To date, various approaches have been developed to prepare nickel phosphide electrocatalysts, which can be mainly classified into five routes according to the different generation routes of phosphine in nickel phosphides, i.e., solution-phase method, thermal phosphidation with hypophosphite, thermal phosphidation with red phosphorus, hydrogen reduction of phosphates, and electrochemical deposition. In this part, progress on the synthesis of nickel phosphide electrocatalysts and corresponding HER performances are comprehensively reviewed and summarized for a comparison of the preparation approaches.
4.1. Solution-Phase Method
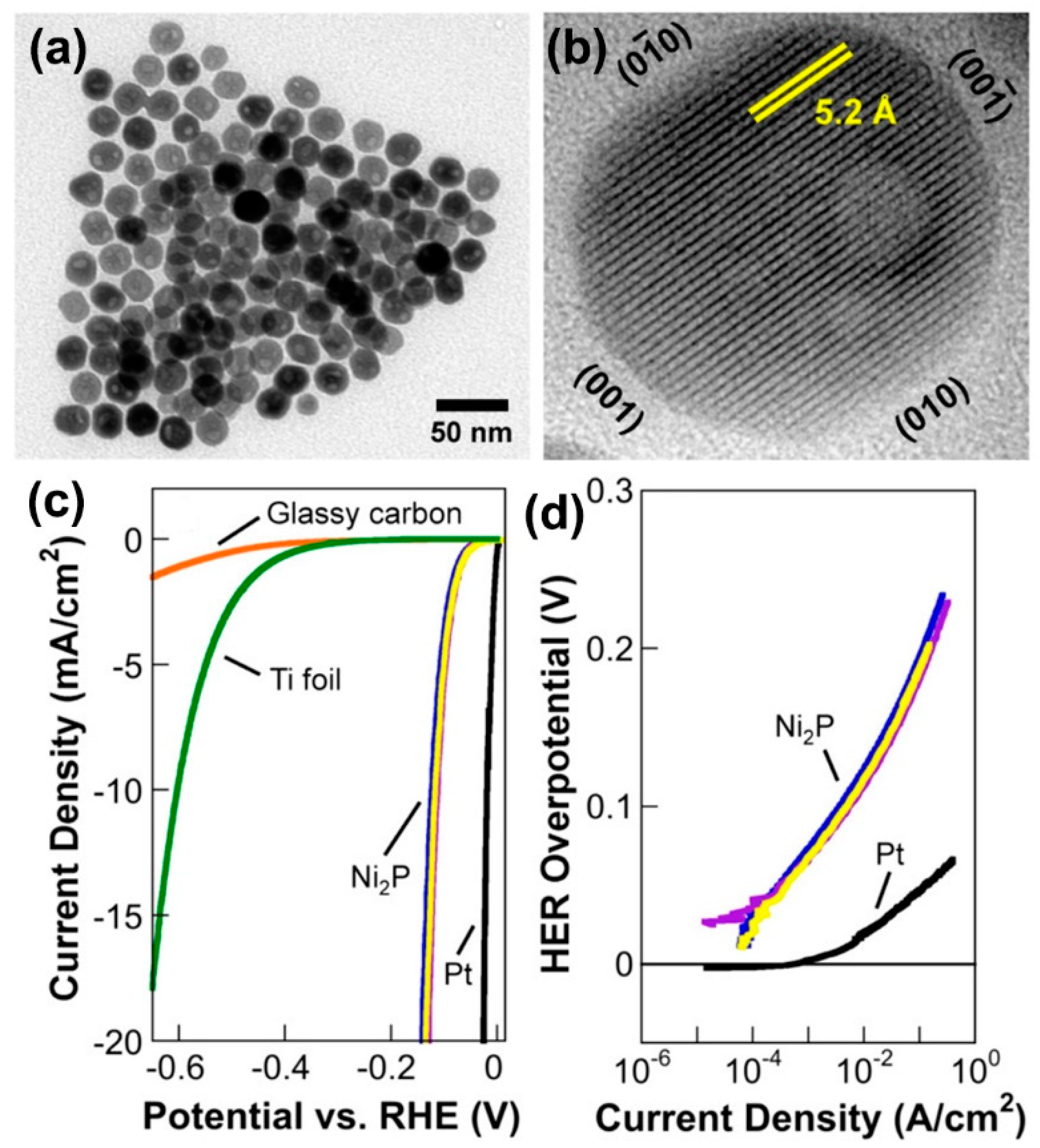
Solution-phase methods, such as the oil-phase reaction, the hydrothermal reaction, and the solvothermal reaction, are generally employed to prepare highly dispersed nanoparticles or nanocrystals. Because of the difficulties in the generation of phosphine species in the environment with oxygen and water, the preparation of nickel phosphide nanostructures is generally carried out in an oil phase solution, which can provide a reduction environment and protect the reaction against oxygen and water. For example, Popczun et al. first proposed a simple solvothermal method to synthesize Ni
2P nanoparticles, which were fulfilled by heating nickel acetylacetonate in 1-octadecene, oleylamine, and trioctylphosphine (TOP) at 320 °C for 2 h [
45]. The Ni
2P nanoparticles were obtained through phosphidation of the in-situ formed Ni nanoparticles with TOP. The transmission electron microscope (TEM) image in
Figure 3a revealed quasi-spherical nanoparticles with an average diameter of 21 ± 2 nm. The high-resolution TEM (HRTEM) image in
Figure 3b clearly showed that the Ni
2P nanoparticles are single crystal and faceted, and the lattice fringes of 5.2 Å correspond to the (100) and (010) planes of Ni
2P. The (001) crystal planes of Ni
2P have been predicted to show higher HER activity. The Ni
2P nanoparticles loaded on a Ti foil electrode exhibit excellent HER performance in 0.5 M H
2SO
4 solution with a low overpotential of 130 mV to achieve a current density of 20 mA/cm
2 (
Figure 3c) and a Tafel slope as low as 46 mV/dec (
Figure 3d). Moon et al. synthesized Ni
2P nanoparticles through a ligand stabilization method [
52], which involves the first preparation of Ni-TOP solution by mixing nickel acetylacetonate and TOP and introducing into the trioctylphosphine oxide (TOPO) under vigorous stirring at 310 °C. The as-prepared Ni
2P electrocatalyst exhibited a high HER activity with a low onset potential for the HER at around −0.02 V vs. RHE and a Tafel slope of 75 mV/dec. Wang et al. reported the preparation of a nanohybrid of carbon nanotubes decorated with Ni
12P
5 nanocrystals (Ni
12P
5/CNT) by in situ one-pot hot-solution methods at a relatively mild temperature [
47]. The Ni
12P
5/CNT nanohybrids showed excellent HER performance with a low overpotential of 129 mV for HER at a current density 10 mA/cm
2 and a small Tafel slope of 56 mV/dec. Similarly, Li et al. reported a simple and straight-forward hydrothermal route to grow Ni
2P nanofilms in situ on the surfaces of carbon nanosheets (CNS), which results in strongly coupled 3D structured nanohybrids (Ni
2P/CNS) [
53]. The as-prepared Ni
2P/CNS exhibits excellent HER performance in acidic solution and alkaline solution.
