Fatty Acid Hydratases: Versatile Catalysts to Access Hydroxy Fatty Acids in Efficient Syntheses of Industrial Interest
Abstract
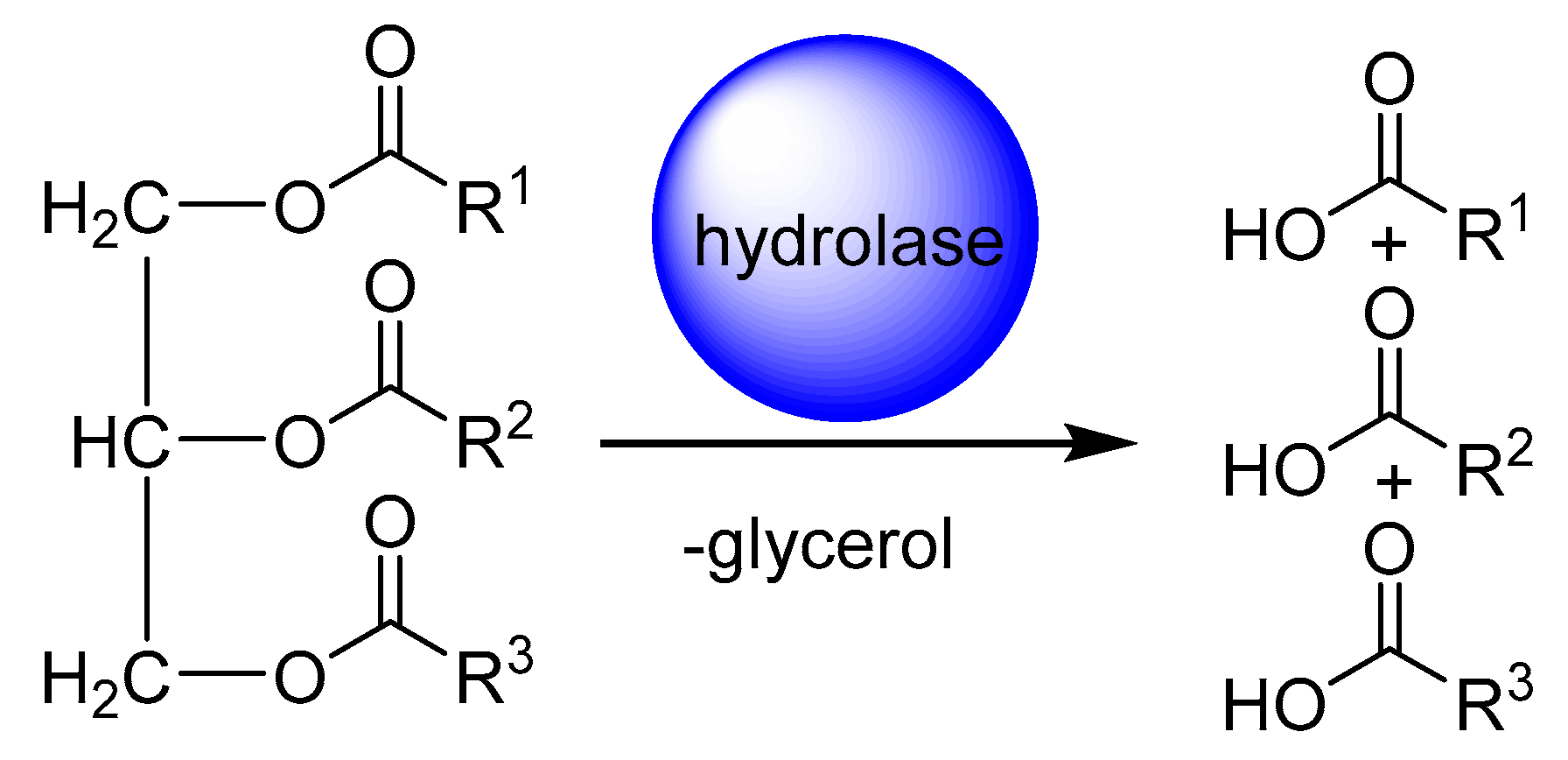
1. Introduction
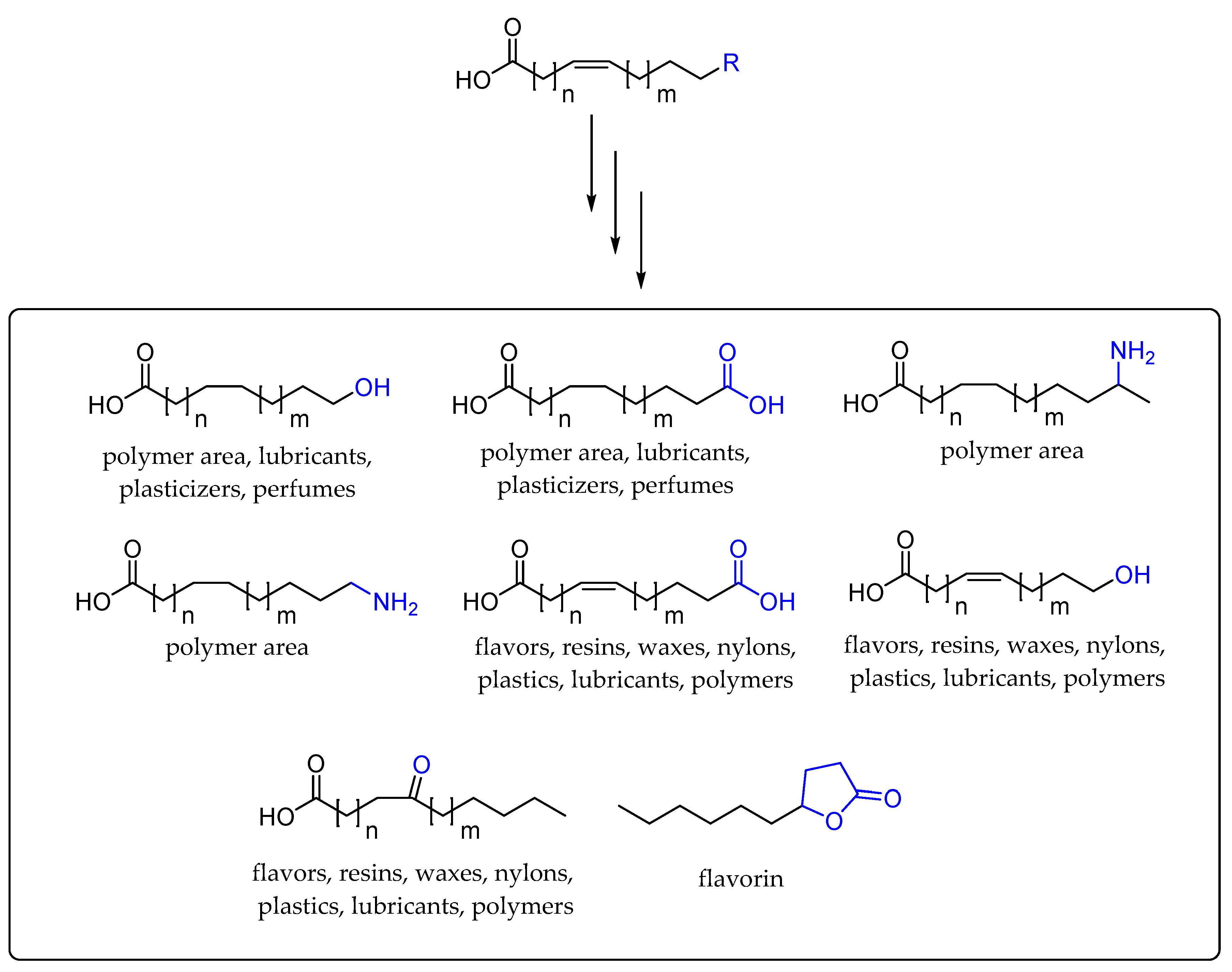
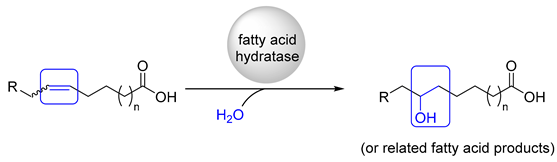
2. Application of Fatty Acid Hydratase in Synthesis and Process Development
3. Cascade Processes Involving Fatty Acid Hydratase
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, C.; Liu, F.; Cai, J.; Xie, U.W.; Long, T.E.; Turner, S.R.; Lyons, A.; Gross, R.A. Polymers from Fatty Acids: Poly (Co-Hydroxyl Tetradecanoic Acid) Synthesis and Physico-Mechanical Studies. ACS Symp. Ser. 2012, 1105, 131–150. [Google Scholar] [CrossRef]
- Świzdor, A.; Panek, A.; Milecka-Tronina, N.; Kołek, T. Biotransformations Utilizing β-Oxidation Cycle Reactions in the Synthesis of Natural Compounds and Medicines. Int. J. Mol. Sci. 2012, 13, 16514–16543. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.; Wubbolts, M.G.; Sanglard, D.; Witholt, B. Production of Chiral Hydroxy Long Chain Fatty Acids by Whole Cell Biocatalysis of Pentadecanoic Acid with an E. Coli Recombinant Containing Cytochrome P450BM-3 Monooxygenase. Tetrahedron Asymmetry 2002, 9, 2832–2844. [Google Scholar] [CrossRef]
- Ashby, R.D.; Solaiman, D.K.Y.; Liu, C.K.; Strahan, G.; Latona, N. Sophorolipid-Derived Unsaturated and Epoxy Fatty Acid Estolides as Plasticizers for Poly (3-Hydroxybutyrate). Am. Oil Chem. Soc. 2016, 93, 347–358. [Google Scholar] [CrossRef]
- Hu, J.; Jin, Z.; Chen, T.Y.; Polley, J.D.; Cunningham, M.F.; Gross, R.A. Anionic Polymerizable Surfactants from Biobased ω-Hydroxy Fatty Acids. Macromolecules 2014, 47, 113–120. [Google Scholar] [CrossRef]
- Mutlu, H.; Meier, M.A.R. Castor Oil as a Renewable Resource for the Chemical Industry. Eur. J. Lipid Sci. Technol. 2010, 112, 10–30. [Google Scholar] [CrossRef]
- Hou, C.T. Biotechnology for Fats and Oils: New Oxygenated Fatty Acids. New Biotechnol. 2009, 26, 2–10. [Google Scholar] [CrossRef]
- Patel, V.R.; Dumancas, G.G.; Viswanath, L.C.K.; Maples, R.; Subong, B.J.J. Castor Oil: Properties, Uses, and Optimization of Processing Parameters in Commercial Production. Lipid Insights 2016, LPI-S40233. [Google Scholar] [CrossRef]
- Knothe, G.; Weisleder, D.; Bagby, M.O.; Peterson, R.E. Hydroxy Fatty Acids through Hydroxylation of Oleic Acid with Selenium Dioxide/Tert.-Butylhydroperoxide. J. Am. Oil Chem. Soc. 1993, 70, 401–404. [Google Scholar] [CrossRef]
- Mountanea, O.G.; Limnios, D.; Kokotou, M.G.; Bourboula, A.; Kokotos, G. Asymmetric Synthesis of Saturated Hydroxy Fatty Acids and Fatty Acid Esters of Hydroxy Fatty Acids. Eur. J. Org. Chem. 2019, 10, 2010–2019. [Google Scholar] [CrossRef]
