Hybrid Technology for DeNOxing by LNT-SCR System for Efficient Diesel Emission Control: Influence of Operation Parameters in H2O + CO2 Atmosphere
Abstract
1. Introduction
2. Results and Discussion
2.1. Evaluation of VNSR/VSCR Ratio in Dry He-Atmosphere
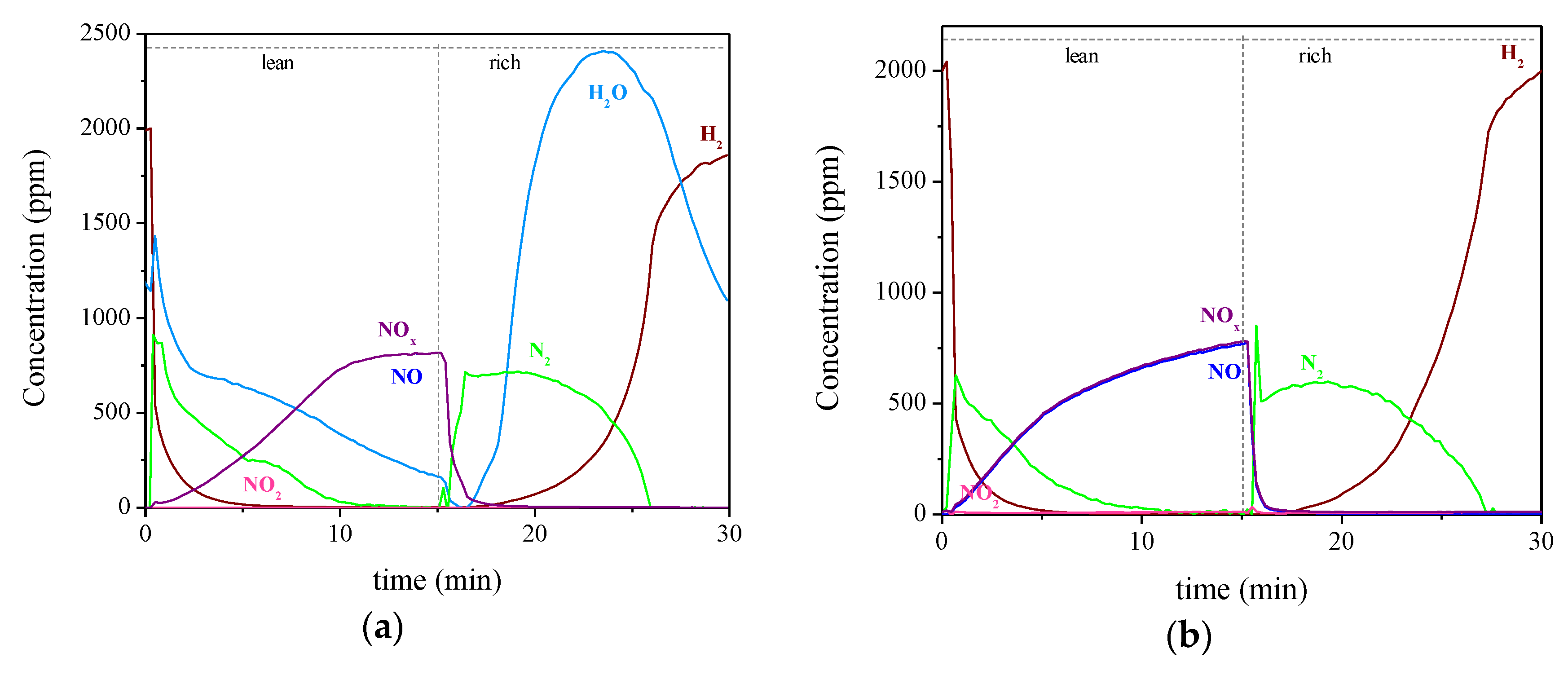
2.2. Influence of H2O and CO2 Presence over NSR-SCR Systems
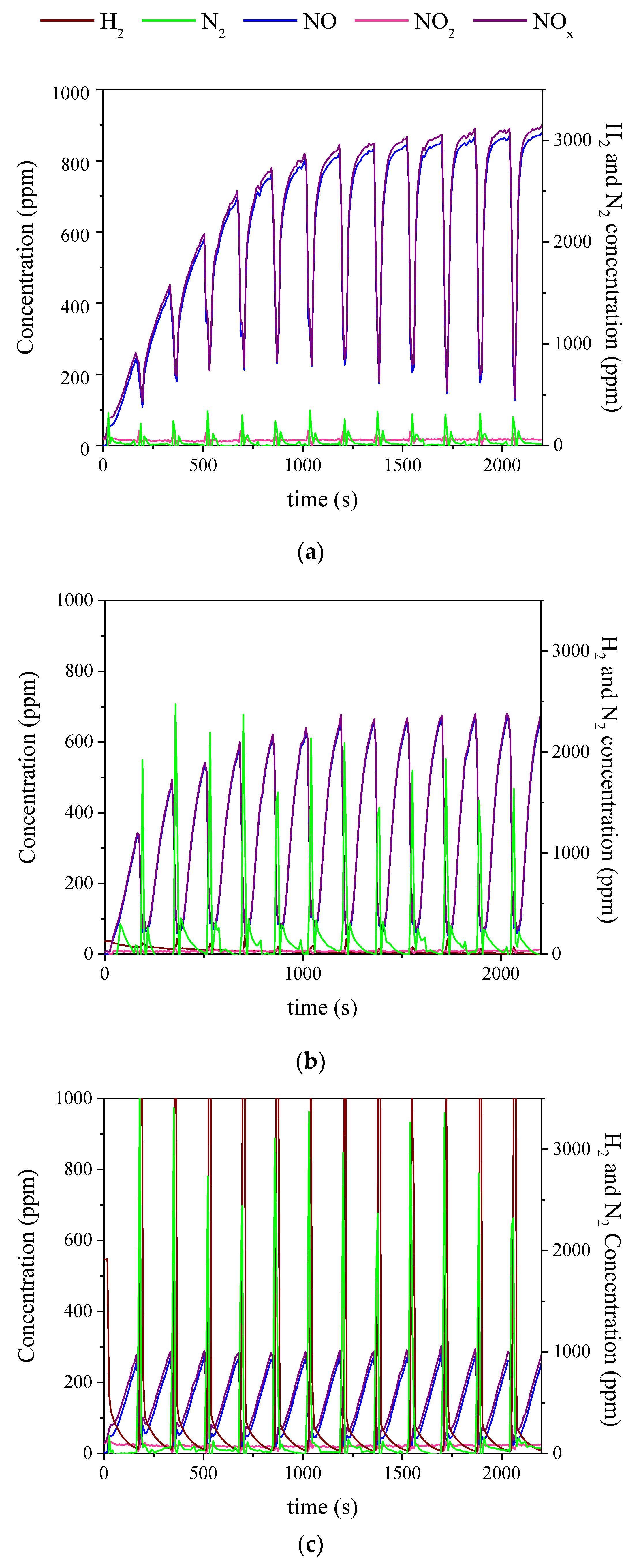
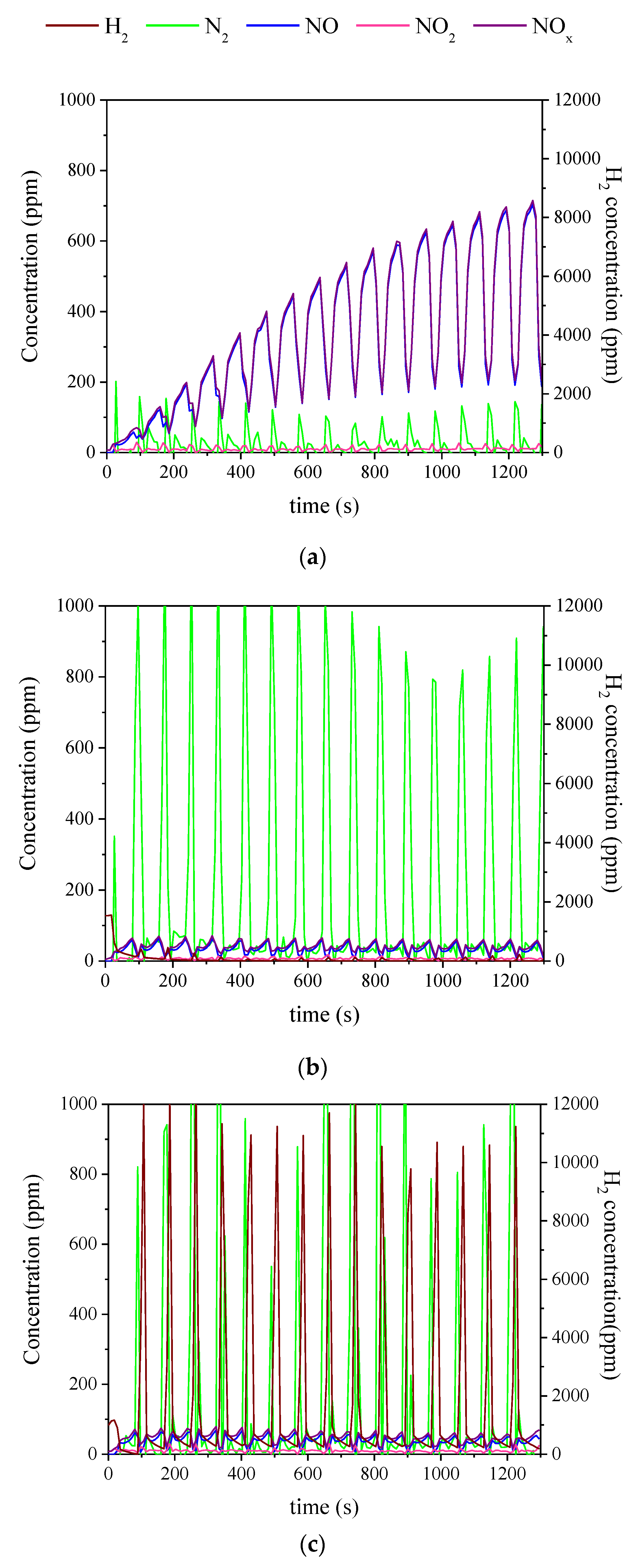
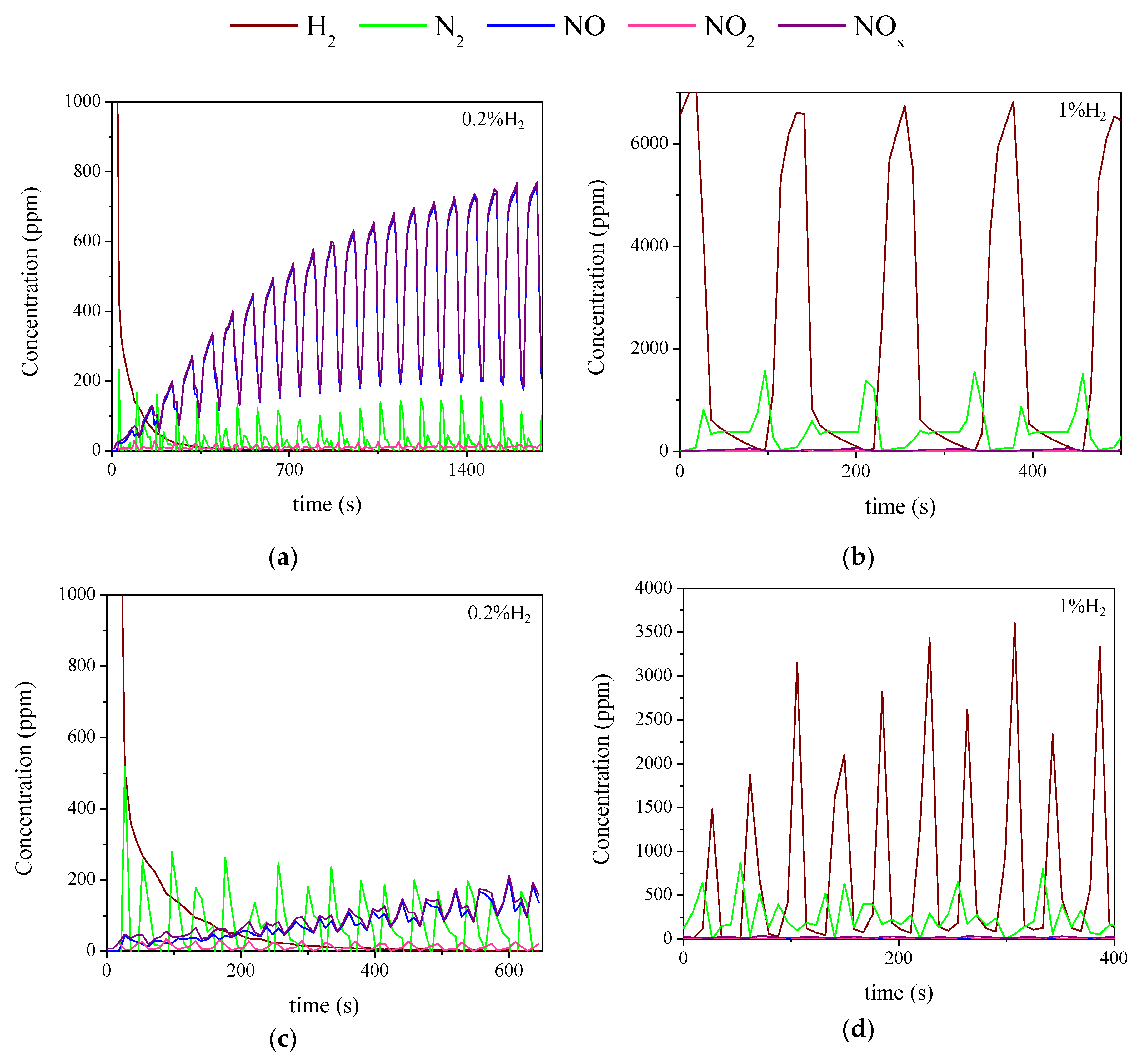
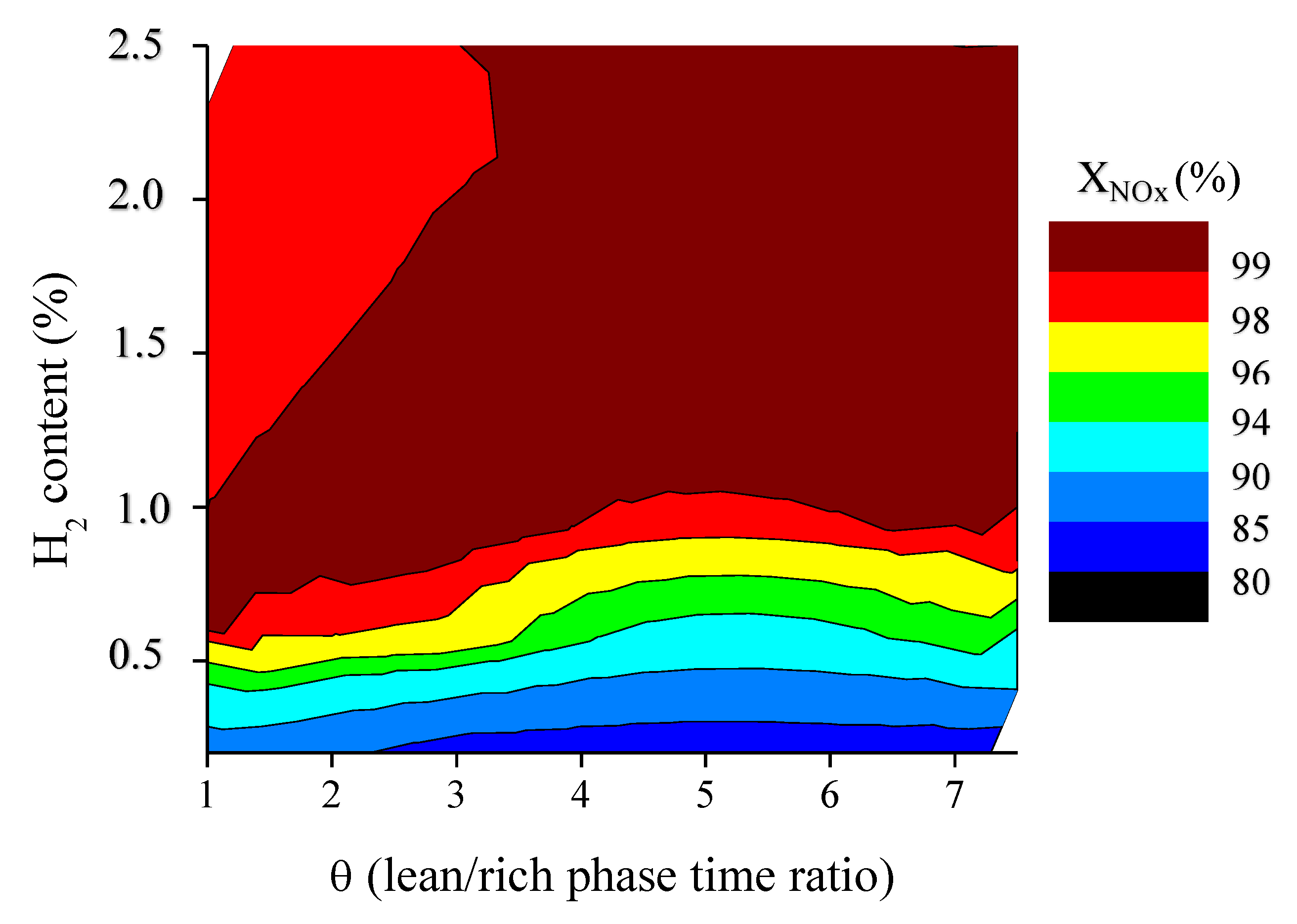
2.3. Influence of the Duration of Lean-Rich Cycles and the Concentration of H2
3. Materials and Methods
3.1. Synthesis of Catalysts
3.2. Catalytic Activity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cortés-Reyes, M.; Herrera, C.; Larrubia, M.A.; Auñón, J.A.; González, M.; Alemany, L.J. Impact of new biofuels on pollutant production and motor performance and study of DeNOx technologies to achieve zero emission in real conditions. Int. J. Innov. Res. Sci. Eng. Technol. 2016, 5, 13082–13088. [Google Scholar]
- Kočí, P.; Plát, F.; Štěpánek, J.; Bártová, Š.; Marek, M.; Kubíček, M.; Schmeißer, V.; Chatterjee, D.; Weibel, M. Global kinetic model for the regeneration of NOx storage catalyst with CO, H2 and C3H6 in the presence of CO2 and H2O. Catal. Today 2009, 147, S257–S264. [Google Scholar] [CrossRef]
- Ting, A.W.-L.; Li, M.; Harold, M.P.; Balakotaiah, V. Fast cycling in a non-isothermal monolithic lean NOx trap using H2 as reductant: Experiments and modeling. Chem. Eng. J. 2017, 326, 419–435. [Google Scholar] [CrossRef]
- Cortés-Reyes, M.; Herrera, C.; Larrubia, M.Á.; Alemany, L.J. Advance in the scaling up of a hybrid catalyst for NSR-SCR coupled systems under H2O + CO2 atmosphere. Catal. Today 2019. [Google Scholar] [CrossRef]
- Kubiak, L.; Castoldi, L.; Lietti, L.; Andonova, S.; Olsson, L. Mechanistic investigation of the reduction of NOx over Pt- and Rh-Based LNT Catalysts. Catalysts 2016, 6, 46. [Google Scholar] [CrossRef]
