Recent Advances in Synthesis and Applications of Carbon-Doped TiO2 Nanomaterials
Abstract
1. Introduction
2. Characterization Techniques for the Formation of Carbon Doping
2.1. XPS Analysis
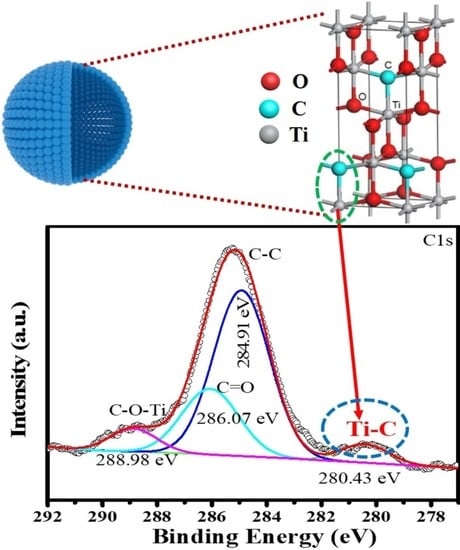
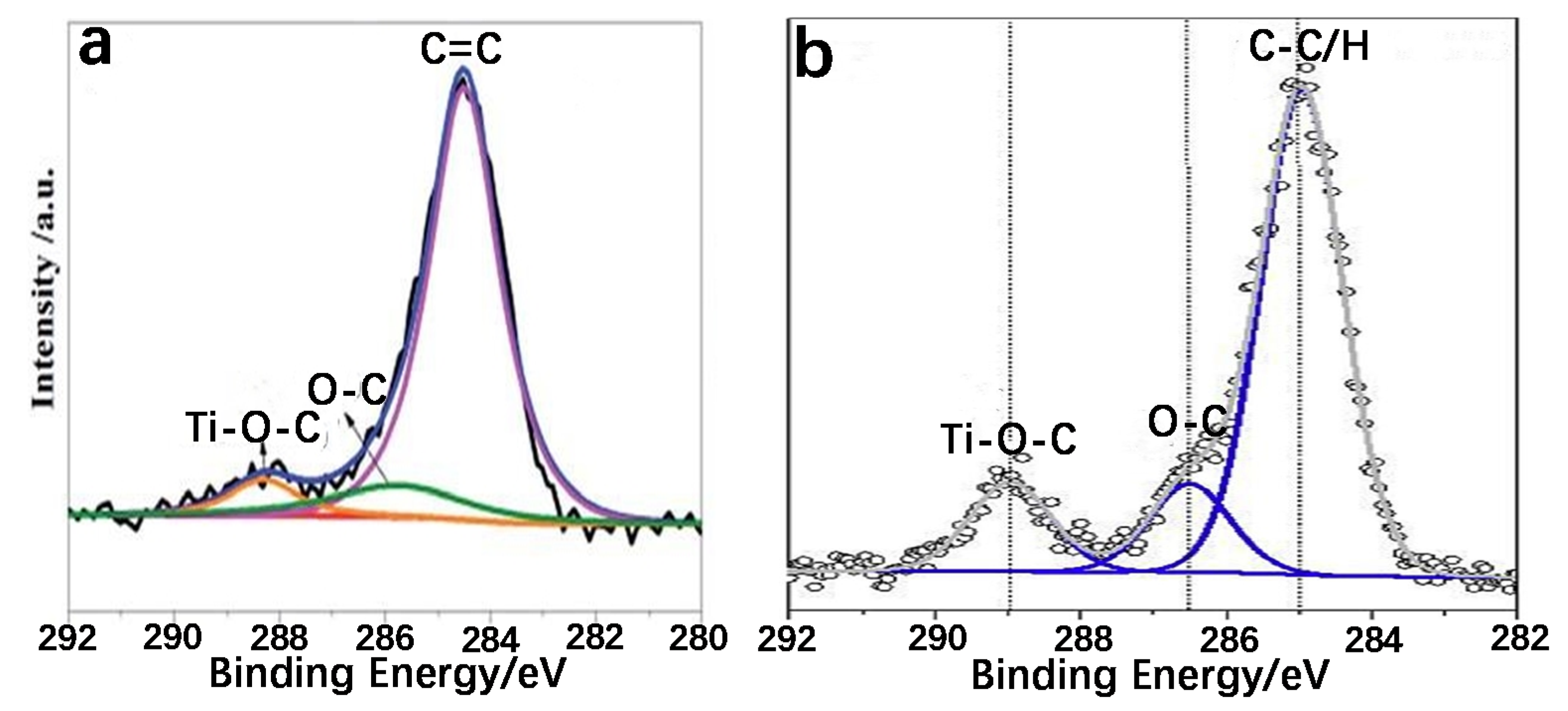
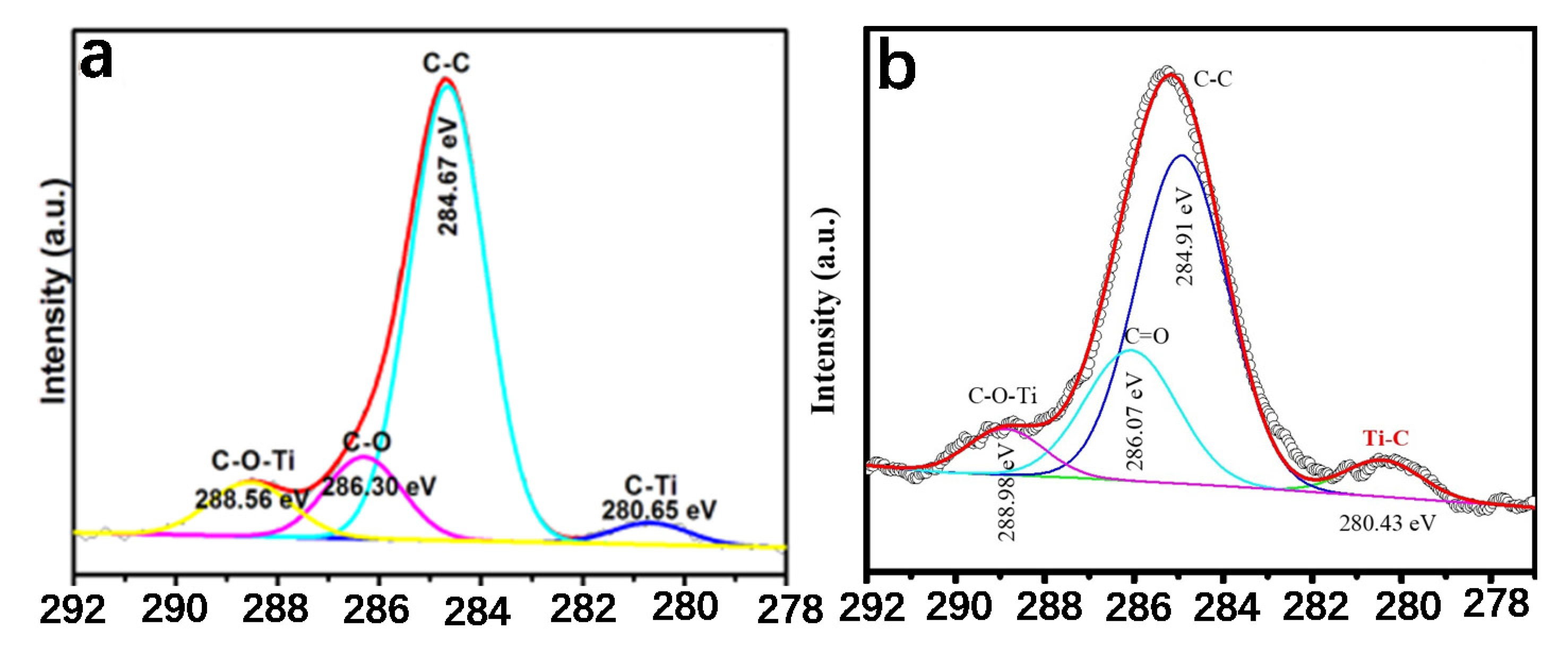
2.1.1. The Existence of Ti–O–C Bond
2.1.2. The Existence of Ti–C Bond
2.2. EPR Analysis
3. Strategies for the Synthesis of C-Doped TiO2 Materials
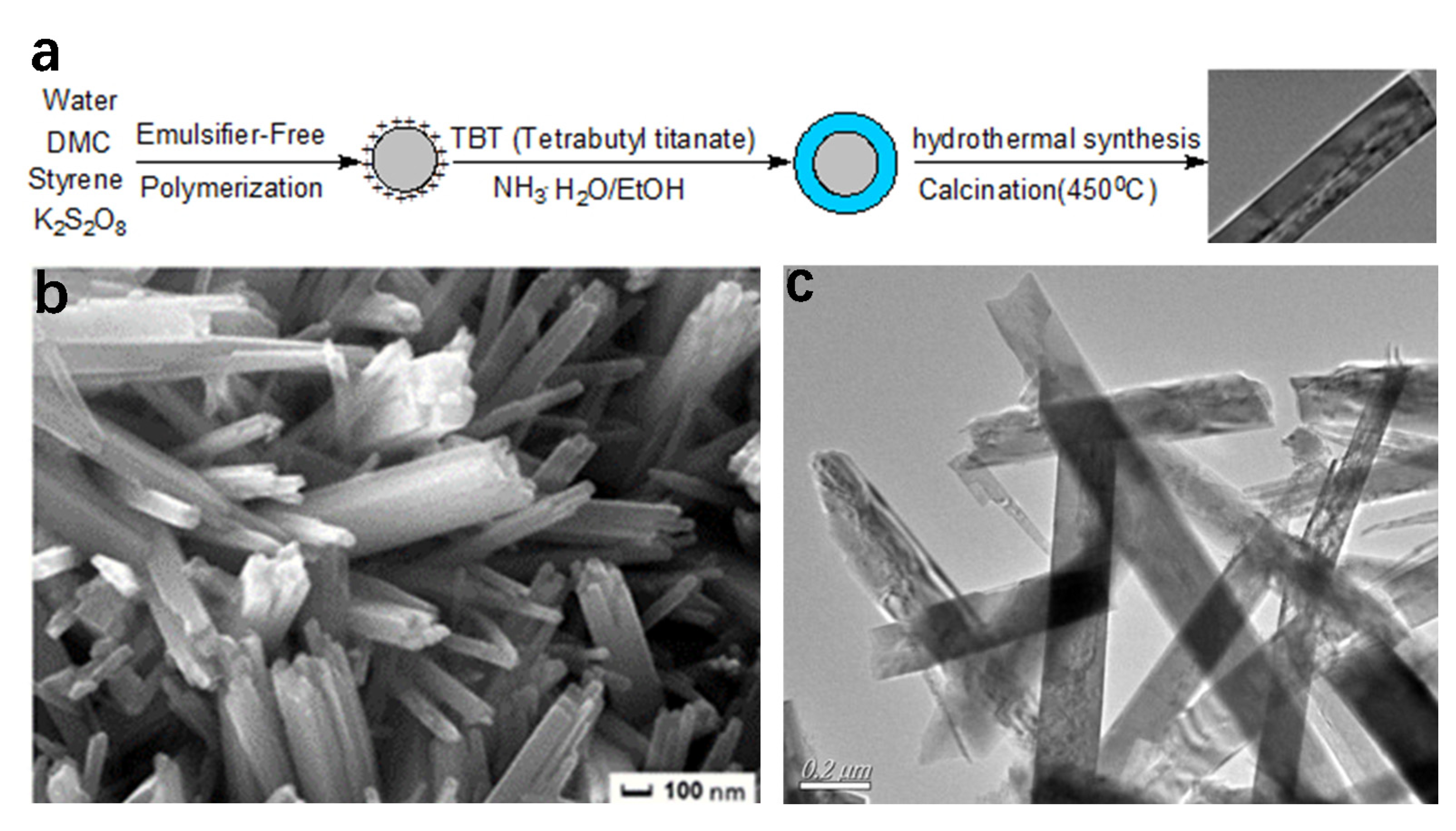
3.1. Hydrothermal Method
3.2. Template-Directed Method
3.3. TiC Calcination
4. Application of C-Doped TiO2 Materials
4.1. The Removal of Organic Pollutants
| Catalyst | Pollutants | Degradation Rate | Enhanced Performance | Reference |
|---|---|---|---|---|
| Carbon-TiO2 nanotubes | Unsymmetrical dimethylhydrazine | 90% | 10% for bare TiO2 | [13] |
| Mesoporous C-TiO2 | Methylthionine chloride | 100% | Improve 10 times than P25 | [75] |
| C-doped anatase TiO2 | Methylene blue | 90% | 3.7 times higher than TiO2 | [66] |
| C-doped ultra-small TiO2 | Toluene | 85% | Less than 60% for bare USTiO2 | [76] |
| C-doped TiO2/α-Fe2O3 heterojunction | Bisphenol A | 79% | 2.7 times higher than pristine TiO2 | [77] |
| C-doped TiO2/anatase (A)/rutile (R) | Nonylphenol | 41% | 8% for undoped TiO2 | [78] |
| C/N-doped TiO2 | Microplastics (MPs) | 71.77 ± 1.88% | Combined effect of pH and temperature driving the photodegradation of MPs | [79] |
| C-TiO2 | Rhodamine B | 83.3% (75 min) | Around 15.0% higher than that of P-25 | [80] |
| Carbon doping and coating of TiO2 | Methylene blue | 85% | 5 times higher than pristine TiO2 | [81] |
| Carbon-doped TiO2 | Caffeic Acid | 92% | High adsorption and degradation | [82] |
| N/C co-doped TiO2 | Fluoroquinolone antibiotics (LEV) | 95.7% | No visible light activity for Degussa P25 | [83] |
| S, N and C doped mesoporous anatase brookite TiO2 | Microcystic toxins | 100% | 12.2% for un-doped TiO2 | [84] |
| TiO2@C microspheres | Congo red | 94% | 2.7 times higher than N-TiO2 | [16] |
| Carbon-doped TiO2 film | Methyl ethyl ketone | 94% | 41% for P25 | [85] |
4.2. Electrochemical Application
5. Summary and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Li, X.; Yu, J.; Jaroniec, M. Hierarchical photocatalysts. Chem. Soc. Rev. 2016, 45, 2603–2636. [Google Scholar] [CrossRef]
- Liu, S.; Tang, Z.; Sun, Y.; Colmenares, J.C.; Xu, Y. One-dimension-based spatially ordered architectures for solar energy conversion. Chem. Soc. Rev. 2015, 44, 5053–5075. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Chen, J.; Liu, Y.; Jiang, Y.; Jiang, C.; Yin, Z.; Xiao, Y.; Cao, S. Silver Nanoparticles Confined in Shell-in-Shell Hollow TiO2 Manifesting Efficiently Photocatalytic Activity and Stability. Chem. Eng. J. 2019, 367, 249–259. [Google Scholar] [CrossRef]
- Shi, Q.; Zhang, Y.; Sun, D.; Zhang, S.; Tang, T.; Zhang, X.; Cao, S. Bi2O3-Sensitized TiO2 Hollow Photocatalyst Drives the Efficient Removal of Tetracyclines under Visible Light. Inorgan. Chem. 2020. [Google Scholar] [CrossRef]
- Qi, D.; Xing, M.; Zhang, J. Hydrophobic Carbon-Doped TiO2/MCF F Composite as a High Performance Photocatalyst. J. Physical. Chem. C 2014, 118, 7329–7336. [Google Scholar] [CrossRef]
- Yin, Z.; Xie, L.; Cao, S.; Xiao, Y.; Chen, G.; Jiang, Y.; Wei, W.; Wu, L. Ag/Ag2O confined visible-light driven catalyst for highly efficient selective hydrogenation of nitroarenes in pure water medium at room temperature. Chem. Eng. J. 2020, 394, 125036. [Google Scholar] [CrossRef]
- Zhou, X.; Wu, J.; Li, Q.; Zeng, T.; Ji, Z.; He, P.; Pan, W.; Qi, X.; Wang, C.; Liang, P. Carbon decorated In2O3/TiO2 heterostructures with enhanced visible light-driven photocatalytic activity. J. Catal. 2017, 355, 26–39. [Google Scholar] [CrossRef]
