Fabrication of a Cu2O-Au-TiO2 Heterostructure with Improved Photocatalytic Performance for the Abatement of Hazardous Toluene and α-Pinene Vapors
Abstract
:1. Introduction
2. Results and Discussion
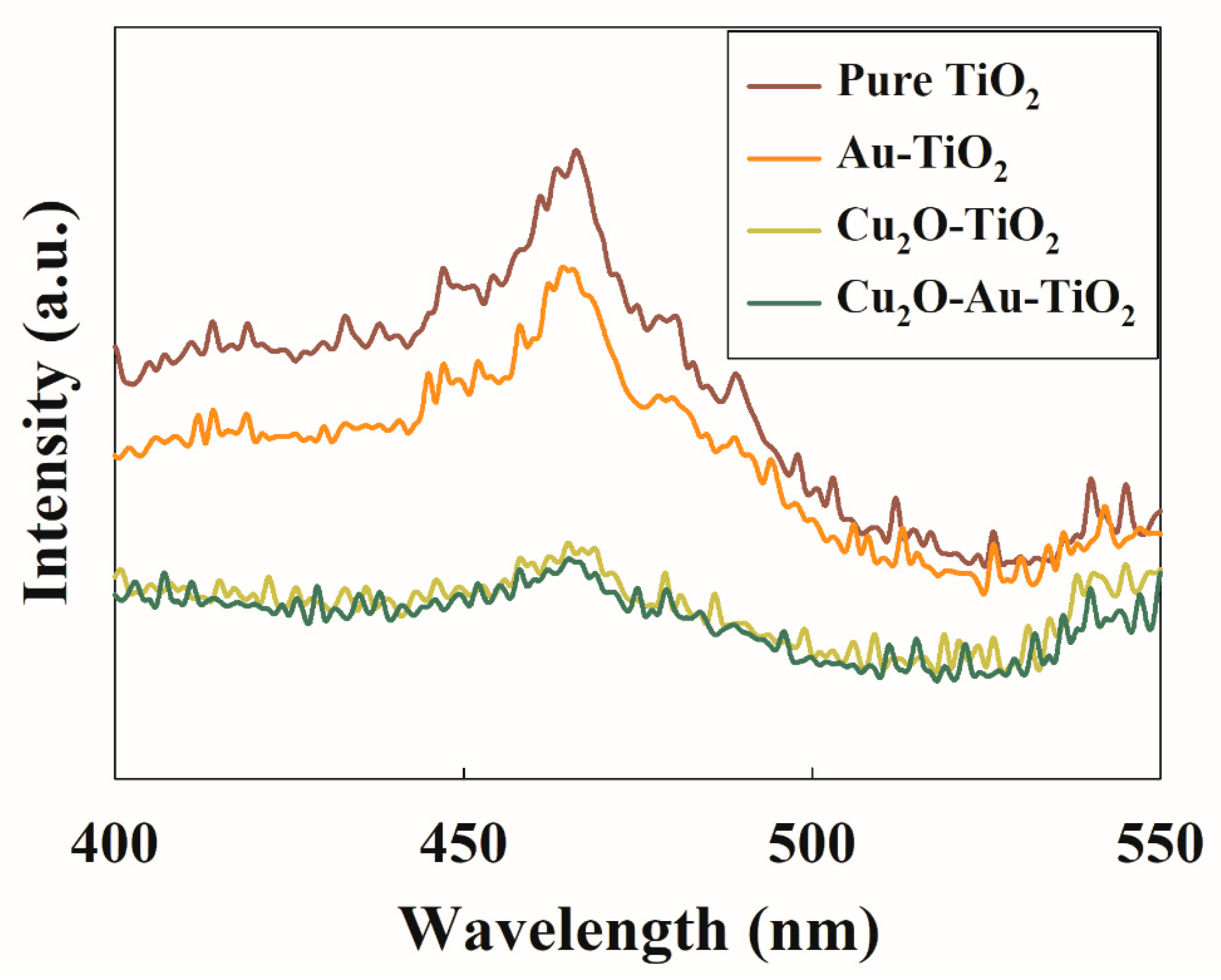
2.1. Characteristics of the Prepared Photocatalysts
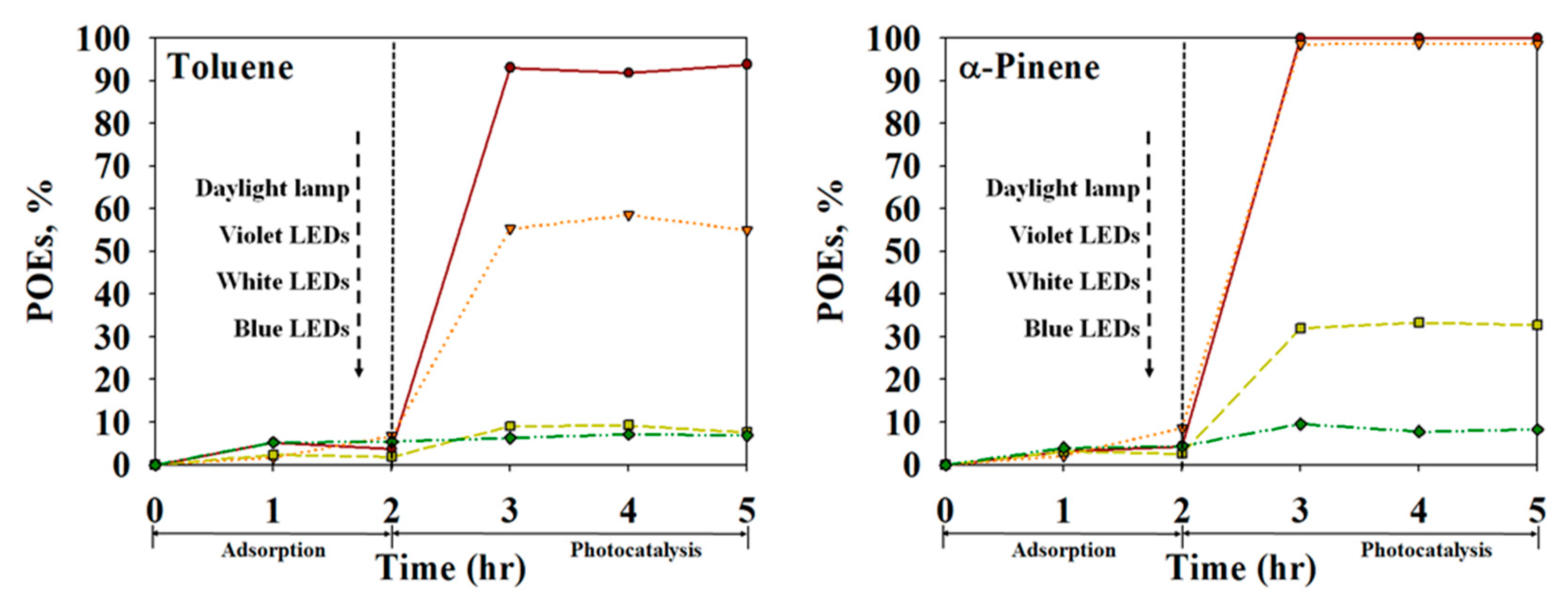
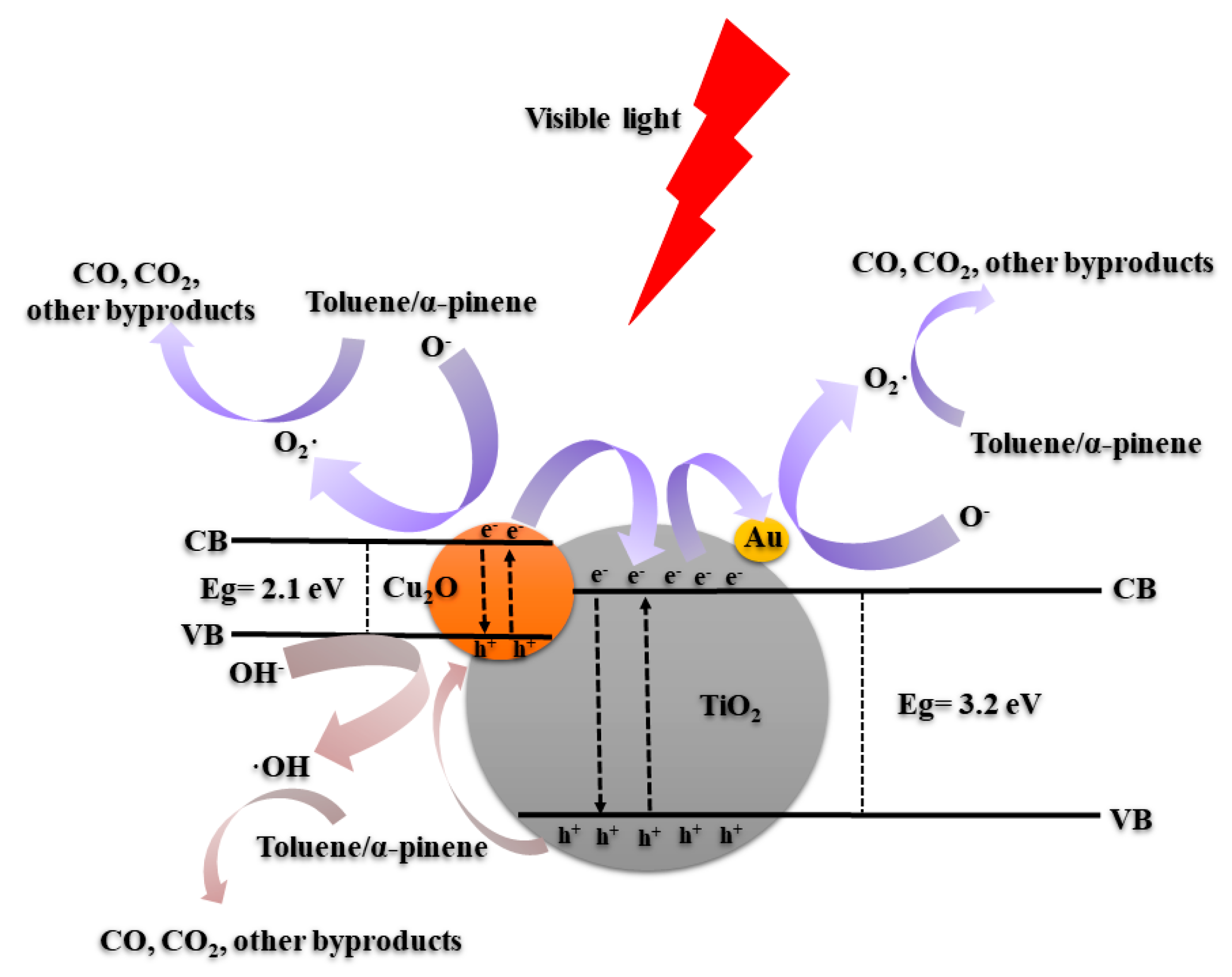
2.2. Photocatalytic Performance
3. Materials and Methods
3.1. Preparation of Photocatalysts
3.2. Characterization
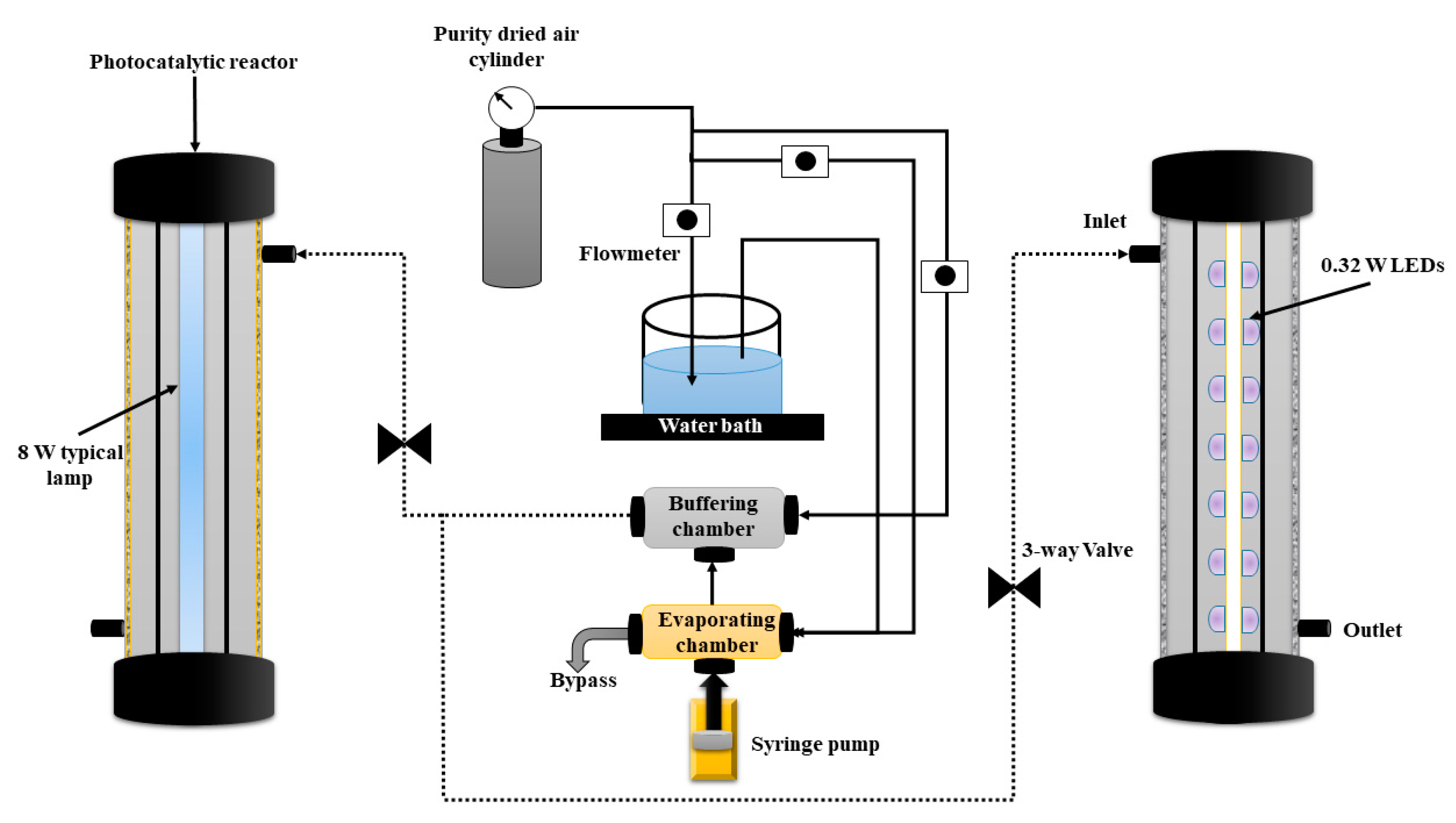
3.3. Evaluation of Photocatalytic Activity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nath, R.K.; Zain, M.F.M.; Jamil, M.l. An environment-friendly solution for indoor air purification by using renewable photocatalysts in concrete: A review. Renew. Sustain. Energy Rev. 2016, 62, 1184–1194. [Google Scholar] [CrossRef]
- Zhu, S.; Xie, X.; Chen, S.-C.; Tong, S.; Lu, G.; Pui, D.Y.H.; Sun, J. Cu-Ni nanowire-based TiO2 hybrid for the dynamic photodegradation of acetaldehyde gas pollutant under visible light. Appl. Surf. Sci. 2017, 408, 117–124. [Google Scholar] [CrossRef]
- Kim, D.J.; Jo, W.K. Mitigation of harmful indoor organic vapors using plug-flow unit coated with 2D g-C3N4 and metallic Cu dual-incorporated 1D titania heterostructure. Chemosphere 2018, 202, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Takigawa, T.; Wang, B.L.; Saijo, Y.; Morimoto, K.; Nakayama, K.; Tanaka, M.; Shibata, E.; Yoshimura, T.; Chikara, H.; Ogino, K.; et