Abstract
Horseradish peroxidase (HRP) enzyme was effectively encapsulated onto an Fe3O4 nanoparticle–polymethyl methacrylate (PMMA) film via the casting method. The HRP was immobilized on the 0.5% Fe3O4Np–PMMA film and characterized by Fourier transform infrared spectroscopy and field emission scanning electron microscopy. Moreover, the reusability, thermal stability, optimum pH, optimum temperature, the influence of metal ions, and the effects of detergent and organic solvent were investigated. After optimizing the immobilization conditions, the highest efficiency of the immobilized enzyme was 88.4% using 0.5% Fe3O4Np–PMMA. The reusability of the immobilized HRP activity was 78.5% of its initial activity after being repeatedly used for 10 cycles. When comparing the free and immobilized forms of the HRP enzyme, changes in the optimum temperature and optimum pH from 30 to 40 °C and 7.0 to 7.5, respectively, were observed. The Km and Vmax for the immobilized HRP were estimated to be 41 mM, 0.89 U/mL for guaiacol and 5.84 mM, 0.66 U/mL for H2O2, respectively. The high stability of the immobilized HRP enzyme was obtained using metal ions, a high urea concentration, isopropanol, and Triton X-100. In conclusion, the applicability of immobilized HRP involves the removal of phenol in the presence of hydrogen peroxide, therefore, it could be a potential catalyst for the removal of wastewater aromatic pollutants.
1. Introduction
Enzymes are macromolecules that catalyze the most complex chemical reactions under particular and defined conditions. However, enzymes also have limitations regarding their non-biological applications such as their industrial applications. Therefore, enzymes for some industrial processes must be immobilized. A technique which has different roles in soluble enzymes. The immobilization of an enzyme is an important technique to minimize the inhibition caused by reaction materials, reaction products, solvents, inhibitors, or any environmental conditions that affect the soluble enzyme. Moreover, enzyme immobilization techniques result in excellent enzyme properties such as high functional and uptake stability, high selectivity, enhanced sensitivity, better short-term response, and extraordinary reproducibility [1,2].
Peroxidases able to oxidize a range of organic compounds via a single oxidation step being initiated by a single electron [3]; they are ubiquitously present in plants, fungi, and vertebrates [4]. Peroxidases oxidize phenols and aromatic amines and convert them to less water-soluble compounds. This ability has potential applications in separating cancer-causing phenols and aromatic amines from industrial waste. Peroxidases may be employed in the bioremediation of xenobiotics (including polycyclic aromatic hydrocarbons and dioxins) and aromatic compounds [5,6,7]. The applicability of peroxidases includes their antioxidant properties [8], as indicators in the food industry [9,10], in the synthesis of conducting materials [11], and as bio-electrodes [12]. Factors that limit the use of the peroxidases as biocatalysts during industrial processes include their weak stability during industrial usage and their inhibitory effects in the presence of high hydrogen peroxide concentrations [13]. Overcoming these limitations promotes the uses of such enzymes in industrial processes. Despite peroxidase being a ubiquitous enzyme, its role as a biocatalyst in industrial and commercial fields is limited due to its instability under operative conditions and inhibition in the presence of high concentrations of the reaction substrate [13]. To be effective in commercial applications, peroxidase development is important. Magnetic nanomaterials have gained much attention regarding the immobilization of proteins/enzymes due to their specific properties, including a high surface area to volume ratio, as well as possessing super-magnetic properties, and their easy separation capabilities under external magnetic fields [14,15]. Fe3O4 is considered to be the most commonly used nanoparticle due to its minimum toxicity and high biocompatibility [16,17]. This study aims to invent a new, cost-effective method for HRP enzyme immobilization by encapsulating horseradish peroxidase (HRP) on an iron magnetic nanoparticle–polymethyl methacrylate (Fe3O4Np–PMMA) film using the casting method to describe the physical characterization and biochemical properties of free and immobilized HRP in order to improve its optimal conditions and retain its activity.
2. Results and Discussion
Nanoparticles provide important remedies for contradictory issues, such as the highest surface area per unit of weight, high enzyme loading, and minimal diffusion limitations, which are usually encountered during enzyme adsorption optimization. Besides the enhanced performance characteristics, the important behaviors of the nanoparticles also highlighted the interesting transition region between homogeneous and heterogeneous catalysis [18]. Magnetic nanomaterials were used in protein immobilization in various capacities [16,19]; for example, non-modified magnetic Fe3O4 nanoparticles were physically immobilized on peroxidase, preserving 55% of original activity after being used 10 times [20]. Figure 1 shows a schematic illustration of HRP encapsulation onto a magnetic Fe3O4Np–PMMA film via casting. Figure 2a shows the effect of nanoparticle concentration on the efficiency of the immobilization. The highest immobilization efficiency (88%) was detected with the 0.5% magnetic Fe3O4Np film. Immobilization efficiency was improved by increasing the proportion of Fe3O4Np to around 0.5% and then decreasing. Jun et al. [21] and Mohamed et al. [22] reported that the peroxidase was immobilized on modified multi-walled carbon nanotubes and amidoximated acrylic polymer with an immobilization efficiency of 81.74% and 70%, respectively. The loss of immobilized-HRP activity when the magnetic Fe3O4Np concentration was increased was attributed to the multiple attachments points of the enzyme to the polymer film [23]. An important benefit of immobilization is that enzyme are easy to reuse. Immobilized HRP enzyme was reused 10 times with the residual activity reducing with repeated use. As Figure 2b shows, the immobilized enzyme on the 0.5% Fe3O4Np–PMMA film retained 78.5% of its original activity after 10 uses, while the immobilized enzymes on the 1% and 2% Fe3O4Np–PMMA films retained 31% and 14% of the initial activity, respectively, after being reused 10 times. Moreover, immobilized HRP on free PMMA was completely inhibited after 10 cycles. This decreased activity was caused by the inactivation of the enzyme, which was induced by protein denaturation and high substrate concentrations [24]. Zhang and Cat [25] and Yu et al. [26] demonstrated that the HRP immobilization on Fe3O4/nanotubes composites and amino-functionalized bacterial cellulose retained 65% and 70% of the original activity after 6 and 10 times, respectively.
