased with the increase of temperature in the range of 283–303 K. It indicated that the adsorption of oxytetracycline on LFC was an endothermic reaction as the adsorption temperature was below 303 K, which might be related to the π–π interaction between LFC and OTC. Since the π–π interaction was the main mechanism of the adsorption, it usually shown endothermic process. The adsorption capacity of OTC decreased at 313 K. It might be related to the increase of desorption caused by the increase of thermal motion of molecular.
The adsorption kinetics of OTC on CN, 2-LFC, 5-LFC and LF were shown in Figure 8. The adsorption process of OTC onto LF was faster than onto CN. The adsorption equilibrium of OTC onto LF was reached in about 30 min. And it was in 120 min onto CN. It was also revealed that the higher the initial mass ratio of LF in the heterojunctions, the shorter the adsorption equilibrium time. The adsorption equilibrium time of OTC onto 2-LFC and 5-LFC was 75 and 60 min, respectively.

Figure 8.
Adsorption kinetics of OTC on CN (a), 2-LFC (b), 5-LFC (c) and LF (d)
The adsorption kinetic parameters of OTC on CN, 2-LFC, 5-LFC and LF were provided in Table 1. The adsorption kinetic curve of CN was fitted by the pseudo second-order model. The adsorption kinetic curves of 2-LFC, 5-LFC and LF were fitted by the pseudo first-order model. The pseudo first-order adsorption kinetic constant (k1) of LF was almost 3 times higher than that of CN, 2-LFC and 5-LFC. Furthermore, for the pseudo second-order adsorption kinetic constant (k2), it was practically 10 times higher than that of CN and 2-LFC. The adsorption capacity of LF was 4 times higher than CN’s. Since the incorporation of 2 wt % of LaFeO3 into CN, the adsorption capacity of OTC was dramatically increased to 8 times high. The enhancement of the adsorption capacity of 2-LFC, 5-LFC and other LFC heterojunctions could attribute to the reheating process in synthetic method. The reheating process led to thermal oxidation etching. It was one of the approaches to manufacture the ultrathin g-C3N4 nanosheets [57], which had higher specific surface area and thus possessed greater adsorption capacity. The ultrathin nanosheets of g-C3N4 were proved above by AFM and HRTEM images.

Table 1.
Adsorption kinetic parameters of OTC on CN, 2-LFC, 5-LFC and LF.
2.3. Adsorption Isotherms of OTC
The adsorption isotherms curve of OTC on CN, 2-LFC, 5-LFC and LF were presented in Figure 9. The adsorption isotherms curves were fitted by the Langmuir and Freundlich models to study the interaction between the absorbent and the adsorbate. The adsorption isotherms parameters of OTC on CN, 2-LFC, 5-LFC and LF were shown in Table 2. Through the comparison of R2 values, the Langmuir model seemed to accord better with the adsorption data of CN, 2-LFC, 5-LFC and LF than the Freundlich model. It demonstrated that the adsorption of OTC on CN, 2-LFC, 5-LFC and LF surface was a monolayer adsorption mode. The saturated adsorption capacity (Q0) of 2-LFC was 101.67 mg g−1, which was 9 times higher than that of CN.
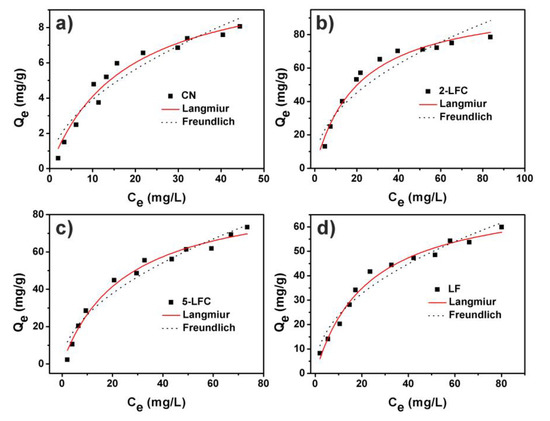
Figure 9.
Adsorption isotherms curve of OTC on CN (a), 2-LFC (b), 5-LFC (c) and LF (d)

Table 2.
Adsorption isotherms parameters of OTC on CN, 2-LFC, 5-LFC and LF.
The three-parameter Dubinin-Astakhov model also has been utilized to fit the experimental adsorption isotherms data (Figure 10). The corresponding values of Qm, EDA, n, and R2 for CN, 2-LFC, 5-LFC and LF were listed in the Table 3. The obtained values of n conformed to the previous literatures and were within the reasonable ranges of 1 to 10 of the Dubinin-Astakhov model [58,59]. The Dubinin-Astakhov results revealed that 2-LFC and 5-LFC had a much higher OTC adsorption capacity than that of CN and LF. It indicated that 2-LFC and 5-LFC had more binding sites available for OTC adsorption. The adsorption capacity of 2-LFC was 84.51 mg g−1, which was 8 times higher than that of CN. The incorporation of LaFeO3 into CN, and the thermal oxidation etching in reheating process was the reason of the enhancement of the saturated adsorption capacity.