- Shao, J.; Sheng, W.; Wang, M.; Li, S.; Chen, J.; Zhang, Y.; Cao, S. In situ synthesis of carbon-doped TiO2 single-crystal nanorods with a remarkably photocatalytic efficiency. Appl. Catal. B Environ. 2017, 209, 311–319. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, J.; Hua, L.; Li, S.; Zhang, X.; Sheng, W.; Cao, S. High photocatalytic activity of hierarchical SiO2@C-doped TiO2 hollow sphere in UV and visible light towards degradation rhodamine B. J. Hazard. Mater. 2017, 340, 309–318. [Google Scholar] [CrossRef]
- Guo, Q.; Zhou, C.; Ma, Z.; Ren, Z.; Fan, H.; Yang, X. Elementary photocatalytic chemistry on TiO2 surfaces. Chem. Soc. Rev. 2016, 45, 3701–3730. [Google Scholar] [CrossRef]
- Zhang, Q.; He, H.; Huang, X.; Yan, J.; Tang, Y.; Wang, H. TiO2@C nanosheets with highly exposed (001) facets as a high-capacity anode for Na-ion batteries. Chem. Eng. J. 2018, 332, 57–65. [Google Scholar] [CrossRef]
- Wang, W.; Xu, D.; Cheng, B.; Yu, J.; Jiang, C. Hybrid carbon@TiO2 hollow spheres with enhanced photocatalytic CO2 reduction activity. J. Mater. Chem. A 2017, 5, 5020–5029. [Google Scholar] [CrossRef]
- Ji, L.; Zhang, Y.; Miao, S.; Gong, M.; Liu, X. In situ synthesis of carbon doped TiO2 nanotubes with an enhanced photocatalytic performance under UV and visible light. Carbon 2017, 125, 544–550. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Z.; Chen, J.; Cheng, L.; Chang, J.; Sheng, W.; Hu, C.; Cao, S. C-doped Hollow TiO2 Spheres: In situ Synthesis, Controlled Shell Thickness, and Superior Visible-light Photocatalytic Activity. Appl. Catal. B Environ. 2015, 165, 715–722. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, X.; Qin, J.; Shen, X.; Yu, R.; Ma, M.; Liu, R. Porous carbon-doped TiO2 on TiC nanostructures for enhanced photocatalytic hydrogen production under visible light. J. Catal. 2017, 347, 36–44. [Google Scholar] [CrossRef]
- Guo, H.; Zheng, Z.; Chen, J.; Weng, W.; Huang, M. Facile template-free one-pot fabrication of TiO2@C microspheres with high visible-light photocatalytic degradation activity. J. Ind. Eng. Chem. 2016, 36, 306–313. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Q.; Yang, J.; Ma, H.; Tade, M.O.; Wang, S.; Liu, J. Facile synthesis of carbon-doped mesoporous anatase TiO2 for the enhanced visible-light driven photocatalysis. Chem. Commun. 2014, 50, 13971–13974. [Google Scholar] [CrossRef]
- Shao, Y.; Cao, C.; Chen, S.; He, M.; Fang, J.; Chen, J.; Li, X.; Li, D. Investigation of nitrogen doped and carbon species decorated TiO2 with enhanced visible light photocatalytic activity by using chitosan. Appl. Catal. B Environ. 2015, 179, 344–351. [Google Scholar] [CrossRef]
- Jia, J.; Li, D.; Wan, J.; Yu, X. Characterization and mechanism analysis of graphite/C-doped TiO2 composite for enhanced photocatalytic performance. J. Ind. Eng. Chem. Res. 2016, 33, 162–169. [Google Scholar] [CrossRef]
- Zhang, P.; Shao, C.; Zhang, Z.; Zhang, M.; Mu, J.; Guo, Z.; Liu, Y. TiO2@carbon core/shell nanofibers: Controllable preparation and enhanced visible photocatalytic properties. Nanoscale 2011, 3, 2943–2949. [Google Scholar] [CrossRef]
- Choi, H.; Kim, J.; Kim, H.; Lee, S.; Lee, Y. Improving the Electrochemical Performance of Hybrid Supercapacitor using Well-organized Urchin-like TiO2 and Activated Carbon. Electrochim. Acta 2016, 208, 202–210. [Google Scholar] [CrossRef]
- Ribeiro, E.; Plantard, G.; Teyssandier, F.; Maury, F.; Sadiki, N.; Chaumont, D.; Goetz, V. Activated-carbon/TiO2 composites preparation: An original grafting by milling approach for solar water treatment applications. J. Environ. Chem. Eng. 2020, 8, 104115. [Google Scholar] [CrossRef]
- Cao, N.; Gu, M.; Gao, M.; Li, C.; Liu, K.; Zhao, X.; Feng, J.; Ren, Y.; Wei, T. A three-layer photocatalyst carbon fibers/TiO2 seed/TiO2 nanorods with high photocatalytic degradation under visible light. Appl. Surf. Sci. 2020, 530, 147289. [Google Scholar] [CrossRef]
