Pyrolytic Formation of TiO2/Carbon Nanocomposite from Kraft Lignin: Characterization and Photoactivities
Abstract
1. Introduction
2. Results and Discussion
2.1. Pyrolysis and Materials Characterization
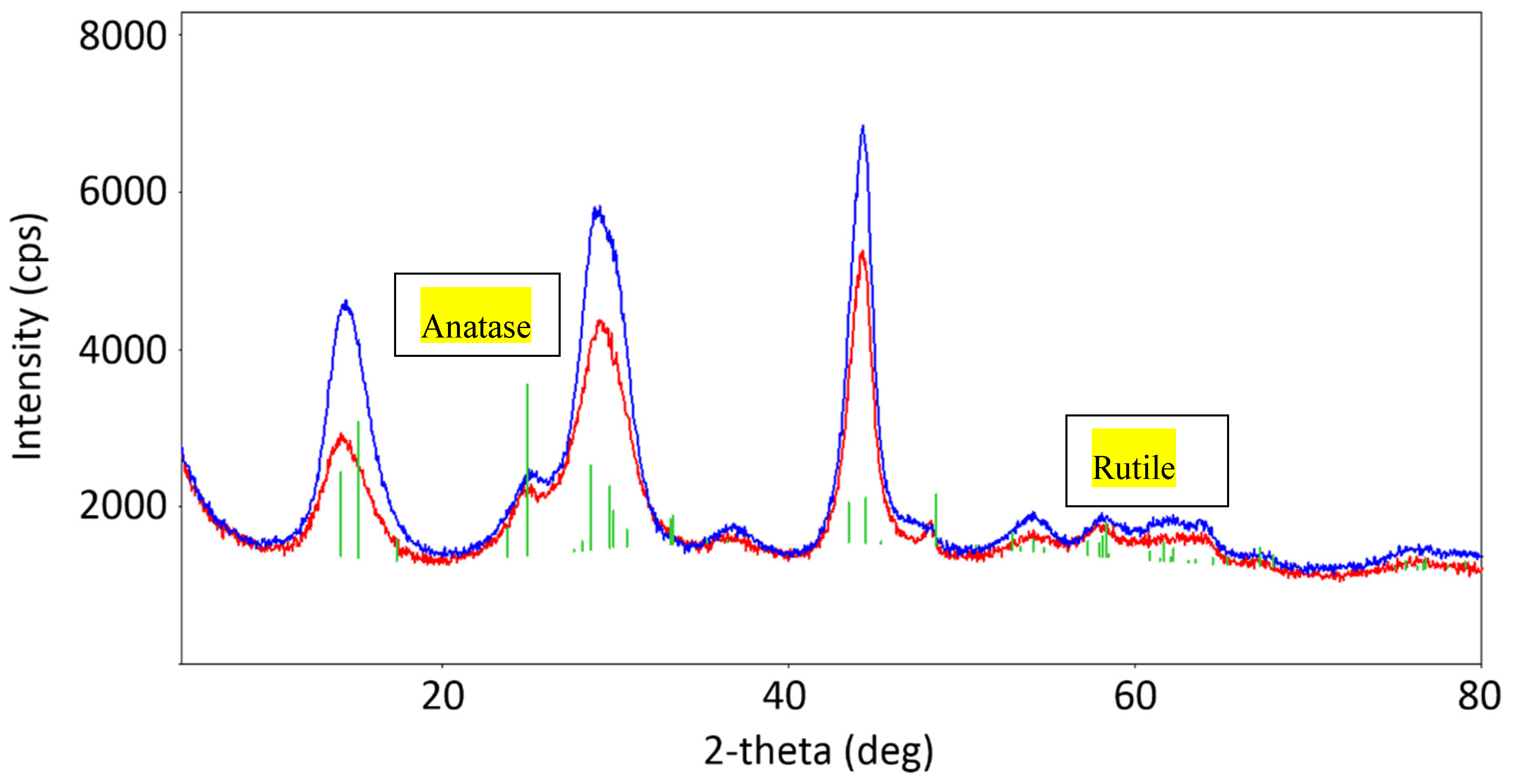
2.1.1. X-ray Powder Patterns
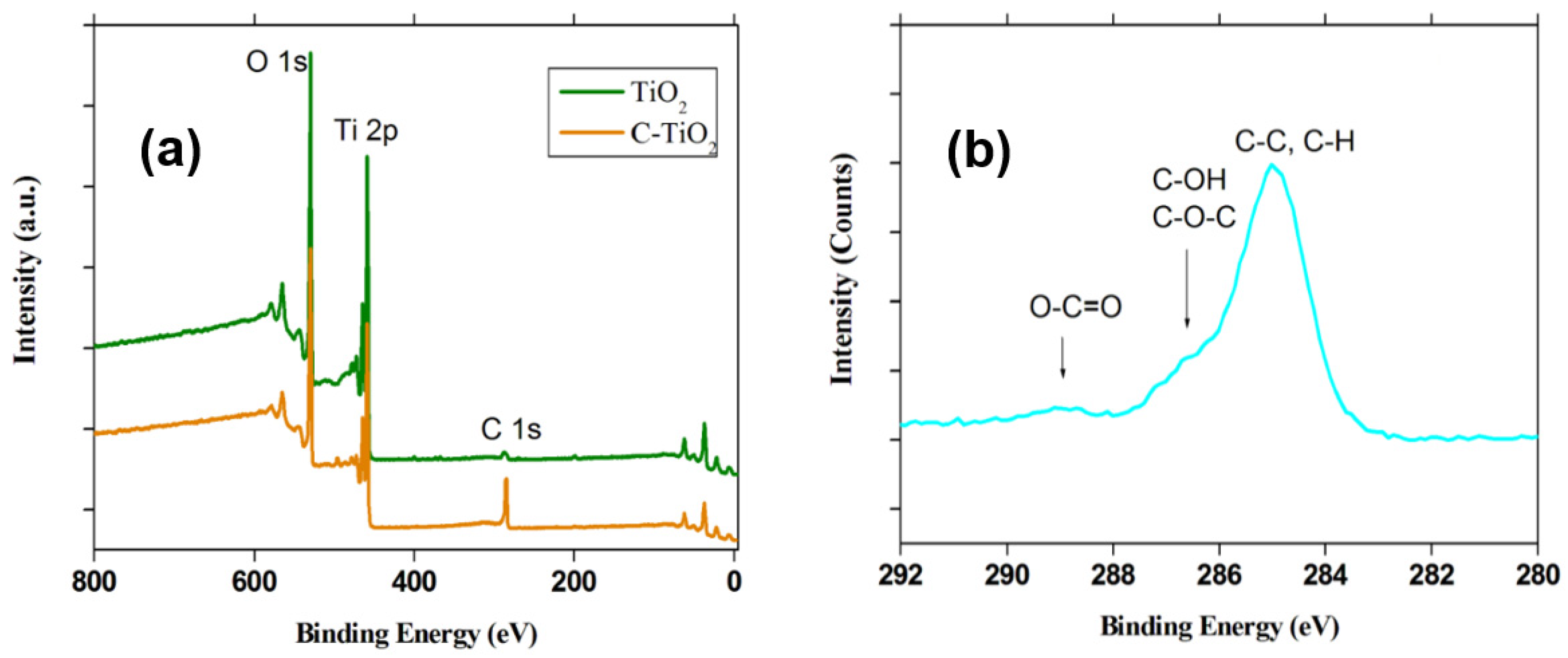
2.1.2. X-ray Photoelectron Spectroscopy (XPS)
2.1.3. Raman Spectral Analysis
2.1.4. Electron Paramagnetic Resonance Spectroscopy
2.1.5. UV-Visible Absorption Spectroscopy
2.1.6. Thermogravimetric Analysis
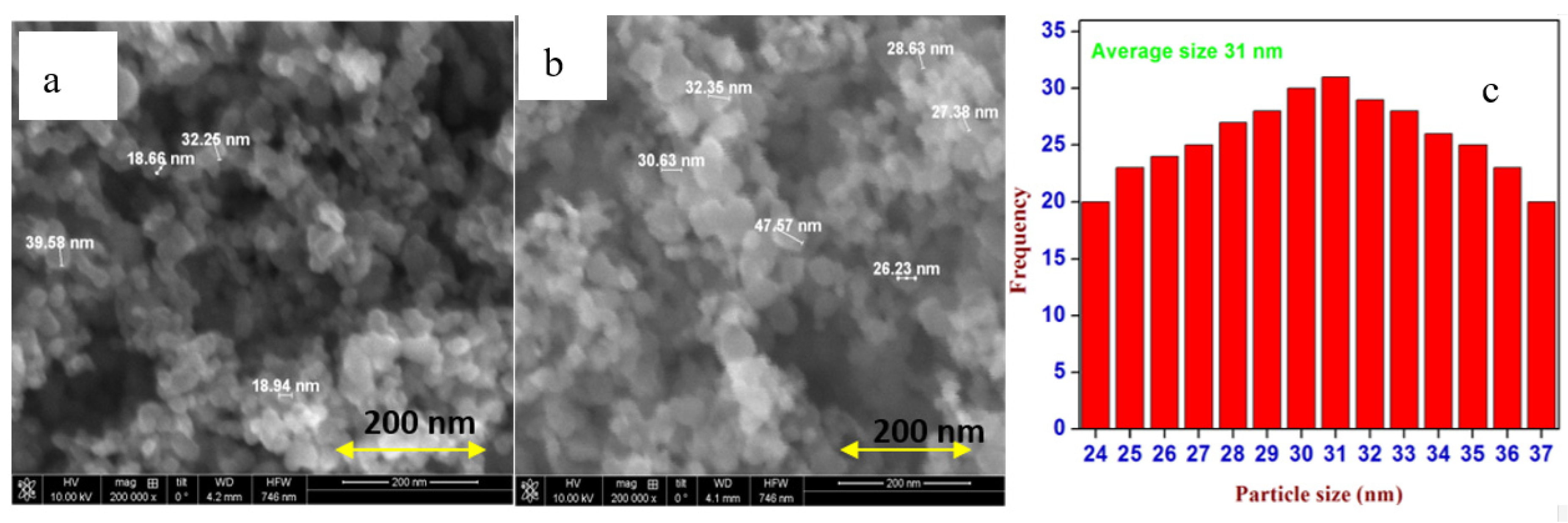
2.1.7. Scanning Electron Microscopic Analysis
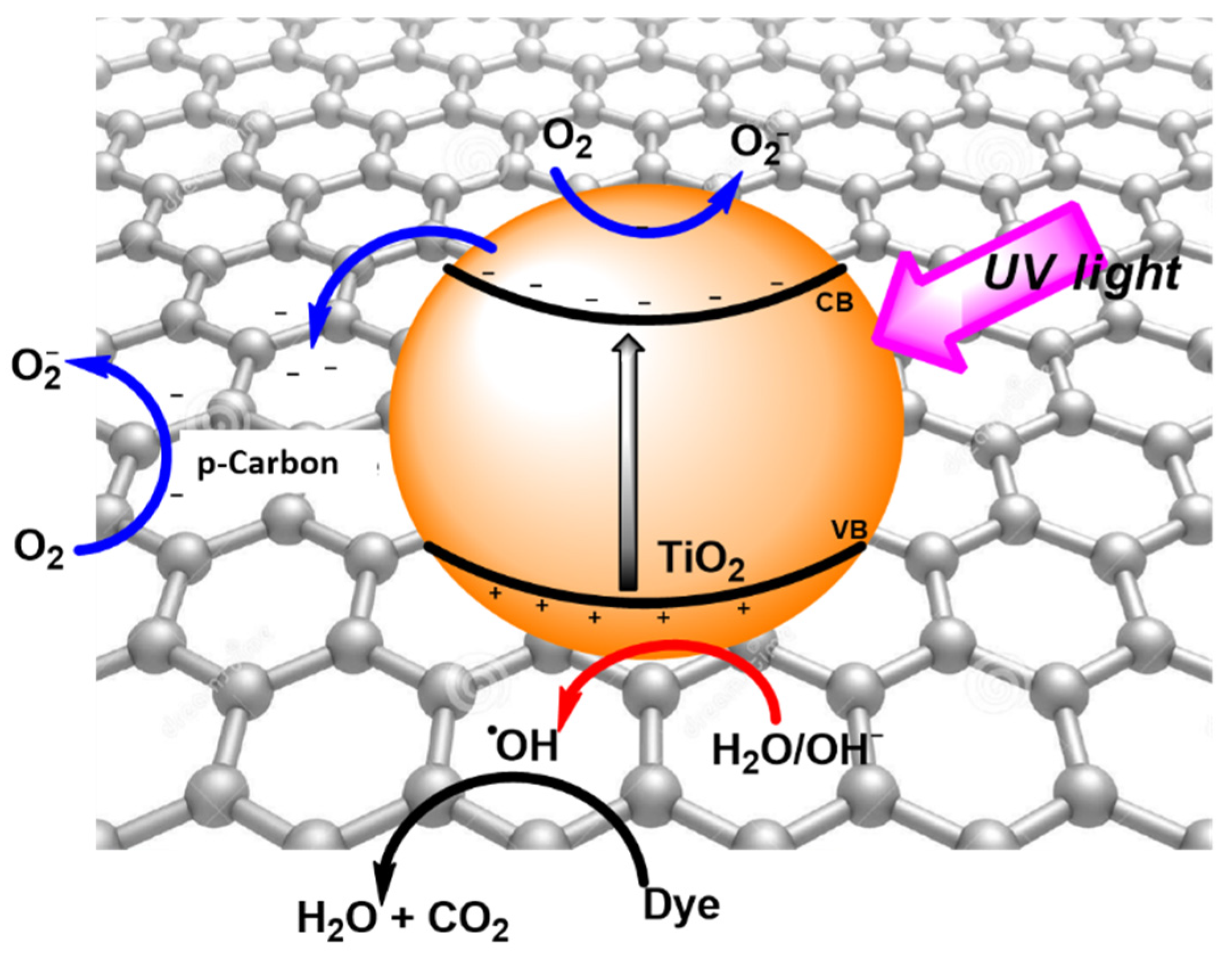
2.2. Photoactivities
2.2.1. Degradation of MB and RhB Dyes under UV Irradiation
2.2.2. Photodegradation of Phenol in the Absence and Presence of O2
2.2.3. Photodegradation of Enrofloxacin in Tap Water under Simulated Solar Light
3. Experimental
3.1. Materials
3.2. High-Vacuum Pyrolysis of Lignin/TiO2
3.3. Characterization of the Materials
3.4. Degradation Procedures and Analyses
4. Concluding Remarks
Supplementary Materials
Supplementary File 1Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yuan, J.; Zhou, S.; Wu, L.; You, B. Organic pigment particles coated with titania via sol–gel process. J. Phys. Chem. B. 2006, 110, 388–394. [Google Scholar] [CrossRef]
- Ohko, Y.; Tatsuma, T.; Fujii, T.; Naoi, K.; Niwa, C.; Kubota, Y.; Fujishima, A. Multicolour photochromism of TiO2 films loaded with silver nanoparticles. Nat. Mater. 2002, 2, 29–31. [Google Scholar] [CrossRef]
