RETRACTED: Experimental Study of CO2 Conversion into Methanol by Synthesized Photocatalyst (ZnFe2O4/TiO2) Using Visible Light as an Energy Source
Abstract
1. Introduction
2. Results
2.1. Characterization
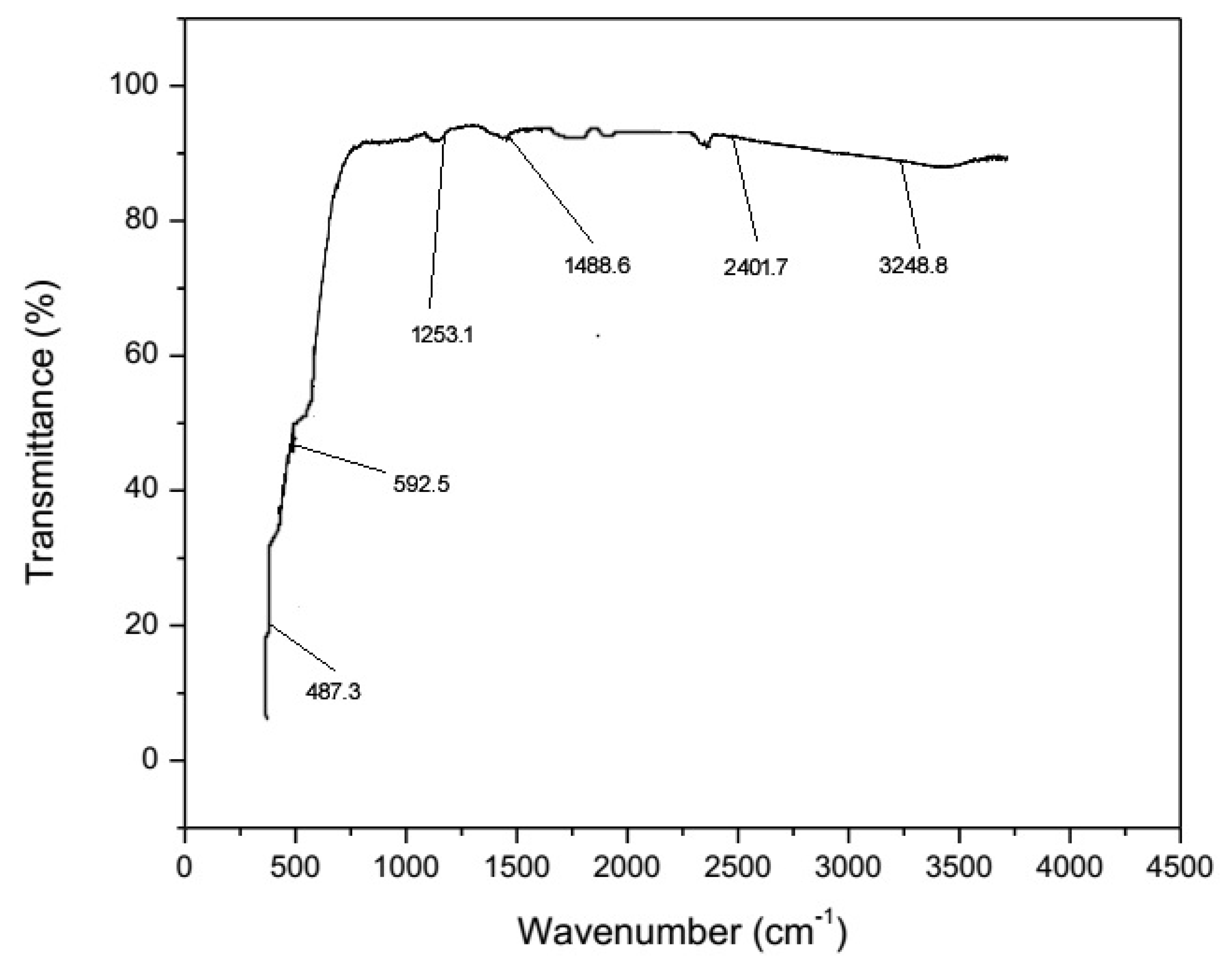
2.1.1. FTIR (Fourier Transform Infrared Spectroscopy)
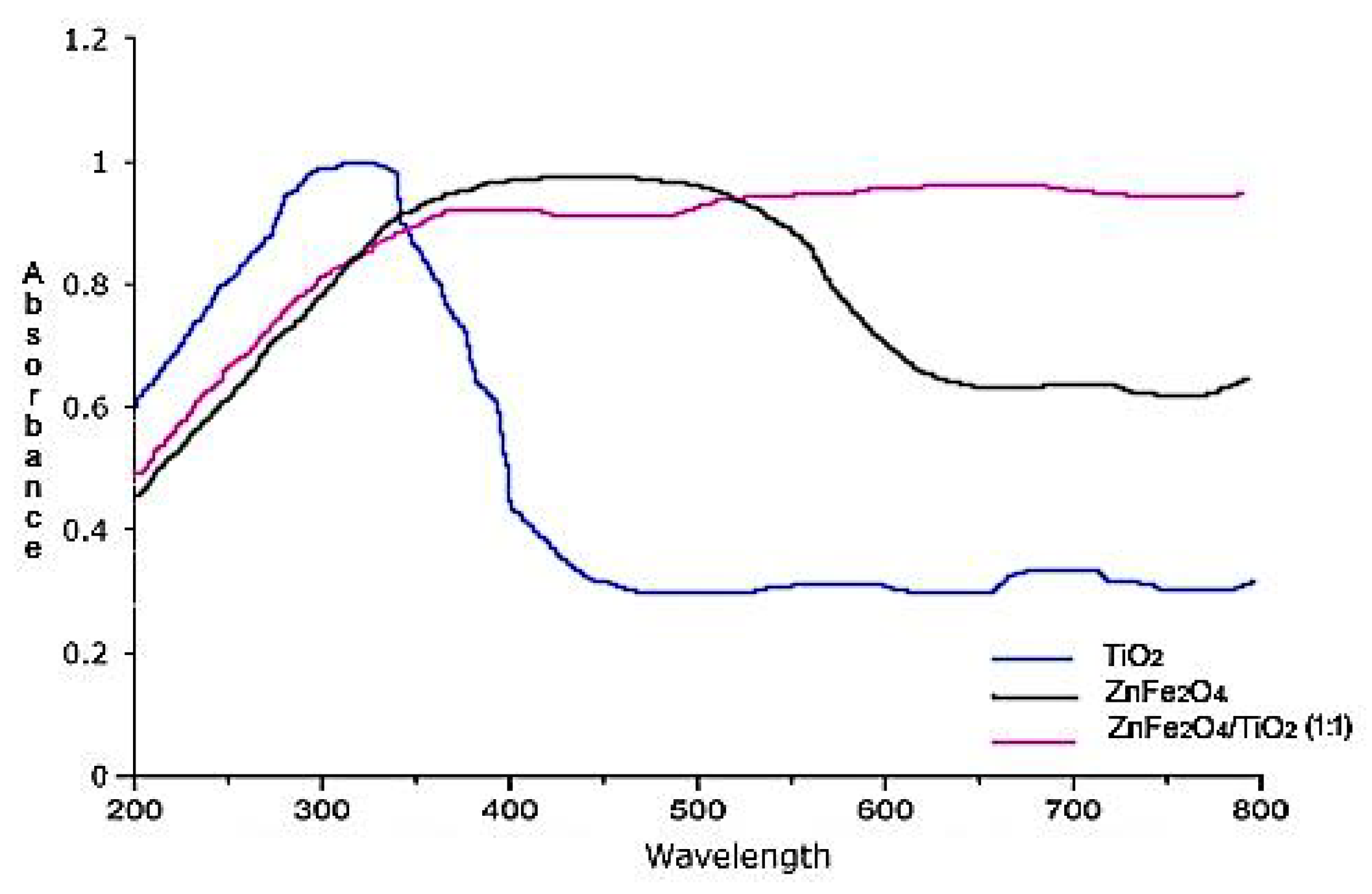
2.1.2. UV–Vis Spectroscopy
