Perovskite-Based Catalysts as Efficient, Durable, and Economical NOx Storage and Reduction Systems
Abstract
1. Introduction
2. Perovskite-Based Catalysts in Automotive Exhaust Catalytic Converters
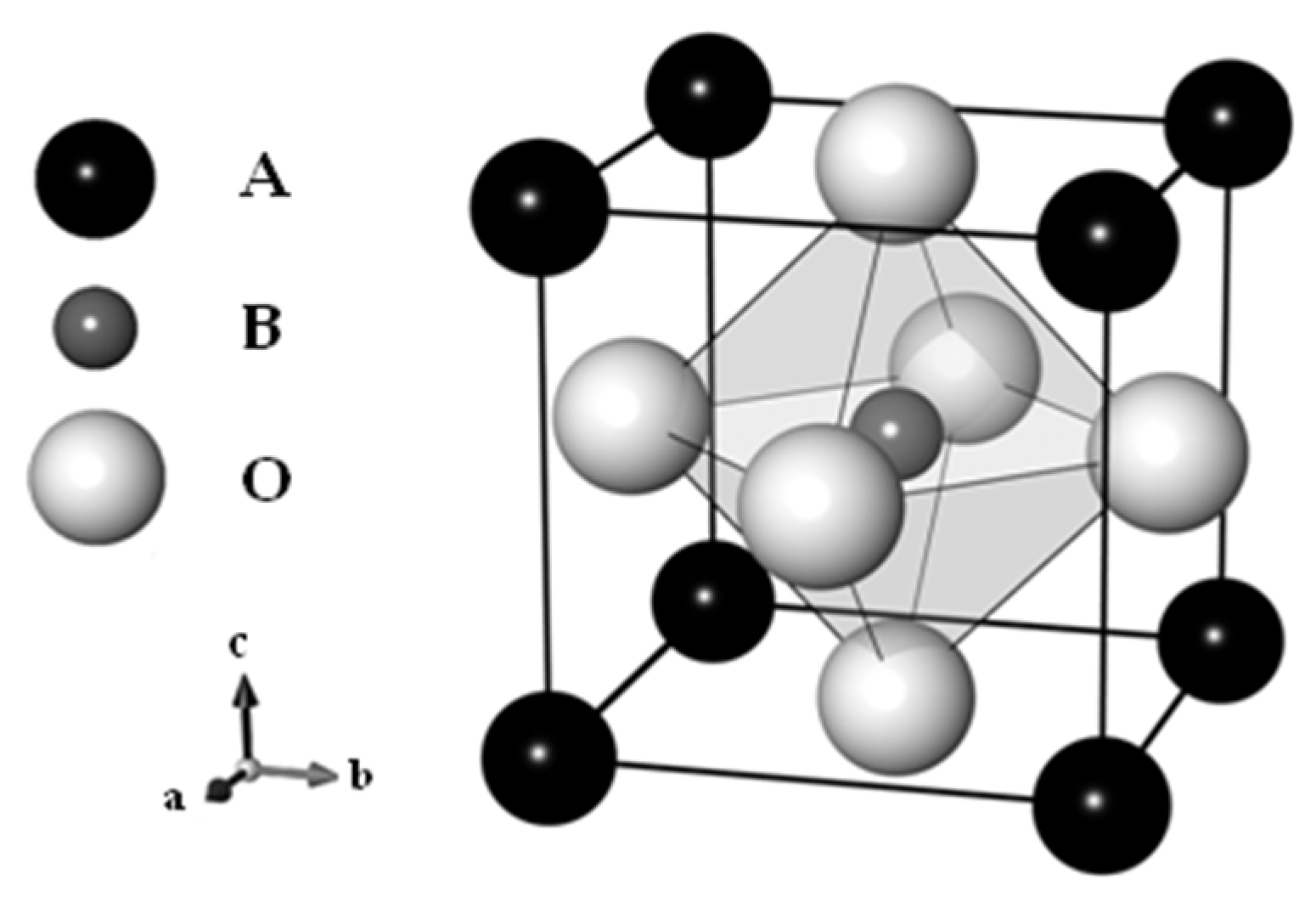
3. Perovskite-Based Catalysts for NSR Technology
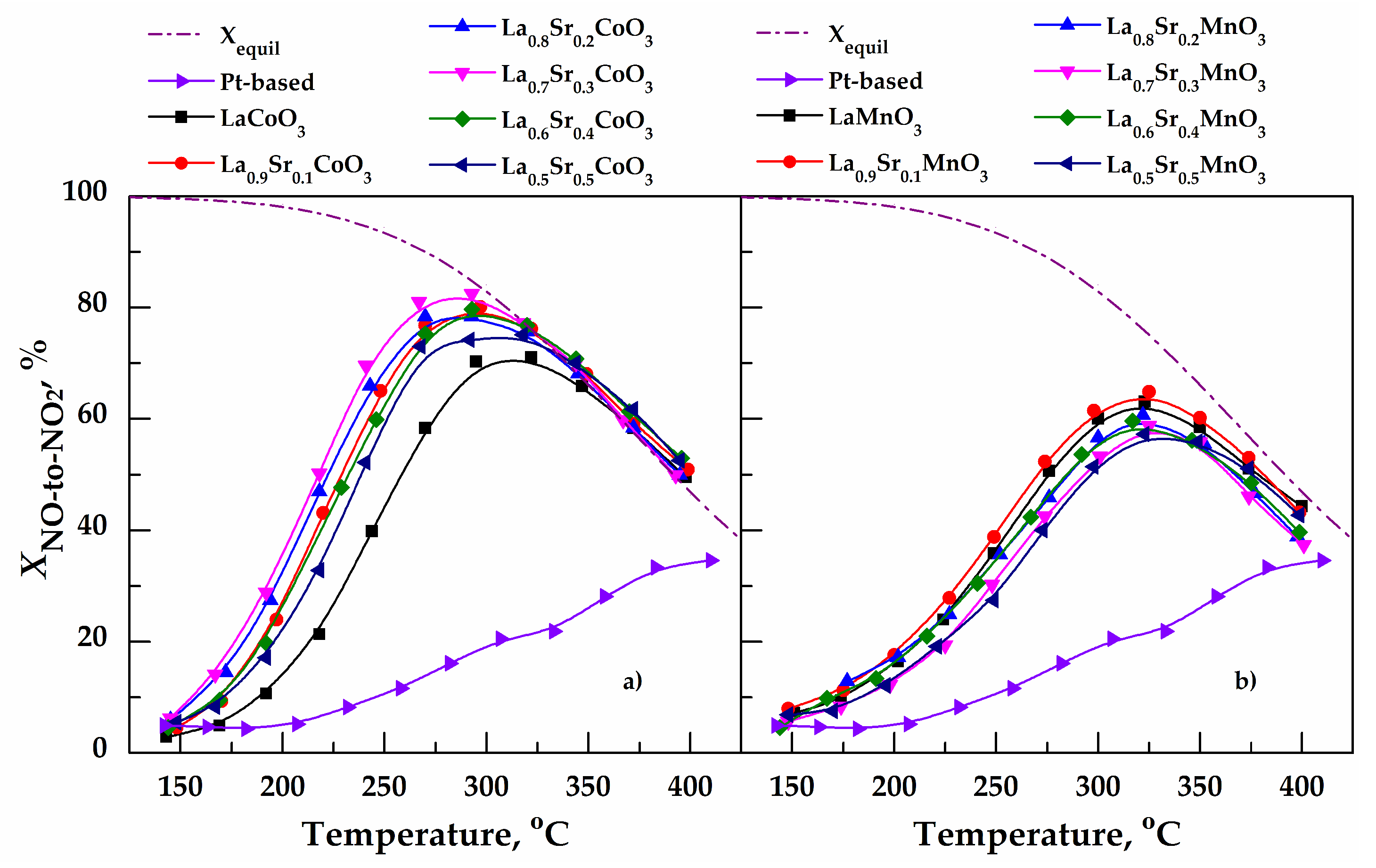
3.1. NO-to-NO2 Conversion
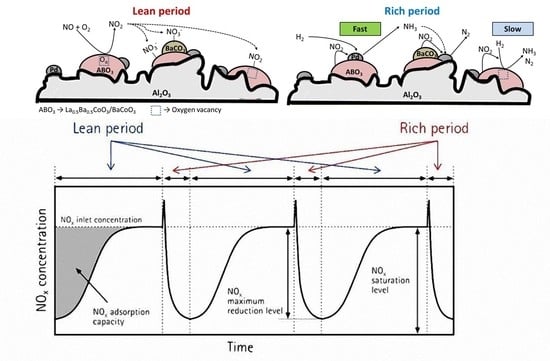
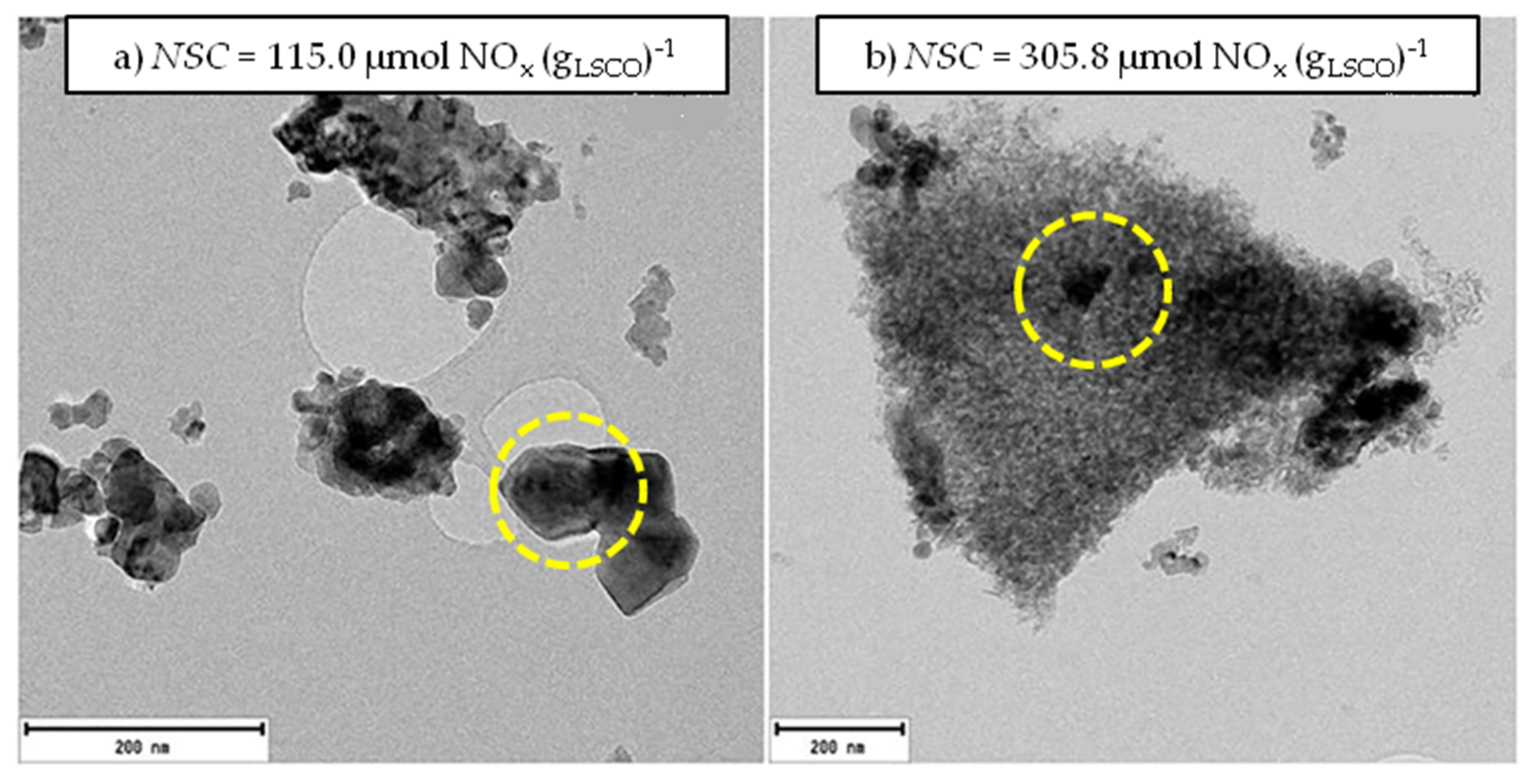
3.2. NOx Adsorption under Oxidizing Conditions
3.3. NOx Reduction
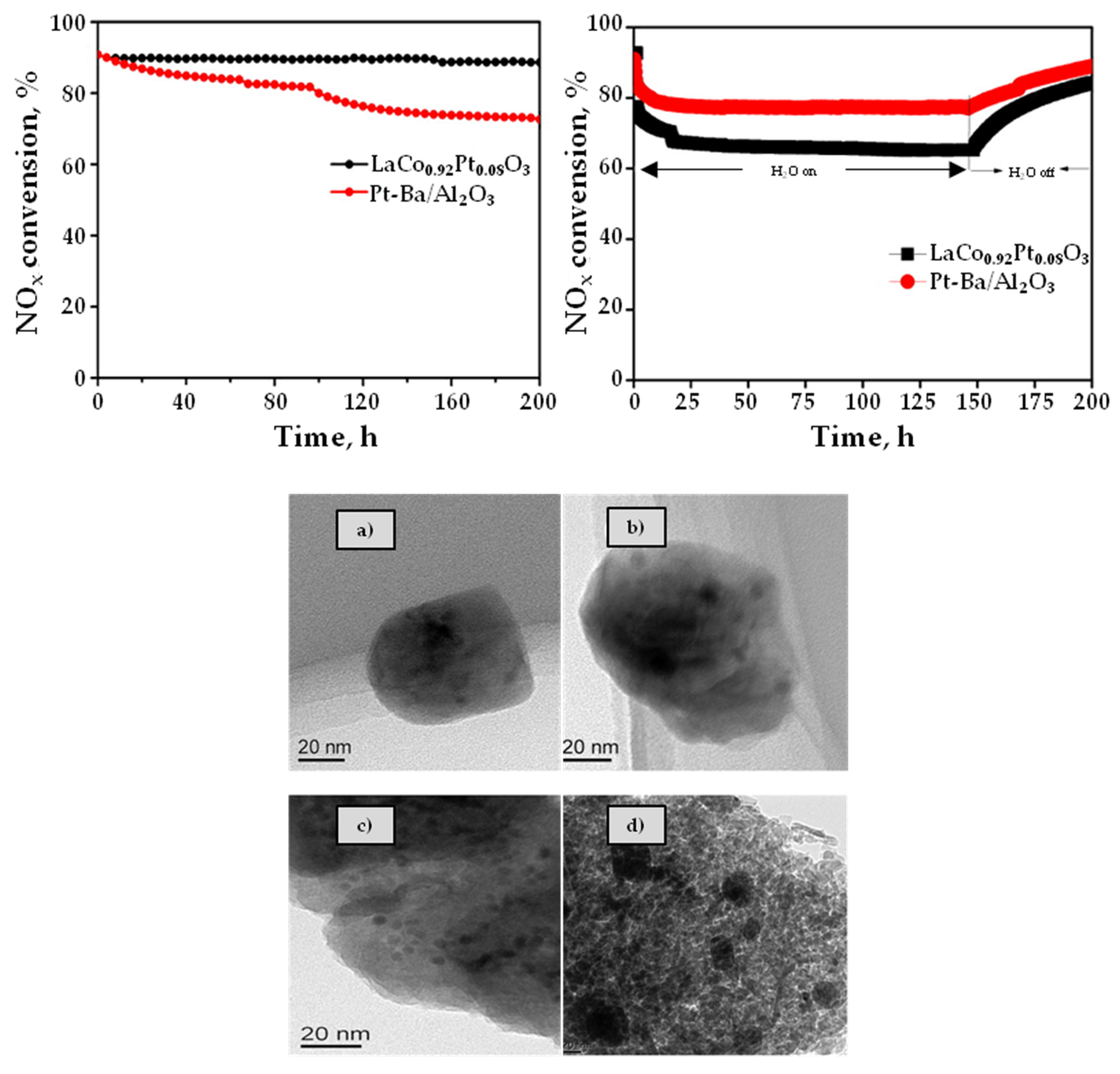
3.4. SO2 and Hydrothermal Resistance
4. Perovskite-Based Catalysts for Combined NSR–SCR Technology
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Srinivasacharya Ayodhya, A.; Gottekere Narayanappa, K. An overview of after-treatment systems for diesel engines. Environ. Sci. Pollut. Res. 2018, 25, 35034–35047. [Google Scholar] [CrossRef]
- Johnson, T. Review of diesel emissions and control. Int. J. Engine Res. 2009, 10, 275–285. [Google Scholar] [CrossRef]
- Granger, P.; Parvulescu, V.I. Catalytic NOx Abatement Systems for Mobile Sources: From Three-Way to Lean Burn after-Treatment Technologies. Chem. Rev. 2011, 111, 3155–3207. [Google Scholar] [CrossRef] [PubMed]
- Tronconi, E.; Nova, I. Urea-SCR Technology for deNOx after Treatment of Diesel Exhausts; Springer: New York, NY, USA, 2014. [Google Scholar]
- González-Velasco, J.R.; Pereda-Ayo, B.; De-La-Torre, U.; Urrutxua, M.; López-Fonseca, R. Coupled NSR-SCR Systems for NOx Removal in Light-Duty Vehicles. ChemCatChem 2018, 10, 2928. [Google Scholar] [CrossRef]
- Takahashi, N.; Shinjoh, H.; Iijima, T.; Suzuki, T.; Yamazaki, K.; Yokota, K.; Suzuki, H.; Miyoshi, N.; Matsumoto, S.; Tanizawa, T.; et al. The new concept 3-way catalyst for automotive lean-burn engine: NOx storage and reduction catalyst. Catal. Today 1996, 27, 63–69. [Google Scholar] [CrossRef]
- Clayton, R.D.; Harold, M.P.; Balakotaiah, V. Performance features of Pt/BaO lean NOx trap with hydrogen as reductant. AIChE J. 2009, 55, 687–700. [Google Scholar] [CrossRef]
- Pereda-Ayo, B.; Duraiswami, D.; Delgado, J.J.; López-Fonseca, R.; Calvino, J.J.; Bernal, S.; González-Velasco, J.R. Tuning operational conditions for efficient NOx storage and reduction over a Pt–Ba/Al2O3 monolith catalyst. Appl. Catal. B Environ. 2010, 96, 329–337. [Google Scholar] [CrossRef]
- Pihl, J.; Parks, J.; Daw, C.; Root, T. Product Selectivity During Regeneration of Lean NOx Trap Catalysts. SAE Tech. Pap. 2006. [Google Scholar] [CrossRef]
- Pereda-Ayo, B.; López-Fonseca, R.; González-Velasco, J.R. Influence of the preparation procedure of NSR monolithic catalysts on the Pt-Ba dispersion and distribution. Appl. Catal. A Gen. 2009, 363, 73–80. [Google Scholar] [CrossRef]
- Pereda-Ayo, B.; Divakar, D.; López-Fonseca, R.; González-Velasco, J.R. Influence of platinum and barium precursors on the NSR behavior of Pt–Ba/Al2O3 monoliths for lean-burn engines. Catal. Today 2009, 147, S244–S249. [Google Scholar] [CrossRef]
- Colombo, M.; Nova, I.; Tronconi, E. A comparative study of the NH3-SCR reactions over a Cu-zeolite and a Fe-zeolite catalyst. Catal. Today 2010, 151, 223–230. [Google Scholar] [CrossRef]
- Ruggeri, M.P.; Selleri, T.; Colombo, M.; Nova, I.; Tronconi, E. Identification of nitrites/HONO as primary products of NO oxidation over Fe-ZSM-5 and their role in the Standard SCR mechanism: A chemical trapping study. J. Catal. 2014, 311, 266–270. [Google Scholar] [CrossRef]
- De-La-Torre, U.; Pereda-Ayo, B.; Moliner, M.; González-Velasco, J.R.; Corma, A. Cu-zeolite catalysts for NOx removal by selective catalytic reduction with NH3 and coupled to NO storage/reduction monolith in diesel engine exhaust aftertreatment systems. Appl. Catal. B Environ. 2016, 187, 419–427. [Google Scholar] [CrossRef]
- De-La-Torre, U.; Pereda-Ayo, B.; Gutiérrez-Ortiz, M.A.; González-Marcos, J.A.; González-Velasco, J.R. Steady-state NH3-SCR global model and kinetic parameter estimation for NOx removal in diesel engine exhaust aftertreatment with Cu/chabazite. Catal. Today 2017, 296, 95–104. [Google Scholar] [CrossRef]