al. Relationship between indoor chemical concentrations and subjective symptoms associated with sick building syndrome in newly built houses in Japan. Int. Arch. Occup. Environ. Health 2010, 83, 225–235. [Google Scholar] [CrossRef]
- Król, S.; Namieśnik, J.; Zabiegała, B. α–Pinene, 3-carene and d-limonene in indoor air of Polish apartments: The impact on air quality and human exposure. Sci. Total Environ. 2014, 468–469, 985–995. [Google Scholar] [CrossRef]
- Jo, W.-K.; Moru, S.; Tonda, S. A green approach to the fabrication of a TiO2/NiAl-LDH core–shell hybrid photocatalyst for efficient and selective solar-powered reduction of CO2 into value-added fuels. J. Mater. Chem. A 2020, 8, 8020–8032. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: Mechanisms and materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Nguyen, D.L.T.; Nguyen, V.H.; Le, T.H.; Vo, D.N.; Trinh, Q.T.; Bae, S.R.; Chae, S.Y.; Kim, S.Y.; Le, Q.V. Recent Advances in TiO2-Based Photocatalysts for Reduction of CO2 to Fuels. Nanomaterials 2020, 10, 337. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.; Shin, K.; Lee, Y.J.; Jung, C.; Lee, H.M. Effects of shell thickness on Ag-Cu2O core-shell nanoparticles with bumpy structures for enhancing photocatalytic activity and stability. Catal. Today 2018, 303, 313–319. [Google Scholar] [CrossRef]
