Benchmarking Perovskite Electrocatalysts’ OER Activity as Candidate Materials for Industrial Alkaline Water Electrolysis
Abstract
1. Introduction
2. Results and Discussion
2.1. Structural and Morphological Analysis
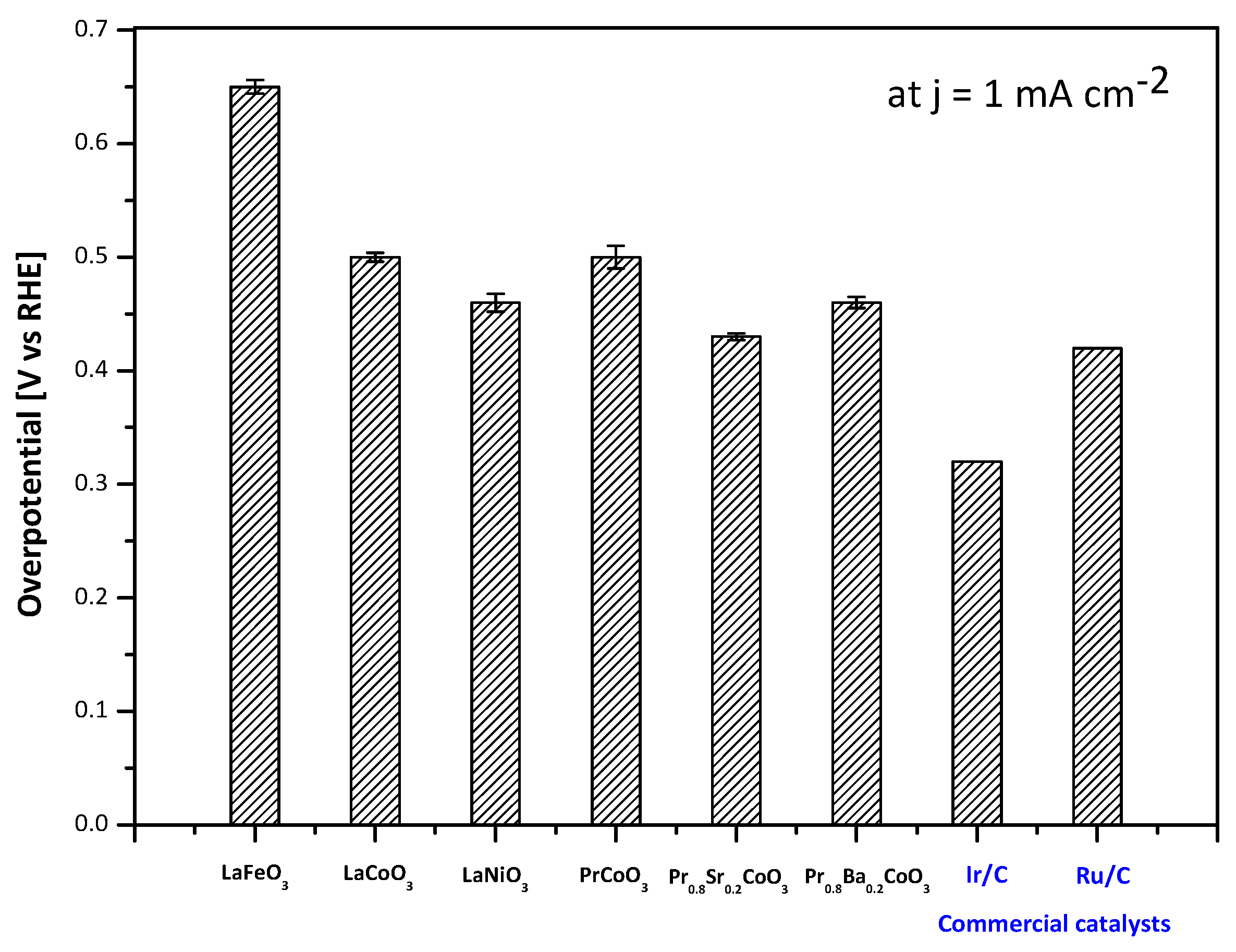
2.2. Electrochemical Performance
3. Materials and Methods
3.1. Synthesis of Perovskite Materials
3.2. Structural and Morphological Characterization of Perovskite Materials
3.3. Electrode Preparation and Electrochemical Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cossar, E.; Barnett, A.O.; Seland, F.; Baranova, E.A. The Performance of Nickel and Nickel-Iron Catalysts Evaluated as Anodes in Anion Exchange Membrane Water Electrolysis. Catalysts 2019, 9, 814. [Google Scholar] [CrossRef]
- Hydrogen Council. How Hydrogen Empowers the Energy Transition; Hydrogen Council: Brussels, Belgium, 2017. [Google Scholar]
- Gielen, D. Hydrogen from Renewable Power Technology Outlook for the Energy Transition; International Renewable Energy Agency: Abu Dhabi, UAE, 2018. [Google Scholar]
- Spiegel, C. Introduction to Electrolyzers. Available online: https://www.fuelcellstore.com/blog-section/introduction-to-electrolyzers (accessed on 1 June 2020).