- Urrutxua, M.; Pereda-Ayo, B.; De La Torre, U.; González-Velasco, J.R. Evaluation of Cu/SAPO-34 Catalysts Prepared by Solid-State and Liquid Ion-Exchange Methods for NOx Removal by NH3-SCR. ACS Omega 2019, 4, 14699–14713. [Google Scholar] [CrossRef] [PubMed]
- González-Velasco, J.R.; López-Fonseca, R.; Pereda-Ayo, B. NSR Technology. In NOx Trap Catalysts and Technologies: Fundamentals and Industrial Applications; The Royal Society of Chemistry: London, UK, 2018; pp. 36–66. [Google Scholar]
- Royer, S.; Duprez, D.; Can, F.; Courtois, X.; Batiot-Dupeyrat, C.; Laassiri, S.; Alamdari, H. Perovskites as Substitutes of Noble Metals for Heterogeneous Catalysis: Dream or Reality. Chem. Rev. 2014, 114, 10292–10368. [Google Scholar] [CrossRef]
- Libby, W.F. Promising Catalyst for Auto Exhaust. Science 1971, 171, 499. [Google Scholar] [CrossRef]
- Voorhoeve, R.J.H.; Remeika, J.P.; Trimble, L.E. Defect Chemistry and Catalysis in Oxidation and Reduction over Perovskite-Type Oxides. Ann. N. Y. Acad. Sci. 2006, 272, 3–21. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, P.; Dai, S. Recent Advances of Lanthanum-Based Perovskite Oxides for Catalysis. ACS Catal. 2015, 5, 6370–6385. [Google Scholar] [CrossRef]
- Zhu, J.; Li, H.; Zhong, L.; Xiao, S.; Xu, X.; Yang, X.; Zhao, Z.; Li, J. Perovskite Oxides: Preparation, Characterizations, and Applications in Heterogeneous Catalysis. ACS Catal. 2014, 4, 2917–2940. [Google Scholar] [CrossRef]
- Kim, C.H.; Qi, G.; Dahlberg, K.; Li, W. Strontium-doped perovskites rival platinum catalysts for treating NOx in simulated diesel exhaust. Science 2010, 327, 1624–1627. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, P.K.; Johnson, D.W.; Schrey, F. Studies of some supported perovskite oxidation catalysts. Mater. Res. Bull. 1974, 9, 1345–1352. [Google Scholar] [CrossRef]
- Onrubia, J.A.; Pereda-Ayo, B.; De-La-Torre, U.; González-Velasco, J.R. Key factors in Sr-doped LaBO3 (B = Co or Mn) perovskites for NO oxidation in efficient diesel exhaust purification. Appl. Catal. B Environ. 2017, 213, 198–210. [Google Scholar] [CrossRef]
- Zhou, C.; Feng, Z.; Zhang, Y.; Hu, L.; Chen, R.; Shan, B.; Yin, H.; Wang, W.G.; Huang, A. Enhanced catalytic activity for NO oxidation over Ba doped LaCoO3 catalyst. RSC Adv. 2015, 5, 28054–28059. [Google Scholar] [CrossRef]
- Zhong, S.; Sun, Y.; Xin, H.; Yang, C.; Chen, L.; Li, X. NO oxidation over Ni–Co perovskite catalysts. Chem. Eng. J. 2015, 275, 351–356. [Google Scholar] [CrossRef]
- Wen, Y.; Zhang, C.; He, H.; Yu, Y.; Teraoka, Y. Catalytic oxidation of nitrogen monoxide over La1−xCexCoO3 perovskites. Catal. Today 2007, 126, 400–405. [Google Scholar] [CrossRef]
- Wang, J.; Su, Y.; Wang, X.; Chen, J.; Zhao, Z.; Shen, M. The effect of partial substitution of Co in LaMnO3 synthesized by sol–gel methods for NO oxidation. Catal. Commun. 2012, 25, 106–109. [Google Scholar] [CrossRef]
- Hong, Z.; Wang, Z.; Li, X. Catalytic oxidation of nitric oxide (NO) over different catalysts: An overview. Catal. Sci. Technol. 2017, 7, 3440–3452. [Google Scholar] [CrossRef]
- Zhu, J.; Thomas, A. ChemInform abstract: Perovskite-type mixed oxides as catalytic material for NO removal. Appl. Catal. B Environ. 2010, 41, 225–233. [Google Scholar] [CrossRef]
- Zhou, C.; Liu, X.; Wu, C.; Wen, Y.; Xue, Y.; Chen, R.; Zhang, Z.; Shan, B.; Yin, H.; Wang, W.G. NO oxidation catalysis on copper doped hexagonal phase LaCoO3: A combined experimental and theoretical study. Phys. Chem. Chem. Phys. 2014, 16, 5106–5112. [Google Scholar] [CrossRef]
- Hong, S.; Lee, G. Simultaneous removal of NO and carbon particulates over lanthanoid perovskite-type catalysts. Catal. Today 2000, 63, 397–404. [Google Scholar] [CrossRef]
- Li, Z.; Meng, M.; Li, Q.; Xie, Y.; Hu, T.; Zhang, J. Fe-substituted nanometric La0.9K0.1Co1−xFexO3−δ perovskite catalysts used for soot combustion, NOx storage and simultaneous catalytic removal of soot and NOx. Chem. Eng. J. 2010, 164, 98–105. [Google Scholar] [CrossRef]
- Liu, J.; Xu, J.; Zhao, Z.; Duan, A.; Jiang, G.; Jing, Y. A novel four-way combining catalysts for simultaneous removal of exhaust pollutants from diesel engine. J. Environ. Sci. 2010, 22, 1104–1109. [Google Scholar] [CrossRef]
- Teraoka, Y.; Kanada, K.; Kagawa, S. Synthesis of La1-xKxMnO perovskite-type oxides and their catalytic property for simultaneous removal of NOx and diesel soot particulates. Appl. Catal. B Environ. 2001, 34, 73–78. [Google Scholar] [CrossRef]
