2D Mesoporous Channels of PMO; a Platform for Cluster-Like Pt Synthesis and Catalytic Activity in Nitrophenol Reduction
Abstract
1. Introduction
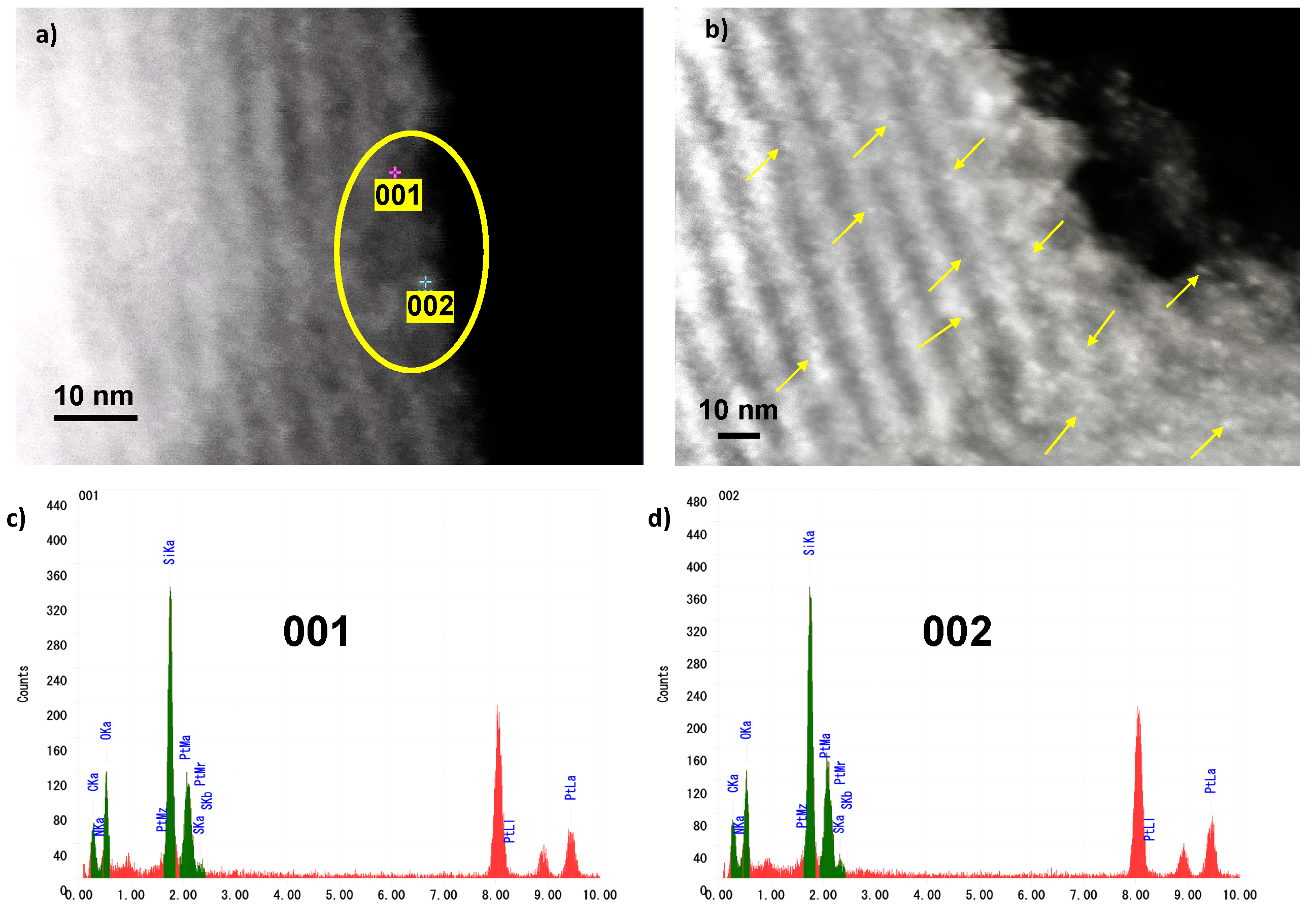
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis of Pt@PMO-TU
3.2. Characterization
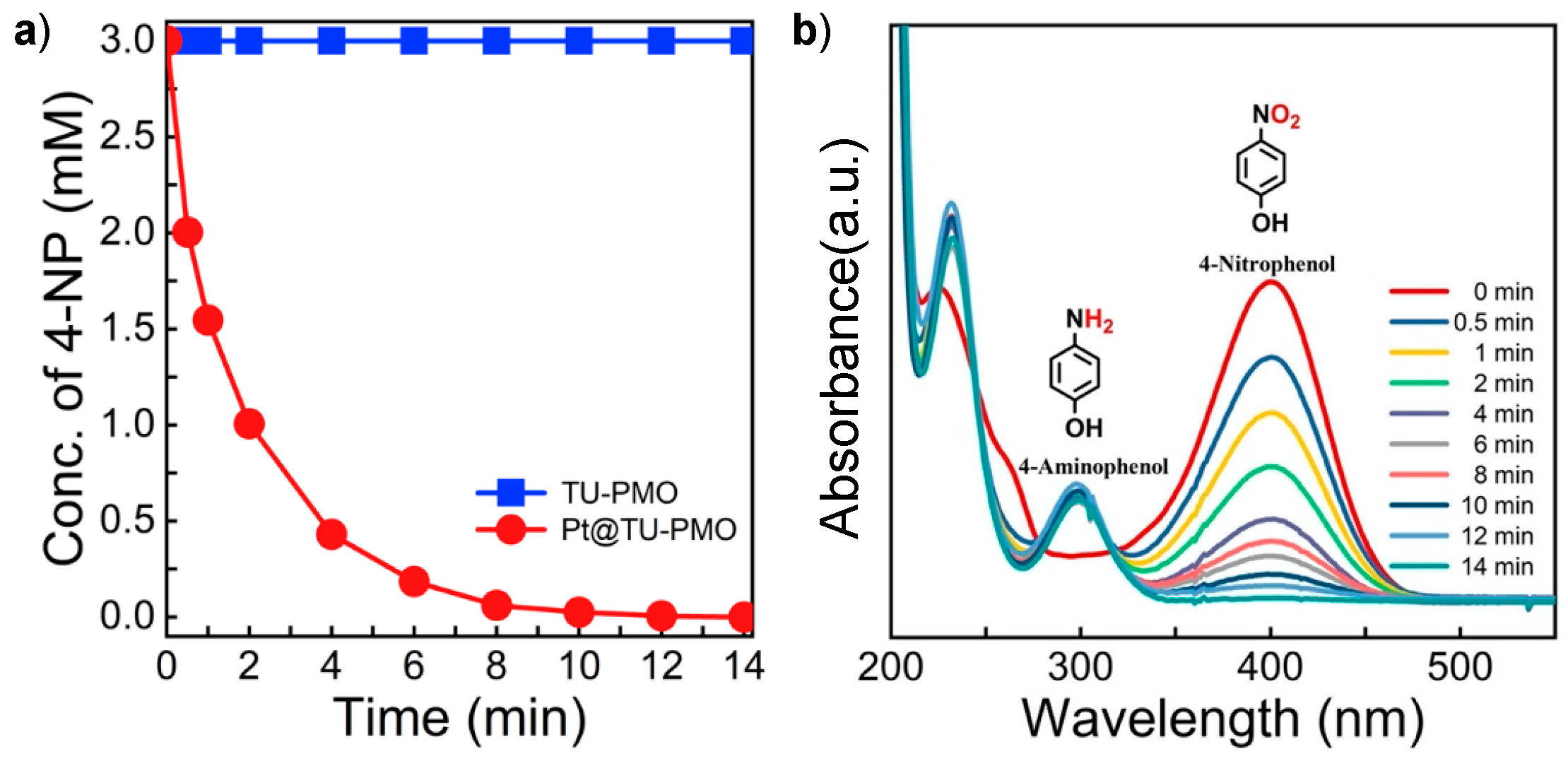
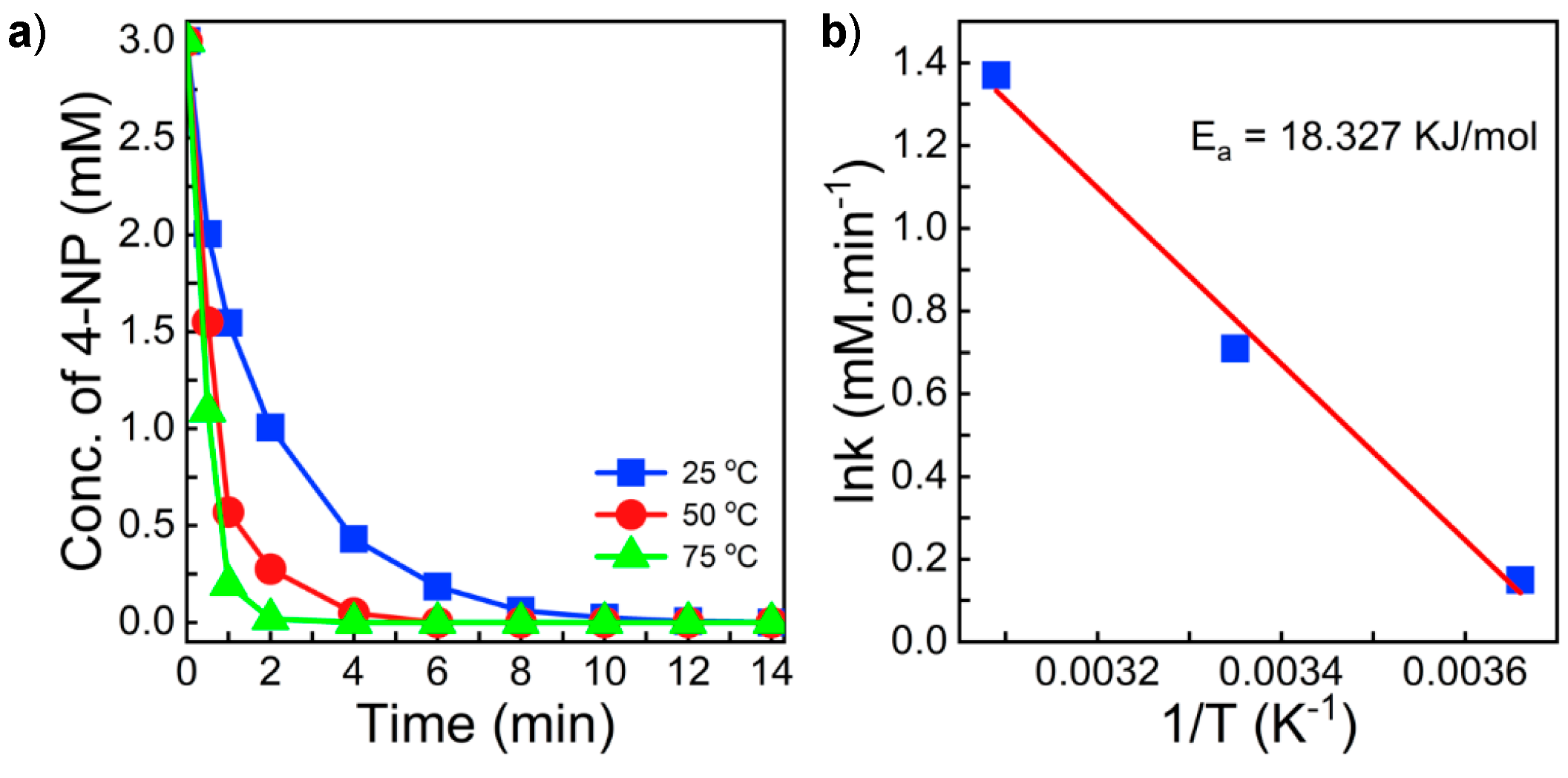
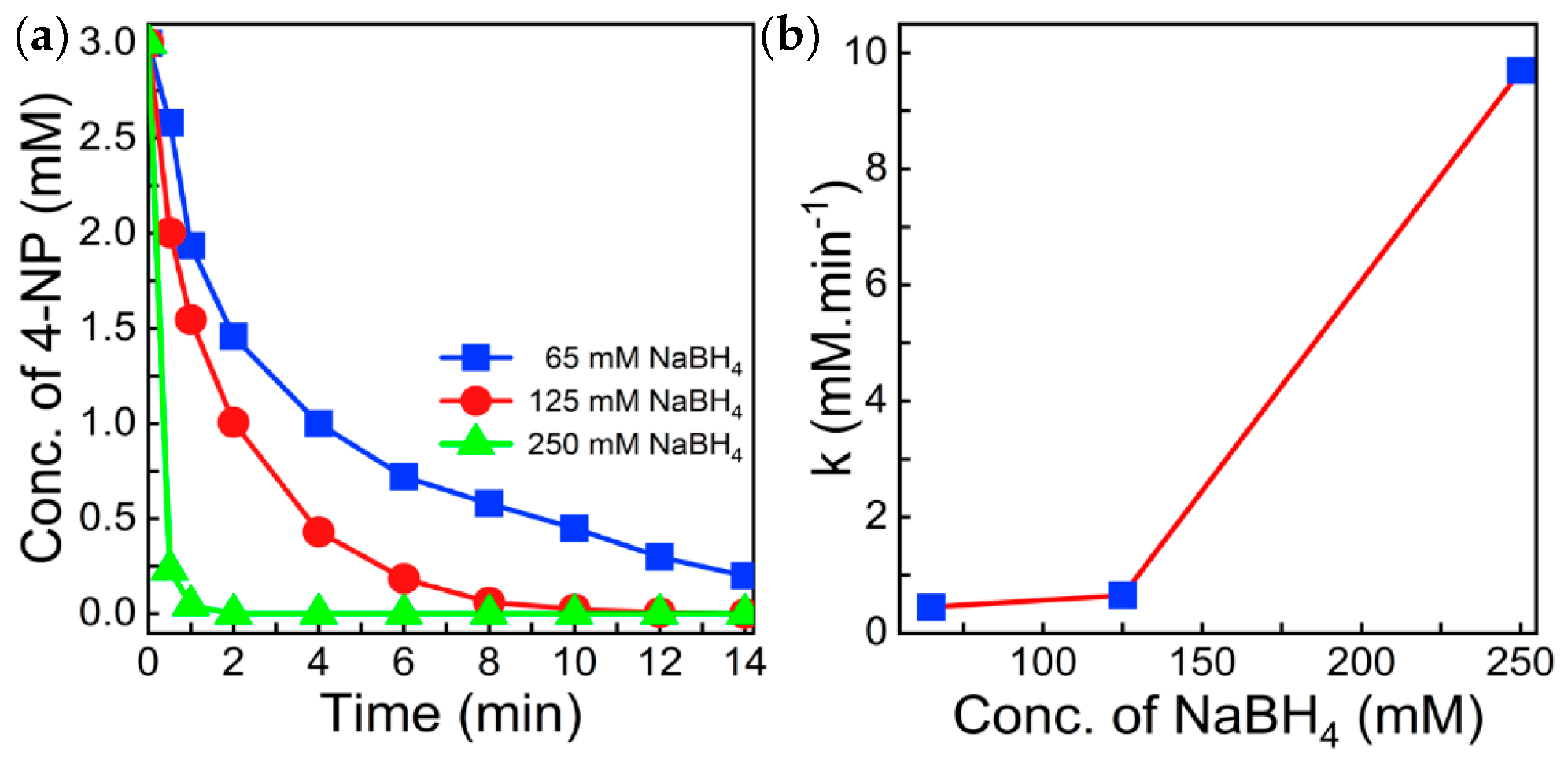
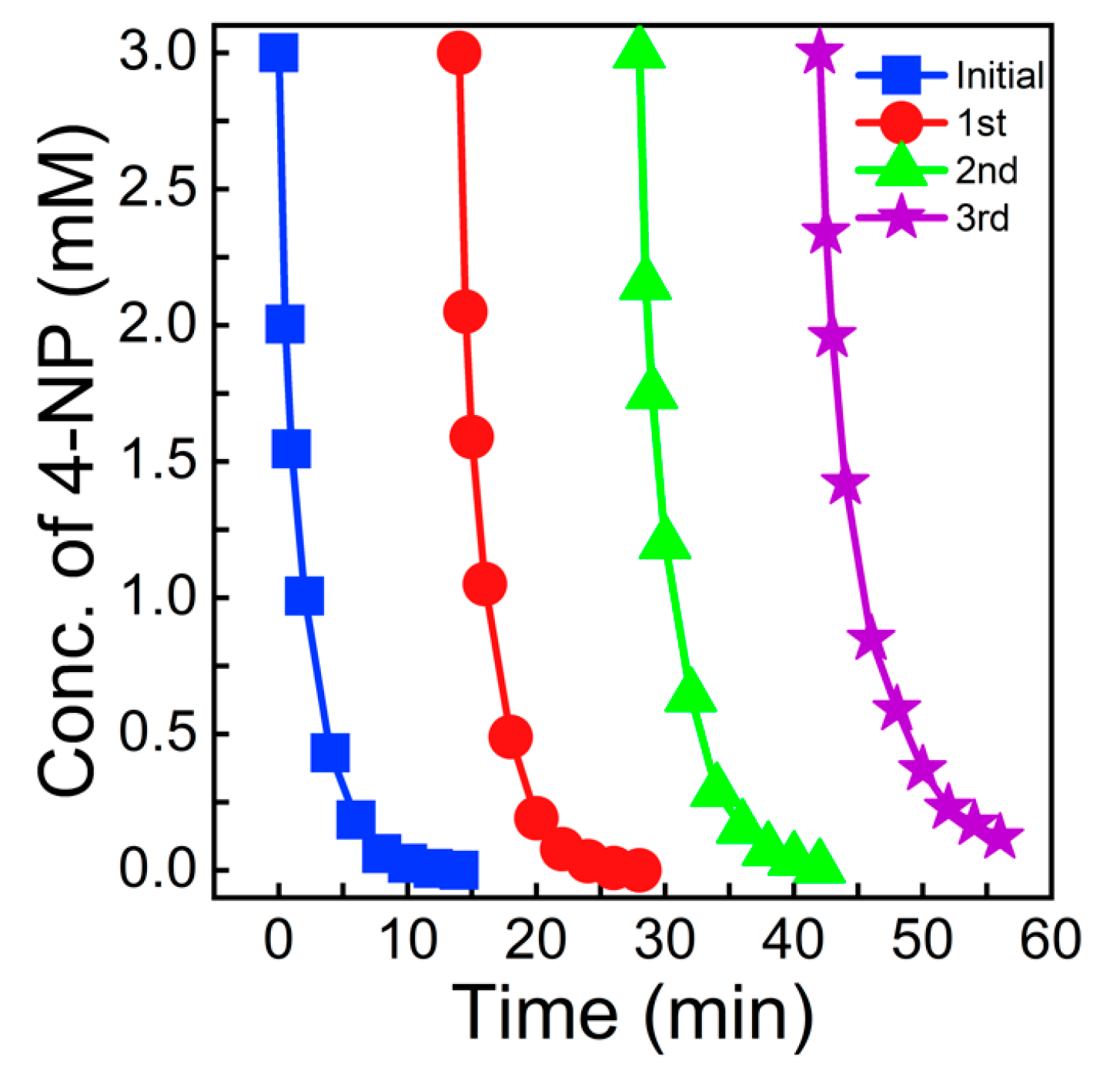
3.3. Catalytic Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- El-Hosainy, H.; El-Sheikh, S.; Ismail, A.; Hakki, A.; Dillert, R.; Killa, H.; Ibrahim, I.; Bahnemann, D. Highly selective photocatalytic reduction of o-dinitrobenzene to o-phenylenediamine over non-metal-doped TiO2 under simulated solar light irradiation. Catalysts 2018, 8, 641. [Google Scholar] [CrossRef]
- Esmat, M.; Farghali, A.A.; El-Dek, S.I.; Khedr, M.H.; Yamauchi, Y.; Bando, Y.; Fukata, N.; Ide, Y. Conversion of a 2D lepidocrocite-type layered titanate into its 1D nanowire form with enhancement of cation exchange and photocatalytic performance. Inorg. Chem. 2019, 58, 7989–7996. [Google Scholar] [CrossRef] [PubMed]
- Doustkhah, E.; Lin, J.; Rostamnia, S.; Len, C.; Luque, R.; Luo, X.; Bando, Y.; Wu, K.C.-W.; Kim, J.; Yamauchi, Y.; et al. Development of sulfonic-acid-functionalized mesoporous materials: synthesis and catalytic applications. Chem. A Eur. J. 2019, 25, 1614–1635. [Google Scholar] [CrossRef] [PubMed]
- Kobya, M.; Soltani, R.D.C.; Omwene, P.I.; Khataee, A. A review on decontamination of arsenic-contained water by electrocoagulation: Reactor configurations and operating cost along with removal mechanisms. Environ. Technol. Innov. 2020, 17, 100519. [Google Scholar] [CrossRef]
