2.1. Batch Operation
The impact of operating temperature on reaction performance was experimentally investigated under batch operation thanks to an easily controllable test under the considered conditions. Methane-driven reduction of ZnO was conducted isothermally at temperatures of 800, 900, and 1000 °C under atmospheric pressure (0.90 bar). A well-grinded packed-bed of ZnO (2 g) was loaded and uniformly spread in the cavity bottom with initial bed height in the range 1.8–2.2 mm (bulk density ~0.5–0.6 g/cm
3), thus avoiding high thermal gradient across its height. A constant mixture of reactive CH
4 (0.1 NL/min) and inert carrier N
2 (0.2 NL/min), resulting in 33% inlet CH
4 concentration, was fed.
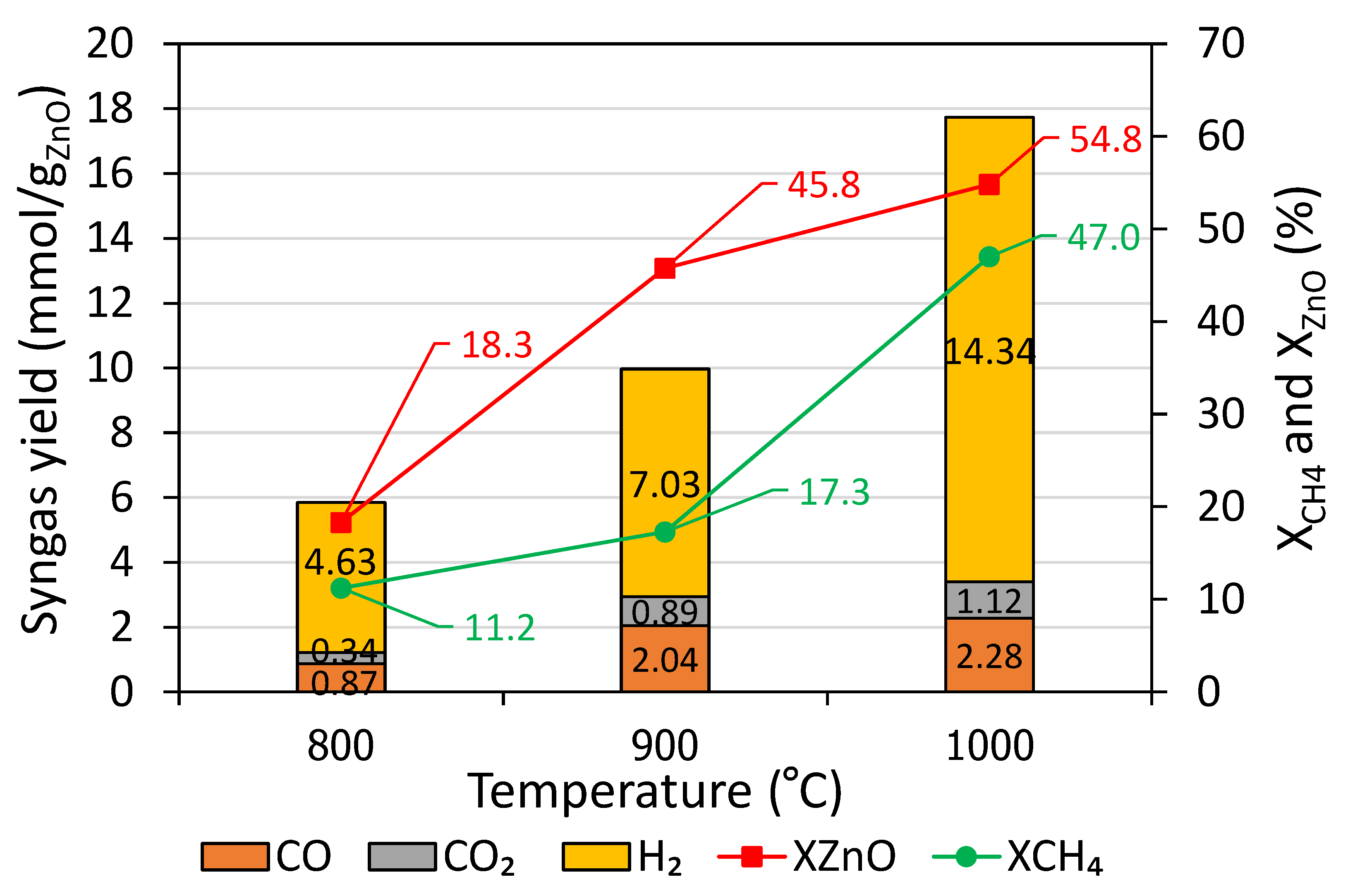
Figure 1 shows syngas yields, methane conversion (X
CH4), and net ZnO conversion (X
ZnO) as a function of temperature. With temperature rising, a remarkable increase in the syngas yield, particularly H
2, was observed. For instance, CO, CO
2, and H
2 increased from 0.87, 0.34, and 4.63 mmol/g
ZnO at 800 °C to 2.28, 1.12, and 14.34 mmol/g
ZnO at 1000 °C, resulting in H
2/CO ratios from 5.32 at 800 °C to 6.29 at 1000 °C. A sharp increase in the H
2 yield at 1000 °C was attributed to the side reaction associated with methane cracking (Equation (11)). In fact, H
2O according to Equation (12) was also formed, but it could not be detected by on-line gas analysis. Besides, H
2O yield can be calculated assuming that it doubles the CO
2 yield according to Equation (12). Because of the strong methane cracking reaction at 1000 °C, X
CH4 attained the maximum value as high as 47.0%. The methane cracking reaction at 1000 °C resulted in the carbon formation, which could adversely affect the performance of the process. Fortunately, this issue can be tackled by decreasing the operating temperature below 1000 °C. Carbon-atom balance (Equation (15)) was applied to determine solid carbon production yield.