2.1.3. FE-SEM Analysis
2.1.4. XRD Spectra
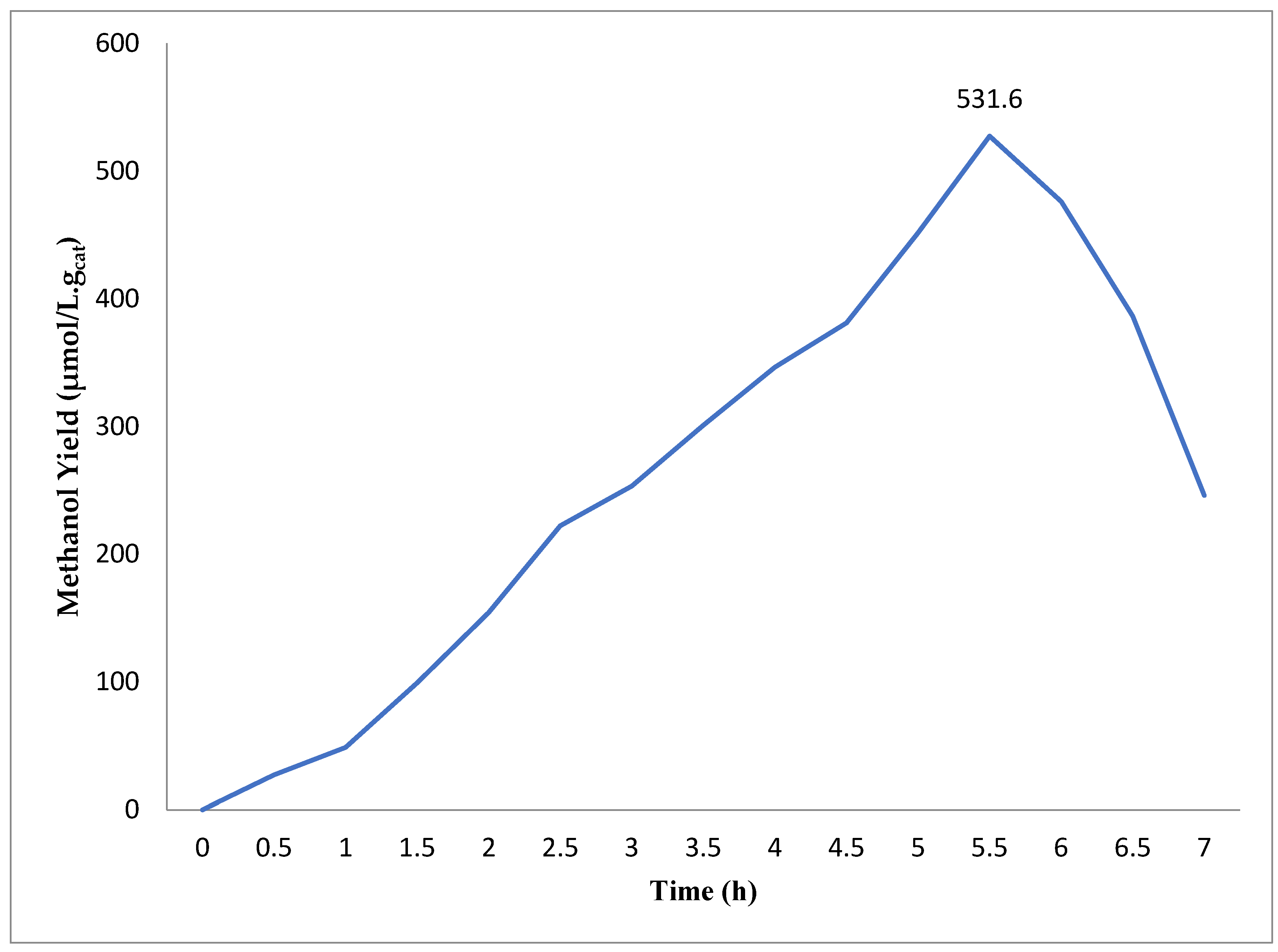
2.2. Conversion of CO2 into Methanol Using Photocatalyst
2.3. Effect of Different Coupling Ratios of ZnFe2O4 with TiO2