- Doustkhah, E.; Ide, Y. Bursting exfoliation of a microporous layered silicate to three-dimensionally meso–microporous nanosheets for improved molecular recognition. ACS Appl. Nano Mater. 2019, 2, 7513–7520. [Google Scholar] [CrossRef]
- Doustkhah, E.; Hasani, M.; Ide, Y.; Assadi, M.H.N. Pd nanoalloys for H2 generation from formic acid. ACS Appl. Nano Mater. 2019. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, R.; He, S.; Li, L.; Wang, X.; Liu, M.; Chen, W. 4-nitrophenol reduction by a single platinum palladium nanocube caged within a nitrogen-doped hollow carbon nanosphere. ChemCatChem 2017, 9, 980–986. [Google Scholar] [CrossRef]
- Zhu, X.Y.; Lv, Z.S.; Feng, J.J.; Yuan, P.X.; Zhang, L.; Chen, J.R.; Wang, A.J. Controlled fabrication of well-dispersed AgPd nanoclusters supported on reduced graphene oxide with highly enhanced catalytic properties towards 4-nitrophenol reduction. J. Colloid Interface Sci. 2018, 516, 355–363. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Q.; Zhang, P.; O’Connor, D.; Varma, R.S.; Yu, M.; Hou, D. One-pot green synthesis of bimetallic hollow palladium-platinum nanotubes for enhanced catalytic reduction of p-nitrophenol. J. Colloid Interface Sci. 2019, 539, 161–167. [Google Scholar] [CrossRef]
- El-Sheikh, S.M.; Ismail, A.A.; Al-Sharab, J.F. Catalytic reduction of p-nitrophenol over precious metals/highly ordered mesoporous silica. New J. Chem. 2013, 37, 2399–2407. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Sun, C.J.; Li, X.; Zhang, W.; Ma, X.; Ren, Y.; Zhang, X. Heterobimetallic metal-organic framework as a precursor to prepare a nickel/nanoporous carbon composite catalyst for 4-nitrophenol reduction. ChemCatChem 2014, 6, 3084–3090. [Google Scholar] [CrossRef]
- Qiao, X.Q.; Zhang, Z.W.; Tian, F.Y.; Hou, D.F.; Tian, Z.F.; Li, D.S.; Zhang, Q. Enhanced catalytic reduction of p-nitrophenol on ultrathin MoS2 nanosheets decorated with noble metal nanoparticles. Cryst. Growth Des. 2017, 17, 3538–3547. [Google Scholar] [CrossRef]