- Hara, M.; Kondo, T.; Komoda, M.; Ikeda, S.; Shinohara, K.; Tanaka, A.; Kondo, J.N.; Domen, K. Cu2O as a photocatalyst for overall water splitting under visible light irradiation. Chem. Commun. 1998, 3, 357. [Google Scholar] [CrossRef]
- Trang, T.N.Q.; Tu, L.T.N.; Man, T.V.; Mathesh, M.; Nam, N.D.; Thu, V.T.H. A high-efficiency photoelectrochemistry of Cu2O/TiO2 nanotubes based composite for hydrogen evolution under sunlight. Compos. B Eng. 2019, 174, 106969. [Google Scholar] [CrossRef]
- Praveen Kumar, D.; Lakshmana Reddy, N.; Mamatha Kumari, M.; Srinivas, B.; Durga Kumari, V.; Sreedhar, B.; Roddatis, V.; Bondarchuk, O.; Karthik, M.; Neppolian, B.; et al. Cu2O-sensitized TiO2 nanorods with nanocavities for highly efficient photocatalytic hydrogen production under solar irradiation. Sol. Energy Mater. Sol. Cells 2015, 136, 157–166. [Google Scholar] [CrossRef]
- Aurirre, M.E.; Zhou, R.; Eugene, A.J.; Guzman, M.I.; Grela, M.A. Cu2O/TiO2 heterostructures for CO2 reduction through a direct Z-scheme: Protecting Cu2O from photocorrosion. Appl. Catal. B Environ. 2017, 217, 485–493. [Google Scholar]
- Sun, Q.; Peng, Y.P.; Chen, H.; Chang, K.L.; Qiu, Y.N.; Lai, S.W. Photoelectrochemical oxidation of ibuprofen via Cu2O-dioped TiO2 nanotube arrys. J. Hazard. Mater. 2016, 319, 121–129. [Google Scholar] [CrossRef]
