Spray-Dried Ni Catalysts with Tailored Properties for CO2 Methanation
Abstract
1. Introduction
2. Results and Discussion
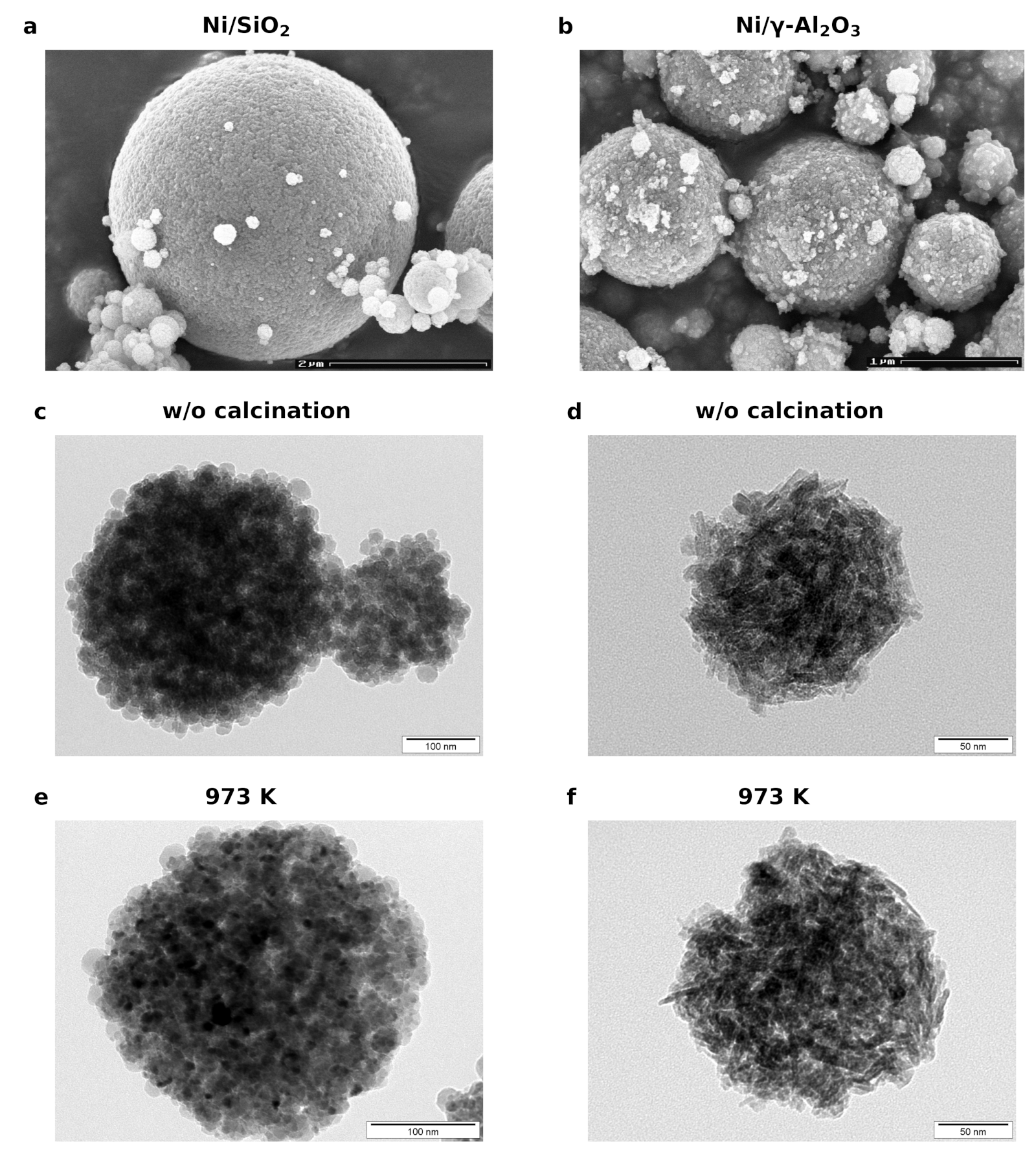
2.1. Morphological Examination
2.2. Elemental Analysis
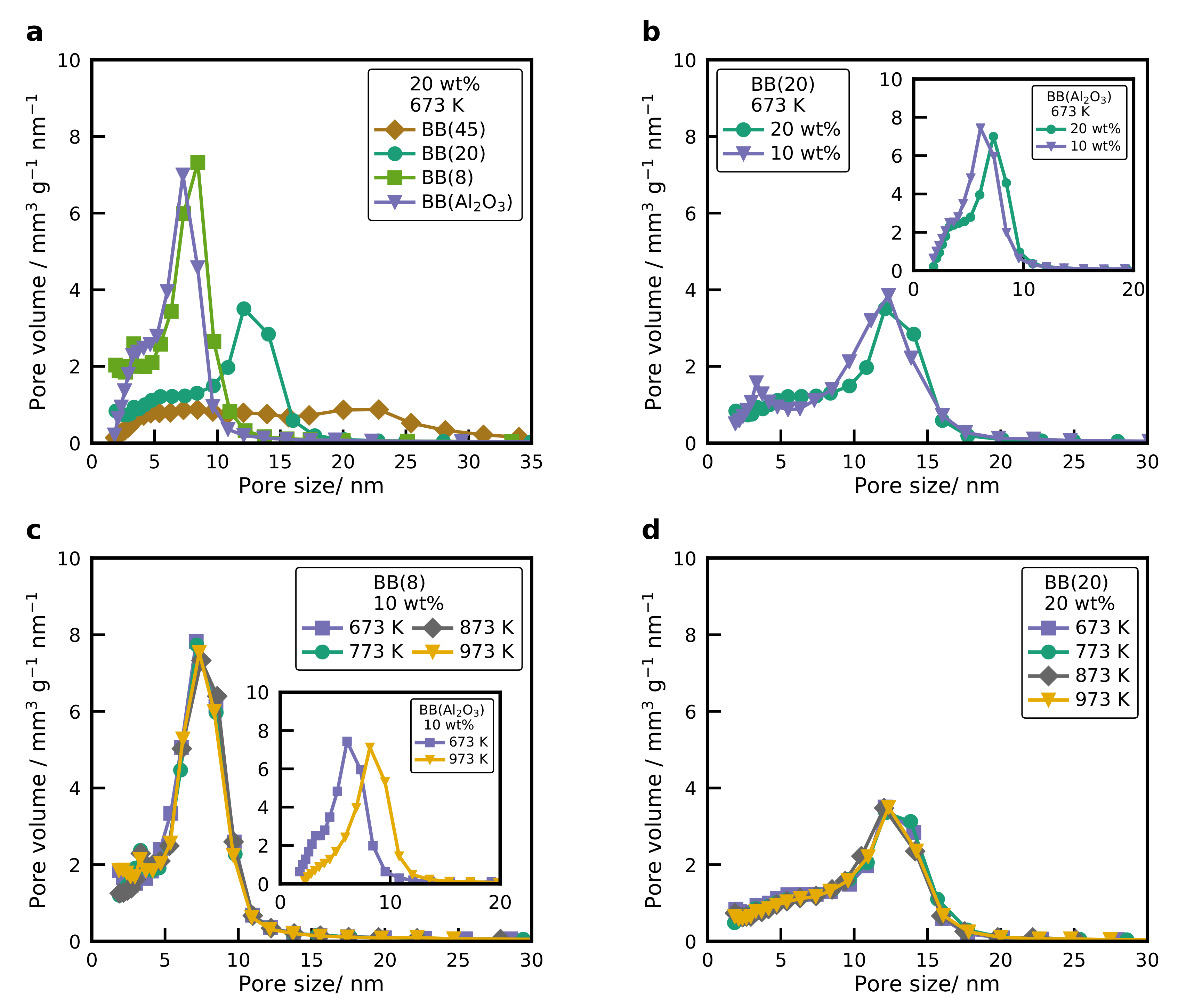
2.3. Physisorption
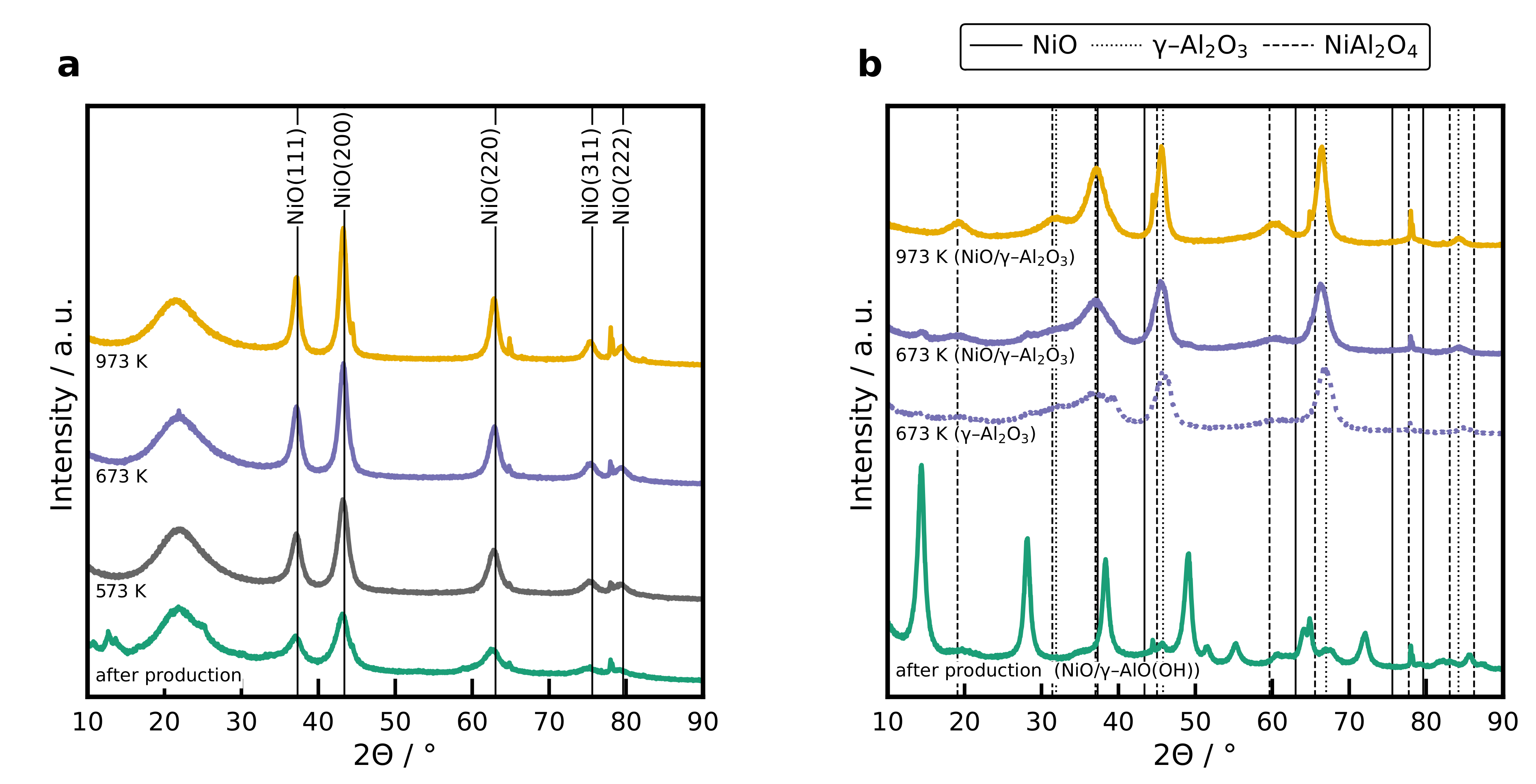
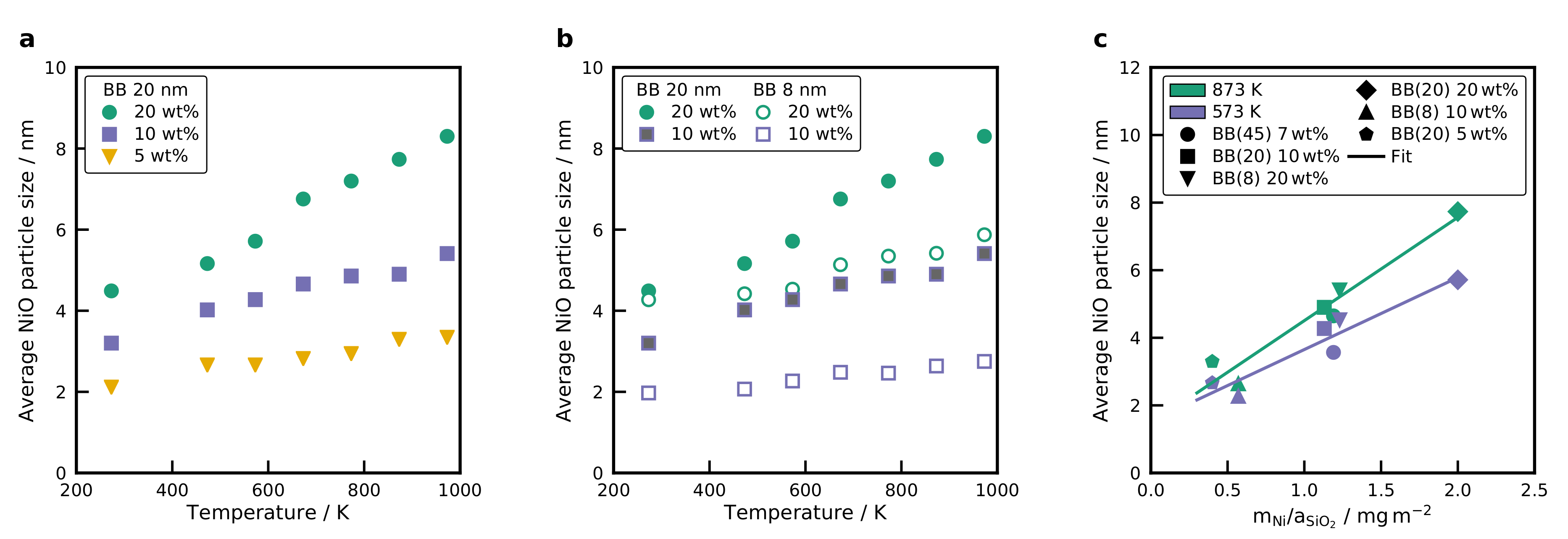
2.4. XRD
2.5. Adsorption and Degree of Reduction
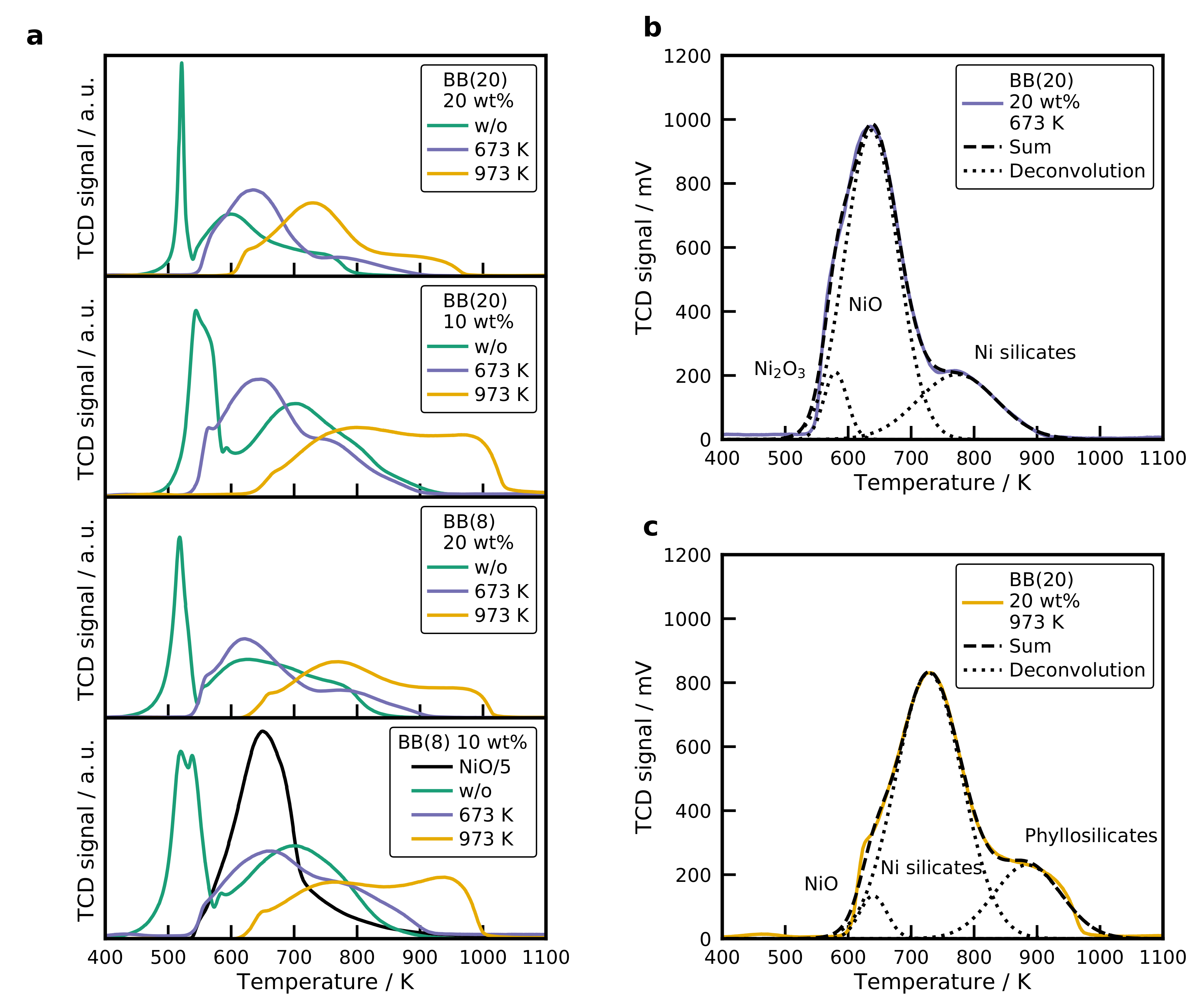
2.6. Temperature-Programmed Reduction
2.7. Temperature-Programmed Desorption
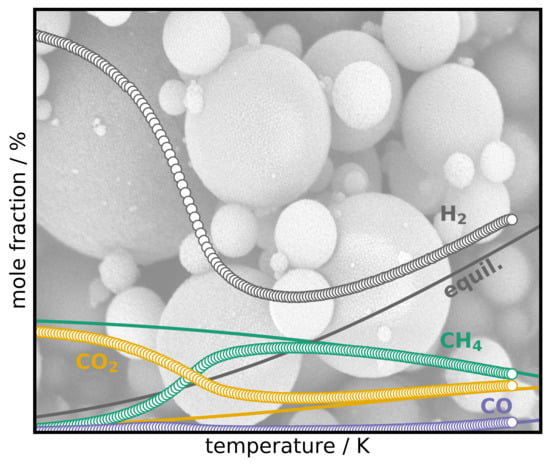
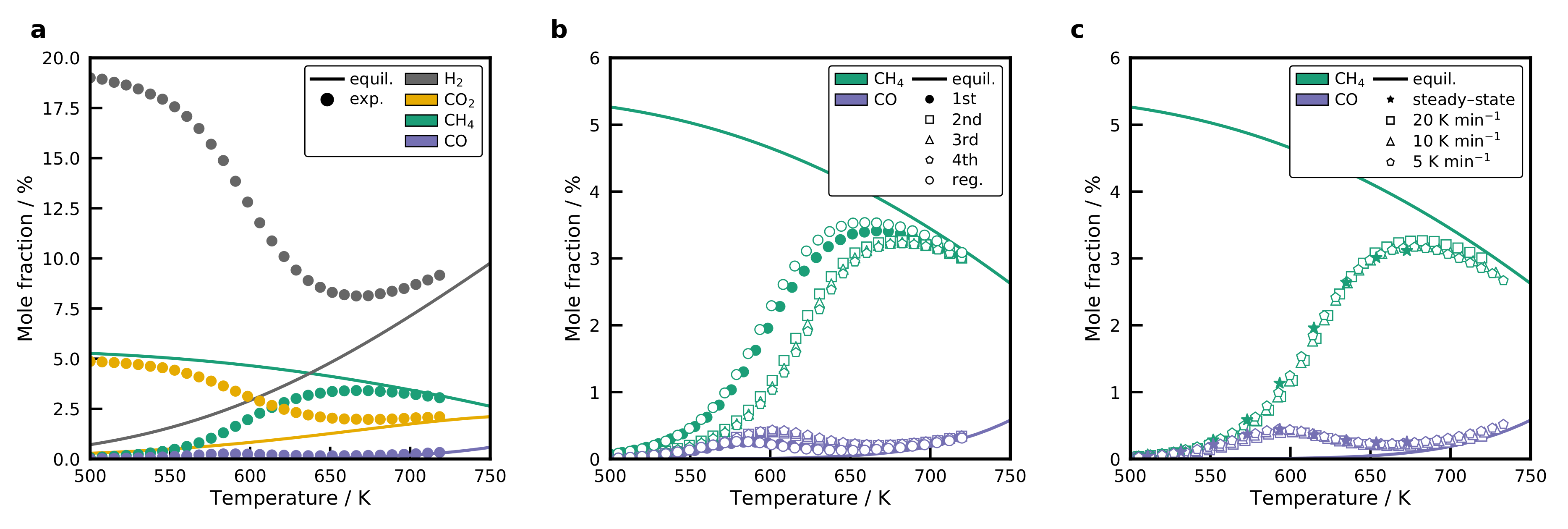
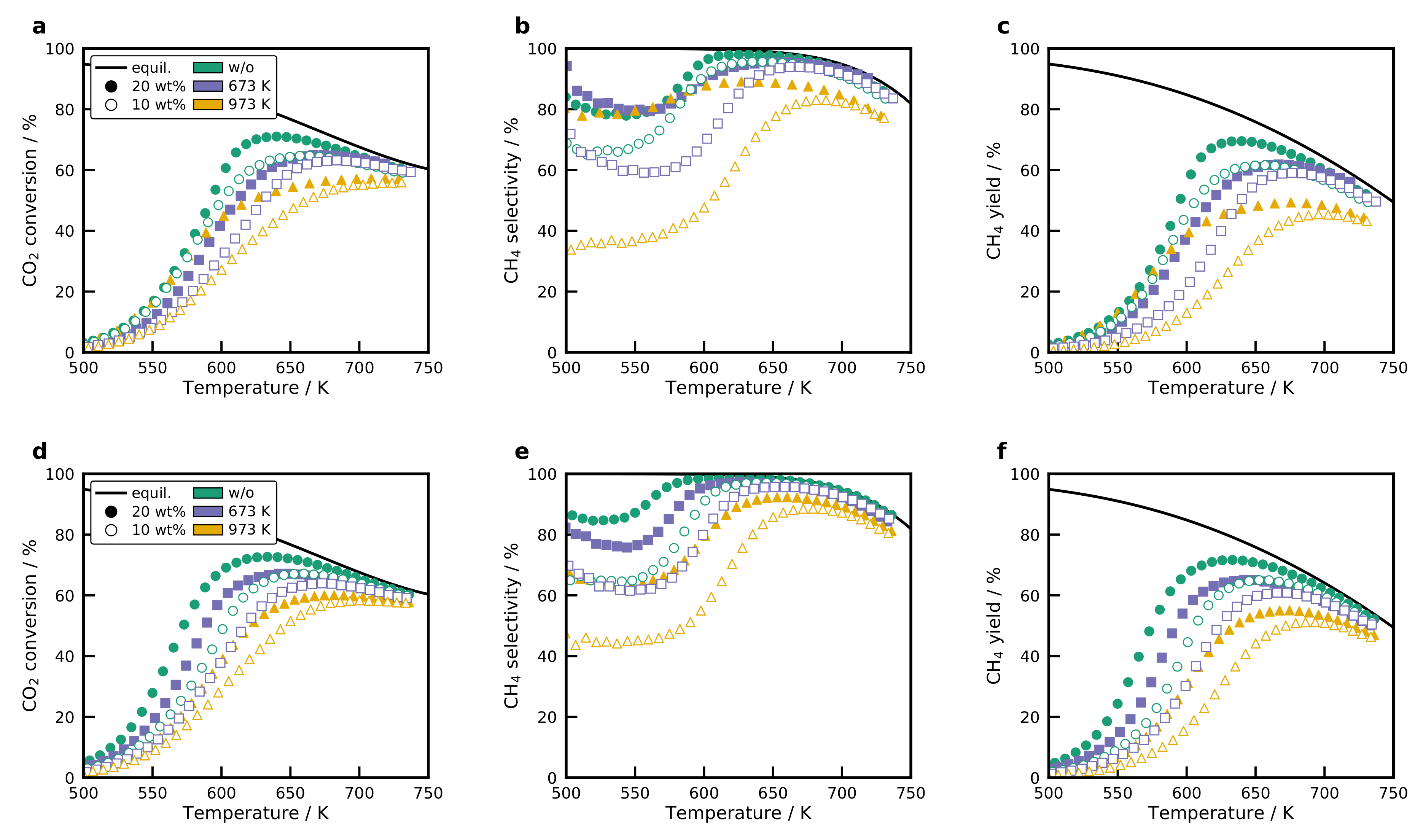
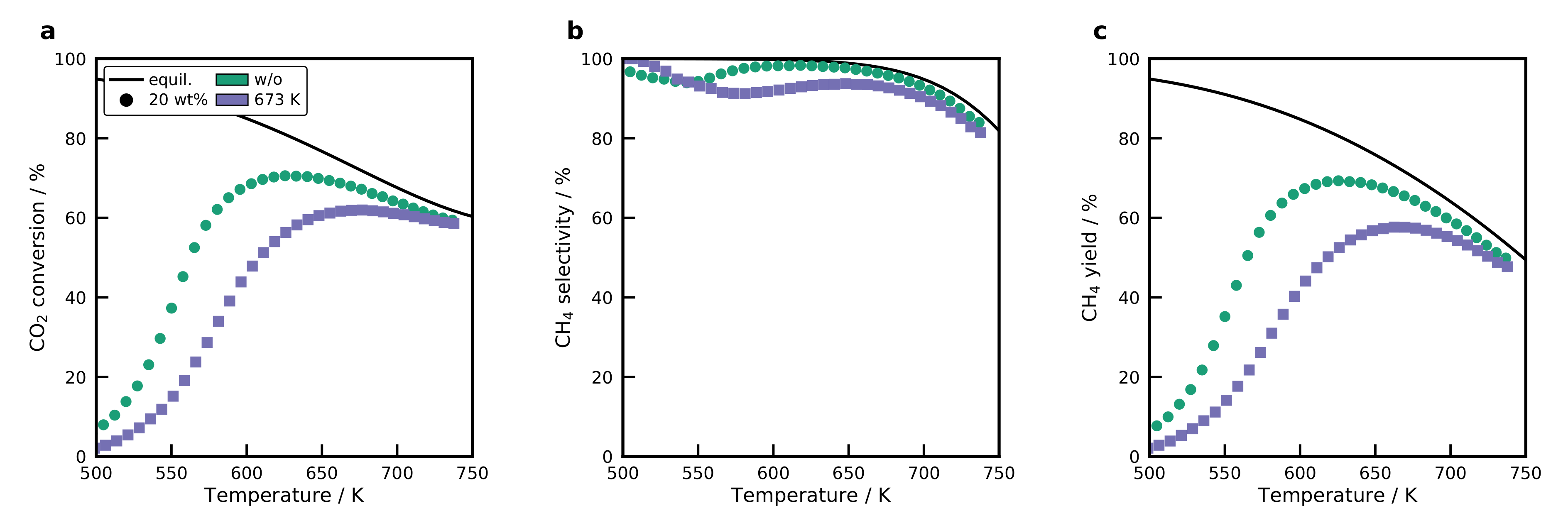
2.8. Activity of the Catalysts
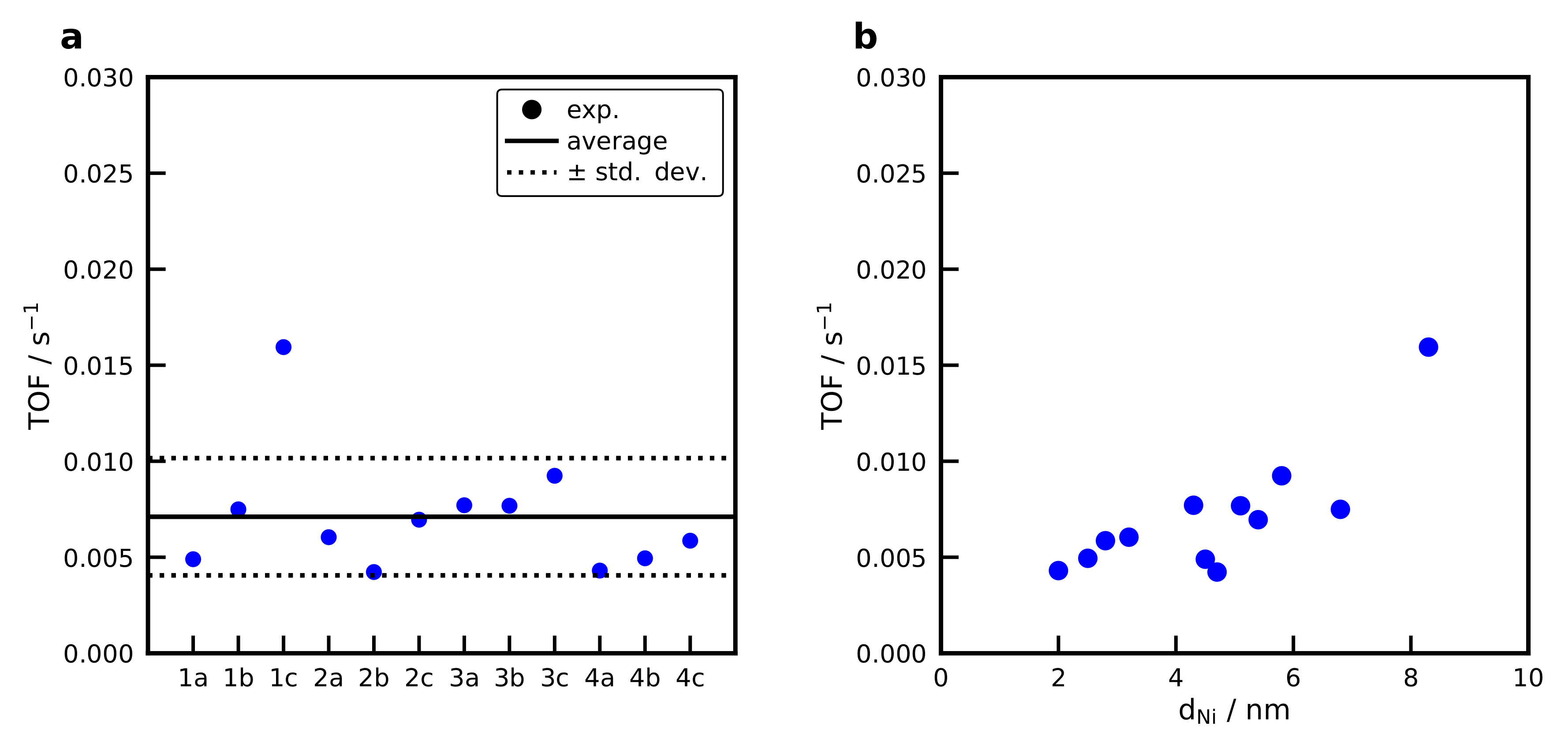
2.9. Activation Energies and Turnover Frequencies
3. Materials and Methods
3.1. Materials
3.2. Experimental Setup and Synthesis of the NiO/SiO2 Nanoparticles
3.3. Physical Characterization
3.4. Chemical Characterization
3.5. Methanation Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| TPR | Temperature-programmed reduction |
| TPD | Temperature-programmed desorption |
| XRD | X-ray diffraction |
| TEM | Transmission Electron Microscopy |
| SEM | Scanning Electron Microscopy |
| TCD | Thermal conductivity detector |
| MS | Mass spectrometer |
| BB | Building-Blocks |
| SAXS | Small-angle X-ray scattering |
| BET | Brunauer-Emmett-Teller method |
| BJH | Barrett-Joyner-Halenda model |
List of Symbols
| Latin symbols | ||
| a | area | m2 |
| D | dispersion | - |
| DOR | degree of reduction | % |
| d | diameter | m |
| f | calibration factor | mol m−2 |
| K | Scherrer form factor | - |
| m | mass | g |
| p | pressure | bar |
| Q | adsorbed amount | mol g−1 |
| R | ideal gas constant | J mol−1 K−1 |
| r | reaction rate | mol s−1 g−1 |
| S | selectivity | - |
| T | temperature | K |
| volume flow | mL min−1 | |
| W | molar mass | g mol−1 |
| w | metal loading | - |
| WHSV | weight hourly space velocity | LN h−1 g−1 |
| X | conversion | - |
| x | molar fraction | - |
| Y | yield | - |
| z | adsorption stoichiometry | - |
| Greek symbols | ||
| α | volume reduction | - |
| β | temperature ramp | K min−1 |
| Θ | Bragg angle | ∘ |
| wavelength | m | |
| density | kg m−3 | |