- Krishna, K.; Makkee, M. Soot oxidation over NOx storage catalysts: Activity and deactivation. Catal. Today 2006, 114, 48–56. [Google Scholar] [CrossRef]
- Gálvez, M.E.; Ascaso, S.; Tobías, I.; Moliner, R.; Lázaro, M.J. Catalytic filters for the simultaneous removal of soot and NOx: Influence of the alumina precursor on monolith washcoating and catalytic activity. Catal. Today 2012, 191, 96–105. [Google Scholar] [CrossRef]
- De La Torre, U.; Pereda-Ayo, B.; Romero-Sáez, M.; Aranzabal, A.; González-Marcos, M.P.; González-Marcos, J.A.; González-Velasco, J.R. Screening of Fe-Cu-zeolites prepared by different methodology for application in NSR-SCR combined DeNOxsystems. Top. Catal. 2013, 56, 215–221. [Google Scholar] [CrossRef]
- Olsson, L.; Wijayanti, K.; Leistner, K.; Kumar, A.; Joshi, S.Y.; Kamasamudram, K.; Currier, N.W.; Yezerets, A. A kinetic model for sulfur poisoning and regeneration of Cu/SSZ-13 used for NH3-SCR. Appl. Catal. B Environ. 2016, 183, 394–406. [Google Scholar] [CrossRef]
- Xu, L.; McCabe, R.W. LNT+in situ SCR catalyst system for diesel emissions control. Catal. Today 2012, 184, 83–94. [Google Scholar] [CrossRef]
- Pereda-Ayo, B.; Duraiswami, D.; González-Velasco, J.R. Control of NOx storage and reduction in NSR bed for designing combined NSR–SCR systems. Catal. Today 2011, 172, 66–72. [Google Scholar] [CrossRef]
- De La Torre, U.; Pereda-Ayo, B.; González-Velasco, J.R. Cu-zeolite NH3-SCR catalysts for NOx removal in the combined NSR–SCR technology. Chem. Eng. J. 2012, 207–208, 10–17. [Google Scholar] [CrossRef]
- Castoldi, L.; Bonzi, R.; Lietti, L.; Forzatti, P.; Morandi, S.; Ghiotti, G.; Dzwigaj, S. Catalytic behaviour of hybrid LNT/SCR systems: Reactivity and in situ FTIR study. J. Catal. 2011, 282, 128–144. [Google Scholar] [CrossRef]