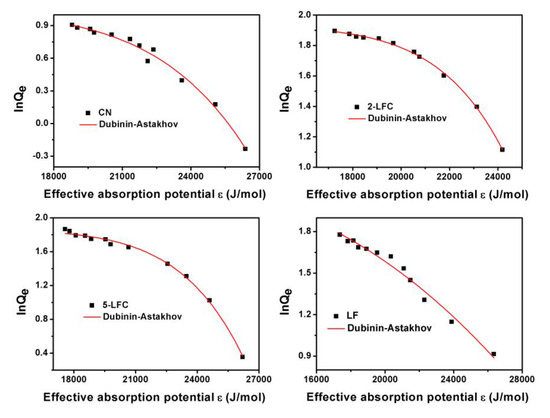
Figure 10.
Adsorption isotherms curve of OTC on CN, 2-LFC, 5-LFC and LF fitted by Dubinin-Astakhov model.

Table 3.
The fitted parameters of OTC on CN, 2-LFC, 5-LFC and LF by Dubinin-Astakhov model.
2.4. Photodegradation of OTC
Figure 11a was the UV-vis spectra of photocatalytic degradation solution of OTC for 2-LFC with different reaction time under visible light irradiation. The intensities of the characteristic absorption peak of OTC at 353 nm were gradually reduced with reaction time in the photocatalytic degradation process. We therefore selected the intensity of the peak at 353 nm to determine the concentration of OTC [9,10]. Photocatalytic degradation of OTC over CN, LF and different mass ratio of LFC heterojunctions under visible light irradiation of 40 W white-light LED was tested and exhibited in Figure 11b. 2-LFC, 5-LFC, 10-LFC and LF had obvious adsorption of OTC, which was consistent with the adsorption experiments results mentioned above. As shown in Figure 11b, 90–94% of OTC was degraded in 180 min over the LFC heterojunctions containing 2 wt % and 5 wt % LaFeO3. As the comparison, only about 40% of OTC was degraded in 180 min for CN, and 60% for other samples. The degradation rate of OTC on the 2-LFC and 5-LFC was markedly promoted. The 2-LFC had the highest photodegradation rate, on which 90% of OTC was degraded after the visible light irradiation of 120 min. The photocatalytic degradation data of OTC was well fitted by the second-order kinetics model (Figure 11c). The second-order reaction rate constant of 2-LFC was the 4.7 and 21.3 times of CN and LF, respectively.
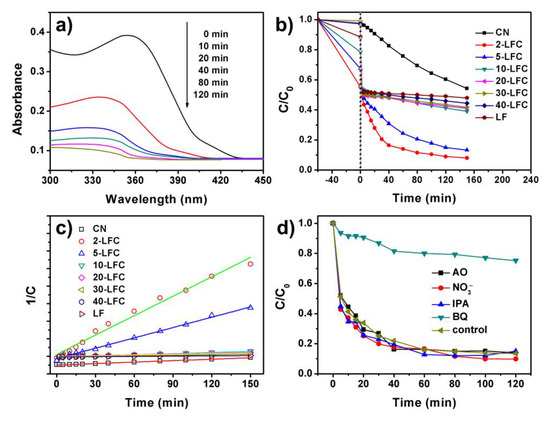
Figure 11.
(a) The time-dependent UV-vis spectra of OTC solution for 2-LFC with reaction time; (b) photocatalytic degradation of OTC over CN, LF and different mass ratio of LFC heterojunctions under visible light irradiation of 40 W white-light LED; (c) corresponding second-order kinetics plots; (d) influence of ammonium oxalate, NaNO3, isopropyl alcohol and benzoquinone on photocatalytic degradation of OTC over 2-LFC.
2-LFC and 5-LFC heterojunctions had higher photocatalytic degradation rate of OTC than CN, LF and other LFCs. UV-vis diffuse reflection spectra of LaFeO3/g-C3N4 heterojunctions reported in our previous work have been proved that LaFeO3/g-C3N4 samples contenting 2 wt % and 5 wt % LaFeO3 had broader absorptions and longer absorption edge wavelengths than that of CN, LF and other LFCs. The absorption intensity of 2-LFC and 5-LFC in the visible-light region was much higher than other LFCs’, which indicated that the 2-LFC and 5-LFC could produce much more photogenerated electron-hole pairs. Based on the transient photocurrent responses and EIS results, it could be deduced that more photogenerated electron-hole pairs and effective charge separation of Z-scheme photocatalytic system made 2-LFC and 5-LFC higher OTC degradation rate.
