Carbon-Based Materials for the Development of Highly Dispersed Metal Catalysts: Towards Highly Performant Catalysts for Fine Chemical Synthesis
Abstract
1. Introduction
2. Carbon Materials as Ideal Supports for Development of Highly Dispersed Metal Catalyst
2.1. Synthetic Strategies and Properties
2.1.1. Impregnation—Wet and Dry Methods
2.1.2. Organometallic Compounds
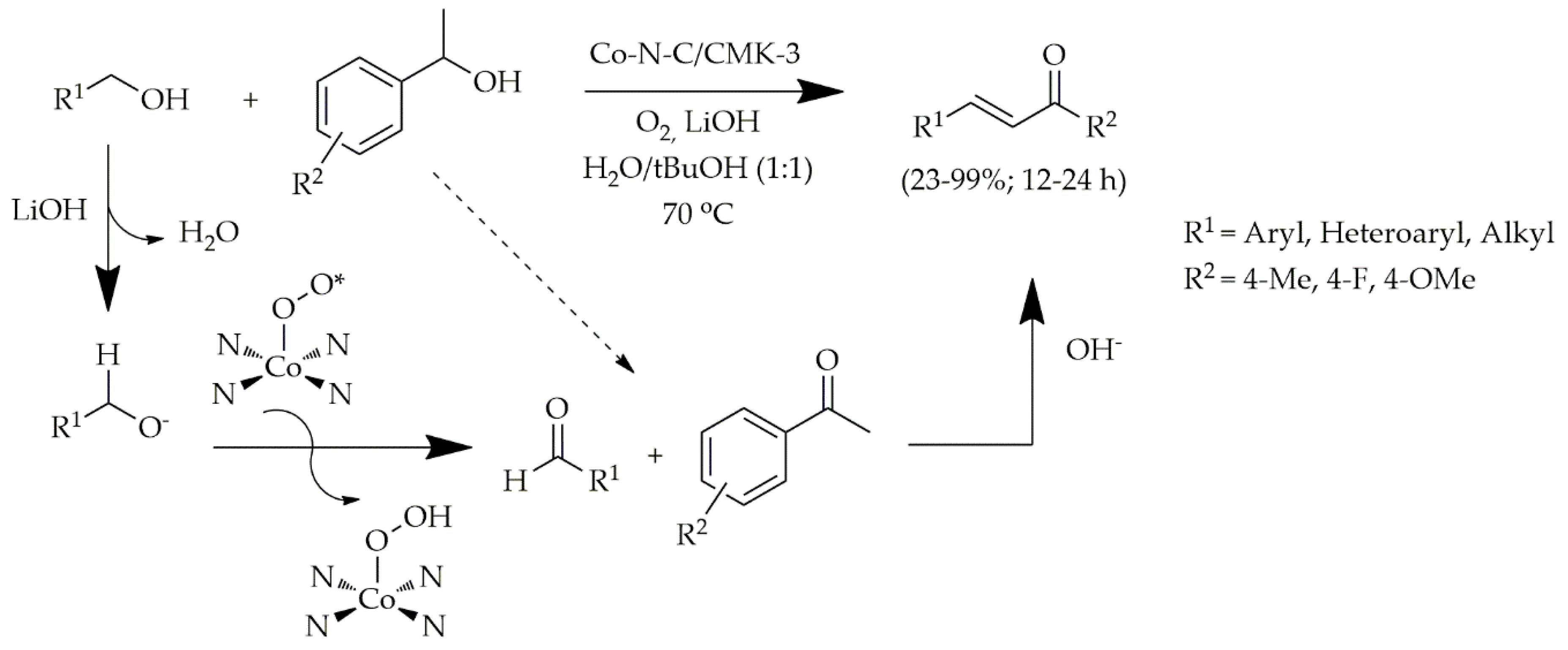
2.1.3. Deposition
Chemical-Vapor-Deposition (CVD)
Atomic-Layer-Deposition (ALD)
2.1.4. Pyrolysis
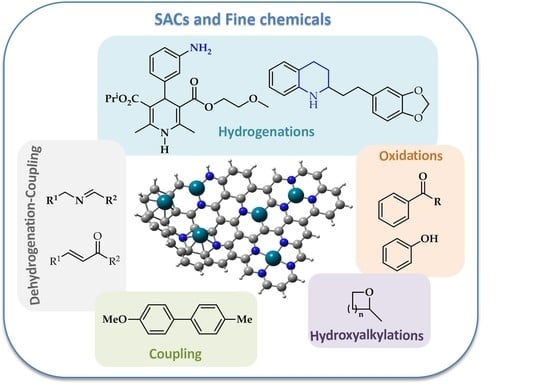
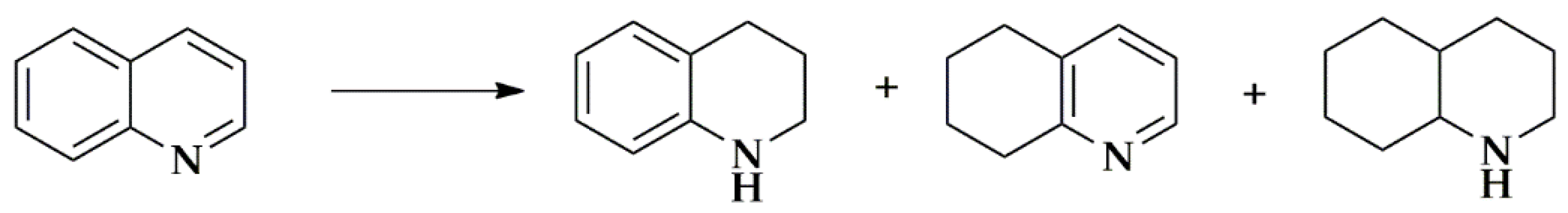
3. Carbon-Supported Metal Single Atom Catalysts. Application in Fine Chemical Synthesis
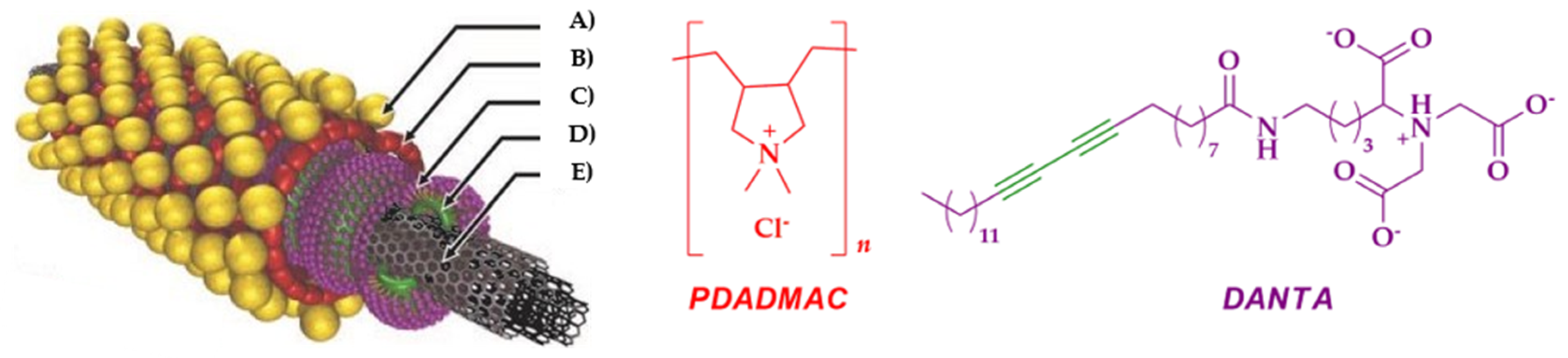
3.1. Carbon Nanotubes
3.2. Graphene and Graphene Oxide
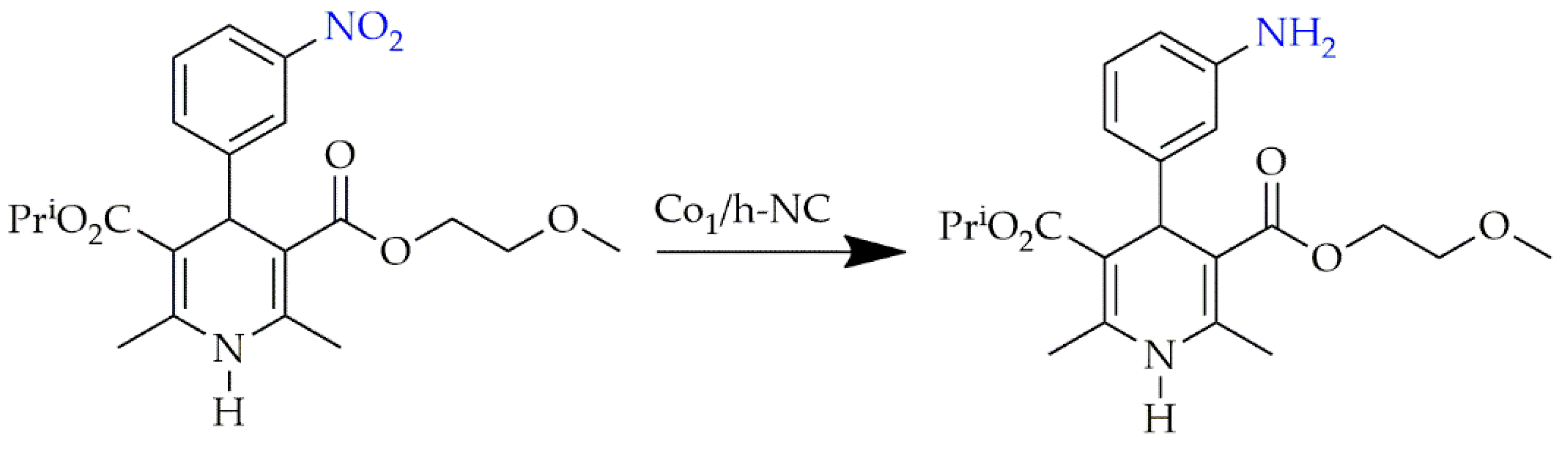
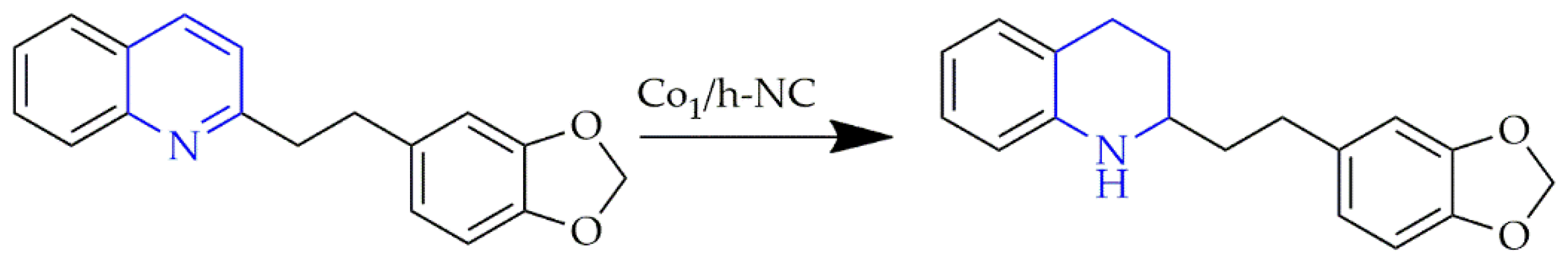
3.3. Carbon Nitride
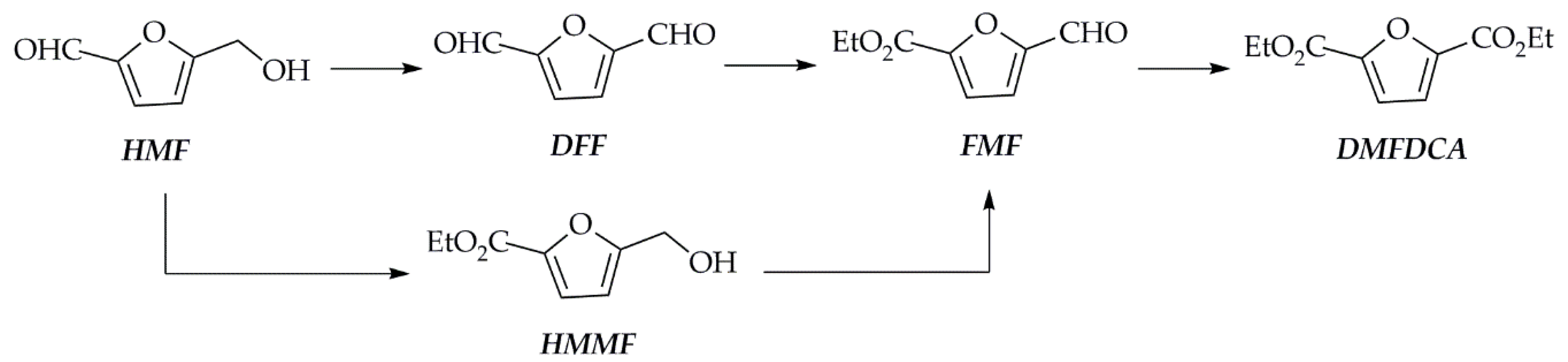
3.4. Carbons from Emerging Precursors: Metal Organic-Frameworks
3.5. Other Porous Carbons
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pérez-Mayoral, E.; Calvino-Casilda, V.; Soriano, E. Metal-supported carbon-based materials: Opportunities and challenges in the synthesis of valuable products. Catal. Sci. Technol. 2016, 6, 1265–1291. [Google Scholar] [CrossRef]
- Serp, P.; Figueiredo, J.L. Carbon Materials for Catalysis; Serp, P., Figueiredo, J.L., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008; ISBN 9780470403709. [Google Scholar]
- Pérez-Mayoral, E.; Calvino-Casilda, V.; Godino, M.; López- Peinado, A.J.; Martín-Aranda, R.M. Porous Catalytic Systems in the Synthesis of Bioactive Heterocycles and Related Compounds. In Green Synthetic Approaches for Biologically Relevant Heterocycles; Brahmachari, G., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 377–408. ISBN 9780128005903. [Google Scholar]
- Pérez-Mayoral, E.; Soriano, E.; Martín-Aranda, R.M.; Maldonado-Hódar, F.J. Mesoporous Catalytic Materials and Fine Chemistry. In Comprehensive Guide for Mesoporous Materials. Volume 1: Synthesis and Characterization; Aliofkhazraei, M., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2015; pp. 83–118. ISBN 978-1-63463-958-3. [Google Scholar]
- Matos, I.; Bernardo, M.; Fonseca, I. Porous carbon: A versatile material for catalysis. Catal. Today 2017, 285, 194–203. [Google Scholar] [CrossRef]
- Yang, X.-F.; Wang, A.; Qiao, B.; Li, J.; Liu, J.; Zhang, T. Single-Atom Catalysts: A New Frontier in Heterogeneous Catalysis. Acc. Chem. Res. 2013, 46, 1740–1748. [Google Scholar] [CrossRef]
- Liu, L.; Corma, A. Metal Catalysts for Heterogeneous Catalysis: From Single Atoms to Nanoclusters and Nanoparticles. Chem. Rev. 2018, 118, 4981–5079. [Google Scholar] [CrossRef] [PubMed]
- Kou, Z.; Zang, W.; Wang, P.; Li, X.; Wang, J. Single atom catalysts: A surface heterocompound perspective. Nanoscale Horiz. 2020, 5, 757–764. [Google Scholar] [CrossRef]
- Christopher, P. Single-Atom Catalysts: Are All Sites Created Equal? ACS Energy Lett. 2019, 4, 2249–2250. [Google Scholar] [CrossRef]
- Liu, J. Catalysis by Supported Single Metal Atoms. ACS Catal. 2017, 7, 34–59. [Google Scholar] [CrossRef]
- Haruta, M. Gold catalysts prepared by coprecipitation for low-temperature oxidation of hydrogen and of carbon monoxide. J. Catal. 1989, 115, 301–309. [Google Scholar] [CrossRef]
- Papp, C. Catalysis at the limit. Nat. Chem. 2018, 10, 995–996. [Google Scholar] [CrossRef]
- Wang, A.; Li, J.; Zhang, T. Heterogeneous single-atom catalysis. Nat. Rev. Chem. 2018, 2, 65–81. [Google Scholar] [CrossRef]
- He, T.; Chen, S.; Ni, B.; Gong, Y.; Wu, Z.; Song, L.; Gu, L.; Hu, W.; Wang, X. Zirconium-Porphyrin-Based Metal-Organic Framework Hollow Nanotubes for Immobilization of Noble-Metal Single Atoms. Angew. Chemie Int. Ed. 2018, 57, 3493–3498. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Díaz, U.; Arenal, R.; Agostini, G.; Concepción, P.; Corma, A. Generation of subnanometric platinum with high stability during transformation of a 2D zeolite into 3D. Nat. Mater. 2017, 16, 132–138. [Google Scholar] [CrossRef]