| surface area | m2 | |
| Subscripts | ||
| calibration | calibration | |
| cat | catalyst | |
| f | form factor | |
| N | normal conditions | |
| Superscripts | ||
| in | inlet | |
| out | outlet | |
References
- Tomiyama, S.; Takahashi, R.; Sato, S.; Toshiaki, S.; Satoshi, Y. Preparation of Ni/SiO2 catalyst with high thermal stability for CO2-reforming of CH4. Appl. Catal. A Gen. 2003, 241, 349–361. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, W.; Wang, Z.; Zhou, X.; Wang, Z.; Liu, C.J. Steam reforming of methane over Ni/SiO2 catalyst with enhanced coke resistance at low steam to methane ratio. Catal. Today 2015, 256, 130–136. [Google Scholar] [CrossRef]
- Huang, X.; Reimert, R. Kinetics of steam reforming of ethane on Ni/YSZ (yttria-stabilised zirconia) catalyst. Fuel 2013, 106, 380–387. [Google Scholar] [CrossRef]
- Xie, Z.; Yan, B.; Lee, J.H.; Wu, Q.; Li, X.; Zhao, B.; Su, D.; Zhang, L.; Chen, J.G. Effects of oxide supports on the CO2 reforming of ethane over Pt-Ni bimetallic catalysts. Appl. Catal. B Environ. 2019, 245, 376–388. [Google Scholar] [CrossRef]
- Sehested, J.; Dahl, S.; Jacobsen, J.; Rostrup-Nielsen, J.R. Methanation of CO over nickel: Mechanism and kinetics at high H2/CO ratios. J. Phys. Chem. B 2005, 109, 2432–2438. [Google Scholar] [CrossRef]
- Nematollahi, B.; Rezaei, M.; Lay, E.N. Preparation of highly active and stable NiO-CeO2 nanocatalysts for CO selective methanation. Int. J. Hydrogen Energy 2015, 40, 8539–8547. [Google Scholar] [CrossRef]
- Rönsch, S.; Schneider, J.; Matthischke, S.; Schlüter, M.; Götz, M.; Lefebvre, J.; Prabhakaran, P.; Bajohr, S. Review on methanation—From fundamentals to current projects. Fuel 2016, 166, 276–296. [Google Scholar] [CrossRef]
- Gao, J.; Liu, Q.; Gu, F.; Liu, B.; Zhong, Z.; Su, F. Recent advances in methanation catalysts for the production of synthetic natural gas. RSC Adv. 2015, 5, 22759–22776. [Google Scholar] [CrossRef]
- Kreitz, B.; Wehinger, G.D.; Turek, T. Dynamic simulation of the CO2 methanation in a micro-structured fixed-bed reactor. Chem. Eng. Sci. 2019, 195, 541–552. [Google Scholar] [CrossRef]
- Kreitz, B.; Friedland, J.; Güttel, R.; Wehinger, G.D.; Turek, T. Dynamic Methanation of CO2—Effect of Concentration Forcing. Chem. Ing. Tech. 2019, 91, 576–582. [Google Scholar] [CrossRef]