- Al-Kahtani, A.A.; Almuqati, T.; Alhokbany, N.; Ahamad, T.; Naushad, M.; Alshehri, S.M. A clean approach for the reduction of hazardous 4-nitrophenol using gold nanoparticles decorated multiwalled carbon nanotubes. J. Clean. Prod. 2018, 191, 429–435. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, Q.; Du, C.; Bu, T.; Liu, Y.; Sun, X.; Luo, W.; Li, R.; Zhao, Y.; Zheng, X.; et al. Three-dimensional Cu/C porous composite: Facile fabrication and efficient catalytic reduction of 4-nitrophenol. J. Colloid Interface Sci. 2019, 553, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.T.; Liu, X.G.; Xing, X.J.; Li, B.; Wang, K.; Chen, S.T.; Wu, Z.; Qiu, D.F. Ordered mesoporous silica cubic particles decorated with silver nanoparticles: A highly active and recyclable heterogeneous catalyst for the reduction of 4-nitrophenol. Dalton Trans. 2019, 48, 2692–2700. [Google Scholar] [CrossRef]
- Guo, S.; Sun, S. FePt nanoparticles assembled on graphene as enhanced catalyst for oxygen reduction reaction. J. Am. Chem. Soc. 2012, 134, 2492–2495. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.; Huang, L.; Zhou, S.; Sheng, X.; Wang, Q.; Zhang, C. Structure and catalytic properties of the Zn-modified ZSM-5 supported platinum catalyst for propane dehydrogenation. Chem. Eng. J. 2015, 270, 352–361. [Google Scholar] [CrossRef]
- Jiang, X.; Han, B.; Zhou, C.; Xia, K.; Gao, Q.; Wu, J. Cu nanoparticles supported on oxygen-rich boron nitride for the reduction of 4-nitrophenol. ACS Appl. Nano Mater. 2018, 1, 6692–6700. [Google Scholar] [CrossRef]
