2.1. Catalysts Characterization
Three bulk molybdenum oxide catalysts (Mo (300), Mo (500) and Mo (700)) were prepared through the calcination of ammonium heptamolybdate tetrahydrate (AHM) at 300, 500, and 700 °C. In addition, a series of alumina-supported molybdenum oxide catalysts (s-Mo (x), where x is the MoO3 content (wt.%)) were obtained from the incipient wetness impregnation of alumina with aqueous AHM solutions and calcination at 800 °C.
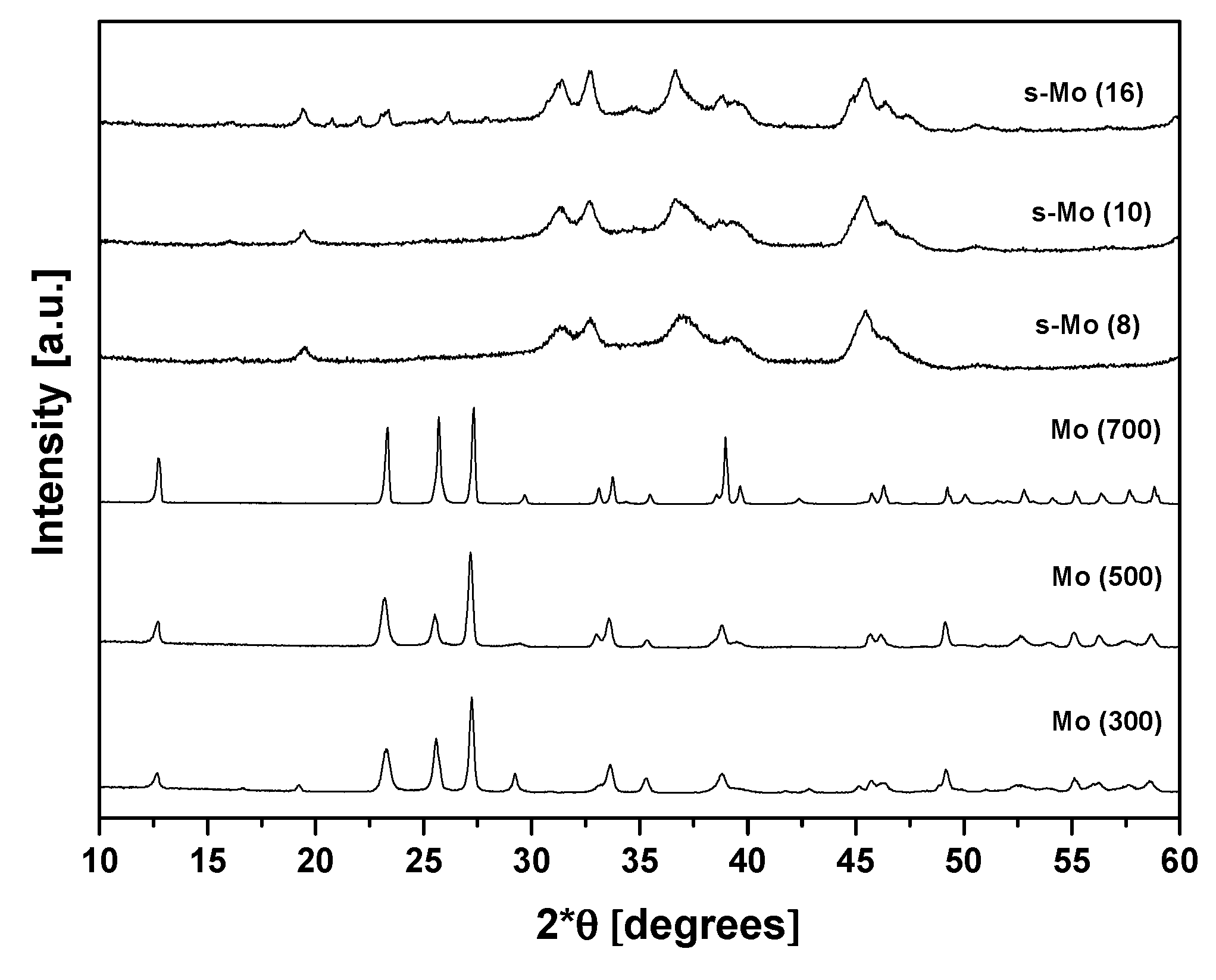
The X-ray diffraction (XRD) patterns of the bulk and a selection of the alumina-supported molybdenum catalysts are shown in
Figure 1. The thermal decomposition AHM is a complex process in which several molybdenum compounds are involved. Chithambararaj et al. [
28] found that ammonium was still present in the crystalline structures at calcination temperatures up to about 300 °C. According to these authors, at relatively low temperatures AHM evolves from [NH
4]
4(Mo
8O
24.8 (O
2)
1.2(H
2O)
2)(H
2O)
4 at 50–75 °C to [NH
4]
8Mo
10O
34 between 100 °C and 200 °C; finally, [NH
4]
4Mo
8O
26 is formed near 300 °C. Then, MoO
2.5(OH)
0.5 is present between 325 and 475 °C that evolves to MoO
2.69(OH)
0.3 at ca. 575 °C, finally resulting MoO
3−x at about 700 °C. It should be noted that molybdenum trioxide can exist both as layered orthorhombic α-MoO
3 and monoclinic β-MoO
3 which has monoclinic rutile type structure formed by edge-sharing MoO
6 octahedra [
29]. The XRD patterns of Mo (300), Mo (500) and Mo (700) show also an evolution as the calcination temperature increases. In the case of Mo (700), a mixture of crystalline phases is present that apparently contains MoO
3 and MoO
2.69(OH)
0.3. On the other hand, Mo (500) seems to contain both MoO
2.5(OH)
0.5 and MoO
2.69(OH)
0.3. As for the Mo (300) sample, in addition to MoO
2.5(OH)
0.5 and MoO
2.69(OH)
0.3, the presence of compounds containing ammonium cannot be ruled out.