To investigate the photocatalytic degradation mechanisms of OTC over 2-LFC under visible light irradiation of 40 W white-light LED, the scavengers, including ammonium oxalate (AO), NaNO3, isopropyl alcohol (IPA) and benzoquinone (BQ), were added in the reaction solution during photocatalytic degradation process [7,13,60]. AO, NaNO3, IPA and BQ were acted as the hole, electron, hydroxyl radical and superoxide radical anions scavenger, respectively. As shown in Figure 11d, the addition of AO, NaNO3 and IPA hardly changed the degradation rate of OTC over 2-LFC under visible light irradiation. These results proposed that hole, electron and hydroxyl radical did not play a major role in OTC degradation over 2-LFC. Meanwhile, the photocatalytic degradation rate of OTC was significantly depressed with the addition of BQ. It suggested that superoxide radical anions were the major active species during the photocatalytic degradation process.
To testify the enhancement of photogenerated charge carrier separation of CN, LF and 2-LFC, The transient photocurrent responses and electrochemical impedance spectroscopy (EIS) were implemented. The transient photocurrent responses were tested for several cycles with chopped irradiation (Figure 12a). The photocurrent densities decreased immediately once the irradiation shut off and returned to stable values while the lamp turned on. The photocurrent intensity of 2-LFC heterojunctions was higher than that of CN and LF. It was a clear demonstration of lower recombination rate and more effective charge separation of 2-LFC than CN and LF [50]. To further verify the higher charge separation efficiency of 2-LFC than CN and LF, EIS was carried out (Figure 12b). The smaller diameter of Nyquist plots indicated the smaller charge transfer resistance. It was found that CN possessed largest diameter and 2-LFC had smallest one, which meant the introduction of LaFeO3 onto the g-C3N4 nanosheets could accelerate the charge transfer [51]. It was beneficial for improving photocatalytic reactivity of 2-LFC.

Figure 12.
(a) The transient photocurrent responses and (b) Nyquist plots of electrochemical impedance spectroscopy of CN, LF and 2-LFC
The AFM, HRTEM images and XPS measurements were identified the formation of LaFeO3/g-C3N4 heterojunctions. The intimate contact interface was clearly proved by HRTEM and XPS results. It was the prerequisite to manufacture the LaFeO3/g-C3N4 heterojunctions. As we confirmed before, LaFeO3/g-C3N4 heterojunctions was the Z-scheme system. Once the intimate contact interface formed, the photoinduced electron of LaFeO3 could recombine with the hole of g-C3N4 through solid-solid interface of heterojunctions. This recombination make the photoinduced electron and hole transferring to and staying on the surface of g-C3N4 and LaFeO3, respectively. Thus, the separation of electron-hole pairs of LaFeO3 and g-C3N4 will be significantly promoted. The enhanced photocatalytic degradation activities of OTC over 2-LFC and 5-LFC were the direct evidence of the highly effective separation of photoinduced electron-hole pairs.
Increasing the specific surface area and broadening pore size distribution of ultrathin g-C3N4 nanosheets and forming the LaFeO3/g-C3N4 heterojunctions seemed to be the feasible methods for enhancing adsorption capacity of OTC for LFC. The reheating process utilized in our synthetic approach led to thermal oxidation etching of bulk g-C3N4 and was a facile method to obtain ultrathin nanosheets structure of g-C3N4. The addition of NH4Cl in the thermal polymerization could get a porous g-C3N4 structure, which further increasing the specific surface area of g-C3N4. The porous structure of g-C3N4 was verified by the TEM images in Figure 2b,c. The formation of LaFeO3/g-C3N4 heterojunctions also was another facile method to improve adsorption capacity of OTC. The incorporation of 2 wt % of LaFeO3 into CN caused 9 times increase of the saturated adsorption capacity of 2-LFC.
3. Materials and Methods
3.1. Sample Preparation
Mesoporous g-C3N4 was synthesized by mixing and heating equal amounts of dicyandiamine and ammonium chloride according to our previous work with some necessary modification [38]. Typically, 10 g of dicyandiamine and 10 g of ammonium chloride were dissolved in 50 mL deionized water and stirred for 60 min. The solution was then evaporated to produce the solid mixture. The mixture was located in porcelain crucible with a cover, heated in air with the heating rate of 4 °C min−1 and calcined at 550 °C for 2 h in a muffle furnace. Mesoporous g-C3N4 was obtained after cooling the as-prepared yellow powder to room temperature.
Various components of LaFeO3/g-C3N4 heterojunctions were prepared by changing the mass ratios of LaFeO3 to g-C3N4. For the fabrication of LaFeO3/g-C3N4 heterojunctions containing 2 wt % of LaFeO3, 0.18 g of La(NO3)3, 0.17 g of Fe(NO3)3 and 2.0 g of mesoporous g-C3N4 were mixed and dissolved in 20 mL of deionized water with stirring for 1 h. After vacuum freeze-drying treatment of the mixture, the obtained powder was heated for 2 h at 500 °C in muffle furnace and cooled to room temperature in air naturally. The mesoporous LaFeO3/g-C3N4 heterojunctions content 2 wt % of LaFeO3 was obtained and donated as 2-LFC. Varying the amounts of LaFeO3, 5-LFC, 10-LFC, 20-LFC, 30-LFC and 40-LFC were prepared. LaFeO3 (LF) was synthesized by heating the mixture of LaFeO3 and La(NO3)3 at 500 °C for 2 h without g-C3N4.