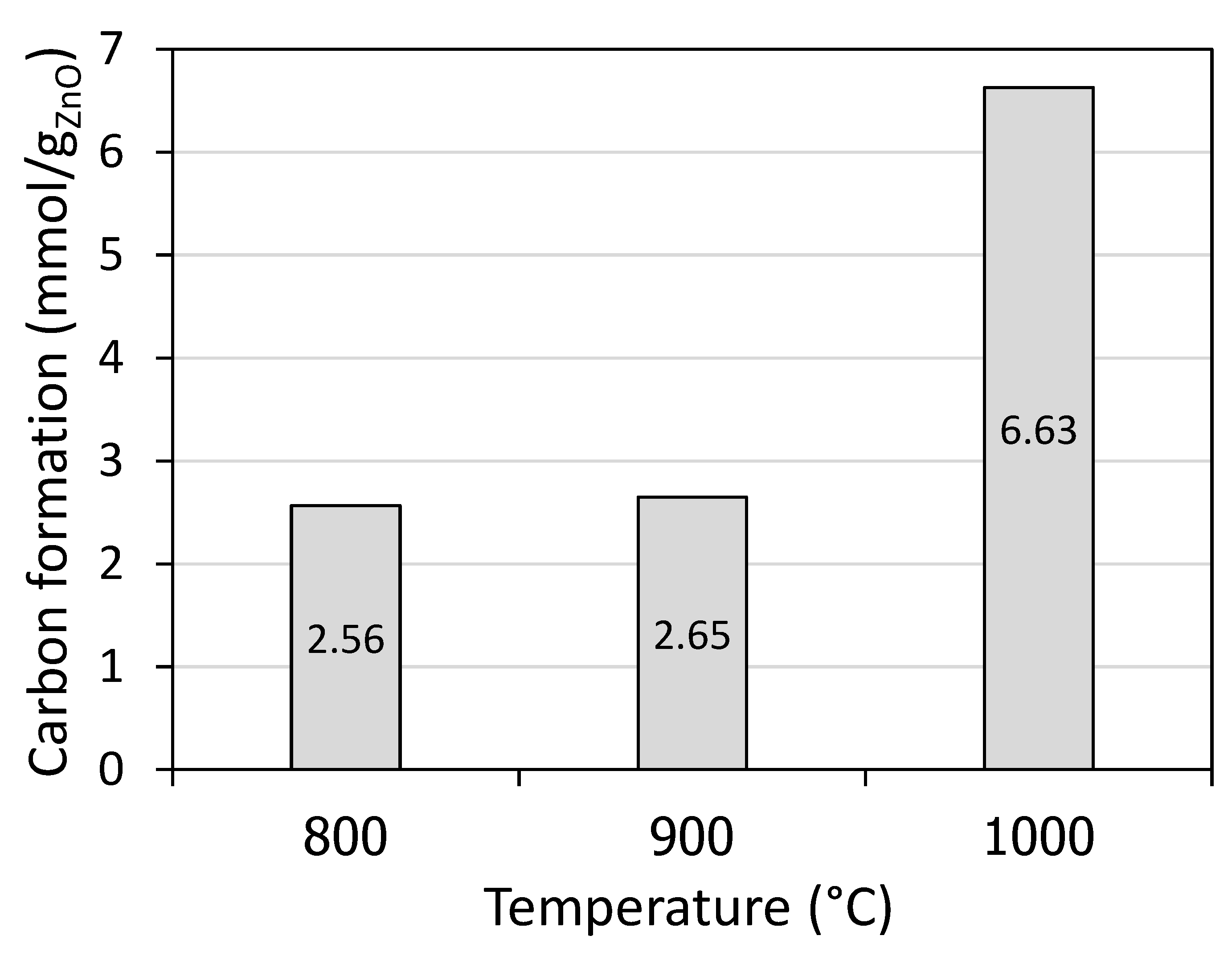
As expected, carbon formation rose considerably when increasing the temperature to 1000 °C. For instance, solid carbon formation increased from 2.56 mmol/g
ZnO at 800 °C to 6.63 mmol/g
ZnO at 1000 °C, according to
Figure 2.
In addition, the maximum XZnO value of 54.8% was reached at 1000 °C. The incomplete ZnO conversion was ascribed to unreacted ZnO remaining in the cavity (also part diffusion in the insulation layer may be possible) and Zn recombination with CO, CO2, and H2O at the outlet cooling zone (inverse reactions of Equations (3)–(5)). In a nutshell, increasing temperature increased syngas yield, XCH4, and XZnO at the expense of favored thermal methane dissociation, especially at 1000 °C, which caused solid carbon deposition. For this reason, temperatures in the range of 900–950 °C were found most recommended for performing methane-driven ZnO reduction under atmospheric pressure.
Besides, the impact of total pressure on reaction performance was also studied since decreasing the total pressure theoretically favors the ZnO reduction reaction [
20,
29]. Experiments were carried out isothermally at reduced pressures (0.15 and 0.45 bar) and atmospheric pressure (0.90 bar) under batch operation. Thanks to the above reported results on the temperature influence, the temperature of 900 °C was chosen for this study in order to relieve the methane cracking issue. Similar to the temperature tests, a packed-bed of ZnO (2 g) was supplied, and 0.1 NL/min of methane flow rate was fed once the desired operating temperature was stable.