- Lee, S.; Cho, I.-S.; Lee, J.H.; Kim, D.H.; Kim, D.W.; Kim, J.Y.; Shin, H.; Lee, J.-K.; Jung, H.S.; Park, N.-G. Two-step sol–gel method-based TiO2 nanoparticles with uniform morphology and size for efficient photo-energy conversion devices. Chem. Mater. 2010, 22, 1958–1965. [Google Scholar] [CrossRef]
- Bao, S.J.; Li, C.M.; Zang, J.F.; Cui, X.Q.; Qiao, Y.; Guo, J. New nanostructured TiO2 for direct electrochemistry and glucose sensor applications. Adv. Funct. Mater. 2008, 18, 591–599. [Google Scholar] [CrossRef]
- Yu, J.; Wang, W.; Cheng, B.; Su, B.-L. Enhancement of photocatalytic activity of mesoporous TiO2 powders by hydrothermal surface fluorination treatment. J. Phys. Chem. C. 2009, 113, 6743–6750. [Google Scholar] [CrossRef]
- Rothenberger, G.; Moser, J.; Gratzel, M.; Serpone, N.; Sharma, D.K. Charge Carrier Trapping and Recombination Dynamics in Small Semiconductor Particles. J. Am. Chem. Soc. 1985, 107, 8054–8059. [Google Scholar] [CrossRef]
- Hu, X.; Li, G.; Yu, J.C. Design, fabrication, and modification of nanostructured semiconductor materials for environmental and energy applications. Langmuir 2009, 26, 3031–3039. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.G.; Devi, L.G. Review on modified TiO2 photocatalysis under UV/visible light: Selected results and related mechanisms on interfacial charge carrier transfer dynamics. J. Phys. Chem. A. 2011, 115, 13211–13241. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, Y.; Xing, M.; Leghari, S.A.K.; Sajjad, S. Development of modified N- doped TiO2photocatalyst with metals, nonmetals and metal oxides. Energy Environ. Sci. 2010, 3, 715–726. [Google Scholar] [CrossRef]