- Kreitz, B.; Brauns, J.; Wehinger, G.D.; Turek, T. Modeling the Dynamic Power–to–Gas Process: Coupling Electrolysis with CO2 Methanation. Chem. Ing. Tech. 2020, 43, 20332. [Google Scholar] [CrossRef]
- Frontera, P.; Macario, A.; Ferraro, M.; Antonucci, P. Supported Catalysts for CO2 Methanation: A Review. Catalysts 2017, 7, 59. [Google Scholar] [CrossRef]
- Ashok, J.; Pati, S.; Hongmanorom, P.; Tianxi, Z.; Junmei, C.; Kawi, S. A review of recent catalyst advances in CO2 methanation processes. Catal. Today 2020. [Google Scholar] [CrossRef]
- Wei, W.; Jinlong, G. Methanation of carbon dioxide: An overview. Front. Chem. Sci. Eng. 2011, 5, 2–10. [Google Scholar] [CrossRef]
- Pan, Q.; Peng, J.; Sun, T.; Wang, S.; Wang, S. Insight into the reaction route of CO2 methanation: Promotion effect of medium basic sites. Catal. Commun. 2014, 45, 74–78. [Google Scholar] [CrossRef]
- Aziz, M.; Jalil, A.A.; Triwahyono, S.; Mukti, R.R.; Taufiq-Yap, Y.H.; Sazegar, M.R. Highly active Ni-promoted mesostructured silica nanoparticles for CO2 methanation. Appl. Catal. B Environ. 2014, 147, 359–368. [Google Scholar] [CrossRef]
- Chen, C.S.; Budi, C.S.; Wu, H.C.; Saikia, D.; Kao, H.M. Size-Tunable Ni Nanoparticles Supported on Surface-Modified, Cage-Type Mesoporous Silica as Highly Active Catalysts for CO2 Hydrogenation. ACS Catal. 2017, 7, 8367–8381. [Google Scholar] [CrossRef]
- Ye, R.P.; Gong, W.; Sun, Z.; Sheng, Q.; Shi, X.; Wang, T.; Yao, Y.; Razink, J.J.; Lin, L.; Zhou, Z.; et al. Enhanced stability of Ni/SiO2 catalyst for CO2 methanation: Derived from nickel phyllosilicate with strong metal-support interactions. Energy 2019, 188, 116059. [Google Scholar] [CrossRef]
- Zhu, P.; Chen, Q.; Yoneyama, Y.; Tsubaki, N. Nanoparticle modified Ni-based bimodal pore catalysts for enhanced CO2 methanation. RSC Adv. 2014, 4, 64617–64624. [Google Scholar] [CrossRef]
- Huang, Y.J.; Schwarz, J.A. The effect of catalyst preparation on catalytic activity: I. The catalytic activity of Ni/Al2O3 catalysts prepared by wet impregnation. Appl. Catal. 1987, 30, 239–253. [Google Scholar] [CrossRef]