- Lindholm, A.; Sjövall, H.; Olsson, L. Reduction of NOx over a combined NSR and SCR system. Appl. Catal. B Environ. 2010, 98, 112–121. [Google Scholar] [CrossRef]
- Forzatti, P.; Lietti, L.; Nova, I.; Tronconi, E. Diesel NOx aftertreatment catalytic technologies: Analogies in LNT and SCR catalytic chemistry. Catal. Today 2010, 151, 202–211. [Google Scholar] [CrossRef]
- Can, F.; Courtois, X.; Royer, S.; Blanchard, G.; Rousseau, S.; Duprez, D. An overview of the production and use of ammonia in NSR+SCR coupled system for NOx reduction from lean exhaust gas. Catal. Today 2012, 197, 144–154. [Google Scholar] [CrossRef]
- Harold, M.P. NOx storage and reduction in lean burn vehicle emission control: A catalytic engineer’s playground. Curr. Opin. Chem. Eng. 2012, 1, 303–311. [Google Scholar] [CrossRef]
- Lietti, L.; Artioli, N.; Righini, L.; Castoldi, L.; Forzatti, P. Pathways for N2 and N2O formation during the reduction of NOx over Pt–Ba/Al2O3 LNT catalysts investigated by labeling isotopic experiments. Ind. Eng. Chem. Res. 2012, 51, 7597–7605. [Google Scholar] [CrossRef]
- Bonzi, R.; Lietti, L.; Castoldi, L.; Forzatti, P. NOx removal over a double-bed NSR-SCR reactor configuration. Catal. Today 2010, 151, 376–385. [Google Scholar] [CrossRef]
- Forzatti, P.; Lietti, L. The reduction of NOx stored on LNT and combined LNT–SCR systems. Catal. Today 2010, 155, 131–139. [Google Scholar] [CrossRef]
- Corbos, E.C.; Haneda, M.; Courtois, X.; Marecot, P.; Duprez, D.; Hamada, H. Cooperative effect of Pt–Rh/Ba/Al and CuZSM-5 catalysts for NOx reduction during periodic lean-rich atmosphere. Catal. Commun. 2008, 10, 137–141. [Google Scholar] [CrossRef]
- Corbos, E.C.; Haneda, M.; Courtois, X.; Marecot, P.; Duprez, D.; Hamada, H. NOx abatement for lean-burn engines under lean–rich atmosphere over mixed NSR-SCR catalysts: Influences of the addition of a SCR catalyst and of the operational conditions. Appl. Catal. A Gen. 2009, 365, 187–193. [Google Scholar] [CrossRef]