In the case of the supported catalysts (calcined at 800 °C), no crystalline molybdenum compound could be identified in the XRD patterns of the samples containing less than 16 wt.% MoO
3 which suggests that the supported species are very well dispersed over the alumina support. However, new diffraction peaks appeared between 20 and 26° (2θ) in the XRD pattern of the s-Mo (16) sample that could correspond to aluminum molybdate (Al
2(MoO
4)
3) and to a series of polyoxo Mo species, as reported in the literature for 16 wt.% MoO
3 supported on alumina and calcined at different temperatures between 527 and 827 °C [
26]. Kitano et al. [
30] investigated a series of alumina-supported molybdenum catalysts calcined at 800 °C containing between 5 and 30 wt.% MoO
3. No XRD peaks corresponding to Mo compounds were found in the diffraction patterns of the catalysts with MoO
3 loadings below 11 wt.% whereas for contents above 13 wt.% Al
2(MoO
4)
3 could be detected. The authors estimated at 10 wt.% the MoO
3 loading required to form a surface monolayer. It was proposed that two-dimensional molybdenum oxide domains and some three-dimensional MoO
3 clusters were formed on 11 wt.% MoO
3/Al
2O
3 whereas the MoO
3 clusters were transformed into aluminum molybdate at higher metallic contents. As can be seen, the XRD results reveal a complex nature of the Mo catalysts that is due to the rich chemistry of this metal.
As expected for the bulk catalysts, these solids showed low specific surface areas that decreased from 17.0 m
2/g for Mo (300) to 4.9 m
2/g for Mo (500) and finally 1.6 m
2/g for Mo (700) as the AHM calcination temperature increased. As for the supported catalysts, their specific surface areas (S
BET) are compiled in
Table 1; all the samples, included the support, were calcined at 800 °C.
It can be seen that a gradual decrease of the S
BET takes place as the MoO
3 loading increases up to 13 wt.%. However, the additional decrease of specific surface area taking place upon an additional MoO
3 loading increase up to 16 wt.% is low resulting in a S
BET of 44 m
2/g for s-Mo (16), which represents a decrease of 60% with respect to the alumina support. This indicates a considerable blockage of the porous network, which is not accompanied by the formation of detectable Mo crystalline phases until a 16 wt.% MoO
3 loading is reached, as evidenced by the XRD results. Following the calculations performed by Kitano et al. [
30], who assigned a cross-sectional area of 0.22 nm
2 to the octahedral MoO
6 unit, the molybdenum oxide required to form a monolayer over the alumina support used in this work can be estimated at about 11 wt.%. Taking into account these results it could be suggested that in the s-Mo (6) to s-Mo (13) series of samples, Mo oxide seems to be present mainly in the form of a two-dimensional structure well dispersed over the support surface.
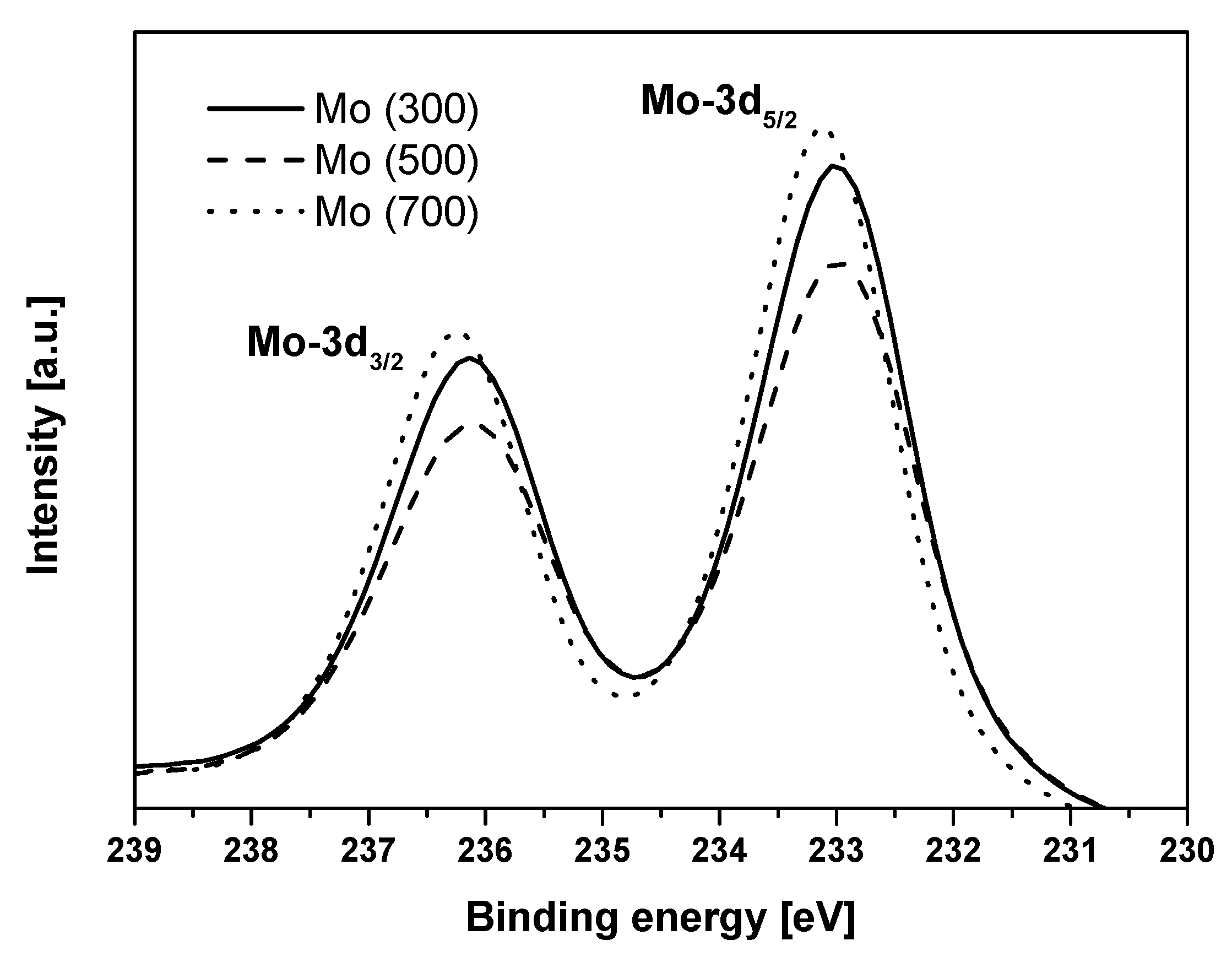
The X-ray photoelectron spectroscopy (XPS) results of the bulk molybdenum catalysts are shown in
Figure 2.