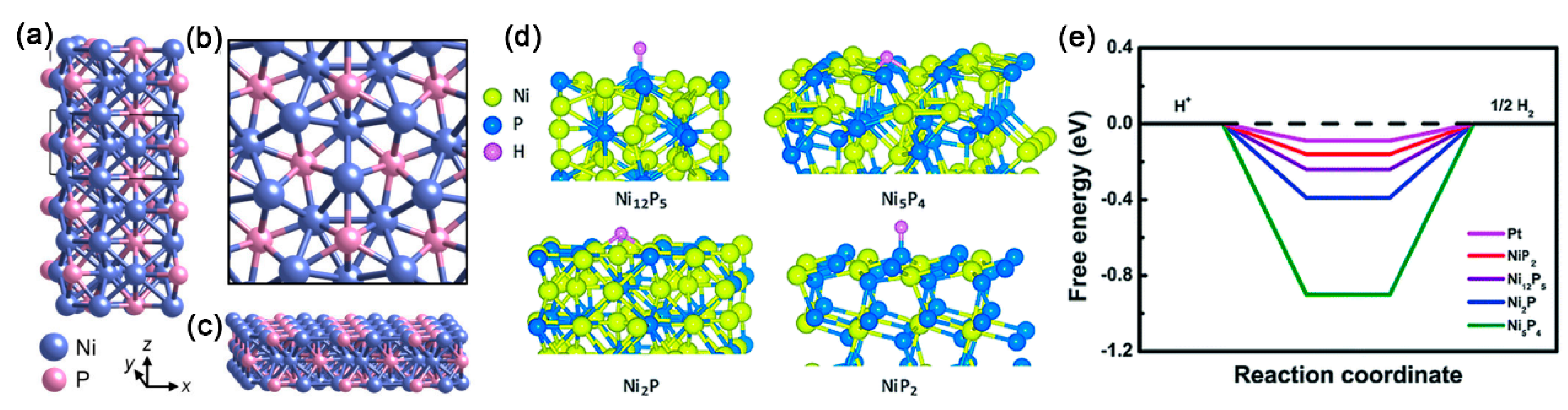
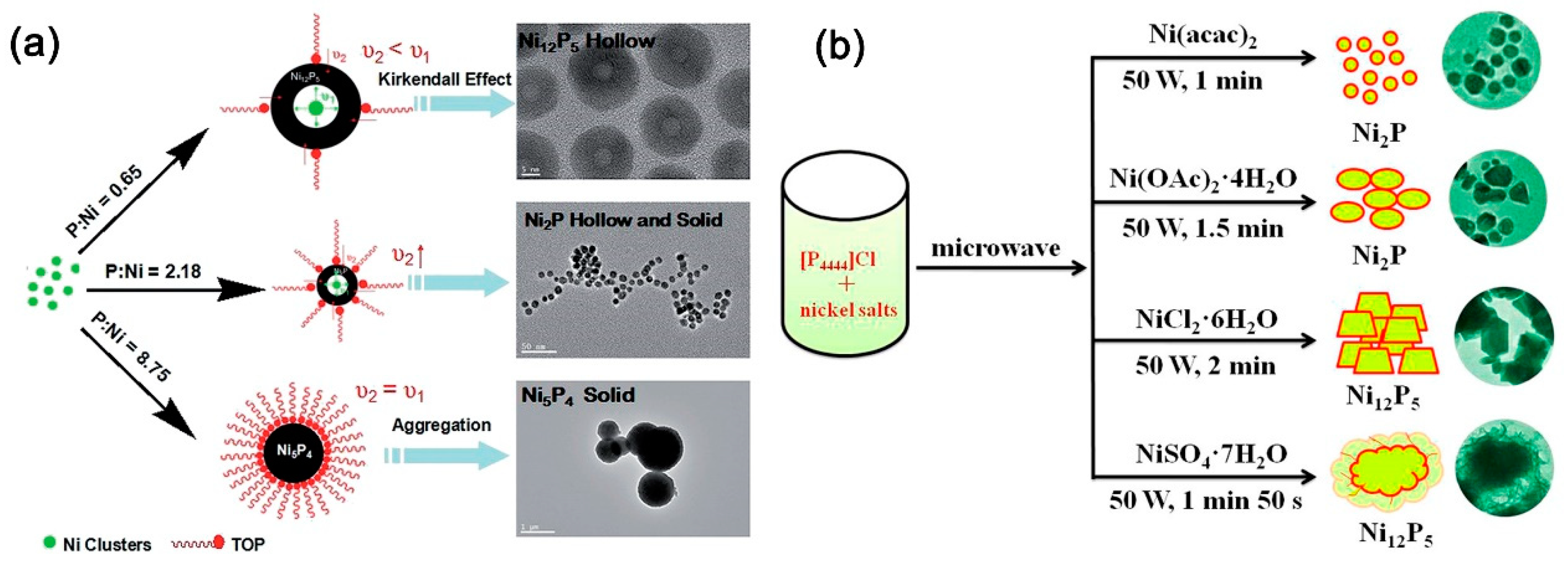
Besides nanoparticles and nanocrystals, some other nickel phosphide nanostructures have also been successfully fabricated through the solution phase method for electrocatalytic HER, and the structure-dependent catalytic performance was also studied. For example, Li et al. synthesized nanostructured Ni
2P with different morphologies (nanorods and nanoparticles) by a one-step solution phase route in which the mixture of TOPO and TOP was used as solvent, but their electrocatalytic HER performances were not fully compared [
54]. Pan et al. synthesized nickel phosphide (Ni
2P) nanoparticles (NPs) with different sizes through thermal decomposition of bis(triphenylphosphine) nickel dichloride (BTND) single source precursor in the presence of oleylamine by controlling the reaction temperature, and the Ni
2P NPs with a small size exhibit higher electrocatalytic activity due to the larger electrochemical active surface area and higher conductivity [
55]. They also reported the synthesis of monodispersed nickel phosphide nanocrystals (NCs) with different phases (Ni
12P
5, Ni
2P, and Ni
5P
4) via the thermal decomposition approach using nickel acetylacetonate as the nickel source, TOP as the phosphorus source, and oleylamine in 1-octadecene as the reductant. As shown in
Figure 4a, the phases of the nickel phosphide NCs could be easily controlled by changing the P/Ni precursor ratio. The as-synthesized nickel phosphide NCs exhibited good catalytic HER performance, and the HER activity showed a tendency of Ni
12P
5 < Ni
2P < Ni
5P
4. The superior catalytic activity is attributed to the higher positive charge of Ni and a stronger ensemble effect of P in Ni
5P
4 NCs. Zhang et al. proposed a facile approach for fabricating nanostructured Ni
2P and Ni
12P
5 by employing phosphonium-based ionic liquid, tetrabutylphosphonium chloride as a novel phosphorus source and reaction medium, upon microwave heating in 1–2 min or conventional heating at 350 °C for 3 h. As shown in
Figure 4b, controlling counter anions of various nickel salts could conveniently tune the phase of as-synthesized nickel phosphides. When the Ni(acac)
2 and Ni(OAc)
2·4H
2O are used as Ni sources, the products are Ni
2P nanoparticles, while the sources of NiCl
2·6H
2O and NiSO
4·7H
2O produce Ni
12P
5 nanocrystals. The as-synthesized Ni
2P nanoparticles presented higher catalytic efficiency than Ni
12P
5. Ni
2P nanoparticles prepared from Ni(acac)
2 require an overpotential of only 102 mV to reach 10 mA/cm
2 with a small Tafel slope of 46 mV/dec. This novel ionic liquid-mediated method for the preparation of nickel phosphides shows advantages, that is, it can be operated by microwave heating in a short period of time, which is of particular interest from the viewpoint of energy saving, fast synthesis, and easy operation.
Nickel phosphide nanoparticles with different Ni/P ratios can be synthesized by solution-phase reactions, which usually need polymer binders like Nafion to adhere catalysts to the electrodes for HER. Polymer binders generally increase electrical resistance and may reduce active sites, which results in reduced effective catalytic activity and affects long-term stability. Therefore, efforts are devoted to developing approaches for directly growing nickel phosphides on conductive substrates through solution-phase reactions. Wang et al. reported an easy one-step route for the fabrication of an integrated nickel phosphide nanorods/nickel (Ni
2P-NRs/Ni) electrode by direct phosphidation of a commercially available Ni foam current collector under solvothermal conditions using red phosphorus (P) as the precursor [
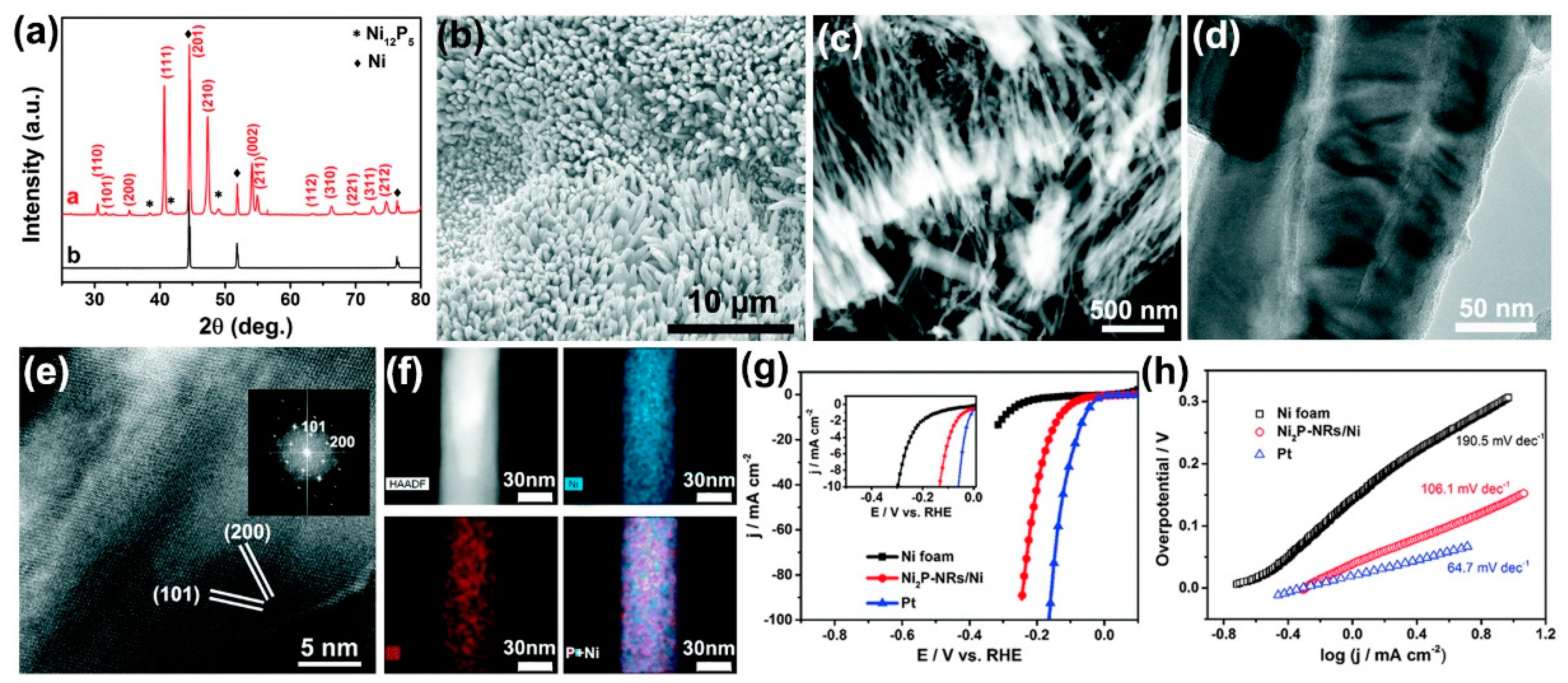
57]. The XRD pattern of the solvothermally-derived product in
Figure 5a can be indexed as a hexagonal Ni
2P phase with little admixture of a tetragonal Ni-rich Ni
12P
5 phase and un-reacted Ni foam underneath. The SEM image in
Figure 5b clearly shows the morphology of the nickel phosphides on Ni foam. Densely and vertically arranged NR arrays have formed on the entire surface of the nickel foam ligament.