- De La Torre, U.; Urrutxua, M.; Pereda-Ayo, B.; González-Velasco, J.R. On the Cu species in Cu/beta catalysts related to DeNOx performance of coupled NSR-SCR technology using sequential monoliths and dual-layer monolithic catalysts. Catal. Today 2016, 273, 72–82. [Google Scholar] [CrossRef]
- Can, F.; Courtois, X.; Berland, S.; Seneque, M.; Royer, S.; Duprez, D. Composition dependent performance of alumina-based oxide supported WO3 catalysts for the NH3-SCR reaction and the NSR + SCR coupled process. Catal. Today 2015, 257, 41–50. [Google Scholar] [CrossRef]
- Colombo, M.; Nova, I.; Tronconi, E. A comparative study of the NH3-SCR reactions over a Cu-zeolite and a Fe-zeolite catalyst. Catal. Today 2010, 151, 223–230. [Google Scholar] [CrossRef]
- Martínez-Franco, R.; Moliner, M.; Franch, C.; Kustov, A.; Corma, A. Rational direct synthesis methodology of very active and hydrothermally stable Cu-SAPO-34 molecular sieves for the SCR of NOx. Appl. Catal. B Environ. 2012, 127, 273–280. [Google Scholar] [CrossRef]
- De La Torre, U.; Pereda-Ayo, B.; Moliner, M.; González-Velasco, J.R.; Corma, A. Cu-zeolite catalysts for NOx removal by selective catalytic reduction with NH3 and coupled to NO storage/reduction monolith in diesel engine exhaust aftertreatment systems. Appl. Catal. B Environ. 2016, 187, 419–427. [Google Scholar] [CrossRef]
- Yu, L.; Zhong, Q.; Zhang, S. Research of copper contained SAPO-34 zeolite for NH3-SCR DeNOx by solvent-free synthesis with Cu-TEPA. Microporous Mesoporous Mater. 2016, 234, 303–309. [Google Scholar] [CrossRef]
- Ma, L.; Cheng, Y.; Cavataio, G.; McCabe, R.W.; Fu, L.; Li, J. Characterization of commercial Cu-SSZ-13 and Cu-SAPO-34 catalysts with hydrothermal treatment for NH3-SCR of NOx in diesel exhaust. Chem. Eng. J. 2013, 225, 323–330. [Google Scholar] [CrossRef]