- Doustkhah, E.; Rostamnia, S.; Imura, M.; Ide, Y.; Mohammadi, S.; Hyland, C.J.T.; You, J.; Tsunoji, N.; Zeynizadeh, B.; Yamauchi, Y. Thiourea bridged periodic mesoporous organosilica with ultra-small Pd nanoparticles for coupling reactions. RSC Adv. 2017, 7, 56306–56310. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, Y.; Zhou, Y.; Zhang, C.; Zhang, H.; Zhao, S.; Fang, J.; Huang, M.; Sheng, X. Synthesis of ordered mesoporous La2O3-ZrO2 composites with encapsulated Pt NPs and the effect of La-dopping on catalytic activity. J. Colloid Interface Sci. 2017, 503, 178–185. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Shojaei, A.F.; Karimi, F.; Tabatabaeia, K.; Shakeri, S. Au nanoparticle loaded with 6-thioguanine anticancer drug as a new strategy for drug delivery. J. Nanostruct. 2018, 8, 417–424. [Google Scholar]
- Yola, M.L.; Atar, N.; Eren, T.; Karimi-Maleh, H.; Wang, S. Sensitive and selective determination of aqueous triclosan based on gold nanoparticles on polyoxometalate/reduced graphene oxide nanohybrid. RSC Adv. 2015, 5, 65953–65962. [Google Scholar] [CrossRef]
- Baghayeri, M.; Namadchian, M.; Karimi-Maleh, H.; Beitollahi, H. Determination of nifedipine using nanostructured electrochemical sensor based on simple synthesis of Ag nanoparticles at the surface of glassy carbon electrode: Application to the analysis of some real samples. J. Electroanal. Chem. 2013, 697, 53–59. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Sheikhshoaie, I.; Samadzadeh, A. Simultaneous electrochemical determination of levodopa and piroxicam using a glassy carbon electrode modified with a ZnO-Pd/CNT nanocomposite. RSC Adv. 2018, 8, 26707–26712. [Google Scholar] [CrossRef]
- Wu, S.; Liu, J.; Ye, Y.; Tian, Z.; Li, P.; Cai, Y.; Lin, Y.; Liang, C. In-situ reactive loading of platinum onto tin oxide nanocrystals with superior catalytic performance for hydrogenation of 4-nitrophenol. Appl. Surf. Sci. 2019, 471, 469–474. [Google Scholar] [CrossRef]