Figure 5c,d are typical dark- and bright-field TEM micrographs, which unambiguously confirm the rod-like shape of the grown Ni
2P. The HRTEM image in
Figure 5e shows that the measured inter-planar spacings of 0.28 and 0.25 nm correspond to the distance of (101) and (200) crystal planes of hexagonal Ni
2P, respectively. Corresponding EDX elemental mapping images in
Figure 5f further indicate that both Ni and P elements are uniformly distributed on the as-prepared electrode. According to the data in polarization curves (
Figure 5g) and Tafel plots (
Figure 5h), when used as an integrated cathode in acidic medium, the as-fabricated Ni
2P-NRs/Ni electrode exhibits remarkable electrocatalytic activity toward HER with a small overpotential of 131 mV to attain the current density of 10 mA/cm
2 and a Tafel slope of 106.1 mV/dec. Furthermore, the electrode also shows reasonably good long-term stability. The novel fabrication method reported is scalable and can be extended to obtain other integrated transition metal phosphide/transition metal electrodes, and, therefore, represents an important development toward water electrolyzer cathode materials. Yu et al. fabricated a 3D carbon-coated nickel phosphide nanosheets array on the surface of Ni foam via a simple one-step solvothermal procedure with red phosphorus as the P source [
58]. The as-prepared C@Ni
8P
3 exhibits excellent HER activity and extraordinary duration in both acidic and basic media. Shi et al. demonstrated the growth of Ni
2P nanosheets on Ni foam by using a low concentration of TOP as a P source [
59]. The Ni
2P/Ni, acting as a robust 3D self-supported super-aerophobic hydrogen-evolving cathode, shows superior catalytic performance, stability, and durability in aqueous media over a wide pH value of 0−14. Lai et al. successfully synthesized a rice-shape nanocrystalline Ni
5P
4 on Ni foam by a simple one-step hydrothermal process with sodium hypophosphite as the P source [
46]. The Ni
5P
4/NF showed excellent HER performance with a low overpotential of 64 mV at a current density of 10 mA/cm
2 and a small Tafel slope of 64 mV/dec. Some other studies on the preparation of nickel phosphide nanostructures with various morphologies have also been reported through a one-step hydrothermal method with different phosphorus compounds as the P source (e.g., TOP, sodium hypophosphite, and red phosphorus). All the morphologies, precursors, and corresponding HER performance in acidic or alkaline electrolyte are summarized in
Table 1 (acidic) and
Table 2 (alkaline) for comparison. Based on the above results, it can be easily found that the solution-phase method generally produces nickel phosphide nanoparticles with different compositions or crystal phases, which can act as model catalysts to study and compare the HER performance between different compositions, crystal phases, or facets and are useful to make catalyst inks for practical applications in membrane electrodes. However, it is difficult to control the morphologies of the nickel phosphides and some of the precursors are high cost for practical use.
4.2. Thermal Phosphidation with Hypophosphite
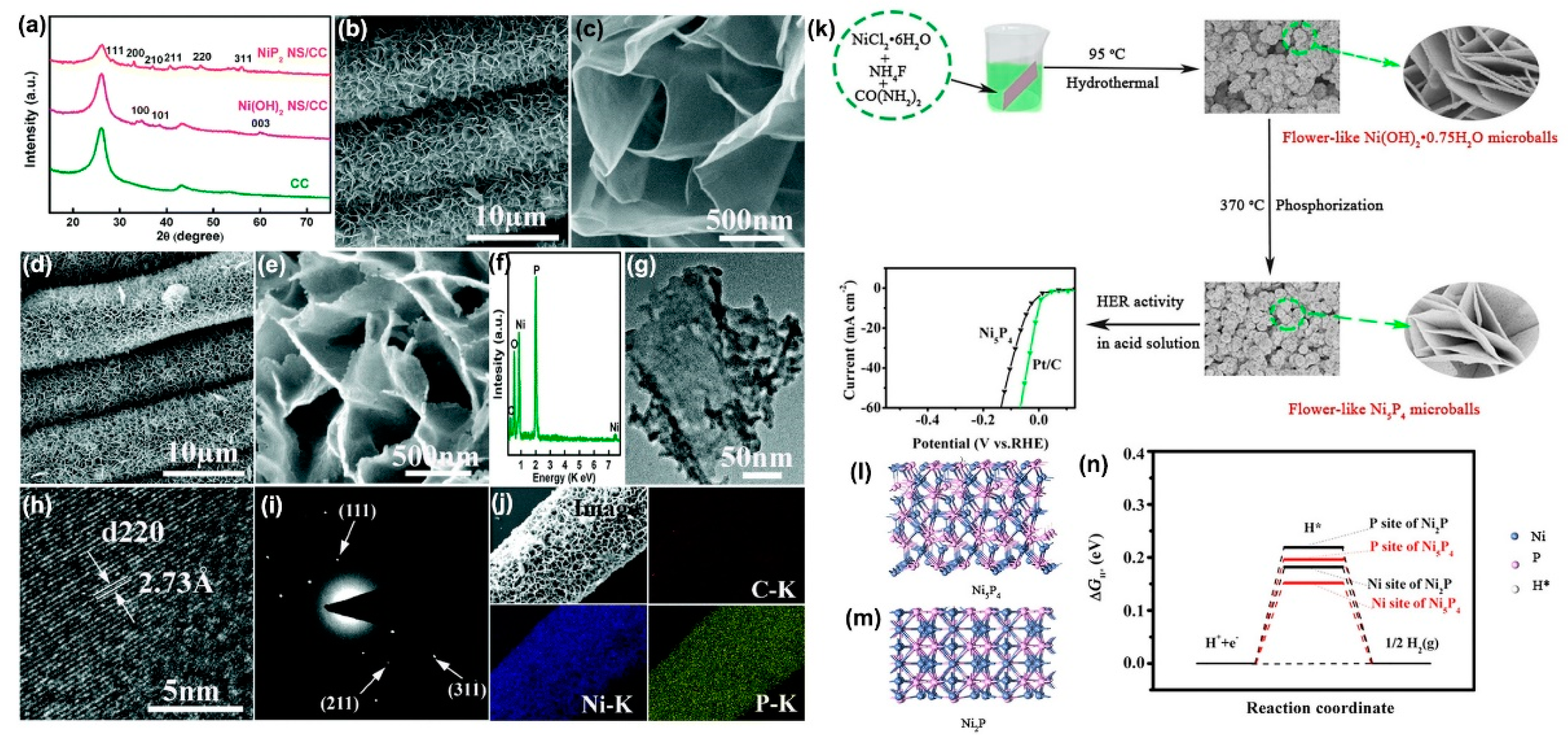
Although the solution-phase methods could produce nickel phosphide nanoparticles or nanocrystals with efficient HER activities, it requires effective immobilization of these catalysts on conductive support electrodes using a polymer binder such as Nafion or polytetrafluoroethylene (PTFE) for practical use. These polymer binders generally increase series resistance and may block active sites and inhibit diffusion, which leads to reduced catalytic HER activity. As a result, it is of significant importance to develop a binder-free HER cathode by directly growing the active nickel phosphide catalysts on current collectors. Jiang et al. reported a two-step strategy toward this direction to construct P-rich NiP
2 nanosheet arrays supported on carbon cloth (NiP
2 NS/CC), which involved the first step to grow Ni(OH)
2 nanosheet arrays on CC (Ni(OH)
2 NS/CC) hydrothermally followed by converting into NiP
2 NS/CC by a low-temperature phosphidation reaction with hypophosphite [
76]. The X-ray diffraction (XRD) pattern in
Figure 6a clearly indicates the crystal phase of Ni
2P. The SEM images of Ni(OH)
2 NS/CC in
Figure 6b,c and NiP
2 NS/CC in
Figure 6d,e indicate that the Ni(OH)
2 nanosheet precursor on carbon cloth can be converted into NiP
2 NS/CC while the structures and morphologies remain unchanged. The corresponding energy dispersive X-ray (EDX) spectrum in
Figure 6f shows the atomic ratio between Ni and P to be close to 1:2. The TEM (
Figure 6g), HRTEM (
Figure 6h), and selected-area electron diffraction (SAED) (
Figure 6i) images further confirmed the morphology and crystal phase of the NiP
2 nanosheet. The corresponding EDX elemental mapping images in
Figure 6j further indicate that both Ni and P elements are uniformly distributed in the whole NiP
2 nanosheet arrays. The NiP
2 NS/CC electrode exhibits high catalytic performance in acidic solutions with overpotentials of 75 and 204 mV to afford current densities of 10 and 100 mA/cm
2, respectively, a Tafel slope of 51 mV/dec, an exchange current density of 0.26 mA/cm
2, and nearly 100% Faradaic efficiency. Similarly, Cao et al. reported the synthesis of a hollow Ni
2P microsphere structure via a facile two-step process, including hydrothermal synthesis of Ni(OH)
2 followed by phosphidation with NaH
2PO
2 under argon [
77]. Wang et al. successfully synthesized flower-like nickel phosphide microballs (Ni
5P
4-MBs) on the surface of a Ti foil substrate by a facile hydrothermal preparation of precursors followed by a post-phosphidation process with hypophosphite (
Figure 6k). All possible Ni and P active sites of nickel phosphide were investigated by DFT simulation at a different hydrogen coverage (
Figure 6l,m,n). According to the DFT calculation results, the P site of Ni
5P
4 and NiP
2 had an endothermic ΔG
H* of 0.196 and 0.219 eV, respectively, which implies an energetically unfavorable interaction with hydrogen. Therefore, the Ni site of Ni
5P
4 is the main catalytically active site for the HER. The flower-like Ni
5P
4 microballs electrocatalyst exhibits excellent activity for the HER with a low overpotential of 35.4 mV to reach a current density of 10 mA/cm
2 and a small Tafel slope of 48 mV/dec in acidic solution. By employing a similar strategy, various structures and morphologies of nickel-containing precursors have been prepared through hydrothermal methods, and then are chemically converted into nickel phosphide electrocatalysts by a low-temperature phosphidation reaction using hypophosphite as a P source. The structures and morphologies and corresponding electrocatalytic HER performances in acidic or alkaline electrolyte are summarized in
Table 3 and
Table 4, respectively.