In all cases, the XP spectra are characterized by two well-resolved contributions at binding energies of 233.0–233.1 and 236.2–236.3 eV that can be assigned to the Mo 3d
5/2 and Mo 3d
3/2 spin-orbit components of Mo (VI), respectively. The splitting energy of this doublet (3.2 eV) agrees well with the values reported in the literature for Mo (VI) [
31]. The presence of molybdenum in oxidation states other than Mo (VI) seems to be not significant though corresponding 3d
5/2 spectral lines have been reported at significantly lower binding energy values (231.6 eV for Mo (V)) [
32]. Choi and Thompson indicated values for the Mo 3d
5/2 line of polycrystalline MoO
3 between 231.6 and 232.7 eV [
31]. Baltrusaitis et al. [
29] compiled a series of values for Mo (VI) taken from the literature and the binding energies reported were 233.0–233.2 eV (Mo 3d
5/2) and 236.1–236.3 eV (Mo 3d
3/2), which coincide with the values found in this work. These values were measured for a layer of oxide thermally developed on molybdenum metal under controlled O
2 pressure [
33] as well as for alumina-supported cobalt-molybdena catalysts [
34].
Regarding acidity, it was first measured through temperature-programmed desorption of NH
3 (NH
3-TPD), a technique that is not capable of distinguishing between Brönsted and Lewis sites due to the strong basicity of ammonia. As a matter of fact, NH
3 can adsorb on both Lewis and Brönsted sites of MoO
3 as molecular NH
3 and ammonium ion, respectively, though adsorption on Lewis sites is more favorable energetically [
35]. The NH
3-TPD patterns corresponding to the alumina support and a selection of the alumina-supported catalysts are included in
Figure 3.
A main peak showing a maximum within the 147–150 °C temperature range characterizes the patterns. The peaks are broad and show a long tail that extends up to 300–600 °C, depending on the case. Very small peaks centered at about 240 °C and 375 °C can be observed in the alumina NH
3-TPD pattern. It is also apparent that baseline drifting complicated the integration of the peaks, which resulted in the specific acidity values compiled in
Table 1. It can be seen that the specific acidity tends to decrease as the molybdenum oxide content increases. This result suggests that the acidity of the solids decreases as the alumina surface becomes covered by molybdenum oxide species. Sankaranarayanan et al. [
24] found similar NH
3-TPD results for a series of alumina-supported molybdenum oxide catalysts calcined at 527 °C, 677 °C and 827 °C and containing 8wt.%, 12wt.% and 16 wt.% MoO
3. The acidity of the samples calcined at 827 °C decreased from 3.8 μmol/m
2 for the sample loaded with 8 wt.% MoO
3 to 2.8 μmol/m
2 for the catalyst containing 16 wt.% MoO
3. This last result can be compared with the value of 2.0 μmol/m
2 obtained in this work for a sample containing 16 wt.% MoO
3 and calcined at 800 °C (see
Table 1). Accurate assessment of the acidity of the unsupported solids through NH
3-TPD was difficult due to the low specific surface area of these materials and the technical limitations of the equipment used (see
Section 3) to load high amounts of sample. Their specific acidity was somewhat lower than that of the supported solids, ranging between 0.6 μmol/m
2 for Mo (300) and 2.0 μmol/m
2 for Mo (500) and Mo (700).