- Rath, P.C.; Saikia, D.; Mishra, M.; Kao, H.M. Exceptional catalytic performance of ultrafine Cu2O nanoparticles confined in cubic mesoporous carbon for 4-nitrophenol reduction. Appl. Surf. Sci. 2018, 427, 1217–1226. [Google Scholar] [CrossRef]
- Esmat, M.; Farghali, A.A.; Khedr, M.H.; El-Sherbiny, I.M. Alginate-based nanocomposites for efficient removal of heavy metal ions. Int. J. Biol. Macromol. 2017, 102, 272–283. [Google Scholar] [CrossRef]
- Wang, H.; Dong, Z.; Na, C. Hierarchical carbon nanotube membrane-supported gold nanoparticles for rapid catalytic reduction of p-nitrophenol. ACS Sustain. Chem. Eng. 2013, 1, 746–752. [Google Scholar] [CrossRef]
- Lang, R.; Xi, W.; Liu, J.C.; Cui, Y.T.; Li, T.; Lee, A.F.; Chen, F.; Chen, Y.; Li, L.; Li, L.; et al. Non defect-stabilized thermally stable single-atom catalyst. Nat. Commun. 2019, 10, 234. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, Y.; Zhang, Y.; Zhao, S.; Fang, J.; Sheng, X.; Zhang, H. Self-Assembly hierarchical silica nanotubes with vertically aligned silica nanorods and embedded platinum nanoparticles. ACS Sustain. Chem. Eng. 2017, 5, 1578–1585. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, H.; Chen, L.; Miao, S.; Wu, S.; Hao, X.; Zhang, W.; Jia, M. Interfacial synergy of PtPd nanoparticles dispersed on amine-modified ZrSBA-15 in catalytic dehydrogenation of ammonia borane and reduction of p-nitrophenol. J. Phys. Chem. C 2018, 122, 12975–12983. [Google Scholar] [CrossRef]
- Liu, C.; Tan, R.; Yu, N.; Yin, D. Pt-Pd bi-metal nanoparticles captured and stabilized by imine groups in a periodic mesoporous organosilica of SBA-15 for hydrogenation of nitrobenzene. Microporous Mesoporous Mater. 2010, 131, 162–169. [Google Scholar] [CrossRef]
- Karimi, B.; Kabiri Esfahani, F. Unexpected golden Ullmann reaction catalyzed by Au nanoparticles supported on periodic mesoporous organosilica (PMO). Chem. Commun. 2011, 47, 10452–10454. [Google Scholar] [CrossRef] [PubMed]