- Yao, W.; Wang, R.; Yang, X. LaCo1-xPdxO3 Perovskite-Type Oxides: Synthesis, Characterization and Simultaneous Removal of NOx and Diesel Soot. Catal. Lett. 2009, 130, 613–621. [Google Scholar] [CrossRef]
- Wang, K.; Qian, L.; Zhang, L.; Liu, H.; Yan, Z. Simultaneous removal of NOx and soot particulates over La0.7Ag0.3MnO3 perovskite oxide catalysts. Catal. Today 2010, 158, 423–426. [Google Scholar] [CrossRef]
- Dhal, G.C.; Dey, S.; Mohan, D.; Prasad, R. Study of Fe, Co, and Mn-based perovskite-type catalysts for the simultaneous control of soot and NOX from diesel engine exhaust. Mater. Discov. 2017, 10, 37–42. [Google Scholar] [CrossRef]
- Ivanov, D.V.; Sadovskaya, E.M.; Pinaeva, L.G.; Isupova, L.E. Influence of oxygen mobility on catalytic activity of La–Sr–Mn–O composites in the reaction of high temperature N2O decomposition. J. Catal. 2009, 267, 5–13. [Google Scholar] [CrossRef]
- Ivanov, D.V.; Pinaeva, L.G.; Isupova, L.A.; Sadovskaya, E.M.; Prosvirin, I.P.; Gerasimov, E.Y.; Yakovleva, I.S. Effect of surface decoration with LaSrFeO4 on oxygen mobility and catalytic activity of La0.4Sr0.6FeO3−δ in high-temperature N2O decomposition, methane combustion and ammonia oxidation. Appl. Catal. A Gen. 2013, 457, 42–51. [Google Scholar] [CrossRef]
- Gunasekaran, N.; Rajadurai, S.; Carberry, J.J. Catalytic decomposition of nitrous oxide over perovskite type solid oxide solutions and supported noble metal catalysts. Catal. Lett. 1995, 35, 373–382. [Google Scholar] [CrossRef]
- Alini, S.; Basile, F.; Blasioli, S.; Rinaldi, C.; Vaccari, A. Development of new catalysts for N2O-decomposition from adipic acid plant. Appl. Catal. B Environ. 2007, 70, 323–329. [Google Scholar] [CrossRef]
- Pan, K.L.; Yu, S.J.; Yan, S.Y.; Chang, M.B. Direct N2O decomposition over La2NiO4-based perovskite-type oxides. J. Air Waste Manag. Assoc. 2014, 64, 1260–1269. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Dujardin, C.; Lancelot, C.; Dacquin, J.P.; Parvulescu, V.I.; Cabié, M.; Henry, C.R.; Neisius, T.; Granger, P. Catalytic abatement of NO and N2O from nitric acid plants: A novel approach using noble metal-modified perovskites. J. Catal. 2015, 328, 236–247. [Google Scholar] [CrossRef]
- Sun, K.; Xia, H.; Hensen, E.; van Santen, R.; Li, C. Chemistry of N2O decomposition on active sites with different nature: Effect of high-temperature treatment of Fe/ZSM-5. J. Catal. 2006, 238, 186–195. [Google Scholar] [CrossRef]
- Belessi, V.C.; Trikalitis, P.N.; Ladavos, A.K.; Bakas, T.V.; Pomonis, P.J. Structure and catalytic activity of La1−xFeO3 system (x = 0.00, 0.05, 0.10, 0.15, 0.20, 0.25, 0.35) for the NO+CO reaction. Appl. Catal. A Gen. 1999, 177, 53–68. [Google Scholar] [CrossRef]
- Belessi, V.C.; Bakas, T.V.; Costa, C.N.; Efstathiou, A.M.; Pomonis, P.J. Synergistic effects of crystal phases and mixed valences in La–Sr–Ce–Fe–O mixed oxidic/perovskitic solids on their catalytic activity for the NO+CO reaction. Appl. Catal. B Environ. 2000, 28, 13–28. [Google Scholar] [CrossRef]
- Leontiou, A.A.; Ladavos, A.K.; Pomonis, P.J. Catalytic NO reduction with CO on La1−xSrx(Fe3+/Fe4+)O3±δ perovskite-type mixed oxides (x = 0.00, 0.15, 0.30, 0.40, 0.60, 0.70, 0.80, and 0.90). Appl. Catal. A Gen. 2003, 241, 133–141. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, X.; Li, Y.; Shu, Z.; Chen, H.; Shi, J. A simple co-nanocasting method to synthesize high surface area mesoporous LaCoO3 oxides for CO and NO oxidations. Micropor Mesopor. Mater. 2013, 176, 8–15. [Google Scholar] [CrossRef]
- Peter, S.D.; Garbowski, E.; Perrichon, V.; Primet, M. NO reduction by CO over alumina-supported perovskites. Catal. Lett. 2000, 70, 27–33. [Google Scholar] [CrossRef]
- Shen, S.; Weng, H. Comparative Study of Catalytic Reduction of Nitric Oxide with Carbon Monoxide over the La1-xSrxBO3 (B = Mn, Fe, Co, Ni) Catalysts. Ind. Eng. Chem. Res. 1998, 37, 2654–2661. [Google Scholar] [CrossRef]
- Varma, S.; Wani, B.N.; Gupta, N.M. Redox behavior and catalytic activity of La–Fe–V–O mixed oxides. Appl. Catal. A Gen. 2003, 241, 341–348. [Google Scholar] [CrossRef]