- Ortalan, V.; Uzun, A.; Gates, B.C.; Browning, N.D. Direct imaging of single metal atoms and clusters in the pores of dealuminated HY zeolite. Nat. Nanotechnol. 2010, 5, 506–510. [Google Scholar] [CrossRef]
- Cheng, N.; Zhang, L.; Doyle-Davis, K.; Sun, X. Single-Atom Catalysts: From Design to Application. Electrochem. Energy Rev. 2019, 2, 539–573. [Google Scholar] [CrossRef]
- Rivera-Cárcamo, C.; Serp, P. Single Atom Catalysts on Carbon-Based Materials. ChemCatChem 2018, 10, 5058–5091. [Google Scholar] [CrossRef]
- Gawande, M.B.; Fornasiero, P.; Zbořil, R. Carbon-Based Single-Atom Catalysts for Advanced Applications. ACS Catal. 2020, 10, 2231–2259. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, X.; Zhou, Z. Carbon-Based Substrates for Highly Dispersed Nanoparticle and Even Single-Atom Electrocatalysts. Small Methods 2019, 3, 1900050. [Google Scholar] [CrossRef]
- Li, J.-C.; Wei, Z.; Liu, D.; Du, D.; Lin, Y.; Shao, M. Dispersive Single-Atom Metals Anchored on Functionalized Nanocarbons for Electrochemical Reactions. Top. Curr. Chem. 2019, 377, 4. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, J.N.; Dang, N.K.; Park, H.J.; Sultan, S.; Kim, M.G.; Haiyan, J.; Lee, Z.; Kim, K.S. Remarkably enhanced catalytic activity by the synergistic effect of palladium single atoms and palladium–cobalt phosphide nanoparticles. Nano Energy 2020, 78, 105166. [Google Scholar] [CrossRef]
- Cao, L.; Luo, Q.; Liu, W.; Lin, Y.; Liu, X.; Cao, Y.; Zhang, W.; Wu, Y.; Yang, J.; Yao, T.; et al. Identification of single-atom active sites in carbon-based cobalt catalysts during electrocatalytic hydrogen evolution. Nat. Catal. 2019, 2, 134–141. [Google Scholar] [CrossRef]
- Liu, Q.; Peng, Y.; Li, Q.; He, T.; Morris, D.; Nichols, F.; Mercado, R.; Zhang, P.; Chen, S. Atomic Dispersion and Surface Enrichment of Palladium in Nitrogen-Doped Porous Carbon Cages Lead to High-Performance Electrocatalytic Reduction of Oxygen. ACS Appl. Mater. Interfaces 2020, 12, 17641–17650. [Google Scholar] [CrossRef] [PubMed]
- Arrigo, R.; Schuster, M. On the High Structural Heterogeneity of Fe-Impregnated Graphitic-Carbon Catalysts from Fe Nitrate Precursor. Catalysts 2019, 9, 303. [Google Scholar] [CrossRef]
- Carlier, S.; Gripekoven, J.; Philippo, M.; Hermans, S. Ru on N-doped carbon supports for the direct hydrogenation of cellobiose into sorbitol. Appl. Catal. B Environ. 2021, 282, 119515. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, H.; Yu, P.; Yuan, Y.; Shahbazian-Yassar, R.; Sheng, Y.; Wu, S.; Tu, W.; Liu, G.; Kraft, M.; et al. Isolated Ni single atoms in nitrogen doped ultrathin porous carbon templated from porous g-C3N4 for high-performance CO2 reduction. Nano Energy 2020, 77, 105158. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, S.; Dou, M.; Zhang, Z.; Wang, F. Photochemically activated atomic ruthenium supported on boron-doped carbon as a robust electrocatalyst for hydrogen evolution. J. Mater. Chem. A 2020, 8, 16669–16675. [Google Scholar] [CrossRef]
- Jin, H.; Sultan, S.; Ha, M.; Tiwari, J.N.; Kim, M.G.; Kim, K.S. Simple and Scalable Mechanochemical Synthesis of Noble Metal Catalysts with Single Atoms toward Highly Efficient Hydrogen Evolution. Adv. Funct. Mater. 2020, 30, 2000531. [Google Scholar] [CrossRef]
- Jeon, I.-Y.; Kim, S.-W.; Shin, S.-H.; Jung, S.-M.; Baek, J.-B. Forming indium-carbon (In–C) bonds at the edges of graphitic nanoplatelets. Mater. Today Adv. 2020, 6, 100030. [Google Scholar] [CrossRef]
- Yan, J.; Tian, X.; Liu, X.-Y.; Zhao, X.; Wang, R.; Zhao, L.-J.; Zhang, X. Correlation between electrochemical properties of the CNTs/AB5 composite hydrogen storage alloys and their catalytic properties for KBH4. Int. J. Hydrogen Energy 2020, 45, 452–463. [Google Scholar] [CrossRef]
- Zhang, L.; Ren, Y.; Liu, W.; Wang, A.; Zhang, T. Single-atom catalyst: A rising star for green synthesis of fine chemicals. Natl. Sci. Rev. 2018, 5, 653–672. [Google Scholar] [CrossRef]