3.2. Catalyst Characterization
Transmission electron microscopy (TEM, JEOL, Tokyo, Japan) and high-resolution-TEM (HRTEM) images were taken on a JEOL-2100F transmission electron microscope. Scanning electron microscopy (SEM, JEOL, Tokyo, Japan) images, energy dispersion spectrum (EDS, JEOL, Tokyo, Japan) and elemental mapping images were measured on JSM-4800F scanning electron microscope. Atomic force microscopy (AFM, Bruker, Billerica, MA, USA) images were tested on a Bruker Multimode 8 AFM system. XPS spectra (ThermoFisher, Waltham Mass, MA, USA) were carried out on a Thermo ESCALAB 250XI X-ray photoelectron spectroscopy spectrometer. X-ray diffraction patterns (XRD, Rigaku Corporation, Tokyo, Japan) patterns of mesoporous g-C3N4, LFC and LF were obtained on a Rigaku Smartlab diffractometer with Cu Kα1 radiation (λ = 1.5406 Å). The Raman spectra (HORIBA, Kyoto, Japan) were analysed on a LabRAM Aramis Raman Microscope excited by 325 nm at room temperature. The surface areas and N2 adsorption desorption isotherms were obtained on an ASAP 2460 Micrometrics instrument (Micrometrics, Londonderry, NH, USA).
3.3. Adsorption and Photocatalytic Activity
The adsorption performances of OTC over mesoporous LaFeO3/g-C3N4 heterojunctions were investigated in the dark. In a typical experiment, 50 mg of LFC, LF or g-C3N4 were dispersed in 100 mL OTC solution at a concentration of 20 mg L−1. The pH of OTC solution was maintained to 7 by adding 0.01 mol L−1 NaOH or HCl solutions. The suspension was sealed in a conical flask with cover, placed in a constant temperature shaker and agitated at 150 rpm in the dark. Aliquots were taken out at certain intervals and separated by centrifugation. The OTC residue remaining in the supernatant was analyzed on a UV-vis spectrophotometer.
The photocatalytic activities of OTC over mesoporous LaFeO3/g-C3N4 heterojunctions were conducted with a 40 W LED as the light source. Firstly, 100 mg of LFC, LF or g-C3N4 were dispersed in 200 mL of 40 mg L−1 OTC solution and stirred in the dark at room temperature for an hour to get the adsorption desorption equilibrium of OTC on the photocatalysts. Then the LED was turned on and triggered photodegradation of OTC. 5 mL of degradation solution was taken out at a given intervals and centrifuged to separate the photocatalysts. The concentration of OTC in the supernatant was analyzed on a UV-vis spectrophotometer.
3.4. Photoelectrochemical Measurement
10 mg photocatalyst and 40 μL 5 wt % Nafion was added in 2 mL ethanol to form a suspension. Then the suspension was dropped onto fluorine-doped tin oxide (FTO) glass with an area of 2 cm2 and implemented as the working electrode. 300 W Xe lamp was the visible light source. 2 cm2 of Pt foil was auxiliary electrode. Ag/AgCl was chose as reference electrode. Electrochemical impedance spectroscopy (EIS) was measured in 0.5 M sodium sulfate aqueous solution.
4. Conclusions
In conclusion, 2-LFC and 5-LFC was certified to be a highly effective adsorbent and photocatalyst for the adsorption and photocatalytic degradation of OTC. To photocatalytic degradation reaction, the adsorption of organic pollutants on the surface of photocatalysts was the prerequisite for mineralization of the pollutants. So the combination of adsorption with photocatalysis should fabricate a class of materials with higher efficiency. Increasing the specific surface area of g-C3N4 and forming the LaFeO3/g-C3N4 heterojunctions enhanced the adsorption capacity of OTC on 2-LFC and 5-LFC. The reheating process and the addition of NH4Cl in the thermal polymerization were the actual measures to get porous g-C3N4 ultrathin nanosheets and to obtain high specific surface area. LaFeO3/g-C3N4 heterojunctions was the Z-scheme system. The intimate contact between LaFeO3 and g-C3N4 could promote the transfer of electron-hole pairs through solid-solid interface of heterojunctions. Thus, the photoinduced electron of LaFeO3 could recombine with the hole of g-C3N4. This recombination make the photoinduced electron and hole transferring to and staying on the surface of g-C3N4 and LaFeO3, respectively. Then, the separation of electron-hole pairs of LaFeO3 and g-C3N4 will be significantly promoted. This work provides a facile method to prepare LaFeO3/g-C3N4 heterojunctions with especially well adsorption and photocatalytic activities for OTC, which can facilitate its practical applications in pollution control.