- Wu, G.; Wang, J.; Thomas, D.F.; Chen, A. Synthesis of F-doped flower-like TiO2 nanostructures with high photoelectrochemical activity. Langmuir 2008, 24, 3503–3509. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.; Tian, Q.; Zhou, H.; Liu, Q.; Liu, P.; Zhong, H. Hierarchical porous TiO2@C hollow microspheres: One-pot synthesis and enhanced visible-light photocatalysis. J. Mater. Chem. 2012, 22, 7036–7042. [Google Scholar] [CrossRef]
- Woan, K.; Pyrgiotakis, G.; Sigmund, W. Photocatalytic carbon-nanotube–TiO2composites. Adv. Mater. 2009, 21, 2233–2239. [Google Scholar] [CrossRef]
- Nor, N.M.; Chung, L.L.; Teong, L.K.; Mohamed, A.R. Synthesis of activated carbon from lignocellulosic biomass and its applications in air pollution control—A review. J. Environ. Chem. Eng. 2013, 1, 658–666. [Google Scholar]
- Farhangi, N.; Medina-Gonzalez, Y.; Chowdhury, R.R.; Charpentier, P.A. Growing TiO2 nanowires on the surface of graphene sheets in supercritical CO2: Characterization and photo efficiency. Nanotechnology 2012, 23, 294005. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Yu, J.; Jaroniec, M. Graphene-based semiconductor photocatalysts. Chem. Soc. Rev. 2012, 41, 782–796. [Google Scholar] [CrossRef]
- Liu, B.; Huang, Y.; Wen, Y.; Du, L.; Zeng, W.; Shi, Y.; Zhang, F.; Zhu, G.; Xu, X.; Wang, Y. Highly dispersive f001g facets-exposed nanocrystalline TiO2 on high quality graphene as a high performance photocatalyst. J. Mater. Chem. 2012, 22, 7484–7491. [Google Scholar] [CrossRef]