- Man, I.C.; Su, H.; Calle-Vallejo, F.; Hansen, H.A.; Martinez, J.I.; Inoglu, N.G.; Kitchin, J.; Jaramillo, T.F.; Nørskov, J.K.; Rossmeisl, J. Universality in Oxygen Evolution Electrocatalysis on Oxide Surfaces. ChemCatChem 2011, 3, 1159–1165. [Google Scholar] [CrossRef]
- Sheffield, J.W.; Martin, K.B.; Folkson, R. Electricity and Hydrogen as Energy Vectors for Transportation Vehicles. In Alternative Fuels and Advanced Vehicle Technologies for Improved Environmental Performance; Woodhead Publishing: Great Britain, UK, 2014; pp. 117–137. [Google Scholar]
- Mergel, J.; Carmo, M.; Fritz, D. Status on Technologies for Hydrogen Production by Water Electrolysis. Trans. Renew. Energy Syst. 2013, 425–450. [Google Scholar] [CrossRef]
- Colli, A.N.; Girault, H.H.; Battistel, A. Non-Precious Electrodes for Practical Alkaline Water Electrolysis. Materials (Basel) 2019, 12, 1336. [Google Scholar] [CrossRef]
- Zeng, K.; Zhang, D. Recent Progress in Alkaline Water Electrolysis for Hydrogen Production and Applications. Prog. Energy Combust. Sci. 2010, 36, 307–326. [Google Scholar] [CrossRef]
- Seetharaman, S.; Balaji, R.; Ramya, K.; Dhathathreyan, K.S.; Velan, M. Graphene Oxide Modified Non-Noble Metal Electrode for Alkaline Anion Exchange Membrane Water Electrolyzers. Int. J. Hydrogen Energy 2013, 38, 14934–14942. [Google Scholar] [CrossRef]