- Köckritz, A.; Martin, A. Oxidation of Unsaturated Fatty Acid Derivatives and Vegetable Oils. Eur. J. Lipid Sci. Technol. 2008, 110, 812–824. [Google Scholar] [CrossRef]
- Kim, K.R.; Oh, H.J.; Park, C.S.; Hong, S.H.; Park, J.Y.; Oh, D.K. Unveiling of Novel Regio-Selective Fatty Acid Double Bond Hydratases from Lactobacillus Acidophilus Involved in the Selective Oxyfunctionalization of Mono- and Di-Hydroxy Fatty Acids. Biotechnol. Bioeng. 2015, 112, 2206–2213. [Google Scholar] [CrossRef] [PubMed]
- Van de Loo, F.; Broun, P.; Turner, S.; Somervillet, C. An oleate 12-hydroxylase from Ricinus communis L. is a fatty acyl desaturase homolog. Proc. Natl. Acad. Sci. USA 1995, 92, 6743–6747. [Google Scholar] [CrossRef] [PubMed]
- Brodkorb, D.; Gottschall, M.; Marmulla, R.; Lüddeke, F.; Harder, J. Linalool Dehydratase-Isomerase, a Bifunctional Enzyme in the Anaerobic Degradation of Monoterpenes. J. Biol. Chem. 2010, 285, 30436–30442. [Google Scholar] [CrossRef] [PubMed]
- Steiger, S.; Mazet, A.; Sandmann, G. Heterologous Expression, Purification, and Enzymatic Characterization of the Acyclic Carotenoid 1, 2-Hydratase from Rubrivivax Gelatinosus. Arch. Biochem. Biophys. 2003, 414, 51–58. [Google Scholar] [CrossRef]
- Bornscheuer, U.T. Enzymes in Lipid Modification. Annu. Rev. Food Sci. Technol. 2018, 9, 116–127. [Google Scholar] [CrossRef]
- Engleder, M.; Pichler, H. On the Current Role of Hydratases in Biocatalysis. Appl. Microbiol. Biotechnol. 2018, 102, 5841–5858. [Google Scholar] [CrossRef]
- Wallen, L.L.; Benedict, R.G.; Jackson, W.R. The Microbiological Production from Oleic of 10-Hydroxystearic Acid from Oleic Acid. Arch. Biochem. Biophys. 1962, 99, 249–253. [Google Scholar] [CrossRef]
- Kim, K.-R.; Oh, D.-K. Production of hydroxy fatty acids by microbial fatty acid-hydroxylation enzymes. Biotechnol. Adv. 2013, 31, 1473–1485. [Google Scholar] [CrossRef]
- Wallen, L.L.; Davis, E.N.; Wu, Y.V.; Rohwedder, W.K. Stereospecific Hydration of Unsaturated Fatty Acids by Bacteria. Lipids 1971, 6, 745–750. [Google Scholar] [CrossRef]
- Niehaus, W.G.; Schroepfer, G.J. The Reversible Hydration of Oleic Acid to 10D-Hydroxystearic Acid. Biochem. Biophys. Res. Commun. 1965, 21, 271–275. [Google Scholar] [CrossRef]
- Volkov, A.; Liavonchanka, A.; Kamneva, O.; Fiedler, T.; Goebel, C.; Kreikemeyer, B.; Feussner, I. Myosin Cross-Reactive Antigen of Streptococcus Pyogenes M49 Encodes a Fatty Acid Double Bond Hydratase That Plays a Role in Oleic Acid Detoxification and Bacterial Virulence. J. Biol. Chem. 2010, 285, 10353–10361. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Liang, N.Y.; Curtis, J.M.; Gänzle, M.G. Characterization of Linoleate 10-Hydratase of Lactobacillus plantarum and Novel Antifungal Metabolites. Front. Microbiol. 2016, 7, 1561. [Google Scholar] [CrossRef] [PubMed]