- Li, G.; Hu, L.; Hill, J.M. Comparison of reducibility and stability of alumina-supported Ni catalysts prepared by impregnation and co-precipitation. Appl. Catal. A Gen. 2006, 301, 16–24. [Google Scholar] [CrossRef]
- Ashok, J.; Ang, M.L.; Kawi, S. Enhanced activity of CO2 methanation over Ni/CeO2-ZrO2 catalysts: Influence of preparation methods. Catal. Today 2017, 281, 304–311. [Google Scholar] [CrossRef]
- Koschany, F.; Schlereth, D.; Hinrichsen, O. On the kinetics of the methanation of carbon dioxide on coprecipitated NiAl(O)x. Appl. Catal. B Environ. 2016, 181, 504–516. [Google Scholar] [CrossRef]
- Chiarello, G.; Rossetti, I.; Forni, L. Flame-spray pyrolysis preparation of perovskites for methane catalytic combustion. J. Catal. 2005, 236, 251–261. [Google Scholar] [CrossRef]
- Compagnoni, M.; Tripodi, A.; Di Michele, A.; Sassi, P.; Signoretto, M.; Rossetti, I. Low temperature ethanol steam reforming for process intensification: New Ni/MxO-ZrO2 active and stable catalysts prepared by flame spray pyrolysis. Int. J. Hydrogen Energy 2017, 42, 28193–28213. [Google Scholar] [CrossRef]
- Saib, A.M.; Claeys, M.; van Steen, E. Silica supported cobalt Fischer–Tropsch catalysts: Effect of pore diameter of support. Catal. Today 2002, 71, 395–402. [Google Scholar] [CrossRef]
- Borg, Ø.; Eri, S.; Blekkan, E.A.; Storsæter, S.; Wigum, H.; Rytter, E.; Holmen, A. Fischer–Tropsch synthesis over γ-alumina-supported cobalt catalysts: Effect of support variables. J. Catal. 2007, 248, 89–100. [Google Scholar] [CrossRef]
- Song, D.; Li, J. Effect of catalyst pore size on the catalytic performance of silica supported cobalt Fischer–Tropsch catalysts. J. Mol. Catal. A Chem. 2006, 247, 206–212. [Google Scholar] [CrossRef]
- Koirala, R.; Pratsinis, S.E.; Baiker, A. Synthesis of catalytic materials in flames: Opportunities and challenges. Chem. Soc. Rev. 2016, 45, 3053–3068. [Google Scholar] [CrossRef]
- Gradon, L.; Balgis, R.; Hirano, T.; Rahmatika, A.M.; Ogi, T.; Okuyama, K. Advanced aerosol technologies towards structure and morphologically controlled next-generation catalytic materials. J. Aerosol Sci. 2020, 149, 105608. [Google Scholar] [CrossRef]
- Martínez Arias, A.; Weber, A.P. Aerosol synthesis of porous SiO2-cobalt-catalyst with tailored pores and tunable metal particle size for Fischer-Tropsch synthesis (FTS). J. Aerosol Sci. 2019, 131, 1–12. [Google Scholar] [CrossRef]