- Cortés-Reyes, M.; Finocchio, E.; Herrera, C.; Larrubia, M.A.; Alemany, L.J.; Busca, G. A study of Cu-SAPO-34 catalysts for SCR of NOx by ammonia. Microporous Mesoporous Mater. 2017, 241, 258–265. [Google Scholar] [CrossRef]
- Ting, A.W.-L.; Harold, M.P.; Balakotaiah, V. Elucidating the mechanism of fast cycling NOx storage and reduction using C3H6 and H2 as reductants. Chem. Eng. Sci. 2018, 189, 413–421. [Google Scholar] [CrossRef]
- De-La-Torre, U.; Pereda-Ayo, B.; Moliner, M.; González-Marcos, J.A.; Corma, A.; González-Velasco, J.R. Optimal operating conditions of coupled sequential NOx storage/reduction and Cu/CHA selective catalytic reduction monoliths. Top. Catal. 2017, 60, 30–39. [Google Scholar] [CrossRef]
- Kota, A.S.; Luss, D.; Balakotaiah, V. Modeling studies on lean NOx reduction by a sequence of LNT-SCR bricks. Ind. Eng. Chem. Res. 2012, 51, 6686–6696. [Google Scholar] [CrossRef]
- Al-Harbi, M.; Epling, W.S. Effects of different regeneration timing protocols on the performance of a model NOx storage/reduction catalyst. Catal. Today 2010, 151, 347–353. [Google Scholar] [CrossRef]
- Cortés-Reyes, M.; Herrera, M.C.; Pieta, I.S.; Larrubia, M.A.; Alemany, L.J. In situ TG-MS study of NOx and soot removal over LNT model catalysts. Appl. Catal. A Gen. 2016, 523, 193–199. [Google Scholar] [CrossRef]
- Righini, L.; Kubiak, L.; Morandi, S.; Castoldi, L.; Lietti, L.; Forzatti, P. n-Heptane As a Reducing Agent in the NOx Removal over a Pt–Ba/Al2O3 NSR Catalyst. ACS Catal. 2014, 4, 3261–3272. [Google Scholar] [CrossRef]
- Seo, C.-K.; Kim, H.; Choi, B.; Lim, M.T. The optimal volume of a combined system of LNT and SCR catalysts. J. Ind. Eng. Chem. 2011, 17, 382–385. [Google Scholar] [CrossRef]