- Rosberg-Cody, E.; Liavonchanka, A.; Göbel, C.; Ross, R.P.; O’Sullivan, O.; Fitzgerald, G.F.; Feussner, I.; Stanton, C. Myosin-Cross-Reactive Antigen (MCRA) Protein from Bifidobacterium Breve Is a FAD-Dependent Fatty Acid Hydratase Which Has a Function in Stress Protection. BMC Biochem. 2011, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Engleder, M.; Pavkov-Keller, T.; Emmerstorfer, A.; Hromic, A.; Schrempf, S.; Steinkellner, G.; Wriessnegger, T.; Leitner, E.; Strohmeier, G.A.; Kaluzna, I.; et al. Structure-Based Mechanism of Oleate Hydratase from Elizabethkingia Meningoseptica. ChemBioChem 2015, 16, 1730–1734. [Google Scholar] [CrossRef] [PubMed]
- Hiseni, A.; Arends, I.W.C.E.; Otten, L.G. New Cofactor-Independent Hydration Biocatalysts: Structural, Biochemical, and Biocatalytic Characteristics of Carotenoid and Oleate Hydratases. ChemCatChem 2015, 7, 29–37. [Google Scholar] [CrossRef]
- Villeneuve, P.; Muderhwa, J.M.; Graille, J.; Haas, M.J. Customizing Lipases for Biocatalysis: A Survey of Chemical, Physical and Molecular Biological Approaches. J. Mol. Catal. B Enzym. 2000, 9, 113–148. [Google Scholar] [CrossRef]
- Goldberg, K.; Schroer, K.; Lütz, S.; Liese, A. Biocatalytic Ketone Reduction—A Powerful Tool for the Production of Chiral Alcohols-Part II: Whole-Cell Reductions. Appl. Microbiol. Biotechnol. 2007, 76, 249–255. [Google Scholar] [CrossRef]
- Breuer, M.; Ditrich, K.; Habicher, T.; Hauer, B.; Keßeler, M.; Stürmer, R.; Zelinski, T. Industrial Methods for the Production of Optically Active Intermediates. Angew. Chem. Int. Ed. 2004, 43, 788–824. [Google Scholar] [CrossRef]
- Strohmeier, G.A.; Pichler, H.; May, O.; Gruber-Khadjawi, M. Application of Designed Enzymes in Organic Synthesis. Chem. Rev. 2011, 111, 4141–4164. [Google Scholar] [CrossRef]
- Abdelraheem, E.M.M.; Busch, H.; Hanefeld, U.; Tonin, F. Biocatalysis explained: From pharmaceutical to bulk chemical production. React. Chem. Eng. 2019, 4, 1878–1894. [Google Scholar] [CrossRef]
- Jemli, S.; Ayadi-Zouari, D.; Hlima, H.B.; Bejar, S. Biocatalysts: Application and engineering for industrial purposes. Crit. Rev. Biotechnol. 2016, 36, 246–258. [Google Scholar] [CrossRef] [PubMed]
- Liese, A.; Seelbach, K.; Wandrey, C. Industrial Biotransformations; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar] [CrossRef]
- Yamada, H.; Nagasawa, T. Production of Useful Amides by Enzymatic Hydration of Nitriles. Agric. Chem. 1990, 613, 142–154. [Google Scholar] [CrossRef]
- Miyazawa, T. Enzymatic Resolution of Amino Acids via Ester Hydrolysis. Amino Acids 1999, 16, 191–213. [Google Scholar] [CrossRef] [PubMed]