Author Contributions
Photocatalysts preparation, K.X. and L.R.; photocatalysts characterization, X.Y., Z.D. and G.F.; photocatalytic experiments, K.X., S.Q., J.L., K.L., S.P. and L.R.; writing—review and editing, R.L. and J.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundations of China (Grant No. 21865006), the First-Class Discipline Construction Project in Guizhou Province-Public Health and Preventive Medicine (No. 2017[85]), and the National Training Programs of Innovation and Entrepreneurship for Undergraduates (20195200119).
Acknowledgments
We thank the Center for Basic Medical Sciences (Guizhou Medical University) for its instrumental and technical support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yang, J.; Chen, D.; Zhu, Y.; Zhang, Y.; Zhu, Y. 3D-3D porous Bi2WO6/graphene hydrogel composite with excellent synergistic effect of adsorption-enrichment and photocatalytic degradation. Appl. Catal. B Environ. 2017, 205, 228–237. [Google Scholar] [CrossRef]
- Zhang, Q.Q.; Ying, G.G.; Pan, C.G.; Liu, Y.S.; Zhao, J.L. Comprehensive Evaluation of Antibiotics Emission and Fate in the River Basins of China: Source Analysis, Multimedia Modeling, and Linkage to Bacterial Resistance. Environ. Sci. Technol. 2015, 49, 6772–6782. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, Z.; Zhu, H. Adsorption and photocatalytic degradation of Sulfamethoxazole by a novel Composite hydrogel with visible light irradiation. Appl. Catal. B Environ. 2017, 217, 603–614. [Google Scholar] [CrossRef]
- Cetecioglu, Z.; Ince, B.; Gros, M.; Rodriguez-Mozaz, S.; Barceló, D.; Orhon, D.; Ince, O. Chronic impact of tetracycline on the biodegradation of an organic substrate mixture under anaerobic conditions. Water Res. 2013, 47, 2959–2969. [Google Scholar] [CrossRef]
- Wang, D.; Jia, F.; Wang, H.; Chen, F.; Fang, Y.; Dong, W.; Zeng, G.; Li, X.; Yang, Q.; Yuan, X. Simultaneously efficient adsorption and photocatalytic degradation of tetracycline by Fe-based MOFs. J. Colloid Interface Sci. 2018, 519, 273–284. [Google Scholar] [CrossRef]
- Regulska, E.; Rivera-Nazario, D.M.; Karpinska, J.; Plonska-Brzezinska, M.E.; Echegoyen, L. Zinc Porphyrin-Functionalized Fullerenes for the Sensitization of Titania as a Visible-Light Active Photocatalyst: River Waters and Wastewaters Remediation. Molecules 2019, 24, 1118. [Google Scholar] [CrossRef]
- Zhao, C.; Pelaez, M.; Duan, X.; Deng, H.; O’Shea, K.; Fatta-Kassinos, D.; Dionysiou, D.D. Role of pH on photolytic and photocatalytic degradation of antibiotic oxytetracycline in aqueous solution under visible/solar light: Kinetics and mechanism studies. Appl. Catal. B Environ. 2013, 134–135, 83–92. [Google Scholar] [CrossRef]
- Lian, L.; Lv, J.; Lou, D. Synthesis of Novel Magnetic Microspheres with Bimetal Oxide Shell for Excellent Adsorption of Oxytetracycline. ACS Sustain. Chem. Eng. 2017, 5, 10298–10306. [Google Scholar] [CrossRef]
- Wen, X.J.; Niu, C.G.; Zhang, L.; Zeng, G.M. Fabrication of SnO2 Nanopaticles/BiOI n-p Heterostructure for Wider Spectrum Visible-Light Photocatalytic Degradation of Antibiotic Oxytetracycline Hydrochloride. ACS Sustain. Chem. Eng. 2017, 5, 5134–5147. [Google Scholar] [CrossRef]
- Li, R.; Jia, Y.; Wu, J.; Zhen, Q. Photocatalytic degradation and pathway of oxytetracycline in aqueous solution by Fe2O3-TiO2 nanopowder. RSC Adv. 2015, 5, 40764–40771. [Google Scholar] [CrossRef]
- Pereira, J.H.O.S.; Queirós, D.B.; Reis, A.C.; Nunes, O.C.; Borges, M.T.; Boaventura, R.A.R.; Vilar, V.J.P. Process enhancement at near neutral pH of a homogeneous photo-Fenton reaction using ferricarboxylate complexes: Application to oxytetracycline degradation. Chem. Eng. J. 2014, 253, 217–228. [Google Scholar] [CrossRef]
