Ethylene Oligomerization over Nickel Supported Silica-Alumina Catalysts with High Selectivity for C10+ Products
Abstract
1. Introduction
2. Results and Discussion
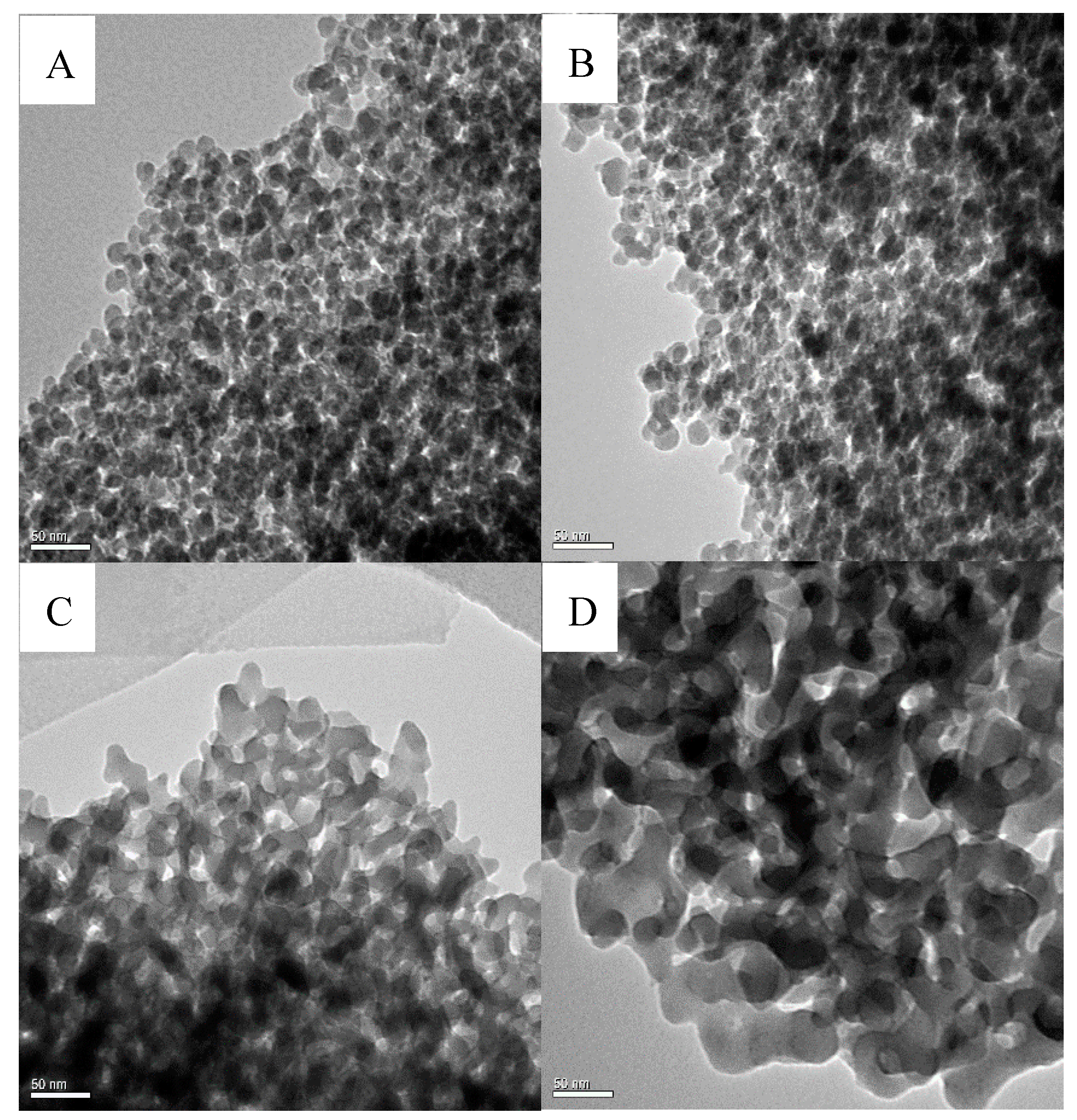
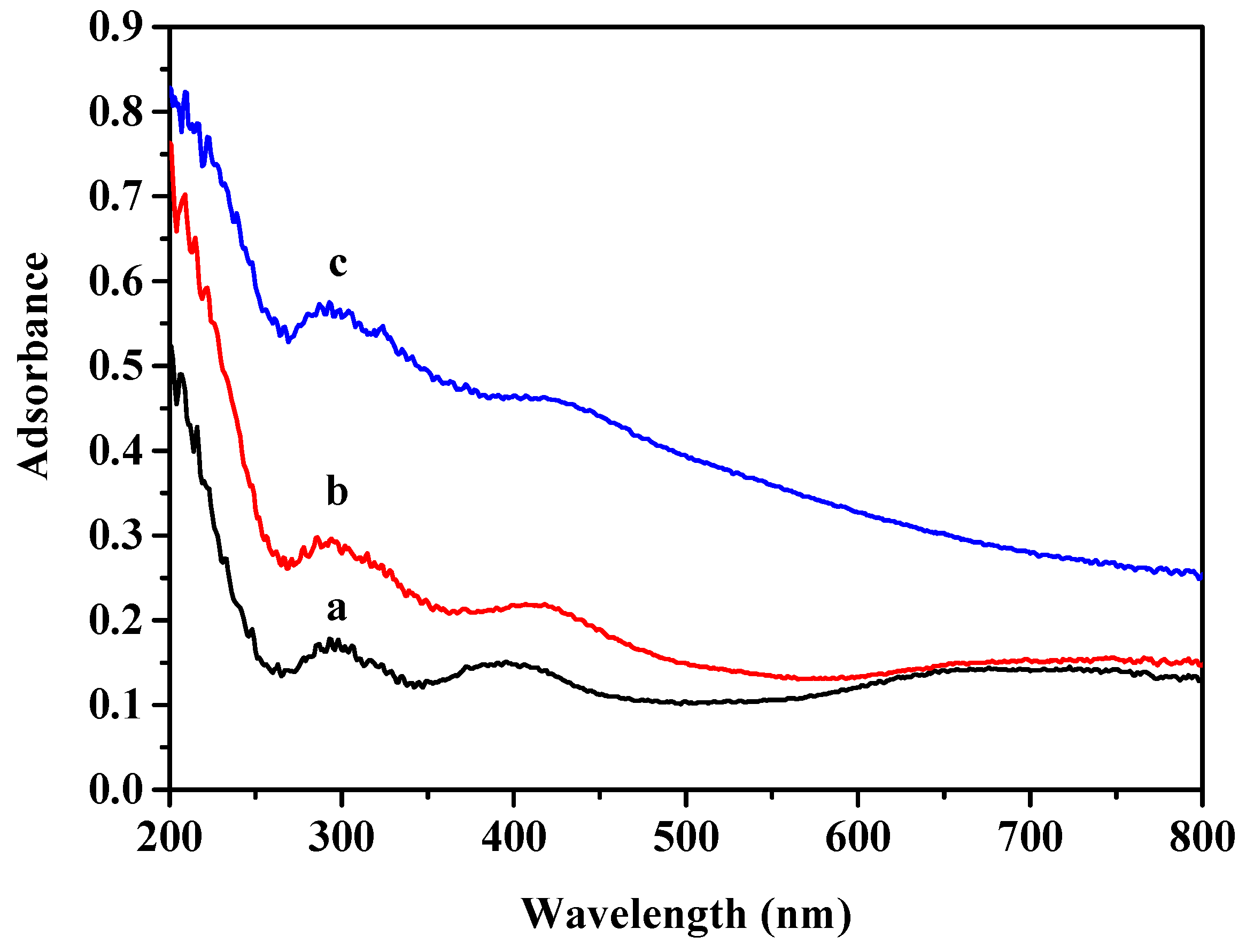
2.1. The Physicochemical Properties of Ni/Si-Al Catalysts
2.2. Ethylene Oligomerization Reaction Tests
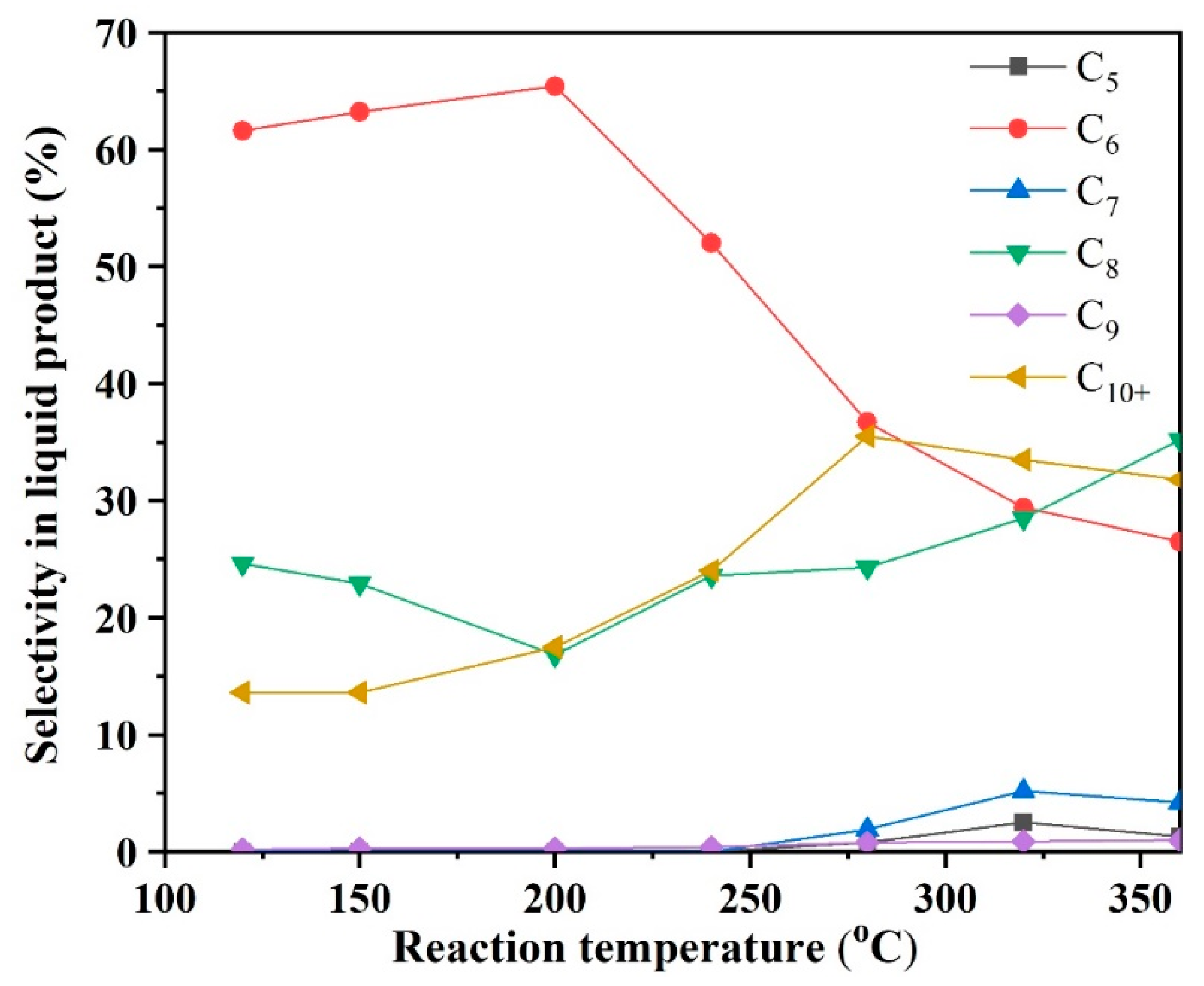
2.2.1. The Effect of Reaction Temperature
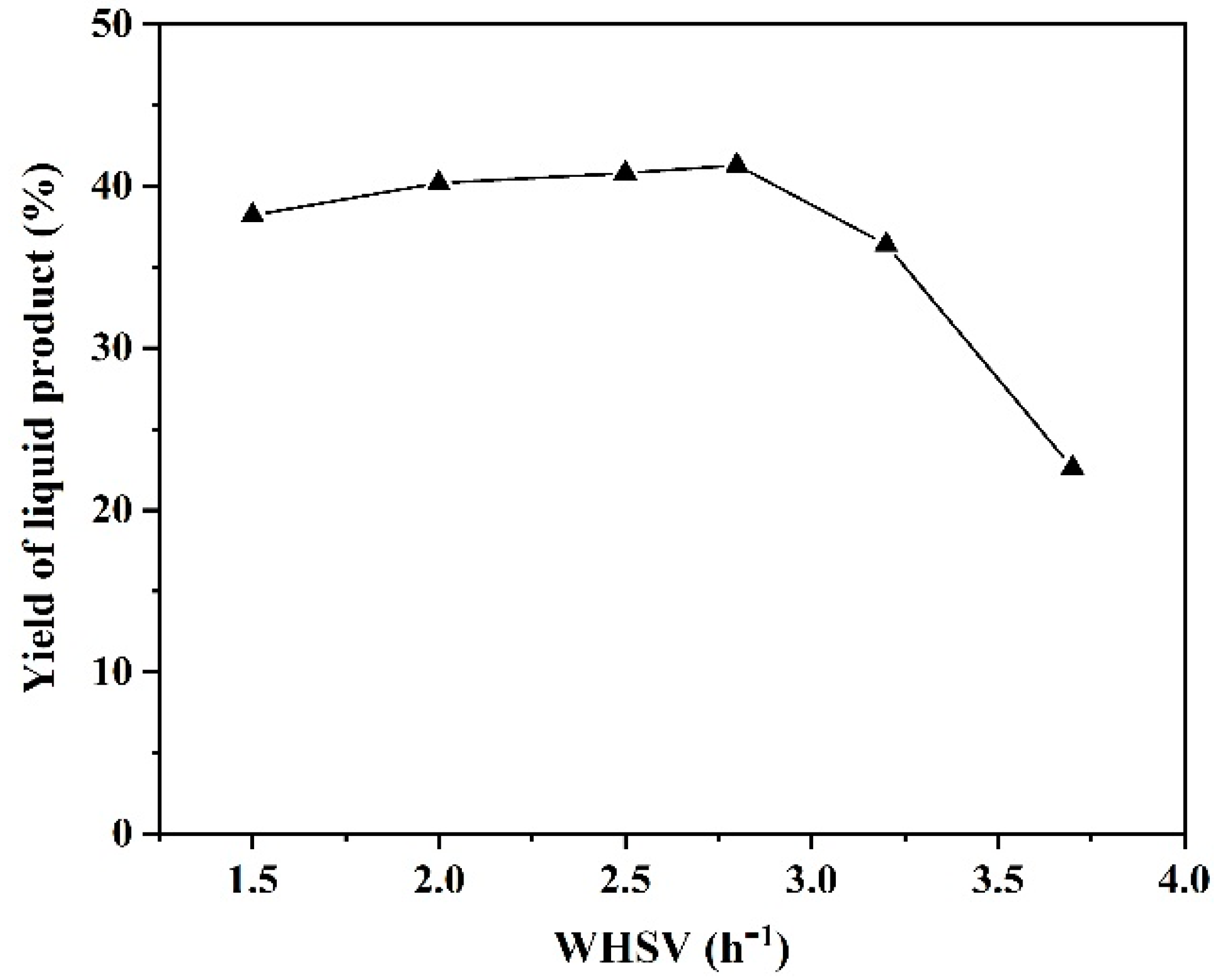
2.2.2. The Effect of Weight Hourly Space Velocity (WHSV)
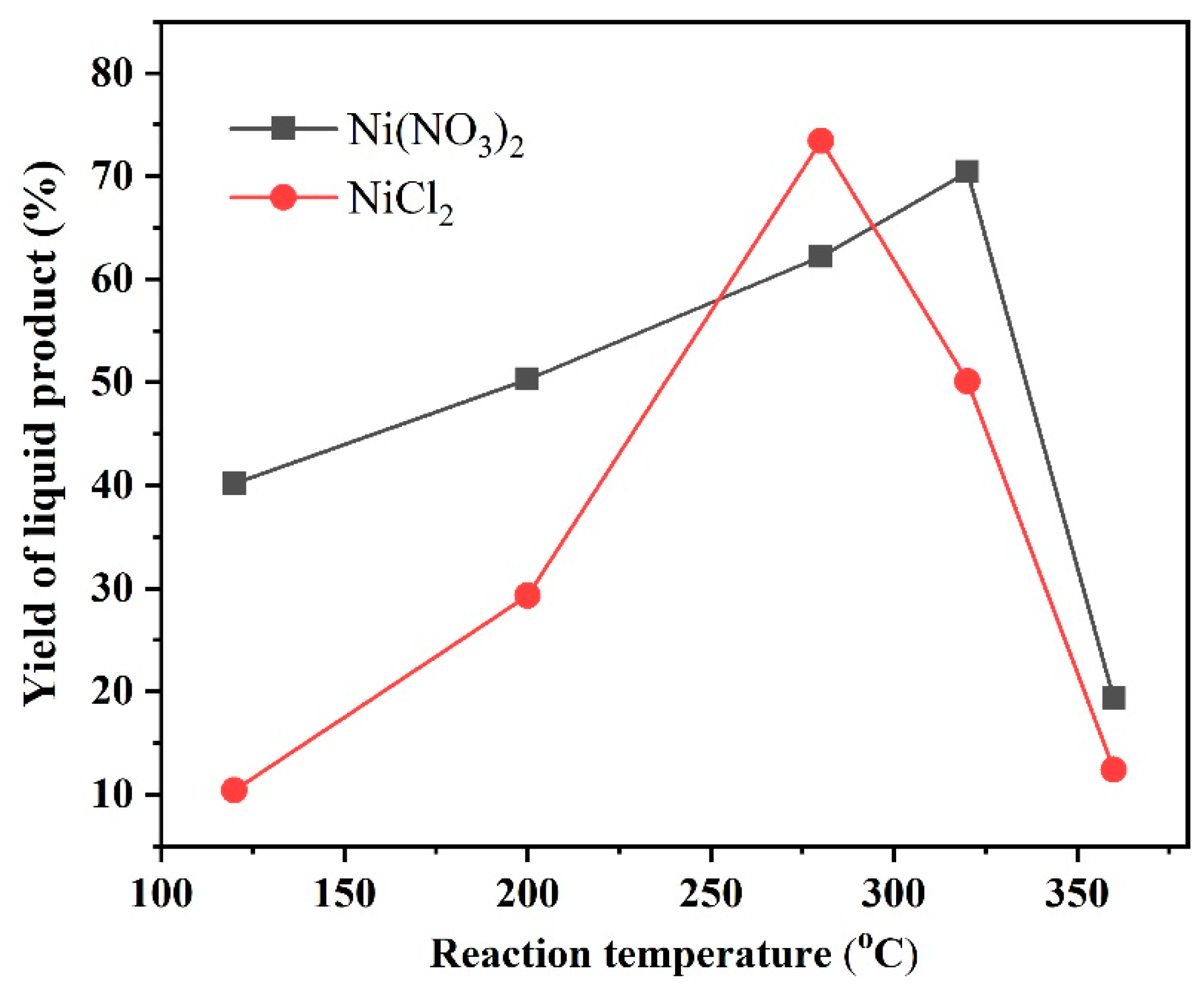
2.2.3. The Effect of the Ni Precursors
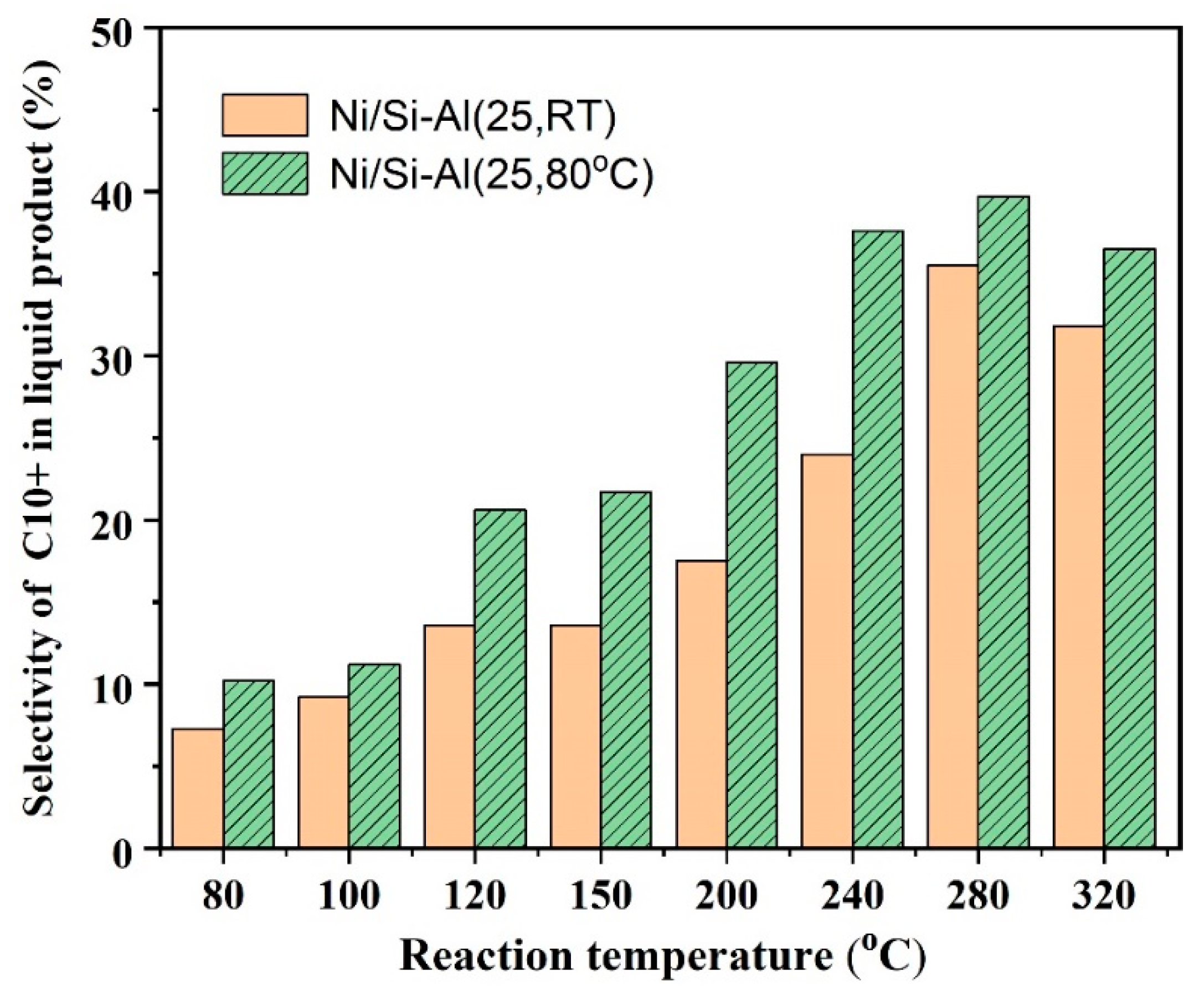
2.2.4. The Effect of Aging Temperature
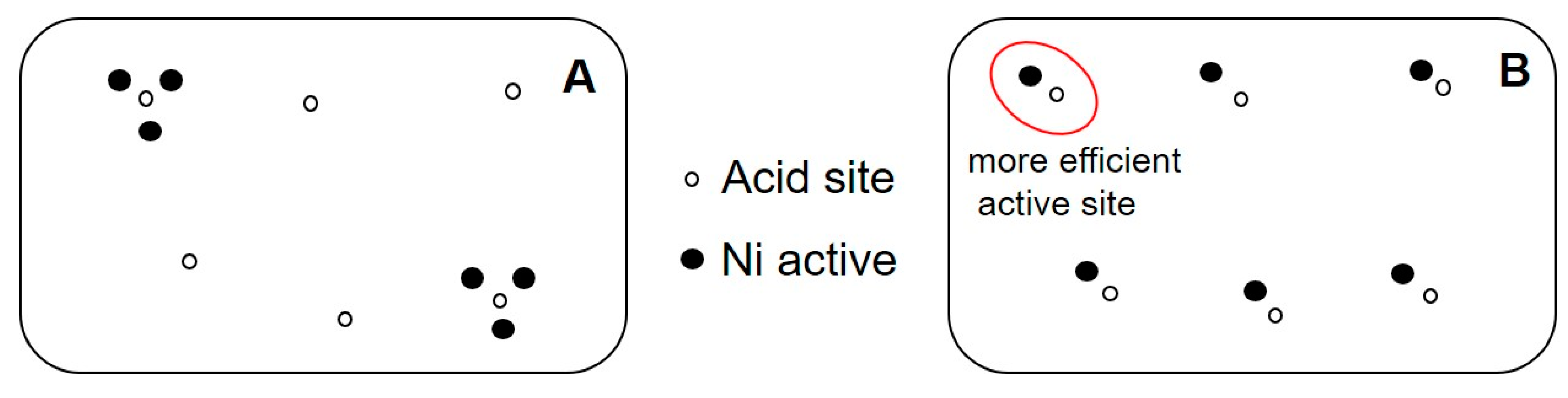
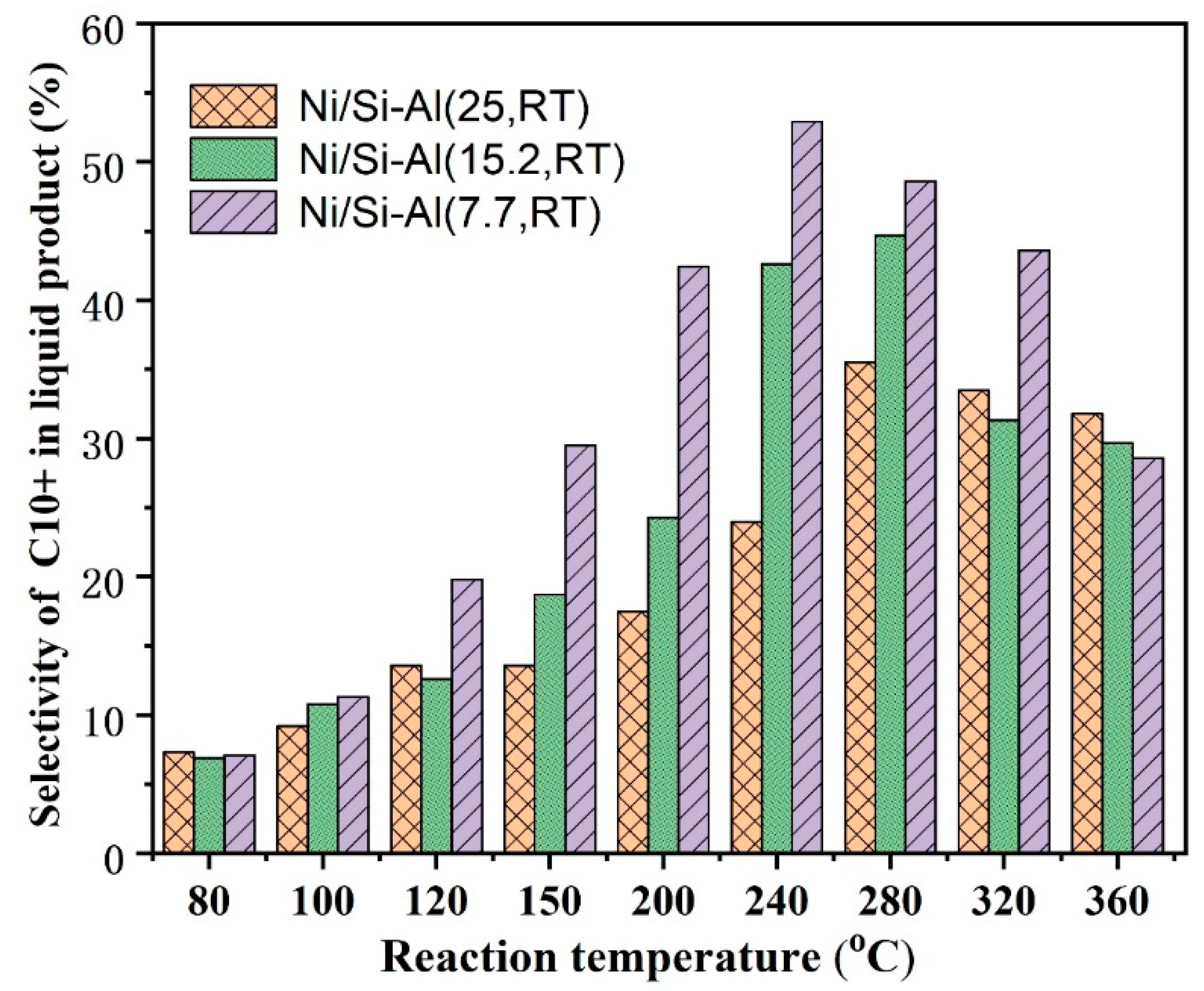
2.2.5. The Effect of the Ratio of Si/Al
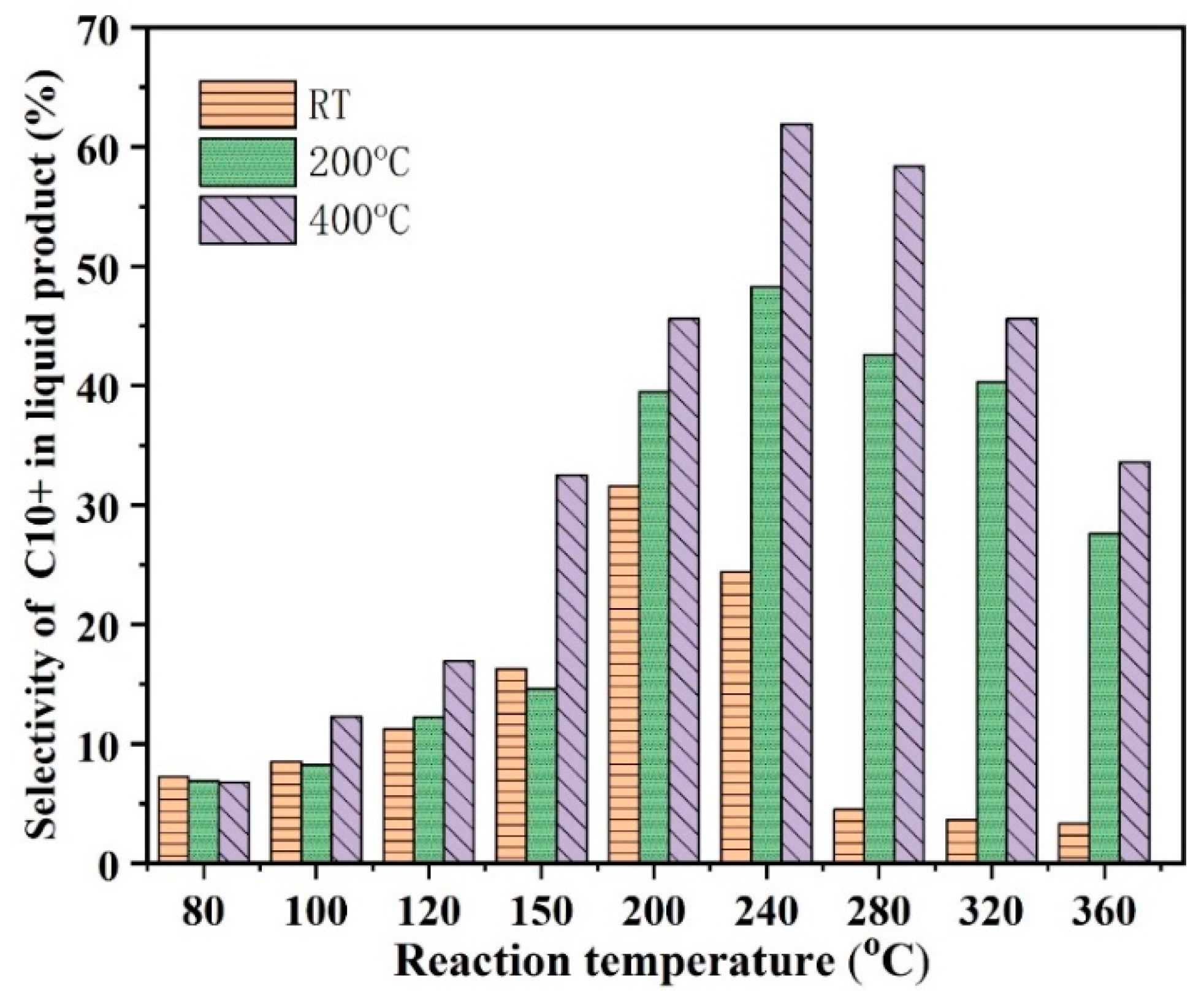
2.2.6. The Effect of Activation Temperature
3. Materials and Methods
3.1. Preparation of Silica–Alumina Supports
3.2. Preparation of Nickel Supported Silica–Alumina by Ion-Exchange Method
3.3. Characterizations
3.4. Catalytic Experiment
4. Conclusion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bond, J.Q.; Alonso, D.M.; Wang, D.; West, R.M.; Dumesic, J.A. Integrated Catalytic Conversion of Gamma-Valerolactone to Liquid Alkenes for Transportation Fuels. Science 2010, 327, 1110–1114. [Google Scholar] [CrossRef] [PubMed]
- Shahinuzzaman, M.; Yaakob, Z.; Ahmed, Y. Non-sulphide zeolite catalyst for bio-jet-fuel conversion. Renew. Sustain. Energy Rev. 2017, 77, 1375–1384. [Google Scholar] [CrossRef]