- Brugging, A.; Roos, E.C.; De Vroom, E. Penicillin Acylase in the Industrial Production of β-Lactam Antibiotics. Org. Process Res. Dev. 1998, 2, 128–133. [Google Scholar] [CrossRef]
- Joo, Y.C.; Seo, E.S.; Kim, Y.S.; Kim, K.R.; Park, J.B.; Oh, D.K. Production of 10-Hydroxystearic Acid from Oleic Acid by Whole Cells of Recombinant Escherichia Coli Containing Oleate Hydratase from Stenotrophomonas Maltophilia. J. Biotechnol. 2012, 158, 17–23. [Google Scholar] [CrossRef]
- Fabritius, D.; Schäfer, H.J.; Steinbüchel, A. Identification and Production of 3-Hydroxy-Δ9-Cis-1, 18-Octadecenedioic Acid by Mutants of Candida Tropicalis. Appl. Microbiol. Biotechnol. 1996, 45, 342–348. [Google Scholar] [CrossRef]
- El-Sharkawy, S.H.; Yang, W.; Dostal, L.; Rosazza, J.P.N. Microbial Oxidation of Oleic Acid. Appl. Environ. Microbiol. 1992, 58, 2116–2122. [Google Scholar] [CrossRef]
- Hudson, J.A.; MacKenzie, C.A.M.; Joblin, K.N. Conversion of Oleic Acid to 10-Hydroxystearic Acid by Two Species of Ruminal Bacteria. Appl. Microbiol. Biotechnol. 1995, 44, 1–6. [Google Scholar] [CrossRef]
- Kaneshiro, T.; Kuo, T.M.; Nakamura, L.K. Conversion of Unsaturated Fatty Acids by Bacteria Isolated from Compost. Curr. Microbiol. 1999, 38, 250–255. [Google Scholar] [CrossRef]
- Koritala, S.; Hou, C.T.; Hesseltine, C.W.; Bagby, M.O. Microbial Conversion of Oleic Acid to 10-Hydroxystearic Acid. Appl. Microbiol. Biotechnol. 1989, 32, 299–304. [Google Scholar] [CrossRef]
- Shinha, T.; Ahuja, R. Bacteremia due to Elizab. Meningoseptica 2015, 2, 13–15. [Google Scholar] [CrossRef]
- Bevers, L.E.; Pinkse, M.W.H.; Verhaert, P.D.E.M.; Hagen, W.R. Oleate Hydratase Catalyzes the Hydration of a Nonactivated Carbon-Carbon Bond. J. Bacteriol. 2009, 191, 5010–5012. [Google Scholar] [CrossRef]
- Ryan, R.P.; Monchy, S.; Cardinale, M.; Taghavi, S.; Crossman, L.; Avison, M.B.; Berg, G.; van der Lelie, D.; Dow, J.M. The versatility and adaptation of bacteria from the genus Stenotrophomonas. Nat. Rev. Microbiol. 2019, 7, 514–525. [Google Scholar] [CrossRef]
- Kim, B.N.; Yeom, S.J.; Oh, D.K. Conversion of Oleic Acid to 10-Hydroxystearic Acid by Whole Cells of Stenotrophomonas Nitritireducens. Biotechnol. Lett. 2011, 33, 993–997. [Google Scholar] [CrossRef]
- Gallegos-Monterrosa, R.; Maróti, G.; Bálint, B.; Kovács, A.T. Draft Genome Sequence of the Soil Isolate Lysinibacillus fusiformis M5, a Potential Hypoxanthine Producer. Genome Announc. 2016, 4, e01272-16. [Google Scholar] [CrossRef]
- Kim, B.N.; Joo, Y.C.; Kim, Y.S.; Kim, K.R.; Oh, D.K. Production of 10-Hydroxystearic Acid from Oleic Acid and Olive Oil Hydrolyzate by an Oleate Hydratase from Lysinibacillus fusiformis. Appl. Microbiol. Biotechnol. 2012, 95, 929–937. [Google Scholar] [CrossRef]