Recently, metal–organic frameworks (MOFs) have been proven to be ideal precursors for preparation of a variety of porous nanostructured carbon/metal composites with perfect dispersity of active sites, which can be employed to fabricate nickel phosphide electrocatalysts for the HER. For example, Yan et al. demonstrated the formation of ultrasmall nickel phosphide nanocrystals anchored on reduced graphene oxide (Ni
2P/rGO) by implanting Ni-containing MOFs (MOF-74-Ni) on graphene oxide followed by phosphidation with hypophosphite (
Figure 7a) [
108]. The Ni
2P/rGO can be used as highly efficient bifunctional electrocatalysts for overall water splitting. The high magnification SEM image (
Figure 7d) and TEM image (
Figure 7e) of Ni
2P/rGO show that the Ni
2P nanocrystals are uniformly anchored on rGO surfaces. The Ni
2P/rGO exhibited excellent catalytic performance for both HER and OER in 1.0 M KOH. Furthermore, an electrolyzer employing Ni
2P/rGO as a bifunctional catalyst in both the cathode and anode generated 10 mA/cm
2 at a voltage of 1.61 V with excellent stability. Veeramani et al. reported the preparation of a new class of nickel phosphide/graphitic carbon (Ni
2P@GC) hybrid through a two-step strategy [
93]. They first synthesized Ni-based MOFs (Ni-BTC MOFs) as precursors, and the phosphidation was conducted with sodium hypophosphite to convert Ni-BTC MOFs to Ni
2P@GC, which is schematically illustrated in
Figure 7b. The SEM image in
Figure 6h indicates a stacked layer-like morphology of the precursor Ni-BTC MOF, which was changed to Ni
2P nanoparticles on GC after the phosphidation reaction but maintained the overall morphologies (
Figure 7i). Similarly, He et al. synthesized nickel phosphide@carbon (Ni
2P@C) composites with unique nanorod array morphology via a facile phosphidation process using Ni(II)-zeolitic imidazolate framework-67 (ZIF-67-Ni) as the precursor (
Figure 7c) [
82]. The Ni
2P@C nanorod array exhibited the highest electrocatalytic activity toward HER in 0.5 M H
2SO
4 solution with a low overpotential of 186 mV at the current density of 10 mA/cm
2 and high durability. By using the same strategy, they also synthesized a hexagonal micro-rod of MOF-74-Ni first via a facile and ultrafast microwave-assisted approach, which was then utilized as a precursor for preparation of a porous Ni
2P/C composite. The precursor of the MOF-74-Ni structure (
Figure 7f) possesses a hexagonal rod structure with smooth facets and uniform size distribution. Interestingly, the surface of the rod became very rough and numerous pores appeared after the phosphidation reaction with hypophosphite (
Figure 7g). The as-prepared Ni
2P/C exhibited excellent electrocatalytic activity toward HER with a low onset potential of 94 mV, long-term stability, and a Tafel slope of 113.2 mV/dec. Some other works have also demonstrated the preparation of nickel phosphide electrocatalysts for HER by phosphidation of MOF precursors with hypophosphite at a relatively low temperature, which are summarized in
Table 3 and
Table 4 based on the acidic or alkaline electrolyte.
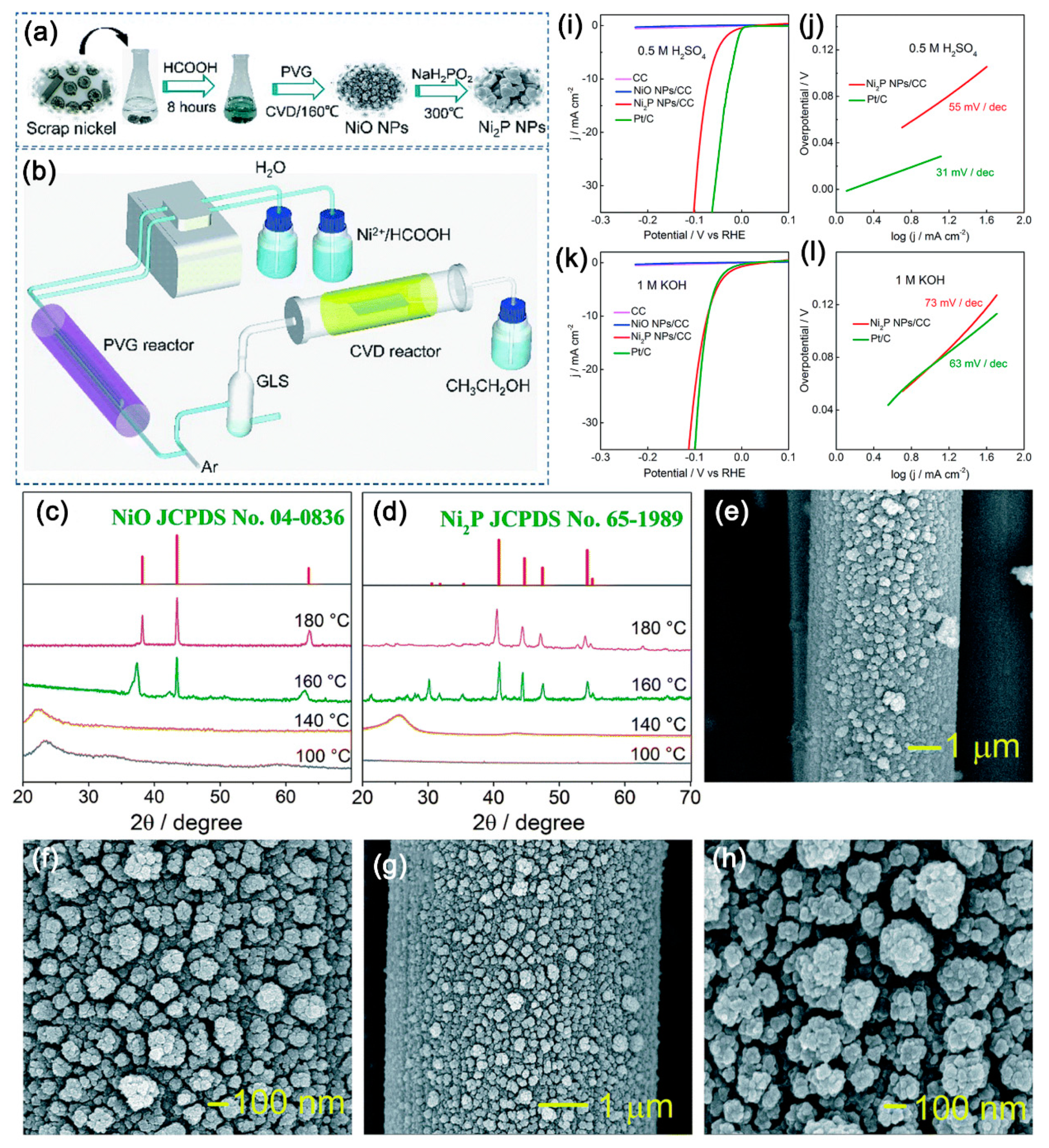
In addition, in spite of that transition, metal nickel has a high abundance and much lower cost as a source for preparation of nickel phosphide electrocatalysts for large-scale hydrogen production through electrochemical water splitting. It may be a more environmentally-friendly and cost-effective way if Ni waste and scrap can be recovered for preparing nickel phosphide electrocatalysts. However, it remains a challenge because of their large amounts of impurities. Recently, Zheng et al. proposed a simple method to synthesize 3D Ni
2P nanoparticles from scrap nickel on carbon cloth (Ni
2P NPs/CC) [
110]. As shown in
Figure 8a,b, they first dissolved nickel waste in formic acid solution by a simple ultrasonic method. Then NiO nanoparticles were fabricated by photochemical vapor generation and CVD. Lastly, the target product of Ni
2P nanoparticles (NPs) was obtained by phosphidation at a low temperature with hypophosphite. The XRD patterns of NiO NPs (
Figure 8c) and Ni
2P NPs/CC (
Figure 8d) at different temperatures demonstrated the formation of each crystal phase. It is intriguing that the NiO NPs consist of relatively regular nanoparticles that are 200–300 nm in size (
Figure 8e,f). After phosphidation, the surface is composed of relatively loose nanoparticles with a coral-like morphology, as shown in
Figure 8g,h. The polarization curves in
Figure 8i,k and Tafel plots in
Figure 8j,l, respectively, suggested as-prepared Ni
2P NPs possessed significant electrocatalytic performance and excellent stability in both acidic and alkaline media toward the HER with low overpotentials of 69 and 73 mV at 10 mA/cm
2 and small Tafel slopes of 55 and 73 mV/dec, respectively. This strategy is simpler, more cost-effective, and more environmentally-friendly when compared to conventional fabrication methods, which shows potentials in the preparation of multi-functional HER electrocatalysts from more abundant elements for efficient utilization of scrap transition metals.
4.3. Thermal Phosphidation with Red Phosphorus
Although the rational design and growth of nickel compound precursors followed by thermal phosphidation with hypophosphite show potentials to prepare nickel phosphides electrocatalysts with controllable morphologies for efficient HER. The PH
3 gases generated during the process (Note: PH
3 gases will be released from sodium hypophosphite at ~300 °C [
111,
112]) are toxic and dangerous to staff. Therefore, gentler phosphidation methods are necessary to lower the risk. A relatively low-toxic red phosphorus powder instead of hypophosphite was employed as a P source in the phosphidation reaction. Ledendecker et al. first demonstrated the simple preparation of highly ordered Ni
5P
4 nanoarchitectures directly on Ni foil through thermal phosphidation with red phosphorus powder at 550 °C for 1 h under an inert atmosphere [
113]. The synthesized Ni
5P
4 nano-architecture electrode exhibits outstanding HER performance in a strong acidic electrolyte. Many interesting morphologies of nickel phosphides prepared by a direct phosphidation reaction method between Ni foam and red phosphorus have been reported because commercial nickel foam holds great promise as a starting material for preparing efficient catalysts due to its unique 3D structure, abundance of macropores, high conductivity, and low price. For example, Wang et al. [
114], Mishra et al. [
115], and Cai et al. [
116] reported the very simple and straight-forward method to fabricate self-supported multi-phase nickel phosphide (e.g., Ni
5P
4-NiP
2 or Ni
5P
4-Ni
2P) nanosheet array cathodes for electrocatalytic HER by placing red phosphorus and commercial nickel foams upstream and downstream of a ceramic crucible, respectively, in the argon atmosphere. In acidic medium, these nickel phosphide nanosheet arrays exhibit very stable and excellent HER performance. Notably, the placement of red phosphorus powder and Ni foam upstream and downstream separately (denoted as F-B method) makes the phosphidation reaction relatively insufficient and low reproducible because the concentration of the P vapor in the reaction tube is significantly influenced by many factors, such as the carry-gas flow rate, the diameter of the tube, and the temperature control. In order to address such issues, we developed a facile, effective, and reproducible phosphidation method by placing a Ni foam above the red phosphorus powder at a distance of 1 cm inside a covered ceramic crucible (denoted as B-U method), which sufficiently allowed the phosphidation reaction [
117]. As-prepared self-supported hierarchical porous nickel phosphide (Ni
5P
4/NiP
2) nanosheets exhibited significantly improved HER performance compared to the structures prepared by the general F-B method.