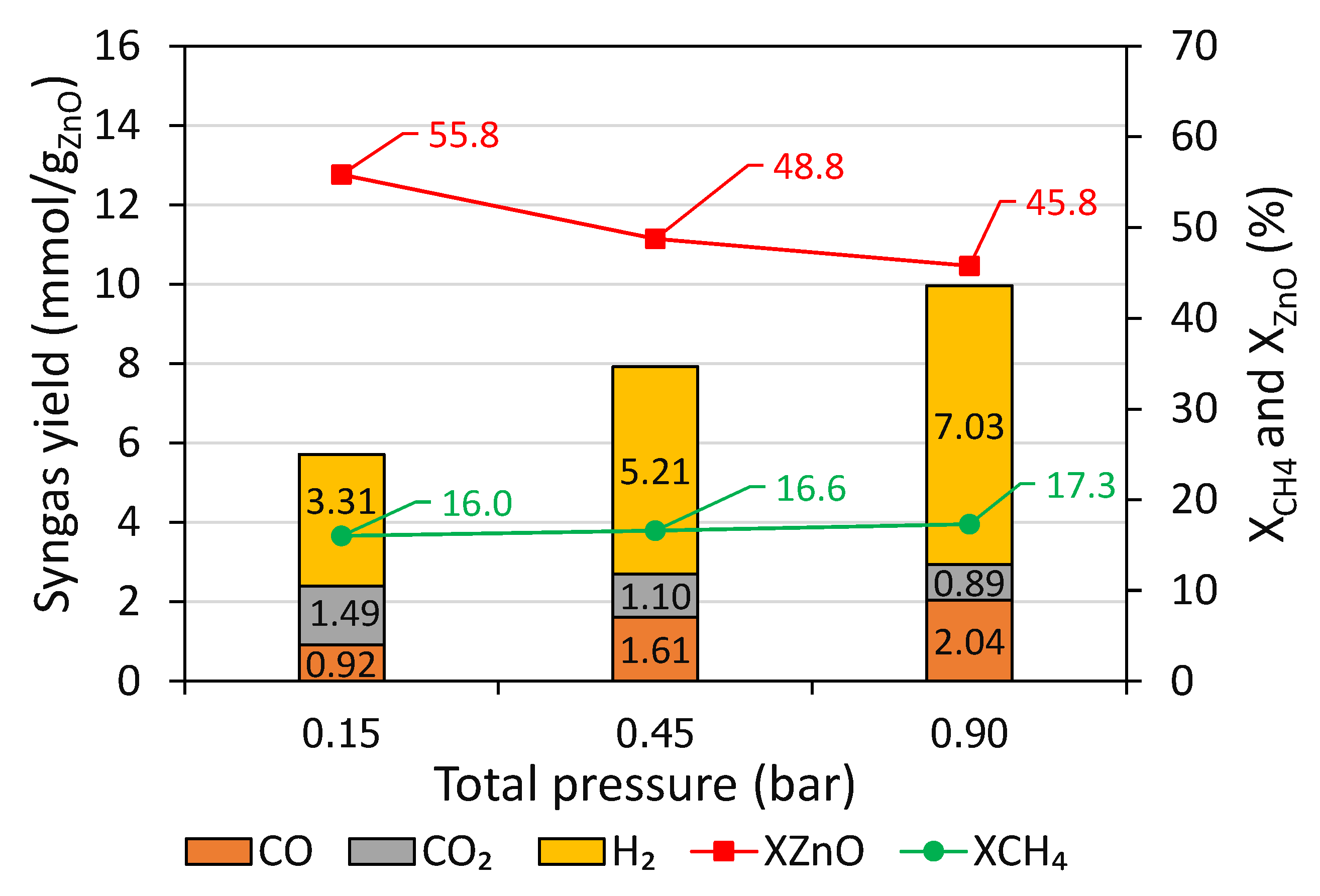
Figure 3 shows that CO and H
2 yields dropped with decreasing total pressure, from 2.04 and 7.03 mmol/g
ZnO at 0.90 bar to 0.92 and 3.31 mmol/g
ZnO at 0.15 bar, resulting in a H
2/CO ratio from 3.44 at 0.90 bar compared to 3.60 at 0.15 bar. In contrast, a considerable increase in the CO
2 yield when reducing the pressure was observed, from 0.89 mmol/g
ZnO at 0.90 bar to 1.49 mmol/g
ZnO at 0.15 bar. These variations can be attributed to the fact that as the pressure decreased, gas velocity increased (by a factor x6 when decreasing pressure from 0.90 to 0.15 bar), which led to a decrease in the gas residence time. Actually, decreasing pressure enhanced reduction extent (as reflected by the increase of X
ZnO) at the expense of decreased gas residence time. As a result, solid-gas reactions (Equations (2)–(7)), gas phase reactions (Equations (8)–(10)), and thermal methane dissociation (Equation (11)) were negatively affected (due to possible kinetic limitations), leading to decreased H
2 and CO and increased CO
2. The discharged oxygen from ZnO was, therefore, recovered rather in the form of CO
2 than CO, thereby explaining the increase of both CO
2 and X
ZnO. Since the oxygen supply remains identical because the same amount of ZnO is used in the tests at different pressures, the higher CO
2 yield observed when decreasing pressure may be the result of kinetic limitations. CO
2 is mainly formed via reaction of ZnO with CO (Equation (4)) and then the Boudouard equilibrium determines the CO and CO
2 proportion. Thus, the main reason for higher CO
2 yield is because the gas residence time is lowered when decreasing total pressure, which in turn limits the CO
2 conversion via the Boudouard reaction (Equation (7)).
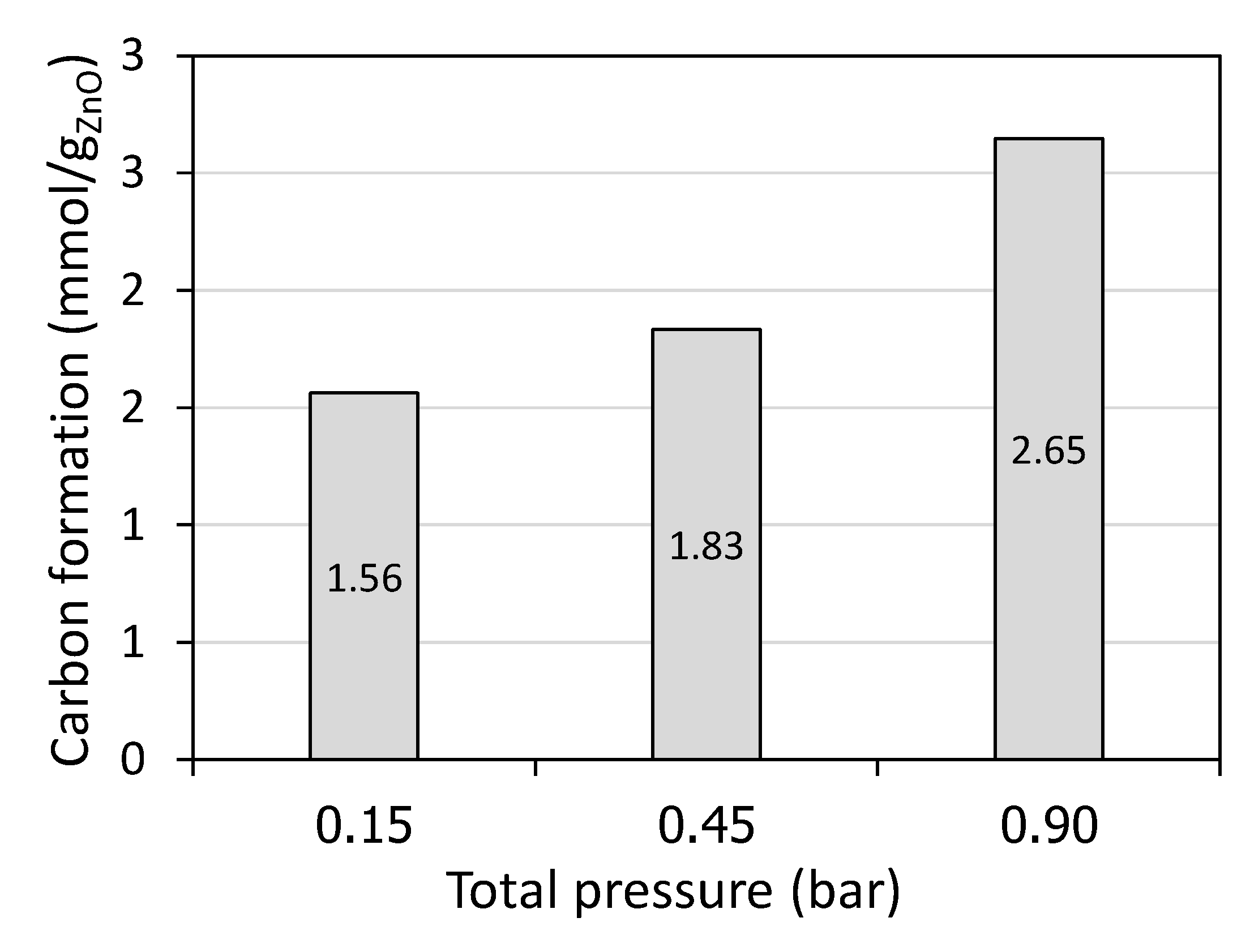
In addition, the drop in the X
CH4 with a decrease in the total pressure also resulted from the increase of the gas velocity (gas residence time decrease), in agreement with the decline of solid carbon formation (from 2.65 mmol/g
ZnO at 0.90 bar to 1.56 mmol/g
ZnO at 0.15 bar according to
Figure 4), thus pointing out the less favorable methane cracking reaction. Importantly, with diminishing the total pressure, X
ZnO was enhanced from 45.8% at 0.90 bar to 55.8% at 0.15 bar, revealing a positive impact of lowering the total pressure on net ZnO conversion, in agreement with both thermodynamics (production of Zn vapor and syngas is favored at low pressure [