- Jeon, E.Y.; Lee, J.H.; Yang, K.M.; Joo, Y.C.; Oh, D.K.; Park, J.B. Bioprocess Engineering to Produce 10- Hydroxystearic Acid from Oleic Acid by Recombinant Escherichia Coli Expressing the Oleate Hydratase Gene of Stenotrophomonas Maltophilia. Process Biochem. 2012, 47, 941–947. [Google Scholar] [CrossRef]
- Seo, M.H.; Kim, K.R.; Oh, D.K. Production of a Novel Compound, 10, 12-Dihydroxystearic Acid from Ricinoleic Acid by an Oleate Hydratase from Lysinibacillus fusiformis. Appl. Microbiol. Biotechnol. 2013, 97, 8987–8995. [Google Scholar] [CrossRef]
- De Angelis, M.; Gobbetti, M. Lactobacillus spp.: General Characteristics. Ref. Modul. Food Sci. 2016. [Google Scholar] [CrossRef]
- Kishino, S.; Takeuchi, M.; Park, S.B.; Hirata, A.; Kitamura, N.; Kunisawa, J.; Kiyono, H.; Iwamoto, R.; Isobe, Y.; Arita, M.; et al. Polyunsaturated Fatty Acid Saturation by Gut Lactic Acid Bacteria Affecting Host Lipid Composition. Proc. Natl. Acad. Sci. USA 2013, 110, 17808–17813. [Google Scholar] [CrossRef]
- Yang, B.; Chen, H.; Song, Y.; Chen, Y.Q.; Zhang, H.; Chen, W. Myosin-Cross-Reactive Antigens from Four Different Lactic Acid Bacteria Are Fatty Acid Hydratases. Biotechnol. Lett. 2013, 35, 75–81. [Google Scholar] [CrossRef]
- Volkov, A.; Khoshnevis, S.; Neumann, P.; Herrfurth, C.; Wohlwend, D.; Ficner, R.; Feussner, I. Crystal Structure Analysis of a Fatty Acid Double-Bond Hydratase from Lactobacillus Acidophilus. Acta Cryst. Sect. D Biol. Cryst. 2013, 69, 648–657. [Google Scholar] [CrossRef]
- Jo, Y.S.; An, J.U.; Oh, D.K. γ-Dodecelactone Production from Safflower Oil via 10-Hydroxy-12 (z)-Octadecenoic Acid Intermediate by Whole Cells of Candida Boidinii and Stenotrophomonas Nitritireducens. J. Agric. Food Chem. 2014, 62, 6736–6745. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Lee, S.H.; Kim, K.R.; Park, J.B.; Oh, D.K. Production of 13S-Hydroxy-9 (Z)-Octadecenoic Acid from Linoleic Acid by Whole Recombinant Cells Expressing Linoleate 13-Hydratase from Lactobacillus acidophilus. J. Biotechnol. 2015, 208, 1–10. [Google Scholar] [CrossRef]
- Hirata, A.; Kishino, S.; Park, S.-B.; Takeuchi, M.; Kitamura, N.; Ogawa, J. A Novel Unsaturated Fatty Acid Hydratase toward C16 to C22 Fatty Acids from Lactobacillus acidophilus. J. Lipid Res. 2015, 56, 1340–1350. [Google Scholar] [CrossRef]
- Takeuchi, M.; Kishino, S.; Park, S.B.; Hirata, A.; Kitamura, N.; Saika, A.; Ogawa, J. Efficient Enzymatic Production of Hydroxy Fatty Acids by Linoleic Acid Δ9 Hydratase from Lactobacillus Plantarum AKU 1009a. J. Appl. Microbiol. 2016, 120, 1282–1288. [Google Scholar] [CrossRef]