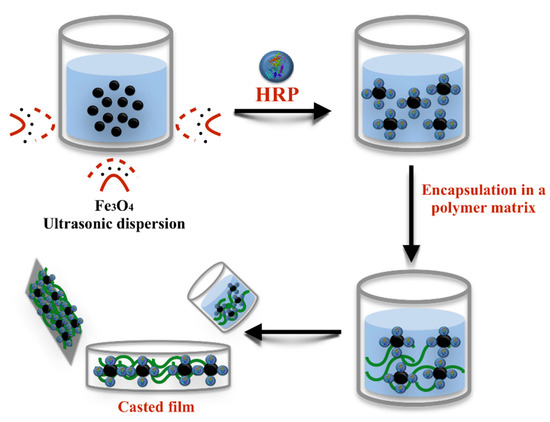
Figure 1.
Schematic illustration of horseradish peroxidase (HRP) encapsulation in to a magnetic Fe3O4 nanoparticle–polymethyl methacrylate (PMMA) (Fe3O4–PMMA) film via the casting method.
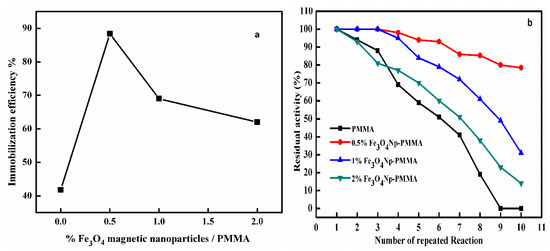
Figure 2.
Effect of Fe3O4 magnetic nanoparticle concentration (a) and number of times (b) the HRP enzyme immobilized on Fe3O4 Np was reused.
As shown in Figure 3 (full-scale A) and Figure 3B,C (expanded regions), the attenuated total reflection ATR-FTIR was measured for the pure (Fe3O4Np, PMMA) and mixed samples (Fe3O4Np–PMMA and HRP–Fe3O4Np–PMMA) to determine whether the IR data of the pure components changed after casting. The characteristic bands of Fe3O4 appeared at 557 cm−1 due to the Fe–O stretching vibration and at 3393 cm-1 due to the O–H stretching vibration of the absorbed water. On the other hand, the characteristic bands of PMMA appeared at 2994, 2950, and 2841 cm-1 due to the stretching vibrations of C–H present in CH3 and CH2, respectively. A strong band due to the carbonyl ester of the PMMA appeared at 1724 cm−1. Other characteristic bands of PMMA appeared with high intensity in the range of 1145–1240 cm−1 are due to the C–O–C stretching vibration. Other typical PMMA peaks appeared at 1435, 1058, 986, 841, and 771 cm−1. Upon mixing Fe3O4 with PMMA using a solvent casting method as presented in the experimental section, the fact that some bands could not be observed due to the Fe3O4 components may have been due to its involvement in functional group interactions and/or the low concentration. However, the bands due to the PMMA in the composites appeared with high intensity but with a slight shift compared with the non-mixed PMMA sample. Furthermore, mixing HRP with Fe3O4Np-PMMA nanocomposites resulted in a lower peak intensity with a slight shift of the bands, indicating the immobilization of HRP on the Fe3O4Np-PMMA film.
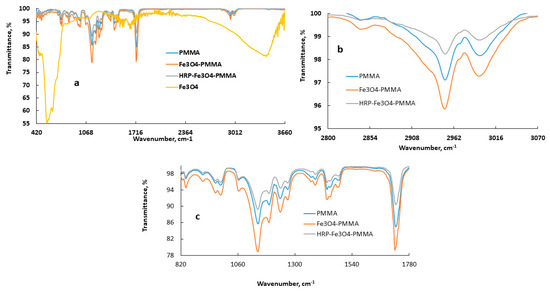
Figure 3.
ATR-FTIR spectra of iron magnetic nanoparticles (Fe3O4), polymethyl methacrylate (PMMA), iron magnetic nanoparticles with polymethyl methacrylate (Fe3O4-PMMA) and horseradish peroxidase encapsulated onto iron magnetic nanoparticles with polymethyl methacrylate (HRP-Fe3O4Np-PMMA) samples (a) (full scale), and ATR-FTIR of expanded regions for PMMA, Fe3O4-PMMA and HRP-Fe3O4-PMMA (b,c).
The SEM morphological micrographs samples of Fe3O4Np, Fe3O4Np embedded into a PMMA film, and HRP encapsulated in an Fe3O4Np–PMMA film are shown in Figure 4. Figure 4a shows that the pristine Fe3O4 nanoparticles were polydispersed and significantly aggregated. Figure 4b shows that the Fe3O4Nps in the PMMA matrix were fairly well dispersed, indicated by the white dots. This sheet contained very rough Fe3O4Np surfaces and a strong interface with the polymer. The sonication and fabrication processes before casting triggered this distribution. Figure 4c displays various magnification images of the casted film after HRP immobilization. The film showed a very rugged, fractured surface, which may have been due to the Fe3O4Np enzyme encapsulation. The Energy-dispersive X-ray (EDX) patterns of the Fe3O4Np/PMMA–HRP are shown in Figure 4d. Fe3O4 encapsulations within the polymer film and restricted X-ray beam penetration depth were due to the poor iron signal.