- Li, X.; Lei, H.; Guo, X.; Zhao, X.; Ding, S.; Gao, X.; Zhang, W.; Cao, R. Graphene-Supported Pyrene-Modified Cobalt Corrole with Axial Triphenylphosphine for Enhanced Hydrogen Evolution in pH 0–14 Aqueous Solutions. ChemSusChem 2017, 10, 4632–4641. [Google Scholar] [CrossRef]
- Kharisov, B.I.; Kharissova, O.V.; Vázquez Dimas, A.; Gómez De La Fuente, I.; Peña Méndez, Y. Review: Graphene-supported coordination complexes and organometallics: Properties and applications. J. Coord. Chem. 2016, 69, 1125–1151. [Google Scholar] [CrossRef]
- Campisciano, V.; Gruttadauria, M.; Giacalone, F. Modified Nanocarbons for Catalysis. ChemCatChem 2019, 11, 90–133. [Google Scholar] [CrossRef]
- Choi, B.; Lee, J.; Lee, S.; Ko, J.-H.; Lee, K.-S.; Oh, J.; Han, J.; Kim, Y.-H.; Choi, I.S.; Park, S. Generation of Ultra-High-Molecular-Weight Polyethylene from Metallocenes Immobilized onto N-Doped Graphene Nanoplatelets. Macromol. Rapid Commun. 2013, 34, 533–538. [Google Scholar] [CrossRef]
- Su, H.; Wu, S.; Li, Z.; Huo, Q.; Guan, J.; Kan, Q. Co(II), Fe(III) or VO(II) Schiff base metal complexes immobilized on graphene oxide for styrene epoxidation. Appl. Organomet. Chem. 2015, 29, 462–467. [Google Scholar] [CrossRef]
- Sabounchei, S.J.; Hosseinzadeh, M.; Zarepour-jevinani, M.; Ghanbari, B. Monodentate palladium(0)–[60]fullerene complexes of diphosphine ligands as efficient and sustainable nanocatalysts for the Mizoroki–Heck coupling reaction of aryl chlorides. New J. Chem. 2017, 41, 9701–9709. [Google Scholar] [CrossRef]
- Kumar, R.; Gravel, E.; Hagège, A.; Li, H.; Jawale, D.V.; Verma, D.; Namboothiri, I.N.N.; Doris, E. Carbon nanotube–gold nanohybrids for selective catalytic oxidation of alcohols. Nanoscale 2013, 5, 6491. [Google Scholar] [CrossRef]
- Queffélec, C.; Schlindwein, S.H.; Gudat, D.; Silvestre, V.; Rodriguez-Zubiri, M.; Fayon, F.; Bujoli, B.; Wang, Q.; Boukherroub, R.; Szunerits, S. Wilkinson-Type Immobilized Catalyst on Diamond Nanoparticles for Alkene Reduction. ChemCatChem 2017, 9, 432–439. [Google Scholar] [CrossRef]
- Ribeiro, A.P.C.; Martins, L.M.D.R.S.; Carabineiro, S.A.C.; Buijnsters, J.G.; Figueiredo, J.L.; Pombeiro, A.J.L. Heterogenized C-Scorpionate Iron(II) Complex on Nanostructured Carbon Materials as Recyclable Catalysts for Microwave-Assisted Oxidation Reactions. ChemCatChem 2018, 10, 1821–1828. [Google Scholar] [CrossRef]
- Pour, S.R.; Abdolmaleki, A.; Dinari, M. Immobilization of new macrocyclic Schiff base copper complex on graphene oxide nanosheets and its catalytic activity for olefins epoxidation. J. Mater. Sci. 2019, 54, 2885–2896. [Google Scholar] [CrossRef]
- Ren, R.; Bi, S.; Wang, L.; Zhao, W.; Wei, D.; Li, T.; Xu, W.; Liu, M.; Wu, Y. Terpyridine-based Pd(II)/Ni(II) organometallic framework nano-sheets supported on graphene oxide—Investigating the fabrication, tuning of catalytic properties and synergetic effects. RSC Adv. 2020, 10, 23080–23090. [Google Scholar] [CrossRef]
- Sánchez-Page, B.; Pérez-Mas, A.M.; González-Ingelmo, M.; Fernández, L.; González, Z.; Jiménez, M.V.; Pérez-Torrente, J.J.; Blasco, J.; Subías, G.; Álvarez, P.; et al. Influence of graphene sheet properties as supports of iridium-based N-heterocyclic carbene hybrid materials for water oxidation electrocatalysis. J. Organomet. Chem. 2020, 919, 121334. [Google Scholar] [CrossRef]
- Serp, P.; Kalck, P.; Feurer, R. Chemical Vapor Deposition Methods for the Controlled Preparation of Supported Catalytic Materials. Chem. Rev. 2002, 102, 3085–3128. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Niu, Z.; Chen, J. Single Atoms on Graphene for Energy Storage and Conversion. Small Methods 2019, 3, 1800443. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, G.; Gauquelin, N.; Chen, N.; Zhou, J.; Yang, S.; Chen, W.; Meng, X.; Geng, D.; Banis, M.N.; et al. Single-atom Catalysis Using Pt/Graphene Achieved through Atomic Layer Deposition. Sci. Rep. 2013, 3, 1775. [Google Scholar] [CrossRef]
- Wang, Y.; Mao, J.; Meng, X.; Yu, L.; Deng, D.; Bao, X. Catalysis with Two-Dimensional Materials Confining Single Atoms: Concept, Design, and Applications. Chem. Rev. 2019, 119, 1806–1854. [Google Scholar] [CrossRef]