Representative FTIR spectra of adsorbed pyridine corresponding to bulk (Mo (500)) and Al
2O
3-supported catalysts (s-Mo (8)) are shown in
Figure 4. Pyridinium ions adsorbed over Brönsted sites exhibit characteristic bands at wavenumbers of 1546–1548 cm
−1 (ν
19b), and 1638–1640 cm
−1 (ν
8a) [
36,
37]. The spectra of Mo (500) and s-Mo (8) show weak and broad bands at 1634 and 1638 cm
−1 as well as at 1532 and 1542 cm
−1, respectively. These bands are ascribed to Brönsted sites that due to their different nature appear at different wavenumbers. As for the Lewis sites on molybdenum oxide, bands of adsorbed pyridine at 1451 (ν
19b) and 1611 cm
−1 (ν
8a) have been reported [
37]. These values are similar to the ones reported for adsorbed pyridine on Al
3+ in alumina surface: 1450 and 1615 cm
−1 [
36]. The spectrum of the alumina-supported catalyst shows a weak band at 1616 cm
−1 and another very intense absorption signal at 1442 cm
−1 that seems compatible with pyridine adsorption on acidic Lewis sites. In the case of the bulk molybdenum oxide catalyst, the band at 1443 cm
−1 is much weaker and the broad band observed at a wavelength of 1606 cm
−1 could correspond to the one reported at 1611 cm
−1 [
37]. This difference in intensity indicates a lower number of Lewis sites on the surface of Mo(500) catalyst. Moreover, the position of the band corresponding to the (ν
8a) vibration mode of pyridine adsorbed on Lewis sites is used as an indicator of the Lewis strength. Comparing spectra in
Figure 4, we can conclude that acidic Lewis sites are stronger in the supported catalyst (1616 cm
−1 compared to 1606 cm
−1 in the Mo(500)) [
38]. It is interesting to stress that an intense band at 1594 cm
−1, absent in the Mo(500) spectrum, is detected in the supported catalyst after pyridine adsorption. This band may be ascribed to species formed by interaction of the probe molecule via N-atom with a weakly acidic H from the surface (denoted as HPy) [