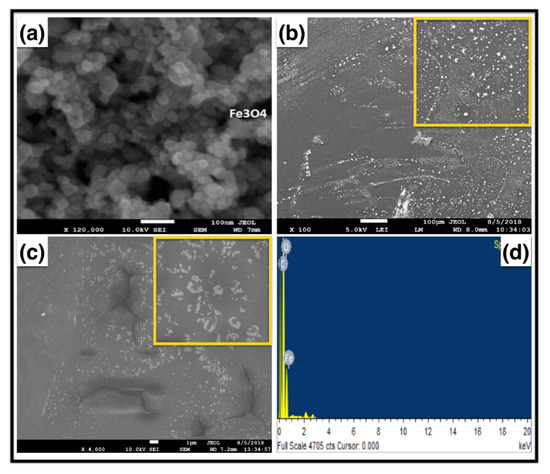
Figure 4.
FESEM images of (a) Fe3O4 Np, (b) the Fe3O4Np–PMMA film, (c) the HRP–Fe3O4 Np–PMMA film, and (d) the SEM–Energy-dispersive X-ray (EDX) spectra of the HRP–Fe3O4 Np–PMMA film.
Figure 5 presents the storage stability of the HRP immobilized on the 0.5% Fe3O4Np–PMMA film, since this sample exhibited the best immobilization efficiency. The immobilized enzyme was kept in refrigerator at 4 °C for 3 months. As shown in the results, the enzyme retained about 59% of its activity after 2 months of storage, decreasing to 28.8% after 3 months of storage, while the activity of the free enzyme decreased to 7.3% after 2 months of storage and was inhibited completely after 75 days of storage. Darwesh et al. [27] reported that the peroxidase immobilization on magnetic nanoparticles retained 40% of the initial activity after stored for 90 days at 4 °C, while it lost all activity after 120 days at the same temperature. Relative to the free form, the immobilized HRP on the 0.5% Fe3O4Np–PMMA film was more efficient regarding the oxidation of o-dianisidine, guaiacol, o-phenylenediamine, p-aminoantipyrine, pyrogallol, and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) (Table 1), therefore, enhanced oxidation was observed in immobilized HRP. The order of the oxidation activity was as follows: o-phenylenediamine > o-dianisidine > p-aminoantipyrine > pyrogallol > ABTS. These results indicated that the immobilization of the enzyme did not affect the substrate’s binding site in the enzyme. Similar findings were reported by Mohamed et al. [20].
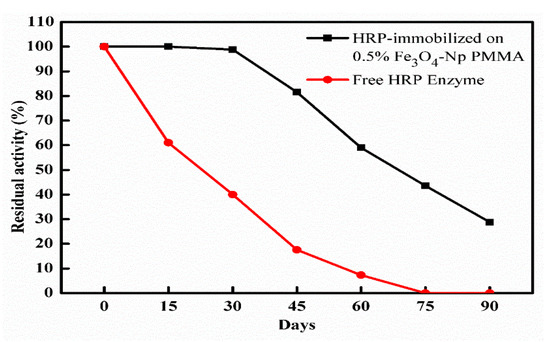
Figure 5.
Storage stability of free and immobilized HRP at 4 °C.

Table 1.
Substrate specificity of free and immobilized HRP on a 0.5% Fe3O4Np–PMMA film. The level of activity with the guaiacol was regarded as 100% activity.
Using different concentrations of H2O2 (1.6–9.7mM) and guaiacol (20–58.8 mM) as the substrates, the effect of substrates concentration was studied in terms of the rate of catalysis by the immobilized and free enzyme forms. A Lineweaver–Burk plot for the immobilized and free enzyme forms was calculated using the maximum reaction velocity (Vmax) and the Michaelis constant (Km). Figure 6 and Table 2 show the apparent Vmax and Km values for the immobilized HRP after encapsulation into the Fe3O4Np–PMMA film and the free enzyme. Relative to free HRP, the Km value of the immobilized HRP increased about 1.32-fold and 1.37-fold for H2O2 and guaiacol, respectively. A higher Km under post-immobilization indicated a lower affinity of the immobilized HRP in comparison to the free enzyme toward the substrate. This difference in the Km value may have been due to loss of enzyme flexibility, which is crucial for substrate binding, due to steric hindrance of the active site and/or by diffusional resistance during substrate transport [28].
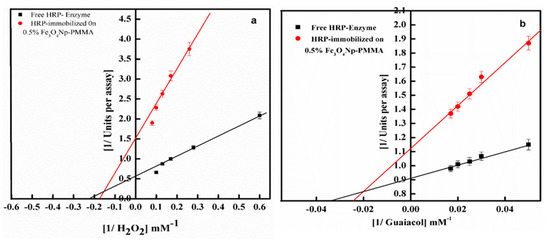
Figure 6.
Lineweaver–Burk plot showing the reaction velocity of free HRP and HRP immobilized on 0.5% Fe3O4Np–PMMA with varying H2O2 (a) and guaiacol (b) concentrations.

Table 2.