- Han, X.; Sun, W.; Zhao, C.; Shi, R.; Wang, X.; Liu, S.; Li, Z.; Ren, J. Synthesis of dimethyl carbonate on single Cu atom embedded in N-doped graphene: Effect of nitrogen species. Mol. Catal. 2017, 443, 1–13. [Google Scholar] [CrossRef]
- Du, P.; Hu, K.; Lyu, J.; Li, H.; Lin, X.; Xie, G.; Liu, X.; Ito, Y.; Qiu, H.-J. Anchoring Mo single atoms/clusters and N on edge-rich nanoporous holey graphene as bifunctional air electrode in Zn−air batteries. Appl. Catal. B Environ. 2020, 276, 119172. [Google Scholar] [CrossRef]
- O’Neill, B.J.; Jackson, D.H.K.; Lee, J.; Canlas, C.; Stair, P.C.; Marshall, C.L.; Elam, J.W.; Kuech, T.F.; Dumesic, J.A.; Huber, G.W. Catalyst Design with Atomic Layer Deposition. ACS Catal. 2015, 5, 1804–1825. [Google Scholar] [CrossRef]
- Huang, X.; Xia, Y.; Cao, Y.; Zheng, X.; Pan, H.; Zhu, J.; Ma, C.; Wang, H.; Li, J.; You, R.; et al. Enhancing both selectivity and coking-resistance of a single-atom Pd1/C3N4 catalyst for acetylene hydrogenation. Nano Res. 2017, 10, 1302–1312. [Google Scholar] [CrossRef]
- Yan, H.; Cheng, H.; Yi, H.; Lin, Y.; Yao, T.; Wang, C.; Li, J.; Wei, S.; Lu, J. Single-Atom Pd1/Graphene Catalyst Achieved by Atomic Layer Deposition: Remarkable Performance in Selective Hydrogenation of 1,3-Butadiene. J. Am. Chem. Soc. 2015, 137, 10484–10487. [Google Scholar] [CrossRef]
- Li, Z.; Ge, R.; Su, J.; Chen, L. Recent Progress in Low Pt Content Electrocatalysts for Hydrogen Evolution Reaction. Adv. Mater. Interfaces 2020, 7, 2000396. [Google Scholar] [CrossRef]
- Nayak, P.; Jiang, Q.; Kurra, N.; Wang, X.; Buttner, U.; Alshareef, H.N. Monolithic laser scribed graphene scaffolds with atomic layer deposited platinum for the hydrogen evolution reaction. J. Mater. Chem. A 2017, 5, 20422–20427. [Google Scholar] [CrossRef]
- Zhang, Z.; Deng, L.; Zhao, Z.; Zhao, Y.; Yang, J.; Jiang, J.; Huang, G.; Mei, Y. Nickel nanograins anchored on a carbon framework for an efficient hydrogen evolution electrocatalyst and a flexible electrode. J. Mater. Chem. A 2020, 8, 3499–3508. [Google Scholar] [CrossRef]
- Liang, Z.; Qu, C.; Xia, D.; Zou, R.; Xu, Q. Atomically Dispersed Metal Sites in MOF-Based Materials for Electrocatalytic and Photocatalytic Energy Conversion. Angew. Chemie Int. Ed. 2018, 57, 9604–9633. [Google Scholar] [CrossRef]
- Niu, S.; Yang, J.; Qi, H.; Su, Y.; Wang, Z.; Qiu, J.; Wang, A.; Zhang, T. Single-atom Pt promoted Mo2C for electrochemical hydrogen evolution reaction. J. Energy Chem. 2020. [Google Scholar] [CrossRef]
- Hu, X.; Luo, G.; Zhao, Q.; Wu, D.; Yang, T.; Wen, J.; Wang, R.; Xu, C.; Hu, N. Ru Single Atoms on N-Doped Carbon by Spatial Confinement and Ionic Substitution Strategies for High-Performance Li–O2 Batteries. J. Am. Chem. Soc. 2020, 142, 16776–16786. [Google Scholar] [CrossRef]
- Hou, Y.; Liang, Y.-L.; Shi, P.-C.; Huang, Y.-B.; Cao, R. Atomically dispersed Ni species on N-doped carbon nanotubes for electroreduction of CO2 with nearly 100% CO selectivity. Appl. Catal. B Environ. 2020, 271, 118929. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.; Li, Y.; Lan, X.; Ali, B.; Wang, T. Highly Efficient Hydrogenation of Nitroarenes by N-Doped Carbon-Supported Cobalt Single-Atom Catalyst in Ethanol/Water Mixed Solvent. ACS Appl. Mater. Interfaces 2020, 12, 34021–34031. [Google Scholar] [CrossRef]
- Chen, X.; Ma, D.-D.; Chen, B.; Zhang, K.; Zou, R.; Wu, X.-T.; Zhu, Q.-L. Metal–organic framework-derived mesoporous carbon nanoframes embedded with atomically dispersed Fe–N active sites for efficient bifunctional oxygen and carbon dioxide electroreduction. Appl. Catal. B Environ. 2020, 267, 118720. [Google Scholar] [CrossRef]
- Han, S.-G.; Ma, D.-D.; Zhou, S.-H.; Zhang, K.; Wei, W.-B.; Du, Y.; Wu, X.-T.; Xu, Q.; Zou, R.; Zhu, Q.-L. Fluorine-tuned single-atom catalysts with dense surface Ni-N4 sites on ultrathin carbon nanosheets for efficient CO2 electroreduction. Appl. Catal. B Environ. 2021, 283, 119591. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Z.; Jiang, J.; Chen, W.; Liao, F.; Ge, X.; Zhou, X.; Chen, M.; Li, R.; Xue, Z.; et al. In-situ polymerization induced atomically dispersed manganese sites as cocatalyst for CO2 photoreduction into synthesis gas. Nano Energy 2020, 76, 105059. [Google Scholar] [CrossRef]