- Hu, X.Y.; Zhou, K.; Chen, B.Y.; Chang, C.T. Graphene/TiO2/ZSM-5 composites synthesized by mixture designwere used for photocatalytic degradation of oxytetracycline undervisible light: Mechanism and biotoxicity. Appl. Surf. Sci. 2016, 362, 329–334. [Google Scholar] [CrossRef]
- Jo, W.K.; Kumar, S.; Isaacs, M.A.; Lee, A.F.; Karthikeyan, S. Cobalt promoted TiO2/GO for the photocatalytic degradation of oxytetracycline and Congo Red. Appl. Catal. B Environ. 2017, 201, 159–168. [Google Scholar] [CrossRef]
- Cheng, L.; Tian, Y.; Zhang, J. Construction of p-n heterojunction film of Cu2O/a-Fe2O3 for efficiently photoelectrocatalytic degradation of oxytetracycline. J. Colloid Interface Sci. 2018, 526, 470–479. [Google Scholar] [CrossRef]
- Liu, Y.; He, X.; Fu, Y.; Dionysiou, D.D. Degradation Kinetics and Mechanism of Oxytetracycline by Hydroxyl Radicalbased Advanced Oxidation Processes. Chem. Eng. J. 2016, 284, 1317–1327. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Chen, Z.; Hu, J.; Li, S.; Wang, Z.; Liu, J.; Wang, X. Semiconductor heterojunction photocatalysts: Design, construction, and photocatalytic performances. Chem. Soc. Rev. 2014, 43, 5234–5244. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yun, J.H.; Luo, B.; Butburee, T.; Peerakiatkhajohn, P.; Thaweesak, S.; Xiao, M.; Wang, L. Recent progress on visible light responsive heterojunctions for photocatalytic applications. J. Mater. Sci. Technol. 2017, 33, 1–22. [Google Scholar] [CrossRef]
- Paracchino, A.; Laporte, V.; Sivula, K.; Graetzel, M.; Thimsen, E. Highly active oxide photocathode for photoelectrochemical water reduction. Nat. Mater. 2011, 10, 456–461. [Google Scholar] [CrossRef]
- Kuang, Y.B.; Jia, Q.X.; Nishiyama, H.; Yamada, T.; Kudo, A.; Domen, K. A front-illuminated nanostructured transparent BiVO4 photoanode for >2% efficient water splitting. Adv. Energy Mater. 2016, 6, 1501645. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.; Lin, M.; Long, J.; Zhang, Z.; Lin, H.; Wu, J.C.; Wang, X. Monolayered Bi2WO6 nanosheets mimicking heterojunction interface with open surfaces for photocatalysis. Nat. Commun. 2015, 6, 8340–8348. [Google Scholar] [CrossRef]
- Zhang, X.; Du, Y.; Zhou, Z.; Guo, L. A simplified method for synthesis of band-structure-controlled (CuIn)xZn2(1-x)S2 solid solution photocatalysts with high activity of photocatalytic H2 evolution under visible-light irradiation. Int. J. Hydrog. Energy 2010, 35, 3313–3321. [Google Scholar] [CrossRef]
- Lu, Y.R.; Yin, P.F.; Mao, J.; Ning, M.J.; Zhou, Y.Z.; Dong, C.K.; Ling, T.; Du, X.W. Stable Inverse Opal Structure of Cadmium Chalcogenide for Efficient Water Splitting. J. Mater. Chem. A 2015, 3, 18521–18527. [Google Scholar] [CrossRef]
- Bhachu, D.S.; Moniz, S.J.A.; Sathasivam, S.; Scanlon, D.O.; Walsh, A.; Bawaked, S.M.; Mokhtar, M.; Obaid, A.Y.; Parkin, I.P.; Tang, J.; et al. Bismuth oxyhalides: Synthesis, structure and photoelectrochemical activity. Chem. Sci. 2016, 7, 4832–4841. [Google Scholar] [CrossRef] [PubMed]
- Cui, P.; Wang, J.; Wang, Z.; Chen, J.; Xing, X.; Wang, L.; Yu, R. Bismuth oxychloride hollow microspheres with high visible light photocatalytic activity. Nano Res. 2016, 9, 593–601. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible-light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Z.; Zhu, Y. Enhanced Visible-Light-Driven Photocatalytic Disinfection Performance and Organic Pollutant Degradation Activity of Porous g-C3N4 Nanosheets. ACS Appl. Mater. Interfaces 2017, 9, 27727–27735. [Google Scholar] [CrossRef]
- Martin, D.J.; Qiu, K.; Shevlin, S.A.; Handoko, A.D.; Chen, X.; Guo, Z.; Tang, J. Highly Efficient Photocatalytic H2 Evolution from Water using Visible Light and Structure-Controlled Graphitic Carbon Nitride. Angew. Chem. Int. Ed. 2014, 53, 9240–9245. [Google Scholar] [CrossRef]
- Ye, L.; Wu, D.; Chu, K.H.; Wang, B.; Xie, H.; Yip, H.Y.; Wong, P.K. Phosphorylation of g-C3N4 for enhanced photocatalytic CO2 reduction. Chem. Eng. J. 2016, 304, 376–383. [Google Scholar] [CrossRef]