- Vermeiren, P.; Adriansens, W.; Moreels, J.P.; Leysen, R. Evaluation of the Zirfon® Separator for Use in Alkaline Water Electrolysis and Ni-H2 Batteries. Int. J. Hydrogen Energy 1998, 23, 321–324. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Sato, E. Electrocatalytic Properties of Transition Metal Oxides for Oxygen Evolution Reaction. Mater. Chem. Phys. 1986, 14, 397–426. [Google Scholar] [CrossRef]
- Hong, W.T.; Risch, M.; Stoerzinger, K.A.; Grimaud, A.; Suntivich, J.; Shao-Horn, Y. Toward the Rational Design of Non-Precious Transition Metal Oxides for Oxygen Electrocatalysis. Energy Environ. Sci. 2015, 8, 1404–1427. [Google Scholar] [CrossRef]
- Burke, M.S.; Enman, L.J.; Batchellor, A.S.; Zou, S.; Boettcher, S.W. Oxygen Evolution Reaction Electrocatalysis on Transition Metal Oxides and (Oxy) Hydroxides: Activity Trends and Design Principles. Chem. Mater. 2015, 27, 7549–7558. [Google Scholar] [CrossRef]
- Suntivich, J.; May, K.J.; Gasteiger, H.A.; Goodenough, J.B.; Shao-Horn, Y. A Perovskite Oxide Optimized for Oxygen Evolution Catalysis from Molecular Orbital Principles. Science (80-.) 2011, 334, 1383–1385. [Google Scholar] [CrossRef] [PubMed]
- Bockris, J.O.; Otagawa, T. The Electrocatalysis of Oxygen Evolution on Perovskites. J. Electrochem. Soc. 1984, 131, 290. [Google Scholar] [CrossRef]