39]. Therefore, the new Brönsted sites (unable to protonate pyridine) appear on the supported catalyst, probably due to the interaction of MoO
3 with the alumina. It is concluded that both, bulk and supported molybdenum oxide catalysts, have Brönsted and Lewis acid sites, though they are much more abundant and stronger in the case of the supported solids, which is logical due to the presence of the alumina support. Moreover, new Brönsted sites are detected in the supported solid that are not present in the unsupported one.
2.2. Catalytic Performance
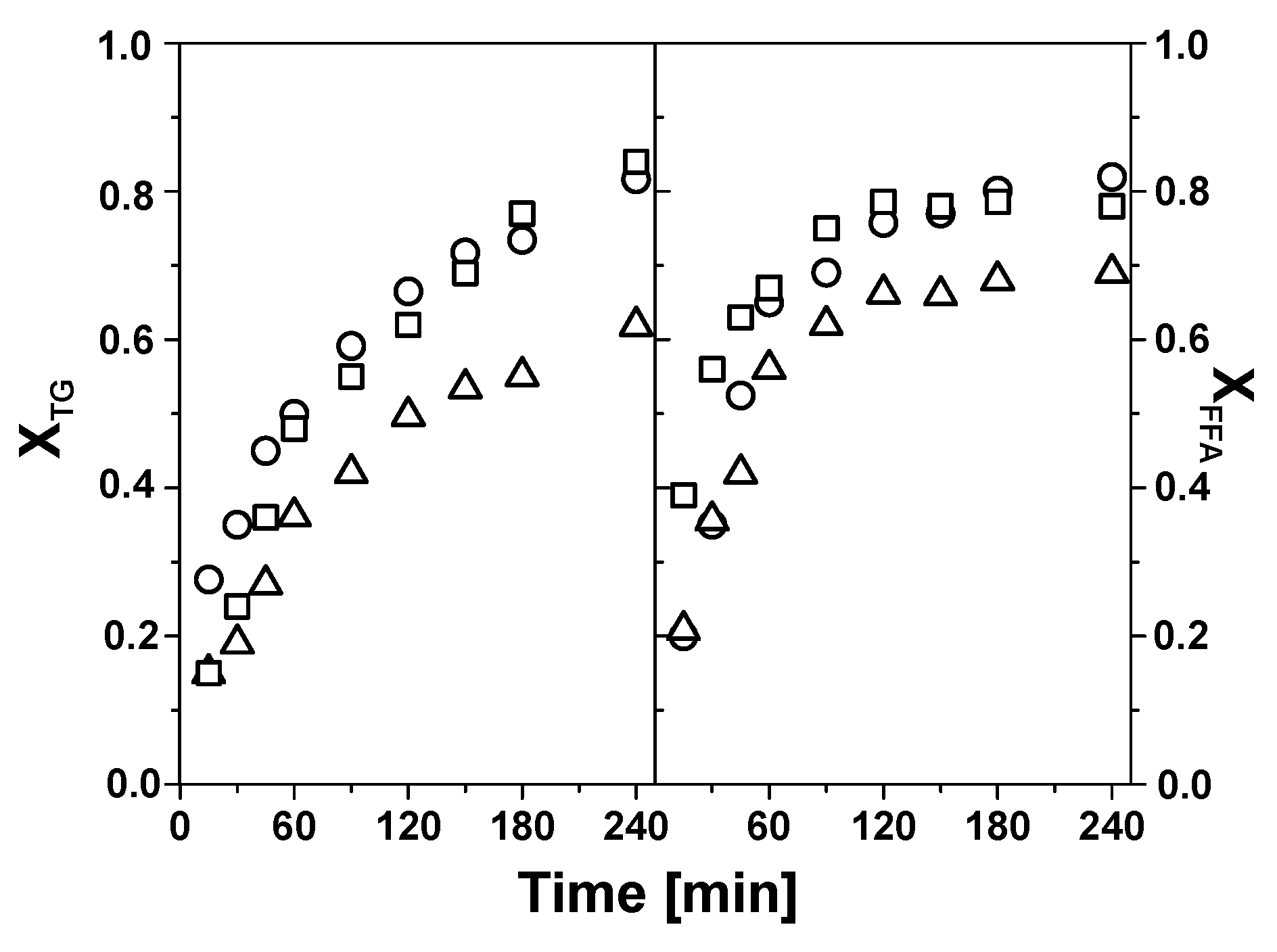
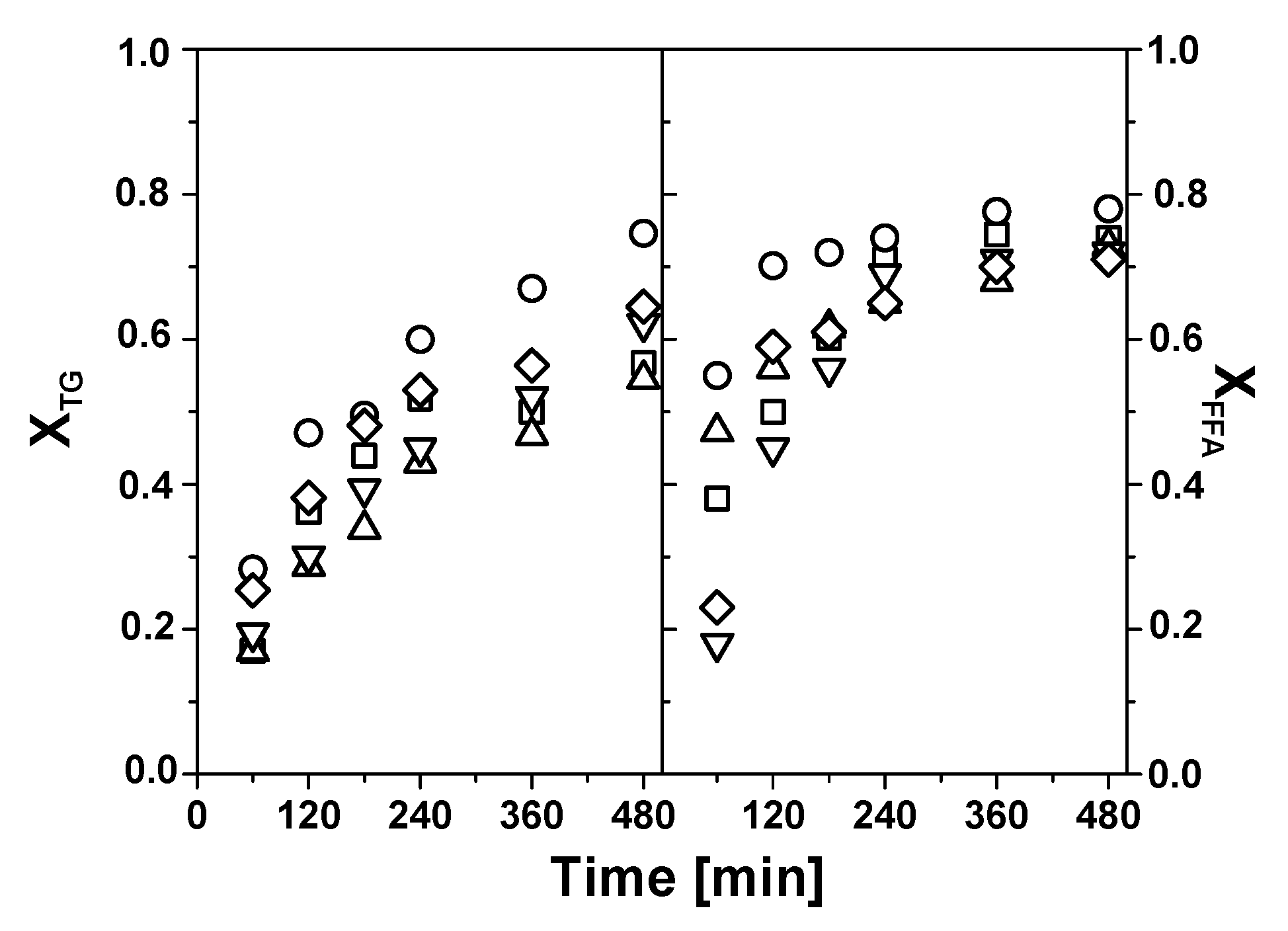
Figure 5 shows the evolution of the conversion of triglycerides (X
TG) and FFAs (X
FFA) with reaction time for the unsupported Mo (300), Mo (500), and Mo (700) catalysts.
It can be seen that both triglycerides and FFAs reach high degrees of conversion (between about 60 and 80%) after 240 min of reaction under the conditions indicated (see
Figure 5 caption). The conversion over Mo (300) and Mo (500) are similar, about 80–85% and 78–82% for the triglycerides and FFAs, respectively, after 240 min of reaction. The conversion over Mo (700) is lower, about 60% (triglycerides) and 70% (FFAs) compared to its counterparts calcined at lower temperatures. As the catalyst concentration is fixed on a mass basis (2 wt.%) it is likely that the main cause of these results is the large decrease of the specific surface area experienced by Mo (700) upon calcination. It is also noteworthy that, irrespective of the catalyst used, X
TG gradually increases with reaction time, whereas FFAs conversion shows a faster initial increase until about 120 min of reaction, with a much slower increase taking place afterward.