- Jan, O.; Song, K.; Dichiara, A.; Resende, F.L.P. Ethylene Oligomerization over Ni-H beta Heterogeneous Catalysts. Ind. Eng. Chem. Res. 2018, 57, 10241–10250. [Google Scholar] [CrossRef]
- Stöcker, M. Methanol-to-hydrocarbons: Catalytic materials and their behavior. Microporous Mesoporous Mater. 1999, 29, 3–48. [Google Scholar] [CrossRef]
- Haw, J.F.; Song, W.G.; Marcus, D.M.; Nicholas, J.B. The Mechanism of Methanol to Hydrocarbon Catalysis. Acc. Chem. Res. 2003, 36, 317–326. [Google Scholar] [CrossRef]
- Finiels, A.; Fajula, F.; Hulea, V. Nickel-based solid catalysts for ethylene oligomerization—A review. Catal. Sci. Technol. 2014, 4, 2412–2426. [Google Scholar] [CrossRef]
- Al-Jarallah, A.; Anabtawi, J.; Siddiqui, M.; Aitani, A.; Al-Sa’Doun, A. Ethylene dimerization and oligomerization to butene-1 and linear α-olefins. Catal. Today 1992, 14, 1–121. [Google Scholar] [CrossRef]
- Sarkar, A.; Seth, D.; Ng, F.T.T.; Rempel, G.L. Selective Oligomerization of lsobutene on Lewis Acid Catalyst: Kinetic Modeling. Ind. Eng. Chem. Res. 2014, 53, 18982–18992. [Google Scholar] [CrossRef]
- Hartmann, M.; Kevan, L. Transition-metal ions in aluminophosphate and silicoaluminophosphate molecular sieves: Location, interaction with adsorbates and catalytic properties. Chem. Rev. 1999, 99, 635–664. [Google Scholar] [CrossRef]
- Fliedel, C.; Ghisolfi, A.; Braunstein, P. ChemInform Abstract: Functional Short-Bite Ligands: Synthesis, Coordination Chemistry, and Applications of N-Functionalized Bis(diaryl/Dialkylphosphino)amine-type Ligands. Chem. Rev. 2016, 47, 9237–9304. [Google Scholar] [CrossRef]