- Schmid, J.; Steiner, L.; Fademrecht, S.; Pleiss, J.; Otte, K.B.; Hauer, B. Biocatalytic Study of Novel Oleate Hydratases. J. Mol. Catal. B Enzym. 2016, 133, 243–249. [Google Scholar] [CrossRef]
- Choi, J.H.; Seo, M.J.; Lee, K.T.; Oh, D.K. Biotransformation of Fatty Acid-Rich Tree Oil Hydrolysates to Hydroxy Fatty Acid-Rich Hydrolysates by Hydroxylases and Their Feasibility as Biosurfactants. Biotechnol. Bioprocess Eng. 2017, 22, 709–716. [Google Scholar] [CrossRef]
- Meier, M.A.R.; Metzger, J.O.; Schubert, U.S. Plant Oil Renewable Resources as Green Alternatives in Polymer Science. Chem. Soc. Rev. 2007, 36, 1788–1802. [Google Scholar] [CrossRef]
- Heo, S.H.; Hou, C.T.; Kim, B.S. Production of Oxygenated Fatty Acids from Vegetable Oils by Flavobacterium sp. Strain DS5. New Biotechnol. 2009, 26, 105–108. [Google Scholar] [CrossRef]
- Kang, W.R.; Seo, M.J.; Shin, K.C.; Park, J.B.; Oh, D.K. Gene Cloning of an Efficiency Oleate Hydratase from Stenotrophomonas Nitritireducens for Polyunsaturated Fatty Acids and Its Application in the Conversion of Plant Oils to 10-Hydroxy Fatty Acids. Biotechnol. Bioeng. 2017, 114, 74–82. [Google Scholar] [CrossRef]
- Jeon, E.Y.; Seo, J.H.; Kang, W.R.; Kim, M.J.; Lee, J.H.; Oh, D.K.; Park, J.B. Simultaneous Enzyme/Whole-Cell Biotransformation of Plant Oils into C9 Carboxylic Acids. ACS Catal. 2016, 6, 7547–7553. [Google Scholar] [CrossRef]
- Kim, S.K.; Park, Y.C. Biosynthesis of ω-Hydroxy Fatty Acids and Related Chemicals from Natural Fatty Acids by Recombinant Escherichia Coli. Appl. Microbiol. Biotechnol. 2019, 103, 191–199. [Google Scholar] [CrossRef]
- Song, J.W.; Lee, J.H.; Bornscheuer, U.T.; Park, J.B. Microbial Synthesis of Medium-Chain α, ω-Dicarboxylic Acids and ω-Aminocarboxylic Acids from Renewable Long-Chain Fatty Acids. Adv. Synth. Catal. 2014, 356, 1782–1788. [Google Scholar] [CrossRef]
- Oh, H.J.; Kim, S.U.; Song, J.W.; Lee, J.H.; Kang, W.R.; Jo, Y.S.; Kim, K.R.; Bornscheuer, U.T.; Oh, D.K.; Park, J.B. Biotransformation of Linoleic Acid into Hydroxy Fatty Acids and Carboxylic Acids Using a Linoleate Double Bond Hydratase as Key Enzyme. Adv. Synth. Catal. 2015, 357, 408–416. [Google Scholar] [CrossRef]
- Lee, D.S.; Song, J.W.; Voß, M.; Schuiten, E.; Akula, R.K.; Kwon, Y.U.; Bornscheuer, U.; Park, J.B. Enzyme Cascade Reactions for the Biosynthesis of Long Chain Aliphatic Amines from Renewable Fatty Acids. Adv. Synth. Catal. 2019, 361, 1359–1367. [Google Scholar] [CrossRef]
- Wu, Y.X.; Pan, J.; Yu, H.L.; Xu, J.H. Enzymatic Synthesis of 10-Oxostearic Acid in High Space-Time Yield via Cascade Reaction of a New Oleate Hydratase and an Alcohol Dehydrogenase. J. Biotechnol. X 2019. [Google Scholar] [CrossRef]