- Wang, X.; Dong, H.; Hu, Z.; Qi, Z.; Li, L. Fabrication of a Cu2O/Au/TiO2 composite film for efficient photocatalytic hydrogen production from aqueous solution of methanol and glucose. Mater. Sci. Eng. B 2017, 219, 10–19. [Google Scholar] [CrossRef]
- Sinatra, L.; LaGrow, A.P.; Peng, W.; Kirmani, A.R.; Amassian, A.; Idriss, H.; Bakr, O.M. A Au/Cu2O–TiO2 system for photo-catalytic hydrogen production. A pn-junction effect or a simple case of in situ reduction? J. Catal. 2015, 322, 109–117. [Google Scholar] [CrossRef]
- Li, J.-M.; Tsao, C.-W.; Fang, M.-J.; Chen, C.-C.; Liu, C.-W.; Hsu, Y.-J. TiO2-Au-Cu2O Photocathodes: Au-Mediated Z-Scheme Charge Transfer for Efficient Solar-Driven Photoelectrochemical Reduction. ACS Appl. Nano Mater. 2018, 1, 6843–6853. [Google Scholar] [CrossRef]
- Hoque, M.; Guzman, M.; Hoque, M.A.; Guzman, M.I. Photocatalytic Activity: Experimental Features to Report in Heterogeneous Photocatalysis. Materials 2018, 11, 1990. [Google Scholar] [CrossRef] [Green Version]
- Kisch, H.; Bahnemann, D. Best Practice in Photocatalysis: Comparing Rates or Apparent Quantum Yields? J. Phys. Chem. Lett. 2015, 6, 1907–1910. [Google Scholar] [CrossRef]
- Yu, J.G.; Hai, Y.; Jaroniec, M. Photocatalytic hydrogen production over CuO-modified titania. J. Colloid Interf. Sci. 2011, 357, 223–228. [Google Scholar] [CrossRef]
- Castillo-López, D.N.; Pal, U. Green synthesis of Au nanoparticles using potato extract: Stability and growth mechanism. J. Nanopart. Res. 2014, 16, 2571. [Google Scholar] [CrossRef]