- Wojciechowski, B. The temperature scanning reactor I: Reactor types and modes of operation. Catal. Today 1997, 36, 167–190. [Google Scholar] [CrossRef]
- Asprey, S. The temperature scanning reactor III: Experimental procedures and data processing. Catal. Today 1997, 36, 209–226. [Google Scholar] [CrossRef]
- Wojciechowski, B.; Asprey, S.P. Kinetic studies using temperature-scanning: The oxidation of carbon monoxide. Appl. Catal. A Gen. 2000, 190, 1–24. [Google Scholar] [CrossRef]
- Liebner, C.; Wolf, D.; Baerns, M.; Kolkowski, M.; Keil, F.J. A high-speed method for obtaining kinetic data for exothermic or endothermic catalytic reactions under non-isothermal conditions illustrated for the ammonia synthesis. Appl. Catal. A Gen. 2003, 240, 95–110. [Google Scholar] [CrossRef]
- Vogt, C.; Wijten, J.; Madeira, C.L.; Kerkenaar, O.; Xu, K.; Holzinger, R.; Monai, M.; Weckhuysen, B.M. Alkali Promotion in the Formation of CH4 from CO2 and Renewably Produced H2 over Supported Ni Catalysts. ChemCatChem 2020, 12, 2792–2800. [Google Scholar] [CrossRef]
- Le, T.A.; Kim, T.W.; Lee, S.H.; Park, E.D. Effects of Na content in Na/Ni/SiO2 and Na/Ni/CeO2 catalysts for CO and CO2 methanation. Catal. Today 2018, 303, 159–167. [Google Scholar] [CrossRef]
- Beierlein, D.; Häussermann, D.; Pfeifer, M.; Schwarz, T.; Stöwe, K.; Traa, Y.; Klemm, E. Is the CO2 methanation on highly loaded Ni-Al2O3 catalysts really structure-sensitive? Appl. Catal. B Environ. 2019, 247, 200–219. [Google Scholar] [CrossRef]
- Zeng, L.; Weber, A.P. Aerosol synthesis of nanoporous silica particles with controlled pore size distribution. J. Aerosol Sci. 2014, 76, 1–12. [Google Scholar] [CrossRef]
- Okuyama, K.; Wuled Lenggoro, I. Preparation of nanoparticles via spray route. Chem. Eng. Sci. 2003, 58, 537–547. [Google Scholar] [CrossRef]
- Röhrbein, J.; Arias, A.M.; Weber, A.P. Aerosol-Synthese von porösen Katalysatorpartikeln mit einstellbaren Porengrößen und Katalysatordurchmessern. Chem. Ing. Tech. 2017, 89, 1739–1751. [Google Scholar] [CrossRef]