- Ma, R.; Lin, G.; Ju, Q.; Tang, W.; Chen, G.; Chen, Z.; Liu, Q.; Yang, M.; Lu, Y.; Wang, J. Edge-sited Fe-N4 atomic species improve oxygen reduction activity via boosting O2 dissociation. Appl. Catal. B Environ. 2020, 265, 118593. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, M.; Zhang, X.; Zhao, C.; Wang, H.; Li, S.; Liu, Z. Direct Conversion of Biomass into Compact Air Electrode with Atomically Dispersed Oxygen and Nitrogen Coordinated Copper Species for Flexible Zinc–Air Batteries. ACS Appl. Energy Mater. 2019, 2, 8659–8666. [Google Scholar] [CrossRef]
- Bacsa, R.R.; Cameán, I.; Ramos, A.; Garcia, A.B.; Tishkova, V.; Bacsa, W.S.; Gallagher, J.R.; Miller, J.T.; Navas, H.; Jourdain, V.; et al. Few layer graphene synthesis on transition metal ferrite catalysts. Carbon N. Y. 2015, 89, 350–360. [Google Scholar] [CrossRef]
- Philippe, R.; Morançais, A.; Corrias, M.; Caussat, B.; Kihn, Y.; Kalck, P.; Plee, D.; Gaillard, P.; Bernard, D.; Serp, P. Catalytic Production of Carbon Nanotubes by Fluidized-Bed CVD. Chem. Vap. Depos. 2007, 13, 447–457. [Google Scholar] [CrossRef]
- John, J.; Gravel, E.; Hagège, A.; Li, H.; Gacoin, T.; Doris, E. Catalytic Oxidation of Silanes by Carbon Nanotube-Gold Nanohybrids. Angew. Chemie Int. Ed. 2011, 50, 7533–7536. [Google Scholar] [CrossRef]
- Corma, A.; Concepción, P.; Boronat, M.; Sabater, M.J.; Navas, J.; Yacaman, M.J.; Larios, E.; Posadas, A.; López-Quintela, M.A.; Buceta, D.; et al. Exceptional oxidation activity with size-controlled supported gold clusters of low atomicity. Nat. Chem. 2013, 5, 775–781. [Google Scholar] [CrossRef]
- Zhang, F.; Jiao, F.; Pan, X.; Gao, K.; Xiao, J.; Zhang, S.; Bao, X. Tailoring the Oxidation Activity of Pt Nanoclusters via Encapsulation. ACS Catal. 2015, 5, 1381–1385. [Google Scholar] [CrossRef]
- Lee, E.-K.; Park, S.-A.; Woo, H.; Hyun Park, K.; Kang, D.W.; Lim, H.; Kim, Y.-T. Platinum single atoms dispersed on carbon nanotubes as reusable catalyst for Suzuki coupling reaction. J. Catal. 2017, 352, 388–393. [Google Scholar] [CrossRef]
- Woo, H.; Lee, E.-K.; Yun, S.-W.; Park, S.-A.; Park, K.H.; Kim, Y.-T. Platinum Single Atoms on Carbon Nanotubes as Efficient Catalyst for Hydroalkoxylation. Bull. Korean Chem. Soc. 2017, 38, 1221–1225. [Google Scholar] [CrossRef]
- Grasseschi, D.; Silva, W.C.; Souza Paiva, R.d.; Starke, L.D.; do Nascimento, A.S. Surface coordination chemistry of graphene: Understanding the coordination of single transition metal atoms. Coord. Chem. Rev. 2020, 422, 213469. [Google Scholar] [CrossRef]
- Deng, D.; Chen, X.; Yu, L.; Wu, X.; Liu, Q.; Liu, Y.; Yang, H.; Tian, H.; Hu, Y.; Du, P.; et al. A single iron site confined in a graphene matrix for the catalytic oxidation of benzene at room temperature. Sci. Adv. 2015, 1, e1500462. [Google Scholar] [CrossRef]
- Bakandritsos, A.; Kadam, R.G.; Kumar, P.; Zoppellaro, G.; Medved’, M.; Tuček, J.; Montini, T.; Tomanec, O.; Andrýsková, P.; Drahoš, B.; et al. Mixed-Valence Single-Atom Catalyst Derived from Functionalized Graphene. Adv. Mater. 2019, 31, 1900323. [Google Scholar] [CrossRef]
- Sun, X.; Han, P.; Li, B.; Zhao, Z. Tunable Catalytic Performance of Single Pt Atom on Doped Graphene in Direct Dehydrogenation of Propane by Rational Doping: A Density Functional Theory Study. J. Phys. Chem. C 2018, 122, 1570–1576. [Google Scholar] [CrossRef]
- Li, M.; Wu, S.; Yang, X.; Hu, J.; Peng, L.; Bai, L.; Huo, Q.; Guan, J. Highly efficient single atom cobalt catalyst for selective oxidation of alcohols. Appl. Catal. A Gen. 2017, 543, 61–66. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Y.; Chu, M.; Duan, T.; Meng, C.; Han, Y. Defect stabilized gold atoms on graphene as potential catalysts for ethylene epoxidation: A first-principles investigation. Catal. Sci. Technol. 2016, 6, 1632–1641. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Zhao, J. PtN3-Embedded graphene as an efficient catalyst for electrochemical reduction of nitrobenzene to aniline: A theoretical study. Phys. Chem. Chem. Phys. 2020, 22, 17639–17645. [Google Scholar] [CrossRef]