- Dong, K.; He, J.; Liu, J.; Li, F.; Yu, L.; Zhang, Y.; Zhou, X.; Ma, H. Photocatalytic performance of Cu2O-loaded TiO2/rGO nanoheterojunctions obtained by UV reduction. J. Mater. Sci. 2017, 52, 6754–6766. [Google Scholar] [CrossRef]
- Jo, W.K.; Kim, Y.G.; Tonda, S. Hierarchical flower-like NiAl-layered double hydroxide microspheres encapsulated with black Cu-doped TiO2 nanoparticles: Highly efficient visible-light-driven composite photocatalysts for environmental remediation. J. Hazard. Mater. 2018, 357, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Jo, W.-K.; Kumar, S.; Eslava, S.; Tonda, S. Construction of Bi2WO6/RGO/g-C3N4 2D/2D/2D hybrid Z-scheme heterojunctions with large interfacial contact area for efficient charge separation and high-performance photoreduction of CO2 and H2O into solar fuels. Appl. Catal. B: Environ. 2018, 239, 586–598. [Google Scholar] [CrossRef]
- Kim, M.; Kim, Y.K.; Lim, S.K.; Kim, S.; In, S.-I. Efficient visible light-induced H2 production by Au@CdS/TiO2 nanofibers: Synergistic effect of core–shell structured Au@CdS and densely packed TiO2 nanoparticles. Appl. Catal. B Environ. 2015, 166–167, 423–431. [Google Scholar] [CrossRef]