29]) and previous studies on vacuum MgO carbothermal reduction [
30]. In short, decreasing the total pressure enhanced X
ZnO at the expense of lowered syngas yield but increased CO
2 yield. In addition, continuous operation involving a vacuum may be costly in terms of energy penalty and capital investment. With respect to these results from batch tests, a temperature at 950 °C and a medium pressure at 0.45 bar are advocated to both favor syngas yield and ZnO conversion and alleviate the side methane cracking reaction.
2.2. Continuous Operation
Methane-driven ZnO reduction was also performed with continuous ZnO injection to study both the feasibility in operating this process in a continuous mode and the influence of ZnO feeding rate on reaction performance. Experiments were conducted isothermally under atmospheric pressure (0.90 bar), and the proper temperature of 950 °C was selected to alleviate the methane cracking issue. In each test, 10 g of ZnO powder was supplied, and a constant CH
4/ZnO inlet molar ratio of 1.5 (50% of methane in excess) was fixed because the excess of reducer flow rate is necessary to favor ZnO conversion. The influence of ZnO feeding rates of 0.5 and 1.0 g/min was investigated.
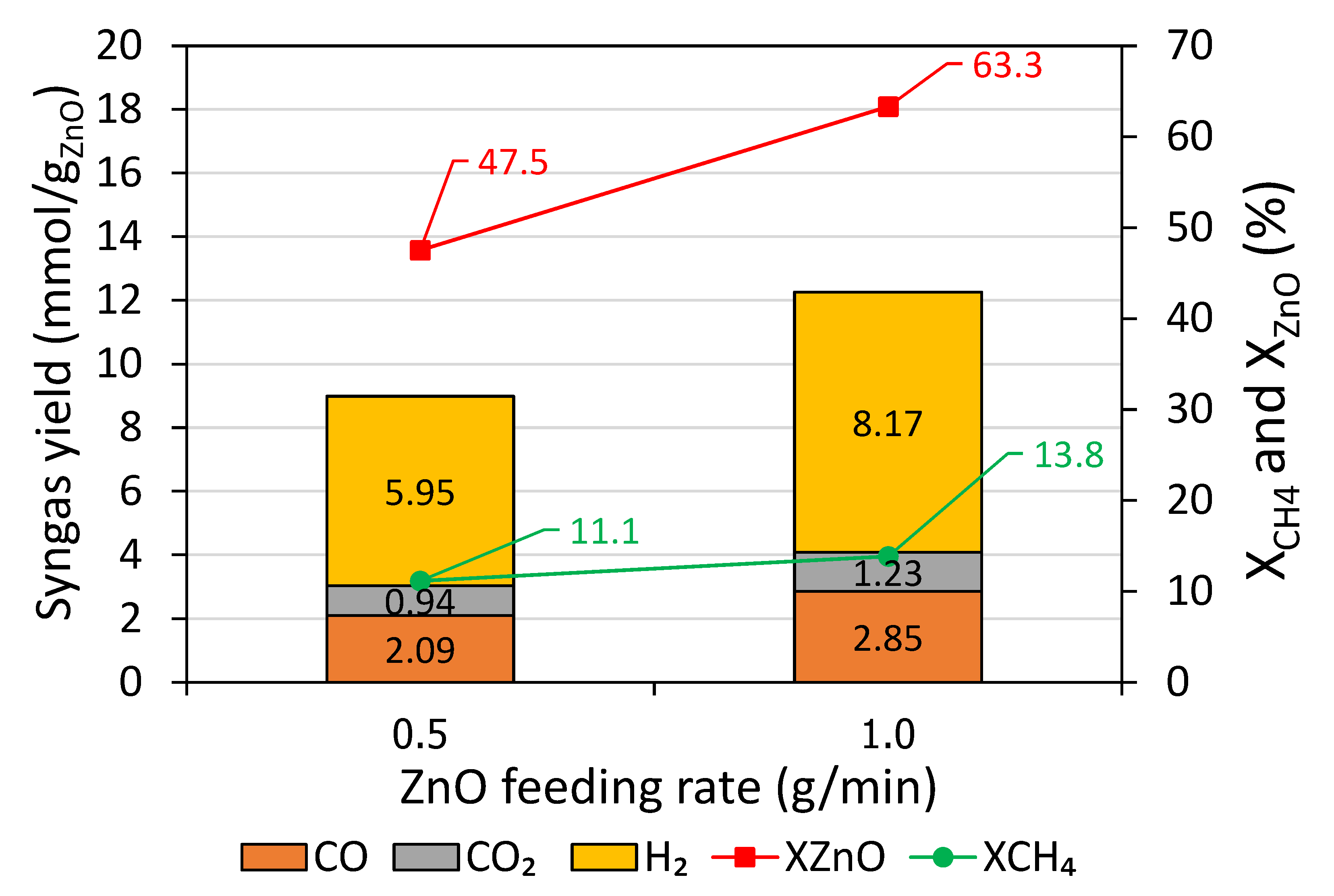
Figure 5 reveals that an increase in the ZnO feeding rate increased syngas yield. For example, CO, CO
2, and H
2 yields were 2.09, 0.94, and 5.95 mmol/g
ZnO at 0.5 g/min compared to 2.85, 1.23, and 8.17 mmol/g
ZnO at 1.0 g/min, resulting in the evolution of total syngas yield from 9.0 mmol/g
ZnO at 0.5 g/min to 12.3 mmol/g
ZnO at 1.0 g/min. Similarly, both X
CH4 and X
ZnO conversion rose significantly from 11.1 and 47.5% at 0.5 g/min to 13.8 and 63.3% at 1.0 g/min, and solid carbon formation also increased from 0.83 mmol/g
ZnO at 0.5 g/min to 1.89 mmol/g
ZnO at 1.0 g/min. This implied that an increase in the ZnO feeding rate promoted ZnO and CH
4 consumptions, leading to an enhancement in the evolved syngas, X
CH4, and X
ZnO. However, rising excessively the ZnO feeding rate would be detrimental because ZnO feeding rate could exceed ZnO consumption rate, which may result in adverse reaction performance and eventually lead to ZnO accumulation. Therefore, the optimal ZnO feeding rate with respect to the maximum X
ZnO conversion need to be further investigated. In short, increasing ZnO feeding rate under a constant over-stoichiometric CH