- Lou, Y.; Wu, H.; Liu, J. Nanocarbon-Edge-Anchored High-Density Pt Atoms for 3-nitrostyrene Hydrogenation: Strong Metal-Carbon Interaction. iScience 2019, 13, 190–198. [Google Scholar] [CrossRef]
- Huang, H.; Wang, X.; Sheng, Y.; Chen, C.; Zou, X.; Shang, X.; Lu, X. Nitrogen-doped graphene-activated metallic nanoparticle-incorporated ordered mesoporous carbon nanocomposites for the hydrogenation of nitroarenes. RSC Adv. 2018, 8, 8898–8909. [Google Scholar] [CrossRef]
- Xi, J.; Sun, H.; Wang, D.; Zhang, Z.; Duan, X.; Xiao, J.; Xiao, F.; Liu, L.; Wang, S. Confined-interface-directed synthesis of Palladium single-atom catalysts on graphene/amorphous carbon. Appl. Catal. B Environ. 2018, 225, 291–297. [Google Scholar] [CrossRef]
- Yan, H.; Lv, H.; Yi, H.; Liu, W.; Xia, Y.; Huang, X.; Huang, W.; Wei, S.; Wu, X.; Lu, J. Understanding the underlying mechanism of improved selectivity in pd1 single-atom catalyzed hydrogenation reaction. J. Catal. 2018, 366, 70–79. [Google Scholar] [CrossRef]
- Almeida Ribeiro, R.S.; Monteiro Ferreira, L.E.; Rossa, V.; Lima, C.G.S.; Paixão, M.W.; Varma, R.S.; Melo Lima, T. Graphitic Carbon Nitride-Based Materials as Catalysts for the Upgrading of Lignocellulosic Biomass-Derived Molecules. ChemSusChem 2020, 13, 3992–4004. [Google Scholar] [CrossRef]
- Verma, S.; Nadagouda, M.N.; Varma, R.S. Porous nitrogen-enriched carbonaceous material from marine waste: Chitosan-derived carbon nitride catalyst for aerial oxidation of 5-hydroxymethylfurfural (HMF) to 2,5-furandicarboxylic acid. Sci. Rep. 2017, 7, 13596. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Antonietti, M. Graphitic carbon nitride “reloaded”: Emerging applications beyond (photo)catalysis. Chem. Soc. Rev. 2016, 45, 2308–2326. [Google Scholar] [CrossRef]
- Huang, J.; Antonietti, M.; Liu, J. Bio-inspired carbon nitride mesoporous spheres for artificial photosynthesis: Photocatalytic cofactor regeneration for sustainable enzymatic synthesis. J. Mater. Chem. A 2014, 2, 7686–7693. [Google Scholar] [CrossRef]
- Yang, S.; Gong, Y.; Zhang, J.; Zhan, L.; Ma, L.; Fang, Z.; Vajtai, R.; Wang, X.; Ajayan, P.M. Exfoliated Graphitic Carbon Nitride Nanosheets as Efficient Catalysts for Hydrogen Evolution Under Visible Light. Adv. Mater. 2013, 25, 2452–2456. [Google Scholar] [CrossRef]
- Mishra, A.; Mehta, A.; Basu, S.; Shetti, N.P.; Reddy, K.R.; Aminabhavi, T.M. Graphitic carbon nitride (g–C3N4)–based metal-free photocatalysts for water splitting: A review. Carbon N. Y. 2019, 149, 693–721. [Google Scholar] [CrossRef]
- Shalom, M.; Gimenez, S.; Schipper, F.; Herraiz-Cardona, I.; Bisquert, J.; Antonietti, M. Controlled Carbon Nitride Growth on Surfaces for Hydrogen Evolution Electrodes. Angew. Chemie 2014, 126, 3728–3732. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, Y.; Shen, Y.; Liu, S.; Zhang, Y. Molecular engineering of polymeric carbon nitride: Advancing applications from photocatalysis to biosensing and more. Chem. Soc. Rev. 2018, 47, 2298–2321. [Google Scholar] [CrossRef]
- Dai, B.; Li, X.; Zhang, J.; Yu, F.; Zhu, M. Application of mesoporous carbon nitride as a support for an Au catalyst for acetylene hydrochlorination. Chem. Eng. Sci. 2015, 135, 472–478. [Google Scholar] [CrossRef]
- Chen, Z.; Vorobyeva, E.; Mitchell, S.; Fako, E.; López, N.; Collins, S.M.; Leary, R.K.; Midgley, P.A.; Hauert, R.; Pérez-Ramírez, J. Single-atom heterogeneous catalysts based on distinct carbon nitride scaffolds. Natl. Sci. Rev. 2018, 5, 642–652. [Google Scholar] [CrossRef]
- Chen, Z.; Mitchell, S.; Krumeich, F.; Hauert, R.; Yakunin, S.; Kovalenko, M.V.; Pérez-Ramírez, J. Tunability and Scalability of Single-Atom Catalysts Based on Carbon Nitride. ACS Sustain. Chem. Eng. 2019, 7, 5223–5230. [Google Scholar] [CrossRef]
- Büchele, S.; Chen, Z.; Mitchell, S.; Hauert, R.; Krumeich, F.; Pérez-Ramírez, J. Tailoring Nitrogen-Doped Carbons as Hosts for Single-Atom Catalysts. ChemCatChem 2019, 11, 2812–2820. [Google Scholar] [CrossRef]