- Wang, W.-S.; Wang, D.-H.; Qu, W.-G.; Lu, L.-Q.; Xu, A.-W. Large ultrathin anatase TiO2nanosheets with exposed f001g facets on graphene for enhanced visible light photocatalytic activity. J. Phys. Chem. C. 2012, 116, 19893–19901. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Suhas, M.; Carrott, P.J.; Ribeiro Carrott, M.M.L. Lignin—From natural adsorbent to activated carbon: A review. Biores. Technol. 2007, 98, 2301–2312. [Google Scholar] [CrossRef]
- Fierro, V.; Torne-Fernandez, V.; Celzard, A. Kraft lignin as a precursor for microporous activated carbons prepared by impregnation with ortho-phosphoric acid: Synthesis and textural characterisation. Microporous Mesoporous Mater. 2006, 92, 243–250. [Google Scholar] [CrossRef]
- Gu, L.; Zhang, H.; Jiao, Z.; Li, M.; Wu, M.; Lei, Y. Glucosamine-induced growth of highly distributed TiO2 nanoparticles on graphene nanosheets as high-performance photocatalysts. RSC Adv. 2016, 6, 67039–67048. [Google Scholar] [CrossRef]
- Sun, M.; Li, W.; Sun, S.; He, J.; Zhang, Q.; Shi, Y. One-step in situ synthesis of graphene-TiO2nanorod hybrid composites with enhanced photocatalytic activity. Mater. Res. Bull. 2015, 61, 280–286. [Google Scholar] [CrossRef]
- Wu, T.; Liu, G.; Zhao, J.; Hidaka, H.; Serpone, N. Photo assisted degradation of dye pollutants. V. Self-photosensitized oxidative transformation of rhodamine-B under visible light irradiation in aqueous TiO2 dispersions. J. Phys. Chem. B 1998, 102, 5845–5851. [Google Scholar] [CrossRef]
- Dong, P.; Wang, Y.; Guo, L.; Liu, B.; Xin, S.; Zhang, J.; Shi, Y.; Zeng, W.; Yin, S. A facile one-step solvothermal synthesis of graphene/rod-shaped TiO2 nanocomposite and its improved photocatalytic activity. Nanoscale 2012, 4, 4641–4649. [Google Scholar] [CrossRef]