- Karimi, B.; Esfahani, F.K. Gold nanoparticles supported on the periodic mesoporous organosilicas as efficient and reusable catalyst for room temperature aerobic oxidation of alcohols. Adv. Synth. Catal. 2012, 354, 1319–1326. [Google Scholar] [CrossRef]
- Karimi, B.; Gholinejad, M.; Khorasani, M. Highly efficient three-component coupling reaction catalyzed by gold nanoparticles supported on periodic mesoporous organosilica with ionic liquid framework. Chem. Commun. 2012, 48, 8961–8963. [Google Scholar] [CrossRef]
- Doustkhah, E.; Mohtasham, H.; Hasani, M.; Ide, Y.; Rostamnia, S.; Tsunoji, N.; Hussein, M. Merging periodic mesoporous organosilica (PMO) with mesoporous aluminosilica (Al/Si-PMO): A catalyst for green oxidation. Mol. Catal. 2019. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, M.; Wang, M.; Li, W.; Wang, C.; Hou, X.; Luan, S.; Wang, Q. Gold/periodic mesoporous organosilicas with controllable mesostructure by using compressed CO2. Langmuir 2018, 34, 3642–3653. [Google Scholar] [CrossRef]
- Karimi, B.; Naderi, Z.; Khorasani, M.; Mirzaei, H.M.; Vali, H. Ultrasmall platinum nanoparticles supported inside the nanospaces of periodic mesoporous organosilica with an imidazolium network: an efficient catalyst for the aerobic oxidation of unactivated alcohols in water. ChemCatChem 2016, 8, 906–910. [Google Scholar] [CrossRef]
- Dai, J.; Zou, H.; Wang, R.; Wang, Y.; Shi, Z.; Qiu, S. Yolk-shell Fe3O4@SiO2@PMO: Amphiphilic magnetic nanocomposites as an adsorbent and a catalyst with high efficiency and recyclability. Green Chem. 2017, 19, 1336–1344. [Google Scholar] [CrossRef]
- Doustkhah, E.; Rostamnia, S.; Zeynizadeh, B.; Kim, J.; Yamauchi, Y.; Ide, Y. Efficient H2 generation using thiourea-based periodic mesoporous organosilica with Pd nanoparticles. Chem. Lett. 2018, 47, 1243–1245. [Google Scholar] [CrossRef]