- Huo, J.; Xue, Y.; Wang, X.; Liu, Y.; Zhang, L.; Guo, S. TiO2/carbon nanofibers doped with phosphorus as anodes for hybrid Li-ion capacitors. J. Power Source 2020, 473, 228551. [Google Scholar] [CrossRef]
- Naoi, K.; Kurita, T.; Abe, M.; Furuhashi, T.; Abe, Y.; Okazaki, K.; Miyamoto, J.; Iwama, E.; Aoyagi, S.; Naoi, W.; et al. Ultrafast Nanocrystalline-TiO2(B)/Carbon Nanotube Hyperdispersion Prepared via Combined Ultracentrifugation and Hydrothermal Treatments for Hybrid Supercapacitors. Adv. Mater. 2016, 28, 6751–6757. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Chen, P.; Li, Z.; Du, P.; Yang, Y.; Li, N.; Xiong, J. Flexible carbon nanotubes/TiO2/C nanofibrous film as counter electrode of flexible quasi-solid dye-sensitized solar cells. Thin Solid Films 2020, 711, 138307. [Google Scholar] [CrossRef]
- Basha, G.M.T.; Srikanth, A.; Venkateshwarlu, B. Effect of reinforcement of carbon nanotubes on air plasma sprayed conventional Al2O3-3%TiO2 ceramic coatings. Mater. Today Proceed 2020, 20, 191–194. [Google Scholar] [CrossRef]
- Wu, H.; Wu, X.; Wang, Z.; Aoki, H.; Kutsuna, S.; Jimura, K.; Hayashi, S. Anchoring titanium dioxide on carbon spheres for high-performance visible light photocatalysis. Appl. Catal. B Environ. 2017, 207, 255–266. [Google Scholar] [CrossRef]
- Yao, J.; Mei, T.; Cui, Z.; Yu, Z.; Xu, K.; Wang, X. Hollow carbon spheres with TiO2 encapsulated sulfur and polysulfides for long-cycle lithium-sulfur batteries. Chem. Eng. J. 2017, 330, 644–650. [Google Scholar] [CrossRef]
- Cheng, C.; Tan, X.; Lu, D.; Wang, L.; Sen, T.; Lei, J.; El-Toni, A.M.; Zhang, J.; Zhang, F.; Zhao, D. Carbon-Dot-Sensitized, Nitrogen-Doped TiO2 in Mesoporous Silica for Water Decontamination through Nonhydrophobic Enrichment-Degradation Mode. Chem. Eng. J. 2015, 21, 17944–17950. [Google Scholar]
- Shahnazi, A.; Nabid, M.R.; Sedghi, R. Synthesis of surface molecularly imprinted poly-o-phenylenediamine/TiO2/carbon nanodots with a highly enhanced selective photocatalytic degradation of pendimethalin herbicide under visible light. React. Funct. Polym. 2020, 151, 104580. [Google Scholar] [CrossRef]
- Zhang, Y.; Foster, C.W.; Banks, C.E.; Shao, L.; Hou, H.; Zou, G.; Chen, J.; Huang, Z.; Ji, X. Graphene-Rich Wrapped Petal-Like Rutile TiO2 tuned by Carbon Dots for High-Performance Sodium Storage. Adv Mater. 2016, 28, 9391–9399. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Kou, X.; Cao, B.; Fang, H. Hierarchical graphene@TiO2 sponges for sodium-ion storage with high areal capacity and robust stability. Electrochim. Acta 2020, 355, 136782. [Google Scholar] [CrossRef]
- Sadeghian, Z.; Hadidi, M.R.; Salehzadeh, D.; Nemati, A. Hydrophobic octadecylamine-functionalized graphene/TiO2 hybrid coating for corrosion protection of copper bipolar plates in simulated proton exchange membrane fuel cell environment. Int. J. Hydrogen Energ. 2020, 45, 15380–15389. [Google Scholar] [CrossRef]
- Wang, G.; Feng, H.; Hu, L.; Jin, W.; Hao, Q.; Gao, A.; Peng, X.; Li, W.; Wong, K.; Wang, H.; et al. An antibacterial platform based on capacitive carbon-doped TiO2 nanotubes after direct or alternating current charging. Nat. Commun. 2018, 9, 2055. [Google Scholar] [CrossRef] [PubMed]
- Zegeye, T.A.; Kuo, C.J.; Wotango, A.S.; Pan, C.; Chen, H.; Haregewoin, A.M.; Cheng, J.; Su, W.; Hwang, B. Hybrid nanostructured microporous carbon-mesoporous carbon doped titanium dioxide/sulfur composite positive electrode materials for rechargeable lithium-sulfur batteries. J. Power Sources 2016, 32, 239–252. [Google Scholar] [CrossRef]
- Trevisan, V.; Olivo, A.; Pinna, F.; Signoretto, M.; Vindigni, F.; Cerrato, G.; Bianchi, C.L. C-N/TiO2 photocatalysts: Effect of co-doping on the catalytic performance under visible light. Appl. Catal. B Environ. 2014, 160, 152–160. [Google Scholar] [CrossRef]