- McGuinness, D.S. Olefin Oligomerization via Metallacycles: Dimerization, Trimerization, Tetramerization, and Beyond. Chem. Rev. 2011, 111, 2321–2341. [Google Scholar] [CrossRef]
- Van Leeuwen, P.W.; Clément, N.D.; Tschan, M.J.-L. New processes for the selective production of 1-octene. Co-Ord. Chem. Rev. 2011, 255, 1499–1517. [Google Scholar] [CrossRef]
- Ittel, S.D.; Johnson, L.K.; Brookhart, M. Late-Metal Catalysts for Ethylene Homo- and Copolymerization. Chem. Rev. 2000, 100, 1169–1204. [Google Scholar] [CrossRef]
- Hengsawad, T.; Srimingkwanchai, C.; Butnark, S.; Resasco, D.E.; Jongpatiwut, S. Effect of Metal-Acid Balance on Hydroprocessed Renewable Jet Fuel Synthesis from Hydrocracking and Hydroisomerization of Biohydrogenated Diesel over Pt-Supported Catalysts. Ind. Eng. Chem. Res. 2018, 57, 1429–1440. [Google Scholar] [CrossRef]
- Wang, W.-C.; Tao, L. Bio-jet fuel conversion technologies. Renew. Sustain. Energy Rev. 2016, 53, 801–822. [Google Scholar] [CrossRef]
- Liu, G.; Yan, B.; Chen, G. Technical review on jet fuel production. Renew. Sustain. Energy Rev. 2013, 25, 59–70. [Google Scholar] [CrossRef]
- Britovsek, G.J.P.; Malinowski, R.; McGuinness, D.S.; Nobbs, J.D.; Tomov, A.K.; Wadsley, A.W.; Young, C.T. Ethylene Oligomerization beyond Schulz—Flory Distributions. ACS Catal. 2015, 5, 6922–6925. [Google Scholar] [CrossRef]
- Keim, W. Oligomerization of Ethylene to alpha-Olefins: Discovery and Development of the Shell Higher Olefin Process (SHOP). Angew. Chem.-Int. Ed. 2013, 52, 12492–12496. [Google Scholar] [CrossRef]