Figure 9a,b clearly show the schematic illustration of the B-U method and F-B method, respectively. The SEM images in
Figure 9c,d suggest the nickel phosphide (B-U method) nanosheets are uniformly covered on the surface and have relatively small areas with sharp edges and nanopores. XRD patterns of the pristine Ni foam, S
B-U, and S
F-B are shown in
Figure 9e, which indicates that the S
F-B shows the Ni
5P
4 phase and unreacted Ni phase, while the S
B-U have two nickel phosphide phases, Ni
5P
4 and NiP
2, which suggests the reliability of the B-U method. The polarization curves in
Figure 9f and Tafel plots in
Figure 9g clearly show that the HER performance of nickel phosphide nanosheets prepared by S
B-U method is better than the one prepared by the S
F-B method. Alternatively, Laursen et al. prepared Ni
3P microparticles by a conventional solid-state reaction, which allowed Ni (s) and P (s) to react in an evacuated quartz tube at 700 °C for 24 h [
48]. The microcrystalline Ni
3P as a noble-metal-free electrocatalyst for HER shows high activity lower than those of Ni
5P
4 and Pt, which are the two most efficient HER catalysts known. Mishra et al. demonstrated the preparation of hierarchical CoP/Ni
5P
4/CoP microsheet arrays via a three-step process [
115], i.e., Ni foam was first directly phosphidated at 500 °C in a tube furnace using red phosphorous to form nickel phosphide nanosheet arrays, which was then soaked in Co-ink (Co(NO
3)
2 and a DMF mixture solution) followed by a second phosphidation at 500 °C. The as-prepared CoP/Ni
5P
4/CoP microsheet arrays require an ultralow overpotential of only 33 mV at a current density of 10 mA/cm
2. Similarly, Chen et al. prepared Ni
5P
4/NiP
2 nanoparticles on Ni
5P
4/NiP
2 nanosheets by first phosphidation of Ni foam with red phosphorus and dipping in a Ni precursor ink followed by the second phosphidation [
118]. As-prepared hierarchical nickel phosphide structures exhibit an overpotential of only 35 mV at a current density of 10 mA/cm
2 in an acidic solution and great stability at a high current density of 1200 mA/cm
2.
Nickel phosphides discussed above were synthesized by direct phosphidation of Ni foam or Ni foil with red phosphorous. Two issues should be considered in practical use when using metal Ni as precursors, i.e., the dissolution behavior of the unphosphidated Ni during electrocatalytic water splitting and the fragility of the nickel phosphide structures. Therefore, an alternative process should be developed to address such issues. Wang et al. reported a convenient and scalable approach for fabricating a self-supported 3D carbon fiber paper electrode deposited with vertically aligned Ni-P nanosheets (NSs) as bifunctional catalysts for HER and OER through electrodeposition of the Ni layer on the surface of carbon microfibers followed by a simple direct phosphidation process [
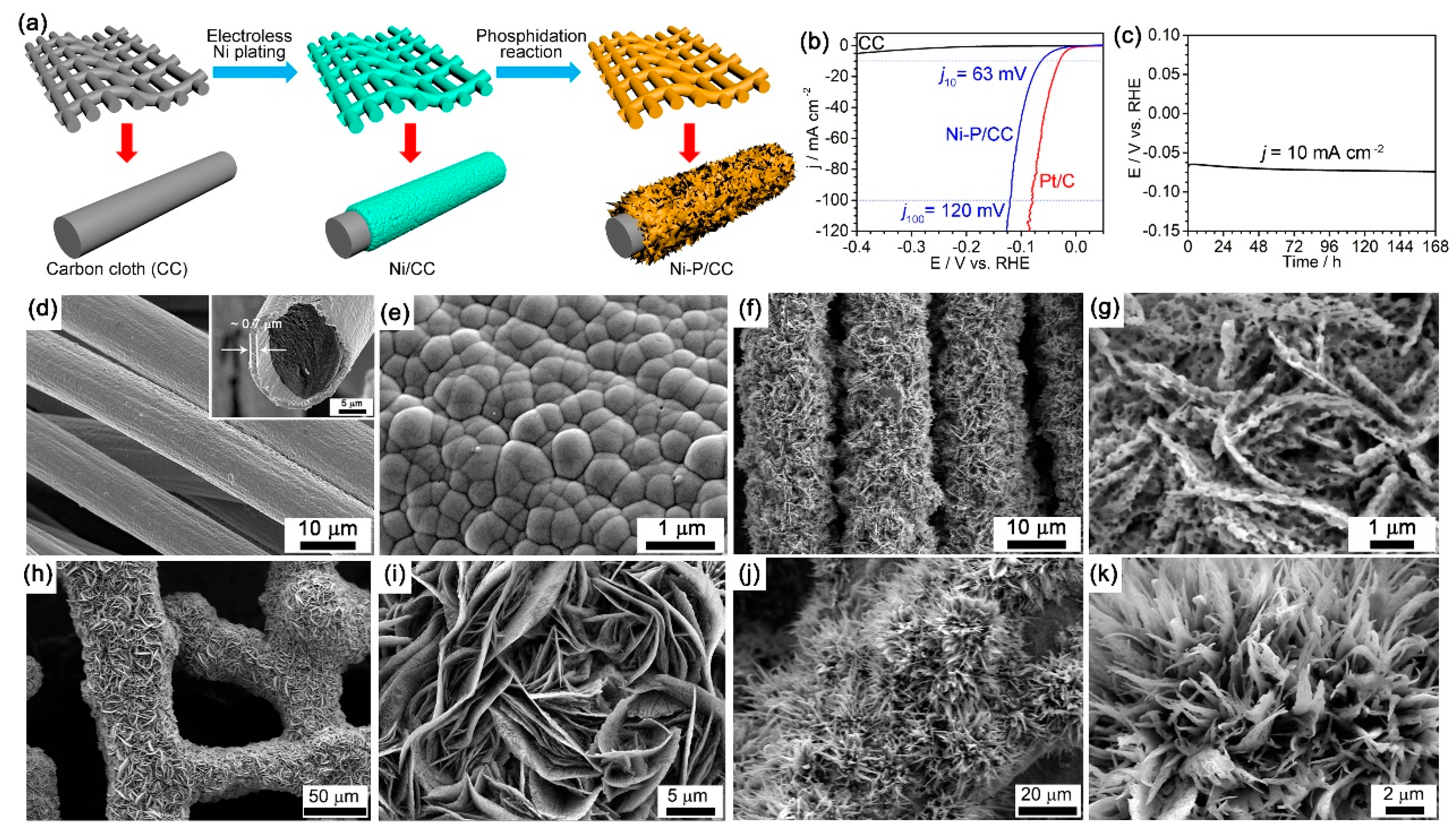
119]. The self-supported 3D electrode can be directly used as either a cathode to drive HER or an anode to expedite OER, which exhibits excellent electrocatalytic activity and outstanding long-term durability. Recently, our group developed a general, facile, and scalable method to prepare nickel phosphide (Ni
5P
4/NiP
2/Ni
2P) porous nanosheets with high electrocatalytic HER activities through electroless Ni plating on arbitrary substrates, which is followed by a simple direct phosphidation reaction [
120]. The preparation of the porous nickel phosphide nanosheets on carbon cloth is schematically shown in
Figure 10a. The polarization curves in
Figure 10b clearly show that the nickel phosphide electrode exhibits excellent HER performance in 0.5 M H
2SO
4 solution with very low overpotentials of 63 mV and 120 mV to achieve current densities of 10 mA/cm
2 and 100 mA/cm
2, respectively. Furthermore, a time-dependent potential curve in
Figure 10c indicates that the electrode exhibits outstanding durability for 168 h under a constant current density of 10 mA/cm
2. The SEM images of Ni/CC-60 and Ni-P/CC-60-450 shown in
Figure 10d,e,f,g, respectively, indicate that the carbon fibers are uniformly covered by high-density novel porous nickel phosphide nanosheets. This strategy was demonstrated in the preparation of porous nickel phosphide nanostructures on leaf vein (LV) and silkworm cocoon (SC), which are both highly active electrocatalysts for HER. The SEM images of Ni-P/LV in
Figure 10h,i show that the nickel phosphide nanosheets are very thin and have many inner pores. The SEM images of Ni-P/SC in
Figure 10j,k indicate that the nickel phosphide nanowires have relatively sharp tips and some pores inside. Therefore, this strategy is a general method to prepare transition-metal compound nanostructures in a facile, stable, and scalable way for various practical applications.