- Doustkhah, E.; Mohtasham, H.; Farajzadeh, M.; Rostamnia, S.; Wang, Y.; Arandiyan, H.; Assadi, M.H.N. Organosiloxane tunability in mesoporous organosilica and punctuated Pd nanoparticles growth; theory and experiment. Microporous Mesoporous Mater. 2019, 293, 109832. [Google Scholar] [CrossRef]
- Zaki, A.H.; El-Shafey, A.; Moatmed, S.M.; Abdelhay, R.A.; Rashdan, E.F.; Saleh, R.M.; Abd-El Fatah, M.; Tawfik, M.M.; Esmat, M.; El-dek, S.I. Morphology transformation from titanate nanotubes to TiO2 microspheres. Mater. Sci. Semicond. Process. 2018, 75, 10–17. [Google Scholar] [CrossRef]
- Jia, W.-G.; Gao, L.-L.; Wang, Z.-B.; Sun, L.-Y.; Han, Y.-F. Synthesis, characterization, and catalytic activities of palladium complexes with phenylene-bridged bis(thione) ligands. Organometallics 2019, 38, 1946–1954. [Google Scholar] [CrossRef]
- Lin, F.; Doong, R. Bifunctional Au−Fe3O4 heterostructures for magnetically recyclable catalysis of nitrophenol reduction. J. Phys. Chem. C 2011, 115, 6591–6598. [Google Scholar] [CrossRef]
- Shi, D.; Zhu, G.; Zhang, X.; Zhang, X.; Li, X.; Fan, J. Ultra-small and recyclable zero-valent iron nanoclusters for rapid and highly efficient catalytic reduction of: p-nitrophenol in water. Nanoscale 2019, 11, 1000–1010. [Google Scholar] [CrossRef]
- Li, M.; Chen, G. Revisiting catalytic model reaction p-nitrophenol/NaBH4 using metallic nanoparticles coated on polymeric spheres. Nanoscale 2013, 5, 11919–11927. [Google Scholar] [CrossRef]




| Catalyst | Conv. (%) | t (min) | Reaction Conditions | Ref. |
|---|---|---|---|---|
| AgPd NCs/rGO | 95.6% | 3 | NaBH4 (5 mM); p-NP (0.7 mM); catalyst (0.05 mg); rt | [8] |
| Pd-PBL | 98.3% | 32 | NaBH4 (1 × 10−4 mmol); p-NP (1 × 10−3 mmol); catalyst (1 mol %); rt | [43] |