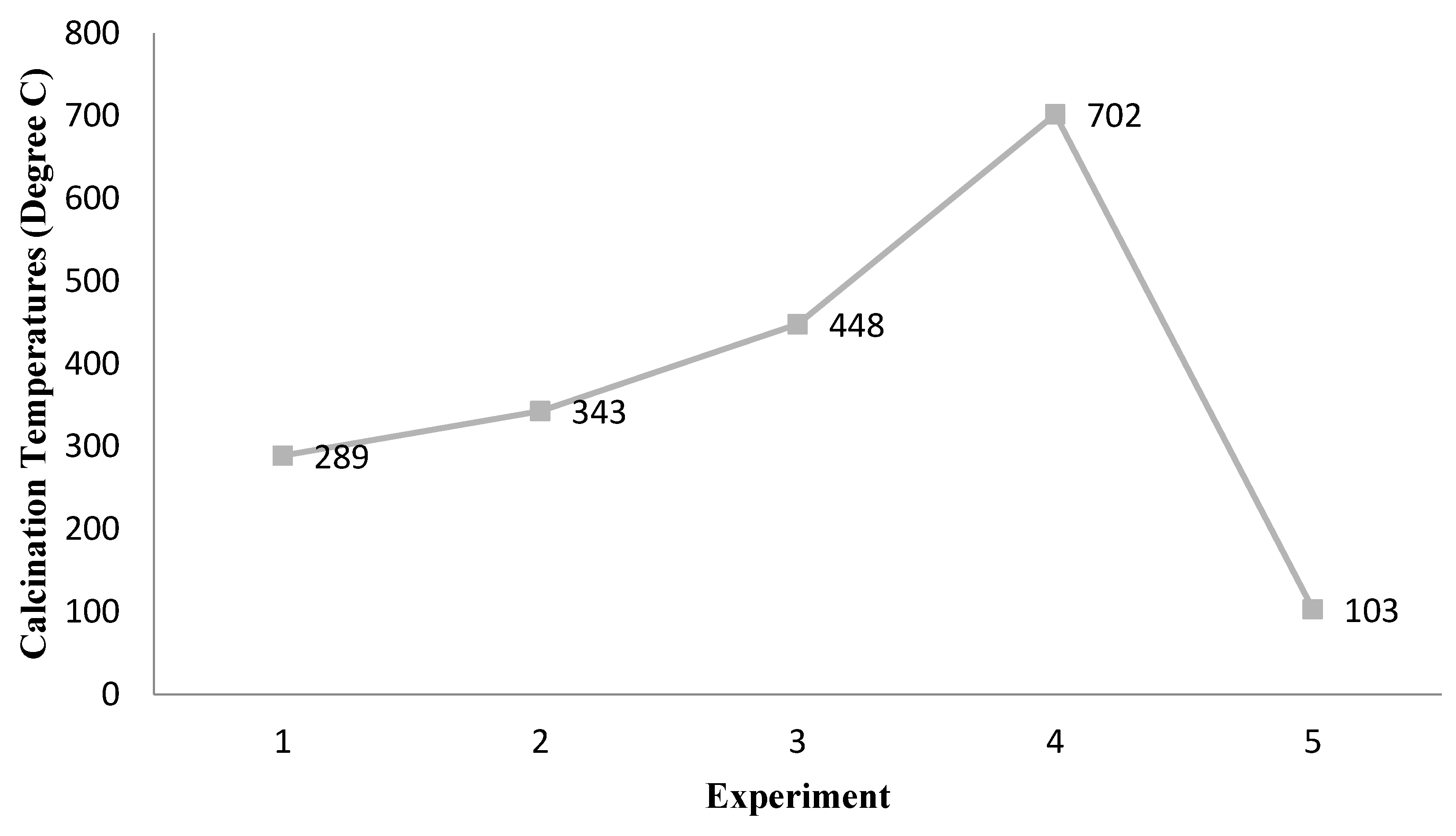
2.4. Variable Calcination Temperature of ZnFe2O4 Effects
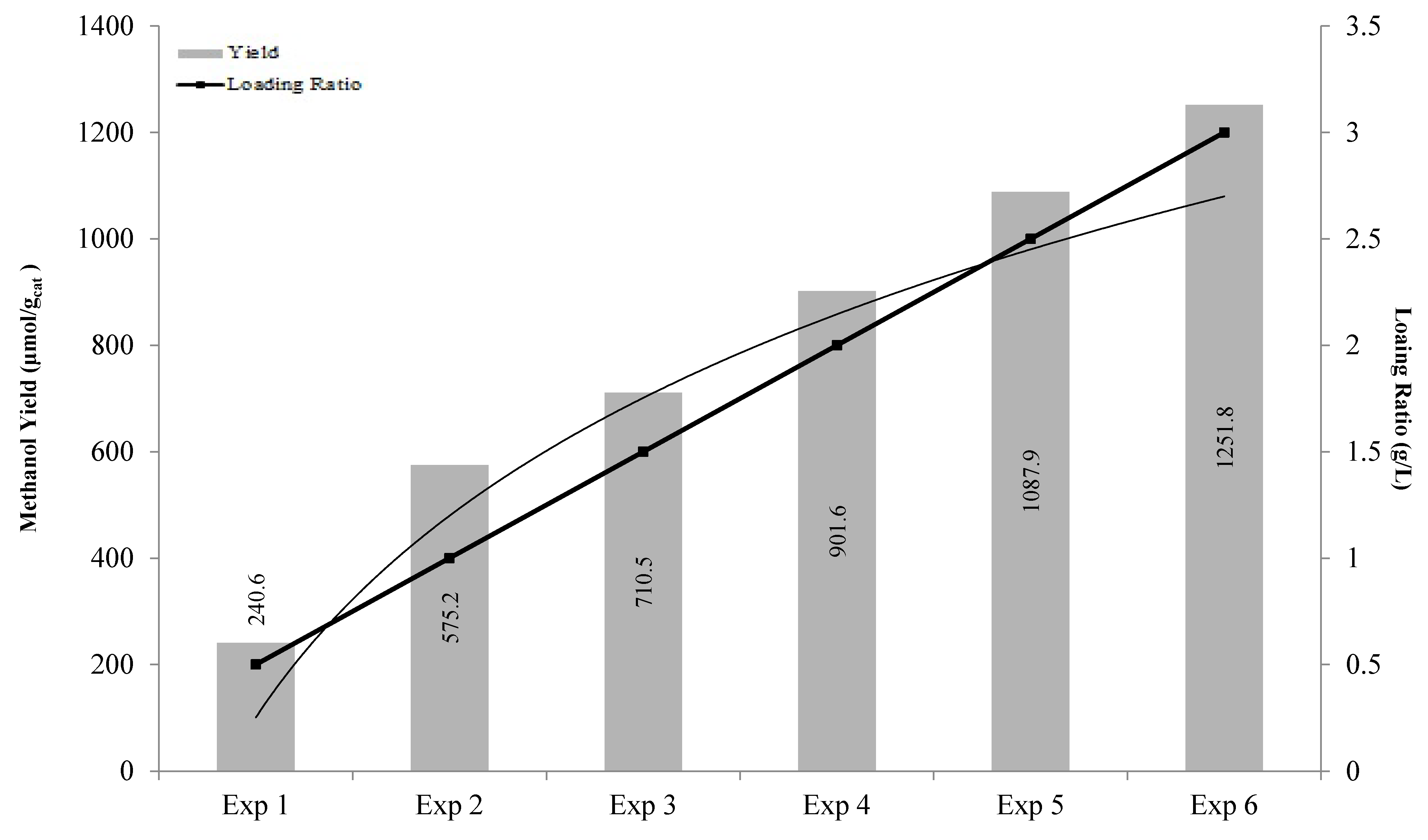
2.5. Effect of Different Catalyst Loading Ratios