4/ZnO molar ratio promoted syngas yield, X
ZnO, as well as X
CH4, thanks to increased ZnO and CH
4 consumptions. With this solar reactor prototype, the feasibility of performing methane-driven ZnO reduction with continuous ZnO injection was established.
2.3. Comparison of Batch and Continuous Operation
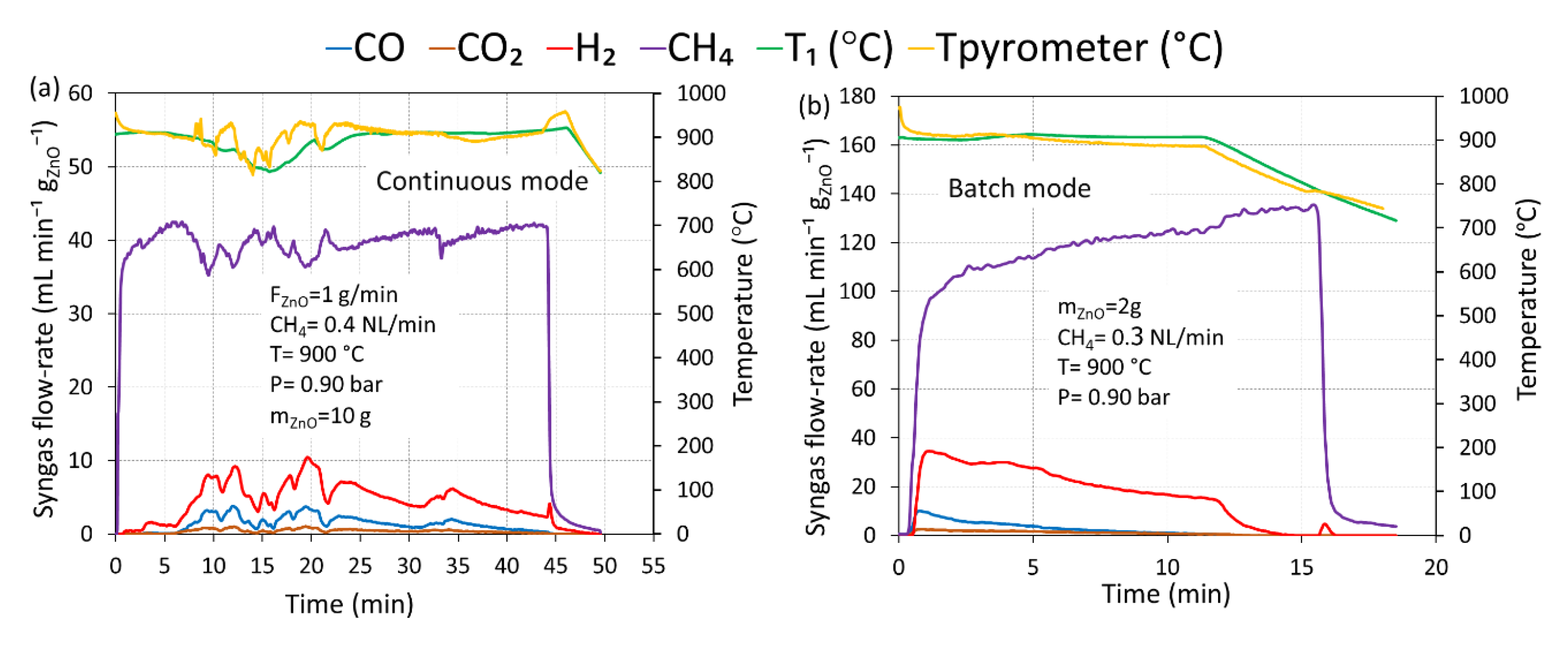
Figure 6 shows the representative syngas production rates’ evolution together with reactor temperatures for both continuous (
Figure 6a) and batch (
Figure 6b) operation at 900 °C under atmospheric pressure (0.90 bar). In this study, a packed bed of ZnO (2 g mass) reacted with a constant methane flow-rate of 0.3 NL/min for the batch test, while ZnO powder (10 g mass) was fed at 1 g/min to react with a methane flow-rate of 0.4 NL/min, resulting in a CH
4/ZnO molar ratio of 1.5, for the continuous test. During continuous operation, the syngas production rates fluctuated constantly and consistently for all the gas species, in response to a change in the reactor temperatures, caused by the varying solar power input resulting from clouds passage. This confirmed that temperature played an important role on the performance of ZnO reduction with methane. Note that only this experiment suffered from the cloud shadowing issue; however, the results can still be reliable and compared. During the initial period (0–5 min of duration), H
2 specie was produced without the presence of CO and CO
2 because of only CH
4 cracking reaction (ZnO was not yet injected and remained in the screw feeder pathway at that time). After that, CO, CO
2, and H
2 were formed once ZnO was injected in the cavity zone, and they reached their maximum level at around 20 min duration. In fact, the theoretical operating time should be approximately in the range 10–15 min (with respect to ZnO mass fed at 1 g/min); however, the actual operating time was above 45 min because of inconstant ZnO feeding rate caused by temporal ZnO feeding blockage and mainly low reaction kinetics (at 900 °C) that cause temporal ZnO accumulation, especially during the possible fall of the ZnO agglomerate in the reactor. Even though after 25 min duration the reactor temperatures remained stable and straightforward, syngas and methane rates evolution still fluctuated, thus denoting the unstable inlet ZnO feeding rate. ZnO powder seemed to be sticky with the screw feeder, which was likely the main cause of compressed ZnO remaining. In contrast to the continuous operation, CO, CO
