Characterization of Protein Hydrolysates from Eel (Anguilla marmorata) and Their Application in Herbal Eel Extracts
Abstract
1. Introduction
2. Results and Discussion
2.1. Enzyme Activity and Degree of Hydrolysis
2.2. Molecular Mass Distribution Profile
2.3. Amino Acid Composition
2.4. Functional Properties of EPHs
2.4.1. Emulsifying Properties
2.4.2. Oil Binding Capacity (OBC)
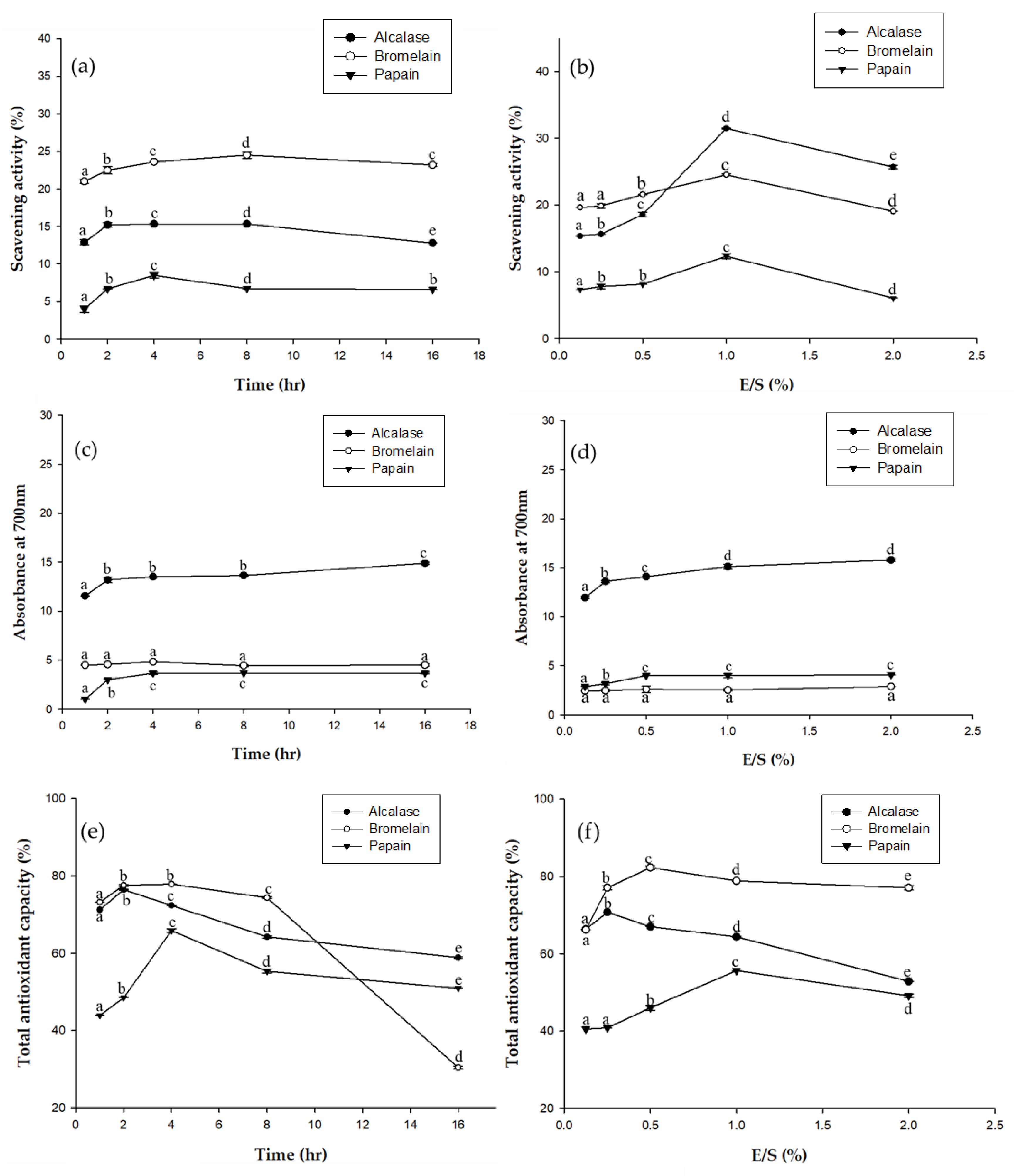
2.5. Antioxidant Properties of EPHs
2.6. Nitrogen Solubility of EPH and Herbal Eel Extracts
2.7. Color of EPH and Herbal Eel Extracts
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Anguilla Marmorata Raw Material Preparation
3.2.2. Anguilla Marmorata Meat Hydrolysate Preparation
3.2.3. Herbal Eel Extracts
3.2.4. Determination of Degree of Hydrolysis (DH)
3.2.5. Molecular Mass Identification
3.2.6. Analysis of Amino Acid Composition
3.2.7. Functional Properties
Nitrogen Solubility of EPH and Herbal Eel Extracts
Emulsifying Properties
3.2.8. Antioxidant Capacity Analysis
Analysis of Reducing Power
Analysis of DPPH Free Radical Scavenging Ability
ABTS Radical-Scavenging Activity Assay
3.2.9. Color of EPH and Herbal Eel Extracts
3.2.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rees Clayton, E.M.; Specht, E.A.; Welch, D.R.; Berke, A.P. Addressing Global Protein Demand Through Diversification and Innovation: An Introduction to Plant-Based and Clean Meat; Melton, L., Shahidi, F., Varelis, P.B.T.-E.o.F.C., Eds.; Academic Press: Oxford, UK, 2019; pp. 209–217. [Google Scholar] [CrossRef]
- Galaz, G.A. Chapter 20—An Overview on the History of Sports Nutrition Beverages; Bagchi, D., Nair, S., Sen, C.K.B.T.-N.a.E.S.P., Eds.; Academic Press: San Diego, CA, USA, 2013. [Google Scholar]
- Kristinsson, H.G. 10—Aquatic food protein hydrolysates. In Woodhead Publishing Series in Food Science, Technology and Nutrition; Shahidi, F.B.T.-M.t.V.o.M.B.-P., Ed.; Woodhead Publishing: Sawston, UK, 2007; 229p. [Google Scholar]
- Egerton, S.; Culloty, S.; Whooley, J.; Stanton, C.; Ross, R.P. Characterization of protein hydrolysates from blue whiting (Micromesistius poutassou) and their application in beverage fortification. Food Chem. 2018, 245, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, J.; Watanabe, S.; Miyai, T.; Sasai, S.; Nishida, M.; Tsukamoto, K. The European eel, Anguilla anguilla (L.), in Japanese waters. Dana 2000, 12, 1–5. [Google Scholar]