2.6. Effect of Catalyst Recycling
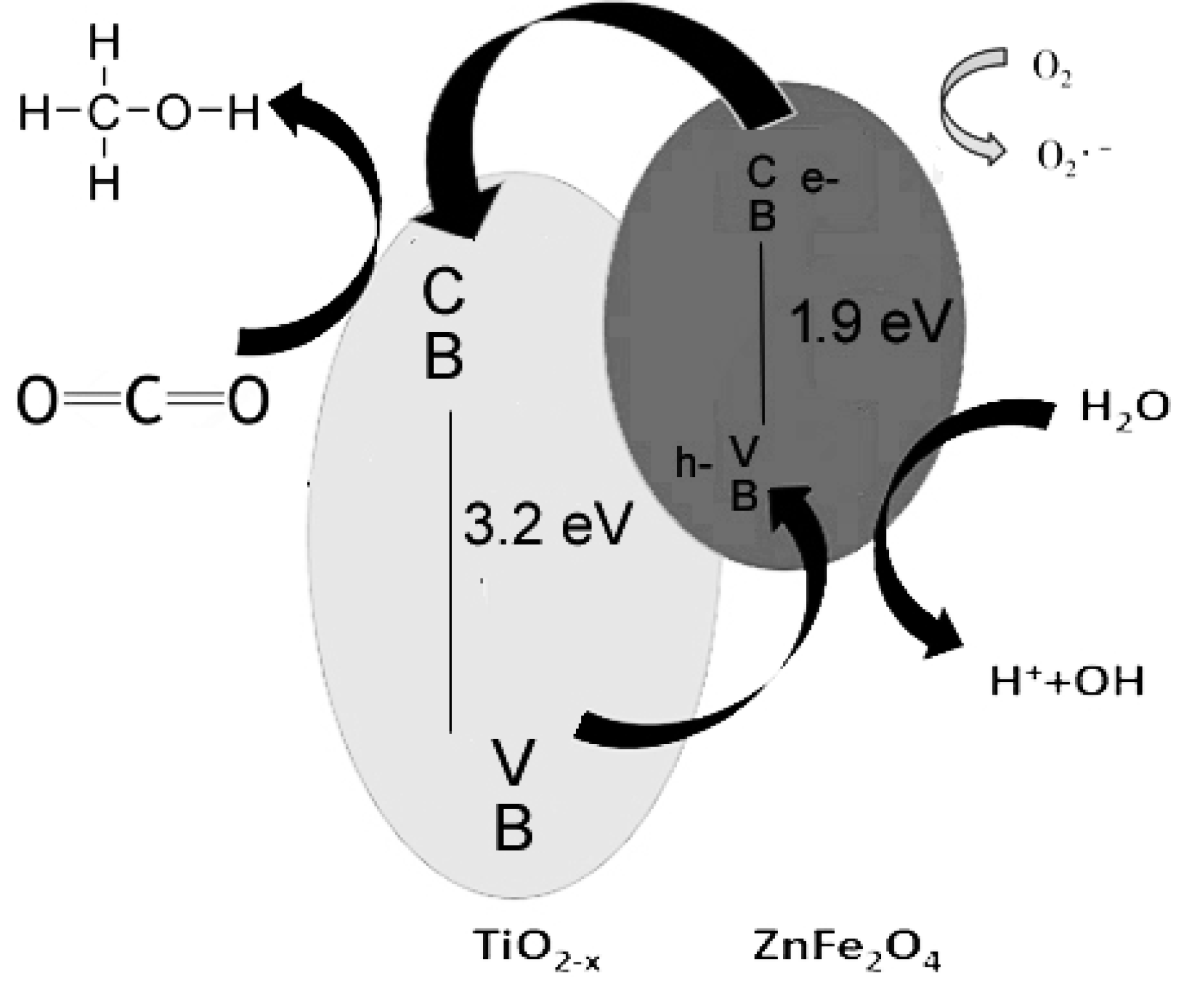
2.7. Proposed Reaction Mechanism
2.8. Comparison with Previous Results
3. Materials and Method