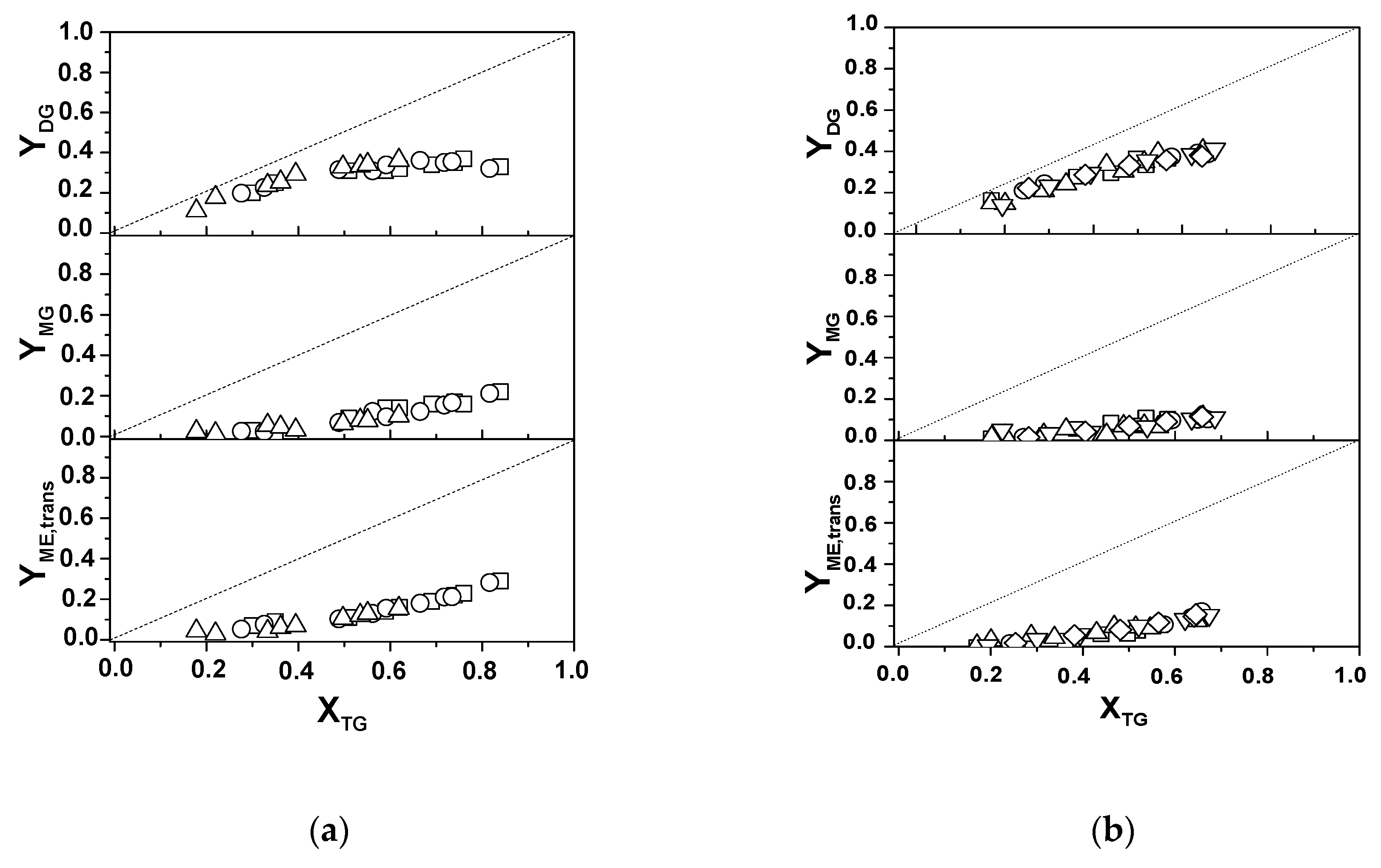
Regarding the yields of the several reaction products,
Figure 6a shows the evolution with X
TG of the diglycerides (Y
DG), monoglycerides (Y
MG), and methyl esters produced by transesterification (Y
ME,trans.) (see Equations (3)–(5) in
Section 3).
The evolution of the products yields is in accordance with the scheme of the triglycerides (TG) methanolysis reaction consisting of three consecutive steps [
4]. In the first step, a molecule of TG is converted into a diglyceride (DG), which then evolves to a monoglyceride (MG) that finally gives glycerol. In each step, a molecule of methanol is consumed and a methyl ester (biodiesel) molecule is formed. This explains why initially, at low X
TG, DG were clearly the most abundant products. As the conversion increases, Y
DG also increases reaching maximum values of about 35% for X
TG within the 60–70% range; then, DG started to decline. The yield of both monoglycerides, and especially methyl esters, increase with the conversion of triglycerides. Maximum biodiesel yields achieved are about 30% and correspond to the highest conversions reached at the end of the catalytic runs (80–85%). It is noteworthy that there are no significant differences among the yields provided by the three unsupported catalysts at similar X
TG, meaning that the selectivity is not affected by the calcination temperature of the unsupported catalysts.
Ferreira Pinto et al. [
23] reported a maximum FAME yield of 64.2% for an unsupported MoO
3 catalyst calcined at 600 °C after 4 h of reaction during the methanolysis of soybean oil acidified with 10 wt.% of oleic acid. Yields were slightly lower (50–56%) for solids calcined at lower temperatures (300–500 °C), and dropped to 43% for MoO
3 calcined at 700 °C. This trend is similar to the one observed in our work. Ferreira Pinto et al. [
23] performed the catalytic tests at considerably higher temperature (150 °C) and methanol to feedstock molar ratio (30:1) than in this work, being the catalyst concentration also higher (5 wt.%), though the vegetable oil contained double FFAs concentration (10 wt.%). These authors found a correlation between the catalytic activity and the acidity of the solids as determined through a titration technique in which aqueous NaOH previously contacted with the catalyst was neutralized with HCl. It was suggested that the changes provoked by the thermal treatment affected the acid properties of the catalysts. The lower biodiesel yields found in this work can be mainly attributed to the milder reaction conditions and lower catalyst concentration employed. On the other hand, the slightly higher specific acidity, developed as the calcination temperature increases, is virtually offset by the accompanying specific surface area decrease.
As for the supported solids,
Figure 7 shows the conversions of triglycerides and FFAs reached by the alumina-supported catalysts loaded with 6 to 16 wt.% MoO
3. It should be noted that experiments carried out with the alumina support under the same reaction conditions provided negligible transesterification and esterification conversions. It merits to be highlighted that the feedstock conversions provided by the bulk catalysts are much higher than those of the supported ones. In this regard, after 240 min of reaction, X
TG and X
FFA reach values between 40–60% and 65–75%, respectively, which are significantly lower than the conversions provided by the unsupported catalysts under similar reaction time (see
Figure 5). Final conversions after 480 min range between 55% and 75% for X
TG and 70–80% for X
FFA. Concerning the effect of the MoO
3 oxide content, it seems that the activity goes through a maximum as the molybdenum oxide content increases. Indeed, both transesterification and esterification conversions increase when passing from s-Mo (6) to s-Mo (8) which is the catalyst providing the highest conversions. Then, the conversions decrease for s-Mo (10), s-Mo (13), and s-Mo (16), which reach relatively similar values.