- Choi, S.O.; Penninger, M.; Kim, C.H.; Schneider, W.F.; Thompson, L.T. Experimental and Computational Investigation of Effect of Sr on NO Oxidation and Oxygen Exchange for La1-xSrxCoO3 Perovskite Catalysts. ACS Catal. 2013, 3, 2719–2728. [Google Scholar] [CrossRef]
- Idriss, H.; Barteau, M.A. Active sites on oxides: From single crystals to catalysts. Adv. Catal. 2000, 45, 261–331. [Google Scholar]
- Mars, P.; van Krevelen, D.W. Oxidations carried out by means of vanadium oxide catalysts. Chem. Eng. Sci. 1954, 3, 41–59. [Google Scholar] [CrossRef]
- Dong, Y.; Xian, H.; Lv, J.; Liu, C.; Guo, L.; Meng, M.; Tan, Y.; Tsubaki, N.; Li, X. Influence of synthesis conditions on NO oxidation and NOx storage performances of La0.7Sr0.3MnO3 perovskite-type catalyst in lean-burn atmospheres. Mater. Chem. Phys. 2014, 143, 578–586. [Google Scholar] [CrossRef]
- Chen, J.; Shen, M.; Wang, X.; Wang, J.; Su, Y.; Zhao, Z. Catalytic performance of NO oxidation over LaMeO3 (Me = Mn, Fe, Co) perovskite prepared by the sol–gel method. Catal. Commun. 2013, 37, 105–108. [Google Scholar] [CrossRef]
- Chen, J.; Shen, M.; Wang, X.; Qi, G.; Wang, J.; Li, W. The influence of nonstoichiometry on LaMnO3 perovskite for catalytic NO oxidation. Appl. Catal. B Environ. 2013, 134–135, 251–257. [Google Scholar] [CrossRef]
- Białobok, B.; Trawczyński, J.; Miśta, W.; Zawadzki, M. Ethanol combustion over strontium- and cerium-doped LaCoO3 catalysts. Appl. Catal. B Environ. 2007, 72, 395–403. [Google Scholar] [CrossRef]
- Dow, W.; Huang, T. Yttria-Stabilized Zirconia Supported Copper Oxide Catalyst: II. Effect of Oxygen Vacancy of Support on Catalytic Activity for CO Oxidation. J. Catal. 1996, 160, 171–182. [Google Scholar] [CrossRef]
- Islam, M.S.; Cherry, M.; Winch, L.J. Defect chemistry of LaBO3 (B = Al, Mn or Co) perovskite-type oxides. Relevance to catalytic and transport behavior. J. Chem. Soc. Faraday Trans. 1996, 92, 479–482. [Google Scholar] [CrossRef]
- Over, H. Surface Chemistry of Ruthenium Dioxide in Heterogeneous Catalysis and Electrocatalysis: From Fundamental to Applied Research. Chem. Rev. 2012, 112, 3356–3426. [Google Scholar] [CrossRef] [PubMed]
- Chien, C.; Shi, J.; Huang, T. Effect of Oxygen Vacancy on CO-NO-O2 Reaction over Yttria-Stabilized Zirconia-Supported Copper Oxide Catalyst. Ind. Eng. Chem. Res. 1997, 36, 1544–1551. [Google Scholar] [CrossRef]
- Ye, J.; Yu, Y.; Meng, M.; Jiang, Z.; Ding, T.; Zhang, S.; Huang, Y. Highly efficient NOx purification in alternating lean/rich atmospheres over non-platinic mesoporous perovskite-based catalyst K/LaCoO3. Catal. Sci. Technol. 2013, 3, 1915–1918. [Google Scholar] [CrossRef]
- Li, X.G.; Dong, Y.H.; Xian, H.; Hernández, W.Y.; Meng, M.; Zou, H.H.; Ma, A.J.; Zhang, T.Y.; Jiang, Z.; Tsubaki, N.; et al. De-NOx in alternative lean/rich atmospheres on La1-xSrxCoO3 perovskites. Energy Environ. Sci. 2011, 4, 3351–3354. [Google Scholar] [CrossRef]
- Li, X.; Chen, C.; Liu, C.; Xian, H.; Guo, L.; Lv, J.; Jiang, Z.; Vernoux, P. Pd-Doped Perovskite: An Effective Catalyst for Removal of NOx from Lean-Burn Exhausts with High Sulfur Resistance. ACS Catal. 2013, 3, 1071–1075. [Google Scholar] [CrossRef]
- Ma, A.; Wang, S.; Liu, C.; Xian, H.; Ding, Q.; Guo, L.; Meng, M.; Tan, Y.; Tsubaki, N.; Zhang, J.; et al. Effects of Fe dopants and residual carbonates on the catalytic activities of the perovskite-type La0.7Sr0.3Co1−xFexO3 NOx storage catalyst. Appl. Catal. B Environ. 2014, 146, 24–34. [Google Scholar] [CrossRef]
- Peng, Y.; Si, W.; Li, J.; Crittenden, J.; Hao, J. Experimental and DFT studies on Sr-doped LaMnO3 catalysts for NOx storage and reduction. Catal. Sci. Technol. 2015, 5, 2478–2485. [Google Scholar] [CrossRef]
- Wang, X.; Qi, X.; Chen, Z.; Jiang, L.; Wang, R.; Wei, K. Studies on SO2 Tolerance and Regeneration over Perovskite-Type LaCo1-xPtxO3 in NOx Storage and Reduction. J. Phys. Chem. C 2014, 118, 13743–13751. [Google Scholar] [CrossRef]
- Liu, X.; Chen, Z.; Wen, Y.; Chen, R.; Shan, B. NO oxidation catalysis on copper dopedhexagonal phase LaCoO3: A combined experimental and theoretical study. Catal. Sci. Technol. 2014, 4, 3687–3696. [Google Scholar]
- Qi, G.; Li, W. Pt-free, LaMnO3 based lean NOx trap catalysts. Catal. Today 2012, 184, 72–77. [Google Scholar] [CrossRef]