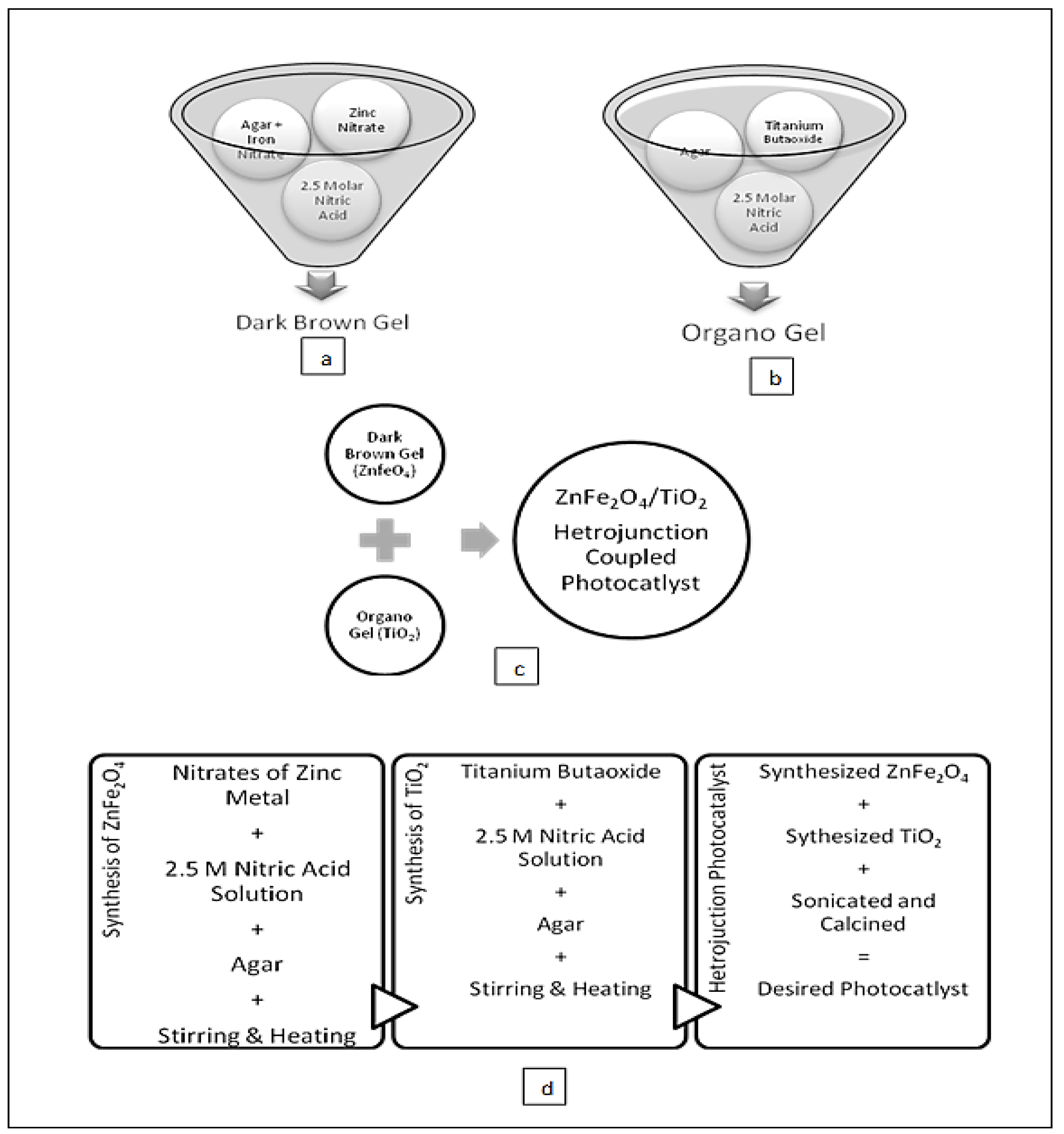
3.1. Synthesis Procedure
3.2. Electron and Hole Pair Formation
- Eg = band gap energy;
- Ec = energy of the conduction band; and
- Ev = energy of the valance band.