- Schlegel, T. Anguilla japonica. FAO 1847, 79, 198–205. [Google Scholar]
- Heinsbroek, L.T.N. A review of eel culture in Japan and Europe. Aquac. Res. 1991, 22, 57–72. [Google Scholar] [CrossRef]
- Minegishi, Y.; Aoyama, J.; Tsukamoto, K.J.M.E. Multiple population structure of the giant mottled eel, Anguilla marmorata. Mol. Ecol. 2008, 17, 3109–3122. [Google Scholar] [CrossRef] [PubMed]
- Jamaluddin, J.; Agustinus, W. Comparative study of mineral content of sidat fish meat (anguilla marmorata quoy gaimard) on yellow eel phase from palu river and lake poso. J. Islamic Pharm. 2018, 3, 8–14. [Google Scholar] [CrossRef]
- Everaert, I.; Stegen, S.; Vanheel, B.; Taes, Y.; Derave, W. Effect of beta-alanine and carnosine supplementation on muscle contractility in mice. Med. Sci. Sports Exerc. 2013. [Google Scholar] [CrossRef]
- Khantaphant, S.; Benjakul, S. Comparative study on the proteases from fish pyloric caeca and the use for production of gelatin hydrolysate with antioxidative activity. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2008, 151, 410–419. [Google Scholar] [CrossRef]
- Klompong, V.; Benjakul, S.; Yachai, M.; Visessanguan, W.; Shahidi, F.; Hayes, K.D. Amino Acid Composition and Antioxidative Peptides from Protein Hydrolysates of Yellow Stripe Trevally (Selaroides leptolepis). J. Food Sci. 2009, 74, C126–C133. [Google Scholar] [CrossRef]
- Hartmann, R.; Meisel, H. Food-derived peptides with biological activity: From research to food applications. Curr. Opin. Biotechnol. 2007, 18, 163–169. [Google Scholar] [CrossRef]
- Sila, A.; Sayari, N.; Balti, R.; Martinez-Alvarez, O.; Nedjar-Arroume, N.; Moncef, N.; Bougatef, A. Biochemical and antioxidant properties of peptidic fraction of carotenoproteins generated from shrimp by-products by enzymatic hydrolysis. Food Chem. 2014, 148, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Mendis, E.; Rajapakse, N.; Kim, S.-K. Antioxidant properties of a radical-scavenging peptide purified from enzymatically prepared fish skin gelatin hydrolysate. J. Agric. Food Chem. 2005, 53, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Kristinsson, H.G.; Rasco, B.A. Fish protein hydrolysates: Production, biochemical, and functional properties. Crit. Rev. Food Sci. Nutr. 2000, 40, 43–81. [Google Scholar] [CrossRef] [PubMed]