- Jo, W.-K.; Moru, S.; Tonda, S. Magnetically responsive SnFe2O4/g-C3N4 hybrid photocatalysts with remarkable visible-light-induced performance for degradation of environmentally hazardous substances and sustainable hydrogen production. Appl. Surf. Sci. 2020, 506, 144939. [Google Scholar] [CrossRef]
- Cheng, W.-Y.; Yu, T.-H.; Chao, K.-J.; Lu, S.-Y. Cu2O-Decorated Mesoporous TiO2 Beads as a Highly Efficient Photocatalyst for Hydrogen Production. ChemCatChem 2014, 6, 293–300. [Google Scholar] [CrossRef]
- Oh, J.T.; Chowdhury, S.R.; Lee, T.l.; Misra, M. Synergetic influence of Au/Cu2O core-shells nanoparticle on optical, photo-electrochemical, and catalytic activities of Au/Cu2O/TiO2 nanocomposite. Dyes Pigm. 2019, 160, 936–943. [Google Scholar] [CrossRef]
- Raj, K.J.A.; Viswanathan, B. Effect of surface area, pore volume and particle size of P25 titania on the phase transformation of anatase to rutile. Indian J. Chem. 2009, 48A, 1378–1382. [Google Scholar]
- Jo, W.K.; Tonda, S. Novel CoAl-LDH/g-C3N4/RGO ternary heterojunction with noTable 2D/2D/2D configuration for highly efficient visible-light-induced photocatalytic elimination of dye and antibiotic pollutants. J. Hazard. Mater. 2019, 368, 778–787. [Google Scholar] [CrossRef]
- Lee, J.Y.; Jo, W.K. Simplified sonochemical preparation of titania embedded with selected metals for purification of benzene and toluene. Ultrason. Sonochem. 2016, 28, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Jo, W.