- Giovannetti, R.; Rommozzi, E.; Zannotti, M.; D’Amato, C.A. Recent advances in graphene-based TiO2 nanocomposites (GTiO2Ns) for photocatalytic degradation of synthetic dyes. Catalysts 2017, 7, 305. [Google Scholar] [CrossRef]
- Jiang, G.; Lin, Z.; Chen, C.; Zhu, L.; Chang, Q.; Wang, N.; Wei, W.; Tang, H. TiO2 nanoparticles assembled on GO nanosheets with high photocatalytic activity for removal of pollutants. Carbon 2011, 49, 2693–2701. [Google Scholar] [CrossRef]
- Gao, Y.; Pu, X.; Zhang, D.; Ding, G.; Shao, X.; Ma, J. Combustion synthesis of GO–TiO2 hybrid materials for photodegradation of methyl orange. Carbon 2012, 49, 4093–4101. [Google Scholar] [CrossRef]
- Sun, L.; Zhao, Z.; Zhou, Y.; Liu, L. Anatase TiO2 nanocrystals with exposed f001g facets on graphene sheets via molecular grafting for enhanced photocatalytic activity. Nanoscale 2012, 4, 613–620. [Google Scholar] [CrossRef]
- Zhang, H.; Lv, X.; Li, Y.; Wang, Y.; Li, J. P25-Graphene Composite as a High Performance Photocatalyst. ACS Nano 2010, 4, 380–386. [Google Scholar] [CrossRef]
- Du, J.; Lai, X.; Yang, N.; Zhai, J.; Kisailus, D.; Su, F.; Wang, D.; Jiang, L. Hierarchically Ordered Macro-Mesoporous TiO2-Graphene Composite Films: Improved Mass Transfer, Reduced Charge Recombination, and Their Enhanced Photocatalytic Activities. ACS Nano 2011, 5, 590–596. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.; Zhang, X.; Zhu, Y. Characterization, uniformity and photocatalytic properties of graphene/TiO2 nanocomposites via Raman mapping. Optics Express 2017, 25, 21496. [Google Scholar] [CrossRef] [PubMed]