- Nikoo, M.; Benjakul, S.; Ahmadi Gavlighi, H.; Xu, X.; Regenstein, J.M. Hydrolysates from rainbow trout (Oncorhynchus mykiss) processing by-products: Properties when added to fish mince with different freeze-thaw cycles. Food Biosci. 2019. [Google Scholar] [CrossRef]
- Gbogouri, G.A.; Linder, M.; Fanni, J.; Parmentier, M. Influence of Hydrolysis Degree on the Functional Properties of Salmon Byproducts Hydrolysates. J. Food Sci. 2004, 69, C615–C622. [Google Scholar] [CrossRef]
- Nielsen, P.M.; Petersen, D.; Dambmann, C. Improved method for determining food protein degree of hydrolysis. J. Food Sci. 2001, 66, 642–646. [Google Scholar] [CrossRef]
- Yang, X.; Wang, G.; Gong, X.; Huang, C.; Mao, Q.; Zeng, L.; Zheng, P.; Qin, Y.; Ye, F.; Lian, B.; et al. Effects of chronic stress on intestinal amino acid pathways. Physiol. Behav. 2019. [Google Scholar] [CrossRef]
- Wang, T.; Zhou, Y.; Cao, S.; Lu, J.; Zhou, Y. Degradation of sulfanilamide by Fenton-like reaction and optimization using response surface methodology. Ecotoxicol. Env. Saf. 2019. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Chen, Y.-W.; Hou, C.-Y. Antioxidant and antibacterial activity of seven predominant terpenoids. Int. J. Food Prop. 2019. [Google Scholar] [CrossRef]
- Kim, S.S.; Ahn, C.B.; Moon, S.W.; Je, J.Y. Purification and antioxidant activities of peptides from sea squirt (Halocynthia roretzi) protein hydrolysates using pepsin hydrolysis. Food Biosci. 2018. [Google Scholar] [CrossRef]
- Zakaria, N.A.; Sarbon, N.M. Physicochemical properties and oxidative stability of fish emulsion sausage as influenced by snakehead (Channa striata) protein hydrolysate. LWT 2018. [Google Scholar] [CrossRef]



| Enzyme | Molecular mass (Da) | |
|---|---|---|
| EPH * | Alcalase | 1056.55 ± 2.60 a |
| Bromelain | 1482.24 ± 3.36 b | |
| Papain | 1554.53 ± 19.22 c |
| Amino Acid | Eel | A | B | P | WHO |
|---|---|---|---|---|---|
| His * | 0.81 | 1.01 | 0.76 | 0.95 | 1.50 |
| Thr * | 0.63 | 2.98 | 2.75 | 0.71 | 2.30 |
| Val * | 0.31 | 1.66 | 3.07 | 2.23 | 3.90 |
| Ile * | 1.87 | 1.93 | 1.27 | 2.15 | 3.00 |
| Leu * | 1.74 | 2.85 | 1.48 | 0.77 | 5.90 |
| Asp | 4.51 | 5.14 | 5.33 | 6.44 | - |
| Glu | 7.18 | 7.10 | 6.96 | 6.95 | - |
| Ser | 0.80 | 1.48 | 2.31 | 0.92 | - |
| Gly | 0.30 | 6.38 | 5.58 | 3.09 | - |
| Ala | 5.83 | 5.03 | 5.02 | 6.37 | - |
| Pro | 1.06 | 1.37 | 0.70 | 1.02 | - |
| Arg | 0.41 | 2.04 | 1.95 | 0.55 | - |