- Khodakov, A.Y.; Griboval-Constant, A.; Bechara, R.; Zholobenko, V.L. Pore Size Effects in Fischer Tropsch Synthesis over Cobalt-Supported Mesoporous Silicas. J. Catal. 2002, 206, 230–241. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- St. O’Neill, H.C.; Dollase, W.A.; Ross, C.R. Temperature dependence of the cation distribution in nickel aluminate (NiAl2O4) spinel: A powder XRD study. Phys. Chem. Miner. 1991, 18, 302–319. [Google Scholar] [CrossRef]
- Zhou, R.S.; Snyder, R.L. Structures and transformation mechanisms of the η, γ and θ transition aluminas. Acta Crystallogr. Sect. B Struct. Sci. 1991, 47, 617–630. [Google Scholar] [CrossRef]
- Cao, A.; Lu, R.; Veser, G. Stabilizing metal nanoparticles for heterogeneous catalysis. Phys. Chem. Chem. Phys. PCCP 2010, 12, 13499–13510. [Google Scholar] [CrossRef]
- Hansen, T.W.; Delariva, A.T.; Challa, S.R.; Datye, A.K. Sintering of catalytic nanoparticles: Particle migration or Ostwald ripening? Accounts Chem. Res. 2013, 46, 1720–1730. [Google Scholar] [CrossRef]
- Cao, A.; Veser, G. Exceptional high-temperature stability through distillation-like self-stabilization in bimetallic nanoparticles. Nat. Mater. 2010, 9, 75–81. [Google Scholar] [CrossRef]
- Petroski, J.M.; Wang, Z.L.; Green, T.C.; El-Sayed, M.A. Kinetically Controlled Growth and Shape Formation Mechanism of Platinum Nanoparticles. J. Phys. Chem. B 1998, 102, 3316–3320. [Google Scholar] [CrossRef]
- Van de Loosdrecht, J.; van der Kraan, A.M.; van Dillen, A.J.; Geus, J.W. Metal-Support Interaction: Titania-Supported and Silica-Supported Nickel Catalysts. J. Catal. 1997, 170, 217–226. [Google Scholar] [CrossRef]
- Zoz, W.; Gonzalez, R. Stabilization and sintering of porous Pt/SiO2: A new approach. Appl. Catal. A Gen. 1993, 102, 181–200. [Google Scholar] [CrossRef]
- Khodakov, A.Y. Enhancing cobalt dispersion in supported Fischer-Tropsch catalysts via controlled decomposition of cobalt precursors. Braz. J. Phys. 2009, 39, 171–175. [Google Scholar] [CrossRef]
- Friedland, J.; Kreitz, B.; Grimm, H.; Turek, T.; Güttel, R. Measuring Adsorption Capacity of Supported Catalysts with a Novel Quasi-Continuous Pulse Chemisorption Method. ChemCatChem 2020, 12, 4373–4386. [Google Scholar] [CrossRef]
- Mile, B.; Stirling, D.; Zammitt, M.A.; Lovell, A.; Webb, M. The location of nickel oxide and nickel in silica-supported catalysts: Two forms of “NiO” and the assignment of temperature-programmed reduction profiles. J. Catal. 1988, 114, 217–229. [Google Scholar] [CrossRef]