- Cao, S.; Liu, B.; Deng, X.; Luo, R.; Chen, H. A novel approach for the preparation of acrylate–siloxane particles with core–shell structure. Polym. Int. 2006, 56, 357–363. [Google Scholar] [CrossRef]
- Yu, S.; Yun, H.; Kim, Y.; Yi, J. Carbon-doped TiO2 nanoparticles wrapped with nanographene as a high performance photocatalyst for phenol degradation under visible light irradiation. Appl. Catal. B Environ. 2014, 144, 893–899. [Google Scholar] [CrossRef]
- Zeng, L.; Song, W.; Li, M.; Zeng, D.; Xie, C. Catalytic oxidation of formaldehyde on surface of H TiO2/H C TiO2 without light illumination at room temperature. Appl. Catal. B Environ. 2014, 147, 490–498. [Google Scholar] [CrossRef]
- Liu, J.; Han, L.; An, N.; Xing, L.; Ma, H.; Cheng, L.; Yang, J.; Zhang, Q. Enhanced visible-light photocatalytic activity of carbonate-doped anatase TiO2 based on the electron-withdrawing bidentate carboxylate linkage. Appl. Catal. B Environ. 2017, 202, 642–652. [Google Scholar] [CrossRef]
- Chen, J.; Qiu, F.; Zhang, Y.; Liang, J.; Zhu, H.; Cao, S. Enhanced supercapacitor performances using C-doped porous TiO2 electrodes. Appl. Surf. Sci. 2015, 356, 553–560. [Google Scholar] [CrossRef]
- Payormhorm, J.; Idem, R. Synthesis of C-doped TiO2 by sol-microwave method for photocatalytic conversion of glycerol to value-added chemicals under visible light. Appl. Catal. A Gen. 2020, 590, 117362. [Google Scholar] [CrossRef]
- Cao, S.; Xue, Z.; Yang, C.; Qin, J.; Zhang, L.; Yu, P.; Wang, S.; Zhao, Y.; Zhang, X.; Liu, R. Insights into the Li+ storage mechanism of TiC@C-TiO2core-shell nanostructures as high performance anodes. Nano Energy 2018, 50, 25–34. [Google Scholar] [CrossRef]
- Liu, G.; He, F.; Zhang, J.; Li, L.; Li, F.; Chen, L.; Huang, Y. Yolk-shell structured Fe3O4@C@F-TiO2 microspheres with surface fluorinated as recyclable visible-light driven photocatalysts. Appl. Catal. B Environ. 2014, 150-151, 515–522. [Google Scholar] [CrossRef]
- Qin, Y.; Zhuang, Y.; Lv, R.; Wang, T.; Wang, W.; Wang, C. Pd nanoparticles anchored on carbon-doped TiO2 nanocoating support for ethanol electrooxidation in alkaline media. Electrochim. Acta 2015, 154, 77–82. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, X.; Sui, L.; Wang, W.; Zhou, X.; Cheng, X.; Gao, S.; Xu, Y.; Huo, L. C-doped TiO2 nanoparticles to detect alcohols with different carbon chains and their sensing mechanism analysis. Sens. Actuat B Chem. 2020, 312, 127942. [Google Scholar] [CrossRef]
- Zou, Y.; Shi, J.; Ma, D.; Fan, Z.; Lu, L.; Niu, C. In situ synthesis of C-doped TiO2@g-C3N4 core-shell hollow nanospheres with enhanced visible-light photocatalytic activity for H2 evolution. Chem. Eng. J 2017, 322, 435–444. [Google Scholar] [CrossRef]
- Yang, C.; Qin, J.; Xue, Z.; Ma, M.; Zhang, X.; Liu, R. Rational design of carbon-doped TiO2 modified g-C3N4 via in-situ heat treatment for drastically improved photocatalytic hydrogen with excellent photostability. Nano Energy 2017, 41, 1–9. [Google Scholar] [CrossRef]
- Dhanasekaran, P.; Selvaganesh, S.; Bhat, S.D. Nitrogen and carbon doped titanium oxide as an alternative and durable electrocatalyst support in polymer electrolyte fuel cells. J. Power Sources 2016, 304, 360–372. [Google Scholar] [CrossRef]
- Han, X.; An, L.; Hu, Y.; Li, Y.; Hou, C.; Wang, H.; Zhang, Q. Ti3-C2 MXene-derived carbon-doped TiO2 coupled with g-C3N4 as the visible-light photocatalysts for photocatalytic H2 generation. Appl. Catal. B Environ. 2020, 265, 118539. [Google Scholar] [CrossRef]
- Qin, Y.; Li, Y.; Lv, R.; Wang, T.; Wang, W.; Wang, C. Enhanced methanol oxidation activity and stability of Pt particles anchored on carbon-doped TiO2 nanocoating support. J. Power Sources 2015, 278, 639–644. [Google Scholar] [CrossRef]