-K.; Kumar, S.; Yadav, P.; Tonda, S. In situ phase transformation synthesis of unique Janus Ag2O/Ag2CO3 heterojunction photocatalyst with improved photocatalytic properties. Appl. Surf. Sci. 2018, 445, 555–562. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.J.; Jo, W.-K. Sustainable treatment of harmful dyeing industry pollutants using SrZnTiO3/g-C3N4 heterostructure with a light source-dependent charge transfer mechanism. Appl. Catal. B Environ. 2019, 242, 171–177. [Google Scholar] [CrossRef]
- Shao, Z.; Zhang, X.; Yang, X.; Zhong, M. Au-Mediated Charge Transfer Process of Ternary Cu2O/Au/TiO2-NAs Nanoheterostructures for Improved Photoelectrochemical Performance. ACS Omega 2020, 5, 7503–7518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.Y.; Choi, J.H. Sonochemical Synthesis of Ce-doped TiO2 Nanostructure: A Visible-Light-Driven Photocatalyst for Degradation of Toluene and O-Xylene. Materials 2019, 12, 1265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.Y.; Jo, W.-K. Three-Dimensional TiO2 Structures Incorporated with Tungsten Oxide for Treatment of Toxic Aromatic Volatile Compounds. Catalysts 2017, 7, 97. [Google Scholar] [CrossRef]
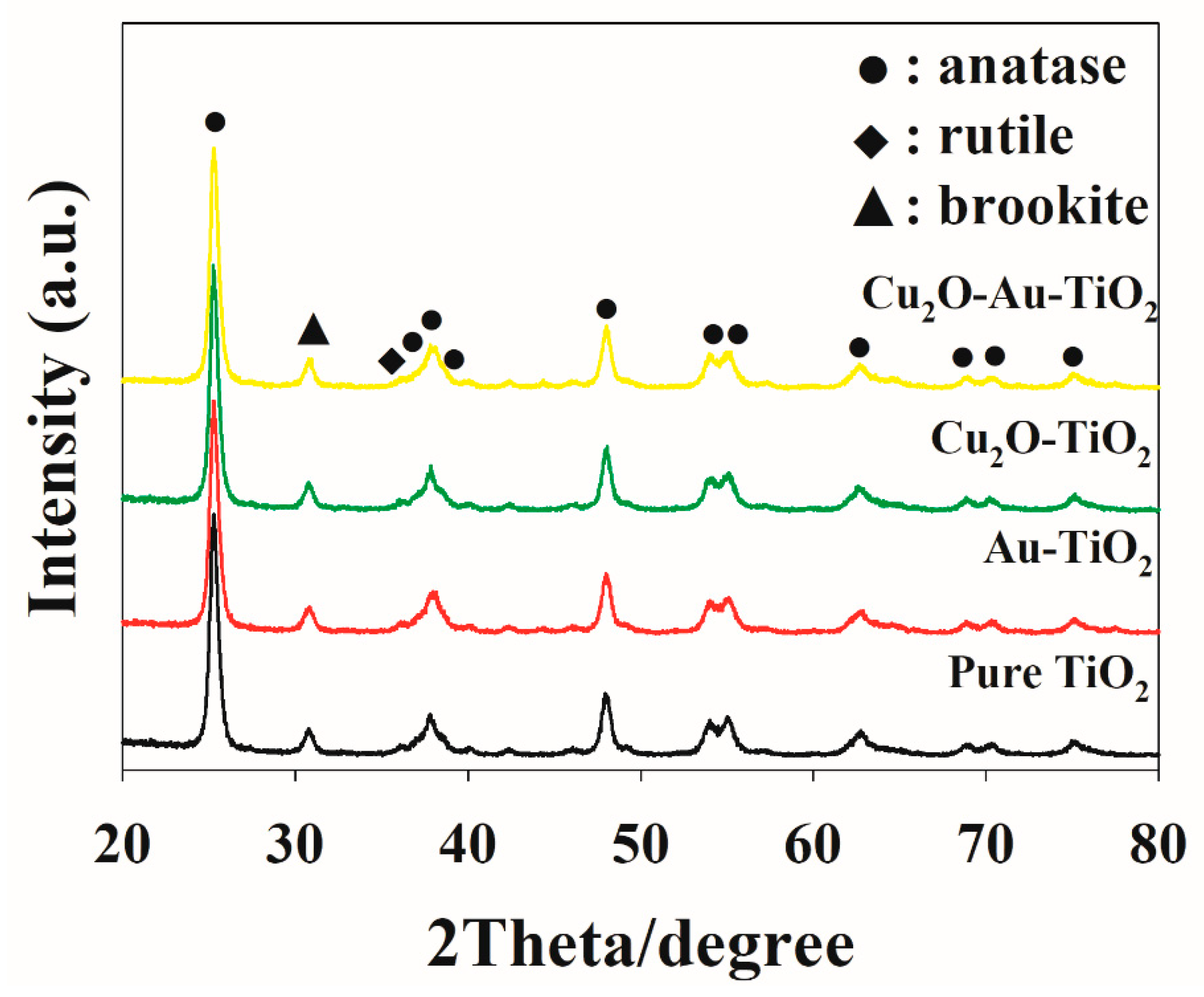
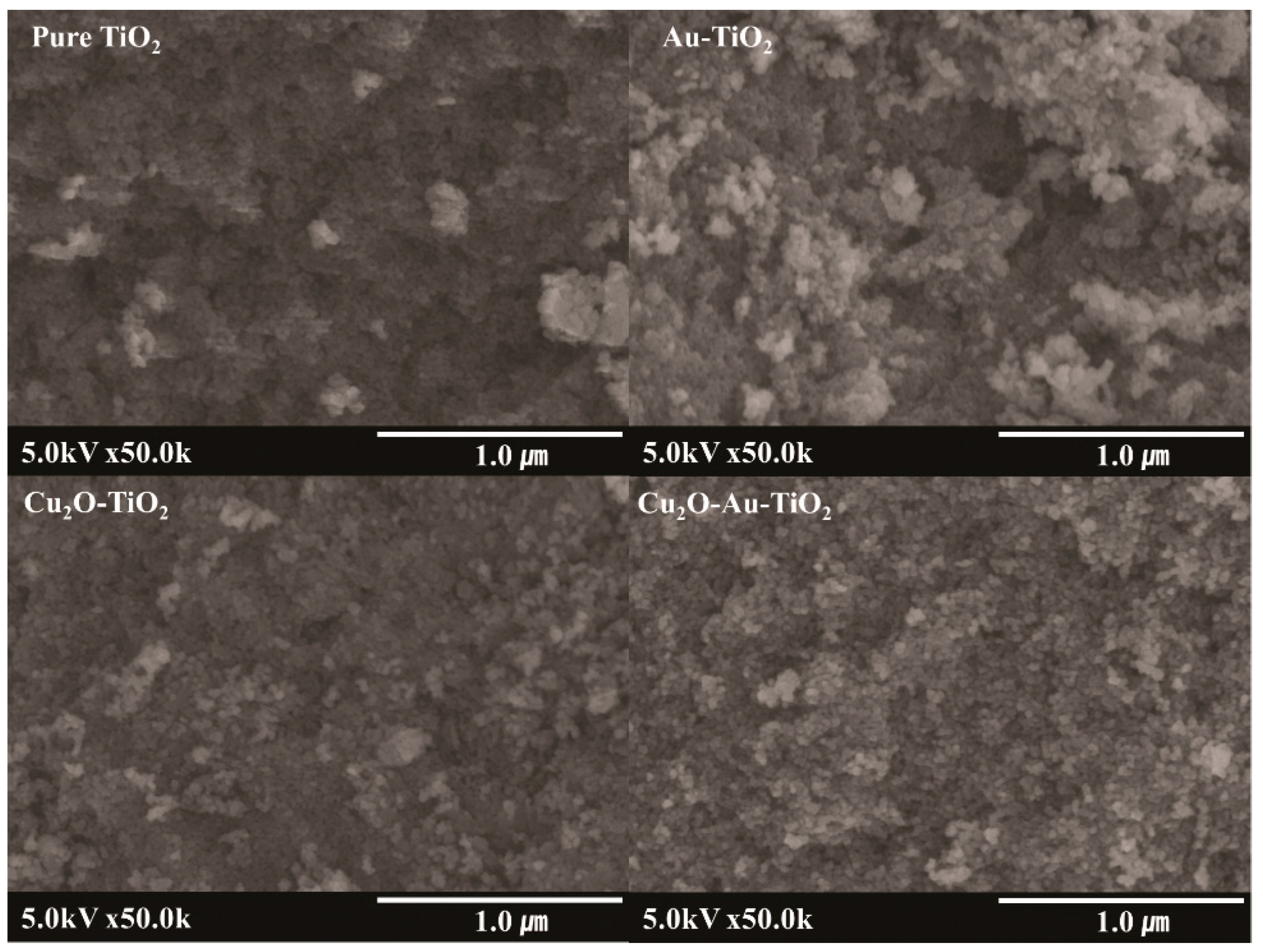
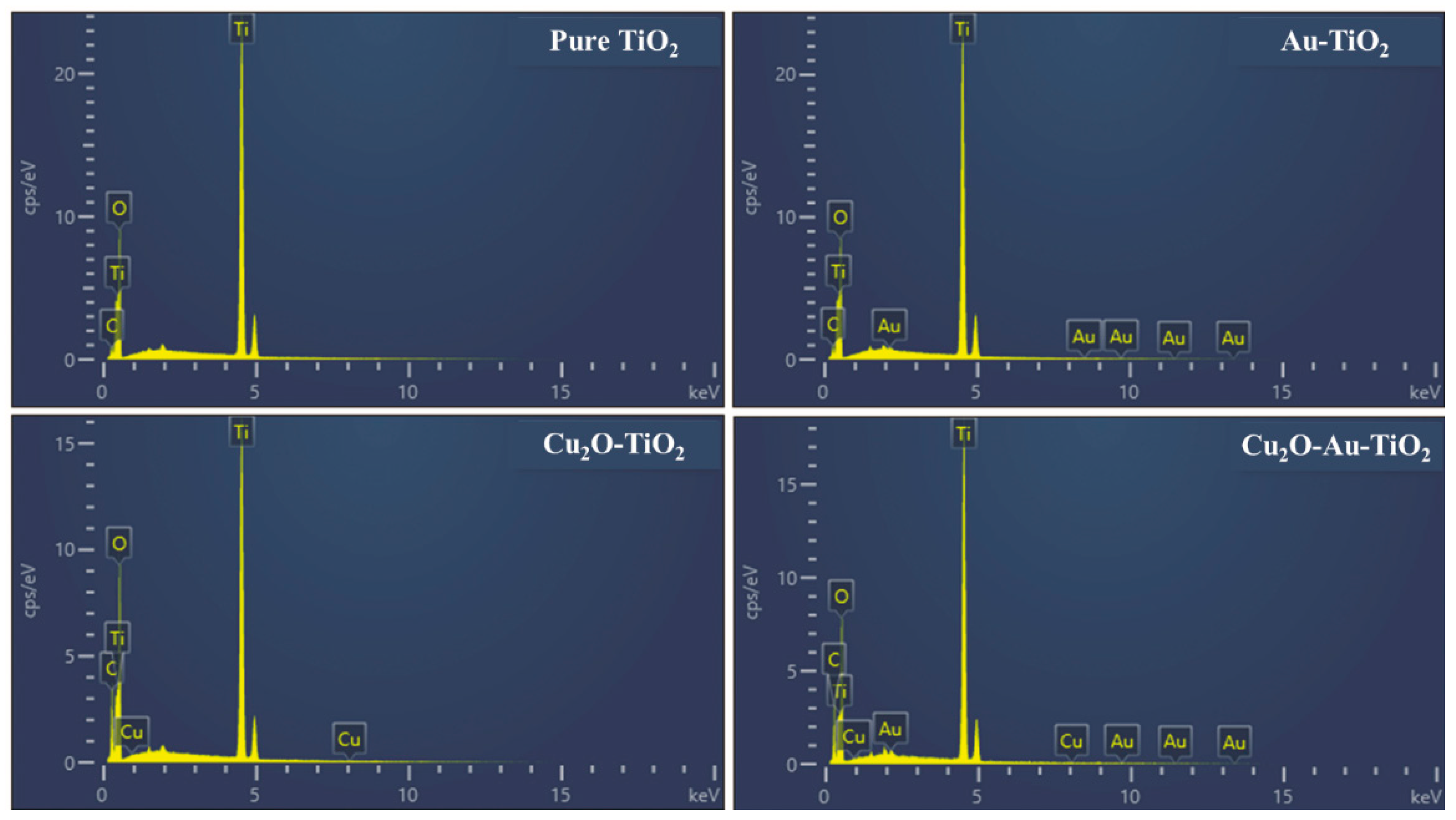
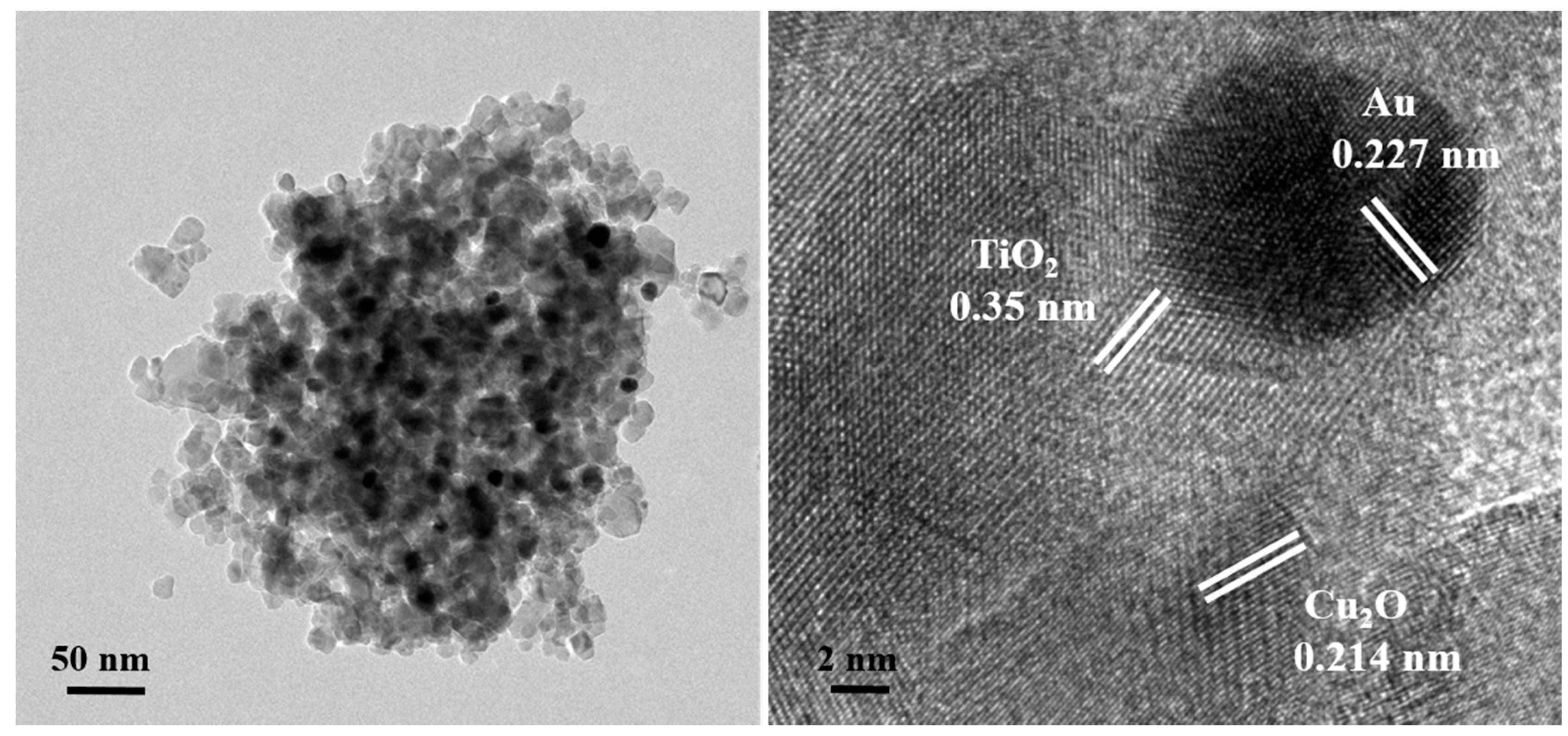
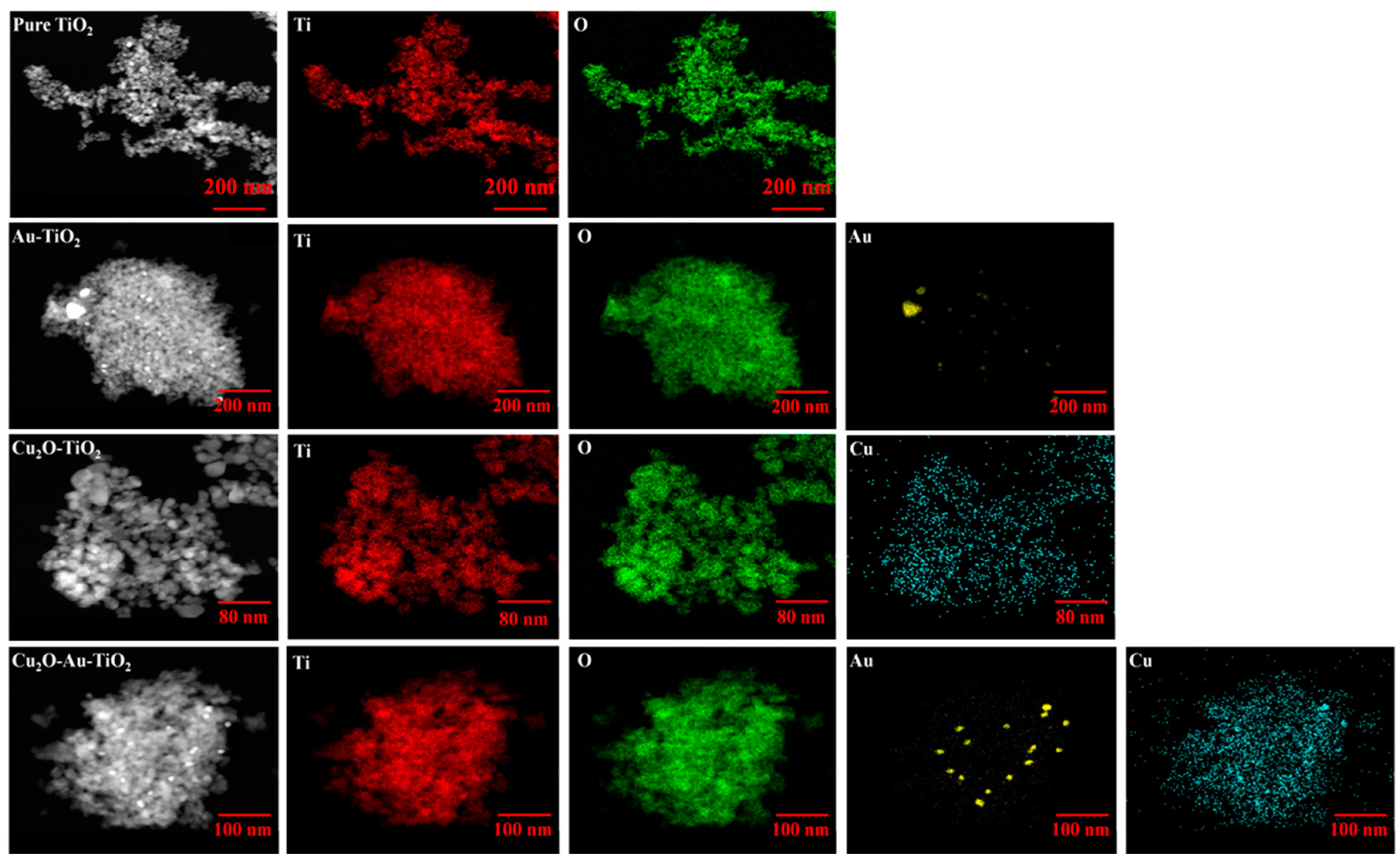
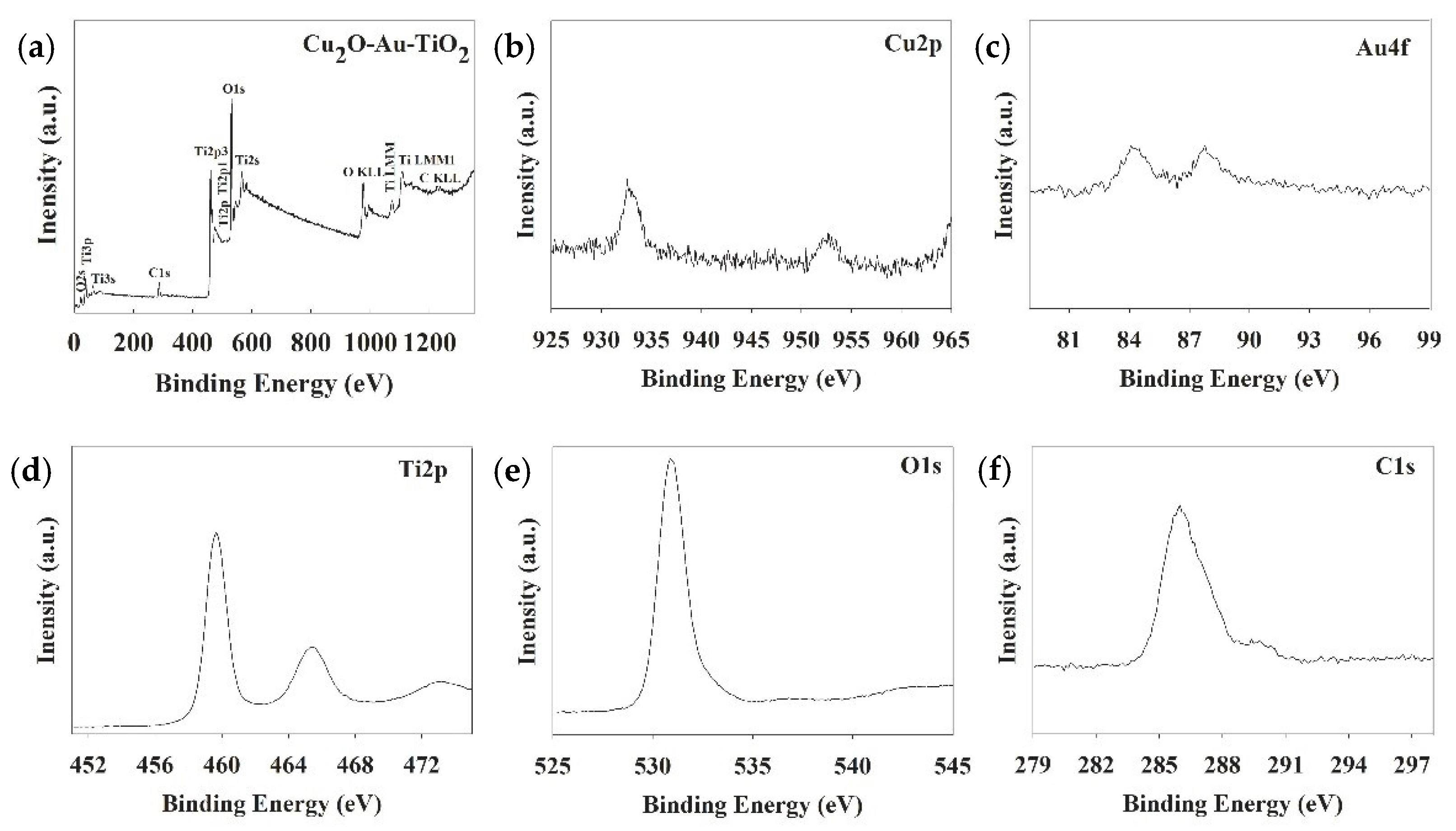
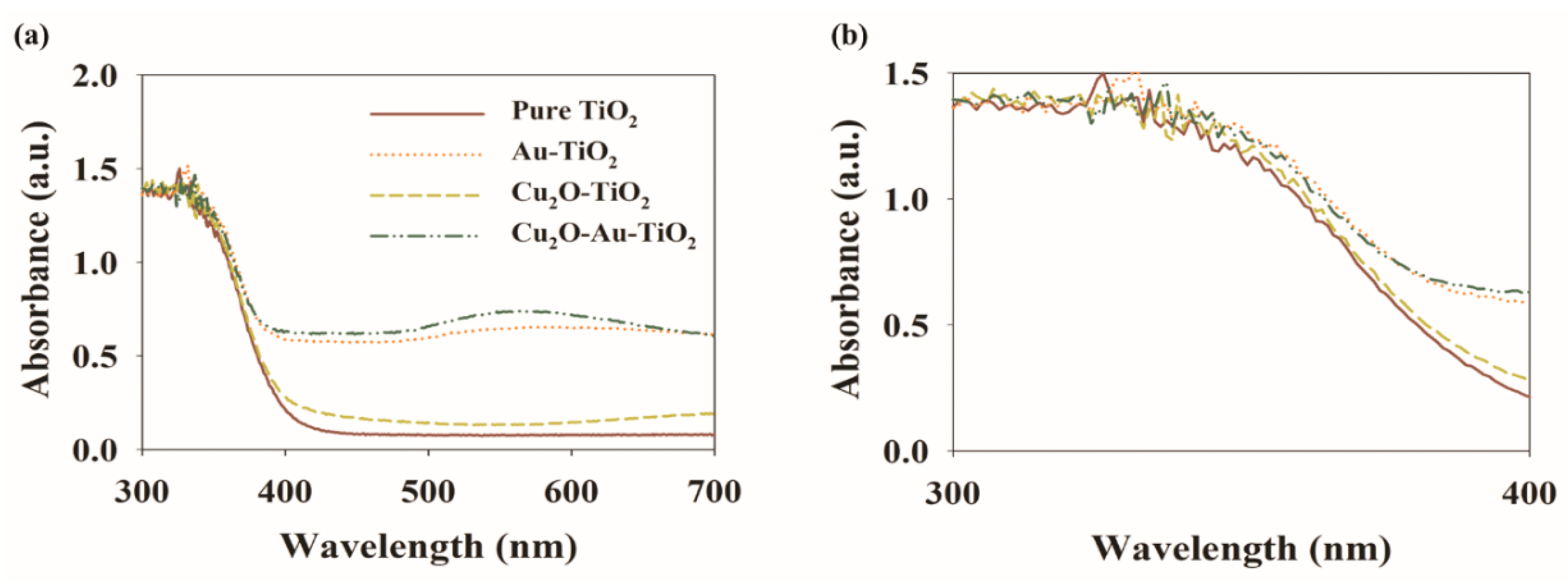