Kinetic parameters of HRP immobilized on a 0.5% Fe3O4Np–PMMA film and free HRP.
In addition to this, a significant decrease in Vmax value was observed for the immobilized enzyme relative to the free enzyme. The Km/Vmax of the immobilized and free HRP forms were 46 and 27.27 for guaiacol, respectively, and 8.84 and 2.5 for H2O2, respectively (Table 2). Such changes in the Km and Vmax values of the immobilized enzymes were due to the noncovalent interaction of the immobilized enzyme with the changed surface of the polymer, which led to an inactive conformation of the enzyme [29,30,31].
The optimal pH values of the immobilized and free HRP forms in the pH range of 4.5–9.0 were evaluated (Figure 7a). The results indicated that the optimum pH value of the immobilized HRP on 0.5% the Fe3O4Np–PMMA film shifted to 7.5. Similar results were reported for immobilized HRP on non-modified Fe3O4 magnetic nanoparticles [20]. Contrarily, HRP immobilized on activated wool showed a broad optimum pH of 7.0–8.0 [32]. The pH moved toward an alkaline value after immobilization, which may have been due to secondary interactions between the enzymes and polymeric matrices [33,34]. Figure 7b shows the effect of changes in temperature on the activity levels of the immobilized and free HRP forms. The optimum temperature of the free enzyme was 30 °C, which shifted to 40 °C for the immobilized enzyme; significantly higher enzyme activity was preserved for the immobilized enzyme at all temperatures between 25 and 80 °C. Similar results were reported for soluble and immobilized peroxidase [32]. The increase in optimal temperature showed that immobilized HRP was resistant to a rise in temperature. The modified physicochemical properties of the enzyme induced the change in the optimum temperature. The thermal stability of the free HRP was 40 °C but, after immobilization, a difference in thermal stability was observed; the immobilized HRP was thermally stable up to 50 °C and retained 62% of its activity at 80 °C, while the free HRP enzyme retained only 25% of its activity at the same temperature (Figure 7c). Similar results were reported for non-modified iron–HRP (50 °C) [20], activated wool–horseradish peroxidase (40 °C) [32], and modified chitosan–HRP (45 °C) [35]. The loss in activity (>80 °C) was probably due to the denaturation of some enzyme components, the degradation of a polymer matrix, and enzyme leaching from the swollen polymer matrix [36]. The thermal stability of the enzymes was found to dramatically increase when bound to the complementary surface of a fairly stable structure in a multipoint manner [37].
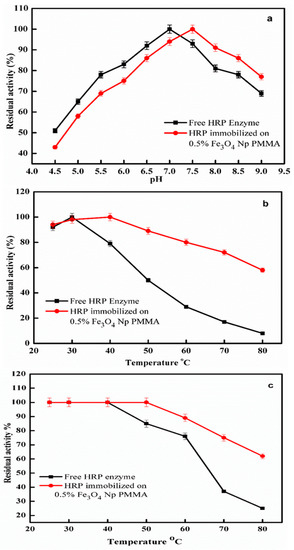
Figure 7.
Optimum pH (a), optimum temperature (b), and thermal stability (c) for free and immobilized–HRP enzymes. Each point represents the mean of three experiments ± S.E.
The influence of metals on the free and immobilized–HRP on a 0.5% Fe3O4Np–PMMA film was evaluated (Table 3). Fe3+ demonstrated high activation in the immobilized HRP relative to the free HRP. Cu2+ also showed similarly high activation effects for the immobilized HRP but had a small inhibitory effect on the free HRP. All of the metal ions studied displayed a moderate inhibitive effect on immobilized HRP compared to free HRP, with the exceptions of Hg2+, Cd2+, and Pb2+, which showed significant inhibitory effects on free HRP. Zn2+ had a strong inhibitory effect on immobilized HRP, but overall inhibited free HRP activity. Therefore, HRP immobilized on an Fe3O4Np–PMMA film was shown to be more resistant than the free form to 5 mM metal ions. The stability of the HRP immobilized on an Fe3O4Np–PMMA film against many metal ions demonstrated that such an enzyme could be used even in the presence of heavy metals to treat aromatic pollutants; similar results were reported by Mohamed et al. and Lobarzewski et al. [20,38].

Table 3.
Effect of metal ions on Free HRP and HRP immobilized on a 0.5% Fe3O4Np–PMMA film.
The effects of some organic materials on immobilized HRP are presented in Table 4. Numerous findings demonstrated that the protein unfolded through a direct interaction between the urea molecule and the peptide backbone via non-covalent interactions involved in the maintenance of the protein’s conformational structure [39,40]. The effect of increasing the urea concentration on free and immobilized HRP activity was investigated. HRP immobilized on the Fe3O4Np–PMMA film showed high resistance toward urea induced inactivation relative to the free form. The immobilized HRP maintained 89% and 58% of its activity at 2.0 and 4.0 M urea, respectively, while the exposure of free HRP enzyme to 2.0 and 4.0 M urea led to a loss in activity of around 36% and 72%, respectively. These findings were in line with the resistance of non-modified Fe3O4 magnetic nanoparticle-HRP [20] and chitosan–HRP [41] against 4.0 M urea. The activity of the immobilized HRP showed high stability when exposed to Triton X-100 compared to the free HRP. Several studies reported that the immobilized peroxidase was significantly stable against denaturation induced by common detergents [20,41,42,43]. The activity levels of free and immobilized HRP were evaluated in the presence of isopropanol, which induced minor inhibition on the activity of immobilized HRP in comparison with free HRP. Similar results were reported by [20,41,42,43].