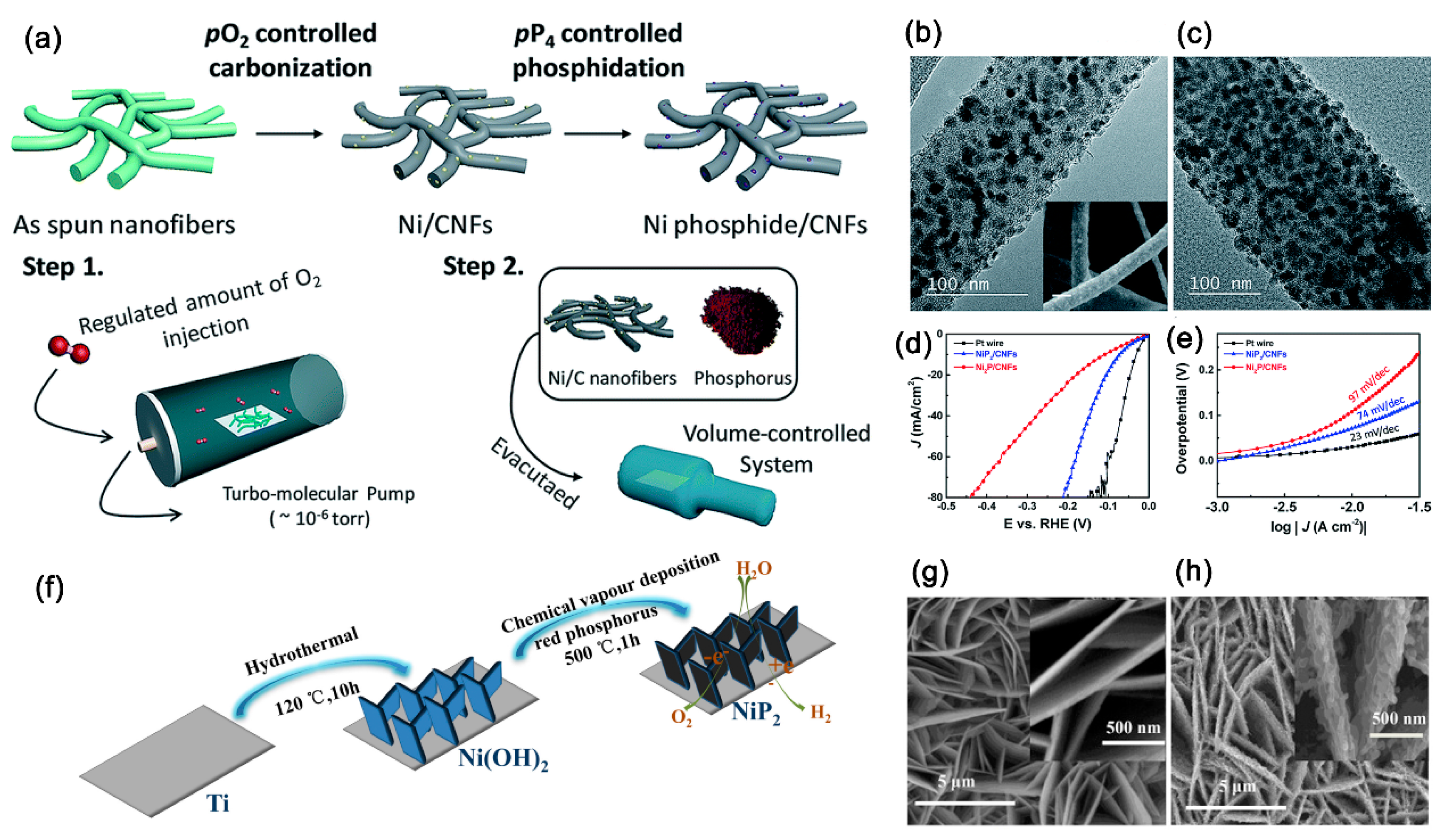
In addition, Ni-containing precursors instead of Ni forms or Ni layer plating can be synthesized by the electrospinning process, the hydrothermal method, or a direct annealing growth method. For instance, Kim et al. successfully prepared nickel phosphide embedded in carbon nanofibers (Ni-P/CNFs) through an electrospinning process followed by a simple direct phosphidation method [
51].
Figure 11a shows the schematic illustration for the synthesis of the Ni-P/CNFs. The TEM images of the Ni/CNFs in
Figure 11b and Ni-P/CNFs in
Figure 11c indicate that the nickel nanoparticles and nickel phosphide nanoparticles are embedded in carbon nanofibers, respectively. The Ni-P/CNFs electrode exhibits excellent HER performance in 0.5 M H
2SO
4 solution with a low overpotential of 71 mV to achieve a current density of 10 mA cm
−2 with a small Tafel slope of 74 mV/dec and excellent catalytic stability over 100 h (
Figure 11d,e). Cao et al. successfully synthesized vertically aligned NiP
2 nanosheets on Ti foil via a facile two-step process [
49]. As shown in
Figure 11f, the precursor of Ni(OH)
2 nanosheets was first prepared on Ti foil through a facile hydrothermal method, and then was transformed into NiP
2 nanosheets by thermal phosphidation with red phosphorus. The SEM images of Ni(OH)
2 nanosheets in
Figure 11g and NiP
2 nanosheets in
Figure 11h indicate that Ni(OH)
2 nanosheets were successfully converted into NiP
2 nanosheets with the structures and morphologies maintaining. Yan et al. proposed an effective synthetic method to obtain self-supported, hierarchical, and edge-rich nickel phosphide nanosheet arrays on nickel foam (Ni
2P NSs-NF), which were successfully employed as a high-efficiency, 3D binder-free electrode for HER. The Ni-precursor on Ni foam was prepared via a facile direct annealing growth method, and then the Ni-precursor was converted into hierarchical edge-rich Ni
2P nanosheet arrays via a direct phosphidation reaction at 500 °C with red phosphorus. The Ni
2P NSs-NF electrode exhibits superior activity for the HER both in alkaline and acidic conditions. Generally, the direct phosphidation reaction with red phosphorus is carried out at a relatively high temperature of ~500 °C. In order to solve this issue, Wu et al. successfully realized the temperature lowering down to 250 °C by using H
2 plasma-activated red phosphorus, which enables the synthesis of self-supported Ni
2P nanosheet (Ni
2P/NF) arrays on commercial nickel foam from a NiO/NF precursor [
121]. The obtained electrode exhibits an excellent hydrogen evolution activity in an acidic electrolyte and also good mechanical strength. The morphologies and HER performance in acidic and alkaline electrolytes of the nickel phosphide electrocatalysts prepared by thermal phosphidation with red phosphorus are summarized in
Table 5 and
Table 6, respectively, for comparison. Although the approach of thermal phosphidation with red phosphorus shows relatively high safety compared to that with hypophosphite and potentials in the preparation of nickel phosphide electrocatalysts with high HER performance, the studies using this method are relatively fewer and mainly focus on the Ni metal precursors. Rare works have involved the control on the structures and morphologies. Therefore, studies are still required to exert the full potentials of this method on the production of nickel phosphide electrocatalysts with controllable structures, morphologies, components, crystal phases, and high HER activities.
4.4. Hydrogen Reduction of Phosphates
Although highly crystallized nickel phosphide electrocatalysts with high HER performance can be prepared by thermal phosphidation with hypophosphite or red phosphorus, the PH
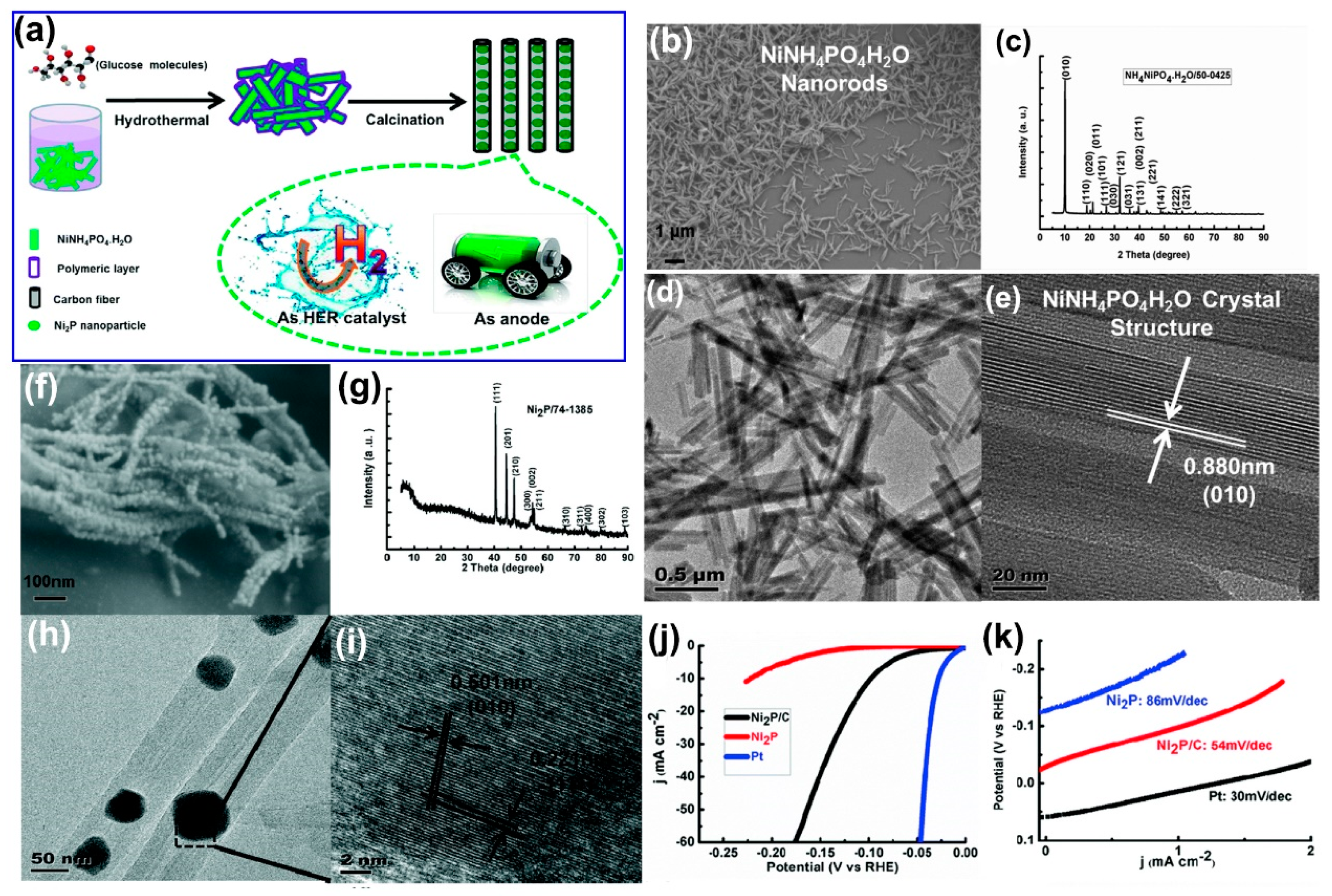
3 gas and P vapor generated during the process are relatively dangerous. Therefore, a safer and facile protocol that can produce highly crystallized nickel phosphide structures with high HER activity is still required. It is well known that hydrogen shows high reducibility under certain conditions. Thus, some researchers have employed hydrogen to reduce phosphates at a higher temperature to produce nickel phosphide structures. For example, Bai et al. demonstrated the preparation of novel peapod-like Ni
2P/C nanocomposites via a two-step process, which is schematically shown in
Figure 12a [
125]. They first prepared NiNH
4PO
4·H
2O nanorods via a facile hydrothermal method at 170 °C for 24 h. The morphology and crystal phase of the NiNH
4PO
4·H
2O nanorods can be clearly known through a SEM image (
Figure 12b), XRD pattern (
Figure 12c), and TEM image (
Figure 12d,e). Subsequently, the crystalline NiNH
4PO
4·H
2O nanorods were converted to peapod-like Ni
2P/C nanocomposites by calcination under hydrogen at 700 °C for 200 min. Through the SEM image (
Figure 12f), the XRD pattern (
Figure 12g), and TEM image (
Figure 12h,i), it can be clearly seen that the NiNH
4PO
4·H
2O nanorods have been successfully transformed into peapod-like Ni
2P/C nanocomposites after calcination. The Ni
2P/C nanocomposites showed excellent electrocatalytic HER activities in 0.5 M H
2SO
4 with a small overpotential of 87 mV to attain the current density of 10 mA/cm
2 and a notably low Tafel slope of 54 mV/dec (
Figure 12j,k). The peapod-like Ni
2P/C nanocomposites can be used not only as an electrocatalytic hydrogen evolution reaction material, but also as an anode material for lithium-ion batteries. Then, by utilizing the similar approach, they successfully fabricated a novel 1-D peapod array of Ni
2P@graphitized carbon fiber composites on Ti foil substrate by hydrothermal growth and polymerization, which is followed by annealing in an H
2 atmosphere [
126]. Miao et al. synthesized a core/shell structured chainmail catalyst of ultrathin P-doped carbon shell-encapsulated nickel phosphides on graphene through a facile solvothermal method following by an annealing treatment in hydrogen atmosphere, which exhibits remarkable electrocatalytic HER activities [
127].