3.3. Experimental Setup and Operating Conditions
- Y = yield
- C = methanol concentration, μmol/L
- V = volume, L
- W = mass of catalyst, g
4. Conclusions
5. Recommendations
Author Contributions
Funding
Conflicts of Interest
References
- Moniz, S.J.A.; Shevlin, S.A.; Martin, D.J.; Guo, Z.-X.; Tang, J. Visible-light driven heterojunction photocatalysts for water splitting–acritical review. Energy Environ. Sci. 2015, 8, 722–765. [Google Scholar] [CrossRef]
- Su, M.; He, C.; Sharma, V.K.; AsiAbou, M.; Xia, D.; Li, X.Z.; Deng, H.; Xiong, Y. Mesoporous zinc ferrite: Synthesis, characterization, and photocatalytic activity with H2O2/visible light. J. Hazard. Mater. 2012, 211, 88–111. [Google Scholar] [CrossRef] [PubMed]
- Ola, O.; Maroto-Valer, M.M. Review of material design and reactor engineering on TiO2 photocatalysis for CO2 reduction. J. Photochem. Photobiol. C Photochem. Rev. 2015, 24, 13–49. [Google Scholar] [CrossRef]
- Tan, C. CO2 utilization. Ind. Mater. 2013, 316, 79–98. [Google Scholar]
- AbdelDayem, H.M.; AbdelDayem, S.S.; Hassan, S.A. Selective methanol oxidation to hydrogen over Ag/ZnO catalysts doped with mono-and bi-rare earth oxides. Ind. Eng. Chem. Res. 2014, 53, 19872–19899. [Google Scholar] [CrossRef]
- Heffer, P.; Prud’homme, M. Fertilizer outlook 2013–2017. In Proceedings of the 81st International Fertilizer Industry Association Conference, Chicago, IL, USA, 20–22 May 2013; pp. 7–9. [Google Scholar]
- Casbeer, E.; Sharma, V.K.; Li, X. Synthesis and photocatalytic activity of ferrites under visible light: A review. Sep. Purif. Technol. 2012, 87, 1–17. [Google Scholar] [CrossRef]
- Karamian, R.; Ghasemlou, F. Total phenolic content, antioxidant and antibacterial activities of three Verbascum species from Iran. J. Med. Plants By-Prod. 2013, 1, 42–56. [Google Scholar]
- Kawamura, S.; Egami, H.; Sodeoka, M. Amino tri-fluoro methylation of olefins via cyclic amine formation: Mechanistic study and application to synthesis of trifluoro methylated pyrrolidines. J. Am. Chem. Soc. 2015, 137, 4859–4878. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Kim, K.; Bogenhagen, D.F. Proliferating cell nuclear antigen-dependent abasic site repair in Xenopus laevis oocytes: An alternative pathway of base excision DNA repair. Mol. Cell. Biol. 1994, 14, 6175–6199. [Google Scholar] [CrossRef]
- Tahir, M.; Amin, N.S. Advances in visible light responsive titanium oxide based photocatalysts for CO2 conversion to hydrocarbon fuels. Energy Convers. Manag. 2013, 76, 192–217. [Google Scholar] [CrossRef]
- Uddin, M.N.; Afrin, R.; Uddin, M.J.; Md. Uddin, M.J.; Alam, A.H.M.K.; Rahman, A.A.; Sadik, G. Vanda roxburghii chloroform extract as a potential source of polyphenols with antioxidant and cholinesterase inhibitory activities: Identification of a strong phenolic antioxidant. BMC Complementary Altern. Med. 2015, 15, 192. [Google Scholar] [CrossRef] [PubMed]
- Rekhila, G.; Bessekhouad, Y.; Trari, M. Visible light hydrogen production on the novel ferrite NiFe2O4. Int. J. Hydrog. Energy 2013, 38, 6331–6348. [Google Scholar] [CrossRef]
- Moualkia, H.; Rekhila, G.; Izerrouken, M.; Mahdjoub, A.; Trari, M. Influence of the film thickness on the photovoltaic properties of chemically deposited CdS thin films: Application to the photodegradation of orange. Mater. Sci. Semicond. Process. 2014, 21, 181–197. [Google Scholar] [CrossRef]
- Li, H.; Lei, Y.; Huang, Y.; Fang, Y.; Xu, Y.; Zhu, X.; Li, X. Photocatalytic reduction of carbon dioxide to methanol by Cu2O/SiCN an ocrystallite under visible light irradiation. J. Nat. Gas. Chem. 2011, 20, 142–151. [Google Scholar]
- Ghosh, A.; Mangalvedhe, N.; Ratasuk, R.; Mondal, B.; Cudak, M.; Visotsky, E.; Thomas, T.A.; Andrews, J.G.; Xia, P.; Jo, H.S.; et al. Heterogeneous cellular networks: From theory to practice. IEEE Commun. Mag. 2012, 50, 52–67. [Google Scholar] [CrossRef]
- Hao, F.; Lakshman, T.V.; Sarit, M.; Song, H. Providing Cloud-Based Services Using Dynamic Network Virtualization. U.S. Patent No. 9,210,065, 22 June 2015. [Google Scholar]
- Kezzim, A.; Nasrallah, N.; Abdi, A.; Trari, M. Visible light induced hydrogen on the novel hetero-system CuFe2O4/TiO2. Energy Convers. Manag. 2011, 52, 2798–2810. [Google Scholar] [CrossRef]