- Posa, V.R.; Annavaram, V.; Koduru, J.R.; Bobbala, P.; Somala, M.V.; Somala, A.R. Preparation of graphene–TiO2 nanocomposite and photocatalytic degradation of Rhodamine-B under solar light irradiation. J. Experim. Nanosci. 2016, 11, 722–736. [Google Scholar] [CrossRef]
- Martins, P.M.; Ferreira, C.G.; Silva, A.R.; Magalhães, B.; Alves, M.M.; Pereira, L.; Marques, P.A.A.P.; Melle-Franco, M.; Lanceros-Méndez, S. TiO2/graphene and TiO2/graphene oxide nanocomposites for photocatalytic applications: A computer modeling and experimental study. Compos. Part B 2018, 145, 39–46. [Google Scholar] [CrossRef]
- Zhang, Q.; Bao, N.; Wang, X.; Hu, X.; Miao, X.; Chaker, M.; Ma, D. Advanced fabrication of chemically bonded graphene/TiO2 continuous fibers with enhanced broadband photocatalytic properties and involved mechanisms exploration. Sci. Rep. 2016, 6, 38066. [Google Scholar] [CrossRef] [PubMed]
- Dondi, D.; Zeffiro, A.; Speltini, A.; Tomasi, C.; Vadivel, D.; Buttafava, A. The role of inorganic sulfur compounds in the pyrolysis of Kraft lignin. J. Anal. Appl. Pyrol. 2014, 107, 53–58. [Google Scholar] [CrossRef]
- Vadivel, D.; Speltini, A.; Zeffiro, A.; Bellani, V.; Pezzini, S.; Buttafava, A.; Dondi, D. Reactive carbons from Kraft lignin pyrolysis: Stabilization of peroxyl radicals at carbon/silica interface. J. Anal. Appl. Pyrol. 2017, 128, 346–352. [Google Scholar] [CrossRef]
- Qiu, B.; Zhou, Y.; Ma, Y.; Yang, X.; Sheng, W.; Xing, M.; Zhang, J. Facile synthesis of the Ti3+ self-dopedTiO2-graphene nanosheet composites with enhanced photocatalysis. Sci. Rep. 2015, 5, 8591. [Google Scholar] [CrossRef]