The tunable compositions and hetero atom-enriched characteristics of inorganic−organic metal phosphonate hybrids make them promising templates and precursors to create high-performance nanomaterials as electrocatalysts for HER by high-temperature reduction. Lv et al. demonstrated the preparation of nitrogen-doped carbon-coated nickel phosphide nanoparticles on Ni foam by hydrogen reduction of the precursors of nickel phosphonates loaded on Ni foam [
128]. Wang et al. reported the in-situ preparation of embedded ultrafine nickel phosphides in N-doped porous carbon nanofibers (Ni
2P@NPCNFs) by electrospinning the precursors, which is followed by a controllable pyrolyzed reduction in an H
2 atmosphere. The Ni
2P@NPCNFs show excellent catalytic performance as an HER catalyst in acidic media, and good durability in neutral and basic media. This method was also demonstrated to prepare Fe
2P@NPCNFs, Co
2P@NPCNFs, and Cu
3P@NPCNFs with the same nanostructure as Ni
2P@NPCNFs. This work provides a general approach for fabrication of transition metal phosphide structures with enhanced conductivity and catalytic activity.
Table 7 summarizes the morphologies, mass loadings, and corresponding electrocatalytic HER performance in an acidic electrolyte of nickel phosphide electrocatalysts prepared through hydrogen reduction of phosphate precursors. As stated above, this hydrogen reduction of the phosphates method does not produce toxic or dangerous PH
3 gas or P vapor during the phosphidation process, and is very suitable for preparation of nickel phosphide electrocatalysts together with conductive supports (e.g., carbon). This is beneficial for the dispersion of the catalysts for high HER activities. However, it should also be noted that it is rather difficult to design or control the morphologies of the final nickel phosphides, and the process requires a relatively high temperature and involves explosive hydrogen gas.
4.5. Electrochemical Deposition
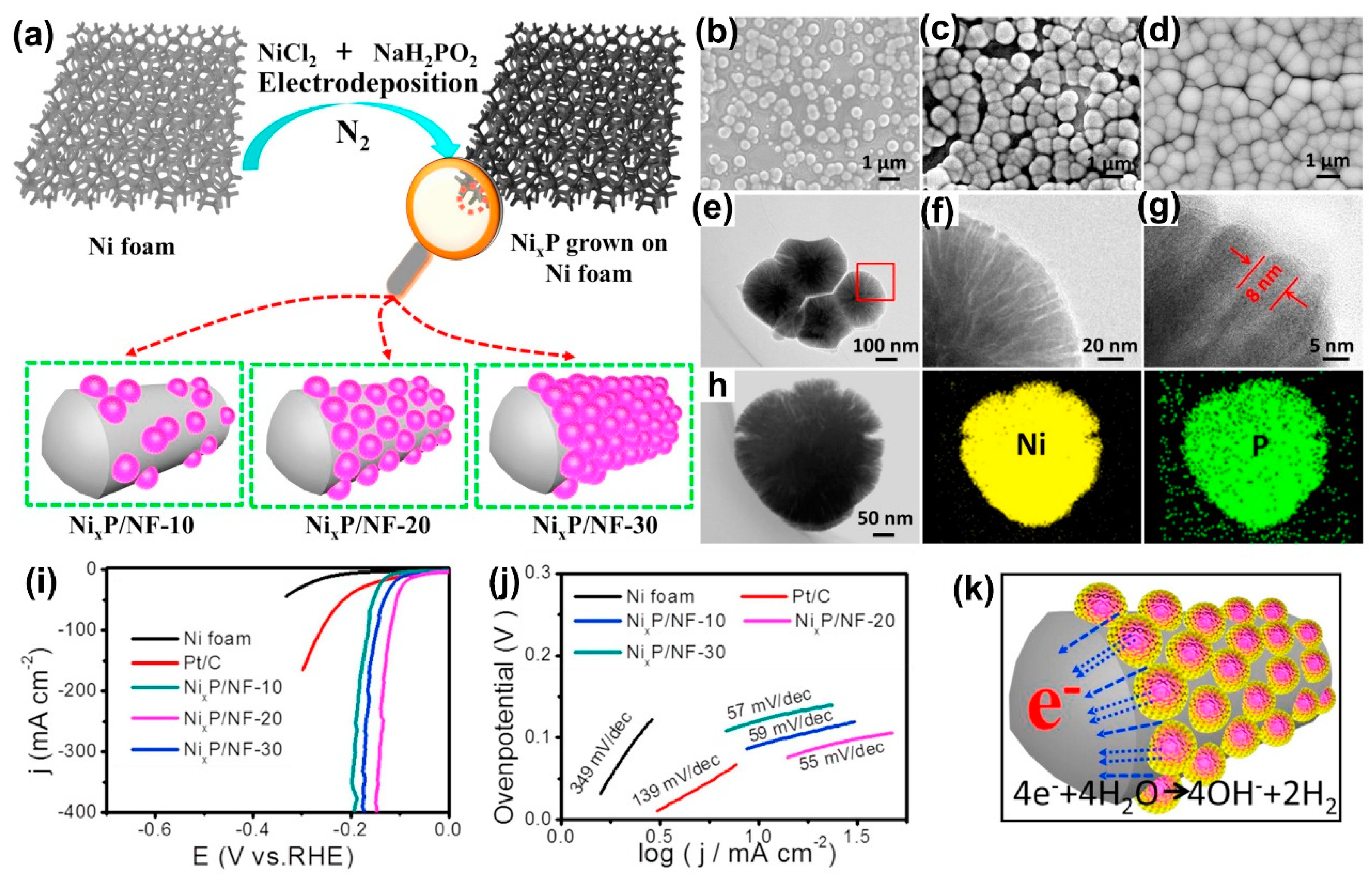
Electrochemical deposition, which involves the migration of the anions and cations in the electrolyte solution under an external electric field and the redox reactions on the electrode surfaces to deposit production layers, is recognized as a facile and mild preparation method due to the conditions generally at room temperature. Generally, it is difficult to obtain highly crystallized products and to control the morphologies through electrochemical deposition. For preparation of nickel phosphides, nickel and phosphorus alloys are generally produced by electrochemical deposition. In the preparation process, nickel-containing salts and phosphorus-containing salts are usually employed as precursors, and a conductive substrate is necessary to act as the electrode to support the depositions. So far, some interesting nickel phosphide structures have been successfully prepared by electrochemical deposition. Cao et al. demonstrated the preparation of hierarchical radial amorphous nickel phosphide (Ni
xP) nanospheres on Ni foam (Ni
xP/NF) at a constant potential of −0.8 V (vs. Ag/AgCl) under a nitrogen atmosphere by using a nickel chloride and sodium hypophosphite electrolyte [
131].
Figure 13a shows a schematic image of the electrodeposition preparation of the hierarchical radial Ni
xP nanospheres on Ni foam at different times. The SEM images in
Figure 13b,d together with the TEM images in
Figure 13e,f,g clearly show the radial microstructure and morphology of the as-prepared Ni
xP nanospheres. The Ni
xP nanosphere has an amorphous phase and is composed of ultrathin nanosheets with a thickness of 8 nm near to the surface. The elemental mapping images in
Figure 13h further verify the homogeneous distribution of Ni and P elements in Ni
xP nanospheres. The polarization curves in
Figure 13i and Tafel plots in
Figure 13j exhibit the electrocatalytic HER performance of the NixP nanospheres. Among them, the Ni
xP/NF-20 electrode exhibits the best HER performance in 1 M KOH solution with very low overpotential of 63 mV to achieve a current density of 10 mA/cm
2, and a Tafel slope as low as 55 mV/dec, which can be attributed to the 3D open-pore Ni foam structures with a high specific surface area and the high conductivity contributed by the in-situ deposition of highly ordered Ni
xP nanospheres on conductive Ni foam.