2, and H
2 production rates in batch operation occurred as soon as methane was injected and reached the production peak simultaneously. They then decreased progressively and smoothly over the entire run duration, and no fluctuation of syngas production rate was observed, denoting a constant ZnO consumption rate and a stability of this system until reaching reaction completion. CO and CO
2 approached zero after 10 min, implying that the ZnO + CH
4 reaction terminated and complete ZnO conversion was reached. After that, H
2 alone was still being formed as a result of thermal methane dissociation reaction (Equation (11)). A sudden decrease in the reactor temperatures after 12 min was because of the shutter closed, which resulted in a sudden drop in the H
2 evolution (and concomitant CH
4 increase). In comparison, the syngas production rates in the continuous test were significantly lower than in the batch test due to low actual ZnO feeding rates (0.25–0.30 g/min). The temperatures given by the thermocouple inside the ZnO bed and by the pyrometer at the bed surface were in close agreement, which confirms the negligible temperature gradient across the bed.
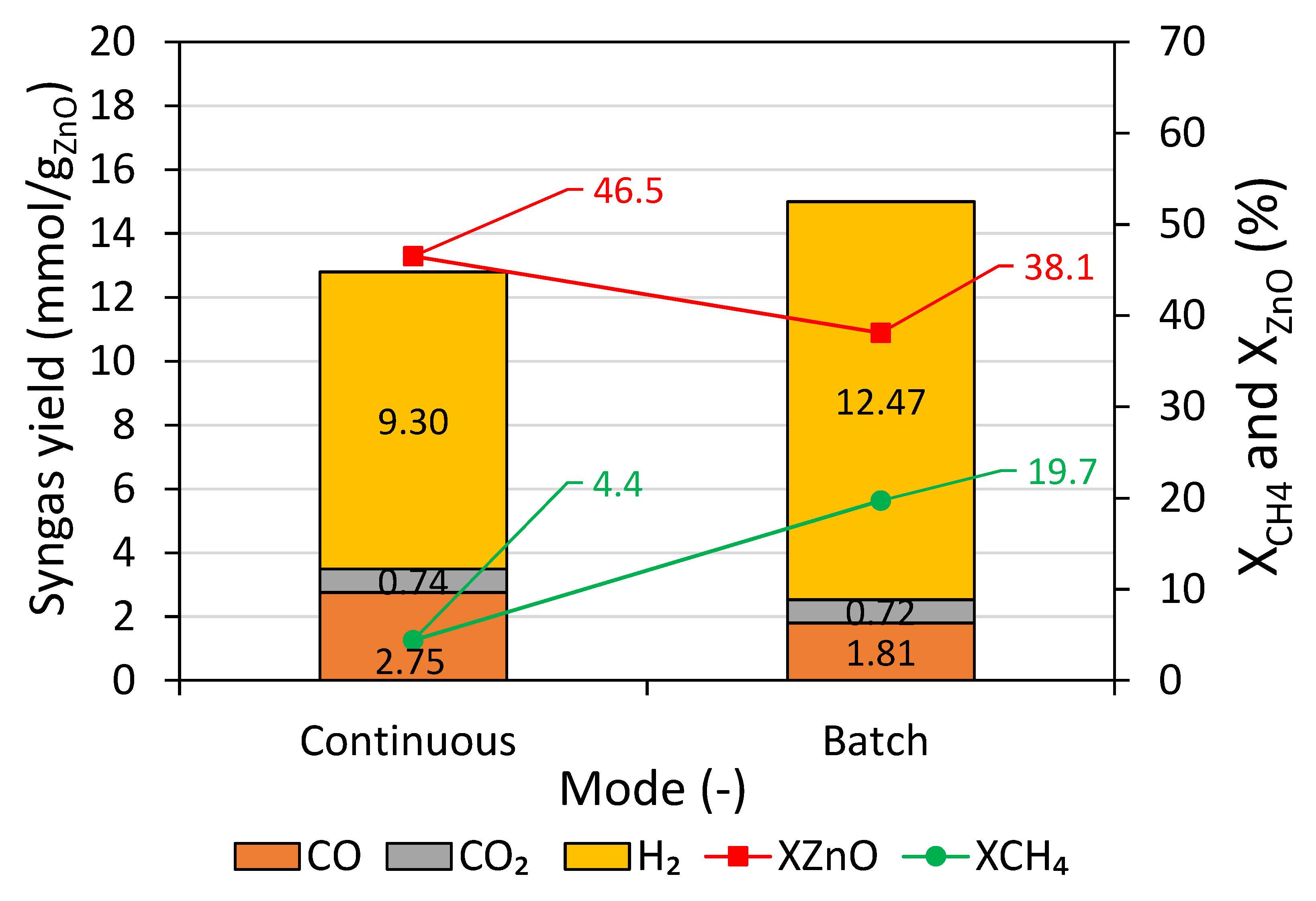
Figure 7 displays syngas yields determined from time integration of the syngas production rates (
Figure 6), along with X
CH4 and X
ZnO. It was found that the batch test resulted in stronger methane cracking, reflected by both higher H
2 yield (12.47 mmol/g
ZnO), solid carbon formation (8.86 mmol/g
ZnO), and X
CH4 (19.7%). A higher H
2 yield in a batch test led to higher total syngas yield. The continuous test resulted in more favorable ZnO + CH
4 reaction, as indicated by higher CO yield (2.75 mmol/g
ZnO) and X
ZnO (46.5%) and lesser methane cracking, reflected by much lower solid carbon formation (1.5 mmol/g
ZnO), possibly thanks to the low ZnO feeding rate and rapid conversion rate. It was possible that the lower X
ZnO in the batch test was due to a too high methane flow rate (0.3 NL/min) with respect to the amount of ZnO packed-bed. It is because when the methane supply rate was considerably higher than the oxygen discharge rate (from ZnO), soot or carbon deposition could fasten at ZnO surface and hinder the solid–gas reaction, leading to lower X
ZnO. Note that ZnO agglomerations remaining in the cavity were observed in both batch and continuous tests.