- Chen, J.; Ge, Y.; Guo, Y.; Chen, J. Selective hydrogenation of biomass-derived 5-hydroxymethylfurfural using palladium catalyst supported on mesoporous graphitic carbon nitride. J. Energy Chem. 2018, 27, 283–289. [Google Scholar] [CrossRef]
- Tian, S.; Wang, Z.; Gong, W.; Chen, W.; Feng, Q.; Xu, Q.; Chen, C.; Chen, C.; Peng, Q.; Gu, L.; et al. Temperature-Controlled Selectivity of Hydrogenation and Hydrodeoxygenation in the Conversion of Biomass Molecule by the Ru1/mpg-C3N4 Catalyst. J. Am. Chem. Soc. 2018, 140, 11161–11164. [Google Scholar] [CrossRef]
- Tian, S.; Gong, W.; Chen, W.; Lin, N.; Zhu, Y.; Feng, Q.; Xu, Q.; Fu, Q.; Chen, C.; Luo, J.; et al. Regulating the Catalytic Performance of Single-Atomic-Site Ir Catalyst for Biomass Conversion by Metal–Support Interactions. ACS Catal. 2019, 9, 5223–5230. [Google Scholar] [CrossRef]
- Chen, Z.; Vorobyeva, E.; Mitchell, S.; Fako, E.; Ortuño, M.A.; López, N.; Collins, S.M.; Midgley, P.A.; Richard, S.; Vilé, G.; et al. A heterogeneous single-atom palladium catalyst surpassing homogeneous systems for Suzuki coupling. Nat. Nanotechnol. 2018, 13, 702–707. [Google Scholar] [CrossRef]
- Kharissova, O.V.; Kharisov, B.I.; Ulyand, I.E.; García, T.H. Catalysis using metal–organic framework-derived nanocarbons: Recent trends. J. Mater. Res. 2020, 35, 2190–2207. [Google Scholar] [CrossRef]
- Han, A.; Wang, B.; Kumar, A.; Qin, Y.; Jin, J.; Wang, X.; Yang, C.; Dong, B.; Jia, Y.; Liu, J.; et al. Recent Advances for MOF-Derived Carbon-Supported Single-Atom Catalysts. Small Methods 2019, 3, 1800471. [Google Scholar] [CrossRef]
- Wei, S.; Li, A.; Liu, J.-C.; Li, Z.; Chen, W.; Gong, Y.; Zhang, Q.; Cheong, W.-C.; Wang, Y.; Zheng, L.; et al. Direct observation of noble metal nanoparticles transforming to thermally stable single atoms. Nat. Nanotechnol. 2018, 13, 856–861. [Google Scholar] [CrossRef]
- Wang, X.; Chen, W.; Zhang, L.; Yao, T.; Liu, W.; Lin, Y.; Ju, H.; Dong, J.; Zheng, L.; Yan, W.; et al. Uncoordinated Amine Groups of Metal–Organic Frameworks to Anchor Single Ru Sites as Chemoselective Catalysts toward the Hydrogenation of Quinoline. J. Am. Chem. Soc. 2017, 139, 9419–9422. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Han, Y.; Zhu, M.; Shang, S.; Li, W. MOF-derived various morphologies of N-doped carbon composites for acetylene hydrochlorination. J. Mater. Sci. 2018, 53, 4913–4926. [Google Scholar] [CrossRef]
- Sun, X.; Olivos-Suarez, A.I.; Osadchii, D.; Romero, M.J.V.; Kapteijn, F.; Gascon, J. Single cobalt sites in mesoporous N-doped carbon matrix for selective catalytic hydrogenation of nitroarenes. J. Catal. 2018, 357, 20–28. [Google Scholar] [CrossRef]
- Feng, Y.; Jia, W.; Yan, G.; Zeng, X.; Sperry, J.; Xu, B.; Sun, Y.; Tang, X.; Lei, T.; Lin, L. Insights into the active sites and catalytic mechanism of oxidative esterification of 5-hydroxymethylfurfural by metal-organic frameworks-derived N-doped carbon. J. Catal. 2020, 381, 570–578. [Google Scholar] [CrossRef]
- Li, X.; Zhang, B.; Fang, Y.; Sun, W.; Qi, Z.; Pei, Y.; Qi, S.; Yuan, P.; Luan, X.; Goh, T.W.; et al. Metal-Organic-Framework-Derived Carbons: Applications as Solid-Base Catalyst and Support for Pd Nanoparticles in Tandem Catalysis. Chem.—A Eur. J. 2017, 23, 4266–4270. [Google Scholar] [CrossRef]
- Niu, X.; Shi, Q.; Zhu, W.; Liu, D.; Tian, H.; Fu, S.; Cheng, N.; Li, S.; Smith, J.N.; Du, D.; et al. Unprecedented peroxidase-mimicking activity of single-atom nanozyme with atomically dispersed Fe–Nx moieties hosted by MOF derived porous carbon. Biosens. Bioelectron. 2019, 142, 111495. [Google Scholar] [CrossRef]
- Lan, G.; Ye, Q.; Zhu, Y.; Tang, H.; Han, W.; Li, Y. Single-Site Au/Carbon Catalysts with Single-Atom and Au Nanoparticles for Acetylene Hydrochlorination. ACS Appl. Nano Mater. 2020, 3, 3004–3010. [Google Scholar] [CrossRef]
- Kochubey, D.I.; Chesnokov, V.V.; Malykhin, S.E. Evidence for atomically dispersed Pd in catalysts supported on carbon nanofibers. Carbon N. Y. 2012, 50, 2782–2787. [Google Scholar] [CrossRef]