| Phe * | 1.12 | 1.19 | 0.29 | 0.98 | - |
| (EAI m2g−1) | ESI (min) | OBC (g/g) | |||||
|---|---|---|---|---|---|---|---|
| EPH conc. | 0.50% | 1% | 2% | 0.50% | 1% | 2% | - |
| Alcalase | 36.8 ± 2.00 a | 19.7 ± 1.00 a | 11.3 ± 0.28 a | 1.11 ± 0.31 a | 2.70 ± 0.20 a | 4.00 ± 0.34 a | 1.58 ± 0.07 a |
| Bromelain | 21.3 ± 1.30 b | 10.2 ± 1.99 b | 8.59 ± 0.87 b | 0.92 ± 0.89 b | 1.92 ± 0.29 b | 2.62 ± 0.44 b | 1.21 ± 0.01 b |
| Papain | 16.2 ± 1.22 c | 8.63 ± 0.58 c | 3.25 ± 0.10 c | 0.81 ± 0.96 c | 1.37 ± 0.75 c | 1.44 ± 0.09 c | 1.12 ± 0.01 b |
| Alcalase | Bromelain | Papain | ||
|---|---|---|---|---|
| EPH | L* | 3.96 ± 0.04 c | 6.37 ± 0.43 b | 8.10 ± 0.18 a |
| a* | 0.42 ± 0.03 b | 0.78 ± 0.02 a | 0.28 ± 0.01 c | |
| b* | 0.74 ± 0.07 c | 2.39 ± 0.02 b | 5.75 ± 0.10 a | |
| Herbal extract with 15% EPH (1.5 g/10 mL) | L* | 2.17 ± 0.00 c | 2.59 ± 0.04 b | 4.52 ± 0.07 a |
| a* | 0.98 ± 0.00 a | 0.62 ± 0.00 c | 0.78 ± 0.11 b | |
| b* | 2.68 ± 0.00 c | 3.75 ± 0.06 b | 5.84 ± 0.05 a |
| Factors | Unit | Symbol | Enzyme | ||
|---|---|---|---|---|---|
| Alcalase | Bromelain | Papain | |||
| Temperature | °C | T | 55 | 55 | 45 |
| Time | h | t | 1.0, 2.0, 4.0, 8.0, 16.0 | ||
| Enzyme concentration | % | E/S | 0.125, 0.25, 0.5, 1.0, 2.0 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, I.-C.; Liao, J.-X.; Ciou, J.-Y.; Huang, L.-T.; Chen, Y.-W.; Hou, C.-Y. Characterization of Protein Hydrolysates from Eel (Anguilla marmorata) and Their Application in Herbal Eel Extracts. Catalysts 2020, 10, 205. https://doi.org/10.3390/catal10020205
Cheng I-C, Liao J-X, Ciou J-Y, Huang L-T, Chen Y-W, Hou C-Y. Characterization of Protein Hydrolysates from Eel (Anguilla marmorata) and Their Application in Herbal Eel Extracts. Catalysts. 2020; 10(2):205. https://doi.org/10.3390/catal10020205
Chicago/Turabian StyleCheng, I-Chun, Jin-Xian Liao, Jhih-Ying Ciou, Li-Tung Huang, Yu-Wei Chen, and Chih-Yao Hou. 2020. "Characterization of Protein Hydrolysates from Eel (Anguilla marmorata) and Their Application in Herbal Eel Extracts" Catalysts 10, no. 2: 205. https://doi.org/10.3390/catal10020205
APA StyleCheng, I.-C., Liao, J.-X., Ciou, J.-Y., Huang, L.-T., Chen, Y.-W., & Hou, C.-Y. (2020). Characterization of Protein Hydrolysates from Eel (Anguilla marmorata) and Their Application in Herbal Eel Extracts. Catalysts, 10(2), 205. https://doi.org/10.3390/catal10020205