- Shen, M.; Zhao, Z.; Chen, J.; Su, Y.; Wang, J.; Wang, X. Effects of calcium substitute in LaMnO3 perovskites for NO catalytic oxidation. J. Rare Earths 2013, 31, 119–123. [Google Scholar] [CrossRef]
- Yoon, D.Y.; Lim, E.; Kim, Y.J.; Kim, J.H.; Ryu, T.; Lee, S.; Cho, B.K.; Nam, I.; Choung, J.W.; Yoo, S. NO oxidation activity of Ag-doped perovskite catalysts. J. Catal. 2014, 319, 182–193. [Google Scholar] [CrossRef]
- Albaladejo-Fuentes, V.; López-Suárez, F.; Sánchez-Adsuar, M.S.; Illán-Gómez, M.J. BaTi0.8Cu0.2O3 Catalysts for NO Oxidation and NOx Storage: Effect of Synthesis Method. Appl. Catal. A Gen. 2017, 60, 220–224. [Google Scholar] [CrossRef]
- Constantinou, C.; Li, W.; Qi, G.; Epling, W.S. NOX storage and reduction over a perovskite-based lean NOX trap catalyst. Appl. Catal. B Environ. 2013, 134–135, 66–74. [Google Scholar] [CrossRef]
- Hodjati, S.; Vaezzadeh, K.; Petit, C.; Pitchon, V.; Kiennemann, A. Absorption/desorption of NOx process on perovskites: Performances to remove NOx from a lean exhaust gas. Appl. Catal. B Environ. 2000, 26, 5–16. [Google Scholar] [CrossRef]
- Hodjati, S.; Petit, C.; Pitchon, V.; Kiennemann, A. Absorption/desorption of NOx process on perovskites: Impact of SO2 on the storage capacity of BaSnO3 and strategy to develop thioresistance. Appl. Catal. B Environ. 2001, 30, 247–257. [Google Scholar] [CrossRef]
- Hodjati, S.; Vaezzadeh, K.; Petit, C.; Pitchon, V.; Kiennemann, A. NOx sorption–desorption study: Application to diesel and lean-burn exhaust gas (selective NOx recirculation technique). Catal. Today 2000, 59, 323–334. [Google Scholar] [CrossRef]
- Milt, V.G.; Querini, C.A.; Miró, E.E.; Ulla, M.A. Abatement of diesel exhaust pollutants: NOx adsorption on Co,Ba,K/CeO2 catalysts. J. Catal. 2003, 20, 424–432. [Google Scholar] [CrossRef]
- Milt, V.G.; Ulla, M.A.; Miró, E.E. NOx trapping and soot combustion on BaCoO3−y perovskite: LRS and FTIR characterization. Appl. Catal. B Environ. 2005, 57, 13–21. [Google Scholar] [CrossRef]
- Xian, H.; Zhang, X.; Li, X.; Li, L.; Zou, H.; Meng, M.; Li, Q.; Tan, Y.; Tsubaki, N. BaFeO3-x Perovskite: An Efficient NOx Absorber with a High Sulfur Tolerance. J. Phys. Chem. C 2010, 114, 11844–11852. [Google Scholar] [CrossRef]
- Xian, H.; Zhang, X.; Li, X.; Li, L.; Zou, H.; Meng, M.; Zou, Z.; Guo, L.; Tsubaki, N. Effect of the calcination conditions on the NOx storage behavior of the perovskite BaFeO3−x catalysts. Catal. Today 2010, 158, 215–219. [Google Scholar] [CrossRef]
- Xian, H.; Li, F.; Li, X.; Zhang, X.; Meng, M.; Zhang, T.; Tsubaki, N. Influence of preparation conditions to structure property, NOx and SO2 sorption behavior of the BaFeO3−x perovskite catalyst. Fuel Process. Technol. 2011, 92, 1718–1724. [Google Scholar] [CrossRef]
- Ge, C.; Li, L.; Xian, H.; Yan, H.; Meng, M.; Li, X. Effects of Ti-doping on the NOx storage and the sulfur resistance of the BaFe1−xTixO3−y perovskite-type catalysts for lean-burn exhausts. Fuel Process. Technol. 2014, 120, 1–7. [Google Scholar] [CrossRef]
- Albaladejo-Fuentes, V.; López-Suárez, F.E.; Sánchez-Adsuar, M.S.; Illán-Gómez, M.J. BaTi1−xCuxO3 perovskites: The effect of copper content in the properties and in the NOx storage capacity. Appl. Catal. A Gen. 2014, 488, 189–199. [Google Scholar] [CrossRef]
- Onrubia-Calvo, J.A.; Pereda-Ayo, B.; De-La-Torre, U.; González-Velasco, J.R. Strontium doping and impregnation onto alumina improve the NOx storage and reduction capacity of LaCoO3 perovskites. Catal. Today 2018, 333, 208–218. [Google Scholar] [CrossRef]
- Neagu, D.; Tsekouras, G.; Miller, D.N.; Ménard, H.; Irvine, J.T.S. In situ growth of nanoparticles through control of non-stoichiometry. Nat. Chem. 2013, 5, 916. [Google Scholar] [CrossRef]
- Peng, Y.; Si, W.; Luo, J.; Su, W.; Chang, H.; Li, J.; Hao, J.; Crittenden, J. Surface tuning of La0.5Sr0.5CoO3 perovskite catalysts by acetic acid for NOx storage and reduction. Environ. Sci. Technol. 2016, 50, 6442–6448. [Google Scholar] [CrossRef]
- Ueda, A.; Yamada, Y.; Katsuki, M.; Kiyobayashi, T.; Xu, Q.; Kuriyama, N. Perovskite catalyst (La, Ba)(Fe, Nb, Pd)O3 applicable to NOx storage and reduction system. Catal. Commun. 2009, 11, 34–37. [Google Scholar] [CrossRef]