| Au/Fe3O4 | 100% | 10 | NaBH4 (0.1 M); p-NP (10 mM); catalyst (2 mg); rt | [44] |
| Au/CNTs membrane | 100% | 15 | NaBH4 (50 mM); p-NP (0.2 mM); catalyst (0.25 mM); rt | [28] |
| Ag–OMS-C | 96% | 2.5 | NaBH4 (0.5 M); p-NP (0.1 mM); catalyst (10 mg); rt | [15] |
| ZVI NCs | 100% | 1 | NaBH4 (3.2 mM); p-NP (0.14 mM); catalyst (0.44 mM); rt | [45] |
| PtPd@N-HCS | 100% | 2 | NaBH4 (0.1 M); p-NP (0.25 mM); catalyst (2 mg); rt | [7] |
| Pt/PMO-TU | 100% | <1 | NaBH4 (250 mM); p-NP (3 mM); catalyst (10 mg); rt | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esmat, M.; Mohtasham, H.; GadelHak, Y.; Mehrebani, R.T.; Tahawy, R.; Rostamnia, S.; Fukata, N.; Khaksar, S.; Doustkhah, E. 2D Mesoporous Channels of PMO; a Platform for Cluster-Like Pt Synthesis and Catalytic Activity in Nitrophenol Reduction. Catalysts 2020, 10, 167. https://doi.org/10.3390/catal10020167
Esmat M, Mohtasham H, GadelHak Y, Mehrebani RT, Tahawy R, Rostamnia S, Fukata N, Khaksar S, Doustkhah E. 2D Mesoporous Channels of PMO; a Platform for Cluster-Like Pt Synthesis and Catalytic Activity in Nitrophenol Reduction. Catalysts. 2020; 10(2):167. https://doi.org/10.3390/catal10020167
Chicago/Turabian StyleEsmat, Mohamed, Hamed Mohtasham, Yasser GadelHak, Reza Tarbiat Mehrebani, Rafat Tahawy, Sadegh Rostamnia, Naoki Fukata, Samad Khaksar, and Esmail Doustkhah. 2020. "2D Mesoporous Channels of PMO; a Platform for Cluster-Like Pt Synthesis and Catalytic Activity in Nitrophenol Reduction" Catalysts 10, no. 2: 167. https://doi.org/10.3390/catal10020167
APA StyleEsmat, M., Mohtasham, H., GadelHak, Y., Mehrebani, R. T., Tahawy, R., Rostamnia, S., Fukata, N., Khaksar, S., & Doustkhah, E. (2020). 2D Mesoporous Channels of PMO; a Platform for Cluster-Like Pt Synthesis and Catalytic Activity in Nitrophenol Reduction. Catalysts, 10(2), 167. https://doi.org/10.3390/catal10020167