- Song, X.; Yang, Q.; Yin, M.; Tang, D.; Zhou, L. Highly efficient pollutant removal of graphitic carbon nitride by the synergistic effect of adsorption and photocatalytic degradation. RSC Adv. 2018, 8, 7260–7268. [Google Scholar] [CrossRef]
- Jiang, L.; Yuan, X.; Zeng, G.; Liang, J.; Wu, Z.; Wang, H.; Zhang, J.; Xiong, T.; Li, H. A facile band alignment of polymeric carbon nitride isotype heterojunctions for enhanced photocatalytic tetracycline degradation. Environ. Sci. Nano 2018, 5, 2604–2617. [Google Scholar] [CrossRef]
- Li, H.; Tu, W.; Zhou, Y.; Zou, Z. Z-Scheme Photocatalytic Systems for Promoting Photocatalytic Performance: Recent Progress and Future Challenges. Adv. Sci. 2016, 3, 1500389. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Tang, L.; Feng, C.; Zeng, G.; Wang, J.; Zhou, Y.; Liu, Y.; Peng, B.; Feng, H. Construction of plasmonic Ag modified phosphorous-doped ultrathin g-C3N4 nanosheets/BiVO4 photocatalyst with enhanced visible-near-infrared response ability for ciprofloxacin degradation. J. Hazard. Mater. 2017, 344, 758–769. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Guo, R.T.; Liu, X.Y.; Pan, W.G.; Wang, Z.Y.; Shi, X.; Tang, J.Y.; Huang, C.Y. Z-Scheme MoS2/g-C3N4 heterojunction for efficient visible light photocatalytic CO2 reduction. Dalton Trans. 2018, 47, 15155–15163. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Wang, P.Q.; Liu, J.Y.; Liu, X.J. Enhanced photocatalytic performance of direct Z-scheme BiOCl- g-C3N4 photocatalysts. RSC Adv. 2014, 4, 19456–19461. [Google Scholar] [CrossRef]
- He, Y.; Zhang, L.; Teng, B.; Fan, M. New Application of Z-Scheme Ag3PO4/g-C3N4 Composite in Converting CO2 to Fuel. Environ. Sci. Technol. 2015, 49, 649–656. [Google Scholar] [CrossRef]
- Cui, L.; Ding, X.; Wang, Y.; Shi, H.; Huang, L.; Zuo, Y.; Kang, S. Facile preparation of Z-scheme WO3/g-C3N4 composite photocatalyst with enhanced photocatalytic performance under visible light. Appl. Surf. Sci. 2017, 391, 202–210. [Google Scholar] [CrossRef]
- Ren, X.; Yang, H.; Gen, S.; Zhou, J.; Yang, T.; Zhang, X.; Cheng, Z.; Sun, S. Controlled growth of LaFeO3 nanoparticles on reduced graphene oxide for highly efficient photocatalysis. Nanoscale 2016, 8, 752–756. [Google Scholar] [CrossRef]
- Xu, K.; Feng, J. Superior photocatalytic performance of LaFeO3/g-C3N4 heterojunction nanocomposites under visible light irradiation. RSC Adv. 2017, 7, 45369–45376. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, H.; Tu, W.; Liu, Y.; Tan, Y.Z.; Yuan, X.; Chew, J.W. Quasi-polymeric construction of stable perovskite-type LaFeO3/g-C3N4 heterostructured photocatalyst for improved Z-scheme photocatalytic activity via solid p-n heterojunction interfacial effect. J. Hazard. Mater. 2018, 347, 412–422. [Google Scholar] [CrossRef]
- Jin, L.; Zhou, X.; Ning, X.; Zhan, L.; Kong, M.; Tan, K.; Li, J.; Lin, Z. Boosting visible light photocatalytic performance of g-C3N4 nanosheets by combining with LaFeO3 nanoparticles. Mater. Res. Bull. 2018, 102, 353–361. [Google Scholar] [CrossRef]
- Ismael, M.; Wu, Y. A facile synthesis method for fabrication of LaFeO3/g-C3N4 nanocomposite as efficient visible-light-driven photocatalyst for photodegradation of RhB and 4-CP. New J. Chem. 2019, 43, 13783–13793. [Google Scholar] [CrossRef]
- Liang, Q.; Jin, Q.J.; Liu, C.; Xu, S.; Li, Z. Constructing a novel p-n heterojunction photocatalyst LaFeO3/g-C3N4 with enhanced visible-light-driven photocatalytic activity. J. Alloy. Compd. 2017, 709, 542–548. [Google Scholar] [CrossRef]
- Ye, Y.; Yang, H.; Wang, X.; Feng, W. Photocatalytic, Fenton and photo-Fenton degradation of RhB over Z-scheme g-C3N4/LaFeO3 heterojunction photocatalysts. Mat. Sci. Semicon. Proc. 2018, 82, 14–24. [Google Scholar] [CrossRef]