Table 4.
Effects of chemical compounds on free HRP and HRP-immobilized on a 0.5% Fe3O4Np–PMMA film.
Enzyme re-usage and the effects of some compounds on the immobilized HRP by encapsulation onto an Fe3O4Np–PMMA film were compared with HRP–immobilized on non-modified Fe3O4Np, which was recently published [20]. Table 5 shows that HRP immobilized by encapsulation onto an Fe3O4Np–PMMA film was able to be reused 10 times, in comparison to 5 times for HRP immobilized on non-modified Fe3O4Np. The two kinds of immobilized enzymes showed high resistance against metals. HRP–immobilized on the Fe3O4Np–PMMA film exhibited higher affinity for the substrate compared with HRP–immobilized on non-modified Fe3O4Np. HRP immobilized on Fe3O4Np–PMMA film showed more resistance to Triton X-100, urea and isopropanol compared with HRP–immobilized on non-modified Fe3O4Np. Phenolic compounds are considered to be a major pollutant in industrial wastewaters, therefore, immobilized and free–HRP were used as catalysts to activate H2O2 to degrade phenol. As shown in Figure 8, after 50 min of incubation, the immobilized–HRP showed higher efficiency regarding the removal of phenol than the free HRP. Similar findings were reported in previous works [22,44].

Table 5.
The number of times the enzyme was reused and the effect of some compounds on HRP–immobilized on 0.5% Fe3O4Np–MMA film and non-modified Fe3O4 magnetic nanoparticles [20].
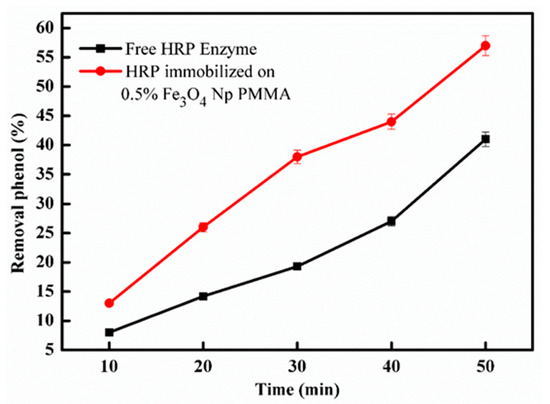
Figure 8.
Effect of incubation time on phenol removal for free and immobilized–HRP. Each point represents the mean of three experiments ± S.E.
3. Experimental Section
3.1. Materials
In this study, we followed the procedure given by Mohamed [45], where horseradish peroxidase (HRP) was purified from horseradish cv. Balady. Materials include dichloromethane (DCM), (puriss. p.a., ACS reagent, reag. ISO, ≥99.9%), polymethyl methacrylate (PMMA) average Mw-966,000) and magnetic Fe3O4–nanopowder (99.99% trace-metal basis) were purchased from Sigma-Aldrich.
3.2. Peroxidase Assay
To evaluate the peroxidase activity, we choose Yuan and Jiang’s method [46]. A total of 1 mL of reaction mixture contained 40 mM guaiacol, 50 mM sodium acetate buffer (pH 5.5), and 8 mM H2O2, in the lowest enzyme preparation quantity. After every 30 s, the absorption disparity due to the oxidation of guaiacol was observed. The operation per one unit of peroxidase was specified as the quantity of enzyme that increased the optical density (O.D) to 1.0 per min under standard assay conditions.
3.3. Procedure for Immobilization
The immobilization supporting materials were prepared by dissolving PMMA in an organic solvent, Dichloromethane (DCM) was doped with different concentrations of the magnetic Fe3O4 nanomaterial. A total of 2 mg of HRP enzyme (50 units, representing 541.8 units/mg of proteins) was mixed with the fabricated supporting matrix at room temperature. The solution was cast in a glass Petri dish and left to dry overnight at ambient temperature to evaporate the solvent. Finally, the film was carefully washed with distilled water to remove all the protein.
To calculate the percentage of relative enzyme activity, the following formula was used:
Relative enzyme activity (%) = activity of immobilized enzyme/initial activity of enzyme × 100%
3.4. Characterization
The morphological characteristics of the supporting material and immobilized enzymes were examined using a field emission scanning electron microscope (FESEM), (JEOL 6700, Japan) at an accelerating voltage of 5 kV. Fourier-transform infrared spectroscopy (FTIR), (Thermo Scientific) was used to probe the chemical compositions of the fabricated samples.
3.5. Reusability of Immobilized Enzymes
The procedure for the reusability of enzyme immobilization was performed by repeating the process for at least 10 cycles. The initial activity of the enzyme was considered to be the control, i.e., 100%, to calculate the remaining enzyme activity
3.6. Physicochemical Characterization of the Enzyme
3.6.1. Optimal Temperature and pH
The optimal pH for the activity of both forms of enzymes was estimated by assaying the enzymatic activity at different pH values (4.5–9). The enzymes were assayed at temperatures from 25 to 80 °C with 100% being the maximum activity used as a reference for the immobilized and free HRP.
3.6.2. Thermal Stability
Free and immobilized HRP enzymes were incubated at different temperatures in the range of 258–0 °C for 15 min in 5 mM sodium acetate buffer at a pH of 6.5 to examine the thermal stability. Then, both forms of the enzyme were cooled for 5 min. H2O2 and guaiacol were added to estimate the enzyme activity, where 100% was considered to be the highest level of activity.