- Lyon, T.J.; Sichau, J.; Dorn, A.; Centeno, A.; Pesquera, A.; Zurutuza, A.; Blick, R.H. Probing Electron Spin Resonance in Monolayer Graphene. Phys. Rev. Lett. 2017, 119, 066802. [Google Scholar] [CrossRef]
- Panich, A.M.; Shames, A.I.; Tsindlekht, M.I.; Yu, V.; Osipov, V.; Patel, M.; Savaram, K.; He, H. Structure and magnetic properties of pristine and Fe-doped micro- and nano-graphenes. J. Phys. Chem. C 2016, 120, 3042–3053. [Google Scholar] [CrossRef]
- Yasse, A.S.; Shahed, U.M. Khan Visible light active carbon modified n-TiO2 for efficient hydrogen production by photoelectrochemical splitting of water. Int. J. Hydrogen Energy 2008, 33, 1118–1126. [Google Scholar]
- Guoxin, H.; Tang, B. Photocatalytic mechanism of graphene/titanate nanotubes photocatalyst under visible-light irradiation. Mater. Chem. Phys. 2013, 138, 608–614. [Google Scholar]
- Cruz, M.; Gomez, C.; Duran-Valle, C.J.; Pastrana-Martínez, L.M.; Faria, J.L.; Silva, A.M.T.; Faraldos, M.; Bahamonde, A. Bare TiO2 and graphene oxide TiO2 photocatalysts on the degradation of selected pesticides and influence of the water matrix. Appl. Surf. Sci. 2017, 416, 1013–1021. [Google Scholar] [CrossRef]
- Takahashi, T.; Sugawara, K.; Noguchi, E.; Sato, T.; Takahashi, T. Band-gap tuning of monolayer graphene by oxygen adsorption. Carbon 2014, 73, 141–145. [Google Scholar] [CrossRef]
- Sturini, M.; Speltini, A.; Maraschi, F.; Rivaglia, E.; Pretali, L.; Malavasi, L.; Profumo, A.; Fasani, E.; Albini, A. Sunlight photodegradation of marbofloxacin and enrofloxacin adsorbed on clay minerals. J. Photochem. Photobiol. A 2015, 299, 103–109. [Google Scholar] [CrossRef]





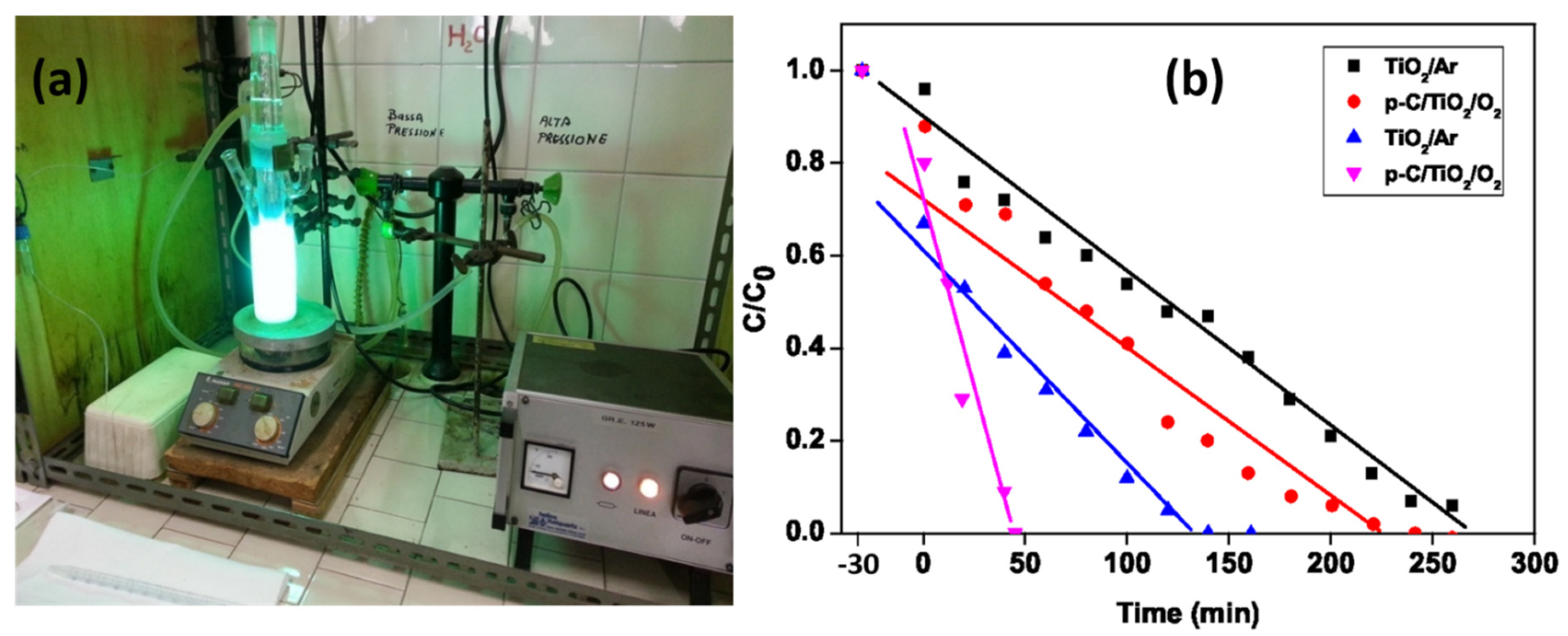
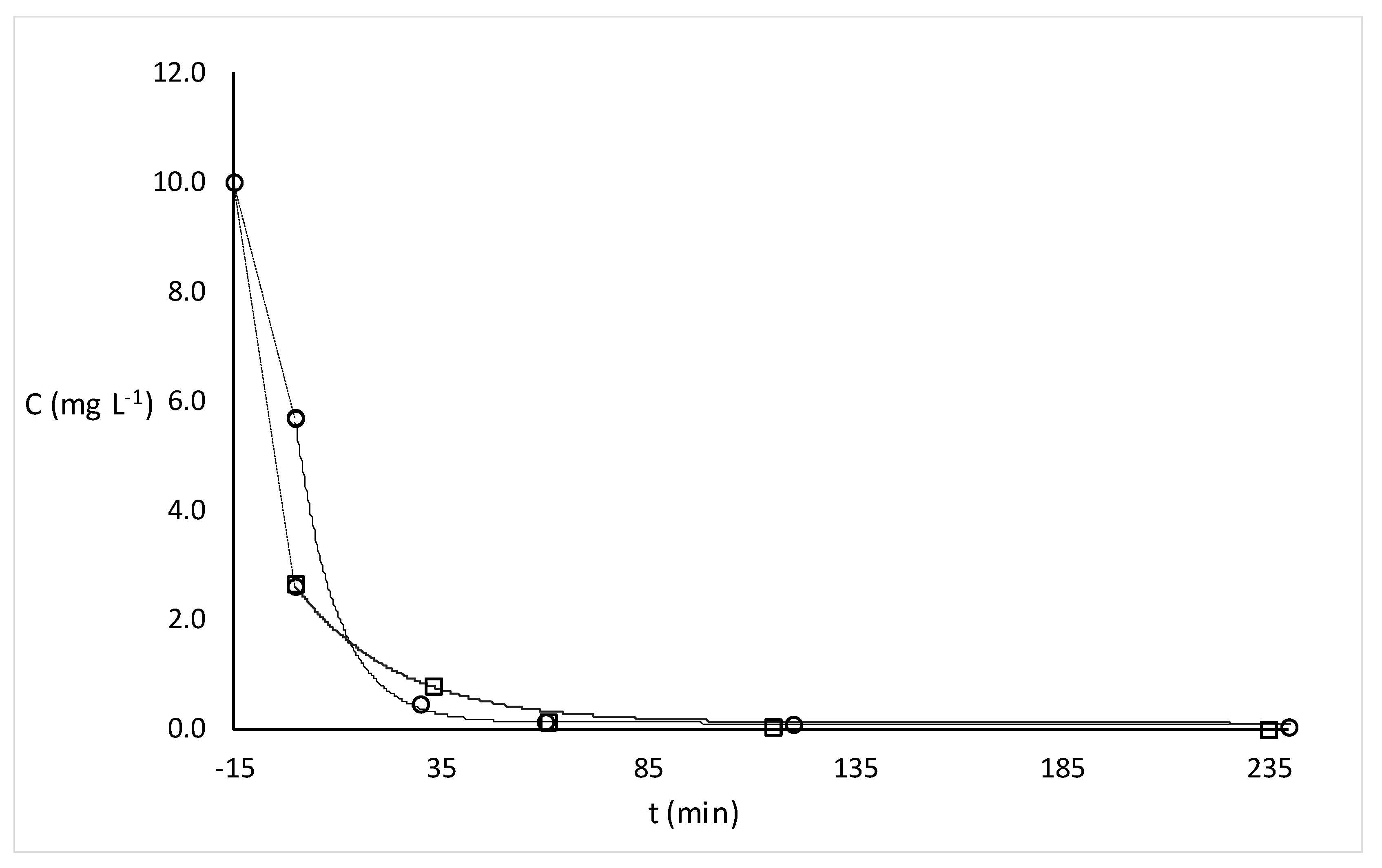