- Aragaw, B.A.; Pan, C.; Su, W.; Chen, H.; Rick, J.; Hwang, B. Facile one-pot controlled synthesis of Sn and C codoped single crystal TiO2 nanowire arrays for highly efficient photoelectrochemical water splitting. Appl. Catal. B Environ. 2015, 163, 478–486. [Google Scholar] [CrossRef]
- Li, Y.; Shen, J.; Li, J.; Liu, S.; Yu, D.; Xu, R.; Fu, W.; Lv, X. Constructing a novel strategy for carbon-doped TiO2 multiple-phase nanocomposites toward superior electrochemical performance for lithium ion batteries and the hydrogen evolution reaction. J. Mater. Chem. A 2017, 5, 7055–7063. [Google Scholar] [CrossRef]
- Ivanov, S.; Barylyak, A.; Besaha, K.; Dimitrova, A.; Krischok, S.; Bund, A.; Bobitski, J. Enhanced lithium ion storage in TiO2 nanoparticles, induced by sulphur and carbon co-doping. J. Power Sources 2016, 326, 270–278. [Google Scholar] [CrossRef]
- Nouri, E.; Mohammadi, M.R.; Lianos, P. Impact of preparation method of TiO2-RGO nanocomposite photoanodes on the performance of dye-sensitized solar cells. Electrochim. Acta 2016, 219, 38–48. [Google Scholar] [CrossRef]
- Guan, D.; Yu, Q.; Xu, C.; Tang, C.; Zhou, L.; Zhao, D.; Mai, L. Aerosol synthesis of trivalent titanium doped titania/carbon composite microspheres with superior sodium storage performance. Nano Res. 2017, 10, 4351–4359. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Cao, S.; Yin, Z.; Cheng, L.; Wu, L. Design and Synthesis of Hierarchical SiO2@C/TiO2 Hollow Spheres for High-Performance Supercapacitors. ACS Appl. Mater. Interfaces 2017, 9, 29982–29991. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, J.; Tang, H.; Xiao, Y.; Qiu, S.; Li, S.; Cao, S. Hierarchically-structured SiO2-Ag@TiO2 hollow spheres with excellent photocatalytic activity and recyclability. J. Hazard. Mater. 2018, 354, 17–26. [Google Scholar] [CrossRef]
- Yin, Z.; Qiu, S.; Chen, W.; Li, H.; Cheng, L.; Cao, S. Highly photocatalytic activity from tri-modified TiO2 hollow spheres. Mater. Let. 2018, 214, 202–204. [Google Scholar] [CrossRef]
- Varnagiris, S.; Medvids, A.; Lelis, M.; Milcius, D.; Antuzevics, A. Black carbon-doped TiO2 films: Synthesis, characterization and photocatalysis. J. Photoch. Photobio. A 2019, 382, 111941. [Google Scholar] [CrossRef]
- Fittipaldi, M.; Gombac, V.; Montini, T.; Fornasiero, P.; Graziani, M. A high-frequency (95 GHz) electron paramagnetic resonance study of B-doped TiO2photocatalysts. Inorg. Chim. Acta 2008, 361, 3980–3987. [Google Scholar] [CrossRef]
- Liu, N.; Chen, X.; Zhang, J.; Schwank, J.W. A review on TiO2-based nanotubes synthesized via hydrothermal method: Formation mechanism, structure modification, and photocatalytic applications. Catal. Today 2014, 225, 34–51. [Google Scholar] [CrossRef]
- Murdoch, M.; Waterhouse, G.; Nadeem, M.; Metson, J.; Keane, M.; Howe, R.; Llorca, J.; Idriss, H. The effect of gold loading and particle size on photocatalytic hydrogen production from ethanol over Au/TiO2 nanoparticles. Nat. Chem. 2011, 3, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Wang, J.; Tafen, D.N.; Wang, H.; Zheng, J.; Lewis, J.P.; Liu, X.; Leonard, S.S. Shape-Enhanced Photocatalytic Activity of Single-Crystalline Anatase TiO2 (101) Nanobelts. J. Am. Chem. Soc. 2010, 132, 6679–6685. [Google Scholar] [CrossRef] [PubMed]
- Matos, J.; Riquelme, J.O.; Poon, P.S.; Montana, R.; Garcia, X.; Campos, K.; Garrido, J.C.H.; Titirici, M.M. C-doped anatase TiO2: Adsorption kinetics and photocatalytic degradation of methylene blue and phenol, and correlations with DFT estimations. J. Colloid Interface Sci. 2019, 547, 14–29. [Google Scholar] [CrossRef]
- Chen, J.; Qiu, F.; Xu, W.; Cao, S.; Zhu, H. Recent progress in enhancing photocatalytic efficiency of TiO2-based materials. Appl. Catal. A Gen. 2015, 495, 131–140. [Google Scholar] [CrossRef]