The findings that the alumina support is not active and that the unsupported catalysts are significantly more active than the alumina-supported ones indicate that the catalytic activity is mainly associated with molybdenum oxide species. Nevertheless, a positive effect of the support in dispersing the active phase is also apparent because the MoO
3 contents were as low as 0.12–0.32 wt.% in the case of the supported catalysts compared with 2 wt.% for the unsupported ones. On the other hand, the acidity of the catalyst as determined by NH
3-TPD does not constitute in this case a suitable measure to predict the activity in the simultaneous transesterification and esterification reactions. This is because alumina showed specific acidities comparable and even higher than those of the supported catalysts (see
Table 1). Notwithstanding, additional effects of the MoO
3-Al
2O
3 interactions on the catalytic activity cannot be ruled out; for instance, the presence of Brönsted acid sites of the [=Mo-O(H)-Al=] bridging type, similar to the ones proposed for MoO
3-Al
2O
3 catalytic systems in other works [
30,
34,
37]. These sites could correspond to the adsorbed pyridinium and HPy species detected by FTIR spectroscopy upon pyridine adsorption over the supported catalysts (see previous section). As the specific surface area decreases with the increase of the MoO
3 content (see
Table 1), the opposite effects of molybdenum oxide on the textural and certain acidic properties could explain that the catalytic activity within the s-Mo (6) to s-Mo (16) series of catalysts reaches a maximum value for an intermediate MoO
3 content, in this case, 8 wt.%.
Sankaranarayanan et al. [
24] found a triglycerides conversion of about 58% (FAME yield of 47%) after 8 h of reaction at 100 °C with methanol to oil molar ratio of 9 using a 16 wt.% MoO
3/Al
2O
3 catalyst calcined at 677 °C at a concentration of 5 wt.%. The feedstock used consisted of refined sunflower oil (non-acidified). This result can be compared with about 64% triglycerides conversion in this work after 8 h of reaction at the same temperature, slightly higher methanol to oil molar ratio (12), but significantly lower concentration (2 wt.%) of the s-Mo (16) catalyst during the methanolysis of sunflower oil acidified with 5 wt.% of FFAs (see
Figure 7). As in our case, Sankaranarayanan et al. [
24] also found that the alumina support was not active for the transesterification reaction within the 60–110 °C range of temperature. As for the effect of the MoO
3 content of the catalysts, these authors found that it depended on the calcination temperature. In this regard, a large increase of activity was found when passing from 12 to 16 wt.% for catalysts calcined at 527 °C that greatly decreased when the calcination temperature increased to 827 °C; moreover, the catalysts calcined at 677 °C were more active than the ones calcined at 827 °C regardless the MoO
3 content [
24]. These results were explained in terms of a higher specific activity of the well-dispersed molybdenum oxide species resulting at relatively low calcination temperatures and the presence of poorly active aluminum molybdate formed at high Mo loadings and calcination temperatures.
The yields of the several products given by the supported catalysts are presented in
Figure 6b. It can be seen that there are no significant differences among the solids, meaning that the MoO
3 content does not affect the catalyst selectivity in the transesterification reaction. The yields of methyl esters were significantly lower than the ones reported by Sankaranarayanan et al. [
24], though it should be remembered that these authors used non-acidified refined sunflower oil. The yields of methyl esters and monoglycerides were also lower than the ones obtained with the unsupported catalysts when the results are compared at the same triglycerides conversion, indicating a different performance, which is ascribed to the different nature of the active sites in both types of solids.
2.3. Catalysts Stability
As it is well-known, a key issue in the field of biodiesel production with heterogeneous catalysts is the chemical stability of the solids in the reaction medium, which is directly connected with both the possibility of reutilizing the catalyst and the quality of the produced biodiesel and glycerol, in particular, the fulfillment of the corresponding standards for their typical uses.
In this work, Mo (500) and s-Mo (8) catalysts were recovered after reaction, thoroughly washed with tetrahydrofuran (THF), which is an excellent solvent for the reaction mixture medium [
40], calcined at their respective original temperatures (500 °C and 800 °C, respectively), and used again. Mo (500) yielded almost the same catalytic activity results than during its first use. XRD and N
2-adsorption data evidenced that no substantial changes took place during reaction. To check the possible occurrence of molybdenum leaching into the reaction mixture, the Mo (500) catalyst was removed after reaction by centrifugation. The upper fraction was transferred to a rotary evaporator to remove the unreacted methanol, resulting in a liquid that acquired an intense blue color that evidenced the presence of molybdenum. The molybdenum concentration was measured by ICP atomic emission spectroscopy resulting in ca. 1000 ppm of Mo. Additionally, Mo (500) was mixed with methanol for 4 h under the typical conditions used in this work. The solid was removed afterwards by centrifugation and the methanol was used in a reaction run. An X
TG value of 75% was obtained after 4 h of reaction, which is only slightly lower than the value of 82% achieved in the presence of the solid catalyst (see
Figure 5). Clearly, leached molybdenum species were capable of homogeneously catalyzing the transesterification and esterification reactions. The fact that the recovered solid gives the same feedstock conversion than the fresh catalyst is not strange provided that the solid represents a sufficiently excess amount with respect to its solubility in the reaction medium, thus guaranteeing the activity during several re-utilization cycles. As a matter of fact, it is likely that the concentration of ca. 1000 ppm of Mo measured is close to the solubility limit of the molybdenum species in the polar phase. Ferreira Pinto et al. [
23] reutilized unsupported MoO
3 in eight consecutive cycles of acidified soybean oil methanolysis. The catalyst was filtered from run to run and was used without washing. A very low loss of activity was observed only during the two last cycles; however, information about Mo leaching was not provided.