- Bockris, J.O.; Otagawa, T. Mechanism of Oxygen Evolution on Perovskites. J. Phys. Chem. 2002, 87, 2960–2971. [Google Scholar] [CrossRef]
- Grimaud, A.; May, K.J.; Carlton, C.E.; Lee, Y.-L.; Risch, M.; Hong, W.T.; Zhou, J.; Shao-Horn, Y. Double Perovskites as a Family of Highly Active Catalysts for Oxygen Evolution in Alkaline Solution. Nat. Commun. 2013, 4, 2439. [Google Scholar] [CrossRef] [PubMed]
- Bertuccioli, L.; Chan, A.; Hart, D.; Lehner, F.; Madden, B.; Standen, E. Development of Water Electrolysis in the European Union; Element Energy: Cambridge, UK, 2014. [Google Scholar]
- Shaner, M.R.; Atwater, H.A.; Lewis, N.S.; McFarland, E.W. A Comparative Technoeconomic Analysis of Renewable Hydrogen Production Using Solar Energy. Energy Environ. Sci. 2016, 9, 2354–2371. [Google Scholar] [CrossRef]
- Otagawa, T.; Bockris, J. Lanthanum Nickelate as Electrocatalyst: Oxygen Evolution. J. Electrochem. Soc. 1982, 129, 2391. [Google Scholar] [CrossRef]
- Soares, C.O.; Silva, R.A.; Carvalho, M.D.; Jorge, M.E.M.; Gomes, A.; Rangel, C.M.; da Silva Pereira, M.I. Oxide Loading Effect on the Electrochemical Performance of LaNiO3 Coatings in Alkaline Media. Electrochim. Acta 2013, 89, 106–113. [Google Scholar] [CrossRef][Green Version]
- Bursell, M.; Pirjamali, M.; Kiros, Y. La0. 6Ca0. 4CoO3, La0. 1Ca0. 9MnO3 and LaNiO3 as Bifunctional Oxygen Electrodes. Electrochim. Acta 2002, 47, 1651–1660. [Google Scholar] [CrossRef]