- Vimonses, V.; Jin, B.; Chow, C.W.K.; Saint, C. An adsorption-photocatalysis hybrid process using multi-functional-nanoporous materials for wastewater reclamation. Water Res. 2010, 44, 5385–5397. [Google Scholar] [CrossRef]
- Lin, Y.; Weng, C.; Chen, F. Key operating parameters affecting photocatalytic activity of visible-light-induced C-doped TiO2 catalyst for ethylene oxidation. Chem. Eng. J. 2014, 248, 175–183. [Google Scholar] [CrossRef]
- Zhang, J.; Vasei, M.; Sang, Y.; Liu, H.; Claverie, J.P. TiO2@carbon photocatalysts: The effect of carbon thickness on catalysis. ACS Appl. Mater. Inter. 2016, 8, 1903–1912. [Google Scholar] [CrossRef]
- Liu, H.; Li, W.; Shen, D.; Zhao, D.; Wang, G. Graphitic carbon conformal, coating of mesoporous TiO2 hollow spheres for high-performance lithium ion battery anodes. J. Am. Chem. Soc. 2015, 137, 13161–13166. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Qiu, S.; Chen, J.; Shao, J.; Cao, S. A Practical Pathway for the preparation of Fe2O3 decorated TiO2 photocatalyst with enhanced visible-light photoactivity. Mater. Chem. Phys. 2017, 190, 53–61. [Google Scholar] [CrossRef]
- Marszewski, M.; Marszewska, J.; Pylypenko, S.; Jaroniec, M. Synthesis of Porous Crystalline Doped Titania Photocatalysts Using Modified Precursor Strategy. Chem. Mater. 2016, 28, 7878–7888. [Google Scholar] [CrossRef]
- Yuan, Y.; Ruan, Z.; Huang, X.; Jiang, Y.; Tan, H. Energy-absorption-based explanation of the TiO2/C photocatalytic activity enhancement mechanism. J. Catal. 2017, 348, 246–255. [Google Scholar] [CrossRef]
- Wei, W.; Yu, C.; Zhao, Q.; Qian, X.; Li, G.; Wan, Y. Synergy effect in photodegradation of contaminants from water using ordered mesoporous carbon-based titania catalyst. Appl. Catal. B Environ. 2014, 146, 151–161. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, Y.; Wu, M.; Szeto, W.; Wang, Y.; Pan, W.; Leung, D.Y.C. Carbon doped ultra-small TiO2 coated on carbon cloth for efficient photocatalytic toluene degradation under visible LED light irradiation. Appl. Surf. Sci. 2020, 527, 146780. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Rahman, N.A.; Zain, M.F.M.; Minggu, L.J.; Kassim, M.B.; Jaafar, J.; Samad, S.; Mastuli, M.S.; Wong, R.J. Hematite microcube decorated TiO2 nanorods as heterojunction photocatalyst with in-situ carbon doping derived from polysaccharides bio-templates hydrothermal carbonization. J. Alloys Compd. 2020, 820, 153143. [Google Scholar] [CrossRef]
- Noorimotlagh, Z.; Kazeminezhad, I.; Jaafarzadeh, N.; Ramezani, Z. Improved performance of immobilized TiO2 under visible light for the commercial surfactant degradation: Role of carbon doped TiO2 and anatase/rutile ratio. Catal. Today 2020, 348, 277–289. [Google Scholar] [CrossRef]
- Maria, C.A.T.; Chiu, J.F.V.; López, J.M.H.; Rosa, J.R.; Barbieri, V.; Siligardi, C.; González, E.I.C. Microplastic pollution reduction by a carbon and nitrogen-doped TiO2: Effect of pH and temperature in the photocatalytic degradation process. J. Hazard. Mater. 2020, 395, 122632. [Google Scholar]
- Zhao, Y.; Li, X.; Tian, C.; Wang, J. Production of carbon-doped titanium dioxide (C-TiO2) from polytitanium-coagulated sludge as an adsorbent or photocatalyst for pollutant removals. J. Clean Prod. 2020, 267, 121979. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Barakat, T.; Wang, T.; Krief, A.; Zeng, Y.; Laboureur, M.; Fusaro, L.; Liao, H.; Su, B. Synergistic effects of carbon doping and coating of TiO2 with exceptional photocurrent enhancement for high performance H2 production from water splitting. J. Energy Chem. 2021, 56, 141–151. [Google Scholar] [CrossRef]
- Venditti, F.; Cuomo, F.; Ceglie, A.; Avino, P.; Russo, M.V.; Lopez, F. Visible Light Caffeic Acid Degradation by Carbon-Doped Titanium Dioxide. Langmuir 2015, 31, 3627–3634. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Yang, W.; Zhang, G.; Yan, L.; Zhang, Y.; Jiang, A.; Xu, H.; Zhou, M.; Liu, Z.; Tang, H.; et al. Alternative synthesis of nitrogen and carbon co-doped TiO2 for removing fluoroquinolone antibiotics in water under visible light. Catal. Today 2020. [Google Scholar] [CrossRef]