- Adamski, A.; Zając, W.; Zasada, F.; Sojka, Z. Copper ionic pairs as possible active sites in N2O decomposition on CuOx/CeO2 catalysts. Catal. Today 2012, 191, 129–133. [Google Scholar] [CrossRef]
- Zabilskiy, M.; Djinović, P.; Erjavec, B.; Dražić, G.; Pintar, A. Small CuO clusters on CeO2 nanospheres as active species for catalytic N2O decomposition. Appl. Catal. B Environ. 2015, 163, 113–122. [Google Scholar] [CrossRef]
- Palella, B.I.; Cadoni, M.; Frache, A.; Pastore, H.O.; Pirone, R.; Russo, G.; Coluccia, S.; Marchese, L. On the hydrothermal stability of CuAPSO-34 microporous catalysts for N2O decomposition: A comparison with CuZSM-5. J. Catal. 2003, 217, 100–106. [Google Scholar] [CrossRef]
- Smeets, P.J.; Sels, B.F.; van Teeffelen, R.M.; Leeman, H.; Hensen, E.J.M.; Schoonheydt, R.A. The catalytic performance of Cu-containing zeolites in N2O decomposition and the influence of O2, NO and H2O on recombination of oxygen. J. Catal. 2008, 256, 183–191. [Google Scholar] [CrossRef]
- Hendershot, R.J.; Vijay, R.; Snively, C.M.; Lauterbach, J. High-throughput study of the influence of H2O and CO2 on the performance of nitrogen storage and reduction (NSR) catalysts. Appl. Surf. Sci. 2006, 252, 2588–2592. [Google Scholar] [CrossRef]
- Lietti, L.; Nova, I.; Forzatti, P. Role of ammonia in the reduction by hydrogen of NOx stored over Pt–Ba/Al2O3 lean NOx trap catalysts. J. Catal. 2008, 257, 270–282. [Google Scholar] [CrossRef]
- Pieta, I.S.; García-Diéguez, M.; Herrera, C.; Larrubia, M.A.; Alemany, L.J. In situ DRIFT-TRM study of simultaneous NOx and soot removal over Pt-Ba and Pt-K NSR catalysts. J. Catal. 2010, 270, 256–267. [Google Scholar] [CrossRef]
- Luo, J.Y.; Epling, W.S. New insights into the promoting effect of H2O on a model Pt/Ba/Al2O3 NSR catalyst. Appl. Catal. B Environ. 2010, 97, 236–247. [Google Scholar] [CrossRef]