As seen in
Figure 8, ZnO was not completely reduced because the operating temperature was quite low (900 °C), and the process was conducted under atmospheric pressure (0.90 bar). The ZnO remaining in the batch test exhibited light yellow agglomerations in a spherical shape, possibly due to chemisorbed soot wrapping their surface, according to
Figure 8b. Note that ZnO remaining in light yellow was only observed when employing the inlet methane flow rate of 0.3 NL/min, presumably caused by a large excess in the inlet methane flow rate. In contrast, ZnO in the continuous test exhibited white compressed pieces resulting from transient sticky ZnO blockage in the screw feeder, according to
Figure 8a. In summary, at considered conditions, operating the process in a continuous mode showed greater performance with respect to higher X
ZnO and CO; however, the unstable inlet ZnO feeding rate remained a challenging issue, which should be further optimized. Higher H
2 exhibited in the batch mode was due to a large excess in the inlet methane flow, which favored methane cracking.
2.4. Characterization of Zn Products
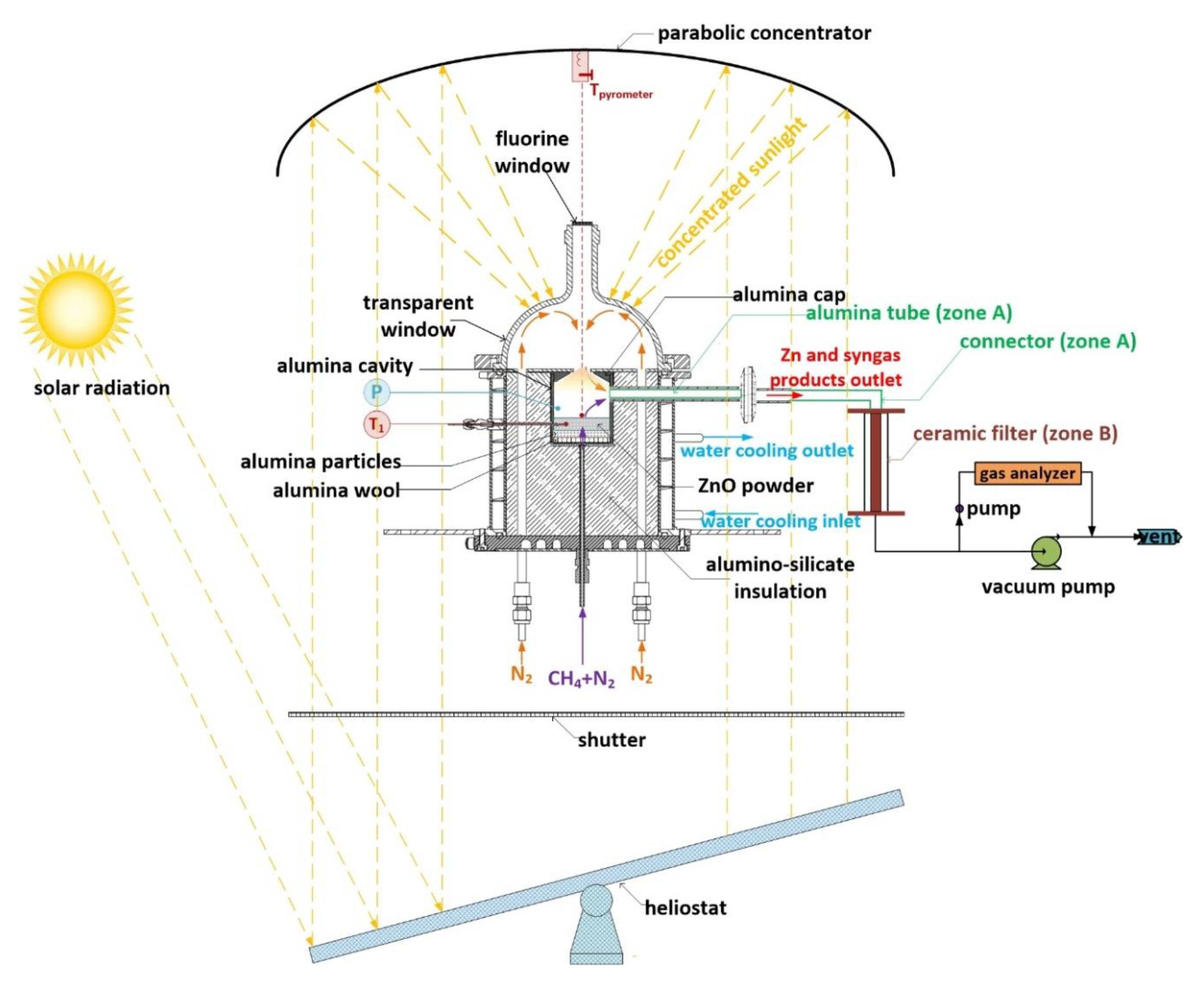
As shown in the next section (Materials and Methods), the outlet components were separated into two zones: (1) zone A represents products from the outlet alumina tube and connector, and (2) zone B represents products from the ceramic porous filter. Therefore, characterization of solid products collected from zones A and B was performed separately. In addition, their structure and morphology were also characterized via X-Ray Diffraction (XRD) and Field Emission Scanning Electron Microscopy (FESEM) analysis.