- Elias, D.C.; Nair, R.R.; Mohiuddin, T.M.; Morozov, S.V.; Blake, P.; Halsall, M.P.; Ferrari, A.C.; Boukhvalov, D.W.; Katsnelson, M.I.; Geim, A.K.; et al. Control of graphene’s properties by reversible hydrogenation: Evidence for graphane. Science 2009, 323, 605–618. [Google Scholar] [CrossRef]
- Kim, M.; Choi, J.-S.; Toops, T.J.; Jeong, E.-S. Coating SiO2 support with TiO2 or ZrO2 and effects on structure and CO oxidation performance of Pt catalysts. Catalysts 2013, 3, 81–108. [Google Scholar] [CrossRef]
- Islam, M.A.; Woon, C.W.; Ethiraj, B.; Cheng, C.K.; Yousuf, A.; Khan, M.M.R. Ultrasound driven biofilm removal for stable power generation in microbial fuel cell. Energy Fuels 2016, 31, 962–979. [Google Scholar] [CrossRef]
- Li, X.; Liu, A.; Chu, D.; Zhang, C.; Du, Y.; Huang, J.; Yang, P. High performance of manganese porphyrin sensitized type CuFe2O4 photocathode for solar water splitting to produce hydrogen in a tandem photoelectrochemical cell. Catalysts 2018, 8, 109. [Google Scholar]
- Akhter, P.; Farkhondehfal, M.A.; Hernández, S.; Hussain, M.; Fina, A.; Saracco, G.; Khan, A.U.; Russo, N. Environmental issues regarding CO2 and recent strategies for alternative fuels through photocatalytic reduction with titania-based materials. J. Environ. Chem. Eng. 2016, 4, 3933–3951. [Google Scholar] [CrossRef]
- Lv, M.; Liu, H. Photocatalytic property and structural stability of CuAl-based layered double hydroxides. J. Solid State Chem. 2015, 227, 229–241. [Google Scholar] [CrossRef]
- Xia, S.; Meng, Y.; Zhou, X.; Xue, J.; Pan, G.; Ni, Z. Ti/ZnO–Fe2O3 composite: Synthesis, characterization and application as a highly efficient photo-electron catalyst for methanol from CO2 reduction. Appl. Catal. B Environ. 2016, 187, 119–136. [Google Scholar] [CrossRef]




| Product | Potential of CO2Reduction (ton CO2/ton of Product) | Market Scale (per Year) | References | |
|---|---|---|---|---|
| Global Demand (million ton, MT) | Market Value (billion $) | |||
| Dimethyl ether (DME) | 1.9 | 6.3 | 3.2 | [4] |
| Dimethyl carbonate (DMC) | 1.47 | 0.24 | 280 | --- |
| Polycarbonate | 0.5 | 3.6 | 14.4 | [4] |
| Methanol | 1.38 | 75 | 36 | [5] |
| Urea | 0.735-0.75 | 198.4 | 59.5 | [6] |
| Sr. No. | Reaction | Thermodynamic Potential V vs. NHE (Normal Hydrogen Electrode) | |
|---|---|---|---|
| V vs. NHE | Reference | ||
| 1 | CO2+ e−→ CO2− | −1.9 | [3] |
| 2 | CO2+ 2H+→ HCOOH | −0.61 | [11] |
| 3 | 2H++ e−→ H2 | −0.41 | [3] |
| Experiments | Loading Ratio (g/L) | Methanol Yield (μmol/gcat) |
|---|---|---|
| 1 | 0.5 | 240.6 |
| 2 | 1 | 575.2 |
| 3 | 1.5 | 710.5 |
| 4 | 2 | 901.6 |
| 5 | 2.5 | 1087.9 |
| 6 | 3 | 1251.8 |
| Photocatalyst | Rate of Methanol Formation (μmol/gcat·h) | Solvent/Electrolyte | Light Source | Reference |
|---|---|---|---|---|
| ZnFe2O4/TiO2 | 141.22 | Na2S, Na2SO3, KOH in Water | 500 W Xenon Lamp | This Study |
| 15% Bi2S3/CdS | 122.6 | NaOH and Na2S in Water | 500 W Xenon Lamp | [23] |
| CeF3/TiO2 | 80 | Water | 500 W Xenon Lamp | [14] |
| Cu2O/SiC | 39 | NaOH and Na2SO3 in Water | 500 W Xenon Lamp | [16] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manzoor, N.; Sadiq, M.; Naqvi, M.; Sikandar, U.; Naqvi, S.R. RETRACTED: Experimental Study of CO2 Conversion into Methanol by Synthesized Photocatalyst (ZnFe2O4/TiO2) Using Visible Light as an Energy Source. Catalysts 2020, 10, 163. https://doi.org/10.3390/catal10020163
Manzoor N, Sadiq M, Naqvi M, Sikandar U, Naqvi SR. RETRACTED: Experimental Study of CO2 Conversion into Methanol by Synthesized Photocatalyst (ZnFe2O4/TiO2) Using Visible Light as an Energy Source. Catalysts. 2020; 10(2):163. https://doi.org/10.3390/catal10020163
Chicago/Turabian StyleManzoor, Numair, Muhammad Sadiq, Muhammad Naqvi, Umair Sikandar, and Salman Raza Naqvi. 2020. "RETRACTED: Experimental Study of CO2 Conversion into Methanol by Synthesized Photocatalyst (ZnFe2O4/TiO2) Using Visible Light as an Energy Source" Catalysts 10, no. 2: 163. https://doi.org/10.3390/catal10020163
APA StyleManzoor, N., Sadiq, M., Naqvi, M., Sikandar, U., & Naqvi, S. R. (2020). RETRACTED: Experimental Study of CO2 Conversion into Methanol by Synthesized Photocatalyst (ZnFe2O4/TiO2) Using Visible Light as an Energy Source. Catalysts, 10(2), 163. https://doi.org/10.3390/catal10020163