- Breuil, P.-A.R.; Magna, L.; Olivier-Bourbigou, H. Role of Homogeneous Catalysis in Oligomerization of Olefins: Focus on Selected Examples Based on Group 4 to Group 10 Transition Metal Complexes. Catal. Lett. 2014, 145, 173–192. [Google Scholar] [CrossRef]
- Dagorne, S.; Fliedel, C. Organoaluminum Species in Homogeneous Polymerization Catalysis. Photophys. Organomet. 2012, 41, 125–171. [Google Scholar]
- Zhang, Q.; Kantcheva, M.; Lana, I.G.D. Oligomerization of Ethylene in a Slurry Reactor Using a Nickel/Sulfated Alumina Catalyst. Ind. Eng. Chem. Res. 1997, 36, 3433–3438. [Google Scholar] [CrossRef]
- Lin, S.; Shi, L.; Zhang, H.; Zhang, N.; Yi, X.; Zheng, A.; Li, X. Tuning the pore structure of plug-containing Al-SBA-15 by post-treatment and its selectivity for C16 olefin in ethylene oligomerization. Microporous Mesoporous Mater. 2014, 184, 151–161. [Google Scholar] [CrossRef]
- Andrei, R.D.; Popa, M.I.; Fajula, F.; Hulea, V. Heterogeneous oligomerization of ethylene over highly active and stable Ni-AlSBA-15 mesoporous catalysts. J. Catal. 2015, 323, 76–84. [Google Scholar] [CrossRef]
- Heydenrych, M.D.; Nicolaides, C.P.; Scurrell, M.S. Oligomerization of Ethene in A Slurry Reactor using A Nickel(II)-exchanged Silica-alumina. Catal. J. Catal. 2001, 197, 49–57. [Google Scholar] [CrossRef]
- Hulea, V. Ni-exchanged AlMCM-41? An efficient bifunctional catalyst for ethylene oligomerization. J. Catal. 2004, 225, 213–222. [Google Scholar] [CrossRef]