- Suen, N.-T.; Hung, S.-F.; Quan, Q.; Zhang, N.; Xu, Y.-J.; Chen, H.M. Electrocatalysis for the Oxygen Evolution Reaction: Recent Development and Future Perspectives. Chem. Soc. Rev. 2017, 46, 337–365. [Google Scholar] [CrossRef]
- Singh, R.N.; Bahadur, L.; Pandey, J.P.; Singh, S.P.; Chartier, P.; Poillerat, G. Preparation and Characterization of Thin Films of LaNiO 3 for Anode Application in Alkaline Water Electrolysis. J. Appl. Electrochem. 1994, 24, 149–156. [Google Scholar] [CrossRef]
- Lopez, K.; Park, G.; Sun, H.-J.; An, J.-C.; Eom, S.; Shim, J. Electrochemical Characterizations of LaMO3 (M = Co, Mn, Fe, and Ni) and Partially Substituted LaNixM1−xO3 (X = 0.25 or 0.5) for Oxygen Reduction and Evolution in Alkaline Solution. J. Appl. Electrochem. 2015, 45, 313–323. [Google Scholar] [CrossRef]
- Sankannavar, R.; Sarkar, A. The Electrocatalysis of Oxygen Evolution Reaction on La1-XCaxFeO3-δ Perovskites in Alkaline Solution. Int. J. Hydrogen Energy 2018, 43, 4682–4690. [Google Scholar] [CrossRef]
- Lee, Y.; Suntivich, J.; May, K.J.; Perry, E.E.; Shao-Horn, Y. Synthesis and Activities of Rutile IrO2 and RuO2 Nanoparticles for Oxygen Evolution in Acid and Alkaline Solutions. J. Phys. Chem. Lett. 2012, 3, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Gorlin, Y.; Jaramillo, T.F. A Bifunctional Nonprecious Metal Catalyst for Oxygen Reduction and Water Oxidation. J. Am. Chem. Soc. 2010, 132, 13612–13614. [Google Scholar] [CrossRef]