- Song, J.W.; Jeon, E.Y.; Song, D.H.; Jang, H.Y.; Bornscheuer, U.T.; Oh, D.K.; Park, J.B. Multistep Enzymatic Synthesis of Long-Chain α, ω-Dicarboxylic and ω-Hydroxycarboxylic Acids from Renewable Fatty Acids and Plant Oils. Angew. Chem. Int. Ed. 2013, 52, 2534–2537. [Google Scholar] [CrossRef]
- Kim, S.U.; Kim, K.R.; Kim, J.W.; Kim, S.; Kwon, Y.U.; Oh, D.K.; Park, J.B. Microbial Synthesis of Plant Oxylipins from γ-Linolenic Acid through Designed Biotransformation Pathways. J. Agric. Food Chem. 2015, 63, 2773–2781. [Google Scholar] [CrossRef]
- Koppireddi, S.; Seo, J.H.; Jeon, E.Y.; Chowdhury, P.S.; Jang, H.Y.; Park, J.B.; Kwon, Y.U. Combined Biocatalytic and Chemical Transformations of Oleic Acid to ω-Hydroxynonanoic Acid and α, ω-Nonanedioic Acid. Adv. Synth. Catal. 2016, 358, 3084–3092. [Google Scholar] [CrossRef]
- Cha, H.J.; Seo, E.J.; Song, J.W.; Jo, H.J.; Kumar, A.R.; Park, J.B. Simultaneous Enzyme/Whole-Cell Biotransformation of C18 Ricinoleic Acid into (R)-3-Hydroxynonanoic Acid, 9-Hydroxynonanoic Acid, and 1, 9-Nonanedioic Acid. Adv. Synth. Catal. 2018, 360, 696–703. [Google Scholar] [CrossRef]
- Farbood, M.I.; Morris, J.A.; McLean, L.B. Fermentation Process for Preparing 10-Hydroxy-C18-Carboxylic Acid and Gamma-Dodecalactone Derivatives. EP078388A25, 1 December 1994. [Google Scholar]
- Park, A.K.; Lee, G.H.; Kim, D.W.; Jang, E.H.; Kwon, H.T.; Chi, Y.M. Crystal structure of oleate hydratase from Stenotrophomonas sp. KCTC 12332 reveals conformational plasticity surrounding the FAD binding site. Biochem. Biophys. Res. Commun. 2018, 499, 772–776. [Google Scholar] [CrossRef]
- Lorenzen, J.; Driller, R.; Waldow, A.; Qoura, F.; Loll, B.; Brück, T. Rhodococcus erythropolis Oleate Hydratase: A New Member in the Oleate Hydratase Family Tree—Biochemical and Structural Studies. ChemCatChem 2018, 10, 407–414. [Google Scholar] [CrossRef]
- Demming, R.M.; Hammer, S.C.; Nestl, B.M.; Gergel, S.; Fademrecht, S.; Pleiss, J.; Hauer, B. Asymmetric Enzymatic Hydration of Unactivated, Aliphatic Alkenes. Angew. Chem. Int. Ed. 2019, 58, 173–177. [Google Scholar] [CrossRef]
- Eser, B.E.; Poborsky, M.; Dai, R.; Kishino, S.; Ljubic, A.; Takeuchi, M.; Jacobsen, C.; Ogawa, J.; Kristensen, P.; Guo, Z. Rational Engineering of Hydratase from Lactobacillus Acidophilus Reveals Critical Residues Directing Substrate Specificity and Regioselectivity. ChemBioChem 2019. [Google Scholar] [CrossRef]
- Engleder, M.; Strohmeier, G.A.; Weber, H.; Steinkellner, G.; Leitner, E.; Müller, M.; Mink, D.; Schürmann, M.; Gruber, K.; Pichler, H. Evolving the Promiscuity of Elizabethkingia Meningoseptica Oleate Hydratase for the Regio- and Stereoselective Hydration of Oleic Acid Derivatives. Angew. Chem. Int. Ed. 2019, 58, 7480–7484. [Google Scholar] [CrossRef]