- Cortés-Reyes, M.; Larrubia, M.Á.; Herrera, C.; Alemany, L.J. Influence of CO2 and H2O co-feeding in the NOx abatement by SCR over an efficient Cu-CHA catalyst. Chem. Eng. Sci. 2019, 201, 373–381. [Google Scholar] [CrossRef]
- Ren, Y.; Harold, M.P. NOx storage and reduction with H2 on Pt/Rh/BaO/CeO2: Effects of Rh and CeO2 in the absence and presence of CO2 and H2O. ACS Catal. 2011, 1, 969–988. [Google Scholar] [CrossRef]
- Chatterjee, D.; Kočí, P.; Schmeißer, V.; Marek, M.; Weibel, M.; Krutzsch, B. Modelling of a combined NOx storage and NH3-SCR catalytic system for Diesel exhaust gas aftertreatment. Catal. Today 2010, 151, 395–409. [Google Scholar] [CrossRef]
- Bártová, Š.; Kočí, P.; Mráček, D.; Marek, M.; Pihl, J.A.; Choi, J.-S.; Toops, T.J.; Partridge, W.P. New insights on N2O formation pathways during lean/rich cycling of a commercial lean NOx trap catalyst. Catal. Today 2014, 231, 145–154. [Google Scholar] [CrossRef]
- Shakya, B.M.; Harold, M.P.; Balakotaiah, V. Modeling and analysis of dual-layer NOx storage and reduction and selective catalytic reduction monolithic catalyst. Chem. Eng. J. 2014, 237, 109–122. [Google Scholar] [CrossRef]
- Cortés-Reyes, M.; Herrera, M.C.; Larrubia, M.A.; Alemany, L.J. Fast ultrasound assisted synthesis of Cu-SAPO-34 for SCR application. Int. J. Innov. Res. Sci. Eng. Technol. 2016, 5, 5540–5550. [Google Scholar]

| Temperature (°C) | Atmosphere | System | Removed NOx (103 mol NOx gNSR−1) | XNOx (%) | SN2 (%) | SNH3 (%) | SN2O (%) |
|---|---|---|---|---|---|---|---|
| 200 | He | NSR | 0.39 | 91 | 94.1 | 5.2 | 0.7 |
| NSR-SCR | 0.51 | 99 | 99.9 | 0 | 0.1 | ||
| H2O + CO2 | NSR | 0.24 | 83 | 90.5 | 9.5 | 0 | |
| NSR-SCR | 0.35 | 84 | 100 | 0 | 0 | ||
| 350 | He | NSR | 0.42 | 88 | 97 | 3 | 0 |
| NSR-SCR | 0.53 | 91 | 100 | 0 | 0 | ||
| H2O + CO2 | NSR | 0.41 | 86 | 100 | 0 | 0 | |
| NSR-SCR | 0.52 | 92 | 100 | 0 | 0 |
| Lean Phase | Rich Phase | |
|---|---|---|
| NO (ppm) | 1000 | - |
| O2 (v/v %) | 3 | - |