- El-Sheikh, S.M.; Zhang, G.; El-Hosainy, H.M.; Ismail, A.A.; O’Shea, K.E.; Falaras, P.; Kontos, A.G.; Dionysiou, D.D. High performance sulfur, nitrogen and carbon doped mesoporous anatase-brookite TiO2 photocatalyst for the removal of microcystin-LR under visible light irradiation. J. Hazard. Mater. 2014, 280, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Shayegan, Z.; Haghighat, F.; Lee, C. Carbon-doped TiO2 film to enhance visible and UV light photocatalytic degradation of indoor environment volatile organic compounds. J. Environ. Chem. Eng. 2020, 8, 104162. [Google Scholar] [CrossRef]
- Shen, Z.; Wang, G.; Tian, H.; Sunarso, J.; Liu, L.; Liu, J.; Liu, S. Bi-layer photoanode films of hierarchical carbon-doped brookite-rutile TiO2 composite and anatase TiO2 beads for efficient dye-sensitized solar cells. Electrochim. Acta 2016, 216, 429–437. [Google Scholar] [CrossRef]
- Elmouwahidi, A.; Garcıa, E.B.; Quiben, J.C.; Cadenas, A.F.P.; Maldonado-Hodar, F.J.; Carrasco-Marın, F. Carbon-TiO2 composites as high-performance supercapacitor electrodes: Synergistic effect between carbon and metal oxide phases. J. Mater. Chem. A 2018, 6, 633–644. [Google Scholar] [CrossRef]
- Zhu, H.; Jing, Y.; Pal, M.; Liu, Y.; Liu, Y.; Wang, J.; Zhang, F.; Zhao, D. Mesoporous TiO2@N-doped Carbon Composite Nanospheres Synthesized by Direct Carbonization of Surfactants after Sol-gel Process for Superior Lithium Storage. Nanoscale 2017, 9, 1539–1546. [Google Scholar] [CrossRef]
- Geng, H.; Ming, H.; Ge, D.; Zheng, J.; Gu, H. Designed fabrication of fluorine-doped carbon coated mesoporous TiO2 hollow spheres for improved lithium storage. Electrochim. Acta 2015, 157, 1–7. [Google Scholar] [CrossRef]
- Zhu, Q.; Xie, C.; Li, H.; Yang, C.; Zeng, D. A novel planar integration ofall-solid-state capacitor and photodetector by an ultra-thin transparentsulfated TiO2 film. Nano Energy 2014, 9, 252–263. [Google Scholar] [CrossRef]
- Yin, Z.; Shao, J.; Tang, W.; Sheng, W.; Sun, D.; Xiao, Y.; Cao, S. Design and synthesis of ‘single-crystal-like’ C-doped TiO2 nanorods for high-performance supercapacitors. Nanotechnology 2020, 31, 275401. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhao, W.; Yang, Z.; Zhang, C.; Dang, F.; Liu, Y.; Jin, F.; Chen, X. A carbon-doped anatase TiO2-Based flexible silicon anode with high-performance and stability for flexible lithium-ion battery. J. Power Sources 2020, 466, 228339. [Google Scholar] [CrossRef]
- Goriparti, S.; Miele, E.; Prato, M.; Scarpellini, A.; Marras, S.; Monaco, S.; Toma, A.; Messina, G.C.; Alabastri, A.; Angelis, F.D.; et al. Direct Synthesis of Carbon-Doped TiO2-Bronze Nanowires as Anode Materials for High Performance Lithium-Ion Batteries. ACS Appl. Mater. Inter. 2015, 7, 25139–25146. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Qiu, F.; Zhang, Y.; Cao, S. Carbon-Doped Hollow Titania with Tuneable Shell Architecture for Supercapacitors. Aust. J. Chem. 2016, 69, 183–190. [Google Scholar] [CrossRef]
- Xiao, Y.; Sun, X.; Li, L.; Chen, J.; Zhao, S.; Jiang, C.; Yang, L.; Cheng, L.; Cao, S. Simultaneous formation of a C/N-TiO2 hollow photocatalyst with efficient photocatalytic performance and recyclability. Chin. J. Catal. 2019, 40, 765–775. [Google Scholar] [CrossRef]
- Hua, Z.; Dai, Z.; Bai, X.; Ye, Z.; Gu, H.; Huang, X. A facile one-step electrochemical strategy of doping iron, nitrogen, and fluorine into titania nanotube arrays with enhanced visible light photoactivity. J. Hazard. Mater. 2015, 293, 112–121. [Google Scholar] [CrossRef]