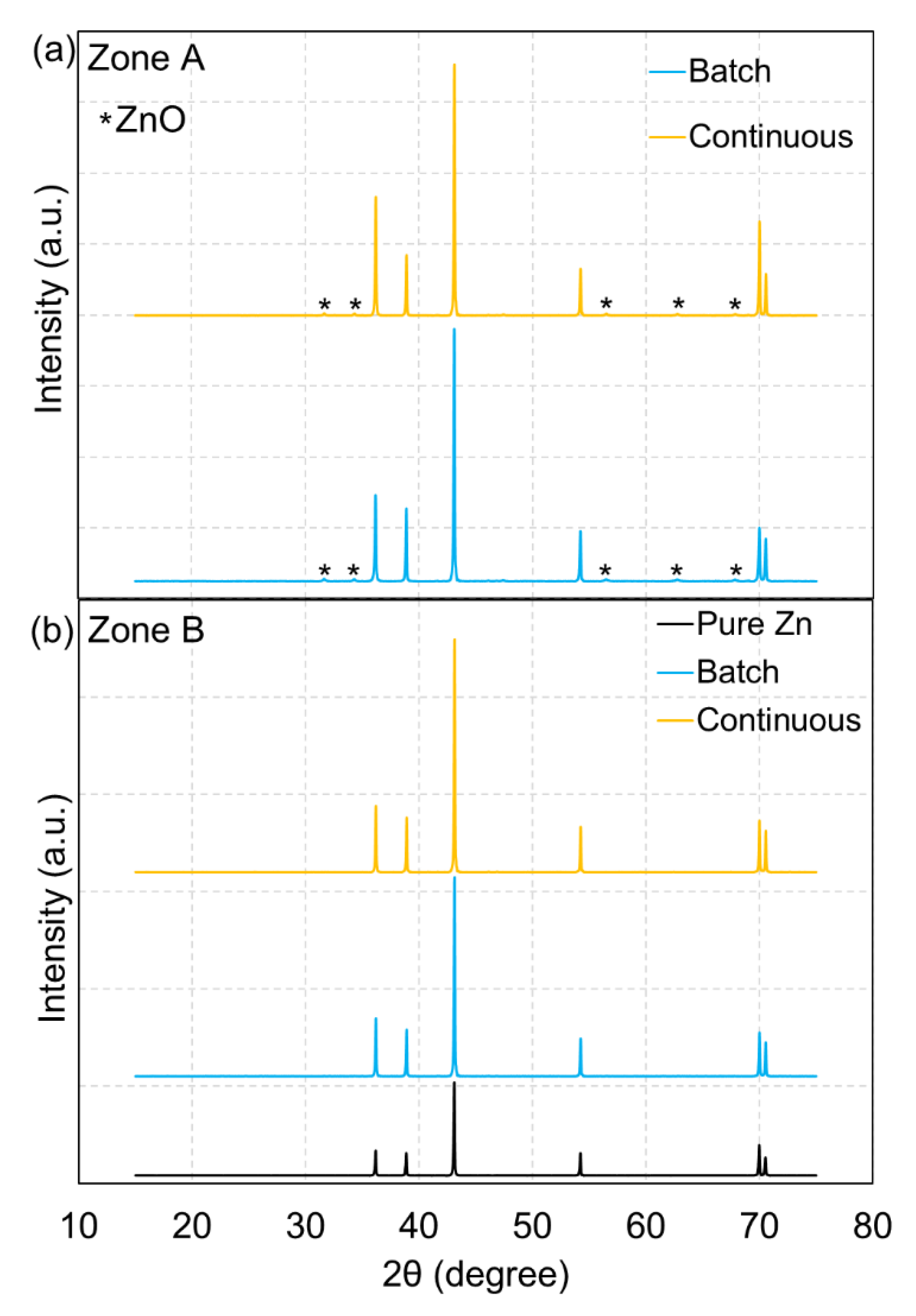
Figure 9 shows representative XRD patterns of the collected products in batch and continuous operation at 900 °C under atmospheric pressure (0.90 bar) compared with a pure Zn reference pattern (the results presented here correspond to the conditions shown in
Figure 6). As a result, clearly defined peaks with high intensity of Zn were revealed in both zone A and zone B, demonstrating high-purity Zn production (with hexagonal close packed crystal structure). However, a very small presence of ZnO was noticed at only zone A where Zn(g) starts to condense (Zn boiling point: 907 °C) (
Figure 9a) in both batch and continuous operation, confirming slight Zn recombination (with CO, CO
2, and H
2O), which was one of the reasons to explain incomplete X
ZnO. In case of ZnO accumulating in zone A in a continuous mode, the stability of the system may be affected due to a ZnO clogging issue. Indeed, this deposition issue may be a drawback when operating in continuous mode due to possible clogging after long processing period, which would require additional system optimization for reducing Zn condensation at the reactor outlet (e.g., quenching using additional inert gas flow at the outlet along the wall to favor condensed products entrainment to the filtering system). After flowing in zone A at the reactor outlet, Zn product particles were then transported with both syngas and inert carrier gas toward the filtering unit (zone B in which temperature was lower than zone A), thereby yielding mainly pure Zn powder (
Figure 9b). In addition, no impact of the operating mode on solid products composition was observed. The mean crystallite size of the solid Zn products in both zone A and zone B was determined using Scherrer’s equation. The largest crystallite sizes of Zn were obtained in zone A in the range of 31–56 nm, followed by zone B in the range of 26–43 nm.
Figure 10 shows FESEM micrographs of the solid products at the reactor outlet (zone A) and ceramic filter (zone B), in which batch operation (
Figure 10a,b) was compared to continuous one (
Figure 10c,d). Overall, FESEM results show a clear microstructure of Zn product and particle size appeared to be microscale. Concerning batch operation, at zone A (
Figure 10a), condensed Zn product grew in plane surfaces as it was in contact and deposed on the inner surface of outlet tube wall. At zone B (
Figure 10b), condensed Zn particles displayed singly scattered hexagonal droplets (surrounded with dispersed soot), in agreement with the Zn crystal structure. Similar to batch operation, condensed Zn obtained from continuous test featured mainly scattered hexagonal grains in a smooth background of soot in both zone A (
Figure 10c) and zone B (
Figure 10d). In comparison, some particle sizes of the Zn product from continuous operation appeared to be bigger than in batch, and fine soot particles wrapping Zn product in continuous mode were less pronounced. The produced Zn metal particles can be further re-oxidized with H
2O or CO
2 in an exothermic reaction to produce pure H
2 or CO by chemical-looping [
31]. According to the study of reactivity of produced Zn via thermogravimetry analysis (TGA) [
29], reoxidation of produced Zn with H
2O/CO
2 was complete (the produced Zn was completely converted back to ZnO), demonstrating that the Zn product can be used as oxygen carrier for the solar chemical-looping reforming of methane. Thus, complete system regeneration via chemical-looping (methane reforming and H
2O/CO
2 splitting) can be achieved.