3.6.3. Kinetic Constant
The kinetic parameters of the free and immobilized HRP enzymes were determined using different concentrations of H2O2 and guaiacol as substrates. The values of the maximum velocity (Vmax) and Michael’s constants (Km) were estimated from Lineweaver–Burk plots.
3.6.4. Substrate specificity
Substrate specificity was determined by incubating the immobilized and free HRP enzymes with different substrates.
3.6.5. Effects of Metal Ions
The effects of different metal ions on the activity of both forms of the peroxidase enzyme were estimated. Enzymes were pre-incubated in 5 mM of various metal ions for 15 min before evaluating the activity of enzyme. The enzyme activity without the presence of a metal ion was considered to be 100%.
3.6.6. Effects of Different Organic Compounds
The enzymatic activity levels of the free and immobilized HRP enzymes were measured using Triton X-100, 2-propanol, and urea. The activity of enzymes unexposed to the compounds was considered to be 100%.
3.6.7. Phenol Removal Determination
A colorimetric assay of the concentration of phenol was performed using the reaction between the samples and 4-aminoantipyrine [47,48]. The reaction mixture (1.0 mL) of the phenolic compound assay included a 50 µL solution of phenol (2.0 mM), which was transferred to a test-tube containing immobilized and/or free enzyme, as well as 700 µL Tris–HCl buffer at 20 mM concentration and a pH of 7.0. The reaction was started with 100 µL of H2O2 solution (4.0 mM). After a designated time, the sample was removed to analyze the phenolic content by adding 50 µL of the 4-aminoantipyrine (2.0 mM), and potassium ferricyanide reagent (6.0 mM) to the Tris–HCl buffer solution (20 mM, pH 7.0). At 510 nm, spectrophotometer absorption was performed at room temperature after 15 min of incubation. To determine the percentage of phenol removed, the reaction of the mixture was left for 2 min intervals. Each set of assays included a blank containing a reagent similar to 4-aminoantipyrine, distilled water, and potassium ferricyanide. The non-enzymatic rate reactions were calculated by observing the rate of phenol degradation by adding H2O2 to the corresponding phenolic compound.
4. Conclusions
The factors that influenced the enzymatic properties of HRP included the chemical and physical characteristics of the support, as well as the process of immobilization. In summary, a magnetic Fe3O4Np–PMMA film was prepared via the casting method and the characteristics of immobilized HRP were detected. HRP was immobilized and encapsulated onto a 0.5% Fe3O4Np–PMMA film, showing very good reusability and a high immobilization yield. From these results, it was concluded that the immobilized HRP encapsulated on the magnetic nanoparticle-polymer was more stable during denaturation induced by pH, urea, metal ions, heat, organic solvent, and detergent. Immobilized HRP could be a powerful agent for the elimination of aromatic compounds from wastewater. The immobilized HRP on magnetic Fe3O4Np–PMMA offered promising results regarding phenol removal and amperometric phenol biosensors.
Author Contributions
Conceptualization, Y.Q.A; Formal analysis, W.H.A., Y.Q.A., and R.M.E.-S.; funding acquisition, W.H.A.; Methodology, W.H.A., Y.Q.A., and R.M.E.; Project administration, Y.Q.A. and R.M.E.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, No. (G-46-130-1440).
Acknowledgments
The author, acknowledges and thanks DSR for their technical and financial support.
Conflicts of Interest
The authors declare no conflict of interest
References
- Sassolas, A.; Blum, L.J.; Leca-Bouvier, B.D. Immobilization strategies to develop enzymatic vbiosensors. Biotechnol. Adv. 2012, 30, 489–511. [Google Scholar] [CrossRef]
- Asgher, M.; Shahid, M.; Kamal, S.; Iqbal, H.M.N. Recent trends and valorization of immobilization strategies and ligninolytic enzymes by industrial biotechnology. J. Mol. Cataly. B-Enzym. 2014, 101, 56–66. [Google Scholar] [CrossRef]
- Klebanoff, S.J. Myeloperoxidase-halide-hydrogen peroxide antibacterial system. J. Bacteriol. 1968, 95, 2131–2138. [Google Scholar] [CrossRef] [PubMed]
- Zamocky, M.; Obinger, C. Molecular phylogeny of heme peroxidases. In Biocatalysis Based on Heme Peroxidases; Torres, E., Ayala, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 7–35. [Google Scholar]
- Hammel, K.E.; Kalyanaraman, B.; Kirk, T.K. Oxidation of polycyclic aromatic hydrocarbons and dibenzo [p]-dioxins by Phanerochaete chrysosporium ligninase. J. Biol. Chem. 1986, 261, 16948–16952. [Google Scholar] [PubMed]
- Klibanov, A.M.; Tu, T.M.; Scott, K.P. Peroxidase-catalyzed removal of phenols from coal-conversion waste waters. Science 1983, 221, 259–261. [Google Scholar] [CrossRef] [PubMed]