- Feng, C.H.; Li, Q.S.; Liu, C.M.; Deng, Y.; Guo, L. Synthesis of Perovskite-Type LaMnO3 under Hydrothermal Conditions. In Materials Science Forum; Trans Tech Publications: Stafa-Zurich, Switzerland, 2005; Volume 475, pp. 4051–4054. [Google Scholar]
- Schmidt, T.J.; Gasteiger, H.A.; Stäb, G.D.; Urban, P.M.; Kolb, D.M.; Behm, R.J. Characterization of High-surface-area Electrocatalysts Using a Rotating Disk Electrode Configuration. J. Electrochem. Soc. 1998, 145, 2354–2358. [Google Scholar] [CrossRef]
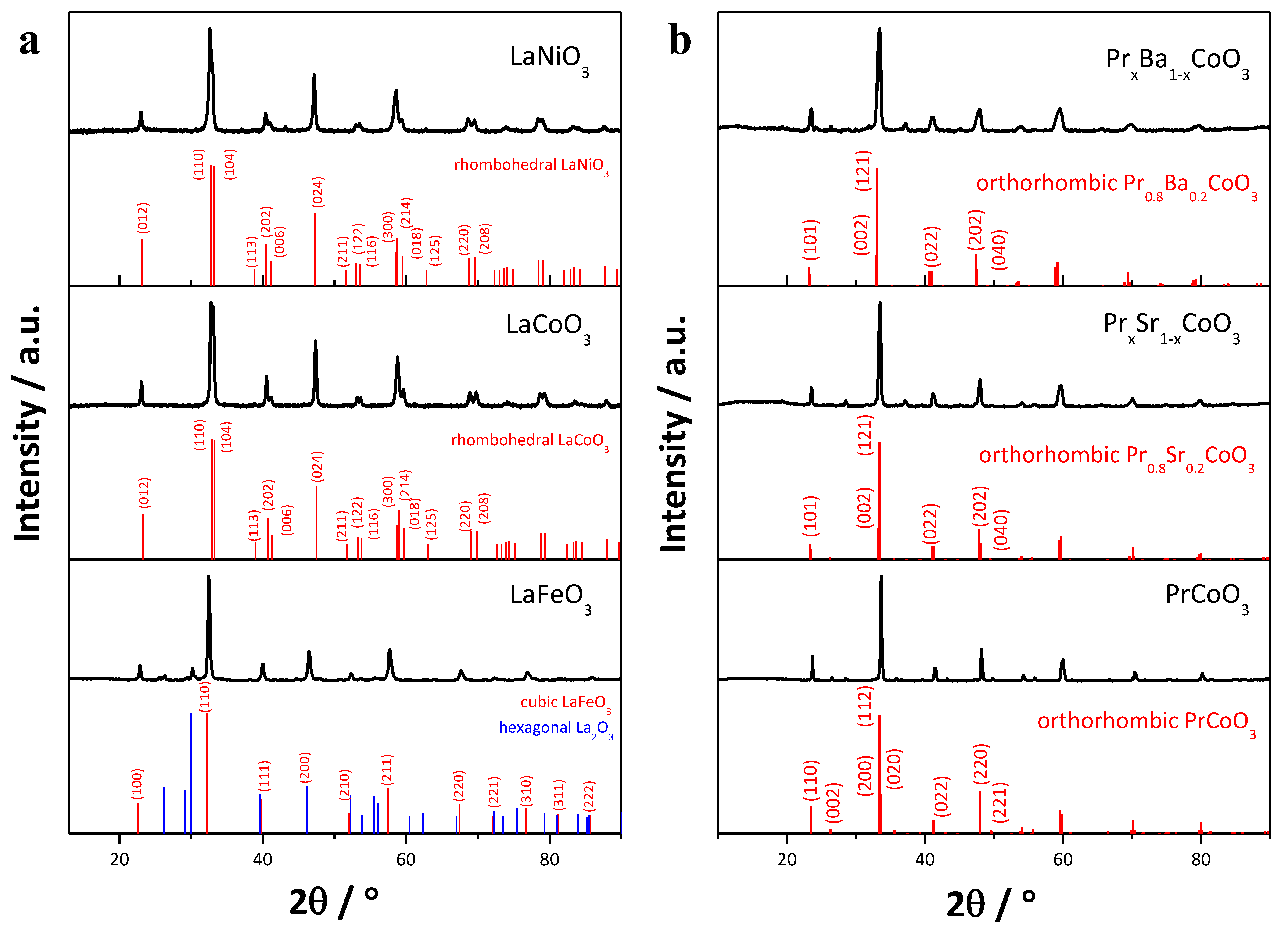
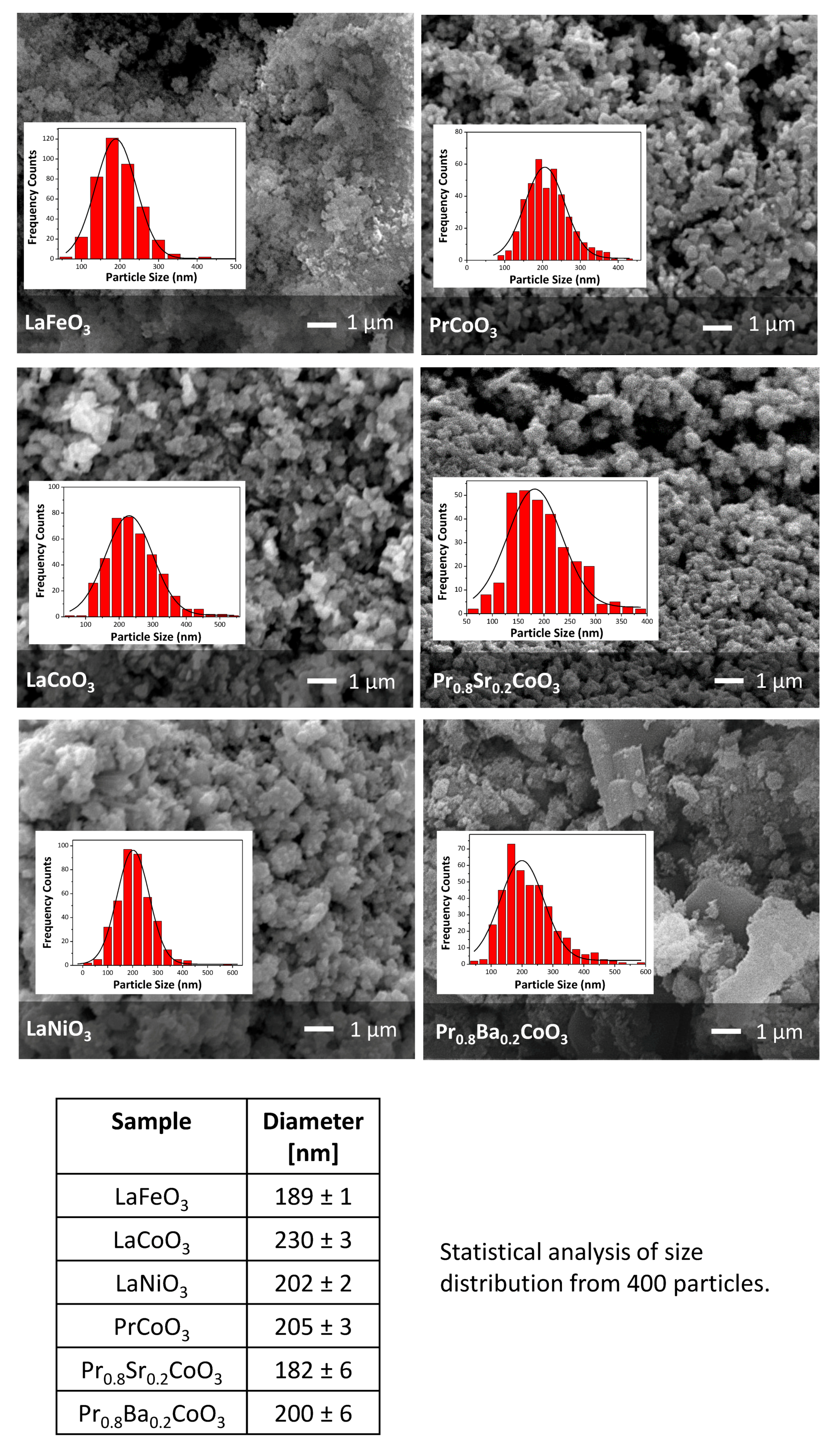
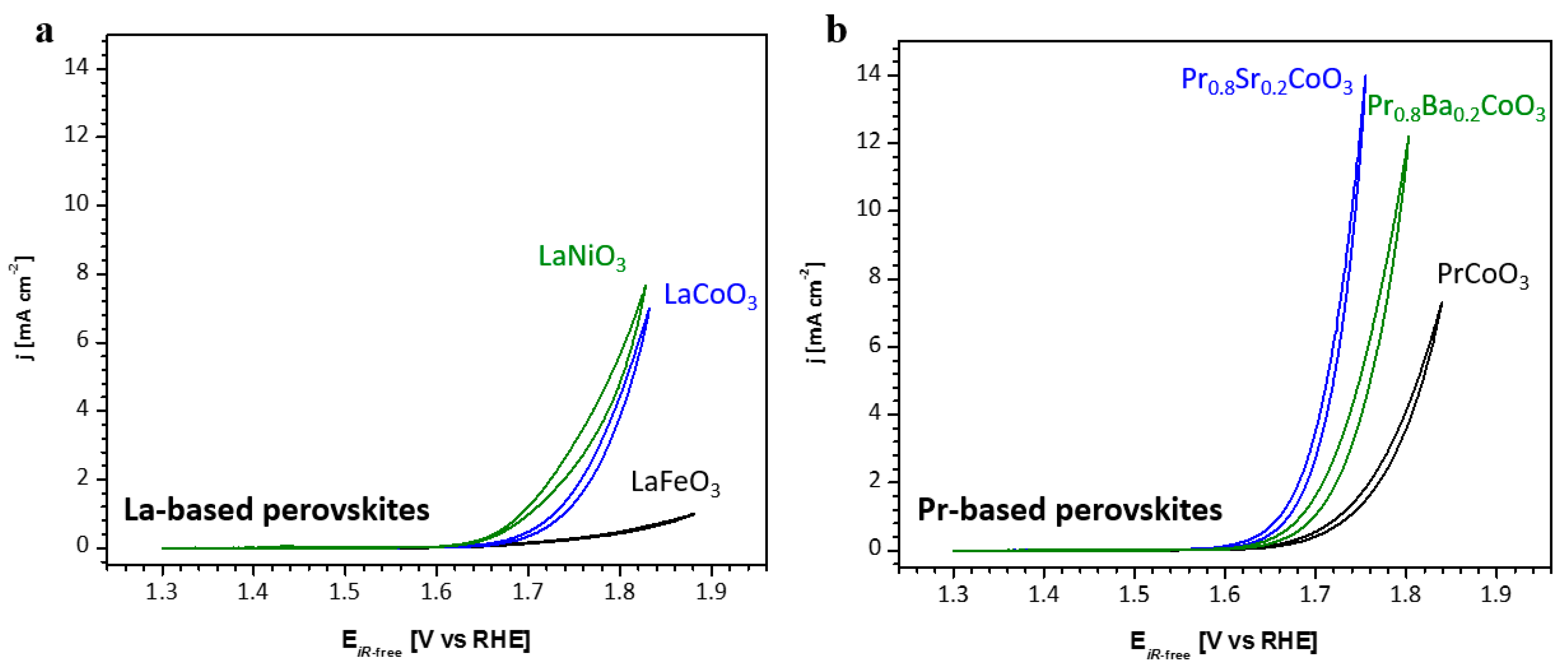
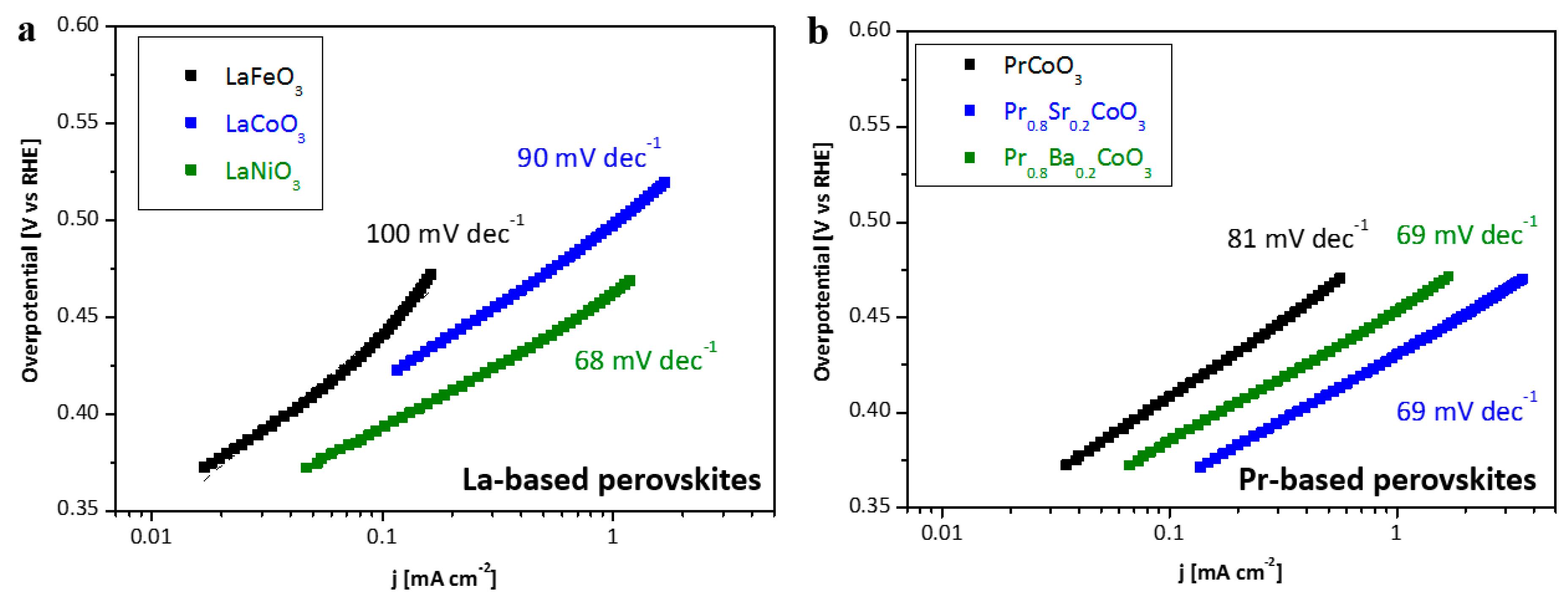
| Space Group | Phase Composition/ vol% | a/Å | b/Å | c/Å | Volume/Å3 | Crystallite Size/nm | χ2/% | |
|---|---|---|---|---|---|---|---|---|
| LaFeO3 | Pm3m | 90.2% cubic LaFeO3; 9.8% hexagonal La2O3 | 3.9300 | 3.9300 | 3.9300 | 60.6988 | 31.4 | 5.33 |
| LaCoO3 | R-3c | 100% rhombohedral LaCoO3 | 5.4364 | 5.4364 | 13.1134 | 335.6294 | 58.2 | 2.34 |
| LaNiO3 | R-3c | 100% rhombohedral LaNiO3 | 5.4456 | 5.4456 | 13.1443 | 337.5681 | 25.5 | 4.35 |
| PrCoO3 | Pnma | 100% orthorhombic PrCoO3 | 5.3459 | 7.5814 | 5.3814 | 218.1059 | 77.1 | 9.88 |