- Tanaka, M.; Itadani, A.; Kuroda, Y.; Iwamoto, M. Effect of Pore Size and Nickel Content of Ni-MCM-41 on Catalytic Activity for Ethene Dimerization and Local Structures of Nickel Ions. J. Phys. Chem. C 2012, 116, 5664–5672. [Google Scholar] [CrossRef]
- Lallemand, M.; Finiels, A.; Fajula, F.; Hulea, V. Catalytic oligomerization of ethylene over Ni-containing dealuminated Y zeolites. Appl. Catal. A Gen. 2006, 301, 196–201. [Google Scholar] [CrossRef]
- Lallemand, M.; Rusu, O.A.; Dumitriu, E.; Finiels, A.; Fajula, F.; Hulea, V. NiMCM-36 and NiMCM-22 catalysts for the ethylene oligomerization: Effect of zeolite texture and nickel cations/acid sites ratio. Appl. Catal. A Gen. 2008, 338, 37–43. [Google Scholar] [CrossRef]
- Heveling, J.; Van Der Beek, A.; De Pender, M. Oligomerization of ethene over nickel-exchanged zeolite y into a diesel-range product. Appl. Catal. 1988, 42, 325–336. [Google Scholar] [CrossRef]
- Wang, X.; Hu, X.; Song, C.; Lux, K.W.; Namazian, M.; Imam, T. Oligomerization of Biomass-Derived Light Olefins to Liquid Fuel: Effect of Alkali Treatment on the HZSM-5 Catalyst. Ind. Eng. Chem. Res. 2017, 56, 12046–12055. [Google Scholar] [CrossRef]
- Avhad, M.; Marchetti, J.M. A review on recent advancement in catalytic materials for biodiesel production. Renew. Sustain. Energy Rev. 2015, 50, 696–718. [Google Scholar] [CrossRef]
- Martinez, A.; Arribas, M.A.; Concepcion, P.; Moussa, S. New bifunctional Ni—H-Beta catalysts for the heterogeneous oligomerization of ethylene. Appl. Catal. A Gen. 2013, 467, 509–518. [Google Scholar] [CrossRef]