- Aitken, M.D. Waste treatment applications of enzymes: opportunities and obstacles. Chem. Eng. J. 1993, 52, 49–58. [Google Scholar] [CrossRef]
- Patel, M.; Day, B.J. Metalloporphyrin class of therapeutic catalytic antioxidants. Trends Pharmacol. Sci. 1999, 20, 359–364. [Google Scholar] [CrossRef]
- Ingram, D.T.; Lamichhane, C.M.; Rollins, D.M.; Carr, L.E.; Mallinson, E.T.; Joseph, S.W. Development of a colony lift immunoassay to facilitate rapid detection and quantification of Escherichia coli O157: H7 from agar plates and filter monitor membranes. Clin. Diagn. Lab. Immunol. 1998, 5, 567–573. [Google Scholar] [CrossRef]
- Wang, X.; Wang, G.; Wang, Z.; Wang, Y.; Huang, R. Purification and characterization of peroxidase from zucchini (Cucurbita pepo L.). J. Food Process. Preserv. 2019, 43, e13977. [Google Scholar] [CrossRef]
- Liu, W.; Kumar, J.; Tripathy, S.; Senecal, K.J.; Samuelson, L. Enzymatically synthesized conducting polyaniline. J. Amer. Chem. Soc. 1999, 121, 71–78. [Google Scholar] [CrossRef]
- Gaspar, S.; Popescu, I.C.; Gazaryan, I.G.; Bautista, A.G.; Sakharov, I.Y.; Mattiasson, B.; Csöregi, E. Biosensors based on novel plant peroxidases: A comparative study. Electrochim. Acta 2000, 46, 255–264. [Google Scholar] [CrossRef]
- Valderrama, B.; Ayala, M.; Vazquez-Duhalt, R. Suicide inactivation of peroxidases and the challenge of engineering more robust enzymes. Chem. Biol. 2002, 9, 555–565. [Google Scholar] [CrossRef]
- Xu, P.; Zeng, G.M.; Huang, D.L.; Feng, C.L.; Hu, S.; Zhao, M.H.; Lai, C.; Wei, Z.; Huang, C.; Xie, G.X.; et al. Use of iron oxide nanomaterials in wastewater treatment: A review. Sci. Environ. 2012, 424, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.W.; Zeng, G.M.; Gong, J.L.; Liang, J.; Xu, P.; Zhang, C.; Huang, B.B. Impact of humic/fulvic acid on the removal of heavy metals from aqueous solutions using nanomaterials: A review. Sci. Environ. 2014, 468, 1014–1027. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jia, S.; Wu, Q.; Ran, J.; Zhang, W.; Wu, S. Studies of Fe3O4-chitosan nanoparticles prepared by co-precipitation under the magnetic field for lipase immobilization. Catal. Commun. 2011, 12, 717–720. [Google Scholar] [CrossRef]
- Gao, J.; Gu, H.; Xu, B. Multifunctional magnetic nanoparticles: Design, synthesis, and biomedical applications. Acc. Chem. Res. 2009, 42, 1097–1107. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, N.; Akgöl, S.; Arısoy, M.; Denizli, A. Reversible adsorption of lipase on novel hydrophobic nanospheres. Sep. Purif. Technol. 2007, 58, 83–90. [Google Scholar] [CrossRef]
- Almulaiky, Y.Q.; Al-Harbi, S.A. A novel peroxidase from Arabian balsam (Commiphora gileadensis) stems: Its purification, characterization and immobilization on a carboxymethylcellulose/Fe3O4 magnetic hybrid material. Int. J. Boil. Macromol. 2019, 133, 767–774. [Google Scholar] [CrossRef]
- Mohamed, S.A.; Al-Harbi, M.H.; Almulaiky, Y.Q.; Ibrahim, I.H.; El-Shishtawy, R.M. Immobilization of horseradish peroxidase on Fe3O4 magnetic nanoparticles. Electron. J. Biotechnol. 2017, 27, 84–90. [Google Scholar] [CrossRef]
- Jun, L.Y.; Mubarak, N.M.; Yon, L.S.; Bing, C.H.; Khalid, M.; Jagadish, P.; Abdullah, E.C. Immobilization of peroxidase on functionalized MWCNTs-buckypaper/polyvinyl alcohol nanocomposite membrane. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef]
- Mohamed, S.A.; Al-Ghamdi, S.S.; El-Shishtawy, R.M. Immobilization of horseradish peroxidase on amidoximated acrylic polymer activated by cyanuric chloride. Int. J. Boil. Macromol. 2016, 91, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.A.; Al-Harbi, M.H.; Almulaiky, Y.Q.; Ibrahim, I.H.; Salah, H.A.; El-Badry, M.O.; El-Shishtawy, R.M. Immobilization of Trichoderma harzianum α-amylase on PPyAgNp/Fe3O4-nanocomposite: Chemical and physical properties. Artif. Cell Nanomed. Biotechnol. 2018, 46, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Ye, P.; Xu, Z.K.; Wu, J.; Innocent, C.; Seta, P. Nanofibrous poly (acrylonitrile-co-maleic acid) membranes functionalized with gelatin and chitosan for lipase immobilization. Biomaterials 2006, 27, 4169–4176. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Cai, X. Immobilization of horseradish peroxidase on Fe3O4/nanotubes composites for Biocatalysis-degradation of phenol. Compos. Interfaces 2019, 26, 379–396. [Google Scholar] [CrossRef]
- Yu, B.; Cheng, H.; Zhuang, W.; Zhu, C.; Wu, J.; Niu, H.; Ying, H. Stability and repeatability improvement of horseradish peroxidase by immobilization on amino-functionalized bacterial cellulose. Process Biochem. 2019, 79, 40–48. [Google Scholar] [CrossRef]