| Strain (Source of Fatty Acid Hydratase) | Product | Conv./% | Product-Amount/g L−1 | PRODUCTIVITY/g L−1 h−1 | Substrate Loading |
|---|---|---|---|---|---|
| Candida tropicalis DSM 3152 (wild-type) [38] | 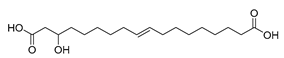 3-hydroxy-Δ9-cis-1,18- octadecenedioic acid | - | 19.4 | 0.8 | 70 mL d−1 |
| Stenotrophomonas nitritireducens (wild-type) [46] | 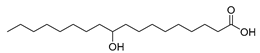 10-hydroxystearic acid | - | 31.5 | 7.9 | 15 g/L |
| Stenotrophomonas maltophilia (exp. in E. coli) [37] |  10-hydroxystearic acid | 98 | 49.0 | 12.3 | 50 g/L |
| Lysinibacillus fusiformis (exp. in E. coli) [48] |  10-hydroxystearic acid | 94 | 40.0 | 384 | 40 g/L |
| Stenotrophomonas maltophilia (exp. in E. coli) [49] | 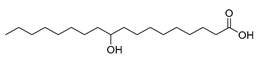 10-hydroxystearic acid | 91 | 46.0 | 197 | 50 g/L |
| Lysinibacillus fusiformis (exp. in E. coli) [50] | 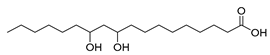 10,12-dihydroxystearic acid | 90 | 13.5 | 108 | 15 g/L |
| Stenotrophomonas nitritireducens (exp. in E. coli) [55] |  10-hydroxy-12(Z)- octadecenoic acid 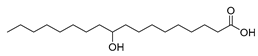 10-hydroxysteric acid | 89; 88 | 5.0, 0.85 | 102, 22 | 7.5 g/L |
| Lactobacillus acidophilus (exp. in E. coli) [56] |  13-hydroxy-9(Z)-octadecenoic acid | 79 | 79.0 | 631 | 100 g/L |
| Lactobacillus plantarum (exp. in E. coli) [58] |  (S)-10-hydoxy-cis-12-octadecenoic acid | 98 | 280.0 | 552 | 90 g/L |
| Stenotrophomonas maltophilia, Lactobacillus acidophilus (exp. in E. coli) [60] | 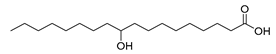 10-monohydroxy fatty acids 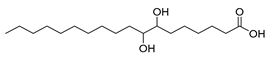 7,8-dihydroxy fatty acids | 65, 81 | 21.7, 13.3 | - | 50 mL (reaction volume) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Löwe, J.; Gröger, H. Fatty Acid Hydratases: Versatile Catalysts to Access Hydroxy Fatty Acids in Efficient Syntheses of Industrial Interest. Catalysts 2020, 10, 287. https://doi.org/10.3390/catal10030287
Löwe J, Gröger H. Fatty Acid Hydratases: Versatile Catalysts to Access Hydroxy Fatty Acids in Efficient Syntheses of Industrial Interest. Catalysts. 2020; 10(3):287. https://doi.org/10.3390/catal10030287
Chicago/Turabian StyleLöwe, Jana, and Harald Gröger. 2020. "Fatty Acid Hydratases: Versatile Catalysts to Access Hydroxy Fatty Acids in Efficient Syntheses of Industrial Interest" Catalysts 10, no. 3: 287. https://doi.org/10.3390/catal10030287
APA StyleLöwe, J., & Gröger, H. (2020). Fatty Acid Hydratases: Versatile Catalysts to Access Hydroxy Fatty Acids in Efficient Syntheses of Industrial Interest. Catalysts, 10(3), 287. https://doi.org/10.3390/catal10030287