| Dye | k (min−1) | % Adsorption in the Dark | ||
|---|---|---|---|---|
| TiO2 | p-C/TiO2 | TiO2 | p-C/TiO2 | |
| MB | 0.047 ± 0.005 | 0.091 ± 0.008 | 48 | 85 |
| RhB | 0.064 ± 0.005 | 0.060 ± 0.007 | 43 | 88 |
| Item | TiO2 (Ar) | TiO2 (O2) | p-C/TiO2 (Ar) | p-C/TiO2 (O2) |
|---|---|---|---|---|
| Rini (10−3 mM min−1) | 3.3 ± 0.1 | 17.6 ± 0.5 | 3.5 ± 0.2 | 4.3 ± 0.3 |
| % adsorption (60 min dark) | 3 | 20 | 11 | 32 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vadivel, D.; Branciforti, D.S.; Speltini, A.; Sturini, M.; Bellani, V.; Malaichamy, I.; Dondi, D. Pyrolytic Formation of TiO2/Carbon Nanocomposite from Kraft Lignin: Characterization and Photoactivities. Catalysts 2020, 10, 270. https://doi.org/10.3390/catal10030270
Vadivel D, Branciforti DS, Speltini A, Sturini M, Bellani V, Malaichamy I, Dondi D. Pyrolytic Formation of TiO2/Carbon Nanocomposite from Kraft Lignin: Characterization and Photoactivities. Catalysts. 2020; 10(3):270. https://doi.org/10.3390/catal10030270
Chicago/Turabian StyleVadivel, Dhanalakshmi, Diego Savio Branciforti, Andrea Speltini, Michela Sturini, Vittorio Bellani, Ilanchelian Malaichamy, and Daniele Dondi. 2020. "Pyrolytic Formation of TiO2/Carbon Nanocomposite from Kraft Lignin: Characterization and Photoactivities" Catalysts 10, no. 3: 270. https://doi.org/10.3390/catal10030270
APA StyleVadivel, D., Branciforti, D. S., Speltini, A., Sturini, M., Bellani, V., Malaichamy, I., & Dondi, D. (2020). Pyrolytic Formation of TiO2/Carbon Nanocomposite from Kraft Lignin: Characterization and Photoactivities. Catalysts, 10(3), 270. https://doi.org/10.3390/catal10030270