- Ng, F.; Creaser, D. Ethylene dimerization over modified nickel exchanged Y-zeolite. Appl. Catal. A Gen. 1994, 119, 327–339. [Google Scholar] [CrossRef]
- Heveling, J.; Nicolaides, C.; Scurrell, M. Catalysts and conditions for the highly efficient, selective and stable heterogeneous oligomerisation of ethylene. Appl. Catal. A Gen. 1998, 173, 1–9. [Google Scholar] [CrossRef]
- Brunauer, S.; Deming, L.S.; Deming, W.E.; Teller, E. On a Theory of the van der Waals Adsorption of Gases. J. Am. Chem. Soc. 1940, 62, 1723–1732. [Google Scholar] [CrossRef]
- Tanabe, K.; Sumiyoshi, T.; Shibata, K.; Kiyoura, T.; Kitagawa, J. A New Hypothesis Regarding the Surface Acidity of Binary Metal Oxides. Bull. Chem. Soc. Jpn. 1974, 47, 1064–1066. [Google Scholar] [CrossRef]
- Grabowski, W. Quantum chemical study of acid-base properties of metal oxides. I. J. Catal. 1980, 61, 103–108. [Google Scholar] [CrossRef]
- Serrano, D.P.; Aguado, J.; Escola, J.M. Developing Advanced Catalysts for the Conversion of Polyolefinic Waste Plastics into Fuels and Chemicals. ACS Catal. 2012, 2, 1924–1941. [Google Scholar] [CrossRef]
- Klier, K.; Ralek, M. Spectra of Zynthetic Zolites Containing Transition Metal Ions—II. Ni2+ Ions in Type A Linde Molecular Sieves. J. Phys. Chem. Solids 1968, 29, 951–957. [Google Scholar] [CrossRef]
- Cornet, D.; Hemidy, J.F.; Mariette, L. Spectroscopic Classification of Interactions between Metallic Support Ions—Case of the Nio-Al2O3 System. Nouv. J. Chim. New J. Chem. 1984, 8, 159–164. [Google Scholar]