Figure 13k illustrates the advantages of Ni
xP/NF-20 electrode during hydrogen evolution process in 1 M KOH, which follows the equation: 4e
− + 4H
2O → 4OH
− + 2H
2 [
132]. Chen et al. reported the preparation of NiP
x nanospheres on carbon cloth by cyclic voltammetry (CV) scanning between 0.2 V and −0.7 V (vs. NHE) at a scan rate of 50 mV/s by using nickel acetate (Ni(ac)
2) and sodium hypophosphite as precursors in acetate buffer solution (pH = 7) [
133]. The NiP
x-coated carbon cloth electrode exhibits good HER performance in phosphate buffer saline (PBS) (pH = 7) solution. Similarly, Liu et al. deposited nickel–phosphorus nanoparticle films on Cu foam (Ni-P/CF) by CV scanning for 15 cycles between −1.0 and −0.3 V at a scan rate of 10 mV/s in electrolyte containing NiSO
4, KOH, and NaOAc [
134]. The Ni-P/CF can deliver a current density of 10 mA/cm
2 at an overpotential of 98 mV for hydrogen production and 325 mV for oxygen generating in 1 M KOH solution. A two-electrode water electrolyzer using Ni-P/CF as cathode and anode produces 10 mA/cm
2 at a cell voltage of 1.68 V with high stability. Sun et al. prepared P-doped nickel (NiP
x) superstructure films on Cu foil by potentio-static deposition (PSD) and potentio-dynamic (PDD) approach, respectively [
135]. By comparing the two deposition methods, it is found that the latter model with a potentio-dynamic control is a valid electrochemical protocol to create crack-free NiP
x films. The PDD-NiP
x film shows a high HER activity under alkaline conditions. The PDD-Ni-10P achieved a low overpotential of 105 mV to deliver a current density of 10 mA/cm
2 with a small Tafel slope of 44.7 mV/dec and excellent catalytic stability for at least 60 h. Tang et al. reported the preparation of Ni-P alloy nanoparticle films on Ni foam by a linear voltammetry scan in the range of 1.2 to 0.2 V (vs. SCE) in the electrolyte solution of nickel sulfate, sodium hypophosphite, and sodium acetate [
136]. The as-prepared Ni-P alloy nanoparticle film electrode can act as an efficient bifunctional water-splitting catalyst in strongly alkaline media, which drives 10 mA/cm
2 at overpotentials of 80 and 309 mV for HER and OER, respectively, and its two-electrode water electrolyzer needs a cell voltage of 1.67 V to achieve 10 mA/cm
2. Cheng et al. fabricated an Ni–P alloy on the plate via pulsed electrodeposition, and the Ni–P alloy with high capacitance performs the best HER catalytic activity [
137]. In addition, Wu et al. fabricated self-supported Ni-P cathode by linear voltammetry scan from −0.3 to −0.9 V (vs Ag/AgCl) at a scan rate of 10 mV s
−1 [
138]. The Ni-P cathode performed a low onset over-potential, good catalytic activity, and long-term stability under neutral and alkaline conditions. The structural information, mass loading, and electrochemical HER performance in the alkaline electrolyte are summarized in
Table 8 for comparison.
Nickel phosphides prepared by the electrochemical deposition method also show remarkable HER activities in acidic solution. For instance, Ren et al. prepared nickel/nickel phosphide nanospheres electrode via in-situ reduction of NiSO
4 and NaH
2PO
2 on carbon cloth substrates using square-wave and CV methods, respectively [
139]. It is found that nickel/nickel phosphide nanospheres prepared with the pulse deposition procedure exhibits higher HER performance than those prepared through the CV approach. As-prepared nickel/nickel phosphide/carbon cloth electrodes exhibit a cathodic current of 10 mA/cm
2 at the overpotential of 164 mV in a 0.5 M H
2SO
4 solution and a Tafel slope of 76 mV/dec. Wasalathanthri et al. prepared amorphous nickel phosphide thin films on Cu foil by electrodeposition at a constant current density of 10 mA/cm
2 for 10 min, which exhibits a good activity and long-term stability in acidic medium, with a potential of −222 mV to achieve 10 mA/cm
2 [
140]. Wang et al. reported the preparation of amorphous sponge-like nickel phosphide-carbon nanotube (Ni
xP/CNT) hybrid electrodes through CV deposition [
143]. These Ni
xP/CNTs exhibit superior catalytic activity for sustained hydrogen evolution in acidic, neutral, and basic media, which is attributed to the 3D porous nanoarchitecture consisting of a highly conductive 3D porous CNT backbone and a well-deposited Ni
xP nanoparticle catalyst. Apart from this, Kim et al. reported that pulse electrodeposition can be used to prepare an amorphous Ni-P catalyst with a high portion of P, which confers acid-resistant properties to the catalyst [
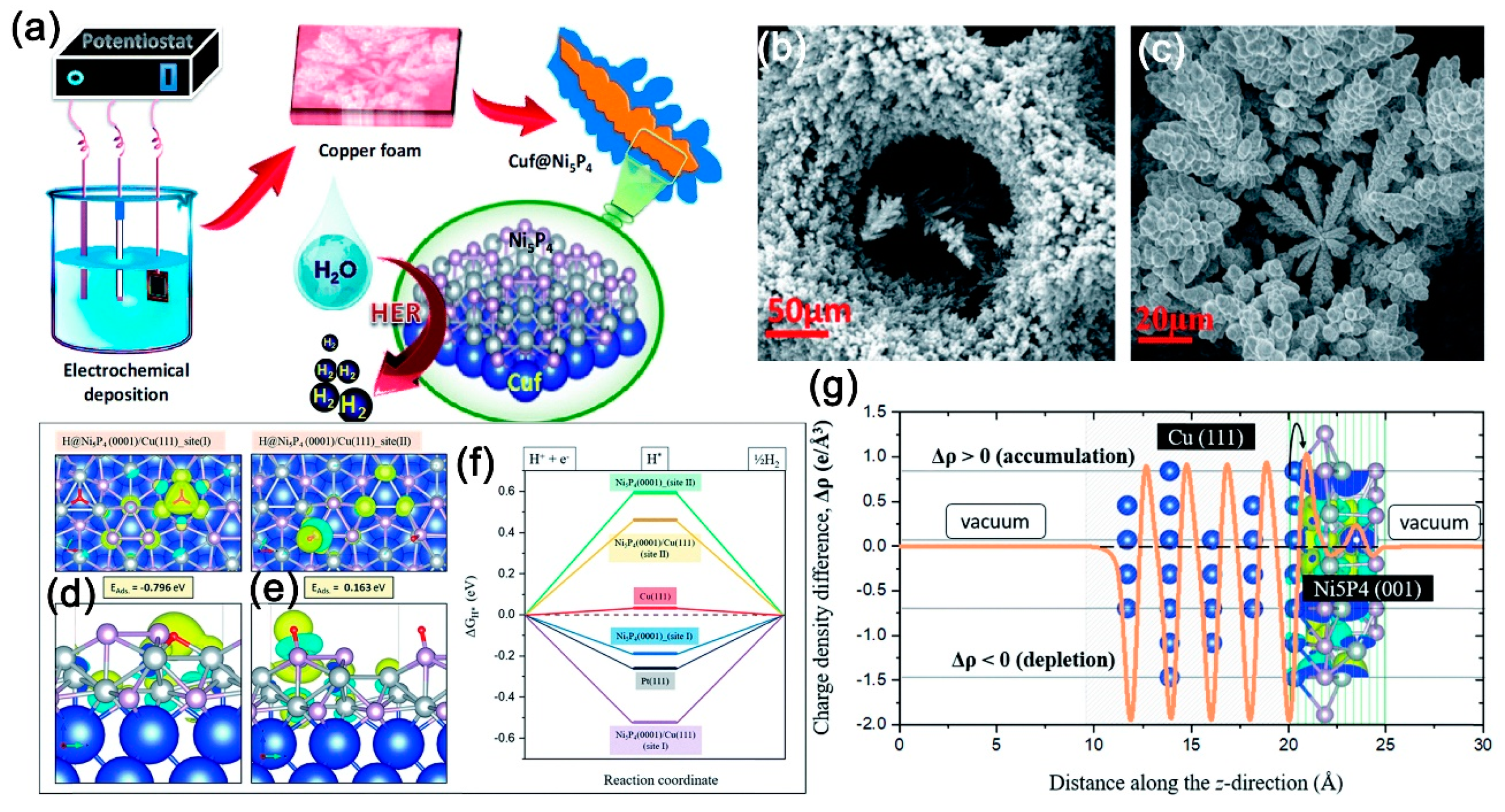
141]. Most of the nickel phosphide catalysts prepared by electrodeposition are amorphous, but Das et al. fabricated copper foam @ single-phase Ni
5P
4 superhydrophilic and aerophobic core–shell nanostructures through a two-step process [
142]. They first prepared a copper foam electrode by implementing a constant current density of 1 A/cm
2. Then, electrodeposition of Ni
5P
4 on copper foam was conducted by implementing a constant potential of −0.8 V.
Figure 14a clearly shows the schematic image of the electrochemical synthesis of the copper foam @ Ni
5P
4 structures. The SEM images in
Figure 14b,c indicate the microstructures and morphologies of the as-prepared Cu@Ni
5P
4. As shown in
Figure 14d,e,f,g, the density functional theory (DFT) model, Ni
5P
4 (0001) and Ni
5P
4 (0001)/Cu (111) surface with H* adsorbed over the three-fold Ni site (site I) exhibited a negative value of DGH*, which favors HER activity. DFT calculations elucidated the origin of a very high negative DGH* in Ni
5P
4 (0001)/Cu (111) upon hydrogen adsorption. This arises due to a localized charge density distribution around the three-fold Ni site of the adsorbate, which is favorable for the HER activity of the catalyst. The super-hydrophilic and aero-phobic nanostructured surface also contributes to the HER performance by reducing the contact resistance and the fast release of air bubbles during the catalysis process. In order to evaluate the HER performance of the materials prepared by electrochemical deposition in an acidic solution, the structural information, mass loading, and electrochemical HER performance in an acidic electrolyte are also summarized in
Table 8. It can be easily found that the electrochemical deposition method for preparation of nickel phosphides shows some apparent advantages such as mild preparation with no need of high temperature, low cost, and high safety with no toxic chemicals. However, there are still some weakness of this method. For instance, it is difficult to control the morphologies and the products are generally amorphous, which may limit the intrinsic activities. Therefore, efforts are still required to address the above issues to obtain stable and highly crystallized nickel phosphide electrocatalysts with high HER activities.