- Tang, W.; Chen, J.; Yin, Z.; Sheng, W.; Lin, F.; Xu, H.; Cao, S. The complete removal of phenolic contaminants from bismuth-modified TiO2 single crystal photocatalyst. Chin. J. Catal. 2021, 42, 347–355. [Google Scholar] [CrossRef]
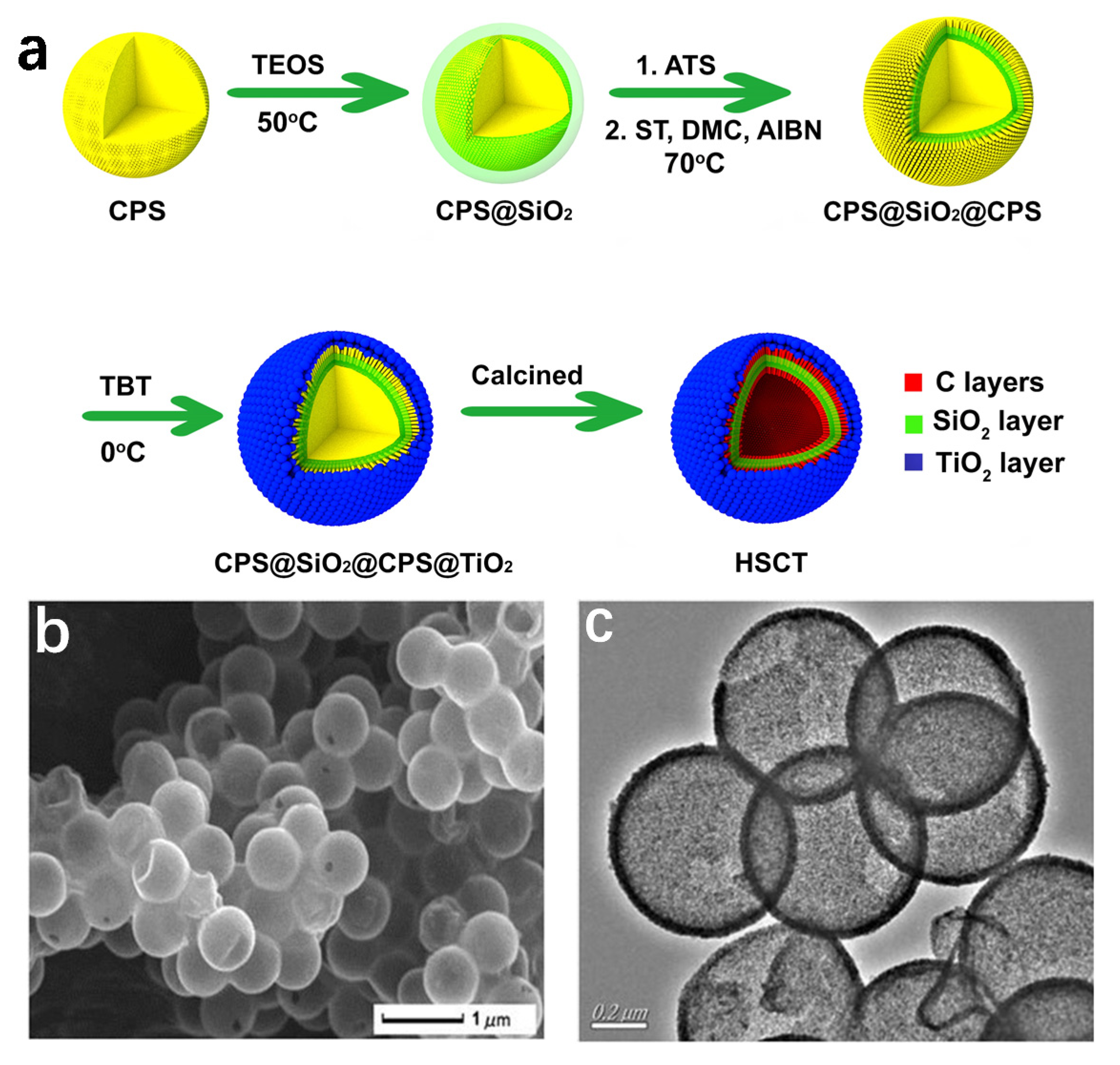
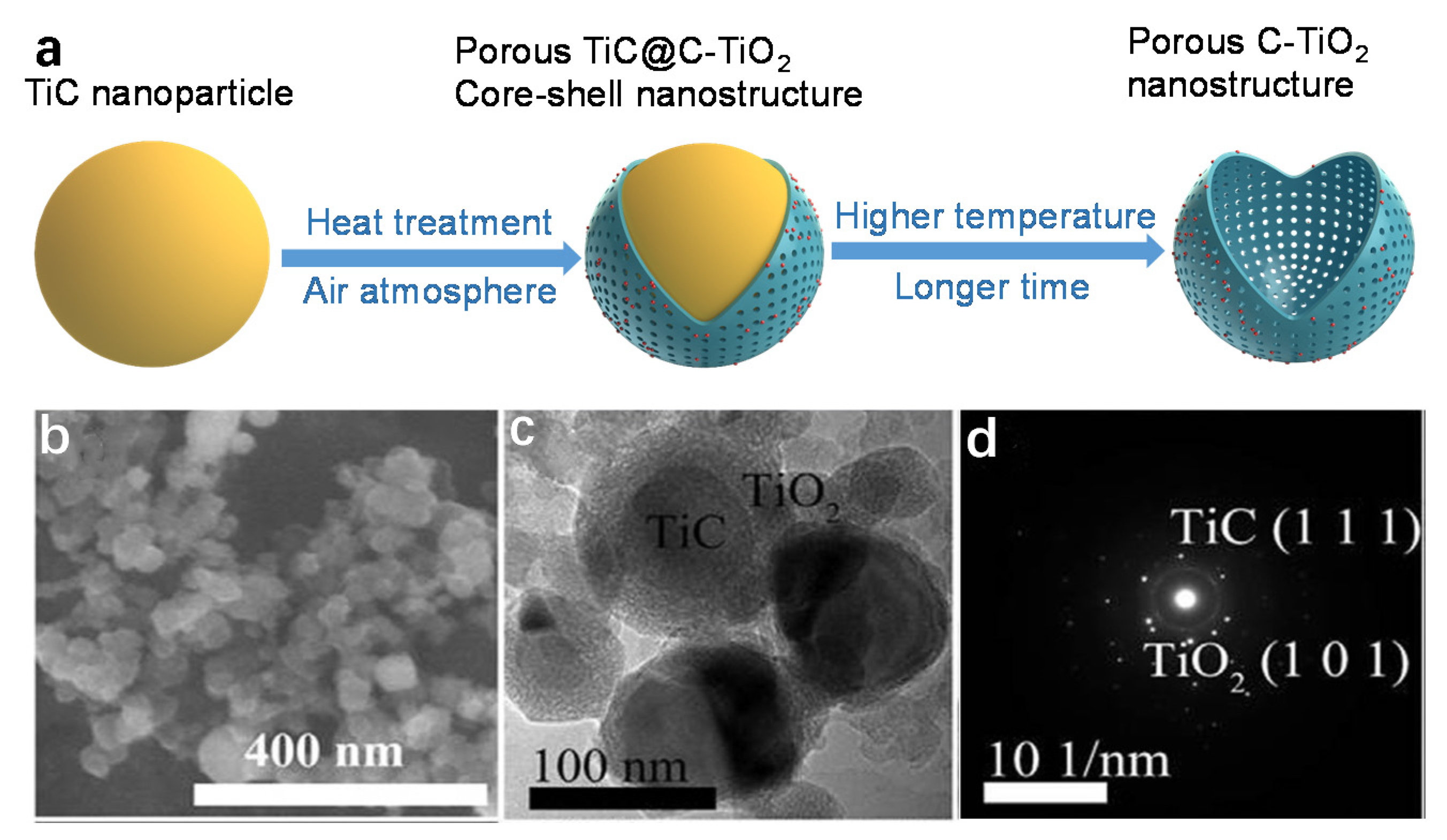
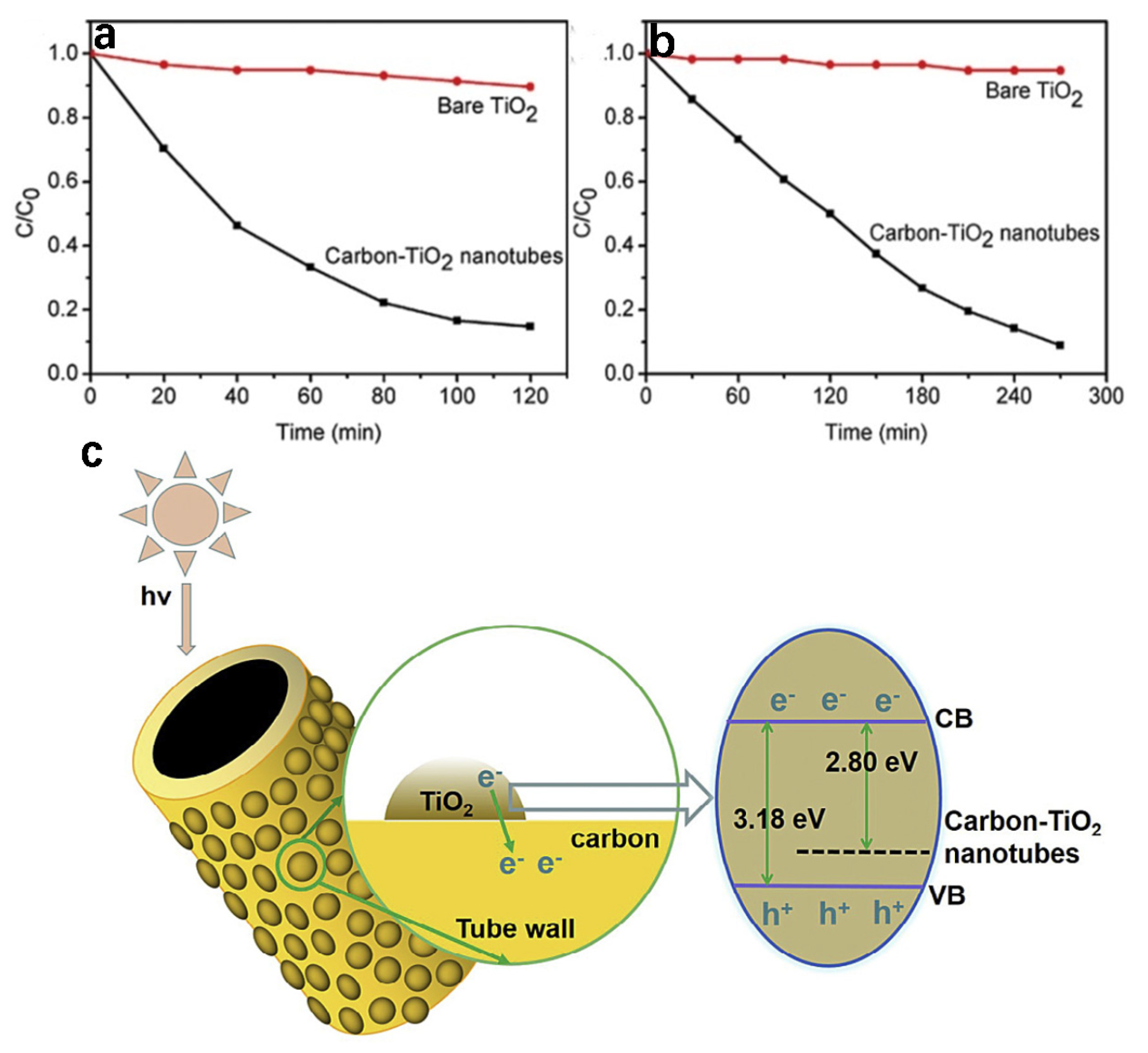
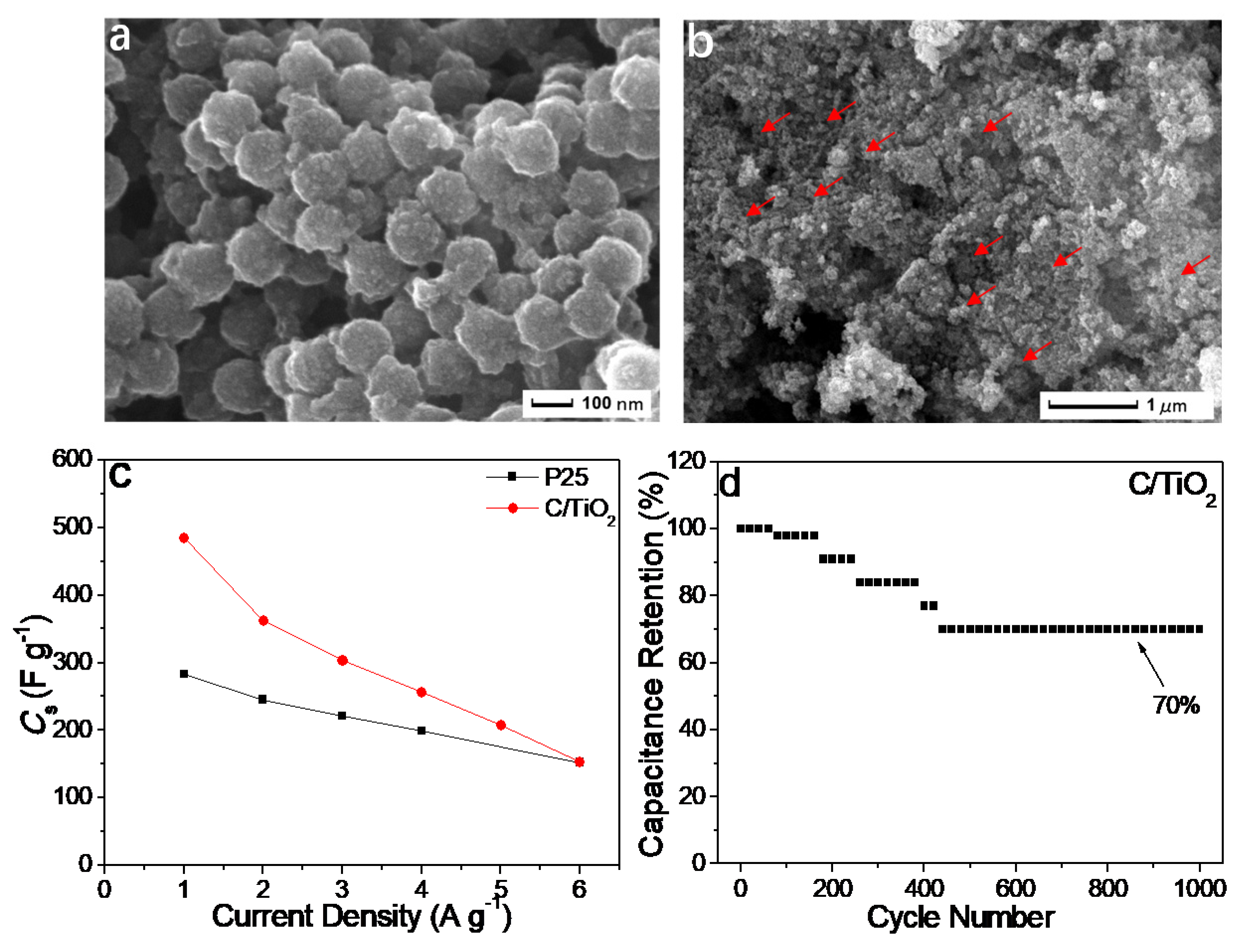
| Catalyst | C1s Peak | Synthesis Methods | Reference |
|---|---|---|---|
| Carbon@TiO2 hollow spheres | Ti–O–C bond | Template-derected method | [12] |
| Carbon-TiO2 nanotubes | Ti–O–C bond | Template-derected method | [13] |
| Carbon-doped TiO2 on TiC structure | Ti–O–C bond | TiC calcination | [15] |
| C-doped TiO2 | Ti–O–C bond | Sol-microwave | [43] |
| Carbon-Doped TiO2 /MCF-F | Ti–O–C bond | Hydrothermal synthesis | [5] |
| TiO2/NCQD composites | Ti–O–C bond | TiC calcination | [44] |
| Fe3O4@C@F-TiO2 | Ti–O–C bond | Hydrothermal synthesis | [45] |
| Pd/TiO2-C | Ti–O–C bond | Solvothermal synthesis | [46] |
| C-doped TiO2 nanoparticles | Ti–C bond | Hydrothermal synthesis | [47] |
| C-doped TiO2@g-C3N4 nanospheres | Ti–C bond | Hydrothermal synthesis | [48] |
| C-TiO2 modified g-C3N4 | Ti–C bond | TiC calcination | [49] |
| MC-Meso C-doped TiO2/S | Ti–C bond | Hydrothermal synthesis | [36] |
| N & C doped TiO2 supported Pt | Ti–C bond | Hydrothermal synthesis | [50] |
| C-TiO2/g-C3N4 composite | Ti–C bond | TiC calcination | [51] |
| C–H–TiO2 | Ti–C bond | TiC calcination | [40] |
| Carbon-doped TiO2 nanorods | Ti–C bond | Template-directed method | [8] |
| SiO2@C-doped TiO2 hollow spheres | Ti–C bond | Template-directed method | [9] |
| C-doped Hollow TiO2 | Ti–C bond | Template-directed method | [14] |
| C-doped porous TiO2 | Ti–C bond | Template-directed method | [42] |
| Electrode Materials | Application Fields | Advantage | Comparative Performance | Stability | Reference |
|---|---|---|---|---|---|
| MC-Meso C-doped TiO2/S | Lithium-sulfur batteries | 802 mAh g−1 | 530 mAh g−1 for mesoporous C-doped TiO2/S | 97.1% after 140 cycles | [36] |
| N&C doped TiO2 supported Pt | Fuel cells | 980 mW cm−2 | 470 mW cm−2 for Pt/TiON-1 | Durability test over 50,000 cycles | [50] |
| Si/TiO2-CC composite | Lithium-ion battery | 3.21 mAh cm−2 | More excellent areal capacity than other silicon composite anodes | Maintain 94.5% after 100 cycles | [92] |
| Carbon-Doped TiO2-Bronze Nanowires | Lithium-ion Batteries | 345 mAh g−1 | 342 mAh g−1 for TB-NWs | Maintain 89% after 1000 cycles | [93] |
| TiO2@C nanosheets | Na-ion batteries | 264.9 mAh g−1 | 170.8 mAh g−1 for pure carbon | After 100 cycles at 100 mA g−1 | [11] |
| S/C co-doped anatase | Lithium ion storage | 210 mAh g−1 | Better electrochemical performance than non-doped TiO2 | 83% capacity retention for 500 cycles | [56] |
| C-doped Hollow TiO2 | Supercapacitor | 418 F g−1 | 283 F g−1 for P25 | 78.1% capacity retention for 10,000 cycles | [94] |
| C-doped porous TiO2 | Supercapacitor | 485 F g−1 | 283 F g−1 for P25 | ~70% capacity retention for 1000 cycles | [42] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hua, L.; Yin, Z.; Cao, S. Recent Advances in Synthesis and Applications of Carbon-Doped TiO2 Nanomaterials. Catalysts 2020, 10, 1431. https://doi.org/10.3390/catal10121431
Hua L, Yin Z, Cao S. Recent Advances in Synthesis and Applications of Carbon-Doped TiO2 Nanomaterials. Catalysts. 2020; 10(12):1431. https://doi.org/10.3390/catal10121431
Chicago/Turabian StyleHua, Li, Zhengliang Yin, and Shunsheng Cao. 2020. "Recent Advances in Synthesis and Applications of Carbon-Doped TiO2 Nanomaterials" Catalysts 10, no. 12: 1431. https://doi.org/10.3390/catal10121431
APA StyleHua, L., Yin, Z., & Cao, S. (2020). Recent Advances in Synthesis and Applications of Carbon-Doped TiO2 Nanomaterials. Catalysts, 10(12), 1431. https://doi.org/10.3390/catal10121431