- Garbarino, G.; Campodonico, S.; Perez, A.R.; Carnasciali, M.M.; Riani, P.; Finocchio, E.; Busca, G. Spectroscopic characterization of Ni/Al2O3 catalytic materials for the steam reforming of renewables. Appl. Catal. A Gen. 2013, 452, 163–173. [Google Scholar] [CrossRef]
- Nikolova, D.; Edreva-Kardjieva, R.; Glurginca, M.; Meghea, A.; Vakros, J.; Voyiatzis, G.A.; Kordulis, C. The Effect of Potassium Addition on the State of the Components in the Oxide Precursor of the (Ni)(Mo)/gamma-Al2O3 Water-gas Shift Catalysts: FT-TR, Diffuse Reflectance and Raman Spectroscopic Studies. Vib. Spectrosc. 2007, 44, 343–350. [Google Scholar] [CrossRef]
- Zanjanchi, M.A.; Ebrahimian, A. Studies on the Solid-state on Exchange of Nickel Ions into Zeolites using DRS Technique. J. Mol. Struct. 2004, 693, 211–216. [Google Scholar] [CrossRef]
- Yang, L.; Xia, K.H. D-orbital for Ni2+ in the Ni(II) Semisepulchrates. Solid State Commun. 1994, 90, 737–739. [Google Scholar] [CrossRef]
- Čapek, L.; Vaněk, L.; Smoláková, L.; Bulánek, R.; Adam, J. The Feasibility of Ni-Alumina Catalysts in Oxidative Dehydrogenation of Ethane. Collect. Czechoslov. Chem. Commun. 2008, 73, 1177–1191. [Google Scholar] [CrossRef]
- Chareonpanich, M.; Teabpinyok, N.; Kaewtaweesub, S. Effect of Nickel Particle Size on Dry Reforming Temperature. In Proceedings of the World Congress on Engineering and Computer Science; International Association of Engineers: Hong Kong, China, 2008; pp. 98–102. [Google Scholar]