- Liu, X.; Jin, A.; Jia, Y.; Xia, T.; Deng, C.; Zhu, M.; Chen, C.; Chen, X. Synergy of adsorption and visible-light photocatalytic degradation of methylene blue by a bifunctional Z-scheme heterojunction of WO3/g-C3N4. Appl. Surf. Sci. 2017, 405, 359–371. [Google Scholar] [CrossRef]
- Kumar, A.S.K.; You, J.G.; Tseng, W.B.; Dwivedi, G.D.; Rajesh, N.; Jiang, S.J.; Tseng, W.L. Magnetically Separable Nanospherical g-C3N4@Fe3O4 as a Recyclable Material for Chromium Adsorption and Visible-Light-Driven Catalytic Reduction of Aromatic Nitro Compounds. ACS Sustain. Chem. Eng. 2019, 7, 6662–6671. [Google Scholar] [CrossRef]
- Wang, T.; Huang, M.; Liu, X.; Zhang, Z.; Liu, Y.; Tang, W.; Bao, S.; Fang, T. Facile one-step hydrothermal synthesis of α-Fe2O3/g-C3N4 composites for the synergistic adsorption and photodegradation of dyes. RSC Adv. 2019, 9, 29109–29119. [Google Scholar] [CrossRef]
- Panneri, S.; Ganguly, P.; Nair, B.N.; Mohamed, A.A.P.; Warrier, K.G.; Hareesh, U.N.S. Copyrolysed C3N4-Ag/ZnO Ternary Heterostructure Systems for Enhanced Adsorption and Photocatalytic Degradation of Tetracycline. Eur. J. Inorg. Chem. 2016, 2016, 5068–5076. [Google Scholar] [CrossRef]
- Guo, W.; Fan, K.; Zhang, J.; Xu, C. 2D/2D Z-scheme Bi2WO6/Porous-g-C3N4 with synergy of adsorption and visible-light-driven photodegradation. Appl. Surf. Sci. 2018, 447, 125–134. [Google Scholar] [CrossRef]
- Xiao, J.; Xie, Y.; Nawaz, F.; Wang, Y.; Du, P.; Cao, H. Dramatic coupling of visible light with ozone on honeycomb-like porous g-C3N4 towards superior oxidation of water pollutants. Appl. Catal. B Environ. 2016, 183, 417–425. [Google Scholar] [CrossRef]
- Li, M.; Zhang, L.; Fan, X.; Wu, M.; Du, Y.; Wang, M.; Kong, Q.; Zhang, L.; Shi, J. Dual synergetic effects in MoS2/pyridine-modified g-C3N4 compositefor highly active and stable photocatalytic hydrogen evolution undervisible light. Appl. Catal. B Environ. 2016, 190, 36–43. [Google Scholar] [CrossRef]
- Zhao, H.; Sun, S.; Jiang, P.; Xu, Z.J. Graphitic C3N4 modified by Ni2P cocatalyst: An efficient, robust and low cost photocatalyst for visible-light-driven H2 evolution from water. Chem. Eng. J. 2017, 315, 296–303. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Wang, P.; Xue, P.; Chen, C.; Diao, D. Structural and tribological behaviors of graphene nanocrystallited carbon nitride films. Appl. Surf. Sci. 2019, 495, 143591. [Google Scholar] [CrossRef]
- Fang, Z.; Hu, Y.; Zhang, W.; Ruan, X. Shell-free three-dimensional graphene-based monoliths for the aqueous adsorption of organic pollutants. Chem. Eng. J. 2017, 316, 24–32. [Google Scholar] [CrossRef]
- Bu, X.; Lu, Y.; Chen, S.; Li, D.; Zhang, Z.; Qian, P. Fabrication of porous carbon nitride foams/acrylic resin composites for efficient oil and organic solvents capture. Chem. Eng. J. 2019, 355, 299–308. [Google Scholar] [CrossRef]
- Li, F.; Liu, S.; Xue, Y.; Wang, X.; Hao, Y.; Zhao, J.; Liu, R.; Zhao, D. Structure Modification Function of g-C3N4 for Al2O3 in the In Situ Hydrothermal Process for Enhanced Photocatalytic Activity. Chem. Eur. J. 2015, 21, 10149–10159. [Google Scholar] [CrossRef]
- Niu, P.; Zhang, L.; Liu, G.; Cheng, H.M. Graphene-Like Carbon Nitride Nanosheets for Improved Photocatalytic Activities. Adv. Funct. Mater. 2012, 22, 4763–4770. [Google Scholar] [CrossRef]
- Yan, B.; Niu, C.H. Adsorption behavior of norfloxacin and site energy distribution based on the Dubinin-Astakhov isotherm. Sci. Total Environ. 2018, 631, 1525–1533. [Google Scholar] [CrossRef]
- Inglezakis, V.J. Solubility-normalized Dubinin-Astakhov adsorption isotherm for ion-exchange systems. Microporous Mesoporous Mater. 2007, 103, 72–81. [Google Scholar] [CrossRef]
- Regulska, E.; Breczko, J.; Basa, A. Pristine and Graphene-Quantum-Dots-Decorated Spinel Nickel Aluminate for Water Remediation from Dyes and Toxic Pollutants. Water 2019, 11, 953. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).