- López-Suárez, F.E.; Illán-Gómez, M.J.; Bueno-López, A.; Anderson, J.A. NOx storage and reduction on a SrTiCuO3 perovskite catalyst studied by operando DRIFTS. Appl. Catal. B Environ. 2011, 104, 261–267. [Google Scholar] [CrossRef]
- Zhao, D.; Gao, Z.; Xian, H.; Xing, L.; Yang, Y.; Tian, Y.; Ding, T.; Jiang, Z.; Zhang, J.; Zheng, L.; et al. Addition of Pd on La0.7Sr0.3CoO3 Perovskite to Enhance Catalytic Removal of NOx. Ind. Eng. Chem. Res. 2018, 57, 521–531. [Google Scholar] [CrossRef]
- Say, Z.; Dogac, M.; Vovk, V.I.; Kalay, Y.E.; Kim, C.H.; Li, W.; Ozensoy, E. Palladium doped perovskite-based NO oxidation catalysts: The role of Pd and B-sites for NOx adsorption behavior via in-situ spectroscopy. Appl. Catal. B Environ. 2014, 154–155, 51–61. [Google Scholar] [CrossRef]
- He, X.; Meng, M.; He, J.; Zou, Z.; Li, X.; Li, Z.; Jiang, Z. A potential substitution of noble metal Pt by perovskite LaCoO3 in ZrTiO4 supported lean-burn NOx trap catalysts. Catal. Commun. 2010, 10, 165–168. [Google Scholar] [CrossRef]
- You, R.; Zhang, Y.; Liu, D.; Meng, M.; Zheng, L.; Zhang, J.; Hu, T. YCeZrO Ternary Oxide Solid Solution Supported Nonplatinic Lean-Burn NOx Trap Catalysts Using LaCoO3 Perovskite as Active Phase. J. Phys. Chem. C 2014, 118, 25403–25420. [Google Scholar] [CrossRef]
- You, R.; Zhang, Y.; Liu, F.; Meng, M.; Jiang, Z.; Zhang, S.; Huang, Y. A series of ceria supported lean-burn NOx trap catalysts LaCoO3/K2CO3/CeO2 using perovskite as active component. Chem. Eng. J. 2015, 260, 357–367. [Google Scholar] [CrossRef]
- Ding, Q.; Xian, H.; Tan, Y.; Tsubaki, N.; Li, X. Mesoporous SiO2-confined La0.7Sr0.3CoO3 perovskite nanoparticles: An efficient NOx adsorber for lean-burn exhausts. Catal. Sci. Technol. 2013, 3, 1493–1496. [Google Scholar] [CrossRef]
- Zahir, M.H.; Suzuki, T.; Fujishiro, Y.; Awano, M. Perovskites with cotton-like morphology consisting of nanoparticles and nanorods: Their synthesis by the combustion method and their NOx adsorption behavior. Appl. Catal. A Gen. 2009, 361, 86–92. [Google Scholar] [CrossRef]
- Du, S.; Wang, S.; Guo, Y.; Lu, X.; Tang, W.; Ding, Y.; Mao, X.; Gao, P. Rational design, synthesis and evaluation of ZnO nanorod array supported Pt:La0.8Sr0.2MnO3 lean NOx traps. Appl. Catal. B Environ. 2018, 236, 348–358. [Google Scholar] [CrossRef]
- Alcalde-Santiago, V.; Davó-Quiñonero, A.; Such-Basáñez, I.; Lozano-Castelló, D.; Bueno-López, A. Macroporous carrier-free Sr-Ti catalyst for NOx storage and reduction. Appl. Catal. B Environ. 2018, 220, 524–532. [Google Scholar] [CrossRef]
- Wen, W.; Wang, X.; Jin, S.; Wang, R. LaCoO3 perovskite in Pt/LaCoO3/K/Al2O3 for the improvement of NOx storage and reduction performances. RSC Adv. 2016, 6, 74046–74052. [Google Scholar] [CrossRef]
- Onrubia-Calvo, J.A.; Pereda-Ayo, B.; Bermejo-López, A.; Caravaca, A.; Vernoux, P.; González-Velasco, J.R. Pd-doped or Pd impregnated 30% La0.7Sr0.3CoO3/Al2O3 catalysts for NOx storage and reduction. Appl. Catal. B Environ. 2019, 259, 118052. [Google Scholar] [CrossRef]
- De-La-Torre, U.; Pereda-Ayo, B.; Onrubia, J.A.; González-Velasco, J.R. Effect of the Presence of Ceria in the NSR Catalyst on the Hydrothermal Resistance and Global DeNOx Performance of Coupled LNT-SCR Systems. Top. Catal. 2018, 61, 1993–2006. [Google Scholar] [CrossRef]
- Seo, C.; Kim, H.; Choi, B.; Lim, M.T.; Lee, C.; Lee, C. De-NOx characteristics of a combined system of LNT and SCR catalysts according to hydrothermal aging and sulfur poisoning. Catal. Today 2011, 164, 507–514. [Google Scholar] [CrossRef]
- Nishihata, Y.; Mizuki, J.; Akao, T.; Tanaka, H.; Uenishi, M.; Kimura, M.; Okamoto, T.; Hamada, N. Self-regeneration of a Pd-perovskite catalyst for automotive emissions control. Nature 2002, 418, 164. [Google Scholar] [CrossRef] [PubMed]
- Ohji, T.; Sekino, T.; Niihara, K. The Science of Engineering Ceramics III; Ceramics Society of Japan: Osaka, Japan, 2006; Volumes 317–318, p. 465. [Google Scholar]
- Kinugasa, Y.; Igarashi, K.; Itou, T.; Suzuki, N.; Yaegashi, T.; Tanaka, T. Device for Purifying Exhaust Gas from Engine. U.S. Patent 5964088, 12 October 1999. [Google Scholar]