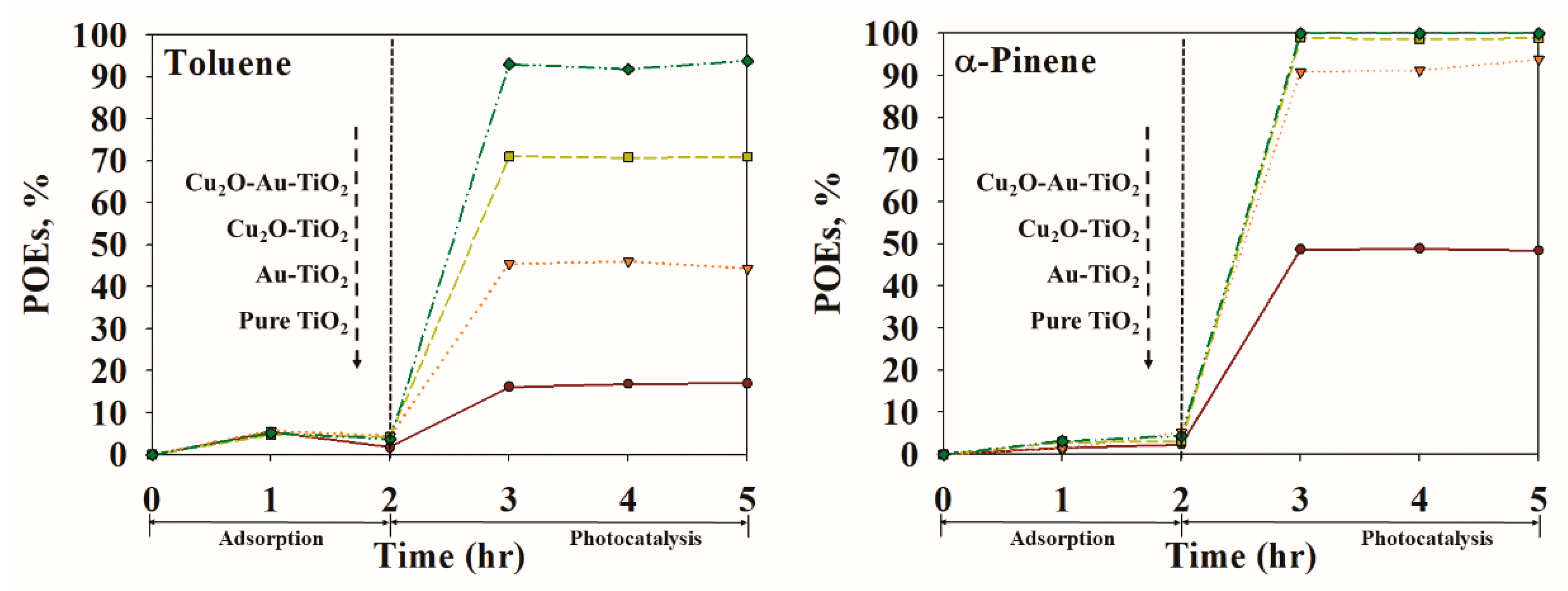
| Photocatalyst | SBET (m2 g−1) | Total Pore Volume (cm3 g−1) | Particle Size (nm) | Pore Size (nm) |
|---|---|---|---|---|
| Pure TiO2 | 58.9 | 0.21 | 24.3 | 13.7 |
| Au-TiO2 | 62.3 | 0.19 | 22.9 | 13.1 |
| Cu2O-TiO2 | 61.2 | 0.22 | 23.3 | 13.9 |
| Cu2O-Au-TiO2 | 61.5 | 0.21 | 23.2 | 13.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.Y.; Choi, J.-H. Fabrication of a Cu2O-Au-TiO2 Heterostructure with Improved Photocatalytic Performance for the Abatement of Hazardous Toluene and α-Pinene Vapors. Catalysts 2020, 10, 1434. https://doi.org/10.3390/catal10121434
Lee JY, Choi J-H. Fabrication of a Cu2O-Au-TiO2 Heterostructure with Improved Photocatalytic Performance for the Abatement of Hazardous Toluene and α-Pinene Vapors. Catalysts. 2020; 10(12):1434. https://doi.org/10.3390/catal10121434
Chicago/Turabian StyleLee, Joon Yeob, and Jeong-Hak Choi. 2020. "Fabrication of a Cu2O-Au-TiO2 Heterostructure with Improved Photocatalytic Performance for the Abatement of Hazardous Toluene and α-Pinene Vapors" Catalysts 10, no. 12: 1434. https://doi.org/10.3390/catal10121434
APA StyleLee, J. Y., & Choi, J.-H. (2020). Fabrication of a Cu2O-Au-TiO2 Heterostructure with Improved Photocatalytic Performance for the Abatement of Hazardous Toluene and α-Pinene Vapors. Catalysts, 10(12), 1434. https://doi.org/10.3390/catal10121434