| H2 (v/v %) | - | 0.2, 1, 2.5 |
| H2O (v/v %) | 1.5 | 1.5 |
| CO2 (v/v %) | 0.3 | 0.3 |
| He | Balance | Balance |
| GHSV (h−1) | 3 × 104 | 3 × 104 |
| Temperature (°C) | 200, 350 | 200, 350 |
| θ = 1 | 20, 60 s | 20, 60 s |
| θ = 3 | 60 s | 20 s |
| θ = 7.5 | 150 s | 20 s |
| H2 (ppm) | T (°C) | 60 s–20 s (θ = 3) | 150 s–20 s (θ = 7.5) | ||
|---|---|---|---|---|---|
| mmolNOx·gNSR−1 | XNOx (%) | mmolNOx·gNSR−1 | XNOx (%) | ||
| 2000 | 200 | 0.44 | 78.6 | 0.61 | 76.3 |
| 350 | 0.46 | 82.5 | 0.62 | 81.3 | |
| 10,000 | 200 | 0.69 | 97.3 | 1.2 | 94.3 |
| 350 | 0.71 | 99.3 | 1.3 | 95.6 | |
| 25,000 | 200 | 0.69 | 97.7 | 1.6 | 97.7 |
| 350 | 0.71 | 99.4 | 1.6 | 97.9 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cortés-Reyes, M.; Herrera, C.; Larrubia, M.Á.; Alemany, L.J. Hybrid Technology for DeNOxing by LNT-SCR System for Efficient Diesel Emission Control: Influence of Operation Parameters in H2O + CO2 Atmosphere. Catalysts 2020, 10, 228. https://doi.org/10.3390/catal10020228
Cortés-Reyes M, Herrera C, Larrubia MÁ, Alemany LJ. Hybrid Technology for DeNOxing by LNT-SCR System for Efficient Diesel Emission Control: Influence of Operation Parameters in H2O + CO2 Atmosphere. Catalysts. 2020; 10(2):228. https://doi.org/10.3390/catal10020228
Chicago/Turabian StyleCortés-Reyes, Marina, Concepción Herrera, María Ángeles Larrubia, and Luis J. Alemany. 2020. "Hybrid Technology for DeNOxing by LNT-SCR System for Efficient Diesel Emission Control: Influence of Operation Parameters in H2O + CO2 Atmosphere" Catalysts 10, no. 2: 228. https://doi.org/10.3390/catal10020228
APA StyleCortés-Reyes, M., Herrera, C., Larrubia, M. Á., & Alemany, L. J. (2020). Hybrid Technology for DeNOxing by LNT-SCR System for Efficient Diesel Emission Control: Influence of Operation Parameters in H2O + CO2 Atmosphere. Catalysts, 10(2), 228. https://doi.org/10.3390/catal10020228