- Chesnokov, V.V.; Kriventsov, V.V.; Malykhin, S.E.; Svintsitskiy, D.A.; Podyacheva, O.Y.; Lisitsyn, A.S.; Richards, R.M. Nature of active palladium sites on nitrogen doped carbon nanofibers in selective hydrogenation of acetylene. Diam. Relat. Mater. 2018, 89, 67–73. [Google Scholar] [CrossRef]
- Feng, Q.; Zhao, S.; Xu, Q.; Chen, W.; Tian, S.; Wang, Y.; Yan, W.; Luo, J.; Wang, D.; Li, Y. Mesoporous Nitrogen-Doped Carbon-Nanosphere-Supported Isolated Single-Atom Pd Catalyst for Highly Efficient Semihydrogenation of Acetylene. Adv. Mater. 2019, 31, 1901024. [Google Scholar] [CrossRef]
- Wu, K.; Zhan, F.; Tu, R.; Cheong, W.-C.; Cheng, Y.; Zheng, L.; Yan, W.; Zhang, Q.; Chen, Z.; Chen, C. Dopamine polymer derived isolated single-atom site metals/N-doped porous carbon for benzene oxidation. Chem. Commun. 2020, 56, 8916–8919. [Google Scholar] [CrossRef]
- Liu, W.; Chen, Y.; Qi, H.; Zhang, L.; Yan, W.; Liu, X.; Yang, X.; Miao, S.; Wang, W.; Liu, C.; et al. A Durable Nickel Single-Atom Catalyst for Hydrogenation Reactions and Cellulose Valorization under Harsh Conditions. Angew. Chemie 2018, 130, 7189–7193. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, L.; Yan, W.; Liu, X.; Yang, X.; Miao, S.; Wang, W.; Wang, A.; Zhang, T. Single-atom dispersed Co–N–C catalyst: Structure identification and performance for hydrogenative coupling of nitroarenes. Chem. Sci. 2016, 7, 5758–5764. [Google Scholar] [CrossRef]
- Long, X.; Li, Z.; Gao, G.; Sun, P.; Wang, J.; Zhang, B.; Zhong, J.; Jiang, Z.; Li, F. Graphitic phosphorus coordinated single Fe atoms for hydrogenative transformations. Nat. Commun. 2020, 11, 4074. [Google Scholar] [CrossRef]
- Huang, R.; Cao, C.; Liu, J.; Zheng, L.; Zhang, Q.; Gu, L.; Jiang, L.; Song, W. Integration of Metal Single Atoms on Hierarchical Porous Nitrogen-Doped Carbon for Highly Efficient Hydrogenation of Large-Sized Molecules in the Pharmaceutical Industry. ACS Appl. Mater. Interfaces 2020, 12, 17651–17658. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, A.; Wang, W.; Huang, Y.; Liu, X.; Miao, S.; Liu, J.; Zhang, T. Co–N–C Catalyst for C–C Coupling Reactions: On the Catalytic Performance and Active Sites. ACS Catal. 2015, 5, 6563–6572. [Google Scholar] [CrossRef]
- Liu, P.; Zheng, N. Coordination chemistry of atomically dispersed catalysts. Natl. Sci. Rev. 2018, 5, 636–638. [Google Scholar] [CrossRef]
- Ma, D.W.; Li, T.; Wang, Q.; Yang, G.; He, C.; Ma, B.; Lu, Z. Graphyne as a promising substrate for the noble-metal single-atom catalysts. Carbon N. Y. 2015, 95, 756–765. [Google Scholar] [CrossRef]

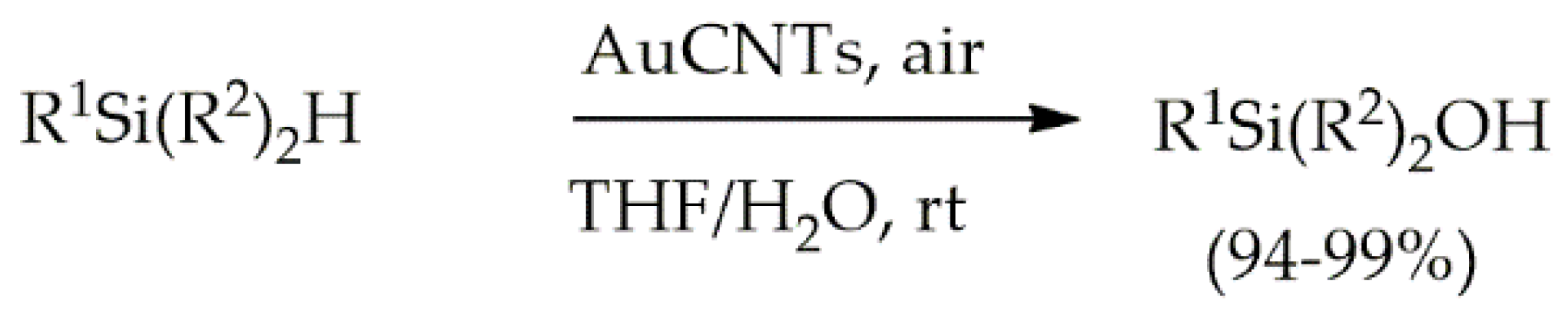

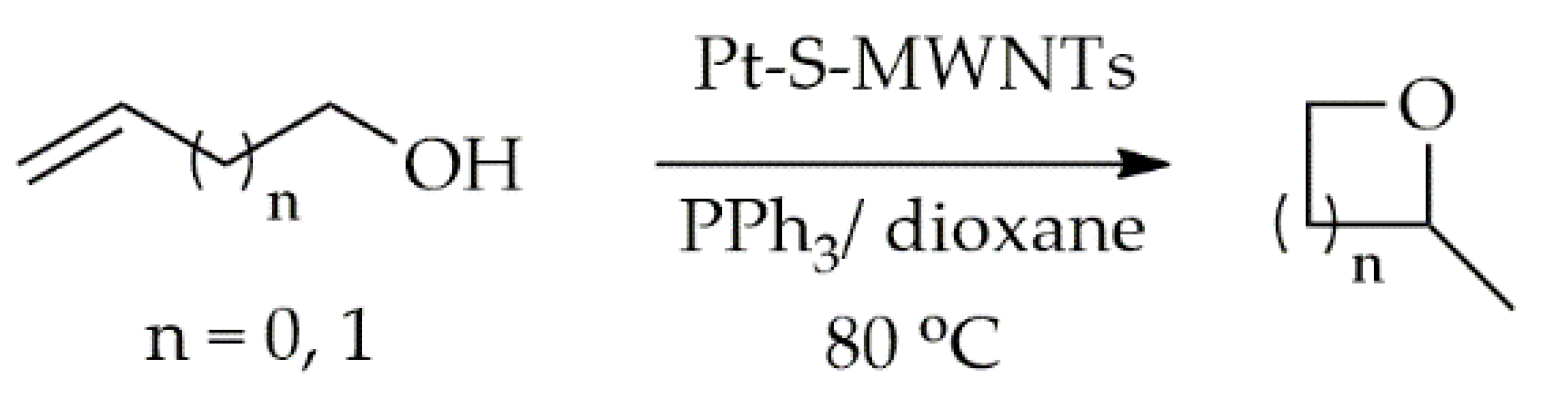
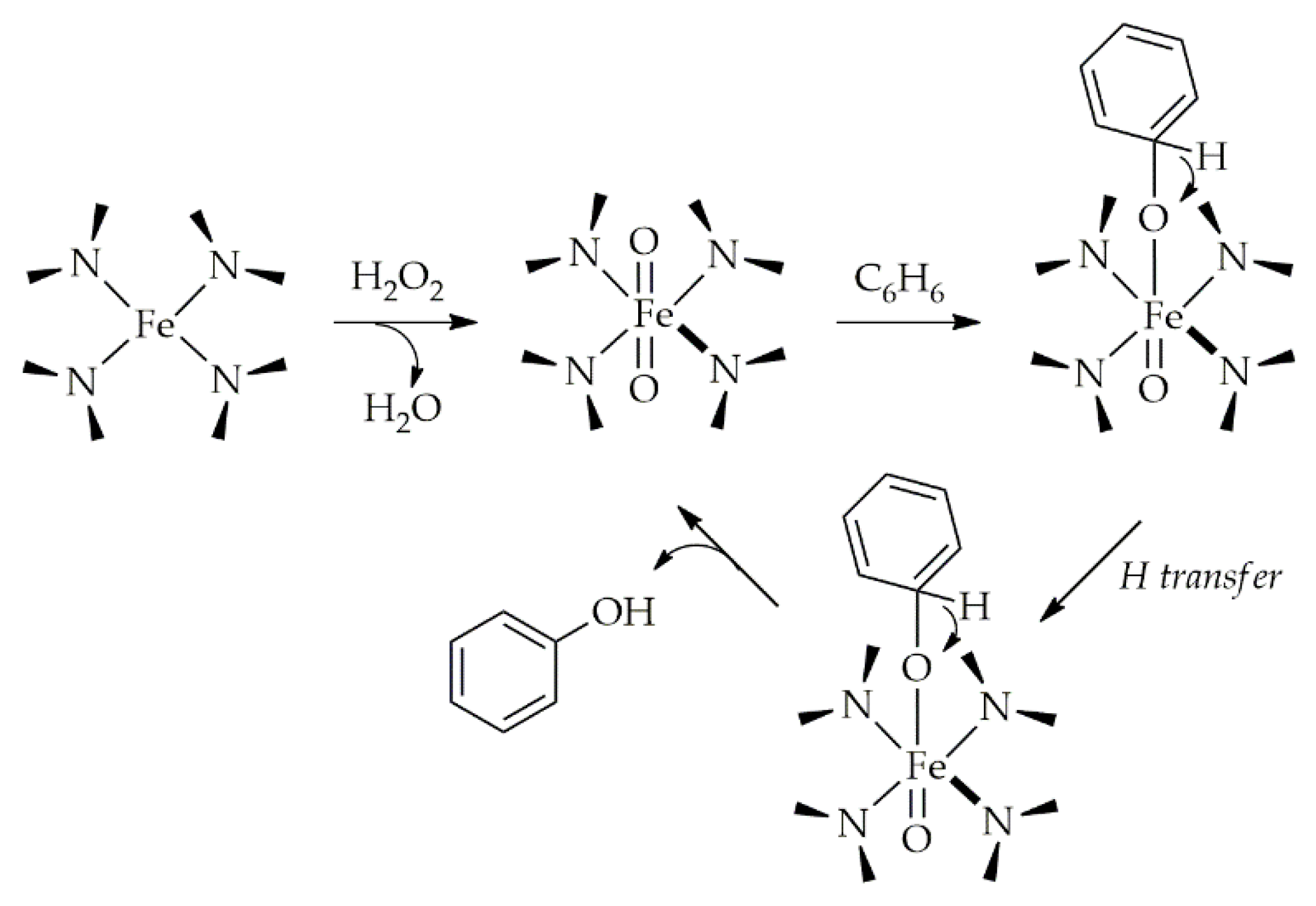
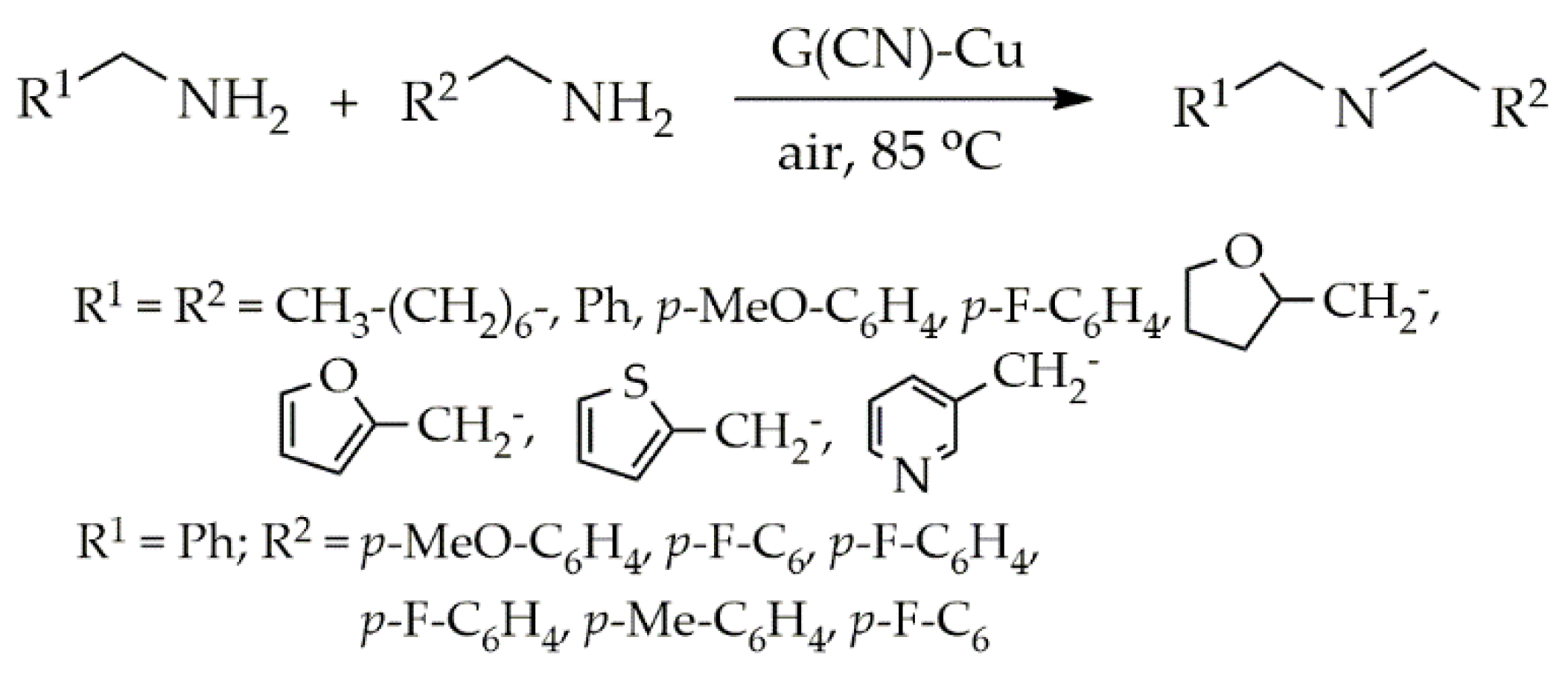
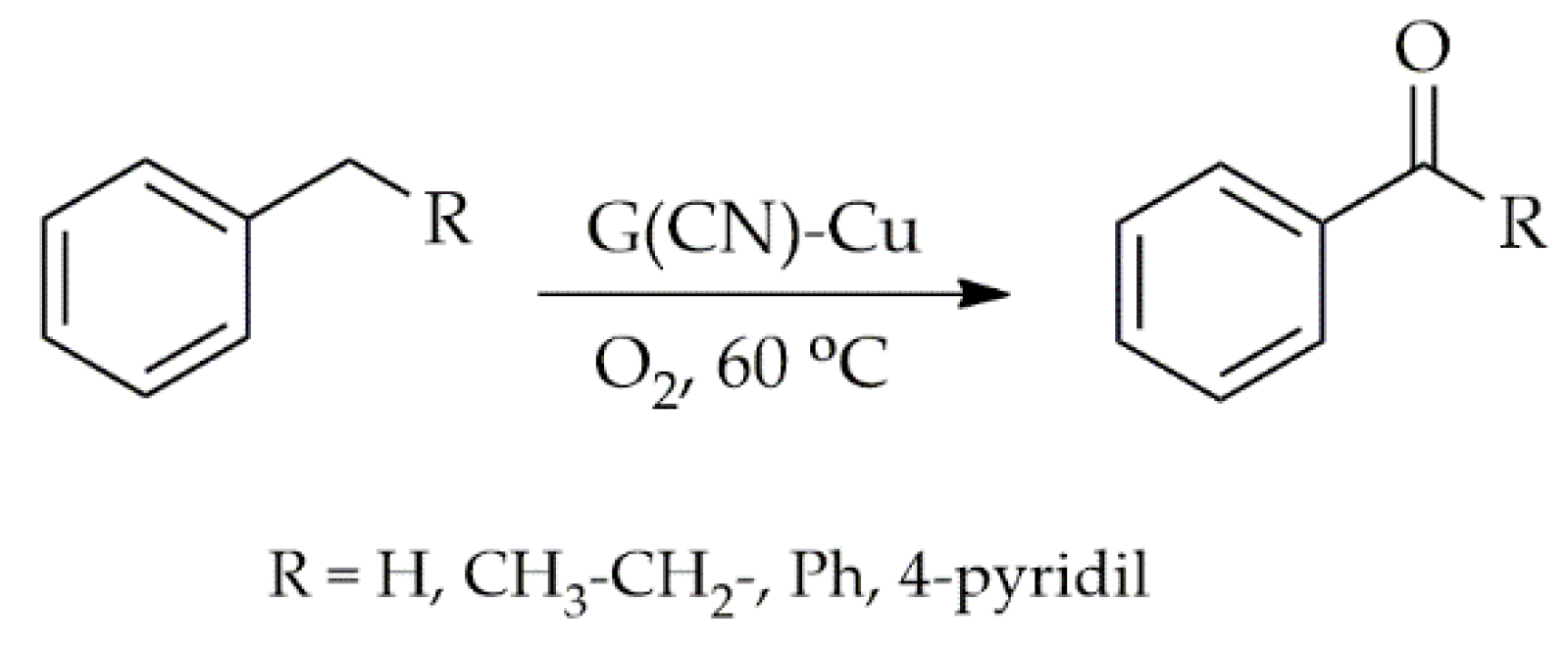

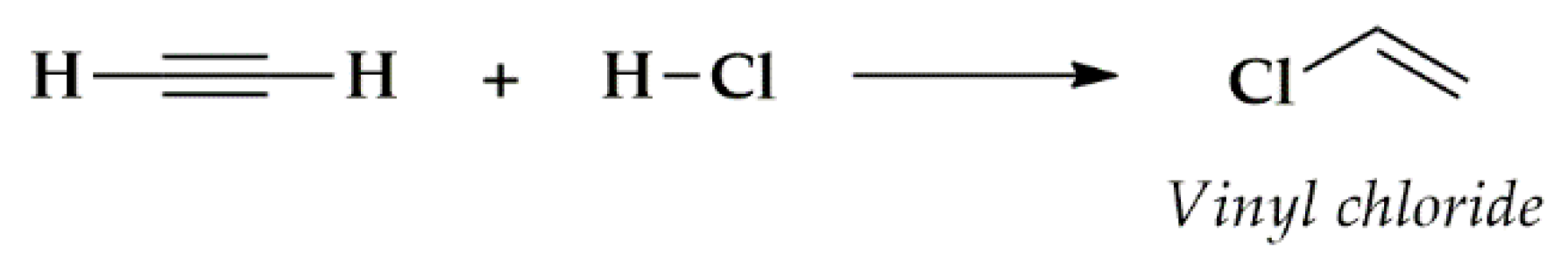


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Mayoral, E.; Matos, I.; Bernardo, M.; Ventura, M.; Fonseca, I.M. Carbon-Based Materials for the Development of Highly Dispersed Metal Catalysts: Towards Highly Performant Catalysts for Fine Chemical Synthesis. Catalysts 2020, 10, 1407. https://doi.org/10.3390/catal10121407
Pérez-Mayoral E, Matos I, Bernardo M, Ventura M, Fonseca IM. Carbon-Based Materials for the Development of Highly Dispersed Metal Catalysts: Towards Highly Performant Catalysts for Fine Chemical Synthesis. Catalysts. 2020; 10(12):1407. https://doi.org/10.3390/catal10121407
Chicago/Turabian StylePérez-Mayoral, Elena, Ines Matos, María Bernardo, Marcia Ventura, and Isabel M. Fonseca. 2020. "Carbon-Based Materials for the Development of Highly Dispersed Metal Catalysts: Towards Highly Performant Catalysts for Fine Chemical Synthesis" Catalysts 10, no. 12: 1407. https://doi.org/10.3390/catal10121407
APA StylePérez-Mayoral, E., Matos, I., Bernardo, M., Ventura, M., & Fonseca, I. M. (2020). Carbon-Based Materials for the Development of Highly Dispersed Metal Catalysts: Towards Highly Performant Catalysts for Fine Chemical Synthesis. Catalysts, 10(12), 1407. https://doi.org/10.3390/catal10121407