- Kim, J.-H.; Suh, D.J.; Park, T.-J.; Kim, K.-L. Effect of metal particle size on coking during CO2 reforming of CH4 over Ni–alumina aerogel catalysts. Appl. Catal. A Gen. 2000, 197, 191–200. [Google Scholar] [CrossRef]

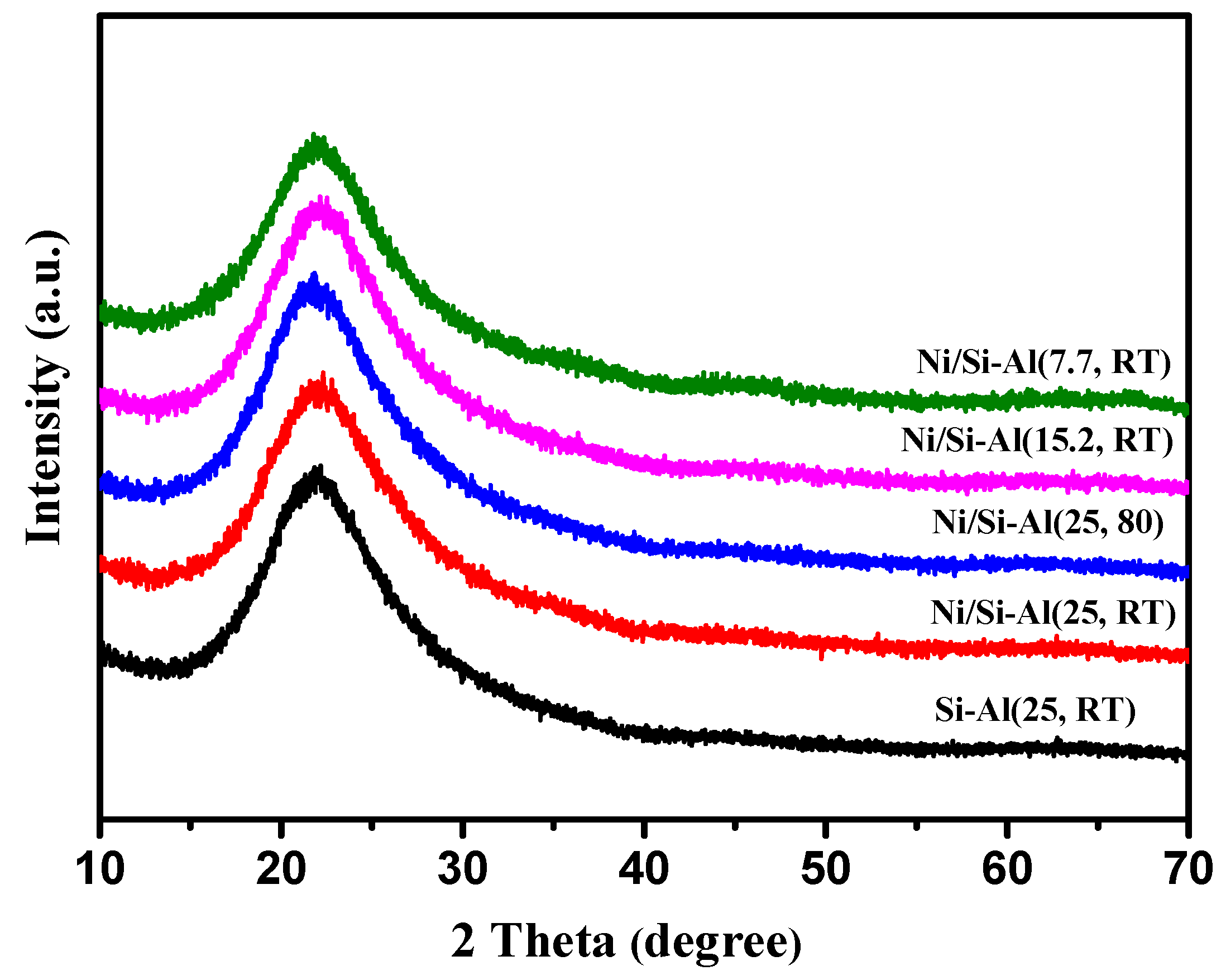
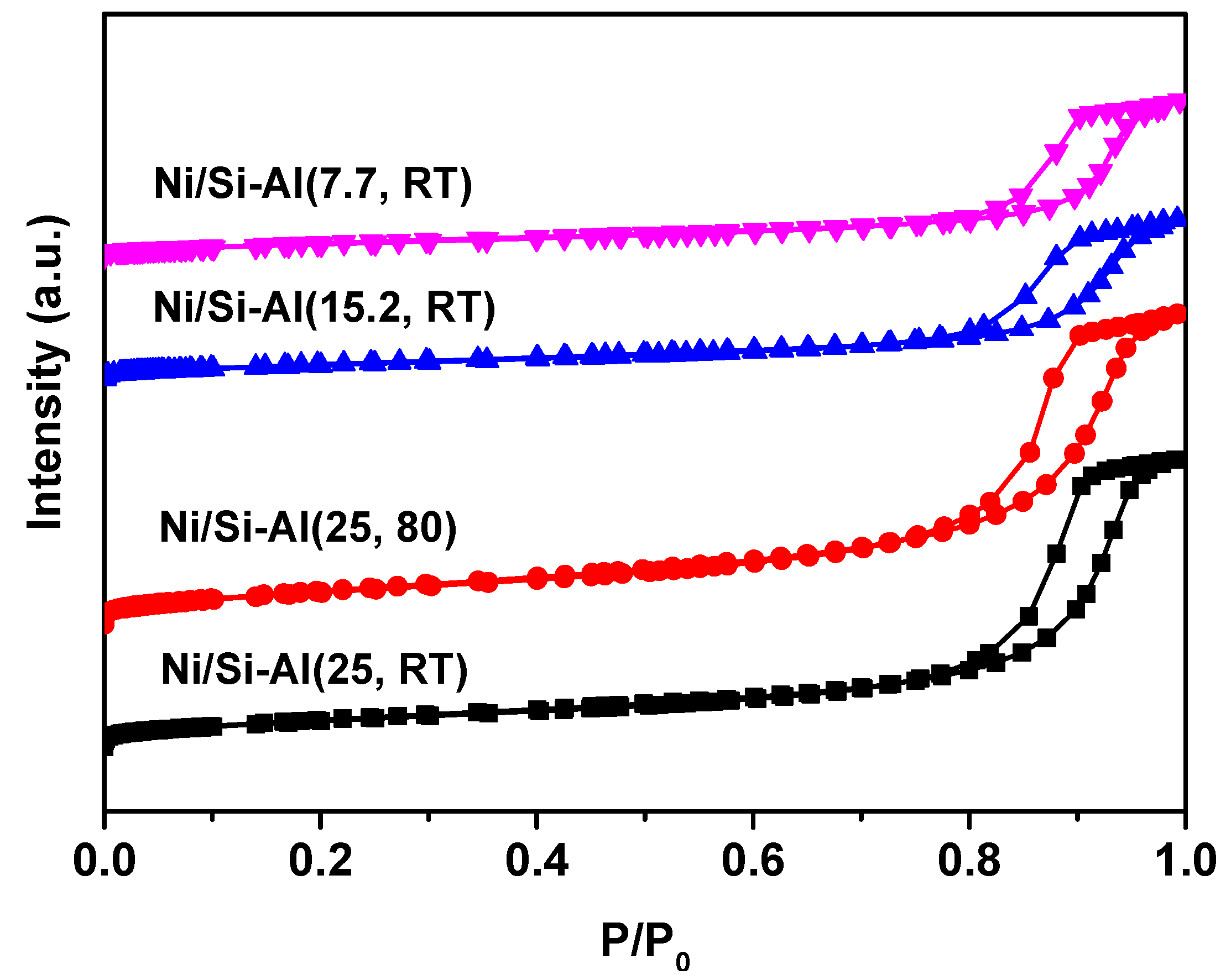
| Catalysts | Ni (%) a | Surface Area (m2/g) b | Vmeso (m3/g) b | Acid Amount (mmol/g) c |
|---|---|---|---|---|
| Ni/Si-Al (25, RT) | 1.36 | 180 | 0.71 | 0.5 |
| Ni/Si-Al (25, 80) | 1.34 | 219 | 0.79 | 0.49 |
| Ni/Si-Al (15.2, RT) | 1.45 | 107 | 0.41 | 0.41 |
| Ni/Si-Al (7.7, RT) | 1.94 | 97 | 0.40 | 0.37 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Li, G.; Wang, Z.; Li, S.; Zhang, M.; Li, X. Ethylene Oligomerization over Nickel Supported Silica-Alumina Catalysts with High Selectivity for C10+ Products. Catalysts 2020, 10, 180. https://doi.org/10.3390/catal10020180
Chen L, Li G, Wang Z, Li S, Zhang M, Li X. Ethylene Oligomerization over Nickel Supported Silica-Alumina Catalysts with High Selectivity for C10+ Products. Catalysts. 2020; 10(2):180. https://doi.org/10.3390/catal10020180
Chicago/Turabian StyleChen, Lei, Guangci Li, Zhong Wang, Shuangju Li, Mingjie Zhang, and Xuebing Li. 2020. "Ethylene Oligomerization over Nickel Supported Silica-Alumina Catalysts with High Selectivity for C10+ Products" Catalysts 10, no. 2: 180. https://doi.org/10.3390/catal10020180
APA StyleChen, L., Li, G., Wang, Z., Li, S., Zhang, M., & Li, X. (2020). Ethylene Oligomerization over Nickel Supported Silica-Alumina Catalysts with High Selectivity for C10+ Products. Catalysts, 10(2), 180. https://doi.org/10.3390/catal10020180