| Pr0.8Sr0.2CoO3 | Pnma | 100% orthorhombic Pr0.8Sr0.2CoO3 | 5.3746 | 7.6079 | 5.4193 | 221.5911 | 76.2 | 7.82 |
| Pr0.8Ba0.2CoO3 | Pnma | 100% rhombohedral Pr0.8Sr0.2CoO3 | 5.3892 | 7.6155 | 5.4536 | 223.5603 | 67.8 | 5.42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matienzo, D.D.; Kutlusoy, T.; Divanis, S.; Bari, C.D.; Instuli, E. Benchmarking Perovskite Electrocatalysts’ OER Activity as Candidate Materials for Industrial Alkaline Water Electrolysis. Catalysts 2020, 10, 1387. https://doi.org/10.3390/catal10121387
Matienzo DD, Kutlusoy T, Divanis S, Bari CD, Instuli E. Benchmarking Perovskite Electrocatalysts’ OER Activity as Candidate Materials for Industrial Alkaline Water Electrolysis. Catalysts. 2020; 10(12):1387. https://doi.org/10.3390/catal10121387
Chicago/Turabian StyleMatienzo, DJ Donn, Tuğçe Kutlusoy, Spyridon Divanis, Chiara Di Bari, and Emanuele Instuli. 2020. "Benchmarking Perovskite Electrocatalysts’ OER Activity as Candidate Materials for Industrial Alkaline Water Electrolysis" Catalysts 10, no. 12: 1387. https://doi.org/10.3390/catal10121387
APA StyleMatienzo, D. D., Kutlusoy, T., Divanis, S., Bari, C. D., & Instuli, E. (2020). Benchmarking Perovskite Electrocatalysts’ OER Activity as Candidate Materials for Industrial Alkaline Water Electrolysis. Catalysts, 10(12), 1387. https://doi.org/10.3390/catal10121387