- Gandhi, H.S.; Cavataio, J.V.; Hammerle, R.H.; Cheng, Y. Catalyst System for NOx and NH3. U.S. Patent 7332135, 19 December 2008. [Google Scholar]
- Urrutxua, M.; Pereda-Ayo, B.; Trandafilovic, L.V.; Olsson, L.; González-Velasco, J.R. Influence of H2, CO, C3H6, and C7H8 as Reductants on DeNOx Behavior of Dual Monoliths for NOx Storage/Reduction Coupled with Selective Catalytic Reduction. Ind. Eng. Chem. Res. 2019, 58, 7001–7013. [Google Scholar] [CrossRef]
- Sakurai, K.; Miyashita, S.; Katumata, Y. Exhaust Purifying System for Internal Combustion Engine. U.S. Patent 2011/0214417 A1, 8 September 2011. [Google Scholar]
- Sakurai, K. Exhaust Purifying System for Internal Combustion Engine. U.S. Patent 0138783 A1, 16 June 2011. [Google Scholar]
- Onrubia-Calvo, J.A.; Pereda-Ayo, B.; De-La-Torre, U.; González-Velasco, J.R. Perovskite-Based Formulations as Rival Platinum Catalysts for NO x Removal in Diesel Exhaust Aftertreatment. In Perovskite Materials, Devices and Integration; Tian, H., Ed.; Intechopen: London, UK, 2019. [Google Scholar]
- Pereda-Ayo, B.; Duraiswami, D.; González-Velasco, J.R. Control of NOx storage and reduction in NSR bed for designing combined NSR–SCR systems. Catal. Today 2011, 172, 66–72. [Google Scholar] [CrossRef]
- De-La-Torre, U.; Pereda-Ayo, B.; Moliner, M.; González-Marcos, J.A.; Corma, A.; González-Velasco, J.R. Optimal Operating Conditions of Coupled Sequential NOx Storage/Reduction and Cu/CHA Selective Catalytic Reduction Monoliths. Top. Catal. 2017, 60, 30–39. [Google Scholar] [CrossRef]

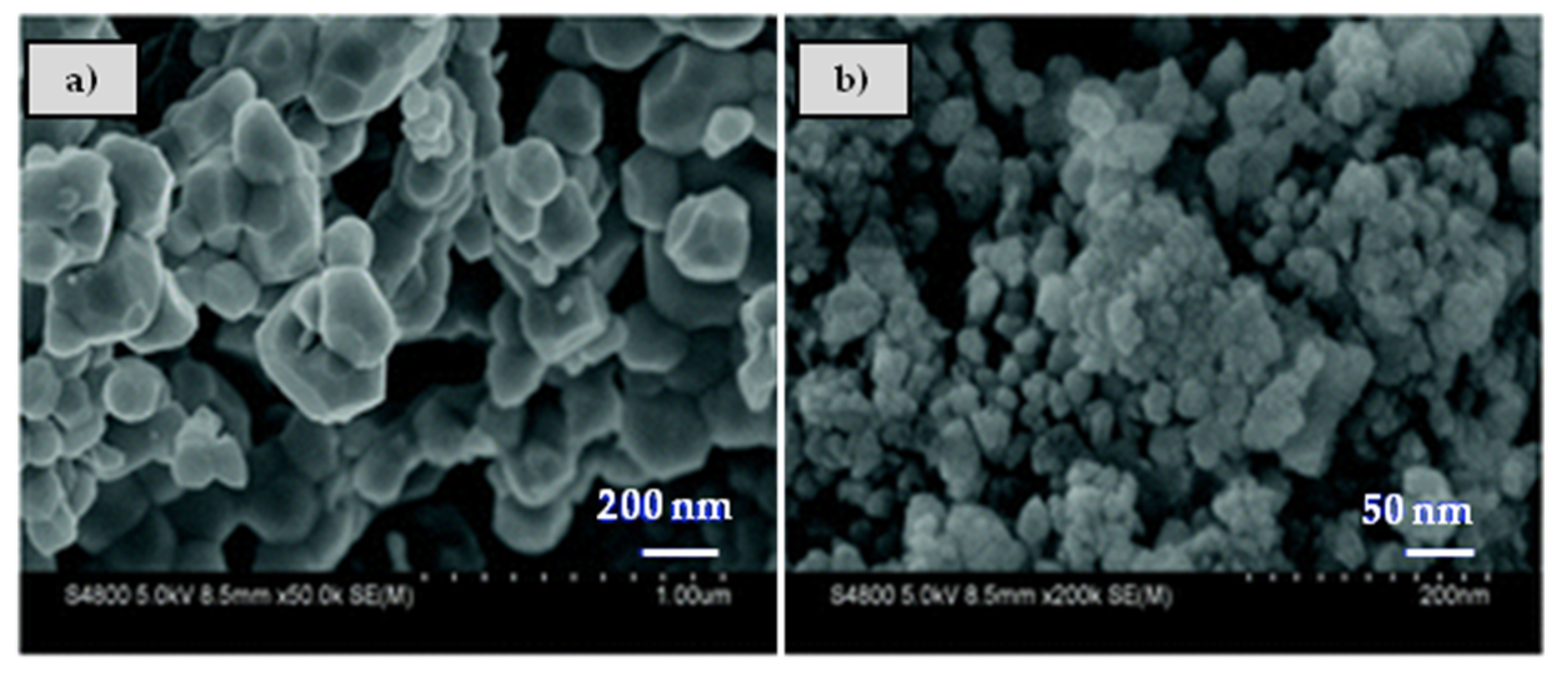
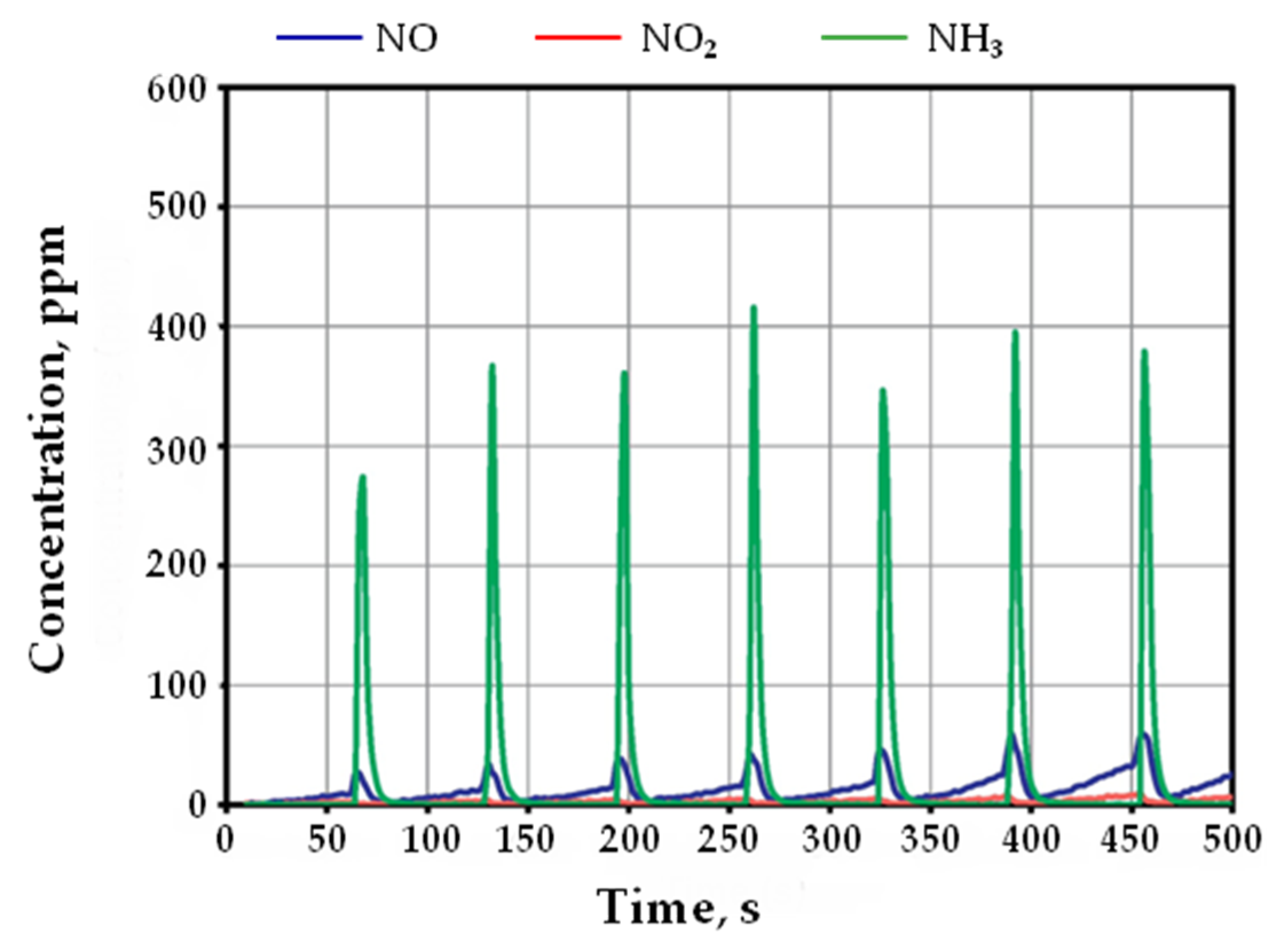
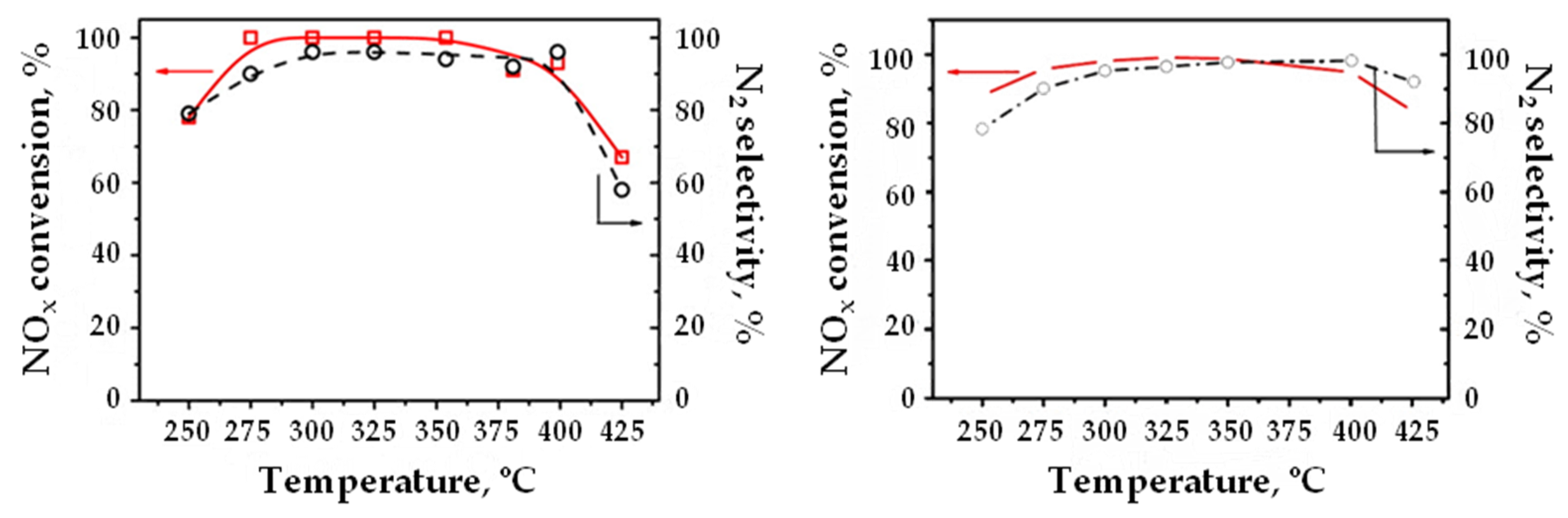
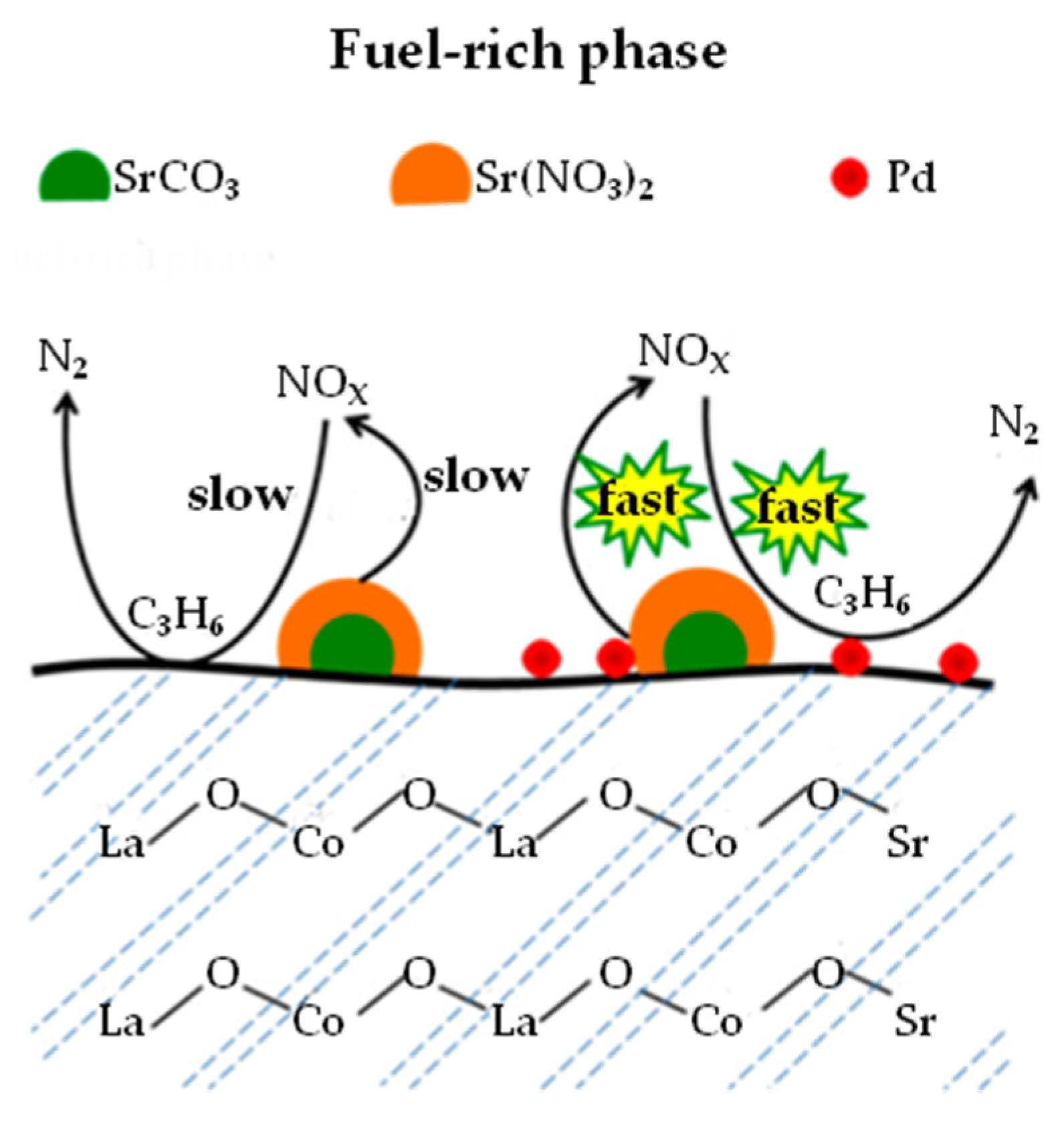
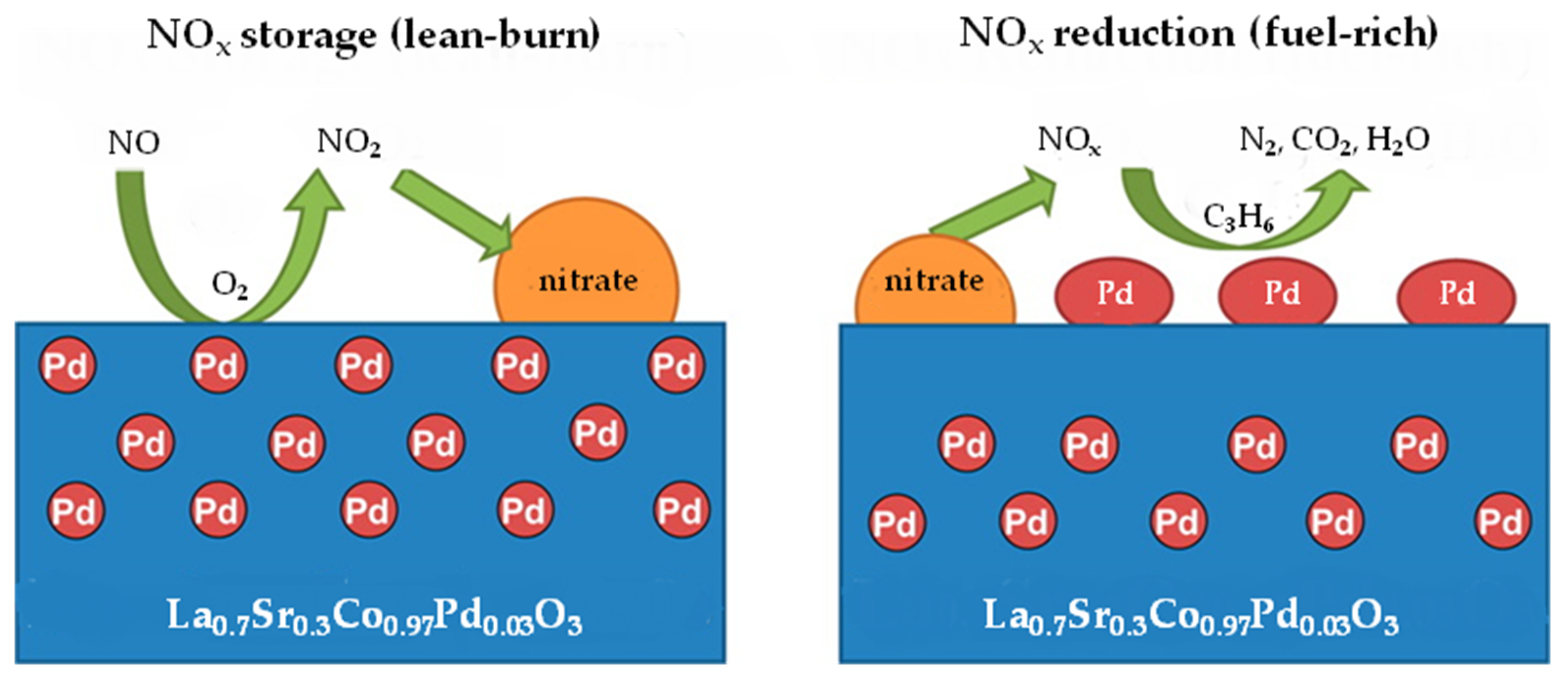


| NSR | SCR | NSR + SCR | NSR–SCR | |
|---|---|---|---|---|
| Principle | The system runs under lean-rich cycles. During lean period NOx is adsorbed on the catalyst, and then is released and reduced in the subsequent rich period. | The SCR catalyst reduces selectively NOx with NH3 generated from an aqueous urea solution. | Operates similarly to NSR system. The SCR unit downstream reduces the NOx with the NH3 produced in the NSR. | Similar operation to NSR system. The NOx diffuses the top SCR layer and generates NH3 in the bottom NSR layer, which then reduces the NOx slipped from the NSR. |
| Model catalyst | Pt–Ba/Al2O3 deposited on a cordierite monolith. | Cu, Fe/Chabazite deposited on a cordierite monolith. | Sequential NSR + SCR double monolith. | Dual layer NSR + SCR single monolith. |
| Advantages | 70–90% efficiency at low loads. More economical for light-duty vehicles. Reductant fluid not required. | Up to 90% NOx conversion efficiency. More economical for heavier vehicles. | High NOx removal efficiency at low temperatures. Reduction of PGM. Reductant fluid not required. | High NOx removal efficiency at low temperatures. Less volume and weight than sequential monoliths. |
| Limitations | Limited NOx storage capacity and NSR efficiency for highway and ascending driving. Need of high amount of PGM. | Low sulfur resistance. Requires on board DEF AdBlue storage tank with heating and injection system. Operational limitations under urban driving conditions. | High cost. Packaging constrains (double monolith). Possible migration of Pt from NSR to SCR. Calibration difficulties. | High cost. Spillover of stored NH3 onto vicinal Pt sites, which limits NOx reduction. Possible migration of Pt from NSR to SCR layer. Calibration difficulties due to its complexity. |
| Formulation | Shape | Feedstream | GHSV, h–1 | T, °C | XNO-to-NO2, % | Ref. |
|---|---|---|---|---|---|---|
| LaCoO3 | powder | [NO] = 100 ppm; [O2] = 10% | 30,000 | 260 | 83.0 | [58] |
| LaCoO3(+) | powder | [NO] = 400 ppm; [O2] = 5% | 80,000 | 350 | 57.9 | [65] |
| La0.9Sr0.1CoO3 | monolith | [NO] = 400 ppm; [O2] = 8% | 30,000 | 300 | 86.0 | [23] |
| La0.7Sr0.3CoO3 | powder | [NO] = 800 ppm; [O2] = 5% | 80,000 | 300 | 74.1 | [66] |
| La0.7Sr0.3CoO3 | powder | [NO] = 650 ppm; [O2] = 6% | 123,500 | 300 | 80.0 | [25] |
| La0.7Sr0.3Co0.97Pd0.03O3 | powder | [NO] = 500 ppm; [O2] = 6.7% | 32,000 | 280 | 87.8 | [67] |
| La0.7Sr0.3Co0.8Fe0.2O3 | powder | [NO] = 750 ppm; [O2] = 5% | 80,000 | 300 | 84.6 | [68] |