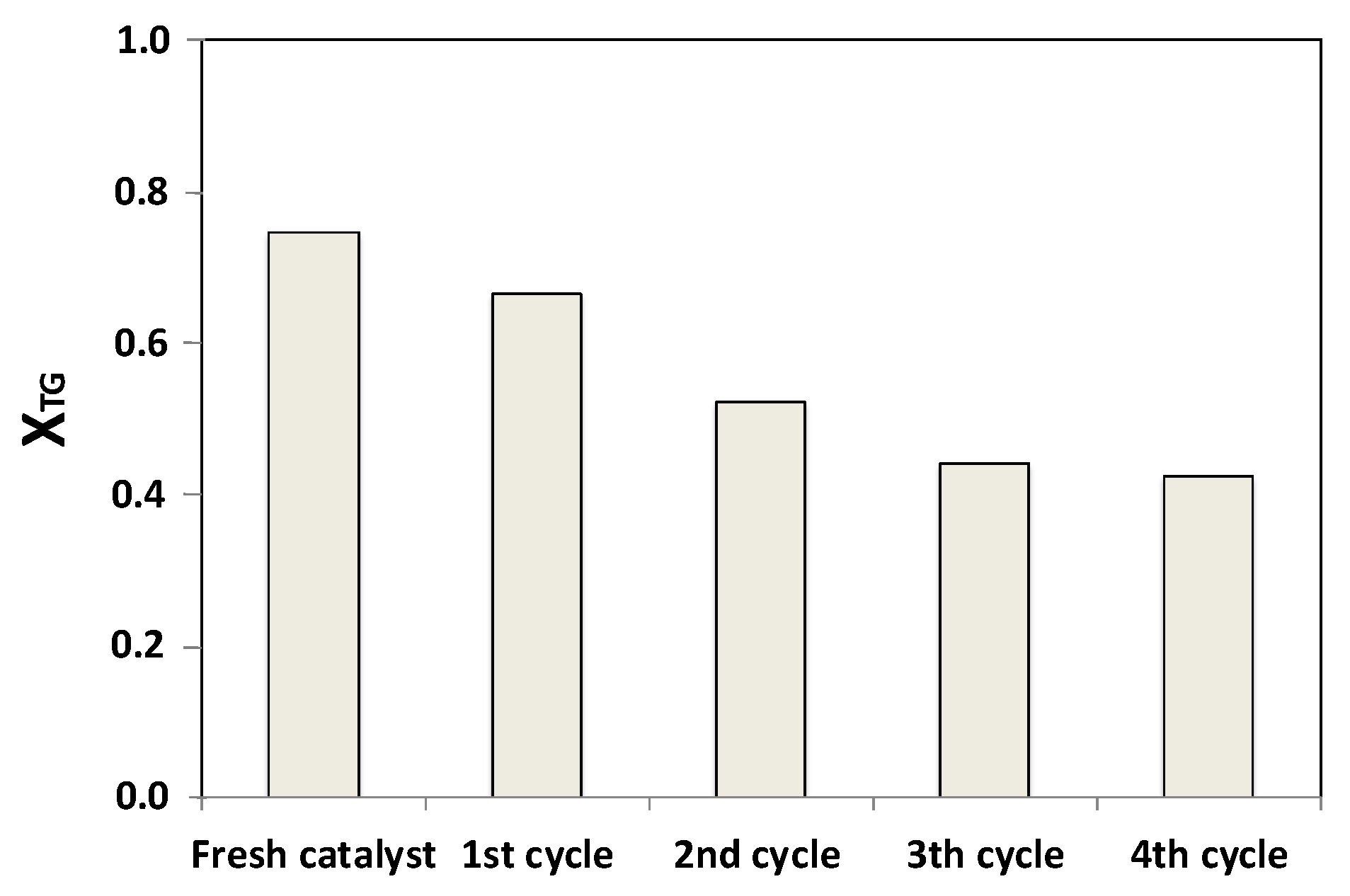
Regarding s-Mo (8), catalyst recovery, washing with THF, calcination, and reuse was repeated four times. The X
TG values recorded after 8 h of reaction are shown in
Figure 8. A gradual decrease of the triglycerides conversion takes place during the first three reaction cycles. The conversion stabilizes at values about 40% after a fourth reaction cycle; a conversion 47% lower than the original value.
As described before for Mo (500), s-Mo (8) was mixed also with methanol for 4 h under the typical conditions used in this work. When used in a reaction run, the recovered methanol yielded XTG and XFFA values of 41% and 46%, respectively, after 4 h of reaction. These conversions were substantially lower than the 60% and 73% values obtained in the presence of fresh s-Mo (8). Mo leaching after the first reaction cycle was investigated following the procedure described above for the unsupported catalyst. Although the Mo concentration could not be measured, the color of the liquid phase resulting after methanol removal evidenced the presence of dissolved Mo. On the whole, the results point to a somewhat improved stability of the supported catalysts compared to the unsupported ones that could be attributed to the interaction established between MoO3 and Al2O3. This interaction has been evidenced by the XRD results that showed the formation of aluminum molybdate, which seems to contribute to an improved dispersion and anchorage of the molybdenum species. This leads to a comparatively high activity for low MoO3 loadings and reduced leaching in comparison with the unsupported catalysts.