- Burattin, P.; Che, M.; Louis, C. Metal Particle Size in Ni/SiO2 Materials Prepared by Deposition-Precipitation: Influence of the Nature of the Ni(II) Phase and of Its Interaction with the Support. J. Phys. Chem. B 1999, 103, 6171–6178. [Google Scholar] [CrossRef]
- Zieliński, J. Reductibility of silica supported nickel oxide. Catal. Lett. 1995, 31, 47–56. [Google Scholar] [CrossRef]
- Ho, S.C.; Chou, T.C. The Role of Anion in the Preparation of Nickel Catalyst Detected by TPR and FTIR Spectra. Ind. Eng. Chem. Res. 1995, 34, 2279–2284. [Google Scholar] [CrossRef]
- Louis, C.; Cheng, Z.X.; Che, M. Characterization of nickel/silica catalysts during impregnation and further thermal activation treatment leading to metal particles. J. Phys. Chem. 1993, 97, 5703–5712. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, J.; Chen, Y.W. Studies of Surface NiO Species in NiO/SiO2 Catalysts using Temperature-programmed Reduction and X-Ray Diffraction. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1992, 88, 2075–2078. [Google Scholar] [CrossRef]
- Burattin, P.; Che, M.; Louis, C. Ni/SiO2 Materials Prepared by Deposition-Precipitation: Influence of the Reduction Conditions and Mechanism of Formation of Metal Particles. J. Phys. Chem. B 2000, 104, 10482–10489. [Google Scholar] [CrossRef]
- Coenen, J.W. Characterization of the standard nickel/silica catalyst EuroNi-1. Appl. Catal. 1989, 54, 65–78. [Google Scholar] [CrossRef]
- Bartholomew, C.H. Chemistry of Nickel-Alumina Catalysts. J. Catal. 1976, 45, 41–53. [Google Scholar] [CrossRef]
- Ewald, S.; Hinrichsen, O. On the interaction of CO2 with Ni-Al catalysts. Appl. Catal. A Gen. 2019, 580, 71–80. [Google Scholar] [CrossRef]
- Goodwin, D.G.; Speth, R.L.; Moffat, H.K.; Weber, B.W. Cantera: An Object-oriented Software Toolkit for Chemical Kinetics, Thermodynamics, and Transport Processes. Version 2.4.0. 2018. Available online: https://www.cantera.org (accessed on 24 August 2020).
- Rusic, B.; Bross, D.H. Active Thermochemical Tables (ATcT) Values Based on ver. 1.122 g of the Thermochemical Network. 2019. Available online: https://atct.anl.gov/ (accessed on 7 August 2020).