- Darwesh, O.M.; Matter, I.A.; Eida, M.F. Development of peroxidase enzyme immobilized magnetic nanoparticles for bioremediation of textile wastewater dye. J. Environ. Chem. Eng. 2019, 7, 102805. [Google Scholar] [CrossRef]
- Sahin, F.; Demirel, G.; Tümtürk, H. A novel matrix for the immobilization of acetylcholinesterase. Int. J. Biol. Macromol. 2005, 37, 148–153. [Google Scholar] [CrossRef]
- Bayramoglu, G.; Akgol, S.; Bulut, A.; Denizli, A.; Arica, M.Y. Covalent immobilization of invertase onto a reactive film composed of 2-hydroxyethylmethacrylate and glycidylmethacrylate: Properties and application in a continuous flow system. Biochem. Eng. J. 2003, 14, 117–126. [Google Scholar] [CrossRef]
- Yahsi, A.; Sahin, F.; Demirel, G.; Tumturk, H. Binary immobilization of tyrosinase by using alginate gel beads and poly(acrylamide-co-acrylic acid) hydrogels. Int. J. Biol. Macromol. 2005, 36, 253–258. [Google Scholar] [CrossRef]
- Kahraman, M.V.; Bayramoglu, G.; Kayaman-Apohan, N.; Gungor, A. UV curable methacrylated/fumaric acid modified epoxy as a potential support for enzyme immobilization. React. Funct. Polym. 2007, 67, 97–103. [Google Scholar] [CrossRef]
- Mohamed, S.A.; Darwish, A.A.; El-Shishtawy, R.M. Immobilization of horseradish peroxidase on activated wool. Process Biochem. 2013, 48, 649–655. [Google Scholar] [CrossRef]
- Arica, M.Y.; Alaeddinoğlu, N.G.; Hasirci, V. Immobilization of glucoamylase onto activated pHEMA/EGDMA microspheres: Properties and application to a packed-bed reactor. Enzyme Microb. Technol. 1998, 22, 152–157. [Google Scholar] [CrossRef]
- Erginer, R.; Toppare, L.; Alkan, S.; Bakir, U. Immobilization of invertase in functionalized copolymermatrices. React. Funct. Polym. 2000, 45, 227–233. [Google Scholar] [CrossRef]
- Monier, M.; Ayad, D.M.; Wei, Y.; Sarhan, A.A. Immobilization of horseradish peroxidase on modified chitosan beads. Int. J. Biol. Macromol. 2010, 46, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Emregul, E.; Sungur, S.; Akbulut, U. Polyacrylamide-gelatine carrier system used for invertase immobilization. Food Chem. 2006, 97, 591–597. [Google Scholar] [CrossRef]
- Martinek, K.; Kilbanov, A.M.; Goldmacher, V.S.; Berezin, I.V. The principles of enzyme stabilization. Biochim. Biophys. Acta. 1977, 485, 1–12. [Google Scholar] [CrossRef]
- Lobarzewsk, J.; Brzyska, M.; Wojcik, A. The influence of metal ions on the soluble and immobilized cytoplasmic cabbage peroxidase activity and its kinetics. J. Mol. Catal. 1990, 59, 373–383. [Google Scholar] [CrossRef]
- Ashraf, H.; Husain, Q. Stabilization of DEAE cellulose adsorbed and glutaraldehyde crosslinked white radish (Raphanus sativus) peroxidase. J. Sci. Ind. Res. 2010, 69, 613–620. [Google Scholar]
- Tanford, C. Theoretical models for the mechanismof denaturation. Adv. Protein Chem. 1970, 24, 1–95. [Google Scholar]
- Mohamed, S.A.; Aly, A.S.; Mohamed, T.M.; Salah, H.A. Immobilization of horseradish peroxidase on non-woven polyester fabric coated with chitosan. Appl. Biochem. Biotechnol. 2008, 144, 169–179. [Google Scholar] [CrossRef]
- Kulshrestha, Y.; Husain, Q. Direct immobilization of peroxidase on DEAE cellulose from ammonium sulphate fractionated proteins of bitter gourd (Momordica charantia). Enzyme Microb. Technol. 2006, 38, 470–477. [Google Scholar] [CrossRef]
- Jamal, F.; Qidwai, T.; Singh, D.; Pandey, P.K. Biocatalytic activity of immobilized poited gourd (Trichosanthes dioica) peroxidase-concanavalin. A complex on calcium alginate pectin gel. J. Mol. Catal. B Enzym. 2012, 74, 125–131. [Google Scholar] [CrossRef]
- Bayramoğlu, G.; Arıca, M.Y. Enzymatic removal of phenol and p-chlorophenol in enzyme reactor: horseradish peroxidase immobilized on magnetic beads. J. Hazard. Mater. 2008, 156, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.A.; Abulnaja, K.O.; Ads, A.S.; Khan, J.A.; Kumosani, T.A. Characterization of an anionic peroxidase from horseradish cv. Balady. Food Chem. 2011, 128, 725–730. [Google Scholar] [CrossRef]
- Yuan, Z.Y.; Jiang, T.J. Horseradish peroxidase. In Handbook of Food Enzymology; Whitaker, J.R., Voragen, A., Wong, D.W.S., Eds.; Marcel Dekker Inc.: New York, NY, USA, 2003; pp. 403–411. [Google Scholar]
- Bhunia, A.; Durant, S.; Wangikar, P.P. Horseradish peroxidase catalyzed degradation of industrially important dyes. Biotechnol. Bioeng. 2001, 72, 562–567. [Google Scholar] [CrossRef]
- Gomez, J.L.; Bodalo, A.; Gomez, E.; Bastida, J.; Hidalgo, A.M.; Gomez, M. Immobilization of peroxidases on glass beads: An improved alternative for phenol removal. Enzyme Microb. Technol. 2006, 39, 1016–1022. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).