| La0.5Sr0.5CoO3 | powder | [NO] = 500 ppm; [O2] = 3% | 120,000(a) | 300 | 55.0 | [69] |
| La0.9Ba0.1CoO3 | powder | [NO] = 400 ppm; [O2] = 10% | 180,000(a) | 265 | 93.0 | [26] |
| La0.8Ce0.2CoO3 | powder | [NO] = 800 ppm; [O2] = 8% | 0.096(b) | 300 | 80.0 | [28] |
| LaCo0.92Pt0.08O3 | powder | [NO] = 280 ppm; [O2] = 8% | 72,000 | 300 | < 80.0(*) | [70] |
| LaCo0.9Cu0.1O3 | powder | [NO] = 400 ppm; [O2] = 10% | 180,000(a) | 310 | 82.0 | [71] |
| LaNi0.7Co0.3O3 | powder | [NO] = 400 ppm; [O2] = 6% | 200,000 | 325 | < 80.0 | [27] |
| LaMnO3 | monolith | [NO] = 400 ppm; [O2] = 8% | 30,000 | 350 | 62.0 | [72] |
| La0.9MnO3 | powder | [NO] = 100 ppm; [O2] = 10% | 30,000 | 296 | 85.0(*) | [59] |
| La0.9Sr0.1MnO3 | powder | [NO] = 650 ppm; [O2] = 6% | 123,500 | 325 | 65.0 | [25] |
| La0.9Sr0.1MnO3 | monolith | [NO] = 400 ppm; [O2] = 8% | 30,000 | 350 | 62.5 | [23] |
| La0.7Sr0.3MnO3 | powder | [NO] = 800 ppm; [O2] = 5% | 80,000 | 350 | 70.2 | [57] |
| La0.9Ca0.1MnO3 | powder | [NO] = 100 ppm; [O2] = 10% | 30,000 | 300 | 82.0 | [73] |
| La0.8Ag0.2MnO3 | powder | [NO] = 400 ppm; [O2] = 8% | 600,000 | 250 | ~ 90.0(*) | [74] |
| LaMn0.9Co0.1O3 | powder | [NO] = 100 ppm; [O2] = 10% | n.a. | 300 | 76.5 | [29] |
| BaTi0.8Cu0.2O3 | powder | [NO] = 500 ppm; [O2] = 6% | n.a. | 400 | 47.0 | [75] |
| Formulation | Feedstream (lean/rich) | GHSV, h–1 | XNOx, %/SN2, % | Ref. |
|---|---|---|---|---|
| 5 wt % K/LaCoO3(+) | [NO] = 400 ppm; [O2] = 5%; [C3H6] = 1000 ppm (180 s)/[C3H6] = 1000 ppm (60 s) | 80,000 | 97.0/97.3 | [65] |
| La0.7Sr0.3CoO3 | NO] = 500 ppm; [O2] = 6.7%; [C3H6] = 1000 ppm (180 s)/[NO] = 500 ppm; [C3H6] = 1000 ppm (60 s) | 80,000 | 71.4/100 | [66] |
| La0.7Sr0.3Co0.97Pd0.03O3 | [NO] = 500 ppm; [O2] = 6.7% (120 s)/[NO] = 500 ppm; [C3H6] = 0.1% (60 s) | 32,000 | > 90.0/> 90.0 | [67] |
| 30 wt % La0.7Sr0.3CoO3/Al2O3 | [NO] = 500 ppm; [O2] = 6%; (150 s)/[NO] = 500 ppm; [H2] = 3%; (20 s) | 123,500 | 46.9/53.3 | [102] |
| 1.5 wt % Pd–30 wt % La0.7Sr0.3CoO3/Al2O3 | [NO] = 500 ppm; [O2] = 6%; (150 s)/[NO] = 500 ppm; [H2] = 3%; (20 s) | 123,500 | 79.2/89.7 | [102] |
| 1.4 wt % Pd/La0.7Sr0.3CoO3 | [NO] = 400 ppm; [O2] = 5%; (50 s)/ [C3H6] = 1000 ppm (10 s) (*) | 120,000(b) | 90.4/n.d. | [92] |
| La0.5Sr0.5CoO3 | [NO] = 500 ppm; [O2] = 5% (120 s)/[NO] = 500 ppm; [C3H6] = 1000 ppm (60 s) | 120,000(b) | 42.4/n.a. | [89] |
| LaCo0.92Pt0.08O3 | [NO] = 280 ppm; [O2] = 8% (120 s)/[NO] = 280 ppm; [H2] = 3.5% (30 s) | 72,000 | 90.0/70.0(*) | [70] |
| 5 wt % K2CO3–20% LaCoO3/S(a) | NO] = 400 ppm; [O2] = 5%; (180 s)/[C3H6] = 1000 ppm (60 s) | 45,000 | 98.2/98.8 | [95] |
| 0.3 wt % Pt–16 wt % K–25 wt % LaCoO3/Al2O3 | [NO] = 500 ppm; [O2] = 8%; (120 s)/ [NO] = 500 ppm; [H2] = 3.5%; (120 s) | n.a. | ~80/90 | [101] |
| LaMnO3 + 4 wt % Pd/Al2O3+2 wt % Rh/CeO2–ZrO2(c) | [NO] = 400 ppm; [O2] = 10% (60 s)/[NO] = 400 ppm; [H2] = 1%; [CO] = 3% (5s) | 25.000 | 85/n.a.(*) | [72] |
| La0.9Sr0.1MnO3 + (1.6 wt % Pd + 0.16 wt % Rh)–20 wt % Ba/CeO2–ZrO2(c) | [NO] = 200 ppm; [O2] = 10% (60 s)/[NO] = 200 ppm; [H2] = 1%; [CO] = 3% (5s) | 50.000 | > 90/n.a.(*) | [23] |
| La0.7Ba0.3Fe0.776Nb0.194Pd0.03O3 | [NO] = 512 ppm; [O2] = 5%; [C3H6] = 200 ppm (54 s)/[NO] = 512 ppm; [CO] = 4% (6 s) | n.a. | 47/n.a. | [90] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onrubia-Calvo, J.A.; Pereda-Ayo, B.; González-Velasco, J.R. Perovskite-Based Catalysts as Efficient, Durable, and Economical NOx Storage and Reduction Systems. Catalysts 2020, 10, 208. https://doi.org/10.3390/catal10020208
Onrubia-Calvo JA, Pereda-Ayo B, González-Velasco JR. Perovskite-Based Catalysts as Efficient, Durable, and Economical NOx Storage and Reduction Systems. Catalysts. 2020; 10(2):208. https://doi.org/10.3390/catal10020208
Chicago/Turabian StyleOnrubia-Calvo, Jon A., Beñat Pereda-Ayo, and Juan R. González-Velasco. 2020. "Perovskite-Based Catalysts as Efficient, Durable, and Economical NOx Storage and Reduction Systems" Catalysts 10, no. 2: 208. https://doi.org/10.3390/catal10020208
APA StyleOnrubia-Calvo, J. A., Pereda-Ayo, B., & González-Velasco, J. R. (2020). Perovskite-Based Catalysts as Efficient, Durable, and Economical NOx Storage and Reduction Systems. Catalysts, 10(2), 208. https://doi.org/10.3390/catal10020208