- Burcat, A.; Ruscic, B. Third Millenium Ideal Gas and Condensed Phase Thermochemical Database for Combustion (with Update from Active Thermochemical Tables); Technical Report; Argonne National Lab. (ANL): Argonne, IL, USA, 2005. [Google Scholar]
- Bjørgum, E.; Chen, D.; Bakken, M.G.; Christensen, K.O.; Holmen, A.; Lytken, O.; Chorkendorff, I. Energetic mapping of Ni catalysts by detailed kinetic modeling. J. Phys. Chem. B 2005, 109, 2360–2370. [Google Scholar] [CrossRef]
- Weatherbee, G.D.; Bartholomew, C.H. Hydrogenation of CO2 on Group VIII Metal: I. Specific Activity of Ni/SiO2. J. Catal. 1981, 68, 67–76. [Google Scholar] [CrossRef]
- Karam, L.; Reboul, J.; El Hassan, N.; Nelayah, J.; Massiani, P. Nanostructured Nickel Aluminate as a Key Intermediate for the Production of Highly Dispersed and Stable Nickel Nanoparticles Supported within Mesoporous Alumina for Dry Reforming of Methane. Molecules 2019, 24, 4170. [Google Scholar] [CrossRef]
- Aziz, M.; Jalil, A.A.; Triwahyono, S.; Saad, M. CO2 methanation over Ni-promoted mesostructured silica nanoparticles: Influence of Ni loading and water vapor on activity and response surface methodology studies. Chem. Eng. J. 2015, 260, 757–764. [Google Scholar] [CrossRef]
- Vrijburg, W.L.; van Helden, J.W.A.; van Hoof, A.J.F.; Friedrich, H.; Groeneveld, E.; Pidko, E.A.; Hensen, E.J.M. Tunable colloidal Ni nanoparticles confined and redistributed in mesoporous silica for CO2 methanation. Catal. Sci. Technol. 2019, 9, 2578–2591. [Google Scholar] [CrossRef]
- Ren, J.; Guo, H.; Yang, J.; Qin, Z.; Lin, J.; Li, Z. Insights into the mechanisms of CO2 methanation on Ni(111) surfaces by density functional theory. Appl. Surf. Sci. 2015, 351, 504–516. [Google Scholar] [CrossRef]
- Vogt, C.; Groeneveld, E.; Kamsma, G.; Nachtegaal, M.; Lu, L.; Kiely, C.J.; Berben, P.H.; Meirer, F.; Weckhuysen, B.M. Unravelling structure sensitivity in CO2 hydrogenation over nickel. Nat. Catal. 2018, 1, 127–134. [Google Scholar] [CrossRef]
- Blaylock, D.W.; Zhu, Y.A.; Green, W.H. Computational Investigation of the Thermochemistry and Kinetics of Steam Methane Reforming Over a Multi-Faceted Nickel Catalyst. Top. Catal. 2011, 54, 828–844. [Google Scholar] [CrossRef]
- Weber, A.P.; Seipenbusch, M.; Kasper, G. Size Effects in the Catalytic Activity of Unsupported Metallic Nanoparticles. J. Nanoparticle Res. 2003, 5, 293–298. [Google Scholar] [CrossRef]
- Ertl, G.; Knözinger, H.; Schüth, F.; Weitkamp, J. Handbook of Heterogeneous Catalysis; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008. [Google Scholar] [CrossRef]


| Property | Unit | 1a | 1b | 1c | 2a | 2b | 2c | 3a | 3b | 3c | 4a | 4b | 4c | Method |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Surface area | m2 g−1 | - | 110 | 96 | - | 111 | 103 | - | 184 | 153 | - | 199 | 181 | BET |
| Pore volume | mm3 g−1 | - | 281 | 269 | - | 290 | 272 | - | 357 | 334 | - | 398 | 375 | BJH |
| Average pore size | - | 9.0 | 9.3 | - | 9.1 | 8.6 | - | 6.4 | 6.6 | - | 6.7 | 6.6 | BJH | |
| Ni loading | wt% | 20.9 | 20.9 | 20.9 | 13.0 | 13.0 | 13.0 | 23.1 | 23.1 | 23.1 | 12.2 | 12.2 | 12.2 | ICP-OES |
| Na content | wt% | 0.01 | 0.01 | 0.01 | <0.003 | <0.003 | <0.003 | 0.12 | 0.12 | 0.12 | 0.15 | 0.15 | 0.15 | ICP-OES |
| K content | wt% | 0.018 | 0.018 | 0.018 | 0.019 | 0.019 | 0.019 | 0.014 | 0.014 | 0.014 | 0.011 | 0.011 | 0.011 | ICP-OES |
| uptake | μmol g−1 | 402 | 181 | 93 | 254 | 171 | 58 | 460 | 271 | 110 | 324 | 205 | 95 | chemisorption |
| Metal surface area | m2 g−1 | 34.3 | 16.3 | 8.9 | 28.1 | 17.6 | 8.4 | 40.5 | 23.8 | 12.0 | 34.6 | 21.1 | 11.3 | chemisorption |
| Average Ni crystal size | 4.1 (4.5) | 8.6 (6.8) | 15.9 (8.3) | 3.1 (3.2) | 5.0 (4.7) | 10.4 (5.4) | 3.9 (4.3) | 6.5 (5.1) | 12.9 (5.9) | 2.4 (2.0) | 3.9 (2.5) | 7.3 (2.8) | chemisorption (XRD) | |
| Ni dispersion | % | 24.6 | 11.7 | 6.3 | 32.4 | 20.2 | 9.7 | 26.2 | 15.4 | 7.8 | 42.4 | 25.9 | 13.9 | chemisorption |
| Degree of reduction | % | 91.9 | 87.0 | 82.7 | 70.8 | 76.6 | 53.9 | 89.2 | 89.2 | 71.4 | 73.5 | 76.3 | 66.2 | chemisorption |
| uptake | μmol g−1 | 78.5 | 26.6 | - | - | - | - | 126.3 | 53.5 | 16.9 | - | - | - | chemisorption |
| Property | Unit | 5a | 5b | 5c | 6a | 6b | 6c | Method |
|---|---|---|---|---|---|---|---|---|
| Surface area | m2 g−1 | - | 178 | 123 | - | 155 | 99 | BET |
| Pore volume | mm3 g−1 | - | 337 | 331 | - | 311 | 293 | BJH |
| Average pore size | - | 5.9 | 8.0 | - | 6.4 | 8.7 | BJH | |
| Ni loading | wt% | 9.0 | 9.0 | 9.0 | 15.1 | 15.1 | 15.1 | ICP-OES |
| Na content | wt% | 0.16 | 0.16 | 0.16 | 0.17 | 0.17 | 0.17 | ICP-OES |
| K content | wt% | 0.0055 | 0.0055 | 0.0055 | 0.0046 | 0.0046 | 0.0046 | ICP-OES |
| uptake | μmol g−1 | 7 | - | - | 143 | 69 | - | chemisorption |
| Metal surface area | m2 g−1 | 5.8 | - | - | 28.3 | 18.1 | - | chemisorption |
| Average Ni crystal size | 14.5 | - | - | 2.9 | 4.5 | - | chemisorption | |
| Ni dispersion | % | 7.1 | - | - | 34.7 | 22.3 | - | chemisorption |
| Degree of reduction | % | 9.6 | - | - | 39.6 | 29.8 | - | chemisorption |
| uptake | μmol g−1 | 140.9 | - | - | 125.6 | - | - | chemisorption |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kreitz, B.; Martínez Arias, A.; Martin, J.; Weber, A.P.; Turek, T. Spray-Dried Ni Catalysts with Tailored Properties for CO2 Methanation. Catalysts 2020, 10, 1410. https://doi.org/10.3390/catal10121410
Kreitz B, Martínez Arias A, Martin J, Weber AP, Turek T. Spray-Dried Ni Catalysts with Tailored Properties for CO2 Methanation. Catalysts. 2020; 10(12):1410. https://doi.org/10.3390/catal10121410
Chicago/Turabian StyleKreitz, Bjarne, Aurina Martínez Arias, Jan Martin, Alfred P. Weber, and Thomas Turek. 2020. "Spray-Dried Ni Catalysts with Tailored Properties for CO2 Methanation" Catalysts 10, no. 12: 1410. https://doi.org/10.3390/catal10121410
APA StyleKreitz, B., Martínez Arias, A., Martin, J., Weber, A. P., & Turek, T. (2020). Spray-Dried Ni Catalysts with Tailored Properties for CO2 Methanation. Catalysts, 10(12), 1410. https://doi.org/10.3390/catal10121410